Abstract
Research on spatial navigation is essential to understanding how mobile species adapt to their environments. Such research increasingly uses virtual environments (VEs) because, although VE has drawbacks, it allows for standardization of procedures, precision in measuring behaviors, ease in introducing variation, and cross-investigator comparability. Developmental researchers have used a wide range of VE testing methods, including desktop computers, gaming consoles, virtual reality, and phone applications. We survey the paradigms to guide researchers’ choices, organizing them by their characteristics using a framework proposed by Girard (2022) in which navigation is reactive or deliberative, and may be tied to sensory input or not. This organization highlights what representations each paradigm indicates. VE tools have enriched our picture of the development of navigation, but much research remains to be done, e.g., determining retest reliability, comparing performance on different paradigms, validating performance against real-world behavior and open sharing. Reliable and valid assessments available on open-science repositories are essential for work on the development of navigation, its neural bases, and its implications for other cognitive domains.
Introduction
Navigation is a central adaptive function for all mobile animals. Survival depends on effective foraging or hunting, avoiding predators, finding mates, and getting to shelter. Research in various domains has expanded our understanding of way finding, from ethological and experimental approaches with various species to neural analyses at levels ranging from individual cells to broad regions mainly in rodents, non-human primates, and humans. Overall, we have amassed a rich empirical investigation, fortunately coupled with a clear idea of the computations required by the task of navigation (Gallistel, 1990). Understanding the development of navigation is a central part of understanding this crucial aspect of adaptive functioning.
Researchers in cognitive development have adopted various views of how children learn to navigate. They range from the Piagetian view that mature spatial representation appears in late childhood (Piaget, 1954) to the nativist claim that babies have the essential elements of the system, later augmented by spatial language (e.g., Shusterman, Ah Lee, & Spelke, 2011). In a more agnostic approach, Siegel and White (1975) proposed that spatial knowledge begins with landmarks, landmarks then linked in sequential routes, and routes linked into survey knowledge, i.e., a cognitive map, although (Montello, 1998) suggested concurrent development of these skills. None of these approaches has emerged as the dominant theoretical framework. Indeed, the very concept of cognitive map, which is embraced by all three kinds of theory in various ways, has proved controversial (e.g., McNamara, Ratcliff, & McKoon, 1984; Shettleworth, 2012; Warren, 2019; see Peer, Brunec, Newcombe, & Epstein, 2021, for an argument that cognitive map formation depends on the environment and the person).
Moving beyond these three approaches to a fourth, there is an increasing focus on how humans come to precisely encode and adaptively combine cues to spatial location (Figure 1; for overviews, see Nardini, 2021; Newcombe & Huttenlocher, 2006; Newcombe, Uttal, & Sauter, 2013). The developmental sequence begins in infancy, as babies acquire motor skills that allow for independent locomotion and decrease their reliance on habit systems. Over childhood, children increasingly use two important navigational systems flexibly: (1) inertial navigation, which draws on information from kinesthesis, proprioception, visual flow, and other sensory systems to perform continuous updating of spatial position; (2) allocentric coding of external information such as landmarks, slope of the ground or the shape of enclosures (Newcombe, 2019; Pullano & Foti, 2022).
Figure 1. Schematic of spatial development across the lifespan.
Darker colors in the gradients represent better navigational accuracy and greater integration of systems. Figure adapted from Nguyen & Tansan, 2022/Open science framework, https://doi.org/10.17605/OSF.IO/PCJR3 (CC BY 4.0).
Initial research on the development of navigation occurred in large-scale environments in the real world. Researchers used large gymnasiums, campuses, suburban developments, or even whole towns to study children’s spatial knowledge (e.g., Allen, Kirasic, Siegel, & Herman, 1979; Anooshian & Young, 1981; Herman & Siegel, 1978; Liben, Myers, Christensen, & Bower, 2013). Despite the ecological validity of real-world spaces, there are substantial drawbacks to this approach. We cannot compare results across labs and evaluate replicability, and factors such as weather and transportation affect our ability to collect the data from the large and diverse samples necessary to evaluate individual differences. Over the past decades, researchers have devised virtual environments (VEs) to address these challenges (e.g., Bullens, Iglói, Berthoz, Postma, & Rondi-Reig, 2010; Laurance, Learmonth, Nadel, & Jake Jacobs, 2003). Truly immersive virtual environments are expensive, cumbersome and can induce motion sickness, although they do allow for physical movement that engages the inertial navigation system. More often, developmental researchers use non-immersive VEs.
The purpose of this article is to provide a guide and highlight the utility of VEs in capturing various navigation approaches in children. Spatial cognition VEs vary in many ways. They assess different aspects of spatial representation, may or may not aim for ecological validity, and may or may not constrain exploration. We organize the paradigms by the specific spatial constructs they assess, using a taxonomy proposed by Girard (2022) in Table 1. In reactive (model-free) encoding with sensory inputs, navigators learn the associations between visual or auditory cues and the goal, as in a target or beacon approach, or memory for sequential turns. Reactive place encoding adds positional information, as when a child finds a toy hidden under sand given a landmark directly overhead or based on entry into the sandbox from a constant point. In deliberative (model-based) encoding, the navigator uses a knowledge store to plan or topological or even metric planning. Stable distal landmarks can enable flexible map-less planning, for example, in a radial maze setup. Building a map is very difficult, but navigators can still use global cues (distal landmarks) to navigate allocentrically as opposed to egocentrically or sequentially. Deliberative place-based coding likely requires more complex environments.
