Abstract
El-Saghier reaction is the novel, general, and green reaction of various amines with ethyl cyanoacetate and ethyl glycinate hydrochloride. A new series of imidazolidin-4-ones and bis-N-(alkyl/aryl) imidazolidin-4-ones was synthesized in a sequential, one-pot procedure under neat conditions for 2 h at 70 °C. Excellent high yields (90–98%) were achieved in a short period of time while avoiding issues related to the hazardous solvents utilized (cost, safety, and pollution). The spectrum analyses and elemental data of the newly synthesized compounds helped us to clarify their structures. The obtained compounds were tested for antibacterial activity in vitro and compared to the standard antibiotic chloramphenicol as the standard, measuring the inhibition zone (nm) and activity index (%). With an antibacterial percentage value of 80.0 against Escherichia coli, N,N′-(propane-1,3-diyl) bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl) acetamide) proved to be the most effective. Antimicrobial activity was confirmed by a molecular docking investigation to investigate how chemicals bind to the bacterial FabH–CoA complex in E. coli (PDB ID: 1HNJ).
1. Introduction
Substituted imidazoles are important moieties constituted in pharmaceuticals, pesticides, and bioactive compounds.1,2 Many imidazoles with a single heterocyclic substituent in the 2-position serve as key intermediates in the synthesis of pharmaceutically active compounds.3 It is vitally necessary to find novel compounds with robust antibacterial capabilities since many clinically relevant illnesses are now resistant to well-known families of antimicrobial reagents. The G protein-coupled receptor antagonist,4 anticancer agents,5 antibacterial activity,6,7 as well as antifungal properties may all be produced chemically by altering the imidazolidine-4-one scaffold.8,9 Antibiotic activity is one of these applications and one of the main areas of study for imidazolidine derivatives.10,11 They were unsuitable for clinical usage due to their mild antibiotic action, though.
The described imidazolidin-4-ones, which are made from primaquine’s amino acid derivatives, have strong gametocytocidal effects on P. berghei. As a result, imidazolidin-4-ones (A) are a unique class of 8-aminoquinoline antimalarials.12 Additionally, several imidazolidinone derivatives with the pharmacophore di-aryl sulfonylurea were created and tested for their anticancer efficacy against a variety of human solid tumors. Due to the absence of methemoglobinemia or hypoglycemia after treatment, imidazolidine-2,4-diones (B) had greater cytotoxic activity than sulfur (C), indicating a distinct metabolic destiny. The chemotherapeutic efficacy of imidazole-4-one derivatives (D) as prospective anticancer medicines has received a lot of attention.13 On the other hand, imidazole-5(4H)-one (E) reacted with active methylene reagents and was predicted to be more effective as an antibacterial agent14 because these compounds have a pyrazole moiety (Figure 1).
Figure 1.
Structures of imidazolidin-4-one and imidazole-4-one literature biological activity analogues (A–E).
It has occasionally been attempted to develop libraries of imidazolidine-4-one and their derivatives due to their great synthetic relevance and diverse spectrum of bioactivities. Numerous synthetic techniques have been developed and improved to manufacture products with high yields, desired quality, and purity.15,16 Considering these findings, the current framework was designed to discover a highly efficient, novel, and one-pot methodology toward the synthesis of imidazolidine-4-ones. The formation of pathogenic bacterial and fungal strains has been tested for by both generated compounds in a preliminary in vitro antimicrobial screening.
2. Results and Discussion
2.1. Chemistry
In keeping with our earlier efforts on the creation of a straightforward, universal technique for heterocyclic chemical synthesis to afford new derivatives,17−22 we report here a fresh approach to the synthesis of imidazole-4-one and/or imidazolidin-4-one derivatives 4a–m through the reaction of different amines with ethylcyanoacetate and ethylglycinate hydrochloride in a sequential, one-pot, procedure under neat condition for 2 h at 70 °C (Scheme 1).
Scheme 1. Synthesis of Imidazole-4-one and/or Imidazolidin-4-one Derivatives 4a–m in a Solvent-Free, Sequential One-Pot Method.
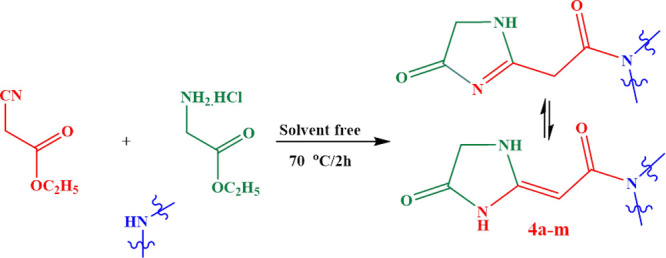
The reaction mechanism involves a nucleophilic attack of the amine group of amines on the carbonyl group of ethylcyanoacetate with subsequent elimination of ethyl alcohol molecules to afford the corresponding cyanoacetamido derivatives I, after which comes the inclusion of the amino group of ethylglycinate hydrochloride on the cyano group (due to delocalization of the lone pair of nitrogen) to afford new intermediate II containing two active methylene groups, ester and imino groups. The active imino group had the opportunity to make another nucleophilic attack into the carbonyl carbon of ester and subsequent ring closure with elimination of another alcohol molecule to afford the desired products (Scheme 2).
Scheme 2. Reaction Mechanism for the Synthesis of N-Cyclohexyl-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl) Acetamide (4f).

To optimize the reaction conditions for the synthesis of imidazolidinones, the utilized catalyst was screened together with the reaction conditions, including the reaction medium, heating method, and reaction time, Table 1. Synthesis of N-cyclohexyl-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4f) was selected as a model reaction for this study and a sequential, consecutive reaction procedure was adopted. Cyclohexylamine was reacted with ethylcyanoacetate and then ethylglycinate hydrochloride was added (after treating with drops of triethylamine) in ethanol under reflux conditions for 4 h. Only 25% of the output included the intended product 4f. We observed both procedures using nuclear magnetic resonance (1H NMR and 13C NMR) spectroscopy and thin-layer chromatography (TLC) to comprehend why the yield of 4f was so low. In the TLC studies, cyclohexylamine was injected into an equimolar mixture of ethyl cyanoacetate and ethyl glycinate hydrochloride, and a yellow spot appeared 5 min later. The reaction mixture was characterized by 1H NMR after the reaction had taken place for 1 h. The 1H NMR and 13C NMR spectra revealed that cyclohexylamine was entirely consumed but that ethyl glycinate hydrochloride did not completely react. Every hour, the combination of the reaction was described, and it was discovered that the rate at which the imidazole product formed was slower than expected. Impurities began to show up when the reaction time exceeded 2 h; however, cyclohexylamine was only totally consumed at a reaction time of 4 h. Considering this information, we propose that the inefficient nucleophilic substitution of the primary amine for the ethyl cyanoacetate is a factor in the reaction’s lower yield. We attempted using 1,4-dioxane or acetonitrile as the solvent in this step to increase its effectiveness, but the reaction times remained lengthy, and the yield did not improve. To improve the amine’s nucleophilicity, acids like AcOH or Lewis acids like CAN were added, but the yield remained poor. Lastly, we completed this stage under neat conditions at different temperatures since it was proposed that this would be able to give effective heating. Surprisingly, at 70 °C, cyclohexylamine was totally consumed in 2 h, no side products formed, and the yield of 4f was greatly enhanced to 90%.
Table 1. Optimization of the Reaction Conditions for the Synthesis of Compound 4f.
| entry | heating mode/solvent | (one-pot)/reaction time (h) | additive | yield (%) |
|---|---|---|---|---|
| 1 | reflux/ethanol | 4 | no | 25 |
| 2 | 80°C/1,4-dioxane | 4 | no | 18 |
| 3 | reflux/acetonitrile | 4 | no | 20 |
| 4 | 70°C/1,4-dioxane | 4 | AcOH | 19 |
| 5 | reflux/1,4-dioxane | 4 | CAN | 21 |
| 6 | reflux/AcOH | 4 | no | 50 |
| 7 | 120°C/neat | 2 | no | traces |
| 8 | 100°C/neat | 2 | no | 45 |
| 9 | 70°C/neat | 2 | LiBr | 80 |
| 10 | 70 °C/neat | 2 | no | 90 |
The mentioned examples use partial data from a published report of imidazolidine-4-one moieties.23−28 Here, the authors discuss and illustrate the design, reaction conditions, solvent, catalyst, yield, and interpretation of a method-comparison study in the literature related to our new method as shown in Table 2. This comparison revealed that this article describes a new and creative class of dimer compounds that combine basic substrates with an advanced structure. The majority of these “monomers” are also fresh substances. Methods from the field of so-called “green chemistry” were applied to their synthesis.
