Highlights
-
•
PSaC and ATPsyn-α genes play a defensive role against SMV infection.
-
•
SMV NIb and NIa-Pro-interacted with GmPSaC and gmatpsyn-α.
-
•
PSaC and ATPsyn-α may interfere with SMV NIb and NIa-Pro-function during SMV infection.
-
•
These genes may be used as resistance-related genes to delay SMV replication.
Keywords: Soybeans, Chloroplast-virus interplay, Plant defense, Soybean mosaic virus, Viral replication
Abstract
To gain a deeper understanding of the molecular mechanisms involved in viral infection and the corresponding plant resistance responses, it is essential to investigate the interactions between viral and host proteins. In the case of viral infections in plants, a significant portion of the affected gene products are closely associated with chloroplasts and photosynthesis. However, the molecular mechanisms underlying the interplay between the virus and host chloroplast proteins during replication remain poorly understood. In our previous study, we made an interesting discovery regarding soybean mosaic virus (SMV) infection in resistant and susceptible soybean cultivars. We found that the photosystem I (PSI) subunit (PSaC) and ATP synthase subunit α (ATPsyn-α) genes were up-regulated in the resistant cultivar following SMV-G7H and SMV-G5H infections compared to the susceptible cultivar. Overexpression of these two genes within the SMV-G7H genome in the susceptible cultivar Lee74 (rsv3-null) reduced SMV accumulation, whereas silencing of the PSaC and ATPsyn-α genes promoted SMV accumulation. We have also found that the PSaC and ATPsyn-α proteins are present in the chloroplast envelope, nucleus, and cytoplasm. Building on these findings, we now characterized protein-protein interactions between PSaC and ATPsyn-α with two viral proteins, NIb and NIa-Pro, respectively, of SMV. Through co-immunoprecipitation (Co-IP) experiments, we confirmed the interactions between these proteins. Moreover, when the C-terminal region of either PSaC or ATPsyn-α was overexpressed in the SMV-G7H genome, we observed a reduction in viral accumulation and systemic infection in the susceptible cultivar. Based on these results, we propose that the PSaC and ATPsyn-α genes play a modulatory role in conferring resistance to SMV infection by influencing the function of NIb and NIa-Pro-in SMV replication and movement. The identification of these photosynthesis-related genes as key players in the interplay between the virus and the host provides valuable insights for developing more targeted control strategies against SMV. Additionally, by utilizing these genes, it may be possible to genetically engineer plants with improved photosynthetic efficiency and enhanced resistance to SMV infection.
1. Introduction
Soybean mosaic virus (SMV), a member of the genus Potyvirus, infects soybeans and is spread by aphids, resulting in severe diseases and significant economic losses around the globe (Hajimorad et al., 2018). The Rsv and Rsc sets of strain-specific NLR-type R-genes, are primarily used to provide genetic resistance to SMV (Widyasari et al., 2020). While Rsc genes that offer resistance to the SC1 to SC22 strains were recorded in China, Rsv genes that confer resistance to the G1 to G7 SMV strains were discovered in the United States. The R gene may result in HR or ER responses depending on the strain and load of the virus (Alazem et al., 2023; Widyasari et al., 2020). There are several other non-NLR host factors for example, GmPP2C3a, GmPAP2.1, PSaC, and ATPsyn-α that have been found to be crucial for resistance, either because they are key components in the signaling cascade that runs downstream of the R-gene or because they regulate immune responses, including plant hormones and RNAi pathways (Bwalya et al., 2022; Seo et al., 2014; Widyasari et al., 2022).
For SMV to infect and replicate on its host, complex molecular interactions between viral proteins and host proteins are required particularly for this kind of virus like other positive-sense single-stranded RNA viruses have a small genome therefore, the host machinery is responsible for the replication of viral genomes (Bwalya and Kim, 2023; Hajimorad et al., 2018; Zhao et al., 2016, 2019; Widyasari et al., 2023). Innumerable host factors of plant-virus interactions have been identified, and interestingly, large proportions of these host factors are chloroplast- and photosynthesis-related proteins (Bhattacharyya and Chakraborty, 2018; Bwalya and Kim, 2023; Yadav et al., 2019; Zhao et al., 2016, 2019). Although photosynthesis is the major function of the chloroplast, its roles clearly extend further than converting light energy into chemical energy. It is evident that plants require more energy from photosynthesis during interactions with pathogens since initiating defense responses requires the energy that photosynthesis provides (Hammerschmidt,1999; Swarbrick et al., 2006). Chloroplast not only provides energy but also plays important roles in the production of reactive oxygen species (ROS), calcium (Ca2+), and several defense-related hormones like salicylic acid (SA), jasmonic acid (JA), and abscisic acid (ABA) that have significant connections to plant immunity (Bhattacharyya and Chakraborty, 2018; Bobik and Burch-Smith, 2015; Bwalya and Kim, 2023; Kozuleva et al., 2011; Nambara and Marion-Poll, 2005; Padmanabhan and Dinesh-Kumar, 2010; Seyfferth and Tsuda, 2014; Stael et al., 2015; Torres et al., 2006; Wasternack, 2007; Wasternack and Hause, 2013; Widyasari et al., 2022; Wildermuth et al., 2001; Yang et al., 2021). Although research on the molecular mechanisms underlying SMV infection of plants has advanced, little is known about how SMV proteins interact with chloroplast-related proteins.
In our previous study (Bwalya et al., 2022), we observed strong upregulation of two chloroplast-related proteins, the photosystem I (PSI) subunit (GmPSaC) and ATP synthase subunit α (GmATPsyn-α), in cultivar L29 in response to SMV-G5H infection, but a weaker response to SMV-G7H. Overexpression of either GmPSaC or GmATPsyn-α in the SMV-G7H genome induced resistance against SMV infection in the susceptible soybean cultivar Lee74, and both proteins were found to localize in the chloroplast envelope, the nucleus, and the cytoplasm. Knockdown of either GmPSaC or GmATPsyn-α significantly reduced their expression, and pSMV-G7H::GFP-infected knockdown plants exhibited a strong GFP signal in the systemic leaves.
