Abstract
The coproporphyrin dependent heme biosynthesis pathway is almost exclusively utilized by Gram-positive bacteria. This fact makes it a worthwhile topic for basic research, since a fundamental understanding of a metabolic pathway is necessary to translate the focus towards medical biotechnology, which is very relevant in this specific case, considering the need for new antibiotic targets to counteract the pathogenicity of Gram-positive superbugs. Over the years a lot of structural data on the set of enzymes acting in Gram-positive heme biosynthesis has accumulated in the Protein Database (www.pdb.org). One major challenge is to filter and analyze all available structural information in sufficient detail in order to be helpful and to draw conclusions. Here we pursued to give a holistic overview of structural information on enzymes involved in the coproporphyrin dependent heme biosynthesis pathway. There are many aspects to be extracted from experimentally determined structures regarding the reaction mechanisms, where the smallest variation of the position of an amino acid residue might be important, but also on a larger level regarding protein-protein interactions, where the focus has to be on surface characteristics and subunit (secondary) structural elements and oligomerization. This review delivers a status quo, highlights still missing information, and formulates future research endeavors in order to better understand prokaryotic heme biosynthesis.
Keywords: Coproporphyrin ferrochelatase, Coproheme decarboxylase, Frataxin, Coproporphyrinogen oxidase, Uroporphyrinogen decarboxylase, Structure determination, Molecular enzymology
Graphical Abstract
1. Introduction
Over the years the structural characterization of macromolecules, especially for proteins, has become an integral tool to understand and perform research on enzymatic reaction mechanisms and protein-protein interaction. This is especially true for enzymes which partake in metabolic and signaling pathways in biological systems, where structural analysis, primarily X-ray crystallography, poses an elementary tool [1], [2].
The aim of this study is to assess the availability and quality of structural data for the enzymes of the coproporphyrin-dependent (CPD) pathway of heme biosynthesis in comparison to the previously well-established protoporphyrin-dependent (PPD) pathway in eukaryotic and Gram-negative organisms. The CPD pathway was discovered in the middle of the 2010 s and is almost exclusively utilized by Gram-positive bacterial strains. It consists partially of unique enzymes and partially of structural orthologues of their PPD counterparts [3], [4], [5], [6], [7]. Why this divergence of the last three steps of heme biosynthesis has evolved in nature is unclear, one hypothesis might be the lack of cellular compartmentalization or periplasmatic space in Gram-positive bacteria [5].
The four final reactions of the PPD pathway and CPD pathway generally resemble each other, but are aligned in a different order, with the exception of a first and common step (Fig. 1): After the original precursor for heme biosynthesis 5-aminolevulinic acid (5-ALA) is synthesized by a set of different enzymes, which are universally conserved across all organisms, it then is used to synthesize uroporphyrinogen III, which leads to the before mentioned first and common step of the PPD and CPD pathway for heme biosynthesis [8], [9]. In a decarboxylation reaction, catalysed by uroporphyrinogen decarboxylase (UroD), carboxyl groups on multiple acetate side chains are removed to yield coproporphyrinogen III [10], [11], [12].
Fig. 1.
Steps of the Gram-positive CPD pathway (A) and Gram-negative/eukaryotic PPD pathway (B) from the common precursor uroporphyrinogen III and a common decarboxylation step, performed by UroD. To highlight the reaction sites on the substrate molecule, green shades mark the respective area. Chemical structures were downloaded from the KEGG compound database (https://www.genome.jp/kegg/compound/), provided by the Bioinformatics Center, Institute for Chemical Research, Kyoto University and the Human Genome Center, Institute of Medical Science, University of Tokyo. The respective identification codes are: C01051 (Uroporphyrinogen III), C03263 (Coproporphyrinogen III), C05770 (Coproporphyrin III), C21284 (Coproheme), C01079 (Protoporphyrinogen IX), C02191 (Protoporphyrin IX) and C00032 (Heme b).
After this initial decarboxylation step the propionates 2 and 4 of the common precursor coproporphyrinogen III are decarboxylated in the PPD pathway by coproporphyrinogen decarboxylase (CgdC) or coproporphyrinogen dehydrogenase (CgdH) [4], [10], [13], [14]. A similar decarboxylation step is performed by the coproheme decarboxylase (ChdC) of the CPD pathway – a mechanistically and structurally completely different enzyme [15], [16], [17], [3]. In the PPD pathway this is followed by the oxidation and therefore aromatization of the tetrapyrrole ring of protoporphyrinogen IX by the protoporphyrinogen oxidase (PgoX), also a key enzyme in the chlorophyll biosynthesis of plants [18], [19], [20]. In the CPD pathway this is the initial step performed by the structurally highly similar coproporphyrinogen oxidase (CgoX) on coproporphyrinogen III [7], [21], [22].
The final step of the PPD pathway consists of the metal insertion into the mature porphyrin macrocycle by the protoporphyrin ferrochelatase (PpfC) [23], [24], [25], [26]. Whereas in the CPD pathway the iron insertion reaction, catalysed by the coproporphyrin ferrochelatase (CpfC), is the penultimate step [27], [28], [29].
A common shared feature of the CPD and PPD pathway is a lack of conservation in the operon organization of the pathway enzymes. The related genes can share operons or cluster through the genome. In Gram-positive genomes those operons usually consist of a few enzymes from the CPD pathway, with the others singled out at different genome sites. The number of gene ensembles in the operon depends on the organism. The genes for 5-ALA and uroporphyrinogen III synthesis form those types of non-conserved operons - the genomic organization of prokaryotic heme biosynthesis clearly lacks a universal pattern [30]. However Gram-positive organisms with a universal operon for heme biosynthesis on one genomic locus do exist, with the leading example of Cutibacterium acnes. An analysis of the Cutibacterium acnes HL096PA1 genome (source: National Center for Biotechnology Information NCBI) show gttR and gsaM (involved in 5-ALA synthesis), pbgS, hmbS and uroS (involved in uroporphyrinogen III synthesis) and finally uroD, cgoX, cpfC and chdC (CPD pathway) all reside next to each other between 371580 and 381804 bp. C. acnes also holds a special place since its ChdC and CpfC enzymes form a fusion protein, linked by a presumably flexible peptide chain [6].
In addition to the enzymes responsible for the synthesis of heme b, support systems for shuttling need to exist. A specific example would be proteinogenic iron transportation to CpfC to counteract the toxicity of free iron ions in the cell. Every pathway, which utilizes iron needs a suitable iron transporting protein to deliver the ions to the desired target [31], [32]. For eukaryotic organisms the iron transporting protein frataxin has been established as a transporter for Fe2+ and Fe3+ through the cell. Mutations in the sequence and the corresponding loss of function of human frataxin are connected to clinical diseases like Friedreich’s ataxia [33], [34], [35]. Meanwhile in Gram-negative bacteria, CyaY, the corresponding protein orthologue to eukaryotic frataxin has been identified for its ability to bind iron and its structural similarity to eukaryotic frataxin and its ability to supply Fe-S cluster proteins with the necessary iron [36], [37], [38]. The ability to bind and transfer iron to target recipients (like CpfC) has been shown for a homologous protein in Gram-positive prokaryotic cells, also annotated as frataxin (or protein with an YdhG domain) in Bacillus subtilis. Despite differences in sequence, a structural and functional conservation to the remaining frataxin family was identified [39], [40], [41]. It should be highlighted that Frataxin seems to fulfill multiple iron transport purposes, given biochemical data. This is also supported by the fact, that it is not necessarily in genomic proximity of any operons or loci containing genes for heme biosynthesis, for example in Firmicute model organism B. subtilis (at 621847 bp in B. subtilis sp. 168) and Actinobacterium Corynebacterium diphtheriae (at 92618 bp in C. diphtheriae strain ATCC 700971 NCTC 13129 Biotype gravis) (source: National Center for Biotechnology Information NCBI). Additionally a homologous gene for frataxin is not present in every organism, which suggests a non - essential role of frataxin in iron transfer processes [41].
The metal binding site and the amount of these binding sites has been discussed in multiple studies for CyaY and eukaryotic frataxin, using structural determination and molecular dynamics simulations [42], [43], [44], [45], [46]. The lack of studies concerning Gram-positive frataxin and if conclusions can be drawn from CyaY and eukaryotic frataxin will be discussed in the respective chapter.
To summarize the known protein structures of the CPD pathway a keyword search of the Protein database (PDB) has been performed. The results are summarized in Table 1. In Table S1 the same information has been expanded by the inclusion of the provided structures of the corresponding/orthologous enzymes of the PPD pathway. This review focuses on structural and functional aspects of enzymes involved in the CPD pathway. An overview of a selection of available structures is presented in Fig. 2. Here, oligomerization status and form of the respective subunits are illustrated in surface mode. CAVER calculations based on the structures and originating from the cofactor binding sites show their conserved location on the protein body regardless of species. Further future challenges in the structural deconvolution of CPD heme biosynthesis next to unraveling enzymatic mechanisms are described as characterizing (i) the important elements of orthologous Gram-positive frataxin proteins in terms of structure and function and (ii) characterizing the interactions of proteins within the pathway by identifying the interacting structural features.
Table 1.