Table 2. Comparison between the Present Method with Respect to Other Methods in the Literature.
Then, if possible, we wanted to streamline the process and do without the solvent. As a result, we performed the reaction at a variety of temperatures and neat conditions, and the outcomes were quite positive. When LiBr was added, the yield did not increase any further. Finally, it was discovered that the reaction was also extremely effective when carried out in conditions free of solvent. As can be seen in Table 3, several imidazole-4-one and/or imidazolidin-4-one derivatives were synthesized under the ideal conditions. Four different secondary amines (including dimethylamine, diethylamine, piperidine, and morpholine), three different primary aliphatic amines (such as ethanolamine, cyclohexylamine, and benzyl amine), six different primary aromatic amines (such as aniline, 1-napthyl amine, o-toluidine, p-methoxyaniline, p-chloroaniline, and p-nitroaniline were used, and the imidazolidine-4-ones were all prepared with a variety of yields. We note the yield increase in the case of the aliphatic primary amine being more than that of the secondary one followed by the aromatic amines. Also, in the case of aromatic amines, the yield was increased by the presence of a donating group in the aromatic ring than a withdrawing group.
Table 3. Synthesis of Imidazole-4-one and/or Imidazolidin-4-one Derivatives 4a–m from Simple Amines in a Sequential, One-Pot Procedure.
Besides the imidazolidine-4-ones, symmetrical bis-N-(alkyl/aryl) imidazolidine-4-ones could also be obtained easily. 1 equiv of the aliphatic and aromatic compound has two amino groups (such as 1,2-diaminoethane, 1,3-diaminopropane, 1,6-diaminohexane, and 1,4-phenylenediamine), 2 equiv of ethyl cyanoacetate, and 2 equiv of ethylglycinate hydrochloric acid, which were mixed and subjected to heat at 70 °C for 2 h, and high yields of the desired products were produced (Scheme 3), and Table 4. Also, we noted that the reaction was completed after 2 h and triturated with cold water, and the yield was improved in the case of the reaction being left overnight and then separated. The method described here offers a good option for the manufacture of these kinds of compounds because it is extremely effective, user- and environment-friendly, and cost-effective.
Scheme 3. Synthesis of bis-N-(Alkyl/Aryl) Acetamido-2-ylidene-4-imidazole Derivatives 6a–d.
Table 4. Formation of Symmetrical Compound (6a–d) under the One-Pot Condition.
There is another optimization in this reaction depending on the molar ration of the reactants, where we find that excellent yields were isolated when we used 1.0 mol of amine, 1.0 mol ethyl cyanoacetate, and 1.20 mole ethylglycinate hydrochloric acid as shown in Table 5.
Table 5. Optimization of the Reaction Molar Ratio for the Synthesis of Compound 4f.
| entry | cyclohexylamine | ethyl cyanoacetate | ethylglycinate hydrochloride | yield (%) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 75 |
| 2 | 1 | 1 | 1.2 | 90 |
| 3 | 1.2 | 1 | 1.2 | 80 |
| 4 | 1 | 1.2 | 1.2 | 80 |
| 5 | 1 | 1 | 1.5 | 85 |
Spectral and elemental analyses of the newly produced chemicals validated their structures. The IR spectra of the obtained compounds showed characteristic bands belong to two C=O group bands, one of amidic group and the other for imidazoline moieties were observed at 1629–1655 and 1652–1712 cm–1, whereas the 1H NMR spectra of imidazoline moieties were observed at 3.56–4.33 ppm as singlet signal belong to methylene group, at 7.63–9.48 ppm as singlet signal belong to NH proton disappeared by D2O. In the 13C NMR spectra of the compounds, C=O group of imidazolines was observed at 170.14–177.44 ppm, the C=N group at 151.55–161.30 ppm, and -CH2 group of imidazoline moieties at 48.64–66.57 ppm. The accuracy range of the data from the elemental analysis was 0.04% (see, Supporting Information Figures S1–S39).
2.2. Antimicrobial Activity
Pathogenic bacteria such as Pseudomonas aeruginosa, Escherichia colias Gram-negative bacteria and Staphylococcus aureus, and Bacillus cereusas Gram-positive bacteria, as well as fungi such as Aspergillus flavus, Trichophyton rubrum, and Candida albicans, have been tested using the well diffusion method.29−31 The antimicrobial experiment was performed with DMSO as a solvent and the results were recorded as the inhibition zone diameter (IZ, mm) at 100 ppm,32,33Table 6.
Table 6. Antimicrobial Data of the Studied Compounds.
| bacteria |
fungi |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-negative bacteria |
Gram-positive bacteria |
Aspergillusflavus |
Trichophytonrubrum |
Candida albicans |
||||||||||
|
Pseudomonas aeruginosa |
Escherichia coli |
Staphylococcus
aureus |
Bacillus cereus |
IZ, nm | % | IZ, nm | % | IZ, nm | % | |||||
| IZ, nm | % | IZ, nm | % | IZ, nm | % | IZ, nm | % | |||||||
| 6b | 14 | 66.7 | 16 | 80.0 | 10 | 52.6 | 12 | 60.0 | 12 | 66.7 | 13 | 68.4 | 12 | 60.0 |
| 4b | 12 | 57.1 | 14 | 70.0 | 12 | 63.2 | 13 | 65.0 | 15 | 83.3 | 15 | 78.9 | 13 | 65.0 |
| 4f | 14 | 66.7 | 12 | 60.0 | 13 | 68.4 | 14 | 70.0 | 14 | 77.8 | 15 | 78.9 | 14 | 70.0 |
| 4g | 11 | 52.4 | 14 | 70.0 | 12 | 63.2 | 13 | 65.0 | 15 | 83.3 | 14 | 73.7 | 13 | 65.0 |
| 4m | 10 | 47.6 | 12 | 60.0 | 10 | 52.6 | 11 | 55.0 | 13 | 72.2 | 13 | 68.4 | 11 | 55.0 |
| 4d | 12 | 57.1 | 12 | 60.0 | 12 | 63.2 | 12 | 60.0 | 13 | 72.2 | 12 | 63.2 | 12 | 60.0 |
| 4c | 13 | 61.9 | 15 | 75.0 | 13 | 68.4 | 14 | 70.0 | 13 | 72.2 | 14 | 73.7 | 14 | 70.0 |
| 6a | 11 | 52.4 | 11 | 55.0 | 9 | 47.4 | 10 | 50.0 | 11 | 61.1 | 12 | 63.2 | 10 | 50.0 |
| 4k | 10 | 47.6 | 10 | 50.0 | 8 | 42.1 | 11 | 55.0 | 10 | 55.6 | 11 | 57.9 | 10 | 50.0 |
| 4j | 11 | 52.4 | 13 | 65.0 | 11 | 57.9 | 12 | 60.0 | 14 | 77.8 | 14 | 73.7 | 12 | 60.0 |
| 4h | 12 | 57.1 | 11 | 55.0 | 9 | 47.4 | 10 | 50.0 | 11 | 61.1 | 12 | 63.2 | 10 | 50.0 |
| 6c | 9 | 42.9 | 10 | 50.0 | 8 | 42.1 | 10 | 50.0 | 10 | 55.6 | 11 | 57.9 | 9 | 45.0 |
| 4e | 9 | 42.9 | 9 | 45.0 | 7 | 36.8 | 10 | 50.0 | 9 | 50.0 | 10 | 52.6 | 9 | 45.0 |
| 4a | 13 | 61.9 | 13 | 65.0 | 11 | 57.9 | 12 | 60.0 | 14 | 77.8 | 13 | 68.4 | 12 | 60.0 |
| 6d | 10 | 47.6 | 10 | 50.0 | 8 | 42.1 | 10 | 50.0 | 10 | 55.6 | 11 | 57.9 | 9 | 45.0 |
| 4i | 11 | 52.4 | 12 | 60.0 | 10 | 52.6 | 12 | 60.0 | 12 | 66.7 | 13 | 68.4 | 12 | 60.0 |
| chloramphenicol | 21 | 20 | 19 | 20 | 18 | 19 | 20 | |||||||
The activity index (%) was determined by comparing the synthesized compounds’ biological activity to that of the gold standard for antibiotics, chloramphenicol, Table 6. Biological activity data show that the produced compounds are effective against the bacteria and fungi studied, Table 6. 6b showed strong action against all the designated bacteria and fungi. The activity index of the named compounds also varied widely from 42.1% for 6c (against Staphylococcus aureus, as Gram-positive bacteria) to 80.0% (against Escherichia coli, as Gram-negative bacteria) in the range of tested bacteria.
