Abstract
Background:
Biliary complications are the leading cause of morbidity and mortality in patients undergo¬ing Liver Transplantation (LT). Post-biliary transplantation strictures (BSs) are a severe problem with a high risk of graft failure. However, management of these BSs has remained controversial, and consid¬erable variability has been reported in Percutaneous Transhepatic Radiological Interventions (PTRIs) related to broad differences in technical procedures.
Objective:
This study aimed to evaluate the efficacy of percutaneous treatments in managing post-LT BSs in a center in Shiraz.
Methods:
PTRIs including balloon dilatation, metallic stent, and internal or internal-external hand-made plastic stent insertion were done for 34 transplanted patients with BSs referring to the Interventional Radiology Unit of Shiraz Namazi Hospital. Technical success rate, patency rates, and complications were evaluated.
Results:
The. In this study, 31 strictures were successfully treated without any significant difference between the anastomotic and non-anastomotic types of stricture (success rate: 91.2%). Based on the results, 12- , 24-, and 36-month primary patency rates were 90.1%, 84.5%, and 76.8%, respectively. The secondary patency rate was 100% at 12 and 24 months and 93.3% at 36 and 60 months. The rate of minor complica¬tions (mild cholangitis and hemobilia) was 6.4%, and no major complications were detected.
Conclusion:
According to the findings, PTRI is an effective method for treating anastomotic and non-anas- tomotic strictures with a high success rate and low complications.
Key Words: Liver transplant, Biliary stricture, Percutaneous transhepatic radiologic intervention
INTRODUCTION
Biliary complications after liver transplantation are the primary cause of mortality and morbidity in liver transplant patients [1] and the second most common cause of graft dysfunction after rejection [2]. These complications include biliary tract stricture, bile leak, duct stone, and sludge [3-5]. Bile duct strictures are the most common biliary complications [3-7]. Strictures are divided into two categories, namely anastomotic (primarily due to ischemia and technique of anastomosis) and non-anastomotic (due to prolonged cold ischemia time, vascular insufficiency, or immunological process) [8-11]. They can also be classified as early (within one month of transplantation, usually related to a technical problem) and late (after one month of transplantation, mainly secondary to vascular insufficiency) [12-14].
There are different radiological methods to detect duct strictures. Routinely, trans-abdominal sonography is the first choice because of its availability, safety, and low cost [15]. However, Computed Tomography (CT) is more sensitive in detecting biliary malignancies [14]. Another method is Magnetic Resonance Imaging (MRI) or, more specifically, Magnetic Resonance Cholangiopancreatography (MRCP) that is safer than CT scan, since it does not use ionizing radiation for imaging. MRCP is also superior in providing high- quality cholangiogram without contrast [16].
To date, no uniform approach is available for managing the above-mentioned biliary complications [17]. Surgery is the primary choice of treatment. Considering the potential risks of surgery, non-surgical therapeutic techniques (endoscopic and interventional radiologic methods) have been introduced recently [18, 19]. However, the efficacy of these treatments has not been precisely clarified. On the other hand, a high variability has been reported in the results of percutaneous interventional techniques for post-liver transplant Biliary Stricture (BS) treatment, which has been related to the differences in technical procedures [20].
The present study aims to evaluate the efficacy (success rate and primary and secondary patency rates) of Percutaneous Transhepatic Radiological Intervention (PTRI) for post-transplantation BSs.
MATERIALS AND METHODS
Study Population
This study was performed at Shiraz Interventional Radiology Unit, and written informed consent was obtained from all the patients. The study was done on all the patients with BS after liver transplantation who presented with pruritus, jaundice, fever, abdominal pain, and an increase in cholestatic enzymes (serum bilirubin and alkaline phosphatase) who were referred to the center for elective PTRI from September 2008 to September 2013. According to the inclusion criteria, 34 patients (20 males and 14 females) were enrolled into the study. BS was confirmed by ultrasonography, MRCP, or Percutaneous Transhepatic Cholangiography (PTC). If biliary stenosis was not confirmed, the patients were excluded from the study. All PTRIs were performed by an experienced interventional radiologist.