Using this taxonomy, we can organize paradigms that have been used to study children’s navigation (Table 2). Enclosed or semi-enclosed maze VEs or open VEs without distal landmarks do not (easily) support the formation of cognitive maps, unless inertial navigation is unusually precise, which is challenging for humans and usually occurs only in simple environments with few turns. They are useful for examining children’s memory for landmarks and specific turn sequences. Environments with distal landmarks, either containing routes, or using open areas on which participants must learn locations, such as a field or open stretch of water offer a stronger basis for cognitive map formation. Cognitive mapping may be best captured by complex naturalistic environments that aim for ecological validity.
Table 2.
Features of navigation VEs and the spatial constructs they measure. Adapted from Nguyen & Newcombe, 2022/Open science framework, https://doi.org/10.17605/OSF.IO/JR8VK (CC BY 4.0).
| Type of VE | Features | Spatial Navigation Approach |
|---|---|---|
| Path Maze | Enclosed maze: no distal landmarks, local landmarks associated with turns, usually narrow hallways with walls |

|
| Semi-enclosed maze: same as enclosed mazes, except the ceiling can be open, usually to a sky |

|
|
| Open maze: includes distal and local landmarks, no covered ceiling, none or very low walls, gives a sense of vista space |

|
|
| Radial/arm maze e.g., figure 1A | distal landmarks, arms coming out from a central point, open at the top | |
| Morris water maze e.g., figure 1B & 1C | local and distal landmarks, boundary around circular arena, no covered ceiling |
|
| Naturalistic city/town e.g., figure 2 | distal and local landmarks, includes buildings and roads |
 
|
 Deliberative with place encoding, ie., mapping Deliberative with place encoding, ie., mapping | ||
 It is possible to map the information from maze VEs, but it is very challenging to execute in a confined structure lacking distal cues.
It is possible to map the information from maze VEs, but it is very challenging to execute in a confined structure lacking distal cues.
 The richness of distal cues in MWM VEs varies, affecting the ease and likelihood of mapping.
The richness of distal cues in MWM VEs varies, affecting the ease and likelihood of mapping.
 Although these environments support mapping, not all individuals may encode this way.
Although these environments support mapping, not all individuals may encode this way.
Path mazes
Maze paradigms can be traversed by reactive navigation with visual sensory inputs (Tables 1 & 2). That is, when navigating corridor paths in an enclosed environment without distal landmarks, participants must focus on recall of the sequence of landmarks and turns, and the association of turns with landmarks (Tlauka & Wilson, 1994). Sometimes landmarks are even absent, so that the focus is simply on turns along the route. Path mazes have been used from 6 years to early adolescence, sometimes with an adult comparison group. Young children often do well, although there is improvement across the elementary school years (Lingwood, Blades, Farran, Courbois, & Matthews, 2015).
Table 1. A taxonomy of navigation strategies.
Adapted from Girard, 2022/FigShare, figshare.https://doi.org/10.6084/m9.figshare.20390742.v2, and adapted with permission (Nguyen & Newcombe, 2022; Open science framework, https://doi.org/10.17605/OSF.IO/JR8VK CC BY 4.0).
| Reactive (Model-free) |
Deliberative (Model-based) |
|
| Sensory Inputs | Stimulus-triggered response Target approach Beacon approach Homing Praxic |
Map-less planning |
| Place Encoding | Place-triggered response |
Topological planning Metric planning 
|
Jansen-Osmann and her group have created several enclosed mazes for children (younger 6–8 years, older 10–12 years) and adults to examine how landmarks are used for way finding, given various manipulations. For instance, participants might learn a route through irregular corridors (without predictable or consistent turns) with pictures of stuffed animals as landmarks; this design makes it more challenging to depend on sequential turns (Jansen-Osmann & Wiedenbauer, 2004). Younger children relied mainly on landmark associations to learn the route, e.g., turn right at the lamp. Memory for landmark associations depended on whether the turn was a decision point, as also found for adults (Janzen & Weststeijn, 2007). Categorization of landmarks did not improve wayfinding in children (or adults), indicating that adding semantic information does not benefit navigation using landmarks (Jansen-Osmann & Fuchs, 2006). Environments without 90° turns only had a detrimental effect on wayfinding behavior in younger children (Jansen-Osmann, Schmid, & Heil, 2007). Landmarks combined with using a map improved spatial knowledge, although children did not profit from learning the map as much as adults did, indicating a possible gap in knowledge for the utility or translation of map information to the task (Jansen-Osmann et al., 2007).
Combining proximal and distal landmarks, Purser et al. (2015) used a maze to compare cognitive functions and route learning in typical and atypical development. Children aged 5–11 years used landmarks to aid in route learning, similar to previous findings. Additionally, success was related to attention and long-term memory, indicating an underlying scaffolding of executive functioning and memory capacity to aid in navigation.
Summary
Maze VEs that capture reactive navigation with sensory inputs show more similarity than difference across the age range from 6 years on. However, younger children may need a more regular spatial structure (right-angle turns, even spacing of paths) to succeed, they may not benefit from maps, and they may do worse in more complex environments or with more perplexing tasks. Nevertheless, by the time they reach school age, children show substantial ability to use landmarks and to learn routes for subsequent way finding (Figure 1).