In addition, when compared to the gold standard for antibiotics, chloramphenicol and the synthesized compounds had impressive antibacterial/antifungal efficacy, Table 6.
2.3. Molecular Docking
Molecular docking research was carried out to determine the interactions and orientations of the synthesized compounds with the active site of the target protein.34−36 Molecular operating environment (MOE) was used to conduct molecular docking on the E. coliFabH–CoA complex (PDB ID: 1HNJ) in this research.37,38 Targeting the fatty acid synthesis receptor FabH allows researchers to assess the drugs’ efficacy against bacteria. The crystal structure of the E. coliFabH–CoA complex was obtained from the Protein Data Bank database (PDB ID: 1HNJ; URL: http://www.rcsb.org).39,40 The binding mechanisms of the named medicines to docking score (S, kcal/mol) and interactions with hydrogen bonds were used to evaluate the target receptor, Figure 2, and Table 7.
Figure 2.
3D and 2D interaction of docked compounds through the 1HNJ active site.
Table 7. Molecular Docking Data.
| ligand | receptor | interaction | distance | E (kcal/mol) | S (kcal/mol) | inhibition constant (Ki) (μM) | |
|---|---|---|---|---|---|---|---|
| 6b | N 5 | MET 207 | H-donor | 3.02 | –1.50 | –8.72 | 0.41 |
| O 9 | CYS 112 | H-acceptor | 2.82 | –0.10 | |||
| O 9 | ASN 274 | H-acceptor | 2.71 | –0.80 | |||
| O 17 | LEU 191 | H-acceptor | 3.06 | –2.50 | |||
| O 24 | ASN 193 | H-acceptor | 3.25 | –1.00 | |||
| 4b | N 3 | GLY 306 | H-acceptor | 3.74 | –0.70 | –6.81 | 2.23 |
| O 8 | THR 190 | H-acceptor | 3.51 | –0.90 | |||
| 4f | N 1 | HIS 244 | H-donor | 3.44 | –0.80 | –7.41 | 3.76 |
| O 10 | ILE 251 | H-acceptor | 3.61 | –0.70 | |||
| O 10 | THR 254 | H-acceptor | 3.29 | –0.70 | |||
| 4g | N 1 | HIS 244 | H-donor | 3.42 | –2.30 | –7.72 | 3.89 |
| C 8 | MET 207 | H-donor | 3.54 | –0.90 | |||
| O 10 | MET 207 | H-donor | 3.37 | –0.20 | |||
| O 10 | ASN 274 | H-acceptor | 3.27 | –1.20 | |||
| 4m | N 6 | ASN 193 | H-acceptor | 3.25 | –1.50 | –7.39 | 7.77 |
| O 19 | GLY 306 | H-acceptor | 3.34 | –0.90 | |||
| 6-ring | ALA 111 | pi-H | 3.89 | –0.60 | |||
| 4d | O 4 | ASN 247 | H-acceptor | 3.45 | –1.20 | –6.98 | 10.34 |
| O 9 | GLY 209 | H-acceptor | 3.52 | –0.80 | |||
| 4c | O 15 | CYS 112 | H-acceptor | 3.64 | –0.70 | –6.34 | |
| N 5 | GLY 186 | H-donor | 3.39 | –2.30 | |||
| O 9 | GLY 186 | H-acceptor | 3.70 | –0.70 | |||
| 6a | O 3 | ALA 111 | H-acceptor | 3.63 | –0.70 | –7.77 | 2.05 |
| O 3 | CYS 112 | H-acceptor | 3.07 | –1.00 | |||
| O 3 | GLY 306 | H-acceptor | 3.32 | –2.00 | |||
| O 16 | ASN 274 | H-acceptor | 3.14 | –1.00 | |||
| O 22 | ASN 247 | H-acceptor | 3.11 | –1.80 | |||
| 4k | O 4 | GLY 306 | H-acceptor | 3.81 | –0.70 | –6.42 | 19.96 |
| O 10 | CYS 112 | H-acceptor | 2.98 | –0.80 | |||
| 4j | O 4 | GLY 306 | H-acceptor | 3.68 | –0.80 | –6.09 | 34.81 |
| O 10 | ASN 193 | H-acceptor | 3.13 | –2.50 | |||
| 4h | N 1 | HIS 244 | H-donor | 3.00 | –1.20 | –7.05 | 6.90 |
| O 10 | MET 207 | H-donor | 3.31 | –0.30 | |||
| O 10 | ASN 274 | H-acceptor | 3.37 | –0.70 | |||
| 6c | O 20 | CYS 112 | H-acceptor | 3.17 | –0.90 | –6.19 | 29.41 |
| O 20 | GLY 306 | H-acceptor | 2.94 | –3.30 | |||
| 4e | O 7 | HIS 244 | H-donor | 2.72 | –2.20 | –5.74 | 62.80 |
| C 11 | CYS 112 | H-donor | 3.73 | –0.80 | |||
| N 12 | PHE 304 | H-donor | 2.96 | –3.20 | |||
| 4a | O 6 | GLY 306 | H-acceptor | 3.03 | –2.50 | –5.60 | 79.51 |
| O 12 | THR 81 | H-acceptor | 3.27 | –1.20 | |||
| 6d | N 20 | THR 81 | H-donor | 3.46 | –0.90 | –7.12 | 6.13 |
| O 25 | PHE 304 | H-acceptor | 3.10 | –0.90 | |||
| O 26 | THR 190 | H-acceptor | 3.65 | –0.60 | |||
| 4i | O 4 | GLY 306 | H-acceptor | 3.46 | –0.90 | –6.12 | 33.10 |
| O 10 | THR 81 | H-acceptor | 3.40 | –0.80 | |||
| 6-ring | ALA 246 | pi-H | 3.84 | –0.60 |
The subject substrates’ extensive hydrogen bonds and hydrophobic interactions with the target receptor are the cause of the subject substrates’ high negative docking scores (S), as shown in Figure 2 and Table 7. This demonstrates how docked substrates are located relatively near to the receptor’s active site.41−43 The inhibitory action in 1HNJ was arranged as follows: 6b > 6a > 4g > 4f > 4m > 6d > 4h > 4d > 4b > 4k > 4c > 6c > 4i > 4j > 4e > 4a. Compound 6b is the most active of the compounds according to the docking studies.
Table 7 displays that the subject compounds showed a strong docking score of −5.40 (4a) to −8.72 (6b) kcal/mol toward the E. coliFabH–CoA complex. A high docking score (−8.72 kcal/mol) suggests that 6b is the most energetic. 6b found five hydrogen bond interactions: N5 with MET 207, O9 with CYS 112, O9 with ASN 274, O17 with LEU 191, and O24 with ASN. When evaluating a molecule for its potential as a hit and lead or therapy candidate, the inhibition constant (Ki value) is an important factor. For a molecule to be called a hit or lead compound, its Ki value must be low, typically in the micromolar (μM) range, because a low Ki value is often indicative of high potency.44−461HNJ domain Ki values for the synthesized compounds ranged from 0.41 (6b) to 79.51, making them all candidates for hits and leads (4b). It appears that 6b, which had the second-lowest Ki value among the produced compounds, could be a potential therapeutic option, Table 7.