All the patients underwent Doppler sonography of the hepatic artery to exclude any vascular insufficiency as the cause of the stricture. The following data were recorded for all the patients: age, sex, pre-transplant diagnosis, type of donor and biliary reconstruction, and time of BS diagnosis after transplantation. Prothrombin Time (PT), Partial Thromboplastin Time (PTT), and platelet were also checked in all the patients.
Doppler Sonography
Under the guide of sonography via right lateral intercostal or subxiphoid access (according to the site of stenosis), skin, subcutaneous tissue, and hepatic capsule were punctured. At first, 10 cc lidocaine 2% (Caspian, Iran) was injected for local anesthesia. Then, the right or left hepatic duct was punctured using a 20-gauge Chiba needle. Under the guidance of fluoroscopy (Siemens, Germany) and by injection of 5 cc half diluted Ultravist (Bayer Pharma AG, Germany) and visualization of the dilated biliary system, J-wire was advanced and the tract was dilated. After that, an 8-F external biliary drainage tube (Bioteque, Taiwan) was placed within the bile duct and cholangiography was done once again. The site of the stricture was confirmed for further management.
Percutaneous Transhepatic Radiologic Intervention
Balloon Dilation
Following the decrease in the serum level of bilirubin, the patients were scheduled for PTRI (ballooning and stenting). After moderate intravenous conscious sedation with thiopental sodium, midazolam, and morphine sulfate, cholangiography was done via the inserted drainage tube. Then, a 0.035 hydrophilic Terumo wire (Tokyo, Japan) was entered into the bile duct and a 6-F arterial sheath was inserted. With the support of a 5-F vertebral stenosis was crossed with a 0.035 hydrophilic Terumo wire and was dilated with a Passeo balloon catheter (Biotronik, Switzerland). The size of the balloon catheter was selected from 7 mm to 10 mm according to the bile duct size. The balloon was inflated to reach the pressure required to eliminate a balloon waist for about 15 min, so as to overcome the elastic recoil of the duct and control the probable hemorrhage. If pressure was not adequate to reduce the waist, a high-pressure balloon was substituted. After the dilatation of the stricture site, the sphincter of Oddi was dilated with the same balloon in all the patients in order to overcome papillary dysfunction, as a common complication of liver transplantation. Considering the stricture site and type of duct reconstruction, 12-F internal or internal-external biliary drainage catheters were left across the stenosis to preserve the lumen during the healing process.
Internal Plastic Stent
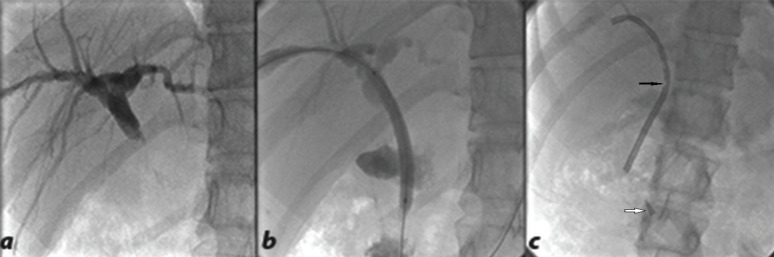
The hand-made internal plastic stent was applied to the choledochocholedochostomy (C-C) type of reconstruction and was derived from modifying the external drainage tube (Bioteque, Taiwan). Its length was measured from the entrance point up to the duodenum (destination point) plus 10 cm (for preventing migration), and hand-made pores were applied on its course for better drainage. The curved end of the stent had to be lodged within the intrahepatic duct to avoid displacement and its such a way that it reduced trauma to the bile duct (Fig 1).
Figure 1.
Internal plastic stent. (a) Percutaneous transhepatic cholangiography showed biliary dilatation due to post-transplantation anastomotic stricture. (b) Balloon dilatation of the stricture site. (c) Hand-made internal plastic stent deployed properly. The black arrow shows the mentioned handmade pore and the white arrow points to the sharp tip of the curved end of the drainage tube that was cut and inserted to reduce duct traumatization on the entrance.
