Abstract
Acquired immune deficiency syndrome (AIDS) is an unadorned disease affected via the human immunodeficiency virus (HIV), which has become the most infectious diseases worldwide. HIV-1 RT has been shown to be present in the cardiac tissue of patients with HIV-associated infective endocarditis, and to be associated with the development of valvular lesions and other cardiac abnormalities. The use of anti-retroviral therapies has helped to control the virus and reduce the incidence of HIV-1 associated infective endocarditis. Though, these treatments have several adjacent effects, and the improvement of drug-resistant stresses of the virus has become a significant challenge in HIV treatment. This study is to identify A. lebbeck phytoconstituents with HIV-1 RT inhibitory activity for potential therapeutic use against HIV-1 RT associated with infective endocarditis. We performed in silico and in vitro screening of natural cardiovascular phytoconstituents from Albizia lebbeck, a medicinal plant that has been traditionally used for the management of numerous diseases. The in silico results showed that all three compounds (geraldone, luteolin, and isookanin) exhibited affinities of solid binidng to the active amino acids of HIV-1 RT's DNA-polymerase (DNA-p) and Ribonuclease-H (RNA-H) active positions, suggesting their potential as HIV-1 RT inhibitors. In vitro assessment of the three compounds at a concentration of 1 mg/mL revealed that Geraldone exhibited the most effective inhibitory consequence on HIV-1 RT activity (83.45%), followed by Isookanin (75.88%) and Luteolin (66.36%). These findings suggest that these compounds have the potential to inhibit HIV-1 RT associated with infective endocarditis and could assist as main compounds for emerging unique anti-HIV-1 agents. Further studies are needed to confirm the in vitro and in vivo efficacy of these molecules and assess their safety and efficiency as anti-HIV-1 drugs.
Keywords: Albizia lebbeck, HIV-1 reverse transcriptase, ADMET analysis, Geraldone, Isookanin, Luteolin, Infective endocarditis
1. Introduction
HIV remains an important global health problem, with millions of people affected worldwide. HIV-1, the most common strain, is characterized by its ability to rapidly replicate and mutate, leading to the development of drug resistance and challenges in treatment (UNAIDS, 2021). Infective endocarditis is a serious and potentially fatal infection of the heart valves and endocardium, which can be caused by a variety of microorganisms, including bacteria and viruses (Pannecouque et al., 2020). Human immunodeficiency virus (HIV) infection is a known risk factor for infective endocarditis, and HIV-associated infective endocarditis is associated with higher rates of morbidity and mortality than non-HIV-associated infective endocarditis (Macingwane and Ngwenya, 2021). The HIV-1 reverse transcriptase (RT) enzyme plays a critical role in the replication of the virus and has been identified as a potential target for the development of antiretroviral drugs (Yadav et al., 2021). The exploration of natural components from medicinal plants as potential therapeutics for HIV-1 has gained significant attention in recent years. Usual yields have remained a rich source of novel chemical entities with diverse biological activities, including antiviral properties (Noreen et al., 2021).
Albizia lebbeck, commonly known as the “Siris tree,” is a medicinal plant with a long history of traditional use in various indigenous systems of medicine (Nair et al., 2005). It has been employed for the treatment of diverse ailments, including respiratory disorders, gastrointestinal disorders, and skin infections (Bahrami et al., 2017). A. lebbeck has been demonstrated to contain various phytochemical constituents, as flavonoids, alkaloids, phenolics, and saponins, which have demonstrated antiviral activities against different viruses (Bahrami et al., 2017). Current studies have also established the cardiovascular and antiviral activity of A. lebbeck in contradiction of influenza A virus (Liu, 2021) and herpes simplex virus type 1 (Gao et al., 2021), further supporting its potential as a source of novel compounds with antiviral properties. Given the plant's rich history of traditional use and its demonstrated antiviral properties, A. lebbeck presents a promising candidate for investigating its potential as a source of natural compounds for the development of novel anti-HIV therapeutics. Therefore, selecting A. lebbeck as the focus of this study is based on its potential to provide new insights into the development of effective treatments for HIV/AIDS. Moreover, A. lebbeck is widely distributed throughout the tropics and easily accessible, making it a suitable candidate for further investigation of its potential as a source of natural cardiovascular phytoconstituents for the growth of novel anti-HIV molecules.