3. Materials and Methods
3.1. Chemistry
At El-Gomhouria Company for Drugs in Egypt, all the chemicals were readily available for purchase, and they were all used without additional purification. The uncorrected melting points were all determined in open-glass capillaries using a Griffin melting point equipment. On a Perkin Elmer 1430 infrared spectrophotometer, IR spectra were captured. At Sohag University in Egypt, 1H NMR and 13C NMR spectra were recorded using a Jeol-400 MHz NMR-spectrometer (DMSO-d6) and CDCl3 (see the Supporting Information). Tetramethylsilane (TMS) is used as an internal standard, and the chemical shifts are presented in ppm downfield. The Vario El Fab-Nr elemental analyzer underwent micro studies. A Hewlett Packard 5988 spectrometer was used to record the mass spectra (Microanalysis Center, Cairo University, Egypt). It was done by using TLC to monitor the reactions.
3.2. General Procedure for the Synthesis of 4-Imidazolinone and/or 4-Imidazolidinone Derivatives 4a–m
An equimolar mixture of amines 3a–m (0.001 mol) and ethylcyanoacetate 1 (0.001 mol) was fused together for 15 min and then 0.0012 mol of ethyl glycinate hydrochloride 2 (treated with an equimolar amount of triethylamine before addition to activate the amino group) was added and the reaction time of 2 h was completed at 70 °C to obtain the equivalent imidazole-4-one/imidazolidine-4-one 4a–m. The precipitates were recovered by filtration, extensively washed with water, and recrystallized from ethanol.
3.2.1. N,N-Dimethyl-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4a)
Yield (92%), pale yellow needles, mp 150 °C, anal. data: (C7H11N3O2, 169.18), Calcd: C, 49.70; H, 6.55; N, 24.84. Found: C, 49.64; H, 6.60; N, 24.85. IR (νmax, cm–1): 3387 (NH), 2982 (CHaliph), 1722 (CO), 1662 (CO). 1H NMR (DMSO-d6): δ ppm 3.21 (s, 6H, 2CH3), 3.41 (s, 2H, CH2–CO), 3.76 (s, 2H, CH2imidazole), 9.15 (s, 1H, NH, exchangeable by D2O). 13CMR (DMSO-d6): δ ppm 30.15, 41.95, 55.12, 161.23, 166.47, 185.47.
3.2.2. N,N-Diethyl-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4b)
Yield (98%), pale yellow needles, mp 122 °C, Anal. data: (C9H15N3O2, 197.23), Calcd: C, 54.81; H, 7.67; N, 21.30. Found: C, 54.73; H, 7.85; N, 21.20. IR (νmax, cm–1): 3402 (NH), 2975 (CHaliph), 1745 (CO), 1640 (CO). 1H NMR (DMSO-d6): δ ppm 1.21 (t, 6H, J = 7.20 Hz, 2CH3), 3.41 (s, 2H, CH2–CO), 3.76 (s, 2H, CH2imidazole), 4.20 (q, 4H, J = 7.14 Hz, 2CH2), 8.54 (s, 1H, NH, exchangeable by D2O). 13CMR (DMSO-d6): δ ppm 14.40, 45.45, 57.23, 62.00, 158.32, 167.12, 173.55.
3.2.3. (E)-2-(2-Oxo-2-(piperidin-1-yl)ethylidene)imidazolidin-4-one (4c)
Yield (90%), pale yellow needles, mp 160 °C, Anal. data: (C10H15N3O2, 209.24), Calcd: C, 57.40; H, 7.23; N, 20.08. Found: C, 57.33; H, 7.27; N, 20.11. IR (nmax, cm–1): 3240 (NH), 2989 (CHaliph), 1713 (CO), 1653 (CO). 1H NMR (CDCl3): δ ppm 1.19–1.22 (m, 4H, 2CH2pip), 1.52–1.55 (m, 2H, CH2pip), 3.86 (s, 2H, CH2imidazole), 4.06–4.16 (m, 4H, 2CH2-Npip), 5.05 (s, 1H, =CH), 8.86 (s, 1H, NH, exchangeable by D2O), 10.53 (s, 1H, NH, exchangeable by D2O). 13CMR (CDCl3): δ ppm 13.75, 26.08, 31.05, 60.19, 88.77, 155.35, 166.93, 172.16.
3.2.4. (E)-2-(4-Hydroxy-1H-imidazole-2(3H)-ylidene)-1-morpholinoethanone (4d)
Yield (74%), pale yellow needles, mp 148 °C, Anal. data: (C9H13N3O3, 211.22), Calcd: C, 51.18; H, 6.20; N, 19.89. Found: C, 51.11; H, 6.23; N, 19.93. IR (nmax, cm–1): 3238 (NH), 2991 (CHaliph), 1713 (CO), 1653 (CO). 1H NMR (CDCl3): δ ppm 3.73 (s, 1H, OH, exchangeable by D2O), 3.77 (m, 4H, 2CH2–N), 4.21 (m, 4H, 2CH2–O), 5.14 (s, 1H, CH=C–NH), 5.63 (s, 1H, CH=imidazoleC–OH), 9.48 (s, 1H, NH, exchangeable by D2O), 10.64 (s, 1H, NH, exchangeable by D2O). 13C MR (CDCl3): δ ppm 14.27, 31.44, 60.17, 89.14, 155.34, 167.37, 172.18.
3.2.5. (E)-N-(2-Hydroxyethyl)-2-(4-oxoimidazolidin-2-ylidene)acetamide (4e)
Yield (77%), white needles, mp 234 °C, Anal. data: (C7H11N3O3, 185.23), Calcd: C, 57.40; H, 7.23; N, 20.08. Found: C, 57.33; H, 7.27; N, 20.11. IR (νmax, cm–1): 3238 (OH), 3175 (NH), 2989 (CHaliph), 1712 (CO), 1692 (CO). 1H NMR (CDCl3): δ ppm 3.63 (s, 1H, OH, exchangeable by D2O), 3.86 (s, 2H, CH2imidazole), 4.06–4.16 (m, 4H, 2CH2), 5.05 (s, 1H, CH), 5.50 (s, 1H, NH, exchangeable by D2O), 8.70 (s, 1H, NH, exchangeable by D2O), 10.54 (s, 1H, NH, exchangeable by D2O).
3.2.6. N-Cyclohexyl-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4f)
Yield (90%), pale yellow needles, mp 174 °C, Anal. data: (C11H17N3O2, 223.23), Calcd: C, 59.17; H, 7.67; N, 18.82. Found: C, 59.19; H, 7.69; N, 18.78. IR (νmax, cm–1): 3445, 3282 (2NH), 2936 (CHaliph), 1712 (CO), 1631 (CO). 1H NMR (DMSO-d6): δ ppm 1.12–1.76 (m, 10H, 5CH2cyclo), 2.09 (s, 1H, NH, exchangeable by D2O), 3.28 (s, 2H, CH2–CO), 3.51 (m, 1H, CH–NH), 3.56 (s, 2H, CH2imidazole), 8.07 (s, 1H, NH, exchangeable by D2O). 13CMR (DMSO-d6): δ ppm 24.77, 25.56, 25.81, 31.22, 32.55, 48.64, 161.40, 163.83, 171.03.
3.2.7. N-Benzyl-2-(5-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4g)
Yield (85%), pale yellow needles, mp 154 °C, Anal. data: (C21H13N3O2, 231.25), Calcd: C, 62.33; H, 5.67; N, 18.17. Found: C, 62.25; H, 5.71; N, 18.21. IR (nmax, cm–1): 3268, 3205 (2NH), 3053 (CHarom), 2977 (CHaliph), 1747 (CO), 1669 (CO). 1H NMR (DMSO-d6): δ ppm 3.81 (s, 2H, CH2–CO), 3.98 (s, 2H, CH2imidazole), 4.27 (s, 2H, CH2–NH), 7.09–7.62 (m, 5H, CH of Ar), 8.47 (s, 1H, NH, exchangeable by D2O), 10.61 (s, 1H, NH, exchangeable by D2O). 13CNMR (DMSO-d6): δ ppm 41.22, 43.26, 55.02, 126.71, 126.92, 126.99, 128.06, 166.81, 166.94, 182.36.