Internal-external Plastic Stent
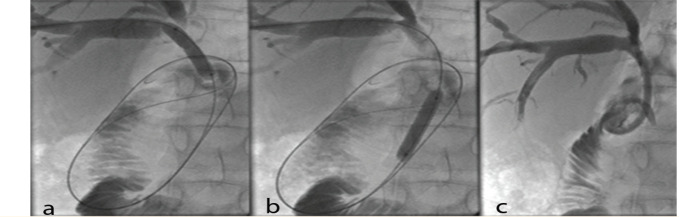
An internal-external plastic stent (Bioteque, Taiwan) was inserted in the Roux-en-y choledochojejunostomy (R-Y) type of reconstruction. It was preferred not to insert an internal stent in this sort of anastomosis, since the endoscope had not accessed the jejunum for removal after the healing process. The stent's internal-external curved end was located within the jejunum, and pores were applied on its course from the proximal end up to the jejunum (Fig 2). It was capped for internal drainage and opened only if fever or bile leakage occurred. The patients were advised to inject 20 cc of normal saline within the stent three times a day for one week and then once a day to prevent stone or sludge formation.
Figure 2.
Internal-external plastic stent. Balloon dilatation of the stricture sites at (a) the proximal part and (b) the distal part of the common bile duct (c) with subsequent internal-external plastic stent insertion.
Metallic Self-expandable Stent
Metallic self-expandable stents were used only in the first cases.
Post PTRI Evaluations
The success or failure rate after PTRI was assessed. Technical success for the intervention was defined as the free passage of the contrast to the bowel and improvement of signs and symptoms as well as liver function tests.
The patients were followed for clinical assessments and laboratory examinations every complications such as sepsis, hemobilia, bile leak, pneumothorax, fistula, and death. Ultrasonography and MRCP were done if recurrence was suspected. Finally, the internal stent was removed endoscopically after six months and the internal-external stent was removed percutaneously after three months when bile duct patency was confirmed. The patients were also assessed in terms of recurrence by primary and secondary patency. Primary patency was defined as the time from the first PTRI to the diagnosis of the recurrence or obstruction, while secondary patency was defined as the total time from the first PTRI to the end of follow-up [21].
Ethical Considerations
This research was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (code: IR.SUMS.MED. REC.1392.6319).
Statistical Analysis
Statistical analysis was performed using the SPSS software, version 16. Fisher's exact test and Kaplan-Meier test were used to analyze the success rate and primary and secondary patency rates, respectively. Additionally, the log-rank test was used to determine the differences between the primary and secondary patency rates and different treatment methods. P-values less than 0.05 were considered statistically significant.
RESULTS
This study was conducted on 34 post-liver transplantation patients [20 males (58.8%) and 14 females (41.2%)] with the mean age of 28 years (range: 2-58 years) who were referred to the Interventional Radiology Unit of Shiraz Namazi Hospital for elective percutaneous management. End-stage liver disease with the underlying cause of cryptogenic (29.4%), autoimmune hepatitis (17.6%), viral hepatitis (B and C) (14.7%), primary sclerosing cholangitis (14.7%), and miscellaneous (23.5%) was the main indication for transplantation. Additionally, 27 (79.4%) cadaveric and 7 (20.6%) living donor transplantations were present with the same frequency of both types of biliary reconstruction; i.e., choledochocholedochostomy (C-C) and Roux-en-y choledochojejunostomy (R-Y) (17 patients in each group).
Among the patients, 24 presented with anastomotic strictures (70.6%), one (2.4%) presented with non-anastomotic stricture, and nine (26.5%) showed both anastomotic and non-anastomotic narrowing. Among the 24 patients with anastomotic strictures, just one site of stricture was delineated; eight patients had two sites and two were complicated with three sites of BSs. The median time interval between liver transplantation and the onset of stricture was 48.25 months (0.5-96). Ultrasonography and MRCP revealed the dilatation of bile ducts above the stricture site in all the patients.
The technical success rate was 91.2%; PTRI step, balloon dilation was done once in all the patients. Then, based on the type of biliary reconstruction and site of stricture, 29.4% (n=10), 41.2% (n=14), and 14.7% (n=5) of the patients underwent internal handmade plastic stent insertion, internal-external plastic stent, and metallic expandable stent, respectively. Two patients were treated solely with balloon dilation, because no elastic recoil was noted after 15 minutes of ballooning. However, the procedure was failed in three patients due to severe strictures that did not allow the passage of the guidewire. All the strictures in this group, which occurred 60 months after liver transplantation, belonged to the R-Y group of reconstruction. After failure, the patients un-derwent surgery for hepato-jejunostomy re-construction.