Known protein structures of the CPD pathway extracted from the protein data base (www.pdb.org). Enzyme nomenclature relates to Daily et al. (2017) [47]. Table includes information about the organism (name, phylum), the crystallized protein (mutation, length) and the structure (PDB-ID, deposition date, R-free, resolution, ligands). Information was updated on 08/05/2023.
| UroD/HemE (Uroporphyrinogen III decarboxylase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Phylum | Mutation | Length | PDB | Date | R-free | Resolution [A°] | Ligand | Notes |
| Bacillus subtilis | Firmicutes | wt | 359 | 2INF | 2006–10–06 | 0.251 | 2.3 | apo | |
| CgoX/HemY (Coproporphyrinogen III oxidase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Phylum | Mutation | Length | PDB | Date | R-free | Resolution [A°] | Ligand | Notes |
| Bacillus subtilis | Firmicutes | wt | 470 | 3I6D | 2009–07–06 | 0.293 | 2.9 | FAD | annoted as PPO |
| Exiguobacterium sibiricum 255–15 | Firmicutes | wt | 475 | 3LOV | 2010–02–04 | 0.241 | 2.06 | FAD | annoted as PPO |
| CpfC/HemH (Coproporphyrin ferrochelatase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Phylum | Mutation | Length | PDB | Date | R-free | Resolution [A°] | Ligand | Notes |
| Bacillus subtilis | Firmicutes | wt | 310 | 1AK1 | 1997–05–28 | 0.243 | 1.9 | apo | |
| Bacillus subtilis | Firmicutes | wt | 306 | 1C9E | 1999–08–02 | 0.255 | 2.3 | Cu2+ N-Methylmesoporphyrin complex | |
| Bacillus subtilis | Firmicutes | wt | 309 | 1DOZ | 1999–12–22 | 0.216 | 1.8 | apo | |
| Bacillus subtilis | Firmicutes | wt | 310 | 1C1H | 2000–03–17 | 0.231 | 1.9 | N-Methylmesoporphyrin | |
| Bacillus subtilis | Firmicutes | wt | 310 | 1N0I | 2002–10–14 | 0.273 | 2 | Cd2+ | |
| Bacillus subtilis | Firmicutes | wt | 310 | 1LD3 | 2003–05–20 | 0.274 | 2.6 | Zn2+ | |
| Bacillus subtilis | Firmicutes | Y13F | 309 | 2AC2 | 2005–07–18 | 0.257 | 2.5 | Zn2+ | |
| Bacillus subtilis | Firmicutes | H183C | 309 | 2AC4 | 2005–07–18 | 0.276 | 2.1 | apo | |
| Bacillus anthracis, str. Ames | Firmicutes | wt | 311 | 2C8J | 2005–12–05 | 0.281 | 2.1 | apo | |
| Bacillus subtilis | Firmicutes | wt | 310 | 2H1V | 2006–05–17 | 0.173 | 1.2 | apo | |
| Bacillus subtilis | Firmicutes | H183A | 310 | 2H1W | 2006–05–17 | 0.259 | 2.6 | apo | |
| Bacillus subtilis | Firmicutes | wt | 310 | 2HK6 | 2006–07–03 | 0.22 | 1.71 | Fe2+ | |
| Bacillus subtilis | Firmicutes | wt | 309 | 2Q2N | 2007–05–29 | 0.25 | 1.8 | Deuteroporphyrin IX 2,4-disulfonic acid dihydrochloride | |
| Bacillus subtilis | Firmicutes | H183C | 309 | 2Q2O | 2007–05–29 | 0.219 | 2.1 | Deuteroporphyrin IX 2,4-disulfonic acid dihydrochloride | |
| Bacillus subtilis | Firmicutes | H183A | 309 | 2Q3J | 2007–05–30 | 0.228 | 2.39 | N-Methylmesoporphyrin | |
| Bacillus subtilis | Firmicutes | Y13M | 310 | 3GOQ | 2009–03–19 | 0.232 | 1.6 | apo | |
| Bacillus subtilis | Firmicutes | wt | 309 | 3M4Z | 2010–03–12 | 0.199 | 1.94 | Co2+ | |
| Listeria monocytogenes | Firmicutes | wt | 312 | 6RWV | 2019–06–06 | 0.201 | 1.64 | apo | |
| Listeria monocytogenes | Firmicutes | wt | 311 | 6SV3 | 2019–09–17 | 0.201 | 1.64 | Coproheme | |
| Listeria monocytogenes | Firmicutes | R45L | 311 | 8AW7 | 2022–08–29 | 0.213 | 2.64 | Coproporphyrin III | |
| Listeria monocytogenes | Firmicutes | wt | 311 | 8AT8 | 2022–12–28 | 0.182 | 1.51 | Coproporphyrin III | |
| Listeria monocytogenes | Firmicutes | wt | 311 | 8BBV | 2022–10–14 | 0.242 | 2.19 | Coproporphyrin III/2 min Fe2+ soak | |
| Listeria monocytogenes | Firmicutes | wt | 311 | 8OMM | 2023–03–31 | 0.233 | 2.15 | Coproporphyrin III/3 min Fe2+ soak | |
| Listeria monocytogenes | Firmicutes | wt | 311 | 8OFL | 2023–04–05 | 0.229 | 2.1 | Coproporphyrin III/4 min Fe2+ soak | |
| ChdC/HemQ (Coproheme decarboxylase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Phylum | Mutation | Length | PDB | Date | R-free | Resolution [A°] | Ligand | Notes |
| Thermus thermophilus HB8* | Deinococcota | wt | 249 | 1VDH | 2004–03–22 | 0.218 | 2 | apo | *Gram - negative |
| Geobacillus stearothermophilus | Firmicutes | wt | 248 | 1T0T | 2004–04–12 | 0.194 | 1.75 | apo | |
| Listeria monocytogenes | Firmicutes | wt | 253 | 4WWS | 2014–11–12 | 0.229 | 2 | apo | |
| Listeria monocytogenes | Firmicutes | wt | 250 | 5LOQ | 2016–08–09 | 0.215 | 1.69 | Coproheme | |
| Geobacillus stearothermophilus | Firmicutes | wt | 248 | 5T2K | 2016–08–23 | 0.176 | 1.8 | Mn-Coproporphoryn III | |
| Listeria monocytogenes | Firmicutes | wt | 250 | 6FXQ | 2018–03–09 | 0.214 | 1.69 | Coproheme, monovinyl monopropionate deuteroheme | |
| Listeria monocytogenes | Firmicutes | wt | 250 | 6FXJ | 2018–03–09 | 0.214 | 1.79 | Coproheme | |
| Corynebacterium dipththeriae | Actinobacter | wt | 237 | 6XUB | 2020–01–17 | 0.227 | 1.78 | monovinyl monopropionate deuteroheme | |
| Corynebacterium dipththeriae | Actinobacter | wt | 237 | 6XUC | 2020–01–17 | 0.223 | 1.87 | Coproheme | |
| Geobacillus stearothermophilus | Firmicutes | wt | 248 | 6VSA | 2020–02–10 | 2.32 | apo | Cryo EM | |
| Geobacillus stearothermophilus | Firmicutes | wt | 248 | 6VSC | 2020–02–11 | 2.6 | apo | Cryo EM | |
| Corynebacterium dipththeriae | Actinobacter | Y135A | 237 | 7Q4G | 2021–10–30 | 0.219 | 1.82 | Coproheme | |
| Corynebacterium dipththeriae | Actinobacter | W183Y | 237 | 7Q4F | 2021–10–30 | 0.182 | 2.15 | Coproheme | |
| Frataxin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Phylum | Mutation | Length | PDB | Date | R-free | Resolution [A°] | Ligand | Notes |
| Bacillus subtilis | Firmicutes | wt | 124 | 2OC6 | 2006–12–20 | 0.217 | 1.75 | apo | Hypothetical protein (NP_388456.1) |
| Lactobacillus casei | Firmicutes | wt | 123 | 2I8D | 2006–09–01 | 0.197 | 1.69 | apo | Hypothetical protein (ZP_00384875.1) |
| Alkalihalobacillus halodurans | Firmicutes | wt | 118 | 2KL4 | 2009–06–30 | apo | NMR | ||
Fig. 2.
Available crystal structures of the CPD pathway in the PDB, found by keyword search. Protein structures shown are (A) UroD, (B) CgoX, (D) CpfC, (D) ChdC and (E) frataxin orthologues, which are possible iron transporters for the pathway. The figure depicts a surface overview and for (A) UroD and (D) ChdC an orientation view of the total structure/oligomer. The initial two letters of the structure titles in bold correspond to the organism of origin and are explained in the text and the glossary. PDB-IDs are depicted below. The cofactors if present are presented as sticks. Additionally, a CAVER calculation (Minimum probe radius = 0.9, Shell depth = 4, Shell radius = 3, Clustering threshold = 3.5) was performed originating from the presumed active site/cofactor binding site to further highlight its location on the protein monomer. CAVER calculations from porphyrin binding sites are depicted in yellow, for CgoX the calculations originated from the bound FAD cofactor in the structure and is depicted in red.
Table 1 and Table S1 include information about the organism of origin, if wild-type or variants were studied, deposition date, R-free and resolution and presence of ligands. It is intended to be a starting point for open research questions concerning mechanism and structures for this set of enzymes. R-free and resolution serve for general quality assessment but not necessarily indicate the fitness of the dataset to tackle the specific question of the respective study.
2. The enzymes of the CPD pathway in detail
2.1. UroD: The common step
The last common step in prokaryotic and eukaryotic heme biosynthesis is catalysed by UroD. It catalyses the decarboxylation of acetate side chains of the intermediate uroporphyrinogen III. It also holds a special place, since it is a non-oxidative decarboxylase, without the utilization of any cofactor. During reaction the 8-propionate substrate uroporphyrinogen III is degraded to a 4-propionate substrate, namely coproporphyrinogen III (Fig. 3A). The relevant propionates are left as methyl groups after decarboxylation [48], [49], [50], [51], [52], [53].
Fig. 3.