3.2.8. 2-(4-Oxo-4,5-dihydro-1H-imidazole-2-yl)-N-phenylacetamide (4h)
Yield (80%), pale yellow needles, mp 169 °C, Anal. data: (C11H11N3O2, 217.22), Calcd: C, 60.82; H, 5.10; N, 19.34. Found: C, 60.82; H, 5.15; N, 19.29. IR (nmax, cm–1): 3311, 3162 (2NH), 3051 (CHarom), 2989 (CHaliph), 1693 (CO), 1627 (CO). 1H NMR (DMSO-d6): δ ppm 3.41 (s, 2H, CH2–CO), 4.09 (s, 2H, CH2imidazole), 7.23–7.70 (m, 5H, CH of Ar), 8.72 (s, 1H, NH, exchangeable by D2O), 10.69 (s, 1H, NH, exchangeable by D2O). 13C NMR (DMSO-d6): δ ppm 27.06, 42.22, 116.36, 119.77, 124.34, 129.31, 138.87, 161.43, 163.05.
3.2.9. N-(Naphthalen-1-yl)-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4i)
Yield (74%), pale yellow needles, mp 218 °C, Anal. data: (C15H13N3O2, 267.28), Calcd: C, 67.40; H, 4.90; N, 15.72. Found: C, 67.42; H, 4.94; N, 15.66. IR (nmax, cm–1): 3464, 3313 (2NH), 3051 (CHarom), 2989 (CHaliph), 1686 (CO), 1630 (CO). 1H NMR (DMSO-d6): δ ppm 3.74 (s, 2H, CH2–CO), 3.91 (s, 2H, CH2imidazole), 6.89–7.56 (m, 8H, CH of Ar + NH exchanged by D2O), 7.95 (s, 1H, NH, exchangeable by D2O). 13CMR (DMSO-d6): δ ppm 56.34, 61.08, 120.88, 121.47, 122.02, 125.20, 126.57, 128.63, 129.37, 129.75, 131.08, 132.91, 142.29, 148.70, 158.46, 166.37.
3.2.10. (E)-2-(4-Oxoimidazolidin-2-ylidene)-N-(o-tolyl)acetamide (4j)
Yield (80%), pale yellow needles, mp 210 °C, Anal. data: (C12H13N3O2, 231.25), Calcd: C, 62.33; H, 5.67; N, 18.17. Found: C, 62.30; H, 5.64; N, 18.23. IR (nmax, cm–1): 3311, 3162 (2NH), 3051 (CHarom), 2989 (CHaliph), 1693 (CO), 1627 (CO). 1H NMR (DMSO-d6): δ ppm 3.63 (s, 3H, CH3), 4.29 (s, 2H, CH2imidazole), 5.68 (s, 1H, =CH), 7.23–7.70 (m, 4H, CH of Ar), 8.12 (s, 2H, 2 NH, exchangeable by D2O), 11.19 (s, 1H, NH, exchangeable by D2O).
3.2.11. (E)-N-(2-Methoxyphenyl)-2-(4-oxoimidazolidin-2-ylidene)acetamide (4k)
Yield (67%), pale yellow needles, mp 275 °C, Anal. data: (C12H13N3O3, 247.25), Calcd: C, 58.29; H, 5.30; N, 16.99. Found: C, 58.31; H, 5.36; N, 16.91. IR (nmax, cm–1): 3302, 3112 (2NH), 3065 (CHarom), 2960 (CHaliph), 1712 (CO), 1643 (CO). 1H NMR (DMSO-d6): δ ppm 3.32 (s, 2H, CH2imidazole), 4.02 (s, 3H, OCH3), 4.80 (s, 1H, =CH), 7.34 (s, 2H, 2NH, exchangeable by D2O), 7.55–7.63 (m, 4H, CH of Ar), 10.13 (s, 1H, NH, exchangeable by D2O).
3.2.12. N-(4-Chlorophenyl)-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4l)
Yield (74%), pale yellow needles, mp 244 °C, Anal. data: (C11H10N4O4, 262.22), Calcd: C, 50.38; H, 3.84; N, 21.37. Found: C, 50.41; H, 3.78; N, 21.40. IR (nmax, cm–1): 3452, 3312 (2NH), 3087 (CHarom), 2974 (CHaliph), 1704 (CO), 1663 (CO). 1H NMR (DMSO-d6): δppm 3.72 (s, 2H, CH2–CO), 4.12 (s, 2H, CH2imidazole), 7.15–7.58 (m, 4H, CH of Ar), 7.37 (s, 1H, NH, exchangeable by D2O), 8.14 (s, 1H, NH, exchangeable by D2O).
3.2.13. N-(4-Nitrophenyl)-2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide (4m)
Yield (61%), pale yellow needles, mp 253 °C, Anal. data: (C11H10N4O4, 262.22), Calcd: C, 50.38; H, 3.84; N, 21.37. Found: C, 50.41; H, 3.78; N, 21.40. IR (nmax, cm–1): 3302, 3112 (2NH), 3065 (CHarom), 2960 (CHaliph), 1712 (CO), 1643 (CO). 1H NMR (DMSO-d6): δ ppm 3.69 (s, 2H, CH2–CO), 4.33 (s, 2H, CH2imidazole), 7.26–7.47 (m, 4H, CH of Ar), 7.37 (s, 1H, NH, exchangeable by D2O), 8.67 (s, 1H, NH, exchangeable by D2O).
3.3. General Procedure for Synthesis of bis-N-(alkyl/aryl) Acetamido-2-ylidene-4-imidazole (6a–d)
A mixture of amines 5a–d (0.001 mol) and ethylcyanoacetate 1 (0.002 mol) fused together for 15 min and then 0.0024 mol of ethyl glycinate hydrochloride 2 (treated with an equimolar amount of triethyl amine before addition to activate the amino group) was added and the reaction time of 2 h was completed at 70 °C to obtain the equivalent bis-N-(alkyl/aryl) acetamido-2-ylidene-4-imidazole. The precipitates were recovered by filtering, carefully washed with water, and recrystallized from ethanol.
3.3.1. (2E,2′E)-N,N’-(Ethane-1,2-diyl)bis(2-(4-oxoimidazolidin-2-ylidene)acetamide) 6a
Yield (96%), pale yellow needles, mp 202 °C, Anal. data: (C12H16N6O4, 308.29), Calcd: C, 46.75; H, 5.23; N, 27.26. Found: C, 46.66; H, 5.28; N, 27.32. IR (nmax, cm–1): 3238, 3162 (2NH), 2974 (CHaliph), 1712 (CO), 1692 (CO). 1H NMR (CDCl3): δ ppm 3.14 (s, 4H, 2CH2–CO), 3.76 (s, 4H, 2CH2–NH), 4.19 (s, 4H, 2CH2imidazole), 9.17 (s, 2H, 2NH, exchangeable by D2O), 10.62 (s, 2H, 2NH, exchangeable by D2O). 13CMR (CDCl3): δ ppm 31.45, 60.17, 89.13, 155.15, 167.36, 172.23.
3.3.2. N,N’-(Propane-1,3-diyl)bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide) 6b
Yield (92%), pale yellow needles, mp 233 °C, Anal. data: (C13H18N6O4, 322.32), Calcd: C, 48.44; H, 5.63; N, 26.07. Found: C, 48.47; H, 5.57; N, 26.10. IR (νmax, cm–1): 3290, 3167 (2NH), 3065 (CHarom), 2971 (CHaliph), 1745 (CO), 1652 (CO). 1H NMR (DMSO-d6): δ ppm 1.40 (m, 2H, CH2), 3.06 (s, 4H, 2CH2–CO), 3.61 (s, 4H, 2CH2imidazole), 3.74 (m, 4H, CH2), 8.02 (s, 1H, NH, exchangeable by D2O), 8.32 (s, 2H, 2NH, exchangeable by D2O), 8.88 (s, 1H, NH, exchangeable by D2O). 13CMR (DMSO-d6): δ ppm 25.67, 29.13, 45.86, 61.91, 154.87, 162.32, 168.30.