In the successful group, all the patients showed the free passage of the contrast agent through the site of the previous stricture into the bowel loop in cholangiography immediately after PTRI. Cholestatic enzymes also improved significantly within three to five days, alleviating the signs and symptoms.
Among the patients, 25 (80.6%) were recurrence-free until the end of the follow-up, but six (19.4%) became symptomatic and showed a rise in cholestatic enzymes, and recurrence was approved after diagnostic imaging.
The primary patency rate was 93.5%, 90.1%, 84.5%, 76.8%, and 64% at 6, 12, 24, 36, and 48 months, respectively. The secondary patency rate was 100% at 12 and 24 months and 93.3% at 36 and 60 months.
No major complications occurred, but two minor complications were noted that were controlled conservatively. No long-term PTRI-related morbidity or mortality was seen, as well. During the study, five irrelevant deaths occurred; three due to infection, one graft rejection, and one head trauma.
In this study, four groups were noted according to the type of the percutaneous intervention (Fig 3):
Figure 3.
Patient classification according to the PTRi success and approach after balloon dilation, number of complication (C) and recurrence (R), mean primary and secondary patency in each group. PTRi= percutaneous transhepatic radiologic intervention, #C= number of cases with complication after PTRi, #R= number of cases with recurrence after PTRi, MPP= mean primary patency, MSP=mean secondary patency
1) Two patients were treated only with balloon dilation with the time intervals of about 4 and 96 months between transplantation and BS, without complications or recurrence within 12- and 24-month follow-ups.
2) Ten patients were managed with balloon dilation and internal plastic stent and just one had the R-Y type of anastomosis. Since the patient was a candidate for hepatojejunostomy reconstruction surgery in the next few months, the internal stent was deployed for the temporary alleviation of the symptoms. One patient became febrile after PTRI and responded to intravenous antibiotic without progression to sepsis. The means of primary and secondary patency in this group were 24.9 and 30.9 months, respectively. Obstruction recurred in two patients after seven months that was re-treated percutaneously with balloon dilation, with a 36-month secondary patency.
3) Fourteen patients underwent balloon dilation and internal-external plastic stent insertion. Among these patients, nine had R-Y and five had C-C anastomosis. Due to the acute angle between the bile duct and duodenum, internal stent insertion was not feasible in these five patients. One recurrence case was noted in this group after two months that was managed percutaneously with balloon dilation with good results until the end of the study (24 months). This patient was complicated with mild hemobilia after the first intervention that was subsided without intervention. In this group, the mean primary patency was 25.07 months and the mean secondary patency was 26.7 months.
4) The metallic expandable stent was inserted after balloon dilatation in five patients among the first cases without complications. In this group, the recurrence rate was 60%; after two years in the first case, after three years in the second case, and after four years in the third case. The first case was managed endoscopically, while the two others were managed per-cutaneously. The secondary patency of these three cases was 48, 45, and 84 months, respectively without further complications.
The results revealed no statistically significant relationships between the technical success rate and the type of anastomosis (p=0.227), site of stricture (p=1), and number of strictures (p=1). Additionally, no significant difference was observed between the cadaveric donor and living donor liver transplantations in terms of the success of PTRI (p=0.511). Nonetheless, a statistically significant decrease was found in the technical success rate with the increase in the time interval between liver transplantation and PTRI (p=0.01). The mean delay time was 13 months in the successful group and 84 months in the failed group. There was no significant relationship between the delay time and recurrence rate (p=0.981).
The results showed no significant difference between different PTRI groups with respect to the primary and secondary patency (Table 1). The results also indicated no significant relationship between the type of PTRI and recurrence (p=0.081) as well as between the kind of anastomosis and recurrence (p=0.571). Moreover, the primary and secondary patency rates were not significantly different regarding the type of anastomosis (p=0.725 and p=0.739, respectively).
Table 1.
Primary and Secondary Patency with Different Types of PTRI.
| PTRI types | Internal plastic stent(n= 10) | Internal-external plastic stent(n= 14) | BD(n= 2) | Metallic stent(n= 5) | P-value |
|---|---|---|---|---|---|
| MPP and MSP | |||||
| MPP (month) | 24.9(11.4-38.3) | 25.07(13.6-36.4) | 18(0-94.2) | 38.6(10.2-66.9) | 0.538 |
| MSP (month) | 30.9(19.5-42.2) | 26.7(16-37.4) | 18(0-94.2) | 52.4(18.3-86.4) | 0.189 |
Note: 95% confidence intervals are shown in parentheses.