In this study, we used a yeast two-hybrid system to identify interactions between SMV viral proteins and soybean host proteins GmPSaC and GmATPsyn-α. Our results demonstrated that nuclear inclusion protein b (NIb) and nuclear inclusion protein a (NIa-Pro) interacted with GmPSaC and GmATPsyn-α, respectively, in the cytoplasm and nucleus, impairing the replication and movement of these viral proteins. We also showed that the C-terminal portion of GmPSaC or GmATPsyn-α is crucial for these interactions. Overexpression of the C-terminal portion of either protein in the SMV-G7H genome reduced viral accumulation and systemic infection.
2. Materials and methods
2.1. Plant materials, growth conditions, and viral infection
Soybean (Glycine max) and Nicotiana benthamiana plants were grown in growth chambers at 25 °C with 70% relative humidity and a 16/8 h photoperiod. To investigate the effects of PSaC and ATPsyn-α mutants on the accumulation of SMV-G7H::GFP in Lee74 plants, plasmids of the infectious clones pSMV-G7H::GFP, pSMV-G7H::GFP::PSaC, pSMV-G7H::eGFP::PSaCΔN&ΔFer, pSMV-G7H::eGFP:: PSaCΔC&ΔN, pSMV-G7H::eGFP:: PSaCΔC&ΔFer4, pSMV-G7H::eGFP::PSaCΔN, pSMV-G7H::eGFP::PSaCΔC, pSMV-G7H::GFP::ATPsyn-α, pSMV-G7H::eGFP::ATPsyn-αΔN&NBD, pSMV-G7H::eGFP::ATPsyn-αΔN&ΔC, pSMV-G7H::eGFP::ATPsyn-αΔC, pSMV-G7H::eGFP::ATPsyn-αΔN, and pSMV-G7H::eGFP::ATPsyn-αΔC &NBD were directly rub-inoculated on Lee74 plants using 10 µg of plasmid per leaf. The upper systemic leaves (SLs) were collected at 14 days post-inoculation (dpi) for RNA and protein extraction.
2.2. RNA extraction and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol (Sigma) following the manufacturer's instructions. A 1 μg quantity of total RNA was used for cDNA synthesis using the GoScript kit (Promega, USA). RT-qPCR was carried out with SYBR-Green (Promega, USA) to measure the relative expression of target genes using the ΔΔCT method. Actin11 was used as an internal control, and the primers used in this study are listed in Table S1. One-sided Student's t-tests (p < 0.05) were used to determine the expression level of eGFP. The experiment was conducted with at least 3 biological replicates.
2.3. Plasmid construction
Total RNA was isolated from leaf samples harvested from soybean plants using TRIzol (Sigma, USA) reagent. The RNA samples were used for cDNA synthesis using the GoSript kit (Promega, USA). The mutants were amplified and cloned were then cloned into a TA vector (pGEM-T Easy, Promega, USA). The clones were confirmed by sequencing with gene-specific primers (Table S1) and then cloned into the pSMV-G7H::GFP infectious clone to generate clones pSMV-G7H::GFP, pSMV-G7H::GFP::PSaC, pSMV-G7H::eGFP::PSaCΔN&ΔFer, pSMV-G7H::eGFP::PSaCΔC&ΔN, pSMV-G7H::eGFP::PSaCΔC&ΔFer4, pSMV-G7H::eGFP::PSaCΔN, pSMV-G7H::eGFP::PSaCΔC, and then pSMV-G7H::GFP::ATPsyn-α, pSMV-G7H::eGFP::ATPsyn-αΔN&NBD, pSMV-G7H::eGFP:: ATPsyn-αΔN&ΔC, pSMV-G7H::eGFP::ATPsyn-αΔC, pSMV-G7H::eGFP::ATPsyn-αΔN, and pSMV-G7H::eGFP::ATPsyn-αΔC &NBD as previously described (Seo et al., 2009).
2.4. Western blotting
Total proteins were extracted from 0.1 g of tissue collected from a pool of inoculated or systemically infected leaves from three plants as described previously (Bwalya et al., 2022). Constructs expressing GFP were detected by protein blot using a polyclonal anti-GFP antibody (Sigma, USA). The primary antibody was bound with the goat anti-rabbit secondary antibody (Bio-Rad, USA). Ponceau-S was used as a loading control.
2.5. Co-immunoprecipitation (Co-IP) assay
For the Co-IP assay, total proteins from N. benthamiana leaves were collected three days after agroinfiltration. Total protein was extracted from 2 g of leaves (a pool of 6 leaves from 3 plants) in extraction buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 M MgCl2, 5 mM DTT, and 5% Nonidet P-40) with protease inhibitor cocktail (Roche, Swiss). Immunoprecipitation was carried out by incubating protein extracts with GFP-Trap beads for 3 h at 4 °C on a gentle rotary shaking. The precipitations were washed four times with cold immunoprecipitation buffer at 4 °C and beads were suspended in 100 µL of extraction buffer, and 35 µL was proportionally mixed with 4 × NuPAGE loading buffer (Thermo-Scientific, USA) and loaded onto a 12% acrylamide SDS-PAGE gel which was then analyzed by immunoblot using anti-mCherry or anti-GFP antibodies as prescribed (Yang et al., 2014).
2.6. Statistical analysis
RT-qPCR was carried out in at least three biological replicates, and each biological replicate was repeated in three technical replicates. In the figure panels with bar graphs, values were compared to that of the mock-treated, uninfected plants (the bar on the left) with one-sided Student's t-tests; * and ** indicate a significant difference at P < 0.05 and < 0.01, respectively. Error bars in the charts are means of the standard deviation of three biological replicates.
2.7. Subcellular localization
ATPsyn-α, PSaC, and chloroplast marker protein EMB1301 were previously cloned into the binary vector pBin61–3HA-mCherry (Bwalya et al., 2022). pBin61-eGFP was constructed by replacing 3HA-mCherry with eGFP, and NIa-Pro, NIb, or chloroplast marker protein EMB130. Agrobacterium infiltration was carried out on N. benthamiana plants using Agrobacterium tumefaciens strain GV3101 at OD600 = 0.5. Samples were collected at 3 dpi for confocal microscopy and protein extraction. A Leica confocal microscope was used to determine the subcellular localization with a 40x lens and a scanning speed of 400 Hz. The ImageJ software evaluated co-localization between proteins using Pearson's correlation coefficient.