(A) General reaction catalysed by UroD, with labeled propionate (P) residues. Remaining methyl groups in the product are labeled (M), side chains changing during reaction are highlighted in green. (B & C) Cofactor binding site overlay of UroD from B. subtilis (2INF) (green), N. tabacum (1J93) (yellow) and H. sapiens (2Q71) (magenta). 2Q71 is a crystal structure with a coproporphyrin III molecule (black) bound, and the hydrogen bonds it forms with nearby residues are highlighted in orange. (B) shows distal site of the cofactor with tyrosine and aspartate residues visible, (C) shows the proximal site of the cofactor with two arginine residues visible.
Currently 27 crystal structures are deposited in the PDB (Table S1). Several mutations and the wild-type of the human enzyme were structurally characterized. Six available structures are UroDs of Gram-negative origin. The single crystal structure of a UroD from Gram-positive heritage was described by Fan et al. in 2008 (2INF) (Table 1, S1). The resolution is at 2.3 Å and the R-free value is 0.251, all relevant amino acid residues discussed below are resolved, which makes it suitable for analysing structure and function and also putting it in context with known crystal structures from different organisms [49].
The enzyme forms homodimers of approximately 80 kDa in total, with a conserved active site cleft surrounded by a flexible region (Fig. 2A) [49], [52]. This structural arrangement as a dimer and with a flexible loop partially covering the active site has been hypothesized to be a feature to keep the reaction intermediate inside, and also to require substrate reorientation for the sequential decarboxylation of the acetate groups [52], [50]. The current state of knowledge suggests a clockwise rotation and order of decarboxylation at physiological concentrations of uroporphyrinogen III [50].
The residues which are assumed to be catalytically important, are mostly conserved in all organisms and consist of an aspartate residue, which seems to be important for binding the pyrrole ring of the substrate (D78). Polar residues (R29, R33, Y154, H322) seem to be of importance for substrate binding and recognition and side chain interactions. H322 is proposed to be decisive for the orientation of the partially decarboxylated product. Besides the histidine residue, the aspartate residue has been discussed being a key residue in catalysis and being involved in the protonation of methylene intermediate side chains, as the final step of forming methyl groups. The tyrosine residue is also assumed to be involved directly or indirectly in protonation and weakening of the carboxyl group linkage [49], [52], [53]. It must be noted that the information on the single residues is only based on the crystal structures of UroD from other organisms, which also partially have a coproporphyrin ligand bound in the active site and molecular modeling approaches were based on them [51], [52]. Comparable data does not exist for any prokaryotic version of the enzyme (Table S1) and a crystal structure with uroporphyrinogen III bound does not exist yet. The active site is depicted in Fig. 3B & C with the most relevant residues shown as lines. As mentioned, the bound cofactor is not uroporphyrinogen III, but rather a coproporphyrin III molecule and all interactions are subsequently discussed in respect to this fact. Overall, the porphyrin is subjected to bended conformation with the aspartate (D78/82/86) residue coordinating the center and the tyrosine residue (Y154/159/164) coordinating propionate 2 on the distal site. (Fig. 3B) All residues align positionally in the crystal structure regardless of the source organism, however the presence of the cofactor in 2Q71 induces conformational differences, given the presence of polar interactions. On the distal site (Fig. 3C) one arginine residue interacts with propionate 4 (R16/25/36) and the other interacts with propionate 7 (R29/32/41) The histidine residue mentioned above is not found near coproporphyrin III and is not included in the Figure. Since literature suggests the sequences and structures of UroDs from the PPD and the CPD pathways align, therefore it seems to follow a similar reaction mechanism, regardless of the organism.
2.2. CgoX: Tetrapyrrole oxidation in the CPD pathway
CgoX catalyses the oxidation of the pyrrole rings and the bonds connecting them, making it an enzymatic reaction affecting the full porphyrin. (Fig. 4) [7], [21]. A graphical analysis of the resolved crystal structures was left out, due to the lack of a porphyrin bound structure of CgoX and therefore no definite knowledge about the important residues for coproporphyrinogen III binding and the subsequent reaction.
Fig. 4.
Reaction catalysed by CgoX. The formed methyl groups which connect the macrocyclic tetrapyrrole ring are labeled by Greek letters (α,β,γ,δ) and marked in green to highlight them as reaction sites.
Currently two crystal structures of CgoX are deposited in the PDB (Table 1, Fig. 1): Both are annotated as PgoX, which is due to their deposition date (2009 and 2010), back then the CPD pathway was not described yet. The crystal structure of CgoX from B. subtilis (3I6D) (BsCgoX) was released by Qin et al. in 2010. Alongside the structure of CgoX from Exiguobacterium sibiricum 255–15 (3LOV) (EsCgoX) the monomeric form of CgoX is confirmed. This monomeric state of the soluble protein is discussed as a unique feature of BsCgoX, compared to e.g.: human CgoX, which forms membrane bound dimers [7]. Both structures have a flavin adenine dinucleotide (FAD) cofactor and 3I6D has an additional acifluorfen inhibitor bound. As it is the case for UroD, most structures deposited in the PDB are homologs originating from eukaryotes or Gram-negative bacterial strains. 13 from 15 structures for the tetrapyrrole oxidizing enzyme in heme biosynthesis are the orthologous protein PgoX from the PPD pathway (Table S1).
Little is known about the exact reaction mechanism, recently a non-conserved aspartate residue in BsCgoX has been shown to be important to maintain an intact H-bonding network in close proximity to the FAD cofactor [54]. The key catalytic residues of CgoX have not been discussed yet – however its ability to utilize coproporphyrinogen III as a substrate has been postulated. Interestingly different bacterial strains that possess CgoX are also able to utilize protoporphyrinogen IX as a substrate in varying degrees [7], [3]. However even before the discovery of the CPD pathway, a higher affinity of coproporphyrinogen III to CgoX was discussed based on the crystal structure of BsCgoX [7]. Since there is no direct homolog in the PPD pathway, CgoX is of particular interest for potential inhibitor studies: Besides acifluorfen, which inhibits PgoX [55], [56] and inefficiently inhibits BsCgoX [7] and CgoX from Staphylococcus aureus (SaCgoX) a variety of other herbicides have shown inhibitory capacity for the enzyme. [3] Furthermore it has been of interest as a target for vaccination against Gram-positive pathogens and other therapies [57], [58]. However, a better understanding of the reaction mechanism of CgoX through structural analysis plays an important role in the development of new anti-bacterial compounds [59].
2.3. CpfC: Metal insertion
Most of the CpfC structures available are from B. subtilis (BsCpfC) and are annotated as HemH, since their depositions backdate (1997 – 2010) to a time when the CPD pathway was not yet discovered. A wide variety of divalent metals were used to find metal binding sites as well as non-physiological porphyrins in the crystallization attempts, which consequently partially bound incorrectly in the pocket of CpfC, but still provided important information [60], [61], [62], [63], [64], [65]. CpfC structures in context of the true physiological role are available from L. monocytogenes, providing all three possible physiological states: apo, coproporphyrin III and iron coproporphyrin III bound (6RWV; 8AT8; 6SV3) (Table 1) [27], [66], [67].
CpfC is a monomer most likely located in the bacterial cytosol, compared to e.g.: human PpfC which is a homodimer containing a [2Fe-2S] cluster in each subunit coordinated by four cysteine residues and is membrane-associated in the mitochondria. [68], [69], [70], [71] 2Fe-2S] clusters seem to be present also in CpfC representatives of the actinobacterial clade for instance C. diphtheriae, but are not conserved within the clade and not present in firmicute CpfCs [72]. The role of the cluster in bacteria is under debate, since the metalation of coproporphyrin III in Firmicutes is performed without it at no catalytic cost [28], [47], [73].
The porphyrin substrate is coordinated by a variety of amino acid residues in the active site by an extensive hydrogen bonding network interacting with the propionate sidechains and is clearly distorted [64], [67], [74]. A distortion of the substrate was postulated and shown to be necessary by QM/MM calculations to be energetically favorable, however they were not performed with the physiological substrate coproporphyrin III, but with different porphyrin analogues [26], [75], [76].
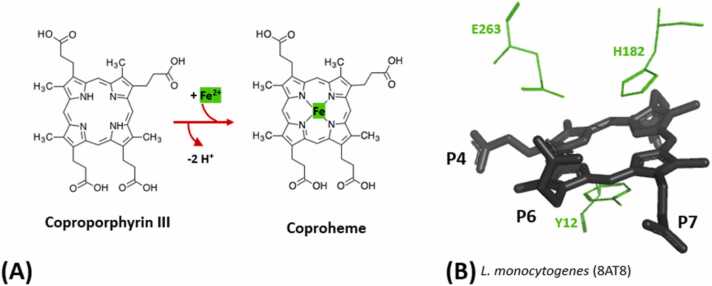
For the iron insertion process two protons have to be abstracted from the pyrrole nitrogen atoms and one electron has to be transferred to oxidize Fe(II) to Fe(III) during the ligation (Fig. 5A). The three prominent amino acid residues are a glutamic acid (E263) and a histidine (H182) on the distal side and a tyrosine (Y12) located on the proximal side (Fig. 5B). Those residues are believed to be important or essential for these tasks. The residues at the distal site are highly conserved throughout all ferrochelatases and it was reported that the histidine variant showed a highly reduced catalytic capability [62], [61], [77], [78], [79]. It is important to mention that these observations were reported using protoporphyrin IX, which has two vinyl instead of two propionate groups. Whether this holds true for the physiological substrate coproporphyrin III remains to be shown. The proximal residue is conserved within the phylogenetic clades. A tyrosine can be found in Firmicutes and a phenylalanine in actinobacterial representatives, whereas in human PpfC a methionine is present [27], [72], [47].