Radial/arm maze
Distal landmarks allow for more global positioning than mazes do, and hence deliberative navigation approaches (Tables 1 & 2). Thus, they enable researchers to study allocentric navigation strategies, e.g., building a birds-eye view depiction of an environment. Participants in virtual arm mazes with distal landmarks usually learn a specified route from a start arm to the goal arm, and then start at arms other than the start arm. Egocentric paths that mimic the trained path would not lead to the goal (e.g., going right every time regardless of the change in departure arm). Allocentric paths lead to the goal based on the distinctions between arms provided by distal cues. In a radial StarMaze, children started using an allocentric strategy between 5 and 7 years, although only 10-year-olds performed comparably to adults (Bullens et al., 2010; Iglói, Zaoui, Berthoz, & Rondi-Reig, 2009). Similarly, in a cross maze (four arms extending from a center square), 5- and 6-year-olds used global positioning information if available, but older children (10 years) used it more consistently (Broadbent, Farran, & Tolmie, 2014). In another setup, called the 4/8VM, navigators start at the center and retrieved four objects located within 4 of the 8 arms of the maze (Figure 2A). In this VE, 8-year-olds predominantly used a spatial strategy, as opposed to a response strategy, but this strategy decreased with age (Bohbot et al., 2012). The reasons for this surprising result are unclear.
Figure 2. Mazes with distal landmarks.
A. The children’s 4/8 VM radial maze from Bohbot et al. (2012). Left shows the overhead view of the maze with the 8 arms and surrounding distal cues. Right shows the first-person viewpoint from the center of the maze. Figure adapted from Bohbot et al. (2012) (CC BY 3.0). B. Overhead (left) and first-person (right) views of the adapted Morris Water Maze from Roome et al. (2017). Distal cues were the colorful mountains around the boundary and the proximal landmark remained inside the arena. Figure adapted from Roome et al., 2022/Open science framework, https://doi.org/10.17605/OSF.IO/WKRBZ (CC BY 4.0). C. The virtual object location task from Rodriguez-Andres et al. (2018). Left shows the overhead view with the proximal landmarks and distal landmarks just beyond the blue boundary wall. Right image shows a child participant’s perspective and the VE setup. Figure adapted from Rodriguez-Andres et al. (2018) (CC BY 4.0).
In a rare departure from desktop VE, (Bécu et al., 2020) used an immersive head-mounted virtual reality setup to test using an allocentric strategy with geometric information (angle between arms) as opposed to landmarks. Participants experienced one of two Y-mazes. One had three equiangular arms (120°) with distal landmarks between the arms. The second had a geometric structure but no landmarks: two 155° and one 50° arms. Adolescents of 10 and 11 years old preferred an egocentric strategy in the non-geometric landmark Y-maze, failing to use the landmark information. However, they did use the geometric information effectively to move away from an egocentric approach. When the geometry of the radial maze arms are the same, even young adolescents fail to take advantage of distal cues, regressing to a more rudimentary response-based strategy.
Summary
Radial/arm mazes provide a structure in which participants can succeed by associating areas of the maze with distinctive distal landmarks. Data suggest that distal landmarks are probably not well utilized until late childhood or early adolescence (Figure 1), e.g., in the StarMaze, children do not use an allocentric strategy reliably until age 10. Variations in the tasks (such as starting point, and the number of arms) may account for the contrasting results on the 4/8 VM, and more systematic probing and comparison of the paradigms is required to resolve the issue. However, real-world situations are seldom as simple as the structure of a radial maze. Next, we highlight a method that addresses how children may use more than one piece of distal information at once, in a continuous space not defined by distinct arms.
Morris water maze
Place learning involves localizing position information using the cues available in the environment and integration is connecting the cues together to help in positioning (Tables 1 & 2). Morris (1984) devised his now-classic Water Maze (MWM) to investigate place navigation in rodents and the effects of hippocampal lesions on the navigation system. Rodents must swim to a platform hidden under the surface of milky water from a variety of entry points within the pool (Morris, 1984, 1981; O’Keefe, Nadel, Keightley, & Kill, 1975). There have been real-world adaptations of the basic idea that involve searching for toys hidden underneath sand or multiple pillows or tiles (e.g., Balcomb, Newcombe, & Ferrara, 2011). Manipulations to this design enable metrics of landmark combination, measuring more holistic retrieval of environmental information.
Technology has allowed us to create VEs that mimic this situation. Numerous versions of this task exist for use with adults (Bierbrauer et al., 2020; Doeller, Barry, & Burgess, 2010; Doeller & Burgess, 2008; Howett et al., 2019; Korthauer et al., 2016; Kunz et al., 2015; Rizk-Jackson et al., 2006; Stangl et al., 2018). The general layout for children consists of a virtual circular arena, often with landmarks inside and distal landmarks just beyond the arena walls. Children 3–4 years inconsistently use place learning to find the invisible target within the arena (Laurance et al., 2003); there was significant improvement in place learning from 5–7 years. By 9–10 years, children performed with adult-like accuracy and often reported using the distal cues to guide their positioning (Laurance et al., 2003).
Negen et al. (2019) examined how 3- to 9-year-olds remember learned locations using the spatial information surrounding them with a virtual reality headset. The two VEs, called Jetty and Arctic, both had proximal landmarks, but only the Jetty also had a distal landscape and a circular boundary. Younger children tend to respond near the associated landmark, but were still imprecise, remembering only the area immediate to the target. Negen et al. modeled three kinds of spatial memory that they found to progress with age: egocentric, single-cue allocentric (e.g., localizing the target by a landmark), and multi-cue allocentric (i.e., localizing the target by a landmark and the boundary). Interestingly, there was multi-cue recall in the absence of distal landmarks (Arctic), suggesting that distal landmarks may not be necessary in this paradigm.
Roome, Sherrill, Coughlin, and Preston (2017) evaluated how different landmark types are used and integrated in children 6–13 years and young adults (Figure 2B). Positional error decreased with age, with the youngest children (6–7-year-olds) relying more on the landmark inside the arena. From 8 years onwards, there was no difference from adults in performance due to landmark type, showing a more flexible use of landmark information and possible integration of multiple cues. Julian, Kamps, Epstein, and Dilks (2019) used a similar task design with Williams Syndrome adults and children (6–10 years), a genetic disorder marked by chromosomal deletion. Children relied on both the proximal landmark and boundary cues, although with more emphasis on the landmark.