3.3.3. N,N’-(Hexane-1,6-diyl)bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide)6c
Yield (94%), pale yellow needles, mp 215 °C, Anal. data: (C16H24N6O4, 364.40), Calcd: C, 52.74; H, 6.64; N, 23.06. Found: C, 52.71; H, 6.59; N, 23.14. IR (νmax, cm–1): 3448, 3290 (2NH), 3067 (CHarom), 2940 (CHaliph), 1725 (CO), 1651 (CO). 1H NMR (DMSO-d6): δ ppm 1.26 (m, 4H, 2CH2), 1.34 (m, 4H, 2CH2), 3.11 (m, 4H, 2CH2), 3.30 (s, 4H, 2CH2–CO), 3.63 (s, 4H, 2CH2imidazole), 8.04 (s, 1H, NH, exchangeable by D2O). 8.23 (s, 2H, 2NH, exchangeable by D2O), 9.91 (s, 1H, NH, exchangeable by D2O). 13CMR (DMSO-d6): δ ppm 25.84, 26.39, 29.13, 45.86, 61.91, 151.02, 162.32, 169.52.
3.3.4. N,N’-(1,4-Phenylene)bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide) 6d
Yield (89%), pale yellow needles, mp 266 °C, Anal. data: (C16H16N6O4, 356.34), Calcd: C, 53.93; H, 4.53; N, 23.58. Found: C, 53.85; H, 4.56; N, 23.63. IR (νmax, cm–1): 3463, 3312 (2NH), 3070 (CHarom), 2979 (CHaliph), 1684 (CO), 1630 (CO). 1H NMR (DMSO-d6): δ ppm 3.76 (s, 4H, 2CH2–CO), 3.98 (s, 4H, 2CH2imidazole), 6.89, 7.19 (s, 2H, 2NH, exchangeable by D2O), 7.20–7.34 (m, 4H, CHarom), 7.66 (s, 2H, 2NH), exchangeable by D2O. 13CMR (DMSO-d6): δ ppm 56.37, 61.01, 126.44, 128.64, 133.07, 148.88, 158.29, 165.68.
3.4. Antimicrobial Activity
The antibacterial and antifungal activity of the produced compounds was measured using the agar well diffusion method,47,48 wherein a volume of the microbial inoculum is dispersed evenly across the entire agar plate surface to inoculate it. Then, a sterile cork borer or tip is used to puncture a hole with a diameter of 6 to 8 mm, and 20 to 100 μL of the antimicrobial agent solution is injected into the well to achieve the necessary concentration. The plates were then kept at 37 °C for 24 h. The diameter of the zone of inhibition formed in the wells after incubation was used to determine antimicrobial efficacy.49,50
3.5. Molecular Docking
Possible binding mechanisms of the investigated drugs against the E. coliFabH–CoA complex receptor (PDB ID: 1HNJ) were investigated using molecular docking. The 3D structure of the receptor of interest was obtained from the Protein Data Bank (http://www.rcsb.org/). The MOE program was used for molecular docking research. Score function (S, kcal/mol) was used to rank the compounds’ binding affinities toward the target receptor.51
4. Conclusions
2-Acetamido-2-ylidene-4-imidazole/bis-N-(alkyl/aryl) acetamido-2-ylidene-4-imidazoles were synthesized via the reaction of different amines with ethylcyanoacetate and ethylglycinate hydrochloric acid via the green reaction condition. Chemical and spectroscopic data allowed for the elucidation of the novel substances. Synthesized compounds were effective in combating the investigations on antibacterial action. The compounds showed an activity index that ranged from 42.1% for N,N’-(hexane-1,6-diyl)bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide) in the case of S. aureus (Gram positive) to 80.0% for, N’-(propane-1,3-diyl)bis(2-(4-oxo-4,5-dihydro-1H-imidazole-2-yl)acetamide) in the case of E. coli (Gram negative). Molecular docking simulations with the E. coliFabH–CoA complex (PDB ID: 1HNJ) were performed to explore the possible interactions of the enzyme with the synthesized compounds. According to the obtained results, the synthesized compounds seemed to be a promising candidate for the progress of antimicrobials.
Acknowledgments
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [grant 3750].
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03767.
All spectra data of IR, 1H NMR, and 13C NMR for the synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Albayati M. R.; Kansız S.; Dege N.; Kaya S.; Marzouki R.; Lgaz H.; Salghi R.; Ali I. H.; Alghamdi M. M.; Chung I.-M. Synthesis, crystal structure, Hirshfeld surface analysis and DFT calculations of 2-[(2, 3-dimethylphenyl) amino]-N’-[(E)-thiophen-2-ylmethylidene] benzohydrazide. J. Mol. Struct. 2020, 1205, 127654. 10.1016/j.molstruc.2019.127654. [DOI] [Google Scholar]
- Mlostoń G.; Celeda M.; Jasiński M.; Urbaniak K.; Boratyński P. J.; Schreiner P. R.; Heimgartner H. 2-Unsubstituted imidazole N-oxides as novel precursors of chiral 3-alkoxyimidazol-2-ylidenes derived from trans-1, 2-diaminocyclohexane and other chiral amino compounds. Molecules 2019, 24, 4398. 10.3390/molecules24234398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramla M. M.; Omar M. A.; El-Khamry A.-M. M.; El-Diwani H. I. Synthesis and antitumor activity of 1-substituted-2-methyl-5-nitrobenzimidazoles. Bioorg. Med. Chem. 2006, 14, 7324–7332. 10.1016/j.bmc.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Handzlik J.; Szymańska E.; Nędza K.; Kubacka M.; Siwek A.; Mogilski S.; Handzlik J.; Filipek B.; Kieć-Kononowicz K. Pharmacophore models based studies on the affinity and selectivity toward 5-HT1A with reference to α1-adrenergic receptors among arylpiperazine derivatives of phenytoin. Bioorg. Med. Chem. 2011, 19, 1349–1360. 10.1016/j.bmc.2010.11.051. [DOI] [PubMed] [Google Scholar]
- Subtel’na I.; Atamanyuk D.; Szymańska E.; Kieć-Kononowicz K.; Zimenkovsky B.; Vasylenko O.; Gzella A.; Lesyk R. Synthesis of 5-arylidene-2-amino-4-azolones and evaluation of their anticancer activity. Bioorg. Med. Chem. 2010, 18, 5090–5102. 10.1016/j.bmc.2010.05.073. [DOI] [PubMed] [Google Scholar]
- Kieć-Kononowicz K.; Szymańska E.; Motyl M.; Holzer W.; Białecka A.; Kasprowicz A. Synthesis, spectral and antimicrobial properties of 5-chloroarylidene aromatic derivatives of imidazoline-4-one. Pharmazie 1998, 53, 680–684. [PubMed] [Google Scholar]
- Matys A.; Podlewska S.; Witek K.; Witek J.; Bojarski A. J.; Schabikowski J.; Otrębska-Machaj E.; Latacz G.; Szymańska E.; Kieć-Kononowicz K.; et al. Imidazolidine-4-one derivatives in the search for novel chemosensitizers of Staphylococcus aureus MRSA: Synthesis, biological evaluation and molecular modeling studies. Eur. J. Med. Chem. 2015, 101, 313–325. 10.1016/j.ejmech.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Szymańska E.; Kieć-Kononowicz K.; Białecka A.; Kasprowicz A. Antimicrobial activity of 5-arylidene aromatic derivatives of hydantoin. Part 2. Farmaco. 2002, 57, 39–44. 10.1016/s0014-827x(01)01172-7. [DOI] [PubMed] [Google Scholar]
- Karolak-Wojciechowska J.; Szymańska E.; Fruziński A.; Kieć-Kononowicz K. Crystallographic studies of (Z) and (E) isomers of 2-amino-5-(2-chlorobenzylidene)-1-methyl-1H-imidazole-4 (5H)-one. J. Mol. Struct. 2010, 966, 14–17. 10.1016/j.molstruc.2009.11.058. [DOI] [Google Scholar]
