Abstract
Activin A, a member of TGF‐β superfamily, has been implicated in the pathogenesis of pulmonary artery hypertension (PAH). PAH due to congenital heart disease (CHD‐PAH) is a major problem in developing countries. Activin A may have a role in PAH development and progression among uncorrected CHD. In this comparative study, serum activin A level was significantly increased in subjects with uncorrected CHD without the presence of PH and were more significantly risen in CHD‐PAH, as compared to control. The utilization of serum activin A measurement seems promising to identify uncorrected CHD patients with PAH development and progression.
Keywords: biomarkers, congenital heart disease, endothelin 1, NT‐pro BNP
INTRODUCTION
Pulmonary artery hypertension (PAH) is characterized by constant pulmonary vasoconstriction, concentrical vascular remodeling, occlusive intimal lesions, luminal thrombosis, and vascular smooth muscle thickening which lead to increased pulmonary artery pressure and resistance. 1 Three common pathways of vascular disarray, namely endothelin, nitric oxide, and prostacyclin pathways, have been recognized which led to successful targeted PAH‐therapy. 1 In addition to these pathways, transforming growth factor beta (TGF‐β) superfamily is known to play a part in PAH pathogenesis, especially in the heritable form of PAH. 2 , 3 Activin A, one of its members, is a glycoprotein which is implicated in the pathogenesis of PAH and mediates TGF‐β superfamily signaling imbalance, bone morphogenetic protein (BMPR)II downregulation, and macrophage activation. 4 In vitro study shows that activin A increases apoptosis in endothelial cells and prevents vascular wall formation. 3 Meanwhile study with pulmonary artery smooth muscle cells from PAH patients treated with activin A inhibitor shows a reduction in cells' growth and proliferation. 5
Among clinical classifications of PAH, congenital heart disease‐associated PAH (CHD‐PAH) is the most common form encountered in developing countries, which is related to the underdiagnosis and lack of treatment of CHD. 6 , 7 Most adults with CHD visit medical services with complaints related to increasing pulmonary artery pressure or pulmonary hypertension (PH). 7 Our previous studies have identified several biomarkers associated with the development and progression of PAH in adult uncorrected CHD patients. 8 , 9 , 10 Since activin A is involved in PAH pathogenesis, we aimed to measure serum activin A level and correlate it with other biomarkers, endothelin‐1, and NT‐pro BNP, in patients with CHD and CHD‐associated PAH to elaborate its potential clinical role into PAH development and progression in these populations.
METHODS
A cross‐sectional method was used for this study. The subjects were patients diagnosed with CHD without PH (CHD‐noPH), CHD‐associated PAH (CHD‐PAH), and idiopathic or hereditary PAH (I/HPAH). The subjects were participants of The COngenital HeARt Disease in adult and Pulmonary Hypertension (COHARD‐PH) registry, 7 from which clinical and hemodynamic parameter data was retrieved. The transthoracic echocardiography (TTE), transesophageal echocardiography (TOE), and right heart catheterization (RHC) were performed for diagnostic modalities. The PAH diagnosis criteria were based on RHC results in accordance with current recommendations. 11 Based on the diagnostic criteria, the subjects were categorized as CHD‐noPH, CHD‐PAH, and I/HPAH. Sample size calculation yielded a minimum of five subjects in each control (no‐PAH) and PAH group for activin A comparison. Therefore, we randomly selected nine subjects from the COHARD‐PH registry in each category for this study. A simple random sampling was performed to select the subjects from the registry. The type of CHD in this study was uncorrected atrial septal defects (ASD). The data of subject characteristics, including biomarker levels, were obtained from the index of diagnosis (after the RHC procedure), before PAH‐specific treatments were given. Nine healthy controls with similar ages and sex were recruited. Among these controls, the TTE was performed to exclude the CHD and measure the estimated pulmonary artery pressure. Those with normal echocardiogram were enrolled for this study, as the control group.
Blood samples for examination of biomarkers, namely NT‐pro BNP, endothelin‐1, and activin A, were withdrawn from the peripheral veins at the time of RHC procedure as part of the COHARD‐PH registry protocol. In controls, the blood samples from peripheral veins were collected. Then, the serum was isolated and frozen at −80⁰C freezer in the Biobank Unit Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada, until biomarkers were assayed. For NT‐proBNP measurement, the electrochemiluminescence immunoassay (Elecsys®ProBNP II) and a Cobas e immunoassay analyzer (Roche Diagnostics) was utilized. For endothelin‐1 measurement, a human endothelin‐1 ELISA kit (RAB1039, Sigma‐Aldrich) and its protocol were applied. For activin A measurement, human/mouse/rat activin A Quantikine® ELISA Kit (DAC00B, R&D Systems) and its protocol were used. The NT‐proBNP measurement was performed in Dr. Sardjito Hospital Central Laboratory. The endothelin‐1 and activin A measurements were conducted in the Integrated Research Laboratory (Laboratorium Riset Terpadu) Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada. This research protocol has been approved by Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada and Dr. Sardjito Hospital, Yogyakarta, Indonesia.