Memory Island (MI; Piper, Acevedo, Craytor, Murray, & Raber, 2010) was designed to characterize place learning in an environment with natural-looking features. MI does not use distal landmarks, although there are very large landmarks that give a sense of stability. Participants learn visible target locations in each quadrant with an aid (flag), then with the target visible only in close proximity (hidden trials), and then with no target during probe trials. The distance traveled to the target did not change with age during hidden trials for 7–10-year-olds. However, children who took a more direct path to the target during hidden trials spent more time in the correct quadrant during probe trials. The most striking improvements were from 9- to 10-years-old.
The virtual object location (VOL) paradigm is another augmented setup that assessed non-cognitive impacts on spatial memory with city-like distal landmarks and playground objects as landmarks (Figure 2C; Rodriguez-Andres, Mendez-Lopez, Juan, & Perez-Hernandez, 2018). Viewing the VE in 3D, 5–6-year-olds performed worse than the 7–12-year-olds at finding the remembered location of objects. Males and children with more videogame experience were more accurate and faster at navigating and children.
Summary
Whereas radial/arm mazes allow success using a distinct association between the arms and distal landmarks, MWM tasks gauge positional information relative to both proximal and distal cues in a continuous space, and thus allow measurement of a combination of landmarks into a framework. Such capabilities seem available by 8–10 years. Does this conclusion apply to more naturalistic environments? In the next section, we examine studies that aim for ecological validity.
Naturalistic environments
Cognitive mapping requires flexible combination of previously mentioned navigation strategies and accurate integration of the available cues. Natural environments imitate the appearance of real-life towns and cities, and tests navigation ability in spaces with naturalistic environmental features. Participants are not limited to inertial navigation and can use local and distal landmarks to build an integrated representation to allow for planning (Tables 1 & 2).
Shortcutting is often used as a metric of cognitive mapping, indicating a detailed knowledge of the environment to connect main paths. Farran et al. (2015) used a city-like maze to show age-related increases in effective shortcutting in typically developing children 5–11-years. Even in an enclosed space with realistic features inside rooms, adolescents 10 to 12 years took significantly more time to reach a room than adults, possibly due to a lack of distal cues and difficulty constructing a map (Murias, Slone, Tariq, & Iaria, 2019). In a museum with rooms, 10-year-olds were performing less like the younger children (7 to 9 years) and more like adults in timing and distance efficiencies, not getting lost, and in effective shortcutting (Burles et al., 2020). Without explicit one-to-one association of a landmark and an intersection or a turn, children must generate those associations with the information available, which seems to hinder precise mapping abilities even in adolescence.
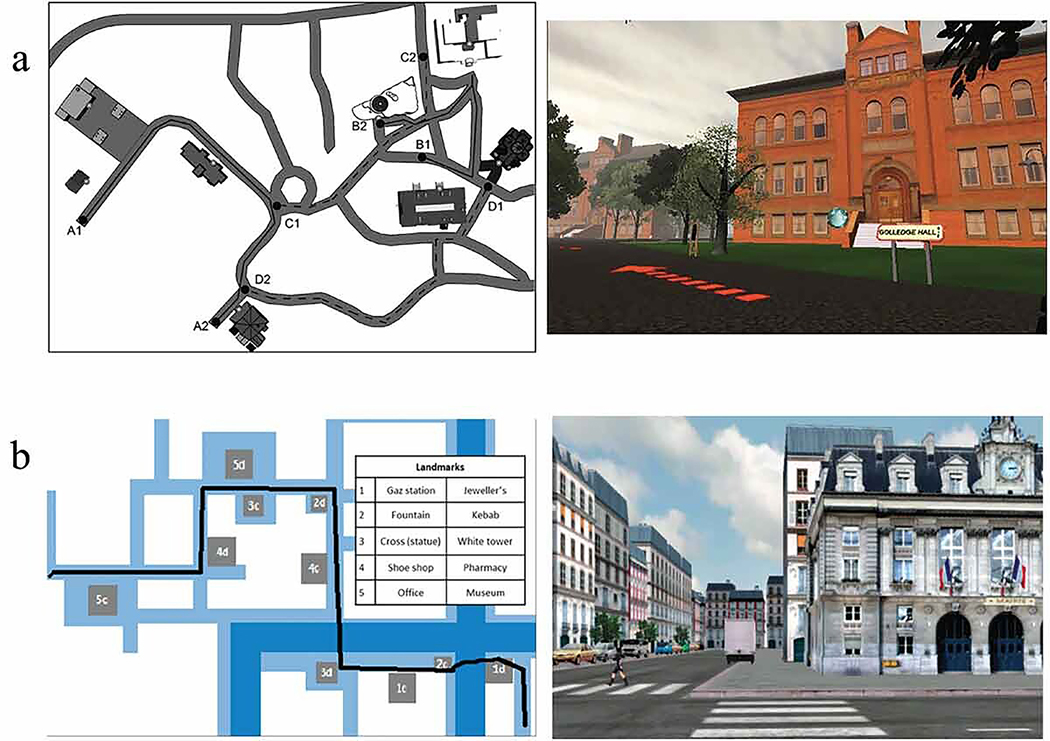
Nazareth, Weisberg, Margulis, and Newcombe (2018) assessed integration ability for landmarks to build a cohesive mental representation of the environment using a VE of a college campus, Virtual Silcton (Weisberg, Schinazi, Newcombe, Shipley, & Epstein, 2014). Participants (8–16 years) learned locations along two main routes and then two connecting routes (Figure 3A). Accurate pointing to buildings within the same route and between the two main routes indicated better integration of the buildings, revealing a mental map. Around age 12 there were similar integration behaviors as adults. Performance was correlated with the Spatial Orientation Test (Hegarty, Kozhevnikov, & Waller, 2004) and the Mental Rotation Test (Vandenberg & Kuse, 1978). A follow-up longitudinal study replicated the cross-sectional findings and found that individual differences also stabilized around age 12 (Brucato, Nazareth, & Newcombe, 2022).