MPP= mean primary patency
MSP= mean Secondary patency
DISCUSSION
Biliary tract stricture is a challenging diagnostic and therapeutic issue after liver transplantation, leading to infection, graft failure, and death [22, 23]. In the previous studies, the median time interval between liver transplantation and BS was 19 months [20]. This measure was obtained as 48.2 months in the present study. The best technique for the man¬agement of BS is controversial, and the choice of treatment depends on the type of anastomotic reconstruction and accessibility of stricture [22]. In one study, surgery was found to be the best treatment for late strictures [5]. Some centers also manage BSs through the surgical approach. However, due to its shortterm morbidity and complications (reported as 26% by Davidson et al. [24] and 64% by Schilitt et al. [25]), it should be performed if less invasive methods such as endoscopic or radiological procedures fail [21].
Up to now, different success rates have been reported for endoscopic treatments: 50% by Pfau et al. [4], 90% by Rizk et al. [26], 100% by Park et al. [27], and 88% by Zoepf et al. [28]. However, this method was accompanied by some problems. For instance, the used plastic stents were needed to repeat replacement with a short interval to prevent obstruction and infection [26, 28]. Additionally, the R-Y type of reconstruction had no chance of endoscopic management.
PTRI has been performed increasingly in the recent years with different success rates, mostly attributed to different techniques [20]. The overall technical success rate was reported as 90% by Giampalma et al. [20], 89% by Zajko et al. [29], 85% by Saad et al. [30], and 78% by Park et al. [27]. In the present study, the success rate was 91.2%, which was higher compared to the previous studies. However, similar to the previous studies [20, 29], the results revealed no significant relationship between the success rate and the type of anastomosis (p=0.227).
In the current research, the overall primary patency rate was 90%, 84.5%, and 76.8% at one, two, and three years, respectively, which were higher than the measures reported by Roumilhac et al. [21] and Zajko et al. [29]. Giampalma et al. also revealed the primary potency of 94%, 79%, and 45% at one, two, and three years, respectively [20].
Roumilhac et al. demonstrated that the secondary patency rate was 88% at one year [21]. In addition, Giampalma et al. showed 94% patency at one and two years and 83% potency at three years. The present study revealed the potency of 100% at one and two years and 93.3% at three years.
In the research carried out by McDonald et al., 13 post-transplant strictures underwent balloon dilation alone with five failure cases [31]. Zajko et al. indicated that the initial success rate of balloon dilation was 89%, with 12% complications [29]. Balloon dilation alone has been found to be successful initially, but with a high rate of recurrence [20]. In the present investigation, balloon dilation alone was done in two patients, revealing excellent response, no recurrence, and 100% patency at one and two years. In the study performed by Roumilhac et al., 14 patients underwent balloon dilation, with the primary patency of 71% at one year and 61.2% at two years [32]. Zajko et al. also showed the potency of 80% at six months among 72 patients [29].
Considering the high recurrence rate associated with balloon dilation alone [28, 32], subsequent stent placement was preferred in the present research in order to increase the patency. Uncovered metallic self-expandable stents were utilized in five patients in early cases. Due to the mentioned problems in the literature (obstruction due to mucosal hyperplasia or stone-sludge) and difficulty with its removal in the case of surgery [33], metallic stents were not used, except for malignant strictures.
In case of a delay for more than five months, a combination of balloon dilation and stent insertion has been found to be more helpful [28]. Culp et al. revealed an 88% success rate for six months with the primary patency rate of 44% at three years and the secondary patency rate of 88% at five years [34]. Roumilhac et al. disclosed a 27% primary patency at two years [21]. On the other hand, Peterson et al. recorded a 100% success rate by using self-expanding stents in refractory strictures, and two of them needed subsequent balloon dilation [35]. Furthermore, Norman et al. obtained the primary patency of 50% and the secondary patency of 80% at 18 months [36]. Due to the previously mentioned problems, Giampalma et al. deployed stents in the strictures that were not responsive to balloon dilation as well as in those that refused surgical revision [20]. In the current research, the primary patency was 100%, 75%, and 50% at one, two, and three years, respectively in the metallic stent group. In addition, the secondary patency was 100% at one year. No migration was detected. In Peterson's research, stent-graft was deployed successfully in eight refractory strictures with two delayed movements. After removal, seven patients recurred a significant stricture [37]. Fortunately, there was no need for surgery in this group in the present study. The insignificant results of the statistical tests might result from the small number of patients.