In silico approaches, including molecular docking, have emerged as powerful tools for predicting the binding affinity and stability of potential drug candidates with target proteins. These computational methods enable the screening of a huge sum of compounds in a time- and cost-effective manner, facilitating the identification of promising leads for further experimental validation (Noreen et al., 2021). By using in silico approaches, researchers can predict the binding affinity and stability of potential drug candidates with target proteins, allowing them to prioritize compounds with the greatest potential for further development. In addition to enabling the identification of new drug candidates, in silico approaches can also be used to optimize the properties of existing compounds, such as improving their pharmacokinetic properties or reducing their toxicity. Developing effective therapeutics that can inhibit the activity of this enzyme is a critical objective in HIV/AIDS research (Noreen et al., 2021). This study aimed to identify phytoconstituents from A. lebbeck that have inhibitory activity against HIV-1 RT associated with infective endocarditis using both in silico and in vitro methods.
2. Materials and methods
2.1. Analysis of the phytochemical profile
Various phytochemicals (Table 1) such as “flavonoids (geraldone, luteolin, and isookanin), saponins (budmunchiamine, N-dimethyl budmunchiamine), alkaloids (2-one-3, 3-dimethyl-4-(1-aminoethyl) azetidin, 2, 4-bis(hydroxylamino)-5-nitropyrimidine), and tannins (leucocyanidin, melacacidin, lebbecacicidin)” present in A. lebbeck remained retrieved as of PubChem “(https://pubchem.ncbi.nlm.nih.gov)” and its 3D structures remained attained. Information about these compounds and their biological effects is freely available on PubChem.
Table 1.
List of Phytocompounds from A. lebbeck.
| Sl.No | Phytochemical | Compounds with chemical formula | PubChem CID | Structure |
|---|---|---|---|---|
| 1 | Flavonoids | Geraldone (C16H12O5) |
5281618 | 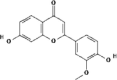 |
| 2 | Luteolin (C15H10O6) |
5280445 |  |
|
| 3 | Isookanin (C15H12O6) |
91196552 |  |
|
| 4 | Saponins | Budmunchiamine (C27H56N4O) | 373662 | 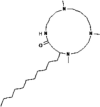 |
| 5 | Albigenin (C29H46O2) |
101280261 | 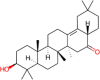 |
|
| 6 | Alkaloids | 2-one-3,3-dimethyl-4-(1-aminoethyl) Azetidin (C7H14N2O) |
541624 | 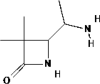 |
| 7 | 2, 4-Bis(hydroxylamino)-5-nitropyrimidine) (C4H5N5O40) | 5364111 | 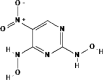 |
|
| 8 | Tannins | Leucocyanidin (C15H14O7) | 71629 | 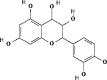 |
| 9 | Rhodiooctanoside (C19H36O10) | 10251759 |  |
|
| 10 | Vicenin-2 (C27H30O15) | 442664 | 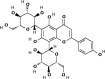 |
For reference values, nevirapine, an initial inhibitor, remained re-docked to the DNA-polymerase spot of HIV-1 RT. In silico molecular docking investigations were performed on compounds that met Lipinski's criteria and remained expected to possess great gastrointestinal absorption and non-toxicity. The phytochemical ligands were subjected to energy minimization using the BIOVIA Discovery Studio 2020 program (BIOVIA, San Diego, CA, USA) and then docked in contradiction of HIV-1 RT using AutoDock 4.2 (Morris, 2009).
2.2. Pharmacokinetic and pharmacodynamics properties
To expect the pharmacokinetic and pharmacodynamic possessions of the identified phytoconstituents, Lipinski's Rule of Five and toxicity parameters evaluation were performed (Lipinski, 2000, Daina et al., 2017). Lipinski's Rule of Five is an extensively used parameter for drug-like molecules that states that a molecule is likely to possess good oral bioavailability and permeability if it chances the subsequent criteria: “molecular weight ≤ 500, calculated logP ≤ 5, number of hydrogen bond donors ≤ 5, and number of hydrogen bond acceptors ≤ 10”. ADMET parameters including “absorption, distribution, metabolism, excretion, and toxicity” remained assessed by the SwissADME web tool (Daina et al., 2017). In addition to identifying potential drug candidates, it is essential to evaluate ADMET possessions to ensure their safety and efficacy as drug molecules. The results of these evaluations will aid in the assortment of appropriate phytoconstitutents for further investigation as potential therapeutics against HIV-1 RT.