- Qian L.; Sun G. Durable and regenerable antimicrobial textiles: Synthesis and applications of 3-methylol-2, 2, 5, 5-tetramethyl-imidazolidin-4-one (MTMIO). J. Appl. Polym. Sci. 2003, 89, 2418–2425. 10.1002/app.12405. [DOI] [Google Scholar]
- Knight B. J.; Stache E. E.; Ferreira E. M. Complementary stereochemical outcomes in proline-based self-regenerations of stereocenters. Org. Lett. 2014, 16, 432–435. 10.1021/ol403320d. [DOI] [PubMed] [Google Scholar]
- El-Saghier A. M.; Abd Allah O. A.; Kadry A. M. Design, Synthesis and Antibacterial Evaluation of some new 3, 5-Diphenylcyclohex-2-en-1-one Derivatives. J. Adv. Chem. 2013, 6, 923. 10.24297/jac.v6i1.5538. [DOI] [Google Scholar]
- Elkanzi N. A.; Kadry A. M.; Ryad R. M.; Bakr R. B.; Ali El-Remaily M. A. E. A. A.; Ali A. M. Efficient and Recoverable Bio-Organic Catalyst Cysteine for Synthesis, Docking Study, and Antifungal Activity of New Bio-Active 3, 4-Dihydropyrimidin-2 (1 H)-ones/thiones Under Microwave Irradiation. ACS Omega 2022, 7, 22839–22849. 10.1021/acsomega.2c02449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghuwainem Y. A.; Abd El-Lateef H. M.; Khalaf M. M.; Abdelhamid A. A.; Alfarsi A.; Gouda M.; Abdelbaset M.; Abdou A. Synthesis, structural, DFT, antibacterial, antifungal, anti-inflammatory, and molecular docking analysis of new VO (II), Fe (III), Mn (II), Zn (II), and Ag (I) complexes based on 4-((2-hydroxy-1-naphthyl) azo) benzenesulfonamide. J. Mol. Liq. 2023, 369, 120936. 10.1016/j.molliq.2022.120936. [DOI] [Google Scholar]
- Araújo M. J.; Bom J.; Capela R.; Casimiro C.; Chambel P.; Gomes P.; Iley J.; Lopes F.; Morais J.; Moreira R.; et al. Imidazolidin-4-one derivatives of primaquine as novel transmission-blocking antimalarials. J. Med. Chem. 2005, 48, 888–892. 10.1021/jm0494624. [DOI] [PubMed] [Google Scholar]
- Shokr E. K.; Kamel M. S.; Abdel-Ghany H.; El- Remaily M. A. E. A. A. A.; Abdou A. Synthesis, characterization, and DFT study of linear and non-linear optical properties of some novel thieno [2, 3-b] thiophene azo dye derivatives. Mater. Chem. Phys. 2022, 290, 126646. 10.1016/j.matchemphys.2022.126646. [DOI] [Google Scholar]
- El-Sayed S. M.; El-Ashmawy M. B.; Bayoumi S. M.; Hassan G. S.; El-Subbagh H. I. New Imidazole-4-one and Imidazolidine-2,4-dione Analogues: Design, Synthesis, Antitumor activity and Molecular Modeling Study. Am. J. Physiol. 2018, 7, 24–41. 10.5455/ajpbp.20180427101802. [DOI] [Google Scholar]
- Gomha S. M.; Hassaneen H. M. Synthesis and antimicrobial activity of some new pyrazoles, fused pyrazolo [3, 4-d]-pyrimidine and 1, 2-dihydroimidazo-[2, 1-c] [1, 2, 4] triazin-6-one derivatives. Molecules 2011, 16, 6549–6560. 10.3390/molecules16086549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R.; Ciofalo M. An updated review on the synthesis and antibacterial activity of molecular hybrids and conjugates bearing imidazole moiety. Molecules 2020, 25, 5133. 10.3390/molecules25215133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saghier A. M.; Mohamed M. A.; Abd-Allah O. A.; Kadry A. M.; Ibrahim T. M.; Bekhit A. A. Green synthesis, antileishmanial activity evaluation, and in silico studies of new amino acid-coupled 1, 2, 4-triazoles. Med. Chem. Res. 2019, 28, 169–181. 10.1007/s00044-018-2274-x. [DOI] [Google Scholar]
- Mohamed M. A.; Abd Allah O. A.; Bekhit A. A.; Kadry A. M.; El-Saghier A. M. Synthesis and antidiabetic activity of novel triazole derivatives containing amino acids. J. Heterocycl. Chem. 2020, 57, 2365–2378. 10.1002/jhet.3951. [DOI] [Google Scholar]
- M M El-Saghier A.; A A Mohamed M.; A Abdalla O.; M Kadry A. Utility of amino acid coupled 1, 2, 4-triazoles in organic synthesis: synthesis of some new antileishmainal agents. Bull. Chem. Soc. Ethiop. 2018, 32, 559–570. 10.4314/bcse.v32i3.14. [DOI] [Google Scholar]
- Satzinger G. Heterocyclen durch Reaktion von Mercapto-und Hydroxycarbonsäureestern mit aktivierten Nitrilen. Justus Liebigs Ann. Chem. 1978, 1978, 473–511. 10.1002/jlac.197819780311. [DOI] [Google Scholar]
- Ceder O.; Stenhede U. On the Structure of Ethyl 4-Imidazolidone-2-acetate. Acta Chem. Scand. 1973, 27, 2221–2223. 10.3891/acta.chem.scand.27-2221. [DOI] [Google Scholar]
- Coburn R. A.; Taylor M. D. Mesoionic purinone analogs. VIII. Synthesis and properties of mesoionic 5-substituted-6-methylimidazo [1, 2-c] pyrimidine-2, 7-diones. J. Heterocycl. Chem. 1982, 19, 567–572. 10.1002/jhet.5570190323. [DOI] [Google Scholar]
- Griffiths G. J.; Hauck M. B.; Imwinkelried R.; Kohr J.; Roten C. A.; Stucky G. C.; Gosteli J. Novel syntheses of 2-butyl-5-chloro-3 H-imidazole-4-carbaldehyde: a key intermediate for the synthesis of the angiotensin II antagonist Losartan. J. Org. Chem. 1999, 64, 8084–8089. 10.1021/jo9824910. [DOI] [PubMed] [Google Scholar]
- Manjunatha A.; Puttaswamy Conversion of imidazoles to imidazolones with chloramine-B: kinetic and mechanistic study. Monatsh. Chem. 2016, 147, 1517–1529. 10.1007/s00706-016-1663-4. [DOI] [Google Scholar]
- Wang M.; Gao R.; Zheng M.; Sang P.; Li C.; Zhang E.; Li Q.; Cai J. Development of bis-cyclic imidazolidine-4-one derivatives as potent antibacterial agents. J. Med. Chem. 2020, 63, 15591–15602. 10.1021/acs.jmedchem.0c00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd Allah O. A.; El-Saghier A. M.; Kadry A. M. Synthesis, Structural Stability Calculation, and Antibacterial Evaluation of Novel 3, 5-Diphenylcyclohex-2-en-1-one Derivatives. Synth. Commun. 2015, 45, 944–957. 10.1080/00397911.2014.994128. [DOI] [Google Scholar]
- Mohamed M. A. A.; Kadry A. M.; Farghaly M. M.; El-Saghier A. M. M. Synthesis, characterization and antibacterial activity of some novel spiro [naphtho [1, 2-e][1, 3] oxazine-3, 4’-pyran] derivatives. J. Pharm. Appl. Chem. 2021, 7, 1–10. 10.18576/jpac/070301. [DOI] [Google Scholar]
- Abd Allah O. A.; El-Saghier A. M.; Kadry A. M.; Seleem A. A. Synthesis and evaluation of some novel curcumin derivatives as anti-inflammatory agents. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 87. [Google Scholar]
- Elkanzi N. A.; Hrichi H.; Salah H.; Albqmi M.; Mali A.; Abdou A. Synthesis, physicochemical properties, biological, molecular docking and DFT investigation of Fe (III), Co (II), Ni (II), Cu (II) and Zn (II) complexes of the 4-[(5-oxo-4, 5-dihydro-1, 3-thiazol-2-yl) hydrazono] methyl} phenyl 4-methylbenzenesulfonate Schiff-base ligand. Polyhedron 2023, 230, 116219. 10.1016/j.poly.2022.116219. [DOI] [Google Scholar]