In the majority of cases in the present investigation, after one attempt of balloon dilation, plastic internal or internal-external stents were inserted according to the accessibility of strictures and the type of anastomosis. The results revealed a primary patency rate of 90% and 78% at six months, one year, and three years and a secondary patency rate of 100% at one and three years in the internal stent group. In the internal-external stent group, the primary patency was 92% at six months, one year, and three years and the secondary patency rate was 100% at two years and 83% at three years. These excellent primary and secondary patency rates were attributed to several factors. Firstly, balloon dilation of the stricture site was done only once to reduce the chance of scar formation, and inflation lasted for 15 minutes. Giampalma et al. inflated the balloon two-three times, each lasting for one-five minutes, and repeated it in several sessions [20]. In another research, Roumilhac et al. did one-three balloon dilations in each patient [34]. Zajko et al. [29] and Saad et al. [30] also performed a maximum of three sessions for each dilatation procedure. Thus, less frequent balloon dilation led to a lower degree of traumatization and, subsequently, fibrosis. Besides, an increase in the time of dilatation was accompanied by a decrease in the chance of hemorrhage, thereby overcoming the elastic recoil. Secondly, internal or internal-external plastic stents were used according to the type of anastomosis and the accessibility of stricture rather than metallic stents or complex surgery. The internal and internal-external stents were removed after six months and three-six months, respectively to maintain the lumen during the healing process. The internal-external drainage was to some ex-tent similar to that performed by Giampalma et al., but they removed the drain when the patient remained asymptomatic without new abnormalities in the liver function test after two weeks of capping [20]. Thirdly, the internal stents were hand-made with a good patency. As mentioned earlier, they were designed not to migrate to the bowel and could be removed by endoscopy whenever needed. These stents have not been used in otherexperiences. Fourthly, dilatation and stent deployment were done with minor trauma to the biliary system by an experienced radiologist. Only one mild hemobilia was noted, which was relieved spontaneously without major procedure-related complications. In one study, 15% complications were noted after PTRI, one requiring the coil embolization of pseudoaneurysm formation and the other needing video-assisted thoracoscopic surgery for hemothorax [27]. Fifthly, washing the internal-external draining tube seemed to be an effective way to decrease the chance of sludge-stone formation and reduce the likelihood of further obstructions. Sixthly, external biliary drainage for at least two days prior to balloon dilation and stent insertion was effective in reducing the procedure-related complications. Seventhly, the low rate of complications (major: 0%, minor: 6.4%) seemed to result from the low risk of traumatization of the biliary system and prophylactic antibiotic administration. Roumilhac et al. encountered 14 complications in 24 patients [34]. Giam- palma et al. also recorded 12% minor and 4% major complications [20].
In the study conducted by Thethy et al., the success rate was lower in late strictures (more than six months) than in early ones [5]. Similarly, the present study findings indicated a significant decrease in the success rate with the increase in the time interval between liver transplantation and PTRI.
In this study, there were no significant differences between different PTRI groups concerning the primary and secondary patency and recurrence rates. This might be associated with the relatively small number of patients in the two groups. Hence, further studies with larger sample sizes are warranted. Additionally, since this was a single-center study with a small number of patients, future evaluations are recommended to be performed on a larger number of patients in other centers.
In conclusion, the study findings demonstrated that PTRI was a safe and efficient management method of post-liver transplant BSs, with high success and primary and secondary patency rates.
CONFLICTS OF INTEREST:
None declared.
FINANCIAL SUPPORT:
None.
ACKNOWLEDGMENTS
The present research project (number 6319) was approved by the Vice-chancellor for Research Affairs of Shiraz University of Medical Sciences, Shiraz, Iran. Hereby, the authors would like to thank Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for her invaluable assistance in editing the manuscript.