Fig. 5.
(A) Reaction catalysed by CpfC. Inserted Fe2+ is highlighted in green. (B) Active site of CpfC from Listeria monocytogenes (8AT8). The cofactor coproporphyrin III is shown in black with visible propionates labeled (P) for orientation. Catalytically important residues are shown as green lines. On the proximal side a tyrosine residue is located and on the distal side a glutamate and a histidine residue are visible.
The path and binding site of the iron to the active site prior to the ligation into coproporphyrin III is of utter interest since it can indicate amino acids involved in the ligation process. In an earlier structure of apo B. subtilis the iron (2HK6) was located between the prominent distal glutamic acid and histidine, but no bound porphyrin was present [61]. In contrast, the QM/MM calculations mentioned above with different porphyrin analogues, suggested that the proximal path is more favorable, as also some investigations of the structurally related human ferrochelatase [26], [69], [80]. There are three new structures available of L. monocytogenes with coproporphyrin III soaked with ferrous iron resolved with different iron occupancies (8BBV; 8OMM; 8OFL, Table 1). These potentially foreshadow an iron approach from the proximal side and conformational changes of the substrate coproporphyrin III during the ligation process. Yet, no proven conclusion can be drawn.
Despite new data and progress, important questions of about this enzymatic reaction remain under investigation: (i) How are the protons abstracted? (ii) From where does the iron approach and (iii) whereto is the electron transferred during the oxidation process of iron?
2.4. ChdC: Terminal two-propionate decarboxylation
Coproheme decarboxylases (ChdCs) catalyse the final step in heme b biosynthesis of monoderm and some diderm bacteria. [47] In this reaction, coproheme is converted to heme b via monovinyl monopropionate deuteroheme (MMD) in two consecutive decarboxylation steps (Fig. 6A). The oxidative decarboxylation of coproheme is in the physiological setting most probably hydrogen peroxide mediated and requires two equivalents of the oxidant (e. g. hydrogen peroxide, in vitro also chlorite and peroxyacetic acid were used) for full conversion of one coproheme to heme b. [81] A tyrosine residue was identified as the catalytically relevant radical site essential for both decarboxylation reactions in ChdCs from the Firmicutes Staphylococcus aureus and Listeria monocytogenes and also from actinobacterial Corynebacterium diphtheriae (Y135 in Fig. 6A) [82], [83]. Additionally in Actinobacteria a histidine residue, which is not conserved in firmicute representatives, was identified to act as a distal base in ChdC from Corynebacterium diphtheriae (H118 in Fig. 6C) [84]. Structural biology was essential in delivering key information on several important details in understanding of the catalytic reaction mechanism of ChdCs. In combination with in-solution spectroscopic studies and computational investigations, X-ray crystallographic data revealed insight, especially into the reorganisation mode of the transiently formed three propionates intermediate MMD. When coproheme is bound the catalytic tyrosine is in close proximity to p2 of pyrrole ring A, whereas after the first decarboxylation step MMD rotates in situ and places p4 of pyrrole ring B close to the catalytic tyrosine. [82], [84], [85], [86], [87].
Fig. 6.
(A) Reaction catalysed by ChdC. Original propionate groups and emerging vinyl groups are labeled (P,V). Sidechains which are reaction sites are marked in green. Included in the reaction is also the three-propionate reaction intermediate MMD. Chemical structure was downloaded from the KEGG compound database (https://www.genome.jp/kegg/compound/), provided by the Bioinformatics Center, Institute for Chemical Research, Kyoto University and the Human Genome Center, Institute of Medical Science, University of Tokyo. The identification code is C22173. (B) Monomeric subunit of ChdC from Corynebacterium diphtheriae (6XUC) with black coproheme cofactor marking the active site. Flexible linker is distinctly colored in cyan (C) Cofactor in the active site with propionates labeled for orientation (P). Visible residues are a histidine and a tyrosine on the distal site and a histidine on the proximal site.
All ChdCs are homopentamers (135–165 kDa) and have a subunit size of 250–300 amino acids that form an N-terminal and a C-terminal ferredoxin-like fold. Only the C-terminal domain has a functional coproheme/heme b binding site (Fig. 6B). While most β-sheets and α-helices overlay nicely between different species, the loop region combining the N- and the C-terminal domain of coproheme decarboxylases shows some differences. This loop defines the putative substrate access channel (for coproheme or heme b, and hydrogen peroxide or chlorite, respectively) (Fig. 6B) [88].
ChdC structures of five organisms are deposited in the PDB at resolutions ranging from 1.69 Å to 2.6 Å (Table 1); from Geobacillus stearothermophilus, Listeria monocytogenes, Thermus thermophilus (1VDH), Thermoplasma acidophilum (3DTZ) and, Corynebacterium diphtheriae.
Apo structures are available from Geobacillus stearothermophilus, solved by X-ray crystallography (GsChdC, 1T0T) and cryo-electron microscopy (6VSC, 6VSA), and LmChdC (4WWS); holo structures from GsChdC in complex with Mn-coproheme (5T2K) and LmChdC in complex with iron coproheme (6FXJ) and the three-propionate intermediate (6FXQ). Recently the structures of coproheme decarboxylase from Corynebacterium diphtheriae were determined in complex with coproheme (6XUC) and in complex with monovinyl monopropionyl deuteroheme (6XUB) along with various mutants from CdChdC [16], [82], [84], [89], [90], [91]. By listing all available deposited structures, it becomes obvious that no structure of any heme b bound ChdC has been solved until now. It would be important to have structural information of all enzymatically relevant states (empty, substrate, intermediate and product bound) of ChdC in hand (apo-ChdC, coproheme-ChdC, MMD-ChdC, heme b ChdC).
3. Cellular iron transport by YdhG/Frataxin like proteins
As mentioned before, the intracellular transfer of iron ions is dependent on protein transporters to protect cells from toxicity. For the CPD pathway this role might be fulfilled by frataxin, an analogous protein to eukaryotic frataxin and an orthologous protein to Gram-negative and prokaryotic CyaY [39], [40], [41]. Little is known so far about the mechanism of Gram-positive frataxin. Studies highlight its capacity to bind and transfer iron to CpfC and other proteins utilizing iron-sulfur clusters [39], [40]. However available structural data is sparse, since the stoichiometry of iron binding, the specificity for iron as a binding partner and the exact location of bound atoms on the protein has not been determined yet. Currently three crystal structures in of apo-forms are available in the PDB (2I8D, 2KL4, 2OC6), all three are of firmicute origin and one of them (2KL4) is an NMR structure. Fig. 7.
Fig. 7.
(A) Crystal structure of BsFrat (2OC6), with highlighted conserved residues in red, conserved antiparallel beta sheet (wheat) and alpha helices (gray). (B) Close Up of conserved antiparallel sheet region (cartoon in wheat), with conserved residues presented as red lines. An additional scheme shows their structural positions in the sheet, according to 2OC6 and the sequence alignment in (C). (C) Results of the multiple sequence alignment of the relevant sequence range, depicted by Berkeley WebLogo. The conserved leucine residue is at alignment position 299, the conserved tyrosine/tryptophan residue at 305, proline at 308 and glycine at 331.
To pre-analyse possible conserved structural elements in Gram-positive frataxin a multiple sequence alignment of 330 sequences of possible candidates of Gram-positive origin has been performed (in MEGA11 by ClustalW algorithm). A key motif in all these proteins seems to be an arrangement of three antiparallel β-strands with conserved residues interspersed (Fig. 8B & C): A leucine residue, a tyrosine or tryptophan residue, which are both polar and aromatic amino acids and a proline and a glycine residue (L34, W40, P42, G49 in B. subtilis). This structural arrangement is found in all three- Gram-positive frataxin structures. It must be noted that Mielcarek and co-workers identified the possible interaction site between frataxin from B. subtilis (BsFrat) and the CpfC from B. subtilis (BsCpfC) by hydrogen-deuterium exchange experiments and showed that the binding site is not necessarily located at the described conserved motif [40]. This highlights the need for more structural data on Gram-positive frataxin, mainly a metal-bound structure to identify the binding site is lacking. Co-crystallization experiments with CpfC can contribute to increase understanding of the role of iron transporters within the CPD pathway.
Fig. 8.
Known frataxin and CyaY crystal structures in cartoon depiction and from the same perspective to allow comparison of metal binding sites. Structures include: Apo-frataxin from Homo sapiens (splitpea) with possible residues involved in metal binding highlighted in red (1EKG), apo-frataxin from Bacillus subtilis (black) (2OC6), Co2+ bound frataxin from Saccharomyces cerevisiae (lightpink) and Co2+ in cyan (3OER), Fe2+ bound frataxin from Saccharomyces cerevisiae in (lightpink) and Fe2+ in orange (4EC2), Co2+ bound CyaY from Escherichia coli (marine) and Co2+ in cyan (2EFF), Eu3+ bound CyaY from Escherichia coli (marine) and Eu3+ in black (2P1X), Co2+ bound CyaY from Psychchromonas ingrahamii (lightorange) and Co2+ in cyan (4LK8), Eu3+ bound CyaY from Psychchromonas ingrahamii (lightorange) and Eu3+ in black (4LP1).
For the iron and metal binding sites, reviewing the variety of metal bound structures from eukaryotic frataxin and CyaY of different origins it might be possible to draw conclusion for Gram-positive frataxin. While sequence homology of Gram-positive frataxin to its relatives is not conserved, a certain degree of structural conservation seems to be maintained across species: When comparing the respective monomers (Fig. 8), the antiparallel sheet conformation, covered by alpha - helices seems to be maintained. This is important, giving the flexible linkers between the sheets and the surface pockets they form with the helices, seem to host metal binding sites frequently (see binding site for Co2+ in 3OER, 2EFF, 4LK8 and Eu3+ binding sites in 2P1X, 4LP1).