2.8. Yeast two-hybrid (Y2H) and X-α-Gal assays
For Y2H assays, ATPsyn-α, PSaC, and their mutants were cloned into pACT2 (AD), and SMV-G5H proteins were cloned into pAS2–1 (BD). Different constructs with the combination of BD and AD vectors were co-transformed into AH109 and grown on plates of SD medium lacking leucine and tryptophan (SD-Leu/-Trp) for 2 d at 30 °C. Single colonies were selected and grown on SD-Trp/-Leu-broth medium until OD600 = 0.5 and then transferred to either SD-Leu/-Trp, or SD-His/-Leu/-Trp, or SD-His/-Leu/-Trp/-Ade agar medium at serial dilutions of 10°, 10−1, 10−2, and 10−3 for 2 d at 30 °C. All protein-protein interactions were confirmed by α-galactosidase activity using X-α-Gal reagent (Clontech) by streaking newly formed co-transformants on SD-His/-Leu/-Trp/-Ade coated with X-α-gal.
3. Results
3.1. Soybean proteins ATPsyn-α and PSaC interact with NIa and NIb, respectively
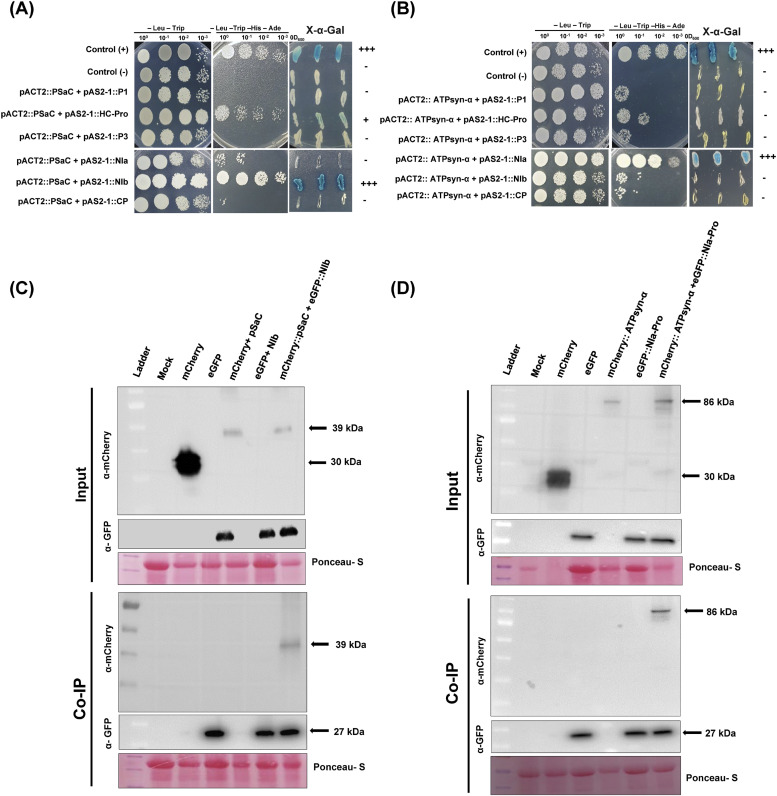
The yeast two-hybrid assay results suggest that GmPSaC interacts with SMV NIb and GmATPsyn-α interacts with SMV NIa-Pro. We observed strong interaction between GmPSaC and Nib by the growth of yeast colonies on media deficient in Leu -Trip -His -Ade and the blue color on X-α-Gal -containing media (Figs. 1A and S1A). Similarly, the strong interaction between GmATPsyn-α and NIa-Pro was confirmed by the growth of yeast colonies on media deficient in -Leu-Trip-His-Ade and then blue color was observed on X-α-Gal -containing media (Figs. 1B and S1B). Co-IP assay with GFP-trap beads confirmed these interactions by showing the immunoprecipitation of GmPSaC and GmATPsyn-α with NIb and NIa-Pro, respectively (Fig. 1C and D). These findings suggest that GmPSaC and GmATPsyn-α may modulate resistance to SMV infection by affecting the function of NIb and NIa-Pro in SMV replication and movement.
Fig. 1.
Interactions between SMV viral proteins and soybean's chloroplast-related proteins. (A) Analysis of interactions between SMV proteins and GmPSaC in the yeast two-hybrid (Y2H) system. (B) Analysis of interactions between SMV proteins and GmATPsyn-α in the Y2H system. Dilutions (10°, 10−1,10−2, and 10−3) of yeast cultures at OD600 = 0.5 were spotted into -Leu -Trip -His -Ade deficient (left) or X-α-Gal -containing (right) plates and grown for 3 d at 28 °C. The symbol of "+” indicates the intensity of the interaction while “-" means no interaction. (C) Co-immunoprecipitation (Co-IP) analysis showing a direct interaction between SMV NIa-Pro-and ATPsyn-α. (D) Co-IP analysis showing a direct interaction between SMV NIb and PSaC.