Figure 3. Natural environments.
A. Virtual Silcton paradigm. Right image shows one of the eight buildings and the city-structure of the VE with general buildings, trees, a road, etc. Figure adapted from Nazareth et al. (2018). B. VE town from Nys et al. (2015), (2018)). The overhead view shows one test route traced in black and a list of landmarks along the route. Figure adapted from Nys et al. (2015) (CC BY 4.0).
With a lifespan (8–100 years) approach, van der Ham, Claessen, Evers, and van der Kuil (2020) developed an online browser task to map a long-term trajectory of multiple spatial abilities. After viewing a video of the route, tests probed for egocentric and allocentric encoding of landmark locations. There were two path assessments: 1) indicate the direction the route continued at a given landmark and 2) decide which two landmarks were closest together. There were age-related improvements in both assessments, with even 13-year-olds doing worse than young adults, providing further evidence that inputs to cognitive mapping are slowly developing.
Nys, Gyselinck, Orriols, and Hickmann (2015) also showed how landmark knowledge enhanced route learning in a virtual town (Figure 3B). Children 6, 7, and 10 years and adults watched one training route and one test route with crossroads and landmarks. Children’s landmark knowledge facilitated route accuracy, but even the oldest children performed worse than adults. This was the only task used to show how concurrently taxing working memory (WM) systems may affect learning in three conditions: no concurrent task and spatial WM and verbal WM concurrent tasks (Nys, Hickmann, & Gyselinck, 2018). Both spatial and verbal WM interfered with route learning for 8–10-year-olds. There was higher interference from spatial tapping on visually learned than verbally learned information. Verbal working memory taxed verbally learned information in the only 8-year-olds. This suggests the possibility that other cognitive factors affect navigation, whether directly related to spatial ability (e.g., spatial orientation and mental rotation in Virtual Silcton) or working memory.
Summary
Cognitive mapping is oftentimes seen as the most “mature” state of navigation, although adults still vary in this ability. VE designs that aim to emulate real-life cognitive mapping and navigation use buildings, roads, and other naturalistic features to create a dynamic environment for navigation. Cognitive mapping based on these studies seems to reach adult levels at about 12 years, a bit later than suggested by paradigms reviewed in previous sections. A pattern of development taking over a decade (Figure 1) suggests that constructing large-scale spatial representations requires the gradual assembly of general skills (e.g., spatial working memory) and specific capacities (e.g., combining information from the allocentric and egocentric systems). More complex environments likely tax those skills and capacities, leading to later ages of maturity.
Conclusion
Navigation development is a well-studied enterprise and is crucial for survival in the real-world. In ideal situations, real-world techniques are the best way to model such development; however, this is not feasible for all researchers, can hinder reproducibility, and present safety concerns for children in larger environments (e.g., cars on roads, crossing the street). Although there are drawbacks to using VEs, as discussed throughout this review, research using VEs has complemented research in real-world environments as a technique for studying how children become able to represent the spatial layout of the world and navigate within it.
We have surveyed environments used with children to date, but also recommend considering several tasks not yet used with children that include engaging visuals, a storyline premise, and video game-like tasks. For example, Sea Hero Quest (Coutrot et al., 2019) is a mobile application game, in which participants sail a boat through an open sea to find treasure and locating a goal, and Spatial Spy (Malanchini et al., 2020) is an open virtual city where participants solve a mystery by collecting clues during tasks on a computer. Using an app would allow for greater reachability to more a diverse sample, potentially increasing sample size, age ranges, and generalizability, which experiments oftentimes lack in the lab.
Although VEs are not suitable for use with infants and toddlers, they have helped us to understand the period from 6 years or so on, with occasional use as young as 3 years (Figure 1). The complexity and task demand in these studies progress with children’s capacity to encode and remember their world. During the decade from preschool to early adolescence, children progressively calibrate the precision with which they encode these sources of spatial information. Learning of routes and landmark associations occur early. They also begin to combine cues adaptively such as proximal and distal landmarks and distance metrics and direction, both within and across systems. Finally, they become able to think flexibly about frames of reference different from their current one (i.e., to perform perspective taking). By the end of this decade, we see adult-level performance on cognitive mapping tasks requiring the integration of visible areas into a cognitive map or graph. We also see adult patterns of individual differences, which are important because people vary a great deal in their way finding skill beyond childhood.
Most of the paradigms discussed here converge on this broad picture of development. They use variations to assess specific hypotheses, e.g., regarding map use, or the advent of Bayesian combination. The abundance of behavioral research is met with scarce understanding of the neural development of navigation. Virtual platforms allow researchers to incorporate advanced neural methods such as EEG, MRI, and brain stimulation. Future VE studies can explore new questions like the transferability of cognitive mapping to other cognitive domains (i.e., social and abstract knowledge) or behavioral comparisons between real-world and virtual encoding.