In general frataxin and CyaY bind different types of metal with different charges [42], [43], [44], [45], [46]. Zn2+ binding in human frataxin has been confirmed by NMR spectroscopy at the same binding sites as Fe2+. The ability of metal loaded frataxin to bind protoporphyrin IX has been shown in the same publication - a feature which highlights the ubiquitous binding modes of frataxin. Whether this is related to any physiological function is not clear. Further, seven possible residues, which change conformation during metal titration and may serve as possible binding sites, are identified by molecular dynamics simulations. Most of them possess polar side chains and are located on the N-terminal alpha helix or the flexible areas in between the sheet conformation (see red residues in Fig. 8, 1EKG). [42] The N-terminal helix and the following first strand is discussed in other publications and for other organisms as well. In a recent publication Rodrigues et al. (2022) highlights the metal binding sites on it for the Drosophila melanogaster frataxin variant [92]. This site for polar and anionic residue site is conserved in yeast frataxin, E. coli CyaY and Gram-positive B. subtilis frataxin. [40], [93], [94].
This suggests in consequence that metal binding of frataxin and frataxin-like proteins is solvent exposed and does not necessarily happen in a 1:1 ratio. Crystal structures from the CyaY family seem to support this conclusion, since they show several metal ions across the molecules with no uniform binding mode even between the two monomers in one unit cell. (see Fig. 8, 2EFF, 2P1X, 4LK8, 4LP1) [43], [44], [93].
Following up on the oligomeric status of frataxin, it has been shown that metal binding supports oligomerization for the apo-monomers of eukaryotic frataxin from S. cerevisiae (see Fig. 8, 3OER, 4EC2): Co2+ binds in the 3-fold axis of a formed trimer, which has been shown by SAXS (Small angle X-ray scattering) measurements, and at the binding site published in the crystal structure (3OER). Also hexameric and dimeric formations have been detected. [46] Fe2+ has a similar effect on the oligomerization status. In addition, SAXS measurements suggest a tetrameric form after Fe2+ binding [45].
Coming back to Gram-positive frataxin, it can be assumed that iron and metal binding generally happens in a similar manner as for eukaryotic frataxin and CyaY. This is mainly based on protein structure similarities. Further biochemical data suggests a 2:1 frataxin:Fe2+ relation, which suggests dimerization as a consequence of metal binding [41]. Therefore a metal-bound structure would not only confirm previous assumption about metal binding sites, but also its oligomerization behavior and its respective conformation.
4. Outlook: Enzyme - enzyme interaction as a future challenge
Crucial open questions concern (i) how the described enzymes interact with each other mechanistically, (ii) in which way they have direct influence on the metabolic flux of precursors to heme b and (iii) what are the tools and support systems that enable functionality in their interaction partners. The exact mode of action of an enzyme interaction strongly depends on the localization within the cell and the corresponding conditions. This also implies the lack of direct enzyme-enzyme interactions in many pathways and the inclusion of a transporter into the pathway [95], [96]. A relevant example for an interactional study including a multi-protein complex is the description of compartmentalized heme biosynthesis in eukaryotes, which utilizes a variety of proteins within and outside of the mitochondrial membrane, who have many possible interaction modes [97], [98].
This highlights that enzyme-enzyme interactions especially in a metabolic pathway are multifaceted and complex problems. In order to study such interactions many things must be considered: (i) Does one deal with a multi-enzyme complex? (ii) Do interactions happen transiently between proteins in the cytosol? (iii) Is substrate shuttling dependent on the proteins interacting or solely on a concentration gradient of the substrate [95]? To tackle those questions and problems for the CPD pathway we suggest a structure-based approach to study enzymatic interactions, with a focus on the interaction between CpfC and ChdC, the last two enzymes of the pathway.
The current state of knowledge is rather limited in order to be able to make elaborate guesses, based on the location of the respective active sites within the protein and their active site architectures. Coproheme shuttling from CpfC to ChdC has been confirmed by steady-state and pre-steady-state assays [5], [66]. Therefore the flexible binding loop covering the ChdC active site is very likely part of the interacting surface, when enzymes collide or bind to each other [15], [88]. However the positioning and possible motion of the loop during the interaction is not clear yet and is a key target for future structure-function studies of the CpfC-ChdC interaction. In previous works it has been shown that the loop length and orientation is highly flexible and important for substrate specificity [15], [88]. Therefore, this loop remains an interesting feature to be investigated regarding putative protein-protein interactions (Fig. 9). Further CpfC and ChdC have shown interaction with IsdG, a heme oxygenase, which seems to be relevant for regulating heme biosynthesis in S. aureus. [99]. Besides the biochemical characterization of this regulatory interaction, a structural investigation of a potential interaction might be of interest for gaining a deeper understanding of the CPD pathway.
Fig. 9.
(A) Active sites of coproporphyrin ferrochelatases and overall monomeric structure, represented for LmCpfC (blue). (B) Active sites and orientation of flexible loop regions for various coproheme decarboxylases and overall pentameric structure with highlighted subunit, represented for LmChdC (magenta).
The most difficult, but key task would be an experimental structure of the interacting enzymes either co-crystallized in complex or using a Cryo-EM based approach. Both methods have been used previously to study protein-protein interactions and can in combination with in vitro kinetic assays and in vivo growth and function-based assays lead to a good overview and understanding of an interaction [100], [101], [102], [103], [104], [105], [106]. Also computational methods, like molecular dynamics simulations, have been a suitable approach to describe the movement and changes of macromolecular arrangements [107], [108]. This includes elaborated docking calculations on AlphaFold2 models, a popular machine learning algorithm, which can accurately predict structures based on protein sequences, although without co-factors and other interaction sites. Protein complex prediction itself is currently not a reliable feature of AlphaFold2. [109], [110], [111], [112]. Such a computational approach could be a valid alternative and add-on to study very transient interactions.
Overall, in order to understand the reactions in a metabolic pathway, enzymatic interactions are key determinants for rate, function and order and are therefore a necessity when attempting to fully understand the CPD pathway.
Supporting
Supporting information contains Table S1, listing all structural data of enzymes involved in either the CPD or the PPD pathway, extracted from the protein data base.
Author statement
The work was written, reviewed and edited by all authors (NF, GP, PGF, TG, SH). Conceptualization of the work was done by NF and SH. Graphical illustration by NF. Data mining by NF.
Funding
This project was funded by the Austrian Science Funds (FWF) projects W1224, P33544, P34934, P36967.
Declaration of Competing Interest
The authors of the submitted manuscript “Structural aspects of enzymes involved in prokaryotic Gram-positive heme biosynthesis” declare no conflict of interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.csbj.2023.07.024.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Poulos T.L. Heme enzyme structure and function. Chem Rev. 2014;114(7):3919–3962. doi: 10.1021/cr400415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y. A glimpse of structural biology through X-Ray crystallography. Cell. 2014;159(5):995–1014. doi: 10.1016/j.cell.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 3.Lobo S.A.L., Scott A., Videira M.A.M., Winpenny D., Gardner M., Palmer M.J., et al. Staphylococcus aureus Haem Biosynthesis: characterisation of the enzymes involved in final steps of the pathway: S. Aureus Haem Biosynthesis: from uroporphyrinogen III to haem. Mol Microbiol. 2015;97(3):472–487. doi: 10.1111/mmi.13041. [DOI] [PubMed] [Google Scholar]
- 4.Celis A.I., DuBois J.L. Making and breaking heme. Curr Opin Struct Biol. 2019;59:19–28. doi: 10.1016/j.sbi.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A.I. Celis J.E. Choby J. Kentro E.P. Skaar J.L. DuBois Control of Metabolite Flux during the Final Steps of Heme b Biosynthesis in Gram-Positive Bacteria 2020. [DOI] [PMC free article] [PubMed]
- 6.Dailey H.A., Gerdes S., Dailey T.A., Burch J.S., Phillips J.D. Noncanonical coproporphyrin-dependent bacterial heme biosynthesis pathway that does not use Protoporphyrin. Proc Natl Acad Sci. 2015;112(7):2210–2215. doi: 10.1073/pnas.1416285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin X., Sun L., Wen X., Yang X., Tan Y., Jin H., et al. Structural insight into unique properties of protoporphyrinogen oxidase from bacillus subtilis. J Struct Biol. 2010;170(1):76–82. doi: 10.1016/j.jsb.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., Kang Z., Chen J., Du G. Optimization of the heme biosynthesis pathway for the production of 5-Aminolevulinic Acid in Escherichia Coli. Sci Rep. 2015;5(1):8584. doi: 10.1038/srep08584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang M., Hong K., Mao Y., Ma H., Chen T., Wang Z. Natural 5-Aminolevulinic acid: sources, biosynthesis, detection and applications. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.841443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips J.D., Whitby F.G., Warby C.A., Labbe P., Yang C., Pflugrath J.W., et al. Crystal structure of the oxygen-dependant Coproporphyrinogen Oxidase (Hem13p) of Saccharomyces Cerevisiae. J Biol Chem. 2004;279(37):38960–38968. doi: 10.1074/jbc.M406050200. [DOI] [PubMed] [Google Scholar]
- 11.Whitby F.G. Crystal structure of human uroporphyrinogen decarboxylase. EMBO J. 1998;17(9):2463–2471. doi: 10.1093/emboj/17.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warby C.A., Phillips J.D., Bergonia H.A., Whitby F.G., Hill C.P. Structural and kinetic characterization of mutant human uroporphyrinogen decarboxylases. Cell Mol Biol. 2010 [PMC free article] [PubMed] [Google Scholar]
- 13.Layer G. Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of radical SAM Enzymes. EMBO J. 2003;22(23):6214–6224. doi: 10.1093/emboj/cdg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Layer G., Verfu¨rth K., Mahlitz E., Jahn D. Oxygen-Independent Coproporphyrinogen-III Oxidase HemN from Escherichia Coli. J Biol Chem. 2002;277(37):34136–34142. doi: 10.1074/jbc.M205247200. [DOI] [PubMed] [Google Scholar]
- 15.Pfanzagl V., Holcik L., Maresch D., Gorgone G., Michlits H., Furtmüller P.G., et al. Coproheme decarboxylases - phylogenetic prediction versus biochemical experiments. Arch Biochem Biophys. 2018;640:27–36. doi: 10.1016/j.abb.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celis A.I., Gauss G.H., Streit B.R., Shisler K., Moraski G.C., Rodgers K.R., et al. Structure-based mechanism for oxidative decarboxylation reactions mediated by amino acids and Heme Propionates in Coproheme Decarboxylase (HemQ) J Am Chem Soc. 2017;139(5):1900–1911. doi: 10.1021/jacs.6b11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.B.R. Streit A.I. Celis K. Shisler K.R. Rodgers G.S. Lukat-Rodgers J.L. DuBois Reactions of Ferrous Coproheme Decarboxylase (HemQ) with O2 and H2O2 Yield Ferric Heme b 2017. [DOI] [PMC free article] [PubMed]
- 18.Rangani G., Salas-Perez R.A., Aponte R.A., Knapp M., Craig I.R., Mietzner T., et al. A Novel Single-Site Mutation in the Catalytic Domain of Protoporphyrinogen Oxidase IX (PPO) Confers Resistance to PPO-Inhibiting Herbicides. Front Plant Sci. 2019;10:568. doi: 10.3389/fpls.2019.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dailey T.A., Dailey H.A. Human Protoporphyrinogen Oxidase: expression, purification, and characterization of the cloned enzyme: human protoporphyrinogen oxidase. Protein Sci. 1996;5(1):98–105. doi: 10.1002/pro.5560050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brzezowski P., Ksas B., Havaux M., Grimm B., Chazaux M., Peltier G., et al. The function of Protoporphyrinogen IX oxidase in chlorophyll biosynthesis requires oxidised plastoquinone in Chlamydomonas Reinhardtii. Commun Biol. 2019;2(1):159. doi: 10.1038/s42003-019-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choby J.E., Skaar E.P. Staphylococcus Aureus Coproporphyrinogen III oxidase is required for aerobic and anaerobic heme synthesis. mSphere. 2019;4(4):e00235–19. doi: 10.1128/mSphere.00235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klimka A., Mertins S., Nicolai A.K., Rummler L.M., Higgins P.G., Günther S.D., et al. Epitope-Specific Immunity against Staphylococcus Aureus Coproporphyrinogen III Oxidase. Npj Vaccin. 2021;6(1):11. doi: 10.1038/s41541-020-00268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira G.C., Franco R., Lloyd S.G., Moura I., Moura J.J.G., Huynh B.H. Structure and function of Ferrochelatase. J Bioenerg Biomembr. 1995;27(2):221–229. doi: 10.1007/BF02110037. [DOI] [PubMed] [Google Scholar]
- 24.Medlock A.E., Carter M., Dailey T.A., Dailey H.A., Lanzilotta W.N. Product release rather than chelation determines metal specificity for ferrochelatase. J Mol Biol. 2009;393(2):308–319. doi: 10.1016/j.jmb.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter G.A., Sampson M.P., Ferreira G.C. Metal Ion substrate inhibition of Ferrochelatase. J Biol Chem. 2008;283(35):23685–23691. doi: 10.1074/jbc.M803372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J., Wen S., Zhou Y., Chao H., Shen Y. Human Ferrochelatase: insights for the Mechanism of Ferrous Iron approaching protoporphyrin IX by QM/MM and QTCP free energy studies. J Chem Inf Model. 2016;56(12):2421–2433. doi: 10.1021/acs.jcim.6b00216. [DOI] [PubMed] [Google Scholar]
- 27.Hofbauer S., Helm J., Obinger C., Djinović‐Carugo K., Furtmüller P.G. Crystal structures and calorimetry reveal catalytically relevant binding mode of coproporphyrin and coproheme in coproporphyrin ferrochelatase. FEBS J. 2020;287(13):2779–2796. doi: 10.1111/febs.15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbs C., Reid J.D., Shepherd M. The Coproporphyrin Ferrochelatase of Staphylococcus Aureus: mechanistic Insights into a Regulatory Iron-Binding Site. Biochem J. 2017;474(20):3513–3522. doi: 10.1042/BCJ20170362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Karadaghi S., Hansson M., Nikonov S., Jönsson B., Hederstedt L. Crystal structure of Ferrochelatase: the terminal enzyme in heme biosynthesis. Structure. 1997;5(11):1501–1510. doi: 10.1016/S0969-2126(97)00299-2. [DOI] [PubMed] [Google Scholar]
- 30.Zamarreño Beas J., Videira M.A.M., Saraiva L.M. Regulation of Bacterial Haem Biosynthesis. Coord Chem Rev. 2022;452 doi: 10.1016/j.ccr.2021.214286. [DOI] [Google Scholar]
- 31.Kozlova E., Sherstyukova E., Sergunova V., Kozlov A., Gudkova O., Inozemtsev V., et al. The toxic influence of excess free iron on red blood cells in the biophysical experiment: an in vitro study. J Toxicol. 2022;2022:1–16. doi: 10.1155/2022/7113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews S.C., Robinson A.K., Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27(2–3):215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 33.Bridwell-Rabb J., Winn A.M., Barondeau D.P. Structure–function analysis of friedreich’s ataxia mutants reveals determinants of frataxin binding and activation of the Fe–S assembly complex. Biochemistry. 2011;50(33):7265–7274. doi: 10.1021/bi200895k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhe-Paganon S., Shigeta R., Chi Y.-I., Ristow M., Shoelson S.E. Crystal structure of human frataxin. J Biol Chem. 2000;275(40):30753–30756. doi: 10.1074/jbc.C000407200. [DOI] [PubMed] [Google Scholar]
- 35.Bencze K.Z., Kondapalli K.C., Cook J.D., McMahon S., Millán-Pacheco C., Pastor N., et al. The structure and function of frataxin. Crit Rev Biochem Mol Biol. 2006;41(5):269–291. doi: 10.1080/10409230600846058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafiei A., Baldir N., Na J., Kim J.H., DeMirci H. Ambient temperature crystal structure of escherichia coli cyay protein displays alternate conformation; preprint. Biochemistry. 2023 doi: 10.1101/2023.03.08.531761. [DOI] [Google Scholar]
- 37.Adinolfi S., Iannuzzi C., Prischi F., Pastore C., Iametti S., Martin S.R., et al. Bacterial Frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat Struct Mol Biol. 2009;16(4):390–396. doi: 10.1038/nsmb.1579. [DOI] [PubMed] [Google Scholar]
- 38.Bou-Abdallah F., Adinolfi S., Pastore A., Laue T.M., Dennis Chasteen N. Iron binding and oxidation kinetics in frataxin CyaY of Escherichia Coli. J Mol Biol. 2004;341(2):605–615. doi: 10.1016/j.jmb.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 39.Albrecht A.G., Landmann H., Nette D., Burghaus O., Peuckert F., Seubert A., et al. The frataxin homologue fra plays a key role in intracellular iron channeling in bacillus subtilis. ChemBioChem. 2011;12(13):2052–2061. doi: 10.1002/cbic.201100190. [DOI] [PubMed] [Google Scholar]
- 40.Mielcarek A., Blauenburg B., Miethke M., Marahiel M.A. Molecular insights into frataxin-mediated iron supply for heme biosynthesis in Bacillus Subtilis. PLOS ONE. 2015;10(3) doi: 10.1371/journal.pone.0122538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi W., Cowan J.A. A structural and functional homolog supports a general role for frataxin in cellular iron chemistry. Chem Commun. 2010;46(5):719–721. doi: 10.1039/B911975B. [DOI] [PubMed] [Google Scholar]
- 42.Bernardo-Seisdedos G., Schedlbauer A., Pereira-Ortuzar T., Mato J.M., Millet O. Protoporphyrin IX Binds to Iron(II)-Loaded and to Zinc-Loaded Human Frataxin. Life. 2023;13(1):222. doi: 10.3390/life13010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noguera M.E., Roman E.A., Rigal J.B., Cousido-Siah A., Mitschler A., Podjarny A., et al. Structural characterization of metal binding to a cold-adapted Frataxin. JBIC J Biol Inorg Chem. 2015;20(4):653–664. doi: 10.1007/s00775-015-1251-9. [DOI] [PubMed] [Google Scholar]