3.2. Analysis of in planta interactions in N. benthamiana
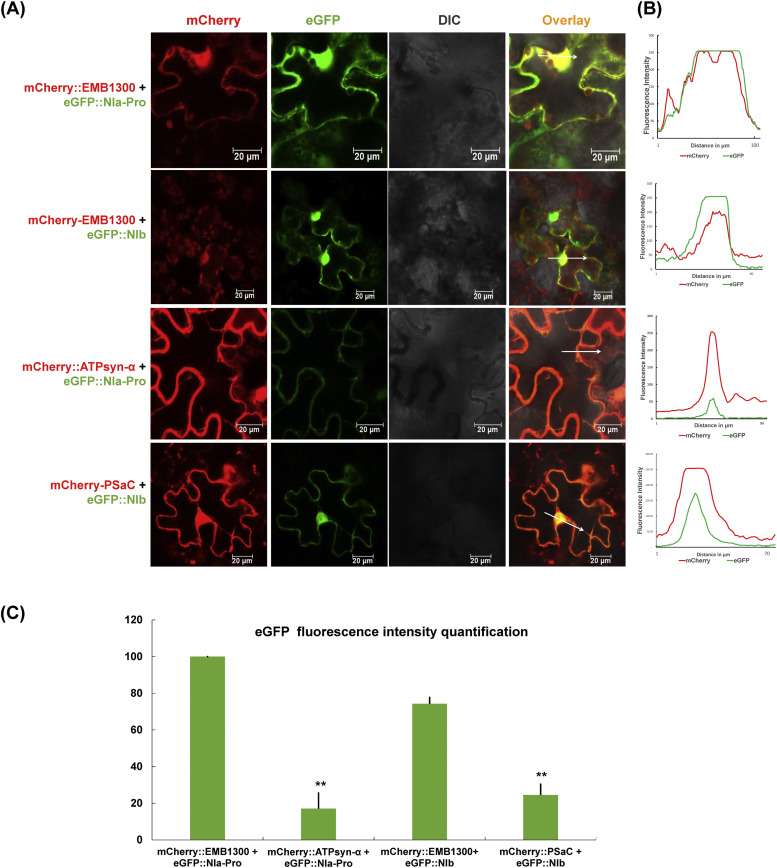
Previously, we confirmed the cellular expression of GmPSaC and GmATPsyn-α in N. benthamiana cells by expressing both genes in the binary vector pBin61-HA-mCherry (Alazem et al., 2020; Bwalya et al., 2022). Both PSaC and ATPsyn-α were present in the nucleus, the cytoplasm, and the chloroplast envelope. We used a chloroplast-localized protein from Arabidopsis, EMB1303 tagged with GFP as a marker protein for confirmation of chloroplast localization (Bwalya et al., 2022; Huang et al., 2009). This study further investigates possible co-localization between the PSaC and SMV NIb, and between ATPsyn-α and SMV NIa-Pro-in planta using a Leica confocal microscope. After co-expression of PSaC::mCherry and ATPsyn-α::mCherry with SMV NIb::GFP and SMV NIa-Pro::GFP in N. benthamiana through Agrobacterium-mediated infiltration, we observed mCherry signal overlapped with GFP signals in the cytoplasm and nucleus (Fig. 2A and B). Our results prove that GmPSaC and GmATPsyn-α co-localized with SMV NIb and NIa-Pro-in N. benthamiana cells. The co-localization PSaC::mCherry and ATPsyn-α::mCherry with SMV NIb::GFP and SMV NIa-Pro::GFP resulted in reduced expression of SMV NIb and NIa-Pro. Further studies are needed to elucidate the precise mechanisms underlying these interactions and their impact on viral pathogenesis.
Fig. 2.
Co-expression of PSaC/ATPsyn-α with NIb or NIa-Pro-in the Nicotiana benthamiana cell. (A) Co-expression of PSaC or ATPsyn-α tagged with mCherry with NIb or NIa-Pro-tagged with a green fluorescent protein (GFP) in the N. benthamiana. The leaves were examined at 3 days post-co-infiltration, and fluorescence was assessed by confocal microscopy. (B) Pearson's coefficient of localization represents the degree of fluorescence coincidence. The intensity of eGFP fluorescence (green line) and mCherry fluorescence (red line) are on the right panels. (C) Statistical analysis was performed to compare eGFP expression levels, based on data from at least three biological replicates. The expression levels of eGFP with co-expression of mCherry::EMB1300 were used as a reference and compared using one-sided Student's t-tests. Symbols * and ** indicate a significant difference at p < 0.05 and p < 0.01, respectively.
3.3. The C-terminal region of either ATPsyn-α or PSaC is crucial for protein-protein interaction
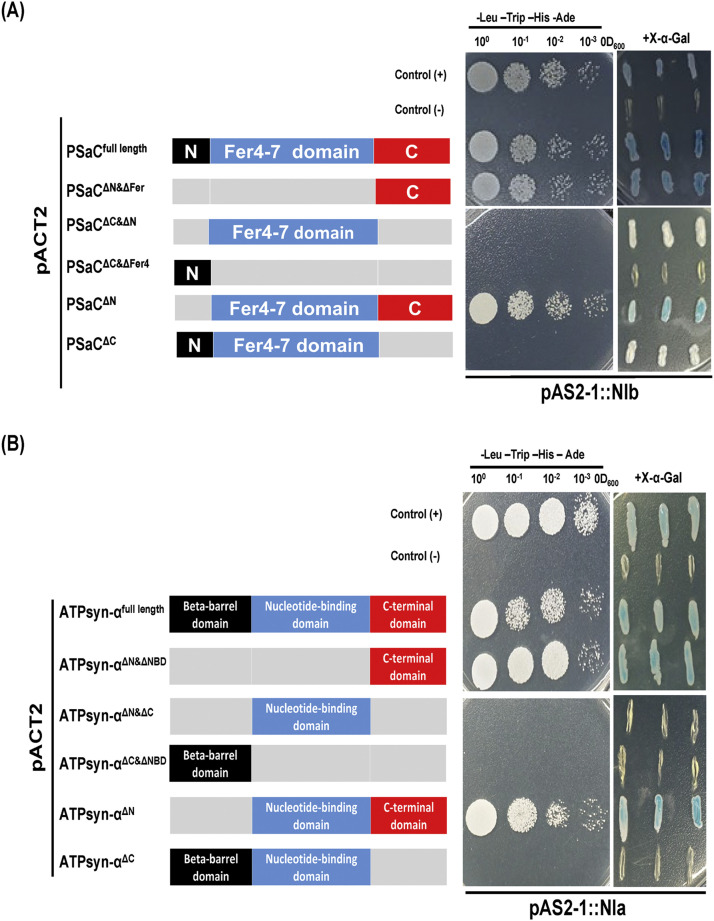
To investigate specific regions of GmPSaC and GmATPsyn-α responsible for interaction, we generated five GmPSaC deletion mutants (Fig. 3A) and five GmATPsyn-α deletion mutants (Fig. 3B). In the Y2H assay, it was determined that two Gm. ATPsyn-α mutants (ATPsyn-αΔN&NBD and ATPsyn-αΔN) and two GmPSaC mutants (PSaC ΔN &ΔFer and PSaCΔN) interacted with NIa-Pro-and with NIb respectively as they grew very well in media lacking Leu-Trip-His-Ade and turned blue on X-α-Gal -containing media (Fig. 3A and B). These interactions, however, were not observed when the mutants without the C-terminal of either ATPsyn-α or PSaC were used (Fig. 3A and B), demonstrating that the C-terminal region of either ATPsyn-α or PSaC is responsible for its interaction with NIa-Pro-and NIb, respectively.
Fig. 3.
Analysis of SMV NIb/ NIa-Pro-interaction with GmPSaC/GmATPsyn-α deletions. (A) Schematic representation of GmPSaC and its deletion mutants used in Y2H assays are depicted in the left diagram and their interaction with NIb on the right. (B) Schematic representation of GmATPsyn-α and its deletion mutants used in Y2H assays are depicted in the left diagram and their interaction with NIa-Pro-on the right. Dilutions (10°, 10−1, 10−2, and 10−3) of yeast cultures at OD600 = 0.5 were spotted into -Leu -Trip -His -Ade deficient (left) or X-α-Gal -containing (right) plates and grown for 3 d at 28 °C. The symbol of "+” indicates the intensity of the interaction while “-" means no interaction.