- Al-Gaber M. A. I.; Abd El-Lateef H. M.; Khalaf M. M.; Shaaban S.; Shawky M.; Mohamed G. G.; Abdou A.; Gouda M.; Abu-Dief A. M. Design, Synthesis, Spectroscopic Inspection, DFT and Molecular Docking Study of Metal Chelates Incorporating Azo Dye Ligand for Biological Evaluation. Materials 2023, 16, 897. 10.3390/ma16030897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saghier A.; Abd El-Halim H.; Abdel-Rahman L. H.; Kadry A. Green synthesis of new trizole based heterocyclic amino acids ligands and their transition metal complexes. Characterization, kinetics, antimicrobial and docking studies. Appl. Organomet. Chem. 2019, 33, e4641 10.1002/aoc.4641. [DOI] [Google Scholar]
- Arafath M. A.; Adam F.; Ahamed M. B. K.; Karim M. R.; Uddin M. N.; Yamin B. M.; Abdou A. Ni (II), Pd (II) and Pt (II) complexes with SNO-group thiosemicarbazone and DMSO: Synthesis, characterization, DFT, molecular docking and cytotoxicity. J. Mol. Struct. 2023, 1278, 134887. 10.1016/j.molstruc.2022.134887. [DOI] [Google Scholar]
- Shaaban S.; Abdou A.; Alhamzani A. G.; Abou-Krisha M. M.; Al-Qudah M. A.; Alaasar M.; Youssef I.; Yousef T. A. Synthesis and in silico investigation of organoselenium-clubbed schiff bases as potential Mpro inhibitors for the SARS-CoV-2 replication. Life 2023, 13, 912. 10.3390/life13040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkanzi N. A.; Ali A. M.; Albqmi M.; Abdou A. New Benzimidazole-Based Fe (III) and Cr (III) Complexes: Characterization, Bioactivity Screening, and Theoretical Implementations Using DFT and Molecular Docking Analysis. Appl. Organomet. Chem. 2022, 36, e6868 10.1002/aoc.6868. [DOI] [Google Scholar]
- Alghuwainem Y. A.; El-Lateef H. M. A.; Khalaf M. M.; Amer A. A.; Abdelhamid A. A.; Alzharani A. A.; Alfarsi A.; Shaaban S.; Gouda M.; Abdou A. Synthesis, DFT, Biological and Molecular Docking Analysis of Novel Manganese (II), Iron (III), Cobalt (II), Nickel (II), and Copper (II) Chelate Complexes Ligated by 1-(4-Nitrophenylazo)-2-naphthol. Int. J. Mol. Sci. 2022, 23, 15614. 10.3390/ijms232415614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmonsef A. H.; El-Saghier A. M.; Kadry A. M. Ultrasound-assisted green synthesis of triazole-based azomethine/thiazolidin-4-one hybrid inhibitors for cancer therapy through targeting dysregulation signatures of some Rab proteins. Green Chem. Lett. Rev. 2023, 16, 2150394. 10.1080/17518253.2022.2150394. [DOI] [Google Scholar]
- Abdou A.; Abdel-Mawgoud A. M. M. Synthesis, structural elucidation, and density functional theory investigation of new mononuclear Fe (III), Ni (II), and Cu (II) mixed-ligand complexes: Biological and catalase mimicking activity exploration. Appl. Organomet. Chem. 2022, 36, e6600 10.1002/aoc.6600. [DOI] [Google Scholar]
- Elkanzi N. A.; Ali A. M.; Hrichi H.; Abdou A. New mononuclear Fe (III), Co (II), Ni (II), Cu (II), and Zn (II) complexes incorporating 4-{[(2 hydroxyphenyl) imino] methyl} phenyl-4-methylbenzenesulfonate (HL): Synthesis, characterization, theoretical, anti-inflammatory, and molecular docking investigation. Appl. Organomet. Chem. 2022, 36, e6665 10.1002/aoc.6665. [DOI] [Google Scholar]
- Hossain M.; Khushy K.; Latif M.; Hossen M. F.; Asraf M. A.; Kudrat-E-Zahan M.; Abdou A. Co (II), Ni (II), and Cu (II) Complexes Containing Isatin-Based Schiff Base Ligand: Synthesis, Physicochemical Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis. Russ. J. Gen. Chem. 2022, 92, 2723–2733. 10.1134/s1070363222120222. [DOI] [Google Scholar]
- Latif M.; Ahmed T.; Hossain M. S.; Chaki B.; Abdou A.; Kudrat-E-Zahan M. Synthesis, Spectroscopic Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis of Ni (II), Zn (II), Sb (III), and U (VI) Metal Complexes Derived from a Nitrogen-Sulfur Schiff Base. Russ. J. Gen. Chem. 2023, 93, 389–397. 10.1134/s1070363223020214. [DOI] [Google Scholar]
- Obrecht A. S.; Urban N.; Schaefer M.; Röse A.; Kless A.; Meents J. E.; Lampert A.; Abdelrahman A.; Müller C. E.; Schmalzing G.; et al. Identification of aurintricarboxylic acid as a potent allosteric antagonist of P2X1 and P2X3 receptors. Neuropharmacology 2019, 158, 107749. 10.1016/j.neuropharm.2019.107749. [DOI] [PubMed] [Google Scholar]
- Abd El-Lateef H. M.; Khalaf M. M.; Kandeel M.; Amer A. A.; Abdelhamid A. A.; Abdou A. New Mixed-ligand Thioether-quinoline complexes of Nickel (II), Cobalt (II), and Copper (II): Synthesis, Structural Elucidation, DFT, antimicrobial activity and Molecular docking exploration. Appl. Organomet. Chem. 2023, 37, e7134 10.1002/aoc.7134. [DOI] [Google Scholar]
- Jarad A. J.; Dahi M. A.; Al-Noor T. H.; El-ajaily M. M.; AL-Ayash S. R.; Abdou A. Synthesis, spectral studies, DFT, biological evaluation, molecular docking and dyeing performance of 1-(4-((2-amino-5-methoxy) diazenyl) phenyl) ethanone complexes with some metallic ions. J. Mol. Struct. 2023, 1287, 135703. 10.1016/j.molstruc.2023.135703. [DOI] [Google Scholar]
- Onawole A. T.; Kolapo T. U.; Sulaiman K. O.; Adegoke R. O. Structure based virtual screening of the Ebola virus trimeric glycoprotein using consensus scoring. Comput. Biol. Chem. 2018, 72, 170–180. 10.1016/j.compbiolchem.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Abdou A. Synthesis, Structural, Molecular Docking, DFT, Vibrational Spectroscopy, HOMO-LUMO, MEP Exploration, antibacterial and antifungal activity of new Fe (III), Co (II) and Ni (II) hetero-ligand complexes. J. Mol. Struct. 2022, 1262, 132911. 10.1016/j.molstruc.2022.132911. [DOI] [Google Scholar]
- Abd El-Lateef H. M.; Khalaf M. M.; Kandeel M.; Amer A. A.; Abdelhamid A. A.; Abdou A. Designing, Characterization, biological, DFT, and molecular docking analysis for new FeAZD, NiAZD, and CuAZD complexes incorporating 1-(2-hydroxyphenylazo)-2-naphthol (H2AZD). Comput. Biol. Chem. 2023, 105, 107908. 10.1016/j.compbiolchem.2023.107908. [DOI] [PubMed] [Google Scholar]
- Balouiri M.; Sadiki M.; Ibnsouda S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. A.; Bekhit A. A.; Abd Allah O. A.; Kadry A. M.; Ibrahim T. M.; Bekhit S. A.; Amagase K.; El-Saghier A. M. Synthesis and antimicrobial activity of some novel 1, 2-dihydro-[1, 2, 4] triazolo [1, 5-a] pyrimidines bearing amino acid moiety. RSC Adv. 2021, 11, 2905–2916. 10.1039/d0ra08189b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.