2.3. Preparation of HIV-1 RT structure
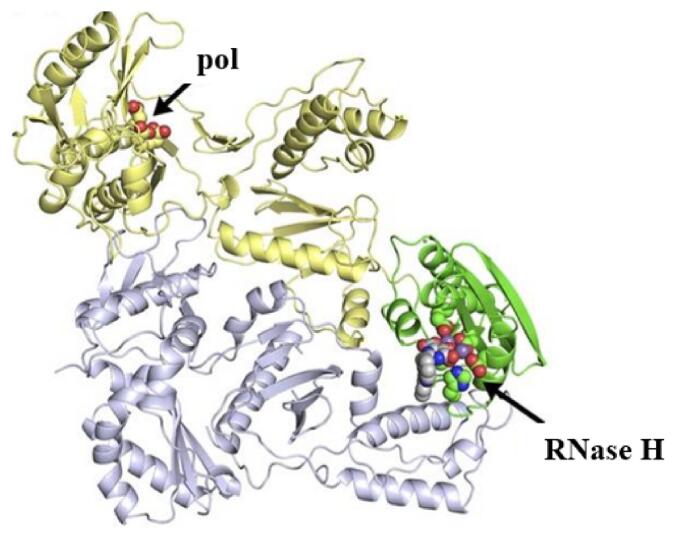
The crystal structure of HIV-1 RT (PDB: 3QIP) was retrieved from the Protein Data Bank (PDB) and prepared for molecular docking studies using AutoDockTools 1.5.6 (Morris, 2009) (Fig. 1). The protein structure was processed by removing water particles then ligands, addition polar hydrogen molecules, assigning Gasteiger charges, and merging non-polar hydrogens. The protein remained then saved in the PDBQT format and used for molecular docking studies. The Protein Data Bank is an extensively used resource for retrieving protein structures (Berman, 2000), and AutoDockTools were used software package for molecular docking studies.
Fig. 1.
Ribbon Diagram of HIV-1 Reverse Transcriptase with Nevirapine and RNA-H Inhibitor (PDB: 3QIP). The p51 subunit is depicted in grey, while the palm, finger, thumb, and connecting subdomains of the p66 subunit are illustrated in yellow. Additionally, the Ribonuclease-H domain is illustrated in green.
2.4. Molecular docking analysis
Molecular docking study was achieved to examine the binding interactions of the phytocompounds of A. lebbeck, p4Y, and nevirapine with HIV-1 RT. The PDBQT files of the protein and ligands were generated using AutoDock 4.2 (Morris, 2009, Mamidala et al., 2022), and the docking simulation was carried out for the DNA-p and RNA-H active locations of HIV-1 RT. The grid boxes and grid centers for the active sites were adjusted to optimize the docking results (Trott and Olson, 2010). The resulting docked structures remained evaluated by the Discovery Studio Visualizer to identify hydrogen bond interactions among the ligands in addition the protein. The use of molecular docking analysis provides a valuable tool for identifying potential lead compounds for drug development against HIV-1 RT (Kitchen, 2020, Kumar et al., 2020).
2.5. HIV-1 reverse transcriptase inhibitor screening
We obtained Nevirapine, Geraldone, Luteolin, and Isookanin from Sigma-Aldrich. All of the test molecules was dissolved in DMSO, and Nevirapine was adjusted to a 200 µM concentration while the compounds were dissolved at a concentration of 1 mg/mL. Using an HIV-1 RT test, colorimetric method, the phytochemicals at 1 mg/mL was evaluated for HIV-1 RT inhibitor in accordance with the manufacturer's instructions. By means of a positive control, Nevirapine, an HIV-1 RT inhibitor, was utilised. The chosen vehicle control was determined to be DMSO (1%, v/v). Using an ELISA plate reader, the colour responses were monitored at 405 and 490 nm.
2.6. Statistical analysis
The mean and standard deviation (SD) of the tentative study performed in triplicate were used to express all data. One-way ANOVA and Dunnett's test were used to analyse the statistical significance using Microsoft Excel. It was deemed significant at p < 0.05.