3.4. The C- terminal of either ATPsyn- α or PSaC is required for resistance
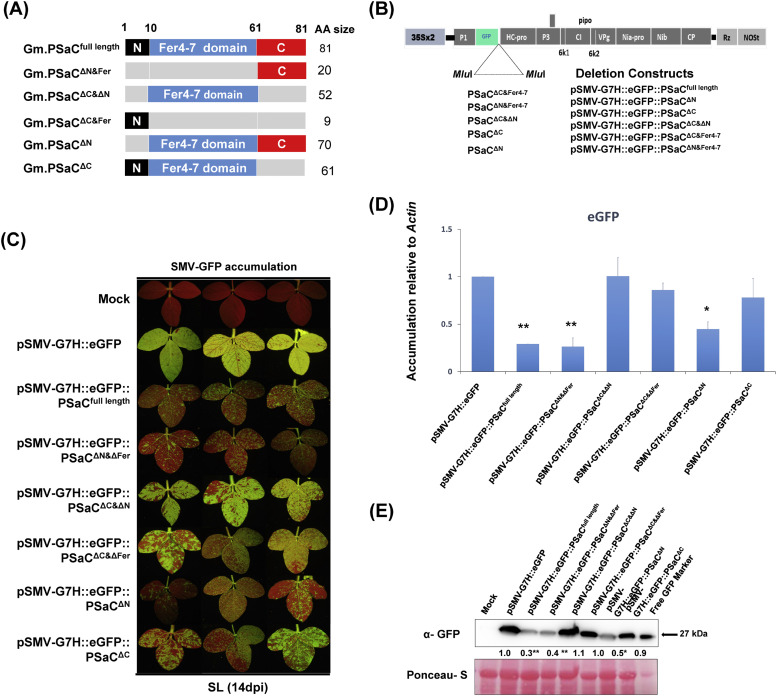
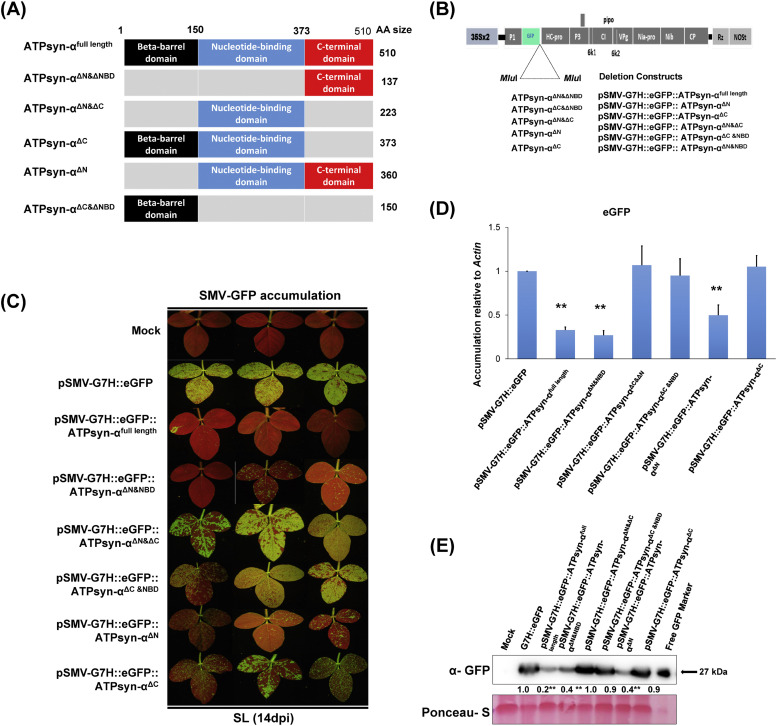
To further investigate the region of GmPSaC and GmATPsyn-α responsible for resistance to SMV, we cloned deletion mutants into pSMV-G7H::GFP and overexpressed them on the susceptible cultivar Lee 74 (Fig. 4A and B; Fig. 5A and B). We discovered that the mutants with N-terminal deletions and middle part deletions of both PSaC and ATPsyn-α did not interfere with the resistance generated by PSaC and ATPsyn-α (Fig. 4C and D; Fig. 5C and D). Moreover, GFP expression showed that virus replication caused by pSMV-G7H::GFP::PSaC and pSMV-G7H::GFP::ATPsyn-α were similar with or without these deletion mutations in the systemic leaves (Fig. 4C and D; Fig. 5C and D). These findings were supported by a western protein blot, which showed that both pSMV-G7H::GFP::PSaC and pSMV-G7H::GFP::ATPsyn-α had similar GFP protein accumulation with and/or without N-terminal and middle portion deletions (Figure 4E; Fig. 5E). However, when we deleted the C-terminal region of either Gm PSaC or Gm ATPsyn-α, overexpression of these mutants resulted in increased GFP expressions in the systemic leaves, indicating the disruption of resistance to SMV-G7H. Therefore, our results indicate that the C- terminus of either ATPsyn-α or PSaC is required for resistance (Fig. 4C–E; Fig. 5C–E).
Fig. 4.
Experimental design, chimera constructions, and effect of GmPSaC full length and deletion mutants. (A) Scheme showing the protocol followed to generate deletion mutants. (B) A schematic diagram showing the genome organization of pSMV-G7H::eGFP on top and deletion constructs below. (C) Green fluorescent protein (GFP) visual levels in the systemically infected leaves (SL) of Lee74 plants. The first unifoliate leaves of 12-day-old seedlings were infected with pSMV-G7H::eGFP, pSMV-G7H::eGFP::PSaC, pSMV-G7H::eGFP::PSaCΔN&ΔFer, pSMV-G7H::eGFP::PSaCΔC&ΔN, pSMV-G7H::eGFP::PSaCΔC&ΔFer4, pSMV-G7H::eGFP::PSaCΔN and pSMV-G7H::eGFP::PSaCΔC. Fourteen days later, the SL from three plants (1, 2, and 3) were photographed under UV light. (D) Relative expression levels of GFP RNA in SL of Lee74 infected with constructs. Actin11 was used as the internal control. (E) Western protein blot for GFP levels in the systemic leaves the SL, and their quantified levels (lower panel). Ponceau S staining of RuBisCO was used on the loading control. Values are means of three biological replicates. Values were compared to that of the corresponding mock-treated plants (the bar on the left) with one-sided Student's t-tests. Symbols * and ** indicate a significant difference at p < 0.05 and p < 0.01, respectively.
Fig. 5.
Experimental design, chimera constructions, and effect of GmATPsyn-α full length and deletion mutants. (A) Scheme showing the protocol followed to generate deletion mutants. (B) Schematic diagram showing the genome organization of pSMV-G7H::eGFP on top and deletion constructs below. (C) GFP visual levels in the systemically infected leaves (SL) of Lee74 plants. The first unifoliate leaves of 12-day-old seedlings were infected with pSMV-G7H::eGFP, pSMV-G7H::eGFP::ATPsyn-α, pSMV-G7H::eGFP::ATPsyn-αΔN&NBD, pSMV-G7H::eGFP::ATPsyn-αΔN&ΔC, pSMV-G7H::eGFP::ATPsyn-αΔC, pSMV-G7H::eGFP::ATPsyn-αΔN and pSMV-G7H::eGFP::ATPsyn-αΔC&NBD. Fourteen days later, the SL from three plants (1, 2, and 3) were photographed under UV light. (D) Relative expression levels of GFP RNA in SL of Lee74 infected with constructs. Actin11 was used as the internal control. (E) Western protein blot for GFP levels in the systemic leaves the SL, and their quantified levels (lower panel). Ponceau S staining of RuBisCO was used on the loading control. Values are means of three biological replicates. Values were compared to that of the corresponding mock-treated plants (the bar on the left) with one-sided Student's t-tests. Symbols * and ** indicate a significant difference at p < 0.05 and p < 0.01, respectively.
3.5. The C-terminal regions of either ATPsyn-α or PSaC are conserved in soybean cultivars, A. thaliana and N. benthamiana
We predicted three-dimensional (3D) structures of both PSaC and ATPsyn-α using I-TASSER (Chengxin et al., 2017; Yang and Zhang, 2015; Zhang et al., 2017; Zheng et al., 2021) (Fig. S2A and B). Next, we aligned the amino acid sequences of the C-terminal regions of either GmPSaC or GmATPsyn-α with homologs from five soybean cultivars: William 82 (W82), Lee74, Somyoungkong (SMK), V94, and L29, as well as orthologs from A. thaliana and N. benthamiana. Amino acid sequence analysis showed that the C-terminal regions of either ATPsyn or PSaC are conserved (Figure S3A and B). It is therefore reasonable to assume that the conserved C-terminal regions of both GmATPsyn-α and PSaC may be necessary for resistance during SMV infection.
3.6. Transient expression of GmPSaC and GmATPsyn-α in N. benthamiana
To analyze the effect of the GmPSaC and GmATPsyn-α genes on G7H accumulation in N. benthamiana, we transiently expressed these two genes (in a binary vector) in N. benthamiana. We co-infiltrated N. benthamiana leaves with G7H-GFP and pBin-3HA-mCherry constructs carrying ATPsyn-α or PSaC and containing an empty vector. Both soybean proteins reduced the amount of SMV-G7H that accumulated in N. benthamiana plants, suggesting that the resistance mechanism controlled by these proteins may be identical in both Glycine max (soybean) and N. benthamiana plants and separate from Rsv3-mediated resistance (Fig. S4) (Bwalya et al., 2022).
4. Discussion
This study was triggered by the analysis of RNA-Seq data. The transcript levels of GmPSaC and GmATPsyn-α temporarily increased at 8 h after infection (Bwalya et al., 2022). In addition, the expression of both proteins on susceptible cultivars delayed SMV accumulation in systemic leaves. Here, we have demonstrated that soybean proteins PSaC and ATPsyn-α directly interact with SMV NIb and NIa-Pro, respectively (Fig. 1A and B). Through Co-IP assays, we found that both GmPSaC and GmATPsyn-α, attached to mCherry, were present in the pulldown fraction and were pulled down with NIb::GFP and NIa-Pro::GFP, respectively (Fig. 1C and D). Furthermore, co-expression of PSaC::mCherry and ATPsyn-α::mCherry with SMV NIb::GFP and SMV NIa-Pro::GFP showed overlapping mCherry and eGFP fluorescence, confirming co-localization in the cytoplasm and nucleus. Interestingly, when two viral proteins, SMV NIb::GFP and SMV NIa-Pro::GFP, were co-expressed with GmATPsyn-α and GmPSaC in N. benthamiana, their expression was lower than when they were co-expressed with the chloroplast-localized protein (EMB1303) from Arabidopsis (Fig. 2). We speculate that the normal movement of NIb and NIa-Pro-to the chloroplast membrane was affected by the interaction with PSaC and ATPsyn-α, leading to low expression of these viral proteins. It's possible that the entry of SMV NIb and NIa-Pro-into the replication complex in the chloroplast membrane was delayed because the expression of SMV NIb and NIa-Pro-was so low when we co-expressed them with GmATPsyn-α and GmPSaC.
Since GmATPsyn-α and GmPSaC are chloroplast proteins, we expected them to be inside the chloroplast, surprisingly in our study we found that GmATPsyn-α and GmPSaC interact with SMV NIa and NIb in the cytoplasm (Fig. 2A and B). Most chloroplast proteins are believed to be synthesized in the cytoplasm, imported, and then targeted to a specific chloroplast compartment (Uniacke et al., 2009). It's conceivable that both SMV NIa and NIb may form a protein complex with GmATPsyn-α and GmPSaC, respectively, and consequently hijack these proteins prior to their entry into the chloroplast to delay virus infection of plants.
The SMV NIb protein was previously found to interact with soybean's poly(A)-binding protein (PABP) (Seo et al., 2007). NIb and PABP interaction has also been reported for another potyvirus, the zucchini yellow mosaic virus (Wang et al., 2000), and its interaction facilitates viral replication. However, unlike the NIb-PABP interaction that promotes viral replication, in our current study, the interaction between SMV NIb and GmPSaC induced a defense response to SMV in the susceptible soybean cultivar Lee 74.