References
- 1.Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol. 2008;14:493. doi: 10.3748/wjg.14.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quiroga S, Sebastià MC, Margarit C, et al. Complications of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics. 2001;21:1085–102. doi: 10.1148/radiographics.21.5.g01se061085. [DOI] [PubMed] [Google Scholar]
- 3.Rerknimitr R, Sherman S, Fogel EL, et al. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224–31. doi: 10.1067/mge.2002.120813. [DOI] [PubMed] [Google Scholar]
- 4.Pfau PR, Kochman ML, Lewis JD, et al. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55–63. doi: 10.1067/mge.2000.106687. [DOI] [PubMed] [Google Scholar]
- 5.Thethy S, Thomson BN, Pleass H, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647–53. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 6.Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol. 2006;41:89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 7.Albert JG. Biliary Complications After Liver Transplantation. Endotherapy in Biliopancreatic Diseases: ERCP Meets EUS: Springer; 2020. pp. 483–8. [Google Scholar]
- 8.Guichelaar MM, Benson JT, Malinchoc M, et al. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885–90. doi: 10.1034/j.1600-6143.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 9.Lemmers A, Pezzullo M, Hadefi A, et al. Biliary cast syndrome after liver transplantation: A cholangiographic evolution study. J Gastroenterol Hepatol. 2021;36:1366–77. doi: 10.1111/jgh.15318. [DOI] [PubMed] [Google Scholar]
- 10.Moench C, Uhrig A, Lohse AW, Otto G. CC chemokine receptor 5Δ32 polymorphism—a risk factor for ischemic-type biliary lesions following orthotopic liver transplantation. Liver Transplant. 2004;10:434–9. doi: 10.1002/lt.20095. [DOI] [PubMed] [Google Scholar]
- 11.Kim YM, Chung TR, Lee DK. High-Level Biliary Strictures After Living-Donor Liver Transplantation. Advanced ERCP for Complicated and Refractory Biliary and Pancreatic Diseases. Springer; 2020. pp. 1–16. [Google Scholar]
- 12.Sanchez-Urdazpal L, Gores GJ, Ward EM, et al. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49–53. doi: 10.1002/hep.1840160110. [DOI] [PubMed] [Google Scholar]
- 13.Magro B, Tacelli M, Mazzola A, et al. Biliary complications after liver transplantation: current perspectives and future strategies. Hepatobiliary Surg Nutr. 2021;10:76. doi: 10.21037/hbsn.2019.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viesca MFY, Arvanitakis M. Early diagnosis and management of malignant distal biliary obstruction: a review on current recommendations and guidelines. Clin Exp Gastroenterol. 2019;12:415. doi: 10.2147/CEG.S195714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Håkansson, Leander P. MR and ultrasound in screening of patients with suspected biliary tract disease. Acta Radiologica. 2002;43:80–6. doi: 10.1080/028418502127347493. [DOI] [PubMed] [Google Scholar]
- 16.Aggag MF, Shehata MSAA, Badawy ZESES. Role of magnetic resonance cholangiopancreatography in evaluation of biliary obstruction. Egypt J Hosp Med. 2019;74:550–7. [Google Scholar]
- 17.Karimian N, Westerkamp AC, Porte RJ. Biliary complications after orthotopic liver transplantation. Curr Opin Organ Transplant. 2014;19:209–16. doi: 10.1097/MOT.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 18.Hintze RE, Adler A, Veltzke W, et al. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepato-gastroenterology. 1997;44:258–62. [PubMed] [Google Scholar]
- 19.Olisov O. Biliary complications after orthotopic liver transplantation. Transplantologiya Russ J Transplant. 2018;2:44–50. [Google Scholar]
- 20.Giampalma E, Renzulli M, Mosconi C, et al. Outcome of post–liver transplant ischemic and nonischemic biliary stenoses treated with percutaneous interventions: The bologna experience. Liver Transplant. 2012;18:177–87. doi: 10.1002/lt.22450. [DOI] [PubMed] [Google Scholar]