As such research accelerates, it needs to embrace the vital and growing effort to create an open science that includes sharing of methods, stimuli, code, and data. Sadly, most VEs are not open access. Sharing requires contacting the researchers directly to obtain the code for the environment, as well as detailed instructions and other specifications. This situation undermines the utility of these paradigms for follow-up or replication studies. There are some exceptions. The van der Ham et al. (2020) task can be accessed online; however, contact with the authors is required to access the data. Virtual Silcton currently is the only openly available and validated study in this review. Silcton is available to researchers as an open-access browser-based experiment (virtualsilcton.com). Virtual methods bypass the constraints of large-scale environment testing, allowing for larger datasets and less time-consuming studies. Over the past few decades, they have enabled us to track significant progressions of navigation development and will continue to shed light on new discoveries. By highlighting the progress garnered by VEs, we hope to encourage more open science initiatives, lessening the time and effort required to build new VEs, promoting replicability within the field, and expanding our understanding beyond behavioral investigation.
Acknowledgments
We appreciatively acknowledge the researchers and labs mentioned in this review for their significant contributions to spatial development and use of innovative methods.
Funding
This work was supported by the National Science Foundation (EHR 1660996 to N.SN.) and the National Institutes of Health (R01 HD099165-02S1 diversity supplement to K.V.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or National Institutes of Health.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed.
References
- Allen GL, Kirasic KC, Siegel AW, & Herman JF (1979). Developmental issues in cognitive mapping: The selection and utilization of environmental landmarks. Child Development, 50(4), 1062–1070. doi: 10.2307/1129332 [DOI] [PubMed] [Google Scholar]
- Anooshian LJ, & Young D (1981). Developmental changes in cognitive maps of a familiar neighborhood. Child Development, 52(1), 341. doi: 10.2307/1129248 [DOI] [Google Scholar]
- Balcomb F, Newcombe NS, & Ferrara K (2011). Finding where and saying where: Developmental relationships between place learning and language in the first year. Journal of Cognition and Development, 12(3), 315–331. doi: 10.1080/15248372.2010.544692 [DOI] [Google Scholar]
- Bécu M, Sheynikhovich D, Ramanoël S, Tatur G, Ozier-Lafontaine A, Sahel JA, & Arleo A (2020). Modulation of spatial cue processing across the lifespan: A geometric polarization of space restores allocentric navigation strategies in children and older adults. BioRxiv. doi: 10.1101/2020.02.12.945808 [DOI] [Google Scholar]
- Bierbrauer A, Kunz L, Gomes CA, Luhmann M, Deuker L, Getzmann S, . . . Axmacher N (2020). Unmasking selective path integration deficits in Alzheimer’s disease risk carriers. Science Advances, 6(35). doi: 10.1126/sciadv.aba1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, McKenzie S, Konishi K, Fouquet C, Kurdi V, Schachar R, . . . Robaey P (2012). Virtual navigation strategies from childhood to senescence: Evidence for changes across the life span. Frontiers in Aging Neuroscience, 4(OCT). doi: 10.3389/fnagi.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent HJ, Farran EK, & Tolmie A (2014). Egocentric and allocentric navigation strategies in Williams syndrome and typical development. Developmental Science, 17(6), 920–934. doi: 10.1111/desc.12176 [DOI] [PubMed] [Google Scholar]
- Brucato M, Nazareth A, & Newcombe NS (2022). Longitudinal development of cognitive mapping from childhood to adolescence. Journal of Experimental Child Psychology, 219, 105412. doi: 10.1016/j.jecp.2022.105412 [DOI] [PubMed] [Google Scholar]
- Bullens J, Iglói K, Berthoz A, Postma A, & Rondi-Reig L (2010). Developmental time course of the acquisition of sequential egocentric and allocentric navigation strategies. Journal of Experimental Child Psychology, 107(3), 337–350. doi: 10.1016/j.jecp.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Burles F, Liu I, Hart C, Murias K, Graham SA, & Iaria G (2020). The emergence of cognitive maps for spatial navigation in 7- to 10-year-old children. Child Development, 91(3). doi: 10.1111/cdev.13285 [DOI] [PubMed] [Google Scholar]
- Coutrot A, Schmidt S, Coutrot L, Pittman J, Hong L, Wiener JM, . . . Spiers HJ (2019). Virtual navigation tested on a mobile app is predictive of real-world wayfinding navigation performance. PLoS ONE, 14(3), e0213272. doi: 10.1371/journal.pone.0213272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, Barry C, & Burgess N (2010). Evidence for grid cells in a human memory network. Nature, 463(7281), 657–661. doi: 10.1038/nature08704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, & Burgess N (2008). Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proceedings of the National Academy of Sciences of the United States of America, 105(15). doi: 10.1073/pnas.0711433105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farran EK, Purser HRM, Courbois Y, Ballé M, Sockeel P, Mellier D, & Blades M (2015). Route knowledge and configural knowledge in typical and atypical development: A comparison of sparse and rich environments. Journal of Neurodevelopmental Disorders, 7(1). doi: 10.1186/s11689-015-9133-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR (1990). The organization of learning, Vol. 336. Cambridge, MA: The MIT Press. [Google Scholar]
- Girard B (2022). The multiple ways to solve a navigation problem, from a modeling point of view (Version2).figshare. 10.6084/m9.figshare.20390742.v2 [DOI]