Potyviral NIa-Pro-is a multifunctional proteinase that participates in several stages of viral infection. NIa-Pro-of papaya ringspot virus has been reported to interact specifically with the eukaryotic translation initiation factor 3 G protein (eIF3G), fructose 1, 6 bisphosphate aldolase class 1 protein (FBPA1), fk 506-binding protein (FK506BP), GTP-binding family protein (GTPBP), methionine sulfoxide reductase B1 protein (MSRB1), and metallothionein-like protein (MTL). Moreover, these proteins which interacted with NIa-Pro-play crucial roles in plant protein translation, biotic and abiotic stress responses, energy metabolism, and signal transduction (Broder et al., 1998; Gao et al., 2012). It is also reported that NIa-Pro-of potato virus Y functions as an elicitor by cleaving host-encoded proteins to elicit the Ry-mediated disease resistance in potatoes via its structural binding to the proteins (Mestre et al., 2000; 2003). In our study, the host proteins GmATPsyn-α interacted with NIa-Pro-and induced resistance to SMV infection.
Other previous reports showed that HC-Pro-of potato virus Y interacted with the ATPsyn-β subunit in Nicotiana tabacum but did not affect the enzymatic activity of ATP synthase, leading to a reduced ATP synthase content in HC-Pro-transgenic plants (Tu et al., 2015). PSaK, a member of the PSI complex, showed a positive role in resistance against plum pox virus (PPV). The cylindrical inclusion protein of PPV interacted with PSaK and interfered with its function (Jimenez et al., 2006). The influence on resistance can vary depending on the viral group, therefore we cannot generalize about how ATPsyn subunits and PSaC affect host plant resistance to viruses.
Host proteins play important roles in the viral infection cycle and can interact with potyviral proteins to allow or overcome viral infection. Understanding the mechanism of how host factors are involved in virus infection may help in developing a managing strategy for SMV infections. Moreover, we have previously reported that the soybean purple acid phosphatase (GmPAP2.1) from L29 binds with SMV P1 protein and induces robust induction of genes that regulate the SA synthesis pathway (Widyasari et al., 2022). Robust induction of SA-related genes triggers high production and accumulation of active SA that activates SAR in the presence of SMV infection.
In this study, we have highlighted important functions of host proteins (PSaC and ATPsyn-α) that interact with viral proteins (NIb and NIa-Pro) elucidating molecular mechanisms of viral infection and host defense. These two host proteins have functions related to the chloroplast, and we know chloroplast organelles are responsible for photosynthesis and have central roles in response to various biotic and abiotic stresses. However, the function of these two genes (PSaC and ATPsyn-α) related to photosynthesis in light harvesting and energy production remains unclear and requires further research.
5. Conclusion
Overall, this study provides a better understanding of the defensive role of GmPSaC and GmATPsyn-α in SMV infection by affecting the functions of NIb and NIa-Pro-in viral replication and movement. GmPSaC and GmATPsyn-α are antiviral host factors that are produced vigorously upon SMV infection and interfere with the functions of SMV NIb and NIa-Pro-in the viral infection cycle, ultimately delaying the development of infection in susceptible cultivars. Furthermore, the findings suggest that GmPSaC and GmATPsyn-α can be utilized as resistance genes to delay SMV replication and can be applied in SMV control strategies and genetically engineered plants with better photosynthetic efficiency and resistance to SMV.
CRediT authorship contribution statement
John Bwalya: Methodology, Software, Investigation, Formal analysis, Writing – original draft. Kristin Widyasari: Methodology, Investigation. Ronny Völz: Methodology, Investigation. Kook-Hyung Kim: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported in part by grants from the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (120080–05–1-HD030), funded by the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea. J.B. and K.W. were supported by research fellowships from the Brain Korea 21 Four Program. The funding sources had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the article for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199205.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Alazem M., Bwalya J., Pai H., Yu J., Cam H.C., Burch-Smith T., Kim K.H. Viral synergism suppresses R gene-mediated resistance by impairing downstream defense mechanisms in soybean. Plant Physiol. 2023:kiad255. doi: 10.1093/plphys/kiad255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alazem M., He M.H., Chang C.H., Cheng N., Lin N.S. Disrupting the homeostasis of high mobility group protein promotes the systemic movement of bamboo mosaic virus. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.597665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya D., Chakraborty S. Chloroplast: the Trojan horse in plant–virus interaction. Mol. Plant Pathol. 2018;19:504–518. doi: 10.1111/mpp.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik K., Burch-Smith T.M. Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 2015;6:781. doi: 10.3389/fpls.2015.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder Y.C., Katz S., Aronheim A. The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 1998;8:1121–1124. doi: 10.1016/s0960-9822(98)70467-1. [DOI] [PubMed] [Google Scholar]
- Bwalya J., Kim K.H. The crucial role of chloroplast-related proteins in viral genome replication and host defense against positive-sense single-stranded RNA viruses. Plant Pathol. J. 2023;39:28–38. doi: 10.5423/PPJ.RW.10.2022.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwalya J., Alazem M., Kim K.H. Photosynthesis-related genes induce resistance against soybean mosaic virus: evidence for involvement of the RNA silencing pathway. Mol. Plant Pathol. 2022;23:543–560. doi: 10.1111/mpp.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chengxin Z., Peter L.F., Yang Z. COFACTOR: improved protein function prediction by combining structure, sequence and protein-protein interaction information. Nucleic. Acids. Res. 2017;45:W291–W299. doi: 10.1093/nar/gkx366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Shen W.T., Yan P., Tuo D.C., Li X.Y., Zhou P. A set of host proteins interacting with papaya ringspot virus NIa-Pro protein identified in a yeast two-hybrid system. Acta Virol. 2012;56:25–30. doi: 10.4149/av_2012_01_25. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R. Induced disease resistance: how do induced plants stop pathogens? Physiol. Mol. Plant Pathol. 1999;55:77–84. [Google Scholar]