- 44.Pastore C., Franzese M., Sica F., Temussi P., Pastore A. Understanding the binding properties of an unusual metal-binding protein - a study of bacterial frataxin: metal-binding properties of CyaY. FEBS J. 2007;274(16):4199–4210. doi: 10.1111/j.1742-4658.2007.05946.x. [DOI] [PubMed] [Google Scholar]
- 45.Söderberg C.A.G., Rajan S., Shkumatov A.V., Gakh O., Schaefer S., Ahlgren E.-C., et al. The molecular basis of iron-induced oligomerization of frataxin and the role of the ferroxidation reaction in oligomerization. J Biol Chem. 2013;288(12):8156–8167. doi: 10.1074/jbc.M112.442285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Söderberg C.A.G., Shkumatov A.V., Rajan S., Gakh O., Svergun D.I., Isaya G., et al. Oligomerization propensity and flexibility of yeast frataxin studied by X-Ray crystallography and small-angle X-Ray scattering. J Mol Biol. 2011;414(5):783–797. doi: 10.1016/j.jmb.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dailey H.A., Dailey T.A., Gerdes S., Jahn D., Jahn M., O’Brian M.R., et al. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev. 2017;81(1) doi: 10.1128/MMBR.00048-16. e00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips J.D., Kushner J.P., Whitby F.G., Hill C.P. Characterization and crystallization of human uroporphyrinogen decarboxylase: crystallization of uroporphyrinogen decarboxylase. Protein Sci. 1997;6(6):1343–1346. doi: 10.1002/pro.5560060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan J., Liu Q., Hao Q., Teng M., Niu L. Crystal structure of uroporphyrinogen decarboxylase from Bacillus Subtilis. J Bacteriol. 2007;189(9):3573–3580. doi: 10.1128/JB.01083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J., Lim C.K. Order of Uroporphyrinogen III Decarboxylation on Incubation of Porphobilinogen and Uroporphyrinogen III with Erythrocyte Uroporphyrinogen Decarboxylase. Biochem J. 1993;289(2):529–532. doi: 10.1042/bj2890529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyckoff E.E., Phillips J.D., Sowa A.M., Franklin M.R., Kushner J.P. Mutational analysis of human Uroporphyrinogen Decarboxylase. Biochim Biophys Acta BBA Protein Struct Mol Enzym. 1996;1298(2):294–304. doi: 10.1016/S0167-4838(96)00148-3. [DOI] [PubMed] [Google Scholar]
- 52.Martins B.M., Grimm B., Mock H.-P., Huber R., Messerschmidt A. Crystal STructure and Substrate Binding Modeling of the Uroporphyrinogen-III decarboxylase from nicotiana tabacum. J Biol Chem. 2001;276(47):44108–44116. doi: 10.1074/jbc.M104759200. [DOI] [PubMed] [Google Scholar]
- 53.Phillips J.D. Structural basis for tetrapyrrole coordination by Uroporphyrinogen Decarboxylase. EMBO J. 2003;22(23):6225–6233. doi: 10.1093/emboj/cdg606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang B., Sun L., Wen X., Xi Z. An Interaction Network in Bacillus Subtilis coproporphyrinogen oxidase is essential for the oxidation of protoporphyrinogen IX. Proteins Struct Funct Bioinforma. 2023:26501. doi: 10.1002/prot.26501. (prot) [DOI] [PubMed] [Google Scholar]
- 55.Witkowski D.A., Halling B.P. Inhibition of plant protoporphyrinogen oxidase by the herbicide acifluorfen-Methyl. Plant Physiol. 1989;90(4):1239–1242. doi: 10.1104/pp.90.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsumoto H., Duke S.O. Acifluorfen-Methyl Effects on Porphyrin Synthesis in Lemna Pausicostata Hegelm. 6746. J Agric Food Chem. 1990;38(11):2066–2071. doi: 10.1021/jf00101a014. [DOI] [Google Scholar]
- 57.Klimka A., Mertins S., Nicolai A.K., Rummler L.M., Higgins P.G., Günther S.D., et al. Epitope-specific immunity against staphylococcus aureus coproporphyrinogen III oxidase. Npj Vaccin. 2021;6(1):11. doi: 10.1038/s41541-020-00268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surdel M.C., Horvath D.J., Lojek L.J., Fullen A.R., Simpson J., Dutter B.F., et al. Antibacterial photosensitization through activation of coproporphyrinogen oxidase. Proc Natl Acad Sci. 2017;114(32) doi: 10.1073/pnas.1700469114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darby E.M., Trampari E., Siasat P., Gaya M.S., Alav I., Webber M.A., et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol. 2022 doi: 10.1038/s41579-022-00820-y. [DOI] [PubMed] [Google Scholar]
- 60.Lecerof D., Fodje M., Hansson A., Hansson M., Al-Karadaghi S. Structural and mechanistic basis of porphyrin metallation by Ferrochelatase. J Mol Biol. 2000;297(1):221–232. doi: 10.1006/jmbi.2000.3569. [DOI] [PubMed] [Google Scholar]
- 61.Hansson M.D., Karlberg T., Rahardja M.A., Al-Karadaghi S., Hansson M. Amino acid residues His183 and Glu264 in Bacillus Subtilis Ferrochelatase direct and facilitate the insertion of metal ion into protoporphyrin IX. Biochemistry. 2007;46(1):87–94. doi: 10.1021/bi061760a. [DOI] [PubMed] [Google Scholar]
- 62.Al-Karadaghi S., Hansson M., Nikonov S., Jönsson B., Hederstedt L. Crystal Structure of Ferrochelatase: the terminal enzyme in heme biosynthesis. Structure. 1997;5(11):1501–1510. doi: 10.1016/S0969-2126(97)00299-2. [DOI] [PubMed] [Google Scholar]
- 63.Lecerof D., Fodje M.N., Alvarez León R., Olsson U., Hansson A., Sigfridsson E., et al. Metal binding to bacillus subtilis ferrochelatase and interaction between metal sites. JBIC J Biol Inorg Chem. 2003;8(4):452–458. doi: 10.1007/s00775-002-0436-1. [DOI] [PubMed] [Google Scholar]
- 64.Shipovskov S., Karlberg T., Fodje M., Hansson M.D., Ferreira G.C., Hansson M., et al. Metallation of the transition-state inhibitor N-methyl mesoporphyrin by ferrochelatase: implications for the catalytic reaction mechanism. J Mol Biol. 2005;352(5):1081–1090. doi: 10.1016/j.jmb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Karlberg T., Hansson M.D., Yengo R.K., Johansson R., Thorvaldsen H.O., Ferreira G.C., et al. Porphyrin binding and distortion and substrate specificity in the ferrochelatase reaction: the role of active site residues. J Mol Biol. 2008;378(5):1074–1083. doi: 10.1016/j.jmb.2008.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabler T., Sebastiani F., Helm J., Dali A., Obinger C., Furtmüller P.G., et al. Substrate Specificity and Complex Stability of Coproporphyrin Ferrochelatase Is Governed by Hydrogen‐bonding Interactions of the Four Propionate Groups. FEBS J. 2022;289(6):1680–1699. doi: 10.1111/febs.16257. [DOI] [PubMed] [Google Scholar]
- 67.Dali A., Gabler T., Sebastiani F., Destinger A., Furtmüller P.G., Pfanzagl V., et al. Active Site Architecture of Coproporphyrin Ferrochelatase with Its Physiological Substrate Coproporphyrin III: propionate interactions and porphyrin core deformation. Protein Sci. 2023;32(1) doi: 10.1002/pro.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Medlock A., Swartz L., Dailey T.A., Dailey H.A., Lanzilotta W.N. Substrate interactions with human ferrochelatase. Proc Natl Acad Sci. 2007;104(6):1789–1793. doi: 10.1073/pnas.0606144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medlock A.E., Najahi-Missaoui W., Shiferaw M.T., Albetel A.N., Lanzilotta W.N., Dailey H.A. Insight into the function of active site residues in the catalytic mechanism of human ferrochelatase. Biochem J. 2021;478(17):3239–3252. doi: 10.1042/BCJ20210460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medlock A.E., Najahi-Missaoui W., Ross T.A., Dailey T.A., Burch J., O’Brien J.R., et al. Identification and characterization of Solvent-Filled channels in human ferrochelatase. Biochemistry. 2012;51(27):5422–5433. doi: 10.1021/bi300598g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dietz J.V., Willoughby M.M., Piel R.B., Ross T.A., Bohovych I., Addis H.G., et al. Mitochondrial Contact Site and Cristae Organizing System (MICOS) machinery supports heme biosynthesis by enabling optimal performance of ferrochelatase. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weerth R.S., Medlock A.E., Dailey H.A. Ironing out the Distribution of [2Fe-2S] Motifs in Ferrochelatases. J Biol Chem. 2021;297(5) doi: 10.1016/j.jbc.2021.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Layer G. Heme biosynthesis in prokaryotes. Biochim Biophys Acta BBA Mol Cell Res. 2021;1868(1) doi: 10.1016/j.bbamcr.2020.118861. [DOI] [PubMed] [Google Scholar]
- 74.Hansson M.D., Lindstam M., Hansson M. Crosstalk between metal ions in bacillus subtilis ferrochelatase. JBIC J Biol Inorg Chem. 2006;11(3):325–333. doi: 10.1007/s00775-006-0080-2. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y., Shen Y., Ryde U. QM/MM study of the insertion of metal ion into protoporphyrin IX by ferrochelatase. J Inorg Biochem. 2009;103(12):1680–1686. doi: 10.1016/j.jinorgbio.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y., Wu J., Ju J., Shen Y. Investigation by MD simulation of the key residues related to substrate-binding and heme-release in human ferrochelatase. J Mol Model. 2013;19(6):2509–2518. doi: 10.1007/s00894-013-1789-9. [DOI] [PubMed] [Google Scholar]