- Hegarty M, Kozhevnikov M, & Waller D (2004). Perspective taking/spatial orientation test. Intelligence, 32(January). doi: 10.1016/j.intell.2004.07.003 [DOI] [Google Scholar]
- Herman JF, & Siegel AW (1978). The development of cognitive mapping of the large-scale environment. Journal of Experimental Child Psychology, 26(3), 389–406. doi: 10.1016/0022-0965(78)90120-0 [DOI] [Google Scholar]
- Howett D, Castegnaro A, Krzywicka K, Hagman J, Marchment D, Henson R, . . . Chan, D. (2019). Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation. Brain, 142(6), 1751–1766. doi: 10.1093/brain/awz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglói K, Zaoui M, Berthoz A, & Rondi-Reig L (2009). Sequential egocentric strategy is acquired as early as allocentric strategy: Parallel acquisition of these two navigation strategies. Hippocampus, 19(12), 1199–1211. doi: 10.1002/hipo.20595 [DOI] [PubMed] [Google Scholar]
- Jansen-Osmann P, & Fuchs P (2006). Wayfinding behavior and spatial knowledge of adults and children in a virtual environments: The role of landmarks. Experimental Psychology, 53(3), 171–181. doi: 10.1027/1618-3169.53.3.171 [DOI] [PubMed] [Google Scholar]
- Jansen-Osmann P, Schmid J, & Heil M (2007). Wayfinding behavior and spatial knowledge of adults and children in a virtual environment: The role of the environmental structure. Swiss Journal of Psychology, 66(1), 41–50. doi: 10.1024/1421-0185.66.1.41 [DOI] [Google Scholar]
- Jansen-Osmann P, & Wiedenbauer G (2004). The representation of landmarks and routes in children and adults: A study in a virtual environment. Journal of Environmental Psychology, 24 (3), 347–357. doi: 10.1016/j.jenvp.2004.08.003 [DOI] [Google Scholar]
- Jansen-Osmann P, Wiedenbauer G, Schmid J, & Heil M (2007). The influence of landmarks and pre-exposure to a structural map during the process of spatial knowledge acquisition: A study with children and adults in a virtual environment. Spatial Cognition and Computation, 7(3), 267–285. doi: 10.1080/13875860701544365 [DOI] [Google Scholar]
- Janzen G, & Weststeijn CG (2007). Neural representation of object location and route direction: An event-related fMRI study. Brain Research, 1165, 116–125. doi: 10.1016/j.brainres.2007.05.074 [DOI] [PubMed] [Google Scholar]
- Julian JB, Kamps FS, Epstein RA, & Dilks DD (2019). Dissociable spatial memory systems revealed by typical and atypical human development. Developmental Science, 22(2). doi: 10.1111/desc.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthauer LE, Nowak NT, Moffat SD, An Y, Rowland LM, Barker PB, . . . Driscoll I (2016). Correlates of virtual navigation performance in older adults. Neurobiology of Aging, 39, 118–127. doi: 10.1016/j.neurobiolaging.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz L, Schröder TN, Lee H, Montag C, Lachmann B, Sariyska R, . . . Axmacher N (2015). Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science, 350 (6259), 430–433. doi: 10.1126/science.aac8128 [DOI] [PubMed] [Google Scholar]
- Laurance HE, Learmonth AE, Nadel L, & Jake Jacobs W (2003). Maturation of spatial navigation strategies: Convergent findings from computerized spatial environments and self-report. Journal of Cognition and Development, 4(2), 211–238. doi: 10.1207/S15327647JCD0402_04 [DOI] [Google Scholar]
- Liben LS, Myers LJ, Christensen AE, & Bower CA (2013). Environmental-scale map use in middle childhood: Links to spatial skills, strategies, and gender. Child Development, 84(6), 2047–2063. doi: 10.1111/cdev.12090 [DOI] [PubMed] [Google Scholar]
- Lingwood J, Blades M, Farran EK, Courbois Y, & Matthews D (2015). The development of wayfinding abilities in children: Learning routes with and without landmarks. Journal of Environmental Psychology, 41, 74–80. doi: 10.1016/j.jenvp.2014.11.008 [DOI] [Google Scholar]
- Malanchini M, Rimfeld K, Shakeshaft NG, McMillan A, Schofield KL, Rodic M, . . . Plomin R (2020). Evidence for a unitary structure of spatial cognition beyond general intelligence. Npj Science of Learning, 5(1). doi: 10.1038/s41539-020-0067-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara TP, Ratcliff R, & McKoon G (1984). The mental representation of knowledge acquired from maps. Journal of Experimental Psychology: Learning, Memory, and Cognition, 10 (4). doi: 10.1037/0278-7393.10.4.723 [DOI] [PubMed] [Google Scholar]
- Montello DR (1998). A new framework for understanding the acquisition of spatial knowledge in large-scale environments. Spatial and Temporal Reasoning in Geographic Information Systems. [Google Scholar]
- Morris RGM (1981). Spatial localization does not require the presence of local cues. Learning and Motivation, 12(2), 239–260. doi: 10.1016/0023-9690(81)90020-5 [DOI] [Google Scholar]
- Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods, 11(1), 47–60. doi: 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- Murias K, Slone E, Tariq S, & Iaria G (2019). Development of spatial orientation skills: An fMRI study. Brain Imaging and Behavior, 13(6), 1590–1601. doi: 10.1007/s11682-018-0028-5 [DOI] [PubMed] [Google Scholar]
- Nardini M (2021). Merging familiar and new senses to perceive and act in space. Cognitive Processing, 22(S1), 69–75. doi: 10.1007/s10339-021-01052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth A, Weisberg SM, Margulis K, & Newcombe NS (2018). Charting the development of cognitive mapping. Journal of Experimental Child Psychology, 170, 86–106. doi: 10.1016/j.jecp.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Negen J, Ali LB, Chere B, Roome HE, Park Y, & Nardini M (2019). Coding locations relative to one or many landmarks in childhood. PLoS Computational Biology, 15(10), e1007380. doi: 10.1371/journal.pcbi.1007380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe NS (2019). Navigation and the developing brain. Journal of Experimental Biology, 222 (Suppl_1). doi: 10.1242/jeb.186460 [DOI] [PubMed] [Google Scholar]