3. Results
3.1. Evaluation pharmacokinetic and pharmacodynamics properties
The assessment of drug likeness property and toxicity parameters for the chosen A. lebbeck compounds was presented in Fig. 2. All of the compounds underwent drug-likeness evaluations and 10 compounds were selected for further analysis. Among the 10 compounds, 6 satisfied Lipinski's criteria, 7 remained predicted to possess great gastrointestinal (GI) absorption, 9 had no AMES toxicity, and 8 had no hepatotoxicity. Based on the in silico results presented in Fig. 2, three compounds (Geraldone, Luteolin, and Isookanin) met Lipinski's criteria and exhibited high absorption of GI, no AMES toxicity, and no hepatotoxicity. To investigate the potential of these three molecules as HIV-1 RT inhibitors, molecular docking simulations were performed.
Fig. 2.
Overlap of Drug-Likeness Criteria for A. lebbeck Compounds: A Venn Diagram Analysis of High Absorption of GI, No AMES Toxicity, No Hepatotoxicity, and Lipinski's Rule Acceptability.
3.2. Binding interactions of phytochemicals against HIV-1 RT DNA-p active site
HIV-1 RT is an attractive pharmacological object for inhibiting the HIV-1 life cycle, and it performs several functions, comprising DNA-p and RNA-H. Current work concentrated on the two active positions of HIV-1 RT, namely the DNA-p and RNA-H domains. The crystal structure of HIV-1 RT co-crystallized by Nevirapine remained analyzed for molecular docking at the DNA polymerase (DNA-p) domain, and Nevirapine was re-docked into the active site to establish reference standards for data elucidation.
The re-docking outcomes presented that Nevirapine bound to the DNA polymerase location through a binding energy of −9.32 kcal/mol. Table 2 shows that three compounds from A. lebbeck, namely Geraldone (–7.84 kcal/mol), Luteolin (–7.4 kcal/mol), and Isookanin (–7.81 kcal/mol), exhibited slightly higher binding energies toward the DNA polymerase position than Nevirapine. These compounds remained docked in the identical pocket as the standard inhibitor, as shown by the ligand-receptor interfaces.
Table 2.
Impact of Molecular Docking of A. lebbeck Phytochemicals on the Active Site of HIV-1 RT DNA-p.
| Sl.No | Compounds | Binding Energy (kcal/mol) | Inhibition constant (nM) | Interactive Amino acid residues |
|---|---|---|---|---|
| 1 | Geraldone | −7.84 | 1.8 | Val179; Leu100; Trp229; Tyr181; Pro95; Leu234; Lys101; Lys103; Lys102; Pro236; Tyr318; Val106; His235; Phe227; Tyr188 |
| 2 | Luteolin | −7.4 | 3.74 | Pro95; Tyr181; Leu100; Lys101; Lys102; Pro236; Tyr318; Lys103; Val106; His235; Leu234; Phe227; Tyr188; Trp229 |
| 3 | Isookanin | −7.81 | 1.9 | Pro95; Trp229; Tyr181; Leu234; Tyr188; Phe227; His235; Val106; Tyr318; Leu100; Pro236; Lys103; Lys102; Lys101 |
| 4 | Nevirapine (original inhibitor) | −9.32 | 148.19 | Lys102; Tyr318; Pro236; His235; Phe227, Leu100; Trp229; Tyr188; Leu234; Tyr181; Val189; Gly190; Lys103; Val106; Val179; Lys101 |
Fig. 3 indicates that the three compounds adopted a binding pattern similar to that of Nevirapine, with Geraldone located in the hydrophobic region and forming π-π stacking interactions with aromatic residues such as Val179, Leu100, Trp229, Tyr181, Pro95, Leu234, Lys101, Lys103, Lys102, Pro236, Tyr318, Val106, His235, and Phe227.
Fig. 3.
2D and 3D Visualization of Ligand-Protein Interactions at the Active Position of HIV-1 RT (PDB: 3QIP) DNA-p for Geraldone, Luteolin, Isookanin, and Nevirapine: A Schematic Illustration of Sticks, Balls, and Hydrogen/Hydrophobic Bonds.