- 77.Kohno H., Okuda M., Furukawa T., Tokunaga R., Taketani S. Site-directed mutagenesis of human ferrochelatase: identification of Histidine-263 as a binding site for metal ions. Biochim Biophys Acta BBA Protein Struct Mol Enzym. 1994;1209(1):95–100. doi: 10.1016/0167-4838(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 78.Hunter G.A., Ferreira G.C. Identification and characterization of an inhibitory metal ion-binding site in ferrochelatase. J Biol Chem. 2010;285(53):41836–41842. doi: 10.1074/jbc.M110.174243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franco R., Ma J.-G., Lu Y., Ferreira G.C., Shelnutt J.A. Porphyrin interactions with wild-type and mutant mouse ferrochelatase. Biochemistry. 2000;39(10):2517–2529. doi: 10.1021/bi991346t. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Shen Y. Is It Possible for Fe2+ to approach protoporphyrin IX from the side of Tyr-13 in Bacillus Subtilis Ferrochelatase? An Answer from QM/MM Study. J Mol Model. 2013;19(2):963–971. doi: 10.1007/s00894-012-1627-5. [DOI] [PubMed] [Google Scholar]
- 81.Hofbauer S., Mlynek G., Milazzo L., Pühringer D., Maresch D., Schaffner I., et al. Hydrogen Peroxide‐mediated Conversion of Coproheme to Heme b by HemQ-Lessons from the First Crystal Structure and Kinetic Studies. FEBS J. 2016;283(23):4386–4401. doi: 10.1111/febs.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Milazzo L., Gabler T., Pühringer D., Jandova Z., Maresch D., Michlits H., et al. Redox cofactor rotates during its stepwise decarboxylation: molecular mechanism of conversion of coproheme to Heme b. ACS Catal. 2019;9(8):6766–6782. doi: 10.1021/acscatal.9b00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Streit B.R., Celis A.I., Moraski G.C., Shisler K.A., Shepard E.M., Rodgers K.R., et al. Decarboxylation involving a Ferryl, propionate, and a Tyrosyl group in a radical relay yields Heme b. J Biol Chem. 2018;293(11):3989–3999. doi: 10.1074/jbc.RA117.000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Michlits H., Lier B., Pfanzagl V., Djinović-Carugo K., Furtmüller P.G., Oostenbrink C., et al. Actinobacterial coproheme decarboxylases use histidine as a distal base to promote compound I formation. ACS Catal. 2020;10(10):5405–5418. doi: 10.1021/acscatal.0c00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu W., Pang Y., Song Y., Li X., Tan H., Chen G. Reorienting mechanism of harderoheme in coproheme decarboxylase-a computational study. Int J Mol Sci. 2022;23(5):2564. doi: 10.3390/ijms23052564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sebastiani F., Risorti R., Niccoli C., Michlits H., Becucci M., Hofbauer S., et al. An active site at work – the role of key Residues in C. Diphteriae Coproheme Decarboxylase. J Inorg Biochem. 2022;229 doi: 10.1016/j.jinorgbio.2022.111718. [DOI] [PubMed] [Google Scholar]
- 87.Sebastiani F., Michlits H., Lier B., Becucci M., Furtmüller P.G., Oostenbrink C., et al. Reaction intermediate rotation during the decarboxylation of coproheme to Heme b in C. Diphtheriae. Biophys J. 2021;120(17):3600–3614. doi: 10.1016/j.bpj.2021.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofbauer S., Pfanzagl V., Michlits H., Schmidt D., Obinger C., Furtmüller P.G. Understanding molecular enzymology of Porphyrin-Binding α + β Barrel Proteins - one fold, multiple functions. Biochim Biophys Acta BBA Proteins Proteom. 2021;1869(1) doi: 10.1016/j.bbapap.2020.140536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hofbauer S., Hagmüller A., Schaffner I., Mlynek G., Krutzler M., Stadlmayr G., et al. Structure and Heme-Binding Properties of HemQ (Chlorite Dismutase-like Protein) from Listeria Monocytogenes. Arch Biochem Biophys. 2015;574:36–48. doi: 10.1016/j.abb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Michlits H., Valente N., Mlynek G., Hofbauer S. Initial steps to engineer coproheme decarboxylase to obtain stereospecific monovinyl, monopropionyl deuterohemes. Front Bioeng Biotechnol. 2022;9 doi: 10.3389/fbioe.2021.807678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bromberg R., Guo Y., Borek D., Otwinowski Z. High-Resolution Cryo-EM reconstructions in the presence of substantial aberrations. IUCrJ. 2020;7(3):445–452. doi: 10.1107/S2052252520002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodrigues A.V., Batelu S., Hinton T.V., Rotondo J., Thompson L., Brunzelle J.S., et al. Drosophila Melanogaster Frataxin: protein crystal and predicted solution structure with identification of the iron-binding regions. Acta Crystallogr Sect Struct Biol. 2023;79(1):22–30. doi: 10.1107/S2059798322011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nair M., Adinolfi S., Pastore C., Kelly G., Temussi P., Pastore A. Solution structure of the bacterial Frataxin Ortholog, CyaY. Structure. 2004;12(11):2037–2048. doi: 10.1016/j.str.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 94.He Y., Alam S.L., Proteasa S.V., Zhang Y., Lesuisse E., Dancis A., et al. Yeast Frataxin solution structure, iron binding, and ferrochelatase interaction. Biochemistry. 2004;43(51):16254–16262. doi: 10.1021/bi0488193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Srivastava D.K., Bernhard S.A. Vol. 28. Elsevier,; 1986. Enzyme–Enzyme Interactions and the Regulation of Metabolic Reaction Pathways; pp. 1–68. (Current Topics in Cellular Regulation). [DOI] [PubMed] [Google Scholar]
- 96.Ovádi J., Srere P.A. Metabolic consequences of enzyme interactions: metabolic enzyme interactions. Cell Biochem Funct. 1996;14(4):249–258. doi: 10.1002/cbf.699. [DOI] [PubMed] [Google Scholar]
- 97.Medlock A.E., Shiferaw M.T., Marcero J.R., Vashisht A.A., Wohlschlegel J.A., Phillips J.D., et al. Identification of the mitochondrial heme metabolism complex. PLOS ONE. 2015;10(8) doi: 10.1371/journal.pone.0135896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piel R.B., Dailey H.A., Medlock A.E. The mitochondrial heme metabolon: insights into the complex(Ity) of Heme synthesis and distribution. Mol Genet Metab. 2019;128(3):198–203. doi: 10.1016/j.ymgme.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Videira M.A.M., Lobo S.A.L., Silva L.S.O., Palmer D.J., Warren M.J., Prieto M., et al. Staphylococcus Aureus Haem Biosynthesis and Acquisition Pathways Are Linked through Haem Monooxygenase IsdG. Mol Microbiol. 2018;109(3):385–400. doi: 10.1111/mmi.14060. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z., Yang L., Zhao X.-E. Co-Crystallization and Structure Determination: an effective direction for Anti-SARS-CoV-2 Drug Discovery. Comput Struct Biotechnol J. 2021;19:4684–4701. doi: 10.1016/j.csbj.2021.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson J.L., Entzminger K.C., Hyun J., Kalyoncu S., Heaner D.P., Morales I.A., et al. Structural and Biophysical Characterization of an Epitope-Specific Engineered Fab Fragment and Complexation with Membrane Proteins: implications for Co-Crystallization. Acta Crystallogr D Biol Crystallogr. 2015;71(4):896–906. doi: 10.1107/S1399004715001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petkun S., Rozman Grinberg I., Lamed R., Jindou S., Burstein T., Yaniv O., et al. Reassembly and Co-Crystallization of a Family 9 processive endoglucanase from its component parts: structural and functional significance of the intermodular linker. PeerJ. 2015;3 doi: 10.7717/peerj.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Faylo J.L., Christianson D.W. Visualizing transiently associated catalytic domains in assembly-line biosynthesis using cryo-electron microscopy. J Struct Biol. 2021;213(4) doi: 10.1016/j.jsb.2021.107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vonck J., Mills D.J. Advances in high-resolution Cryo-EM of oligomeric enzymes. Curr Opin Struct Biol. 2017;46:48–54. doi: 10.1016/j.sbi.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 105.Bonomi M., Pellarin R., Vendruscolo M. Simultaneous determination of protein structure and dynamics using cryo-electron microscopy. Biophys J. 2018;114(7):1604–1613. doi: 10.1016/j.bpj.2018.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu D., Mehdipour A.R., Finke F., Goojani H.G., Groh R.R., Grund T.N., et al. Dissecting the conformational complexity and mechanism of a bacterial Heme Transporter. Nat Chem Biol. 2023 doi: 10.1038/s41589-023-01314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rakers C., Bermudez M., Keller B.G., Mortier J., Wolber G. Computational close up on protein-protein interactions: how to unravel the invisible using molecular dynamics simulations?: computational close up on protein-protein interactions. Wiley Interdiscip Rev Comput Mol Sci. 2015;5(5):345–359. doi: 10.1002/wcms.1222. [DOI] [Google Scholar]
- 108.Cuendet M.A., Michielin O. Protein-protein interaction investigated by steered molecular dynamics: the TCR-PMHC Complex. Biophys J. 2008;95(8):3575–3590. doi: 10.1529/biophysj.108.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bryant P., Pozzati G., Elofsson A. Improved prediction of protein-protein interactions using AlphaFold2. Nat Commun. 2022;13(1):1265. doi: 10.1038/s41467-022-28865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Burke D.F., Bryant P., Barrio-Hernandez I., Memon D., Pozzati G., Shenoy A., et al. Towards a structurally resolved human protein interaction network. Nat Struct Mol Biol. 2023;30(2):216–225. doi: 10.1038/s41594-022-00910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carugo O., Djinović-Carugo K. Structural biology: a golden era. PLOS Biol. 2023;21(6) doi: 10.1371/journal.pbio.3002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material