For statistical analysis, the test of normality for continuous variables was conducted with the Shapiro–Wilk test. For normally distributed variables, their mean differences among groups were tested using a one‐way analysis of variance (ANOVA) test and continued with post hoc analysis, whereas the mean difference between two groups was analyzed with student T‐test. For nonnormally distributed variables, the nonparametric statistics were used. Subsequently, the correlation between continuous variables was analyzed with Pearson or Spearman correlation tests. The statistical significance was accepted at p < 0.05.
RESULTS AND DISCUSSION
The characteristics of each subject group are depicted in Table 1. Subjects with PAH, both CHD‐PAH and I/HPAH, had higher right atrial (RA) area, right ventricular (RV) diameter, tricuspid valve gradient (TVG), and tricuspid regurgitant velocity (TRV), whereas the tricuspid annular plane systolic excursion (TAPSE) value was lower when compared to subjects without PAH, namely CHD‐noPH and controls. The mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance index (PVRi) values, measured by RHC, were higher in PAH subjects. Subjects with I/HPAH had more severe hemodynamic consequences of increased pulmonary artery pressure and resistance, as indicated by reduced RV function (lower TAPSE value) and higher mPAP and PVRi, when compared to CHD‐PAH.
Table 1.
Comparison of characteristics among subjects with congenital heart disease (CHD)‐noPH, CHD‐pulmonary artery hypertension (PAH), and I/HPAH as compared to control.
| Characteristics | Control (n = 9) | CHD‐noPH (n = 9) | CHD‐PAH (n = 9) | I/HPAH (n = 9) | p‐value |
|---|---|---|---|---|---|
| Females | 6 (66.7) | 6 (66.7%) | 9 (100%) | 9 (100%) | 1.0 |
| Age (years) | 26.6 ± 2.7 | 31.6 ± 8.9 | 33.9 ± 9.1 | 36.0 ± 7.6 | 0.069 |
| BMI (kg/m2) | 21.9 ± 2.9 | 20.6 ± 1.7 | 19.1 ± 4.1 | 21.5 ± 3.1 | 0.207 |
| RA area (cm2) | N.M | 17.4 ± 3.2 | 23.5 ± 6.7 | 25.9 ± 5.6 | 0.022 |
| RV diameter (mm) | 30.6 ± 4.1 | 42.8 ± 5.5 | 51.0 ± 9.6 | 53.0 ± 5.3 | <0.001 |
| TAPSE (mm) | N.M | 24.3 ± 4.5 | 20.8 ± 3.0 | 15.1 ± 3.7 | <0.001 |
| TVG (mmHg) | N.M | 40.9 ± 33.0 | 86.8 ± 33.8 | 73.4 ± 13.1 | 0.013 |
| TRV (m/s) | N.M | 2.6 ± 0.3 | 4.6 ± 0.9 | 4.1 ± 0.5 | <0.001 |
| LVEF (%) | 68.6 ± 5.2 | 64.6 ± 9.4 | 74.7 ± 8.5 | 67.4 ± 11.9 | 0.137 |
| mPAP (mmHg) | N.M | 16.67 ± 2.5 | 56.33 ± 8.11 | 57.78 ± 13.70 | <0.001 |
| PVRi (Wood Unit.m2) | N.M | 1.4 ± 0.9 | 14.7 ± 4.2 | 29.1 ± 14.4 | <0.001 |
Note: All the numerical data were shown as mean ± standard deviation (SD), and all the categorical data were shown as number (%).
Abbreviations: BMI, body mass index; LVEF, left ventricle ejection fraction; mPAP, mean pulmonary artery pressure; N.M, not measured; PVRI, pulmonary vascular resistance index; RA, right atrial; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TRV, tricuspid regurgitant velocity; TVG, tricuspid valve gradient.
Table 2 and Figure 1 show the biomarker levels among subject groups. Serum activin A level was incrementally increased in subjects with PAH (both CHD‐PAH and I/HPAH). Subjects with I/HPAH had the highest level of serum activin A. In subjects with CHDs, CHD‐PAH had a significantly higher level of serum activin A as compared to CHD‐noPH. Control subjects had the least serum activin A level. Our study clearly showed that activin A level was increasing in the serum of subjects with PAH, as compared to subjects without PAH. Similar results were previously reported in subjects with IPAH, collagen tissue disease‐associated PAH, portopulmonary hypertension and HIV‐related PAH. 12 Our study broadens the findings by involving CHD‐associated PAH, which also showed significant increasing in serum activin A level. The connection between activin A and PAH has been recognized. Activin A was substantially expressed in endothelial and smooth muscle cells of pulmonary vasculatures 4 , 13 as well as in the lung macrophages, 12 in patients with PAH. It mediates the pulmonary vascular remodeling prevailing in PAH pathogenesis which can be reversed by ACTRIIA‐Fc, a potent activin ligand trap. 13 Six subjects in this study were males. Compared to females there was no significant difference in activin A level.
Table 2.
The comparison of serum biomarkers among subject groups.
| Serum biomarkers | Control (n = 9) | CHD‐noPH (n = 9) | CHD‐PAH (n = 9) | I/HPAH (n = 9) | p‐value |
|---|---|---|---|---|---|
| NT‐pro BNP (pg/mL) | N.M | 153.07 ± 118.54 | 1948.42 ± 2681.07 | 2489.11 ± 2012.64 | 0.001 a |
| Endothelin‐1 (pg/mL) | N.M | 14.20 ± 1.10 | 15.31 ± 1.38 | 14.93 ± 0.91 | 0.207b |
| Activin A (pg/mL) | 274.85 ± 49.42 | 366.02 ± 68.56 | 503.68 ± 179.69 | 695.04 ± 305.12 |
<0.001c 0.01d |
Note: All the numerical data were shown as mean ± standard deviation (SD).
Abbreviation: N.M, not measured.
CHD‐noPH, n = 6; CHD‐PAH, n = 8; I/HPAH, n = 8.
Kruskal–Wallis test.
One‐way analysis of variance (ANOVA) among all groups including control.
One‐way ANOVA excluding control.
Figure 1.
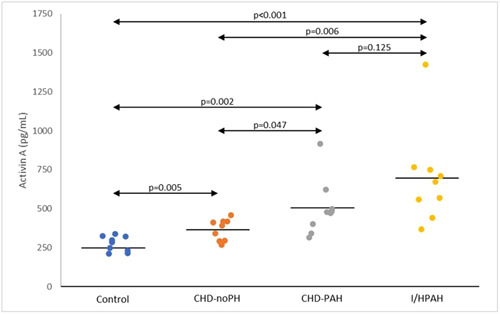
The incremental increase of serum activin A level among subject groups (p value from the Student T test, analyzed between groups).
In subjects with CHD, both CHD‐noPH and CHD‐PAH, serum activin A was significantly correlated with other PAH biomarkers such as NT‐pro BNP with which it is strongly correlated (Figure 2a). It also had a moderate correlation with endothelin‐1, a biomarker implicated in PAH pathogenesis. However, serum activin A did not correlate with mPAP and PVRi in subjects with CHD. In subjects with PAH, both CHD‐PAH and I/HPAH, there was no significant correlation between serum activin A level and other biomarkers and hemodynamic parameters (Table 3). A strong correlation between serum activin A and NT‐proBNP was specifically found in CHD‐PAH (r = 0.900, p < 0.001) (Figure 2b). In other groups, there was no correlation between activin A and NT‐proBNP (CHD‐noPH: r = 0.219, p = 0.571) and I/HPAH: r = −0.390, p = 0.299).
Figure 2.

(a) Positive correlation between activin A and NT‐proBNP levels in subjects with congenital heart disease (CHD) (both CHD‐noPH and CHD‐PAH) (r = 0.876, p < 0.001). (b) Positive correlation between activin A and NT‐proBNP levels in subjects with CHD‐pulmonary artery hypertension (PAH) (r = 0.900, p < 0.001).
Table 3.
The correlation between activin A and other biomarkers and pulmonary artery hypertension (PAH) hemodynamic parameters.
| Parameters |
Congenital heart disease (CHD)‐noPH and CHD‐PAH n = 18 |
CHD‐PAH and I/HPAH n = 18 |
||
|---|---|---|---|---|
| r‐value | p‐value | r‐value | p‐value | |
| NT‐pro BNP | 0.876 | <0.001 | 0.195 | 0.437 |
| Endothelin‐1a | 0.625 | 0.017 | 0.428 | 0.098 |
| mPAP | 0.428 | 0.077 | −0.388 | 0.112 |
| PVRi | 0.464 | 0.060 | −0.203 | 0.419 |
Abbreviations: mPAP, mean pulmonary artery pressure; PVRI, pulmonary vascular resistance index.
CHD‐noPH and CHD‐PAH, n = 14; CHD‐PAH and IPAH, n = 16.
Previous studies showed, among patients with PAH, higher serum activin A was associated with mortality and may serve as an accurate prognostic indicator. 12 , 14 The positive significant correlation between activin A and NT‐pro BNP was shown in subjects with CHD, especially in CHD‐PAH, which may indicate the presence of PAH in this population, whereas among subjects who had already developed PAH there was no correlation found. In an experimental study, activin A promoted endothelin‐1 over expression in pulmonary artery smooth muscle and in human PAH, serum activin A level was significantly correlated with serum endothelin‐1 level. 12 In our study, the significantly positive correlation between activin A and both biomarkers, endothelin‐1 and NT‐pro BNP, was found only in subjects with CHD. It may be an indication of PAH development as a natural history of volume and pressure overload occurring in patients with uncorrected CHD. It is intriguing to suggest that by measuring serum activin A level, we may detect the progression of uncorrected CHD haemodynamic into PAH and activin A as a differentiator between CHD‐no PH and CHD‐PAH.
We concluded from the results of our study that serum activin A level was increased in patients with PAH. The elevated levels of activin A were detected in subjects with CHD without the presence of PH and were increased in CHD with the presence of PAH (CHD‐associated PAH). Furthermore, among CHD patients, serum activin A significantly correlated with other biomarkers implicated in the presence of PAH, namely NT‐pro BNP and endothelin‐1. This finding indicated that activin A may serve as a biomarker for detection of the presence of PAH among subjects with uncorrected CHD. The utilization of serum activin A measurement seems promising since the prevalence of uncorrected CHD is still significantly high in developing countries and PAH is a pathway of the natural history of this disease which has been significantly associated with adverse outcomes and mortality. Further study with a larger sample size and prospective follow‐up is necessary to corroborate these preliminary findings.
AUTHOR CONTRIBUTIONS
Muhammad R. Hadwiono: Concept; analysis and interpretation of data; drafted the article; appoved the published manuscript. Anggoro B. Hartopo: Concept; analysis and interpretation of data; revised the article; approved the published manuscript. Widya Wasityastuti: Concept; revised the article; approved published manuscript. Dyah W. Anggrahini: Acquisition of data; revised the article; approved published manuscript. Gusty R. T. Ryanto: Interpretation of data; revised the article; approved the published manuscript. Noriaki Emoto: Interpretation of data; revised the article; approved the published manuscript. Lucia K. Dinarti: Acquisition of data; revised the article; approved the published manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This research has been approved by Medical and Health Research Ethics Committee of Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada and Dr. Sardjito Hospital, Yogyakarta, Indonesia (Ref: KE/FK/1189/EC/2021)
ACKNOWLEDGMENTS
The authors are grateful to the research assistants who support and maintain the COHARD‐PH registry database: Armalya Pritazahra MD, Andreas Hartanto, MD, Abdul Majid Halim, MD, Cindy Elica Cipta, MD, and Fika Humaeda Assilmi, MD. Authors thank to Ms. Sri Fatmawati and Ms. Sumartiningsih from Laboratorium Riset Terpadu, and Mr. Efri Kurniawan and Ms. Linda Tri from the Biobank Unit, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia who assisted in sample handling and biomarker examination. Some of the data has been used for the completion of Master degree thesis by Dr. Muhammad Reyhan Hadwiono from Master in Biomedical Sciences Program, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada submitted to Universitas Gadjah Mada, Yogyakarta, Indonesia. This research and publication received funding from Universitas Gadjah Mada Program Rekognisi Tugas Akhir (RTA) batch II (no contract: 5722/UN1.P.III/Dit‐Lit/PT.01.05/2022) to Anggoro Budi Hartopo as the Principal Investigator. The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Hadwiono MR, Hartopo AB, Wasityastuti W, Anggrahini DW, Ryanto GRT, Emoto N, Dinarti LK. Increased serum activin A level in congenital heart disease‐associated pulmonary artery hypertension: a comparative study from the COHARD‐PH registry. Pulm Circ. 2023;13:e12280. 10.1002/pul2.12280
REFERENCES
- 1. Balistrieri A, Makino A, Yuan JXJ. Pathophysiology and pathogenic mechanisms of pulmonary hypertension: role of membrane receptors, ion channels and Ca2+ signaling. Physiol Rev. 2023;103(3):1827–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fessel JP, Loyd JE, Austin ED. The genetics of pulmonary arterial hypertension in the post‐BMPR2 era. Pulm Circ. 2011;1(3):305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guignabert C, Humbert M. Targeting transforming growth factor‐β receptors in pulmonary hypertension. Eur Respir J. 2021;57(2):2002341. [DOI] [PubMed] [Google Scholar]
- 4. Ryanto GRT, Ikeda K, Miyagawa K, Tu L, Guignabert C, Humbert M, Fujiyama T, Yanagisawa M, Hirata K, Emoto N. An endothelial activin A‐bone morphogenetic protein receptor type 2 link is overdriven in pulmonary hypertension. Nat Commun. 2021;12(1):1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kudryashova T, Shen Y, Pena A, Cronin E, Okorie E, Goncharov D, Goncharova E. Inhibitory antibodies against activin A and TGF‐β reduce self‐supported, but not soluble factors‐induced growth of human pulmonary arterial vascular smooth muscle cells in pulmonary arterial hypertension. Int J Mol Sci. 2018;19(10):2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dinarti LK, Anggrahini DW, Lilyasari O, Siswanto BB, Hartopo AB. Pulmonary arterial hypertension in Indonesia: current status and local application of international guidelines. Global Heart. 2021;16(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinarti LK, Hartopo AB, Kusuma AD, Satwiko MG, Hadwiono MR, Pradana AD, Anggrahini DW. The COngenital HeARt Disease in adult and Pulmonary Hypertension (COHARD‐PH) registry: a descriptive study from single‐center hospital registry of adult congenital heart disease and pulmonary hypertension in Indonesia. BMC Cardiovasc Disord. 2020;20(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dinarti LK, Hartopo AB, Anggrahini DW, Sadewa AH, Setianto BY, Wahab AS. Profile of endothelin‐1, nitric oxide, and prostacyclin levels in pulmonary arterial hypertension related to uncorrected atrial septal defect: results from a single center study in Indonesia. Cardiol Res Pract. 2020;2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pratama RS, Hartopo AB, Anggrahini DW, Dewanto VC, Dinarti LK. Serum soluble suppression of tumorigenicity‐2 level associates with severity of pulmonary hypertension associated with uncorrected atrial septal defect. Pulm Circ. 2020;10(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartopo AB, Anggrahini DW, Satwiko MG, Damarkusuma A, Pritazahra A, Hadwiono MR, Dewanto VC, Di Somma S, Emoto N, Dinarti LK. Usefulness of combining NT‐proBNP level and right atrial diameter for simple and early noninvasive detection of pulmonary hypertension among adult patients with atrial septal defect. Acta Med Indones. 2022;54(4):556–566. [PubMed] [Google Scholar]
- 11. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. [DOI] [PubMed] [Google Scholar]
- 12. Yndestad A, Larsen KO, Øie E, Ueland T, Smith C, Halvorsen B, Sjaastad I, Skjønsberg OH, Pedersen TM, Anfinsen OG, Damås JK, Christensen G, Aukrust P, Andreassen AK. Elevated levels of activin A in clinical and experimental pulmonary hypertension. J Appl Physiol. 2009;106(4):1356–1364. [DOI] [PubMed] [Google Scholar]
- 13. Yung LM, Yang P, Joshi S, Augur ZM, Kim SSJ, Bocobo GA, Dinter T, Troncone L, Chen PS, McNeil ME, Southwood M, Poli de Frias S, Knopf J, Rosas IO, Sako D, Pearsall RS, Quisel JD, Li G, Kumar R, Yu PB. ACTRIIA‐Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med. 2020;12(543):eaaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guignabert C, Savale L, Boucly A, Thuillet R, Tu L, Ottaviani M, Rhodes CJ, De Groote P, Prévot G, Bergot E, Bourdin A, Howard LS, Fadel E, Beurnier A, Roche A, Jevnikar M, Jaïs X, Montani D, Wilkins MR, Sitbon O, Humbert M. Serum and pulmonary expression profiles of the activin signaling system in pulmonary arterial hypertension. Circulation. 2023;147(24):1809–1822. [DOI] [PubMed] [Google Scholar]