- 21.Roumilhac D, Poyet G, Sergent G, et al. Long-term results of percutaneous management for anastomotic biliary stricture after orthotopic liver transplantation. Liver Transplant. 2003;9:394–400. doi: 10.1053/jlts.2003.50052. [DOI] [PubMed] [Google Scholar]
- 22.Testa G, Malagò M, Broelsch CE. Complications of biliary tract in liver transplantation. World J Surg. 2001;25:1296–9. doi: 10.1007/s00268-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 23.Boraschi P, Donati F, Pacciardi F, et al. Biliary complications after liver transplantation: assessment with MR cholangiopancreatography and MR imaging at 3T device. Europ J Radiol. 2018;106:46–55. doi: 10.1016/j.ejrad.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Davidson BR, Rai R, Nandy A, et al. Results of choledochjejunostomy in the treatment ofbiliary complications after liver transplantation in the era of nonsurgical therapies. Liver Transplant. 2000;6:201–6. doi: 10.1002/lt.500060215. [DOI] [PubMed] [Google Scholar]
- 25.Schlitt HJ, Meier PN, Nashan B, et al. Reconstructive surgery for ischemic-type lesions at the bile duct bifurcation after liver transplantation. Annal Surg. 1999;229 doi: 10.1097/00000658-199901000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizk RS, McVicar JP, Emond MJ, et al. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc. 1998;47:128–35. doi: 10.1016/s0016-5107(98)70344-x. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Kim M-H, Lee SK, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78–85. doi: 10.1067/mge.2003.11. [DOI] [PubMed] [Google Scholar]
- 28.Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Balloon dilatation vs balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transplant. 2006;12:88–94. doi: 10.1002/lt.20548. [DOI] [PubMed] [Google Scholar]
- 29.Zajko AB, Sheng R, Zetti GM, et al. Transhepatic balloon dilation of biliary strictures in liver transplant patients: a 10-year experience. J Vasc Interv Radiol. 1995;6:79–83. doi: 10.1016/s1051-0443(95)71063-6. [DOI] [PubMed] [Google Scholar]
- 30.Saad WE, Saad NE, Davies MG, et al. Transhepatic balloon dilation of anastomotic biliary strictures in liver transplant recipients: the significance of a patent hepatic artery. J Vasc Interv Radiol. 2005;16:1221–8. doi: 10.1097/01.RVI.0000173281.69988.57. [DOI] [PubMed] [Google Scholar]
- 31.McDonald V, Matalon TA, Patel SK, et al. Biliary strictures in hepatic transplantation. J Vasc Interv Radiol. 1991;2:533–8. doi: 10.1016/s1051-0443(91)72237-9. [DOI] [PubMed] [Google Scholar]
- 32.Mahajani R, Cotler S, Uzer M. Efficacy of endoscopic management of anastomotic biliary strictures after hepatic transplantation. Endoscopy. 2000;32:943–9. doi: 10.1055/s-2000-9619. [DOI] [PubMed] [Google Scholar]
- 33.Lopez RR, Cosenza CA, Lois J, et al. Long-term results of metallic stents for benign biliary strictures. Arch Surg. 2001;136:664–9. doi: 10.1001/archsurg.136.6.664. [DOI] [PubMed] [Google Scholar]
- 34.Culp WC, McCowan TC, Lieberman RP, et al. Biliary strictures in liver transplant recipients: treatment with metal stents. Radiology. 1996;199:339–46. doi: 10.1148/radiology.199.2.8668775. [DOI] [PubMed] [Google Scholar]
- 35.Petersen BD, Maxfield SR, Ivancev K, et al. Biliary strictures in hepatic transplantation: treatment with self-expanding Z stents. J Vasc Interv Radiol. 1996;7:221–8. doi: 10.1016/s1051-0443(96)70765-0. [DOI] [PubMed] [Google Scholar]
- 36.Diamond NG, Lee SP, Niblett RL, et al. Metallic stents for the treatment of intrahepatic biliary strictures after liver transplantation. J Vasc Interv Radiol. 1995;6:755–61. doi: 10.1016/s1051-0443(95)71181-2. [DOI] [PubMed] [Google Scholar]
- 37.Petersen BD, Timmermans HA, Uchida BT, et al. Treatment of refractory benign biliary stenoses in liver transplant patients by placement and retrieval of a temporary stent-graft: work in progress. J Vasc Interv Radiol. 2000;11:919–29. doi: 10.1016/s1051-0443(07)61812-0. [DOI] [PubMed] [Google Scholar]