The Isookanin compound exhibited H-bond contacts with Lys101 and Lys103 at distances of 5.49 Å and 3.79 Å, respectively, while Luteolin formed hydrogen–bond interactions with Lys101 at a distance of 5.70 Å, as shown in Fig. 3. These results were consistent with earlier studies (Srivastava, 2013) and demonstrated the favorable positioning of hydrogen bond donor atoms in the vicinity of Lys101′s C O, in accordance with pharmacophore models. Additionally, the hydrogen bond formed between Lys101′s C O and the right linker atom indicated a favorable location for hydrogen bonding, which supported the outcomes of the pharmacophore models.
The docking energies of Geraldone, Luteolin, and Isookanin were −7.84, −7.4, and −7.81 kcal/mol, respectively, which suggests that these compounds have a strong binding attraction to the position of HIV-1 RT DNA-p active.
3.3. Binding interactions of phytochemicals against RNA-H active position of HIV-1 RT
P4Y was re-docked into the binding site of the unique inhibitor for the RNA-H domain of HIV-1 RT. 5.08 kcal/mol was the binding energy among P4Y and the RNA-H. Notably, all three potential entities from A. lebbeck exhibited slightly lower binding energies to the Ribonuclease-H domain than the unique inhibitor. Geraldone displayed the highest binding affinity toward the RNA-H active position by a binding energy of −5.53 kcal/mol, monitored by Isookanin (−5.43 kcal/mol) and Luteolin (−5.17 kcal/mol), as shown in Table 3. These potential phytochemicals were found to dock in the identical pocket as the standard inhibitor based on the ligand-receptor interfaces shown in Fig. 4. Additionally, they interacted with specific of the amino acids present in the P4Y complex by HIV-1 RT.
Table 3.
Effect of molecular docking of phytochemicals from A. lebbeck upon the HIV-1 RNA-H Active Site.
| Sl.No | Compound | Binding Energy (kcal/mol) | Inhibition constant (nM) | Interactive Amino acid residues |
|---|---|---|---|---|
| 1 | Geraldone | −5.53 | 88.09 | Tyr501, Ser499, Glu378, Asn474, Gly444, Ala445, Asp549, Ser553, His539, Asp443, Asp498, Pro537, Val536, Trp535, Gln500 |
| 2 | Luteolin | −5.17 | 163.06 | Asp549, His539, Asp443, Ala538, Pro537, Ser553, Ala445, Ala446, Gly444, Thr477, Glu478, Asn474, Ser499, Asp498, Gln500, Trp535 |
| 3 | Isookanin | −5.43 | 103.9 | Asp498, Gln500, Trp535, Ala538, Pro537, Pro537, Lys540, Ser499, His539 |
| 4 | P4Y (original inhibitor) |
−5.08 | 190.17 | Asp549, Asp443, Asp498, Trp535, Gln500, Ala538, Ser499, Pro537, Try501, Lys540, His539 |
Fig. 4.
2D and 3D Visualization of Ligand-Protein Interactions at the RNA-H Active Position of HIV-1 RT (PDB: 3QIP) for Geraldone, Luteolin, Isookanin, and Nevirapine: A Schematic Illustration of Sticks, Balls, and Hydrogen/Hydrophobic Bonds.
Fig. 4 illustrates the van der Waals and hydrogen bonds interactions that the Geraldone molecule produced with the residues Gln500, Asn474, and Asp549 as well as Tyr501, Ser499, Glu378, Gly444, Ala445, Ser553, His539, Asp443, Asp498, Pro537, Val536 and Trp535. With the residues Asp549, Gly444, Ala538, Trp535 and Asn474, the Luteolin molecule established hydrogen bonds. Other hydrophobic contacts were made with His539, Asp443, Pro537, Ser553, Aala445, Ala446, Thr477, Glu478, Ser499, Asp498 and Gln500. As part of the first inhibitor P4Y contact, the Isookanin molecule also established hydrogen bonds with the residues Asp498, Ala538, Lys540, and His539.
These three putative ligands in A. lebbeck, and our in silico molecular docking results suggested that they may block HIV-1 RT at the active sites of the DNA-p and RNA-H.
3.4. HIV-1 RT inhibitor screening
The three compounds were examined at a concentration of 1 mg/mL to screen for in vitro inhibitory action of HIV-1 RT. Compared to DMSO, all over the drugs significantly inhibited HIV-1 RT activity. Geraldone had the strongest HIV-1 RT inhibitory effect of the three drugs, at 83.45 ± 1.89%, followed by Isookanin (75.8 ± 2.8%) and Luteolin (66.36 ± 2.03%). Nevirapine (200 µM), the positive control, demonstrated an inhibition of 95.77 ± 2.56% (Fig. 5).