- Newcombe N, & Huttenlocher J (2006). Development of spatial cognition. Child Psychology in Practice, 4, 734–776. [Google Scholar]
- Newcombe NS, Uttal DH, & Sauter M (2013). Spatial development. In Zelazo PD Ed., The oxford handbook of developmental psychology (Vol. 1, pp. 563–590). Oxford University Press. doi: 10.1093/oxfordhb/9780199958450.013.0020 [DOI] [Google Scholar]
- Nguyen K, & Newcombe N (2022, August 25). Navigation development taxonomy & organization. doi: 10.17605/OSF.IO/JR8VK [DOI] [Google Scholar]
- Nguyen K, & Tansan M (2022, August 21). Navigation development figure. doi: 10.17605/OSF.IO/PCJR3 [DOI] [Google Scholar]
- Nys M, Gyselinck V, Orriols E, & Hickmann M (2015). Landmark and route knowledge in children’s spatial representation of a virtual environment. Frontiers in Psychology, 5. doi: 10.3389/fpsyg.2014.01522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nys M, Hickmann M, & Gyselinck V (2018). The role of verbal and visuo-spatial working memory in the encoding of virtual routes by children and adults. Journal of Cognitive Psychology, 30(7), 710–727. doi: 10.1080/20445911.2018.1523175 [DOI] [Google Scholar]
- O’Keefe J, Nadel L, Keightley S, & Kill D (1975). Fornix lesions selectively abolish place learning in the rat. Experimental Neurology, 48(1), 152–166. doi: 10.1016/0014-4886(75)90230-7 [DOI] [PubMed] [Google Scholar]
- Peer M, Brunec IK, Newcombe NS, & Epstein RA (2021). Structuring knowledge with cognitive maps and cognitive graphs. Trends in Cognitive Sciences, 25(1), 37–54. doi: 10.1016/j.tics.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J (1954). The construction of reality in a child. Norton. Piaget, J. [Google Scholar]
- Piper BJ, Acevedo SF, Craytor MJ, Murray PW, & Raber J (2010). The use and validation of the spatial navigation memory Island test in primary school children. Behavioural Brain Research, 210(2), 257–262. doi: 10.1016/j.bbr.2010.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullano L, & Foti F (2022). The development of human navigation in middle childhood: A narrative review through methods, Terminology, and fundamental stages. Brain Sciences, 12(8), 1097. MDPI AG. doi: 10.3390/brainsci12081097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser HRM, Farran EK, Courbois Y, Lemahieu A, Sockeel P, Mellier D, & Blades M (2015). The development of route learning in down syndrome, Williams syndrome and typical development: Investigations with virtual environments. Developmental Science, 18(4), 599–613. doi: 10.1111/desc.12236 [DOI] [PubMed] [Google Scholar]
- Rizk-Jackson AM, Acevedo SF, Inman D, Howieson D, Benice TS, & Raber J (2006). Effects of sex on object recognition and spatial navigation in humans. Behavioural Brain Research, 173(2). doi: 10.1016/j.bbr.2006.06.029 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Andres D, Mendez-Lopez M, Juan MC, & Perez-Hernandez E (2018). A virtual object-location task for children: Gender and videogame experience influence navigation; age impacts memory and completion time. Frontiers in Psychology, 9(APR). doi: 10.3389/fpsyg.2018.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roome HE, Sherrill KR, Coughlin CA, & Preston AR (2017). The development of spatial navigation: Importance of cue integration. Cognitive development society Biennial Meeting. Portland, Oregon. [Google Scholar]
- Roome H, Sherrill K, Nguyen K, & Preston A (2022, August 21). MoshiGO VE figure. doi: 10.17605/OSF.IO/WKRBZ [DOI] [Google Scholar]
- Shettleworth SJ (2012). Modularity, comparative cognition and human uniqueness. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1603), 2794–2802. doi: 10.1098/rstb.2012.0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman A, Ah Lee S, & Spelke ES (2011). Cognitive effects of language on human navigation. Cognition, 120(2), 186–201. doi: 10.1016/j.cognition.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AW, & White SH (1975). The development of spatial representations of large-scale environments. Advances in Child Development and Behavior, 10(C). doi: 10.1016/S0065-2407(08)60007-5 [DOI] [PubMed] [Google Scholar]
- Stangl M, Achtzehn J, Huber K, Dietrich C, Tempelmann C, & Wolbers T (2018). Compromised grid-cell-like representations in old age as a key mechanism to explain age-related navigational deficits. Current Biology, 28(7), 1108–1115.e6. doi: 10.1016/j.cub.2018.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlauka M, & Wilson PN (1994). The effect of landmarks on route-learning in a computer-simulated environment. Journal of Environmental Psychology, 14(4), 305–313. doi: 10.1016/S0272-4944(05)80221-X [DOI] [Google Scholar]
- Vandenberg SG, & Kuse AR (1978). Mental rotations, a group test of three-dimensional spatial visualization. Perceptual and Motor Skills, 47(2), 599–604. doi: 10.2466/pms.1978.47.2.599 [DOI] [PubMed] [Google Scholar]
- van der Ham IJM, Claessen MHG, Evers AWM, & van der Kuil MNA (2020). Large-scale assessment of human navigation ability across the lifespan. Scientific Reports, 10(1). doi: 10.1038/s41598-020-60302-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WH (2019). Non-Euclidean navigation. In Journal of Experimental Biology, 222. doi: 10.1242/jeb.187971 [DOI] [PubMed] [Google Scholar]
- Weisberg SM, Schinazi VR, Newcombe NS, Shipley TF, & Epstein RA (2014). Variations in cognitive maps: Understanding individual differences in navigation. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40(3), 669–682. doi: 10.1037/a0035261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed.