Abstract
Introduction:
Coronary computed tomographic angiography (CCTA) reporting has traditionally been operator-dependent, and no precise classification is broadly used for reporting Coronary Artery Disease (CAD) severity. The Coronary Artery Disease Reporting and Data Systems (CAD-RADS) was introduced to address the inconsistent CCTA reports. This systematic review with meta-analysis aimed to comprehensively appraise all available studies and draw conclusions on the prognostic value of the CAD-RADS classification system in CAD patients.
Method:
Online databases of PubMed, Embase, Scopus, and Web of Science were searched until September 19th, 2022, for studies on the value of CAD-RADS categorization for outcome prediction of CAD patients.
Results:
16 articles were included in this systematic review, 14 of which had assessed the value of CAD-RADS in the prediction of major adverse cardiovascular events (MACE) and 3 articles investigated the outcome of all-cause mortality. Our analysis demonstrated that all original CAD-RADS categories can be a predictor of MACE [Hazard ratios (HR) ranged from 3.39 to 8.63] and all categories, except CAD-RADS 1, can be a predictor of all-cause mortality (HRs ranged from 1.50 to 3.09). Moreover, higher CAD-RADS categories were associated with an increased hazard ratio for unfavorable outcomes among CAD patients (p for MACE = 0.007 and p for all-cause mortality = 0.018).
Conclusion:
The evidence demonstrated that the CAD-RADS classification system can be used to predict incidence of MACE and all-cause mortality. This indicates that the implementation of CAD-RADS into clinical practice, besides enhancing the communication between physicians and improving patient care, can also guide physicians in risk assessment of the patients and predicting their prognosis.
Key Words: Coronary artery disease, Risk assessment, CAD-RADS, Reporting and Data System
1. Introduction:
Coronary computed tomographic angiography (CCTA) is an accurate and non-invasive tool with a high negative predictive value, which is increasingly being used for the evaluation of patients with stable angina and acute coronary artery disease (CAD) (1). CCTA provides physicians with utile information on the presence of atherosclerosis, and its characteristics such as the extent and location (2).
CCTA reporting has traditionally been operator-dependent, and no precise classification has been broadly used for summarizing and categorizing CAD severity in this imaging modality (3).
Previously, efforts had been made to design and implement uniform structuralized reporting frameworks for the interpretation of imaging assessments such as breast (BI-RADS), prostate (PI-RADS), liver (LI-RADS), and lung (Lung-RADS) imaging reporting and data systems (4).
To this end, in 2016, the Coronary Artery Disease Reporting and Data Systems (CAD-RADS), a multi-society consensus reached by radiologists and cardiologists, was introduced to address the inconsistent CCTA reports (5).
CAD-RADS has been intended to establish a common lexicon between multiple disciplines involved in patient management. Moreover, the acceptable intra and inter-observer variability of this scoring system contributed to the consistent and efficient patient clinical management and facilitated data gathering for registries with research purposes (6, 7). CAD-RADS provides a precise yet simple representation of CAD severity by classifying patients based on the most severe stenosis identified in CCTA (8). CAD-RADS categorizes the stenosis according to the degree of luminal diameter stenosis, ranging from the absence of any occlusion or plaque (category 0) to total occlusion of at least one coronary artery (category 5). There are also modifiers including N, S, G, and V, which stand for non-diagnostic, stent, graft, and vulnerability, respectively, providing additional details of the CCTA finding (Table 1) (5).
Table 1.
CAD-RADS classification system
| Category | Maximal Stenosis | Interpretation |
|---|---|---|
| CAD-RADS 0 | 0% | No CAD |
| CAD-RADS 1 | 1 - 24% | Minimal nonobstructive |
| CAD-RADS 2 | 25 - 49% | Mild nonobstructive |
| CAD-RADS 3 | 50 - 69% | Moderate stenosis |
| CAD-RADS 4A | 70 - 99% | Severe stenosis |
| CAD-RADS 4B | Left main >50% or 3-vessel ≥ 70% | Severe stenosis |
| CAD-RADS 5 | 100% | Total coronary occlusion |
CAD-RADS: Coronary artery disease-reporting and data system; CAD: Coronary artery disease.
In addition to the previously mentioned advantages of CAD-RADS, its clinical meaningfulness would be pronounced when patient categorization provides guidance on their therapeutic and preventive management measures. To achieve this aim, a preliminary step is to confirm the validity of CAD-RADS categorization for patient prognosis. Previously, several reports have investigated the predictive value of the CAD-RADS categorization system on patient outcomes, consisting of the risk for a major adverse cardiovascular event (MACE) and all-cause mortality. This systematic review with meta-analysis aimed to comprehensively appraise all available studies and draw a conclusion on the predictive value of the CAD-RADS classification system in CAD patients.
2. Methods:
2.1. Study design and setting
This systematic review and meta-analysis was designed to evaluate the predictive value of CAD-RADS in the assessment of outcomes in CAD patients. In this study, PICO was defined as: Patients (P): patients with suspected or known coronary artery disease, Index test (I): CAD-RADS classification tool, Comparison (C): coronary artery disease patients not developing the outcome of the study, Outcome (O): Major adverse cardiovascular event (MACE) and all-cause mortality.
2.2. Search strategy
Appropriate keywords related to the aim of this study were chosen based on MeSH (Medline database) and Emtree (Embase database) terms, a review of the related literature, and consultation with experts in the field. A systematic search was performed using four online databases of PubMed, Embase, Scopus, and Web of Science until September 19th, 2022. The search strategy used for this study is provided in supplementary material 1. Google and Google Scholar search engines and references of the included articles were also reviewed to retrieve any papers that might have been missed.
2.3. Selection criteria
All articles evaluating the value of CAD-RADS for the prediction of outcomes in CAD patients were included in this study. The exclusion criteria were commentaries and editorials, review articles, case reports, case series, and articles not reporting the data of interest.
2.4. Data collection
Two researchers independently reviewed the titles and abstracts of the retrieved articles and full-text screening was performed for possibly relevant articles and appropriate articles were included in the study. The information reported in the included articles was summarized and compiled in a checklist designed according to the criteria of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. Any disagreements were resolved by consulting a third reviewer. The data checklist included article characteristics (name of first author, year of publication, and country of study), study design, studied population, number of patients, number of men, age, studied outcome and number of patients developing the outcome, follow-up duration, reported CAD-RADS category, and the relevant effect size reported for each CAD-RADS category. The effect size of interest was chosen to be hazard ratio and the authors of any articles not providing required information were contacted by email, with a 1-week reminder in order to gain access to their results. Any disagreement between the two reviewers was resolved by the third reviewer.
2.5. Quality and certainty of evidence assessment
The quality of the articles was assessed using the guidelines provided by the Quality Assessment of Prognostic Accuracy Studies (QUAPAS) tool (9). Based on this guideline the articles are assessed according to their risk of bias (in domains of participants, index test, outcome, flow and timing, and analysis) and their applicability (in domains of participants, index test, outcome, and flow and timing). The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) guidelines were used to evaluate the certainty of evidence (10). The certainty of evidence table was designed using GRADEpro online software (www.gradepro.org).
2.6. Statistical analysis
Analyses were performed in the STATA 17.0 statistical software. The predictive value of CAD-RADS for outcomes of CAD patients was recorded as hazard ratio (HR) and 95% confidence interval (CI) and the data were analyzed using the “meta” package. The studies utilizing original CAD-RADS categories (1, 2, 3, 4A, 4B, and 5), with CAD-RADS category 0 as the reference, were included in the meta-analysis. The experiments with reports of combinations of the original CAD-RADS categories into a subset category or continuous variable were excluded from the meta-analysis and have been reported qualitatively. A meta-regression analysis was performed to evaluate the effect of the follow-up duration on the predictive value of CAD-RADS. The Heterogeneity between included studies was evaluated using I2 statistics and Chi-squared test.
Publication bias assessment was not applicable since less than 10 articles were included in each meta-analysis (11).
3. Results:
3.1. Study characteristics
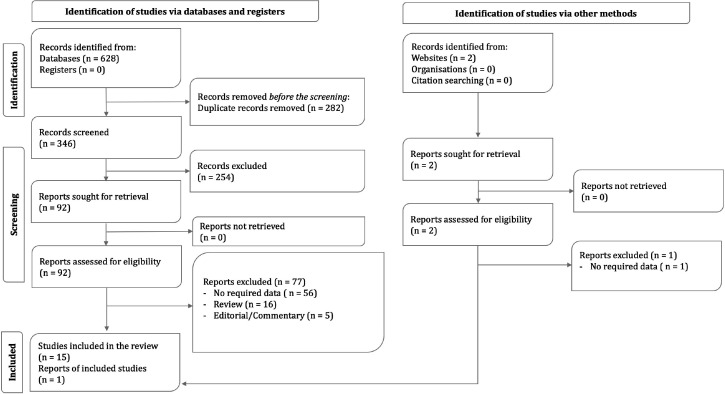
The systematic search of online databases of PubMed, Embase, Scopus, and Web of Science resulted in 346 non-duplicate records. 92 of these records were deemed to be eligible and upon further evaluation, 15 articles were chosen to be included in this study. Two articles were found via manual search, one of which was included. Finally, 16 articles (12-27) were included in this study (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) diagram
All included articles had assessed suspected or known coronary artery disease patients with a low to intermediate probability of CAD. The articles had defined CAD-RADS categories as introduced by Cury et al. (5) and only one article had a slight definition variation (5% difference in categories 1 and 2) (13). The results of analyses did not differ significantly by the inclusion of this article and thus, the record was not excluded. The characteristics of the included studies are demonstrated in Table 2.
Table 2.
Characteristics of included studies
| Study, Year | Design# | Sample size | Age* | Male% (N) | Follow-up | Event% (N) |
|---|---|---|---|---|---|---|
| Major Adverse Cardiac Event (MACE) | ||||||
| Altay, 2021 (12) | Retrospective | 359 | 54.17 | 54.31 (195) | 8 years | 6.96 (25) |
| Bittner, 2020(13) | Retrospective | 3840 | 60.4±8.2 | 48.64 (1868) | 2.08 years | 2.99 (115) |
| Duguay, 2017(20) | Retrospective | 48 | 56 ± 10 | 60.4 (29) | 1.6 year | 29.16 (14) |
| Faber, 2021(21) | Retrospective | 1615 | 59 | 66.62 (1076) | 10.5 years | 3.31 (51) |
| Finck. 2019 (a)(14) | Retrospective | 2011 | 59 ± 11 | 66.03 (1328) | 10 years | 2.88 (58) |
| Lee, 2021(15) | Retrospective | 1492 | 58±6.14 | 50.87 (759) | 3 months 31.5 months |
4.22 (63) 6.90 (103) |
| Maclean, 2022(22) | Retrospective | 720 | 58 [IQR 19] | 62.08 (447) | 5.4 years | 7.5 (54) |
| Mangalesh, 2022(23) | Prospective | 366 | 62 | 70.76 (259) | 2.56 years | 16.39 (60) |
| Senoner, 2020(16) | Prospective | 1430 | 57.9±11.1 | 55.59 (795) | 10.55 years | 3.98 (57) |
| Tang, 2022(17) | Prospective | 511 | 61 [33-94] | 75.92 (388) | 1 year | 6.65 (34) |
| Van Rosendael, 2019(24) | Prospective | 2134 | 54.72 | 49.01 (1046) | 3.6 years | 0.06 (130) |
| Williams, 2020(18) | Retrospective | 1769 | 58±10 | 56.35 (997) | 4.7 years | 2.31 (41) |
| Xie, 2018(19) | Retrospective | 5039 | 59.97 | 63.74 (3212) | 5 years | 15.30 (771) |
| Yamamoto, 2021(25) | Prospective | 133 | 67 ± 11 | 69.92 (93) | 3.33 years | 10.52 (14) |
| All-cause mortality | ||||||
| Finck, 2019 (b)(26) | Retrospective | 1913 | 58.97 | 66.54 (1273) | 9.7 years | 5.17 (99) |
| Huang, 2021(27) | Retrospective | 9625 | 59.8±10.7 | 44.28 (4262) | 4.3 years | 5.61 (540) |
| Senoner, 2020(16) | Prospective | 1430 | 57.9±11.1 | 55.59 (795) | 10.55 years | 7.41 (106) |
| Xie, 2018(19) | Retrospective | 5039 | 59.97 | 63.74 (3212) | 5 years | 6.23 (314) |
*: Age is reported as mean ± SD or median [IQR]. #: All studies are observational.
3.2. Value of CAD-RADS in prediction of MACE
14 out of the 16 included articles assessed the value of CAD-RADS categories for the prediction of MACE (12-25). 10 articles had reports of combinations of the original CAD-RADS categories into a subset or overall category, which were not included in the meta-analysis and are reported separately (13, 15-17, 20-25).
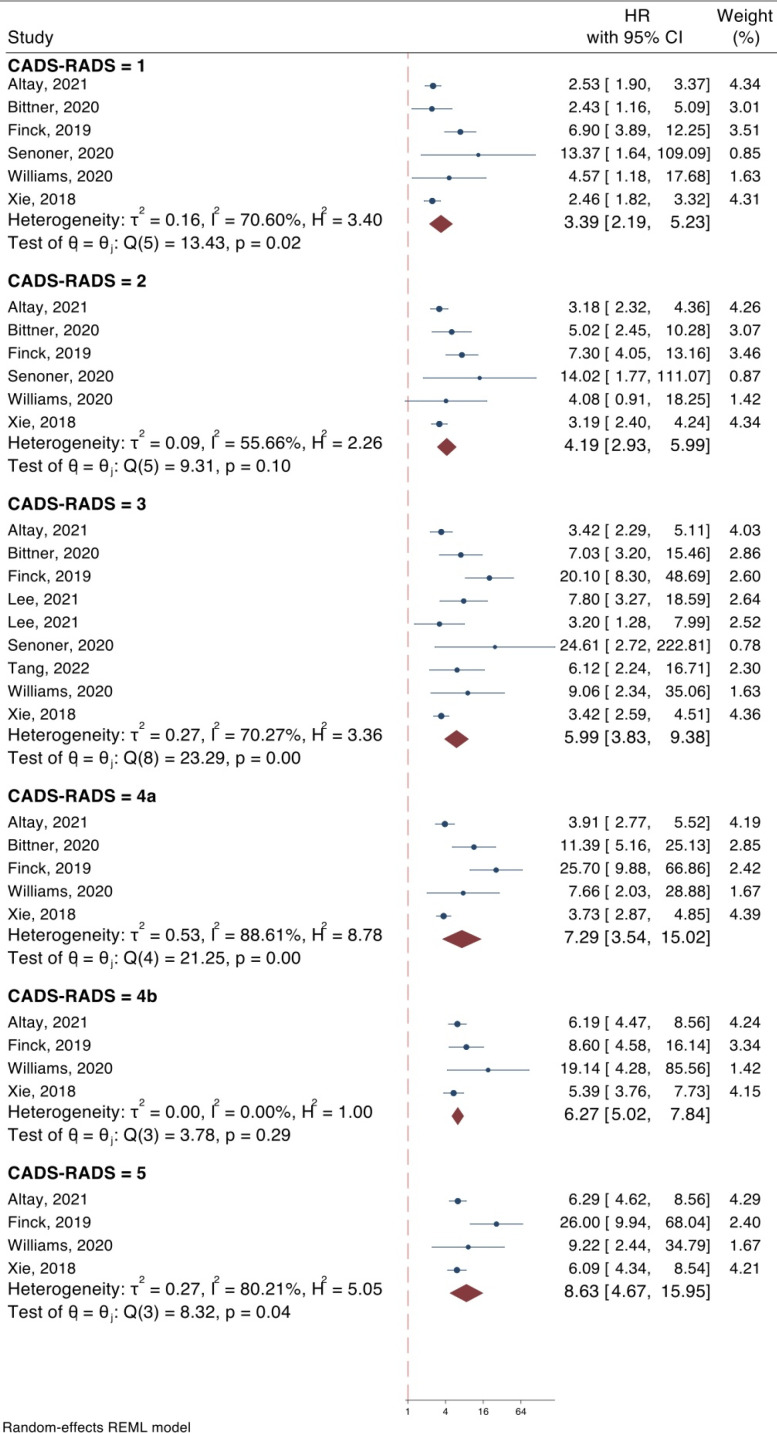
8 articles (12-19) were included in the meta-analysis for the evaluation of the value of original CAD-RADS categorization for the prediction of MACE. The results of our analysis demonstrated that all the original CAD-RADS categories (1, 2, 3, 4A, 4B, 5) can predict MACE in CAD patients. The hazard ratios of CADS-RADS 1, 2, 3, 4a, 4b, and 5 for prediction of MACE were 3.39 (95% CI: 2.19-5.23), 4.19, (95% CI: 2.93-5.99), 5.99 (95% CI: 3.83-9.38), 7.29 (95% CI: 3.54-15.02), 6.27 (95% CI: 5.02-7.84), and 8.63 (95% CI: 4.67-15.95), respectively. All the analyses were statistically significant (p < 0.0001) (Figure 2), with an increase in trend in the risk of MACE across CAD-RADS categories (Regression co-efficient = 0.066; 95% CI: 0.018-0.114; p = 0.007).
Figure 2.
Value of CAD-RADS classification system in prediction of major adverse cardiovascular events in coronary artery disease patients. CAD-RADS: Coronary artery disease-reporting and data system; CI: Confidence interval; HR: Hazard ratio
The follow-up of the studies ranged between 3 months to 10 years. A meta-regression analysis was performed to assess the effect of follow-up duration on the predictive value of CAD-RADS for MACE. The results showed that the difference between follow-up durations had no significant effect on the predictive value of CAD-RADS in any of the categories (Supplementary Table 1).
3.3.Value of CAD-RADS in the prediction of all-cause mortality
4 out of the 16 included articles evaluated the value of CAD-RADS in the prediction of all-cause mortality (16, 19, 26, 27). One article (26) only reported the results for combinations of the original CAD-RADS categories into a subset or overall category, which was not included in the meta-analysis and is reported qualitatively.
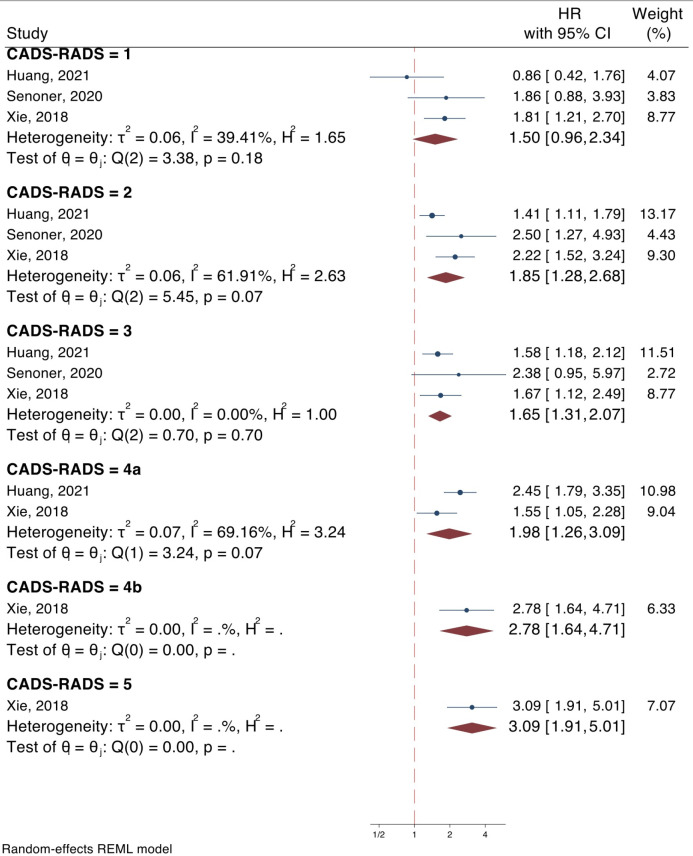
3 articles (16, 19, 27) had assessed the value of the original CAD-RADS categories in the prediction of all-cause mortality. The results of the analysis showed that all CAD-RADS categories, except CAD-RADS 1, can be a predictor of all-cause mortality. The hazard ratios of CADS-RADS 1, 2, 3, 4a, 4b, and 5 for prediction of all-cause mortality were 1.50 (95% CI: 0.96-2.34, p = 0.073), 1.85 (95% CI: 1.28-2.68, p = 0.001), 1.65 (95% CI: 1.31-2.07, p < 0.0001), 1.98 (95% CI: 1.26-3.09, p = 0.003), 2.78 (95% CI: 1.64-4.71, p < 0.0001), and 3.09 (95% CI: 1.91-5.01, p < 0.0001), respectively (Figure 3). The analysis showed an increasing trend in the risk of all-cause mortality across CAD-RADS categories (Regression co-efficient = 0.046; 95% CI: 0.008-0.084; p = 0.018).
Figure 3.
Value of CAD-RADS classification system in prediction of all-cause mortality in coronary artery disease patients. CAD-RADS: Coronary artery disease-reporting and data system; CI: Confidence interval; HR: Hazard ratio
Although it should be noted that the results of CAD-RADS categories 4a, 4b, and 5 should be interpreted with caution due to the limited number of studies in the respective analyses.
The follow-up of the included studies varied between 4 to 10 years. A meta-regression analysis was performed to assess the effect of follow-up length on the predictive value of CAD-RADS for all-cause mortality. Due to the limited number of included studies, the analyses could only be performed on CAD-RADS categories 1, 2, and 3, none of which showed relations to the follow-up duration (Supplementary Table 2).
3.4. Miscellaneous CAD-RADS reporting
10 articles had reports of combinations of the original CAD-RADS categories for the prediction of MACE (13, 15-17, 20-25). Lee et. al (15) demonstrated that a combined category of CAD-RADS 1 and 2 could not predict 3-month (HR = 2.4, 95% CI: 0.8-6.9) and 31.5-month (HR = 1.5, 95% CI: 0.7-3.2) MACE. Van Rosendael et al. (24) reported that moderate CAD (consisting of CAD-RADS 2 and 3) was not a predictor of MACE (HR = 1.95, 95% CI: 1.19-3.2).
Two studies utilized a category of CAD-RADS ≥ 3 with conflicting results. Duguay et al. (20) reported a hazard ratio of 3.12 (95% CI: 1.03-10.17) for this combined category in the prediction of MACE, while Yamamato et al. (25) did not report a predictive value of CAD-RADS for MACE (HR = 1.3, 95% CI: 0.16-11.1).
CAD-RADS 4 (combined category of CAD-RADS 4A and 4B) was utilized by two studies (16, 17), and was shown to be a predictor of MACE (HR = 36.48, 95% CI: 4.94-269.67 and HR = 13.49, 95% CI: 4.809-37.86). 5 experiments had data on a combined category of CAD-RADS 4A, 4B and 5 (15, 23-25). Four of these experiments (15, 23, 24) showed that this combined CAD-RADS category can predict MACE. Two studies combined CAD-RADS categories 4b and 5 and reported conflicting results. Bittner et al. (13) reported a hazard ratio of 21.84 (95% CI: 8.63-55.26) while Yamamoto et al. (25) reported a hazard ratio of 2.6 (95% CI: 0.72-9.2) for the predictive value of this subset category for MACE.
Two studies reported results for the predictive value of overall CAD-RADS. Faber et al. (21) demonstrated that CAD-RADS as a continuous variable can be predictive of MACE in males (HR = 1.97, 95% CI: 1.12-3.45), females (HR = 5.34, 95% CI: 2.42-11.8), patients < 65 years of age (HR = 2.34, 95% CI: 1.23-4.45) and patients ≥ 65 years of age (HR = 2.8, 95% CI: 1.46-5.35). Maclean et al. (22) reported a hazard ratio of 2.96 (95% CI: 2.2-4) for the predictive value of overall CAD-RADS for the prediction of MACE.
Three articles (16, 26, 27) had reported combinations of the original CAD-RADS categories for prediction of all-cause mortality. Senoner et. al (16) reported that CAD-RADS 4 (combined CAD-RADS 4A and 4B) can predict all-cause mortality (HR = 2.97, 95% CI: 1.59-5.57). Huang et. al (27) also reported that combined CAD-RADS 4B and 5 can be predictive of all-cause mortality (HR = 2.761, 95% CI: 1.961-3.887). Finck et al. (26) reported that while an overall CAD-RADS category can be predictive of all-cause mortality in non-diabetic patients (HR = 2.03, 95% CI: 1.44-2.86), it does not predict all-cause mortality in non-diabetic patients (HR = 1.72, 95% CI: 0.98-3.01). Although it should be noted that the diabetic group consisted of only 132 patients. The results of the analyses on the miscellaneous CAD-RADS reporting are demonstrated in table 3.
Table 3.
Results of miscellaneous CAD-RADS reporting
| CAD-RADS category | Study, Year | Hazard Ratio (95% CI) |
|---|---|---|
| Major Adverse Cardiovascular Event (MACE) | ||
| 1 and 2 | Lee, 2021 (15) | 2.4 (0.8-6.9) |
| 1.5 (0.7-3.2) | ||
| 2 and 3 | Van Rosendael, 2019 (24) | 1.95 (1.19-3.2) |
| ≥3 | Duguay, 2017 (20) | 3.12 (1.03-10.17) |
| Yamamoto, 2021 (25) | 1.3 (0.16-11.1) | |
| 4 (4A and 4B) | Senoner, 2020 (16) | 36.48 (4.94-269.67) |
| Tang, 2022 (17) | 13.49 (4.809-37.86) | |
| ≥4 | Lee, 2021 (15) | 1.7 (1.1-39.2) |
| Lee, 2021 (15) | 8.5 (3.7-15.8) | |
| Mangalesh, 2022 (23) | 3.801 (1.58-9.145) | |
| Van Rosendael, 2019 (24) | 2.68 (1.3-5.53) | |
| Yamamoto, 2021 (25) | 1.3 (0.35-5.15) | |
| 4B+5 | Bittner, 2020 (13) | 21.84 (8.63-55.26) |
| Yamamoto, 2021 (25) | 2.6 (0.72-9.2) | |
| Overall | Faber, 2021 (21) | 1.97 (1.12-3.45) male |
| 5.34 (2.42-11.8) female | ||
| 2.34 (1.23-4.45) < 65 years | ||
| 2.8 (1.46-5.35) ≥65 years | ||
| Maclean, 2022 (22) | 2.96 (2.2-4) | |
| All-cause mortality | ||
| 4 (4A and 4B) | Huang, 2021 (27) | 2.761 (1.961-3.887) |
| 4B and 5 | Senoner, 2020 (16) | 2.97 (1.59-5.57) |
| Overall | Finck, 2019 (b) (26) | 2.03 (1.44-2.86) non-diabetic |
| 1.72 (0.98-3.01) diabetic | ||
CAD-RADS: Coronary artery disease-reporting and data system; CI: confidence interval.
3.5. Quality assessment
The risk of bias was evaluated according to the guidelines of QUAPAS. The risk of bias was assessed to be unclear in the domain of patient selection in four studies due to no report of the sampling method. Seven articles were judged to have an unclear risk of bias in the domain of index test due to no mention of the specialty of the assessor. Two articles were evaluated as unclear in risk of bias in the domain of outcome due to no report of MACE definition and the source of gathered data (registries, medical records, etc.). All articles were judged to have a high risk of bias in the flow and timing domain. However, considering that according to guidelines the treatment of coronary artery disease patients varies depending on its severity, clinical management of CAD patients cannot be identical and thus, the risk of bias in the domain of flow and timing was decided to be excluded from the judgment of overall risk of bias. One study was found to have a high risk of bias in the domain of analysis due to a high loss to follow-up rate. Studies were rated as low in all other domains of risk of bias assessment. One study was judged to have unclear applicability in the domain of outcome due to non-informative outcome definition. Studies were assessed to have no concerns in applicability in other domains (Table 4).
Table 4.
Risk of bias assessment
| Study, year | Risk of Bias | Applicability | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Outcome |
Flow
and timing* |
Analysis |
Patient
selection |
Index
test |
Outcome | Flow and timing | ||
| Altay, 2021 (12) | Unclear | Low | Unclear | High | Low | Low | Low | Unclear | Low | Some concern |
| Bittner, 2020 (13) | Low | Low | Low | High | Low | Low | Low | Low | Low | Low |
| Duguay, 2017 (20) | Unclear | Low | Low | High | Low | Low | Low | Low | Low | Some concern |
| Faber, 2021 (21) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Finck, 2019 (a) (14) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Finck, 2019 (b) (26) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Huang, 2021 (27) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Lee, 2021 (15) | Unclear | Low | Low | High | Low | Low | Low | Low | Low | Some concern |
| Maclean, 2022 (22) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Mangalesh, 2022 (23) | Unclear | Low | Unclear | High | Low | Low | Low | Low | Low | Some concern |
| Senoner, 2020 (16) | Low | Low | Low | High | Low | Low | Low | Low | Low | Low |
| Tang, 2022 (17) | Low | Low | Low | High | High | Low | Low | Low | Low | Some concern |
| Van Rosendael, 2019 (24) | Low | Low | Low | High | Low | Low | Low | Low | Low | Low |
| Williams, 2020 (18) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Xie, 2018 (19) | Low | Unclear | Low | High | Low | Low | Low | Low | Low | Some concern |
| Yamamoto, 2021 (25) | Low | Low | Low | High | Low | Low | Low | Low | Low | Low |
*Flow and timing domain was judged to be of high risk of bias due to differences in treatment of coronary artery disease patients; However, considering that according to guidelines the treatment of coronary artery disease patients varies depending on its severity, the bias in flow and timing domain was excluded from the judgment of overall risk of bias.
3.6. Certainty of evidence
All included studies were observational studies and the base level of evidence was set as low. The level of evidence for the outcome of MACE was reduced by two grades due to considerable risk of bias and observed heterogeneity in the analysis. It was increased by two grades due to the observed large magnitude of effect (HR > 2) and possible dose-response gradient observed and thus, the level of evidence for the outcome of MACE was judged to be low. The level of evidence for the outcome of all-cause mortality was decreased by two grades due to the considerable risk of bias and imprecision (wide CIs) and increased by one due to the possible dose-response gradient. The level of evidence for the outcome of all-cause mortality was judged to be very low (Table 5).
Table 5.
Certainty of evidence
| Certainty assessment | Hazard Ratio (95% CI) | Certainty | |||||
|---|---|---|---|---|---|---|---|
| Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ||
| MACE (follow-up: range 1 month to 10 years) | |||||||
| 8 studies | Serious | Seriousa | Not serious | Not serious | Large magnitude of effectPossible dose response gradient |
CAD-RADS 1: 3.39 (95% CI: 2.19-5.23) CAD-RADS 2: 4.19 (95% CI: 2.93-5.99) CAD-RADS 3: 5.99 (95% CI: 3.83-9.38) CAD-RADS 4A: 7.29 (95% CI: 3.54-15.02) CAD-RADS 4B: 6.27 (95% CI: 5.02-7.84) CAD-RADS 5: 8.63 (95% CI: 4.67-15.95) |
⨁⨁◯◯Low |
| All-cause mortality (follow-up: range 6 months to 8 years) | |||||||
| 3 studies | Serious | Not serious | Not serious | Seriousb | Possible dose response gradient |
CAD-RADS 1: 1.50 (95% CI: 0.96-2.34) CAD-RADS 2: 1.85 (95% CI: 1.28-2.68) CAD-RADS 3: 1.65 (95% CI: 1.31-2.07) CAD-RADS 4A: 1.98 (95% CI: 1.26-3.09) CAD-RADS 4B: 2.78 (95% CI: 1.64-4.71) CAD-RADS 5: 3.09 (95% CI: 1.91-5.01) |
⨁◯◯◯ Very low |
CAD-RADS: Coronary artery disease-reporting and data system; MACE: Major adverse cardiac event; CI: Confidence interval.
a. There was considerable heterogeneity among the studies.
b. Wide CIs
4. Discussion:
This systematic review and meta-analysis examined the effectiveness of hierarchical CAD-RADS categorization in predicting MACE and all-cause mortality in CAD patients. According to our analysis, all original CAD-RADS categories can be used as a predictor of MACE, and all categories, except CAD-RADS 1, can be a predictor of all-cause mortality. Moreover, patients with higher CAD-RADS categories had a higher risk of unfavorable outcomes. Our results confirm that CAD-RADS categorization of CCTA findings is valid for prognostication of CAD patients’ outcomes, which is consistent with other scores such as modified Duke index (28).
The results of our analysis demonstrate that CAD-RADS categories 1 and 2, which are representative of non-obstructive CAD patients (less than 50% stenosis), are associated with an increased risk of unfavorable outcomes. Studies have shown that non-obstructive CAD is attributable to MACE in acute coronary syndrome patients treated with percutaneous coronary intervention and that non-obstructive CAD in CCTA should be considered as a clinically important finding (29, 30). This implies that the previous concept of dichotomization of CAD patients into an obstructive and non-obstructive group, may not be informative enough for the prediction of patient outcomes, and preventative and treatment strategy decision-making should not solely rely on the degree of obstruction and various variables such as plaque characteristics should also be taken into account.
Contrary to the results of the meta-analysis of the original CAD-RADS categories, the results of the articles utilizing a combination of CAD-RADS categories were less conclusive on their predictive value due to having wider CIs and reports of insignificant predictive value for higher CAD-RADS categories. However, it should be noted that the references for the analyses of the combined CAD-RADS categories differed among studies and the studies not demonstrating a predictive value for the CAD-RADS category had smaller sample sizes. This finding implies that CAD-RADS might be better utilized as the original separate categories and attempts of combining these categories (even only combining categories 4A and 4B) could reduce the accuracy of the classification system. However, it should be kept in mind that few studies with limited sample sizes had evaluated combinations of CAD-RADS categories.
The included studies had different durations of follow-up, however, our analysis indicated that follow-up had no effect on the predictive value of CAD-RADS for MACE and all-cause mortality.
Faber et al. (21) evaluated the predictive value of CAD-RADS for MACE in male and female populations and demonstrated that CAD-RADS as a continuous variable might be a better predictive tool in the female rather than the male population. Articles have demonstrated that the female CAD population has higher mortality than the male counterparts, which could explain the higher HR reported for the female population (31). Further studies could shed more light on the differences in the predictive value of CAD-RADS in male and female populations.
The fundamental parameter for the categorization of CCTA in CAD-RADS is the luminal diameter of the most stenotic vessel and post-CCTA recommendation on the further diagnostic test and management depends on the mere CAD-RADS category. Unlike other scoring indices such as the Duke index, CAD-RADS ignores the number of involved vessels and the location of the culprit lesion. As a result, the CAD-RADS category cannot replace the CCTA report and should be interpreted in conjunction with the detailed CCTA report. Evidence indicates that along with dimensional parameters of coronary atherosclerotic lesions, plaque characteristics also play a pivotal role in risk prediction of CAD patients (32, 33). CAD-RADS reflects plaque characterization through an extra modifier that assesses the presence of vulnerable or high-risk plaque. In our review, due to the unavailability of data, we could not perform an analysis on the effect of such additional modifiers on patients’ risk of MACE and mortality. It can be assumed that detailed categorization of CCTA findings in addition to CAD-RADS scoring may yield more prognostic information than what is presented in our study. Although the CAD-RADS was not designed to take the place of the complete descriptive reports of CCTA, the lack of incorporation of such contributing factors may render CCTA's rich data obsolete. Nevertheless, the structuralized reporting of CCTA using CAD-RADS and the discrete prognostic value of each category will lead to better patient care by consolidating the communication between referring and interpreting physicians (34).
To improve the representativeness of CAD-RADS categorization, CAD-RADS 2.0 (35) was introduced in 2022, which incorporates parameters reflecting plaque burden and ischemia in the form of modifiers added to stenosis assessment. This could improve the decision-making process for CAD patients by better representing the extent of CAD and the lesions’ characteristics. Yet, CAD-RADS’s shortcoming remains due to the fact that it cannot be used to evaluate the number of involved vessels. This reduces the effectiveness of the CAD-RADS classification system in predicting outcomes and progression in patients with multiple vessels involved (36).
With the latest advancements in image processing, there is potential for the addition of other parameters related to atherosclerosis pathogenesis, including pericardial fat attenuation (37), to enhance the precision of prognostic and diagnostic performance of the CAD-RADS scoring system. In this regard, machine learning was shown to be promising in integrating multiplex CCTA parameters with both other performed test variables and patient characteristics. This would enable individualized and highly accurate risk prediction, which would significantly impact delivering optimal patient care (38, 39).
Moreover, besides risk stratification, the CAD-RADS scoring system recommends further test studies and treatment plans for each CAD-RADS category. Although these recommendations are derived from expert consensus, since there currently are scarce evidence on the treatment strategies of CAD-RADS guidelines, the treating physician should implement an individualized treatment plan for each patient and not solely rely on the recommendations provided by CAD-RADS guidelines. Future studies should investigate the effectiveness of these treatment plans on the disease progression of patients.
5. Limitation
We acknowledge that our study has limitations. The definition of MACE varied between the studies, which limits accurate comparison of the studies. A recent systematic review (40) has shown that only a limited number of studies match the conventional MACE definition of acute myocardial infarction, stroke, and cardiovascular death. Since the definitions vary slightly between studies, all reports of MACE were pooled and analyzed together. Also, not all the included studies specified whether the included patients were acute or chronic CAD patients or whether the patients were symptomatic or asymptomatic, which might lead to different prognoses and treatment plans. Further studies should more accurately specify the patient population and compare the predictive capabilities of CAD-RADS in acute and chronic CAD patients. Also, a major contributor to the outcome of CAD patients is the treatment strategies devised for the patients, which was not reported in the included articles and may vary depending on national and institutional guidelines.
6. Conclusion:
Low to very low levels of evidence demonstrated that the CAD-RADS classification system can be used to predict outcomes of MACE and all-cause mortality. This indicates that the implementation of CAD-RADS into clinical practice, besides enhancing the communication between physicians and improving patient care, can also guide physicians in risk assessment of the patients’ prognosis. Further well-designed clinical trials or prospective cohort studies are needed to provide a high level of evidence for predicting the value of CAD-RADS in CAD patients.
7. Declarations:
7.1. Availability of data and materials
The gathered data and checklist can be provided to qualified researchers with the intent of replicating the procedure and results.
7.2. Conflicting interests
The authors declare that they have no competing interests.
7.3. Funding
This study was funded by Shahid Beheshti University of Medical Sciences (Grant No. 43005143).
7.4. Author contributions
Study design: MY, RM
Data gathering: MR, KA
Analysis: MY, KA
Interpretation of results: all authors
Drafting and revising: all authors
7.5. Acknowledgements
Not applicable.
Supplementary materials
Supplementary Material 1.
Search strategy for the “Evaluation of the value of Coronary Artery Disease – Reporting and Data System (CAD-RADS) in outcome prediction of coronary artery disease patients” (Septemeber 19th, 2022)
|
PubMed:
CAD-RADS [tiab] OR [tiab] CAD RADS[tiab] OR CADRADS[tiab] OR Coronary Artery Disease Reporting and Data System[tiab] OR coronary artery disease reporting[tiab] Embase: ‘Coronary Artery Disease Reporting and Data System’/exp OR ‘CAD-RADS’:ab,ti OR ‘CAD RADS’:ab,ti OR ‘CADRADS’:ab,ti OR ‘Coronary Artery Disease Reporting’:ab,ti Web of Science: (ALL=("Coronary Artery Disease Reporting and Data System" OR "CAD-RADS" OR "CAD RADS" OR "CADRADS" OR "Coronary Artery Disease Reporting")) Scopus: TITLE-ABS-KEY ("Coronary Artery Disease Reporting and Data System" OR "CAD-RADS" OR "CAD RADS" OR "CADRADS" OR "Coronary Artery Disease Reporting") |
Supplementary Table 1.
Meta-regression for evaluation of effect of follow-up on predictive performance of CAD-RADS for major adverse cardiovascular event (MACE)
| Variable | Meta-regression coefficient | 95% Confidence interval | P value |
|---|---|---|---|
| CAD-RADS 1 | 1.00975 | 0.99771, 1.02193 | 0.113 |
| CAD-RADS 2 | 1.00461 | 0.99264, 1.01671 | 0.452 |
| CAD-RADS 3 | 1.00492 | 0.99291, 1.01708 | 0.423 |
| CAD-RADS 4a | 1.00490 | 0.97994, 1.03050 | 0.703 |
| CAD-RADS 4b | 1.00441 | 0.99411, 1.01482 | 0.402 |
| CAD-RADS 5 | 1.01510 | 0.99035, 1.04047 | 0.234 |
CAD-RADS: Coronary artery disease-reporting and data system.
Supplementary Table 2.
Meta-regression for evaluation of effect of follow-up on predictive performance of CAD-RADS for all-cause mortality
| Variable | Meta-regression coefficient | 95% Confidence interval | P value |
|---|---|---|---|
| CAD-RADS 1 | 1.00573 | 0.98776, 1.02402 | 0.534 |
| CAD-RADS 2 | 1.00615 | 0.99350, 1.01897 | 0.342 |
| CAD-RADS 3 | 1.00551 | 0.99266, 1.01853 | 0.402 |
| CAD-RADS 4a | Not assessable due to limited number of studies | ||
| CAD-RADS 4b | Not assessable due to limited number of studies | ||
| CAD-RADS 5 | Not assessable due to limited number of studies | ||
CAD-RADS: Coronary artery disease-reporting and data system.
References
- 1.Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76(10):1226–43. doi: 10.1016/j.jacc.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cordeiro MA, Lima JA. Atherosclerotic plaque characterization by multidetector row computed tomography angiography. J Am Coll Cardiol. 2006;47(8 Suppl):C40–7. doi: 10.1016/j.jacc.2005.09.076. [DOI] [PubMed] [Google Scholar]
- 3.Chandrashekhar Y, Min JK, Hecht H, Narula J. CAD-RADS: A Giant First Step Toward a Common Lexicon? JACC Cardiovasc Imaging. 2016;9(9):1125–9. doi: 10.1016/j.jcmg.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Kumar P, Bhatia M. Coronary Artery Disease Reporting and Data System: A Comprehensive Review. J Cardiovasc Imaging. 2022;30(1):1–24. doi: 10.4250/jcvi.2020.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI) Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10(4):269–81. doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Maroules CD, Hamilton-Craig C, Branch K, Lee J, Cury RC, Maurovich-Horvat P, et al. Coronary artery disease reporting and data system (CAD-RADS(TM)): Inter-observer agreement for assessment categories and modifiers. J Cardiovasc Comput Tomogr. 2018;12(2):125–30. doi: 10.1016/j.jcct.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Abdel Razek AAK, Elrakhawy MM, Yossof MM, Nageb HM. Inter-observer agreement of the Coronary Artery Disease Reporting and Data System (CAD-RADS(TM)) in patients with stable chest pain. Pol J Radiol. 2018;83:e151–e9. doi: 10.5114/pjr.2018.75641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foldyna B, Szilveszter B, Scholtz JE, Banerji D, Maurovich-Horvat P, Hoffmann U. CAD-RADS - a new clinical decision support tool for coronary computed tomography angiography. Eur Radiol. 2018;28(4):1365–72. doi: 10.1007/s00330-017-5105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Mulder F, Leeflang M, Wolff R, Whiting P, Bossuyt PM. QUAPAS: An Adaptation of the QUADAS-2 Tool to Assess Prognostic Accuracy Studies. Ann Intern Med. 2022;175(7):1010–8. doi: 10.7326/M22-0276. [DOI] [PubMed] [Google Scholar]
- 10.Schunemann H, Higgins J, Vist G, Glasziou P, Akl E, Skoetz N, et al. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. Cochrane Handbook for Systematic Reviews of Interventions. 2019:375–402. [Google Scholar]
- 11.Page M, Higgins J, Sterne J. Assessing risk of bias due to missing results in a synthesis. Cochrane Handbook for Systematic Reviews of Interventions . 2019: 349–74. [Google Scholar]
- 12.Altay S. Prognostic Value of Standard Coronary Computed Tomography Angiography Reporting System (CAD-RADS) Indian J Radiol Imaging. 2021;31(1):37–42. doi: 10.1055/s-0041-1729128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittner DO, Mayrhofer T, Budoff M, Szilveszter B, Foldyna B, Hallett TR, et al. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc Imaging. 2020;13(7):1534–45. doi: 10.1016/j.jcmg.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finck T, Hardenberg J, Will A, Hendrich E, Haller B, Martinoff S, et al. 10-Year Follow-Up After Coronary Computed Tomography Angiography in Patients With Suspected Coronary Artery Disease. JACC Cardiovasc Imaging. 2019 (a)12(7 Pt 2):1330–8. doi: 10.1016/j.jcmg.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Kim JY, Han K, Im DJ, Lee KH, Kim TH, et al. Coronary CT Angiography CAD-RADS versus Coronary Artery Calcium Score in Patients with Acute Chest Pain. Radiology. 2021;301(1):81–90. doi: 10.1148/radiol.2021204704. [DOI] [PubMed] [Google Scholar]
- 16.Senoner T, Plank F, Barbieri F, Beyer C, Birkl K, Widmann G, et al. Added value of high-risk plaque criteria by coronary CTA for prediction of long-term outcomes. Atherosclerosis. 2020;300:26–33. doi: 10.1016/j.atherosclerosis.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Tang CX, Qiao HY, Zhang XL, Di Jiang M, Schoepf UJ, Rudzinski PN, et al. Functional CAD-RADS using FFR(CT) on therapeutic management and prognosis in patients with coronary artery disease. Eur Radiol. 2022;32(8):5210–21. doi: 10.1007/s00330-022-08618-5. [DOI] [PubMed] [Google Scholar]
- 18.Williams MC, Moss A, Dweck M, Hunter A, Pawade T, Adamson PD, et al. Standardized reporting systems for computed tomography coronary angiography and calcium scoring: A real-world validation of CAD-RADS and CAC-DRS in patients with stable chest pain. J Cardiovasc Comput Tomogr. 2020;14(1):3–11. doi: 10.1016/j.jcct.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Xie JX, Cury RC, Leipsic J, Crim MT, Berman DS, Gransar H, et al. The Coronary Artery Disease-Reporting and Data System (CAD-RADS): Prognostic and Clinical Implications Associated With Standardized Coronary Computed Tomography Angiography Reporting. JACC Cardiovasc Imaging. 2018;11(1):78–89. doi: 10.1016/j.jcmg.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Duguay TM, Tesche C, Vliegenthart R, De Cecco CN, Lin H, Albrecht MH, et al. Coronary Computed Tomographic Angiography-Derived Fractional Flow Reserve Based on Machine Learning for Risk Stratification of Non-Culprit Coronary Narrowings in Patients with Acute Coronary Syndrome. Am J Cardiol. 2017;120(8):1260–6. doi: 10.1016/j.amjcard.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Faber M, Will A, Hendrich E, Martinoff S, Hadamitzky M. Sex- and age-specific differences in the long-term prognostic value of morphological plaque features detected by coronary computed tomography angiography. J Cardiovasc Comput Tomogr. 2021;15(3):274–80. doi: 10.1016/j.jcct.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Maclean E, Mahon C, Sehmi J, Kanaganayagam G, Ngee TT, Nicol ED. CT multivessel aggregate stenosis score: A novel point-of-care tool for predicting major adverse cardiac events. J Cardiovasc Comput Tomogr. 2022;16(4):350–4. doi: 10.1016/j.jcct.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Mangalesh S, Yadav P, Dudani S, Mahesh NK. Atherogenic index of plasma predicts coronary artery disease severity and major adverse cardiac events in absence of conventional risk factors. Coron Artery Dis. 2022;33(7):523–30. doi: 10.1097/MCA.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 24.van Rosendael AR, Shaw LJ, Xie JX, Dimitriu-Leen AC, Smit JM, Scholte AJ, et al. Superior Risk Stratification With Coronary Computed Tomography Angiography Using a Comprehensive Atherosclerotic Risk Score. JACC Cardiovasc Imaging. 2019;12(10):1987–97. doi: 10.1016/j.jcmg.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto A, Nagao M, Ando K, Nakao R, Fukushima K, Matsuo Y, et al. Risk stratification in coronary artery disease using NH(3)-PET myocardial flow reserve and CAD-RADS on coronary CT angiography. Int J Cardiovasc Imaging. 2021;37(11):3335–42. doi: 10.1007/s10554-021-02312-1. [DOI] [PubMed] [Google Scholar]
- 26.Finck T, Will A, Hendrich E, Martinoff S, Hadamitzky M. Coronary computed tomography angiography as a tool for long-term cardiovascular risk stratification in diabetic patients. Heart Vessels. 2019 (b)34(7):1086–95. doi: 10.1007/s00380-018-01339-0. [DOI] [PubMed] [Google Scholar]
- 27.Huang Z, Zhang S, Jin N, Hu Y, Xiao J, Li Z, et al. Prognostic value of CAD-RADS classification by coronary CTA in patients with suspected CAD. BMC Cardiovasc Disord. 2021;21(1):476. doi: 10.1186/s12872-021-02286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50(12):1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 29.Wang ZJ, Zhang LL, Elmariah S, Han HY, Zhou YJ. Prevalence and Prognosis of Nonobstructive Coronary Artery Disease in Patients Undergoing Coronary Angiography or Coronary Computed Tomography Angiography: A Meta-Analysis. Mayo Clin Proc. 2017;92(3):329–46. doi: 10.1016/j.mayocp.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–91. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 31.Group EUCCS, Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37(1):24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 32.Gossl M, Versari D, Hildebrandt H, Mannheim D, Olson ML, Lerman LO, et al. Vulnerable plaque: detection and management. Med Clin North Am. 2007;91(4):573–601. doi: 10.1016/j.mcna.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30(7):1282–92. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 34.Canan A, Ranganath P, Goerne H, Abbara S, Landeras L, Rajiah P. CAD-RADS: Pushing the Limits. Radiographics. 2020;40(3):629–52. doi: 10.1148/rg.2020190164. [DOI] [PubMed] [Google Scholar]
- 35.Cury RC, Leipsic J, Abbara S, Achenbach S, Berman D, Bittencourt M, et al. CAD-RADS 2 0 - 2022 Coronary Artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI) J Cardiovasc Comput Tomogr. 2022;16(6):536–57. doi: 10.1016/j.jcct.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Szilveszter B, Vattay B, Bossoussou M, Vecsey-Nagy M, Simon J, Merkely B, et al. CAD-RADS may underestimate coronary plaque progression as detected by serial CT angiography. Eur Heart J Cardiovasc Imaging. 2022;23(11):1530–9. doi: 10.1093/ehjci/jeab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398):eaal2658. doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 38.Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256(3):774–82. doi: 10.1148/radiol.10092473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolossvary M, Karady J, Szilveszter B, Kitslaar P, Hoffmann U, Merkely B, et al. Radiomic Features Are Superior to Conventional Quantitative Computed Tomographic Metrics to Identify Coronary Plaques With Napkin-Ring Sign. Circ Cardiovasc Imaging. 2017;10(12):e006843. doi: 10.1161/CIRCIMAGING.117.006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. 2021;21(1):241. doi: 10.1186/s12874-021-01440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The gathered data and checklist can be provided to qualified researchers with the intent of replicating the procedure and results.