Fig. 5.
Inhibitory activities of A. lebbeck on HIV-1 RT.
4. Discussion
The HIV-1 RT is a key pharmacological object for inhibiting the HIV-1 life cycle, and it performs several functions, with Ribonuclease-H activities and DNA polymerase. This investigation concentrated on the two active positions of HIV-1 RT, specifically the DNA-p and RNA-H. The molecular docking results showed that three compounds from A. lebbeck, namely Geraldone, Luteolin, and Isookanin, exhibited slightly higher binding energies toward the DNA polymerase position than Nevirapine. In particular, Geraldone showed the highest binding affinity (−7.84 kcal/mol) among the three compounds. The binding pattern of these compounds was similar to that of Nevirapine, with Geraldone located in the hydrophobic region and forming π-π stacking interactions with several aromatic residues such as Val179, Leu100, Trp229, Tyr181, Pro95, Leu234, Lys101, Lys103, Kys102, Pro236, Tyr318, Val106, His235, and Phe227.
These outcomes are steady with erstwhile outcomes that have identified A. lebbeck as a potential source of anti-HIV-1 agents. For instance, a recent study reported that A. lebbeck extracts showed potent inhibitory efficacies in contradiction of together wild-type and drug-resistant HIV-1 strains associated with infective endocarditis (Kumar, 2021, Gujjeti et al., 2014, Mamidala et al., 2020, Nair, 2013). Moreover, several bioactive compounds isolated from A. lebbeck, including Geraldone, Luteolin, and Isookanin, have been shown to possess anti-HIV-1 activities (Chattopadhyay, 2013, Nair, 2013, Davella and Mamidala, 2021, Kumar et al., 2020). The present study affords molecular understandings into the binding modes and interactions of A. lebbeck compounds with the DNA-p of HIV-1 RT. These findings may facilitate the expansion of novel anti-HIV-1 molecules and contribute to the discovery of new drug leads from natural sources.
The present study investigated the potential of three compounds, namely Geraldone, Isookanin, and Luteolin, from A. lebbeck as inhibitors of HIV-1 RT at the active positions of the DNA polymerase and Ribonuclease-H domains. Molecular docking was performed to analyze their binding energies and interactions through the RNA-H domain of HIV-1 RT. The results showed that Geraldone had the highest binding affinity to the RNA-H active spot with a binding energy of −5.53 kcal/mol, followed by Isookanin and Luteolin. These potential phytochemicals were found to dock in the similar pocket as the standard inhibitor and interacted with specific of the amino acid residues present in the P4Y complex with HIV-1 RT. Additionally, the three compounds exhibited slightly lower binding energies to the RNA-H than the unique inhibitor. These findings suggest that these compounds from A. lebbeck may have the potential to inhibit HIV-1 RT at the active positions of the DNA polymerase and RNA-H. The exploration affords molecular insights into the prospective use of A. lebbeck constituents as anti-HIV-1 agents associated with infective endocarditis and may contribute to the discovery of new drug leads from natural sources.
5. Conclusions
In conclusion, the molecular docking study showed that three compounds, Geraldone, Isookanin, and Luteolin, of A. lebbeck, exhibited high binding affinities to the active sites of the RNA-H and DNA-p of HIV-1 RT. Notably, Geraldone showed the highest binding affinity to the ribonuclease-H active site. Geraldone, Isookanin, and Luteolin, showed potent in vitro inhibitory activity in contradiction of HIV-1 RT with Geraldone demonstrating the strongest effect, indicating their potential as lead molecules on behalf of anti-HIV-1 associated with infective endocarditis drug development. The study affords important insights into the potential use of A. lebbeck as a natural source for developing new drugs against HIV-1 associated with infective endocarditis. However, further experimental validation is required to check the inhibitory activity of these molecules and to determine their safety and efficacy.
Declaration of Competing Interest
The author declares that she has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The author extends her appreciation to the Deputyship for Research and Innovation,Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-017-2).
Footnotes
Peer review under responsibility of King Saud University.