- Hajimorad M.R., Domier L.L., Tolin S.A., Whitham S.A., Saghai M.M.A. Soybean mosaic virus: a successful potyvirus with a wide distribution but restricted natural host range. Mol. Plant Pathol. 2018;19:1563–1579. doi: 10.1111/mpp.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.Z., Zhang X.Y., Yang S.H. A novel chloroplast-localized protein emb1303 is required for chloroplast development in Arabidopsis. Cell Res. 2009;19 doi: 10.1038/cr.2009.84. 1225-1225. [DOI] [PubMed] [Google Scholar]
- Jimenez I., Lopez L., Alamillo J.M., Valli A., Garcia J.A. Identification of a plum pox virus CI- interacting protein from chloroplast that has a negative effect in virus infection. Mol. Plant Microbe Interact. 2006;19:350–358. doi: 10.1094/MPMI-19-0350. [DOI] [PubMed] [Google Scholar]
- Kozuleva M., Klenina I., Proskuryakov I., Kirilyuk I., Ivanov B. Production of superoxide in chloroplast thylakoid membranes ESR study with cyclic hydroxylamines of different lipophilicity. FEBS Lett. 2011;585:1067–1071. doi: 10.1016/j.febslet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Mestre P., Brigneti G., Baulcombe D. An Ry-mediated resistance response in potato requires the intact active site of the NIa proteinase from potato virus Y. Plant J. 2000;23:653–661. doi: 10.1046/j.1365-313x.2000.00834.x. [DOI] [PubMed] [Google Scholar]
- Mestre P., Brigneti G., Durrant M., Baulcombe D. Potato virus Y NIa protease activity is not sufficient for elicitation of Ry-mediated disease resistance in potato. Plant J. 2003;36:755–761. doi: 10.1046/j.1365-313x.2003.01917.x. [DOI] [PubMed] [Google Scholar]
- Nambara E., Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- Padmanabhan M.S., Dinesh-Kumar S.P. All hands on deck-the role of chloroplasts, endoplasmic reticulum, and the nucleus in driving plant innate immunity. Mol. Plant Microbe Interact. 2010;23:1368–1380. doi: 10.1094/MPMI-05-10-0113. [DOI] [PubMed] [Google Scholar]
- Stael S., Kmiecik P., Willems P., Van Der Kelen K., Coll N.S., Teige M., Van Breusegem F. Plant innate immunity–sunny side up? Trends Plant Sci. 2015;20:3–11. doi: 10.1016/j.tplants.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.K., Hwang S.H., Kang S.H., Choi H.S., Lee S.H., Sohn S.H., Kim K.H. Interaction study of soybean mosaic virus proteins with soybean proteins using the yeast two-hybrid system. Plant Pathol. J. 2007;23:281–286. [Google Scholar]
- Seo J.K., Lee H.G., Choi H.S., Lee S.H., Kim K.H. Infectious in vivo transcripts from a full-length clone of soybean mosaic virus strain G5H. Plant Pathol. J. 2009;25:54–61. [Google Scholar]
- Seo J.K., Kwon S.J., Cho W.K., Choi H.S., Kim K.H. Type 2C protein phosphatase is a key regulator of antiviral extreme resistance limiting virus spread. Sci. Rep. 2014;4:5905. doi: 10.1038/srep05905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfferth C., Tsuda K. Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci. 2014;5:697. doi: 10.3389/fpls.2014.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick P.J., Schulze-Lefert P., Scholes J.D. Metabolic consequences of susceptibility and resistance (race-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ. 2006;29:1061–1076. doi: 10.1111/j.1365-3040.2005.01472.x. [DOI] [PubMed] [Google Scholar]
- Torres M.A., Jones J.D., Dangl J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006;141:373–378. doi: 10.1104/pp.106.079467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Jin Y., Ma D., Li H., Zhang Z., Dong J., et al. Interaction between PVY HC-Pro and the NTCF1β- subunit reduces the amount of chloroplast ATP synthase in virus- infected tobacco. Sci. Rep. 2015;5:15605. doi: 10.1038/srep15605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniacke J., Zerges W., Haselkorn R. Chloroplast protein targeting involves localized translation in Chlamydomonas. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1439–1444. doi: 10.1073/pnas.0811268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ullah Z., Grumet R. Interaction between zucchini yellow mosaic potyvirus RNA-dependent RNA polymerase and host poly-(A) binding protein. Virology. 2000;275:433–443. doi: 10.1006/viro.2000.0509. [DOI] [PubMed] [Google Scholar]
- Wasternack C., Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. an update to the 2007 review in annals of botany. Ann. Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Widyasari K., Alazem M., Kim K.H. Soybean resistance to soybean mosaic virus. Plants. 2020;9:219. doi: 10.3390/plants9020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyasari K., Bwalya J., Kim K.H. Binding immunoglobulin 2 functions as a proviral factor for potyvirus infections in Nicotiana benthamiana. Mol. Plant Pathol. 2023;24:179–187. doi: 10.1111/mpp.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyasari K., Tran P.T., Shin J., Son H., Kim K.H. Overexpression of purple acid phosphatase GmPAP2.1 confers resistance to soybean mosaic virus in a susceptible soybean cultivar. J. Exp. Bot. 2022;73:1623–1642. doi: 10.1093/jxb/erab496. [DOI] [PubMed] [Google Scholar]
- Yang J.W., Fu J.X., Li J., Cheng X.L., Li F., Dong J.F., Liu Z.L., Zhuang C.X. A novel co-immunoprecipitation protocol based on protoplast transient gene expression for studying protein-protein interactions in rice. Plant Mol. Biol. Rep. 2014;32:153–161. [Google Scholar]
- Yadav N., Yadav D.K., Yadav S., Khurana S.M. Chloroplast proteins and virus interplay: a pathfinder to crop improvement. Plant Biotechnol. Prog. Genom. Era. 2019:631–665. [Google Scholar]
- Yang F., Xiao K., Pan H., Liu J. Chloroplast: the emerging battlefield in plant–microbe interactions. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.637853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 2015;43:W174–W181. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Xu J., Chen B., Cui W., Zhou Z., Song X., Chen Z., Zheng H., Lin L., Peng J., Lu Y., Deng Z., Chen J., Yan F. Characterization of proteins involved in chloroplast targeting disturbed by rice stripe virus by novel protoplast chloroplast proteomics. Int. J. Mol. Sci. 2019;20:253. doi: 10.3390/ijms20020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Zhang X., Hong Y., Liu Y. Chloroplast in plant-virus interaction. Front. Microbiol. 2016;7:1565. doi: 10.3389/fmicb.2016.01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Zhang C., Li Y., Pearce R., Bell E.W., Zhang Y. Folding non-homology proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Method. 2021;1 doi: 10.1016/j.crmeth.2021.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Freddolino P.L., Zhang Y. COFACTOR: improved protein function prediction by combining structure, sequence and protein-protein interaction information. Nucleic Acids Res. 2017;45:W291–W299. doi: 10.1093/nar/gkx366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.