References
- Bahrami G., Mohammadi S.A., Rahimi-Nasrabadi M. Albizia lebbeck: a short review on chemical constituents and therapeutic properties. Pharmacogn Rev. 2017;11(22):143–152. [Google Scholar]
- Berman H.M. The protein data bank. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassault Systèmes BIOVIA, San Diego, CA, USA. Discovery Studio Visualizer. Retrieved from https://discover.3ds.com/discovery-studio-visualizer-download.
- Chattopadhyay D. A review on the phytochemistry, pharmacology and toxicology studies of A. lebbeck. J. Ethnopharmacol. 2013;148(3):363–383. [Google Scholar]
- Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7(1):42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davella R., Mamidala E. Luteolin: a potential multiple targeted drug effectively inhibits diabetes mellitus protein targets. J. Pharm. Res. Int. 2021;33:161–171. [Google Scholar]
- Gao M. Albizia lebbeck extract inhibits herpes simplex virus type 1 infection by disrupting viral attachment and penetration. Antiviral Res. 2021;191 [Google Scholar]
- Gujjeti R.P., Namthabad S., Mamidala E. HIV-1 reverse transcriptase inhibitory activity of Aerva lanata plant extracts. BMC Infect Dis. 2014;27:12. [Google Scholar]
- Kitchen D.B. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 2020;19(10):589–609. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- Kumar S. Evaluation of anti-HIV-1 activity of Albizia lebbeck and identification of bioactive compounds. J. Ethnopharmacol. 2021;271 [Google Scholar]
- Kumar M.P., Mamidala E., Al-Ghanim K.A., Al-Misned F., Mahboob S. Evaluation of the andrographolides role and its indoleamine 2, 3-dioxygenase inhibitory potential and attendant molecular mechanism against STZ-induced diabetic rats. Saudi J. Biol. Sci. 2020;27(2):713–719. doi: 10.1016/j.sjbs.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44(1):235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Liu Y. Antiviral activity of Albizia lebbeck against influenza A virus through inhibition of viral entry. J. Ethnopharmacol. 2021;266 [Google Scholar]
- Macingwane J., Ngwenya B.N. Identification of active compounds of Albizia lebbeck against HIV-1 reverse transcriptase: a computational study. J. Biomol. Struct. Dyn. 2021;39(14):5300–5311. [Google Scholar]
- Mamidala E., Davella R., Gurrapu S. An in silico approach for identification of inhibitors as a potential therapeutics targeting SARS-Cov-2 protease. Asian J. Pharm. Res. Health Care. 2020:3–9. [Google Scholar]
- Mamidala E., Davella R., Kumar M.P., Swamy S., Abhiav M., Kaimkhani Z.A., Al-Ghanim K.A., Mahboob S. In silico prediction of mozenavir as a potential drug for SARS-CoV-2 infection via binding multiple drug targets. Saudi J. Biol. Sci. 2022;29(2):840–847. doi: 10.1016/j.sjbs.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Computational Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M.P. Anti-HIV-1 activity of the extracts and compounds from the leaves of Albizia lebbeck. J. Ethnopharmacol. 2013;149(3):840–845. [Google Scholar]
- Nair R., Kalariya T., Sumitra C. Antibacterial activity of some selected Indian medicinal flora. Turk. J. Biol. 2005;29(1):41–47. [Google Scholar]
- Noreen Y., Sultana B., Anwar F., Zaman W., Ahmed S., Alam S. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackie virus: a systematic review. Phytother. Res. 2021;35(5):2570–2597. doi: 10.1002/ptr.6024. [DOI] [PubMed] [Google Scholar]
- Pannecouque C., Daelemans D., De Clercq E., Tetko I.V. HIV-1 reverse transcriptase inhibitors: recent advances and future challenges. J Med. Chem. 2020;63(24):15484–15509. [Google Scholar]
- Srivastava M. In silico docking analysis of selected flavonoids as potent inhibitors of human DNA topoisomerase IIα. Nat. Prod. Res. 2013;27(18):1689–1692. [Google Scholar]
- Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS, 2021. Global HIV & AIDS statistics - 2021 fact sheet. Retrieved from https://www.unaids.org/en/resources/fact-sheet.
- Yadav S., Das S., Avvaru B.S., Giri R., Chaudhury S., Sarkar I. HIV-1 reverse transcriptase: a therapeutic target and review of recent advances. Med. Res. Rev. 2021;41(4):2402–2441. [Google Scholar]