Abstract
Background
Hypertensive disorders of pregnancy, gestational diabetes, and having a small‐for‐gestational‐age baby are known to substantially increase a woman's risk of cardiovascular disease. Despite this, evidence for models of care that mitigate cardiovascular disease risk in women with these pregnancy‐related conditions is lacking.
Methods and Results
A 6‐month prospective cohort study assessed the effectiveness of a multidisciplinary Women's Heart Clinic on blood pressure and lipid control in women aged 30 to 55 years with a past pregnancy diagnosis of hypertensive disorders of pregnancy, gestational diabetes, or a small‐for‐gestational age baby in Melbourne, Australia. The co‐primary end points were (1) blood pressure <140/90 mm Hg or <130/80 mm Hg if diabetes and (2) total cholesterol to high‐density lipoprotein cholesterol ratio <4.5. The study recruited 156 women with a mean age of 41.0±4.2 years, 3.9±2.9 years from last delivery, 68.6% White, 20.5% South/East Asian, and 80.5% university‐educated. The proportion meeting blood pressure target increased (69.2% to 80.5%, P=0.004), with no significant change in lipid targets (80.6% to 83.7%, P=0.182). Systolic blood pressure (−6.9 mm Hg [95% CI, −9.1 to −4.7], P<0.001), body mass index (−0.6 kg/m2 [95% CI, −0.8 to −0.3], P<0.001), low‐density lipoprotein cholesterol (−4.2 mg/dL [95% CI, −8.2 to −0.2], P=0.042), and total cholesterol (−4.6 mg/dL [95% CI, −9.1 to −0.2] P=0.042) reduced. Heart‐healthy lifestyle significantly improved with increased fish/olive oil (36.5% to 51.0%, P=0.012), decreased fast food consumption (33.8% to 11.0%, P<0.001), and increased physical activity (84.0% to 92.9%, P=0.025).
Conclusions
Women at high risk for cardiovascular disease due to past pregnancy‐related conditions experienced significant improvements in multiple cardiovascular risk factors after attending a Women's Heart Clinic, potentially improving long‐term cardiovascular disease outcomes.
Registration
URL: https://www.anzctr.org.au; Unique identifier: ACTRN12622000646741.
Keywords: cardiovascular disease, pregnancy, women, Women's Heart Clinic
Subject Categories: Cardiovascular Disease, Lifestyle, Pregnancy, Primary Prevention
Nonstandard Abbreviations and Acronyms
- GD
gestational diabetes
- HDP
hypertensive disorders of pregnancy
- TC
total cholesterol
- WHC
Women's Heart Clinic
Clinical Perspective.
What Is New?
This is the first study evaluating the effectiveness of female‐specific cardiovascular health care services, namely Women's Heart Clinics, in managing cardiovascular disease risk in women with past pregnancy conditions.
We found these centers identified a large proportion of women with undertreated hypertension and hypercholesterolemia, and following a Women's Health Clinic intervention, women had significant improvements in blood pressure, lipids, body mass index, waist circumference, and adherence to a heart‐healthy lifestyle.
What Are the Clinical Implications?
These clear benefits should encourage further research into Women's Heart Clinics globally and a focus on this undermanaged high‐risk female population.
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality globally in both women and men. 1 However, women are less likely than men to have cardiovascular risk factors recognized and treated early in life. 2 , 3 In addition, women can have sex‐specific conditions that identify them as high risk for CVD such as a history of premature menopause (before age 40 years) as well as pregnancy‐associated conditions of gestational hypertension/preeclampsia (termed hypertensive disorders of pregnancy [HDP]), 4 , 5 , 6 gestational diabetes (GD) 7 , 8 , 9 , 10 and having small‐for‐gestational age (SGA) babies. 11 , 12 , 13 , 14 , 15 These common pregnancy‐related conditions fall under the umbrella of “risk‐enhancing factors,” whereby they independently identify women at high future risk for atherosclerotic CVD. Unfortunately, there is a lack of awareness of the role these pregnancy‐related conditions play in a woman's CVD risk assessment, both in patients and their health care providers. 16 , 17 , 18 As a result, women with past pregnancy‐related complications are often lost to follow‐up without ongoing monitoring of blood pressure (BP) or screening for type 2 diabetes (T2D). 19 , 20 , 21 This is particularly evident in young women and underserved populations such as lower socioeconomic and ethnic minority groups. 22 , 23
The American College of Cardiology guidelines on the primary prevention of CVD, stress the importance of incorporating a pregnancy history. 24 The 2022 update to the Australian CVD risk calculator 25 provides information on the impact of pregnancy‐related conditions for CVD risk in women for the first time. Although these tools serve to improve health care provider awareness, they do not offer specific guidance on what additional cardiovascular care should be provided. With both a high burden and a rising rate of HDP 26 and GD, 27 , 28 there is an unmet need to develop interventions that target women postpartum to improve detection and treatment of cardiovascular risk factors.
Interventions focused on prevention of CVD in women with these past pregnancy conditions are scarce. A systematic review found only 2 randomized clinical trials for women with HDP; one was an online education program for lifestyle advice 29 ; and similarly for women with GD, interventions have largely been lifestyle advice alone. 30 , 31 Women's Heart Clinics (WHCs) have become established in several tertiary institutions in the United States. 32 , 33 , 34 The driver for this model of care is the recognition that heart disease in women has been historically underrecognized and undertreated by health care providers 35 and that women are largely unaware of their risk. 22 However, this health care service is rarely used in other countries and supportive evidence is lacking. 36 We therefore aimed to assess the efficacy of a multidisciplinary WHC on cardiovascular risk factor control in women with past pregnancy‐related conditions of HDP (gestational hypertension and preeclampsia), GD, or SGA babies.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design
This was a prospective before and after cohort study, aiming to recruit 150 women with a past pregnancy‐related condition, assessed at baseline and 6 months, after attending a WHC at 1 of 3 cardiovascular sites in Melbourne, Australia. All women gave their written informed consent. The trial was approved by the Monash Health Human Research Ethics Committee (NMA/ERM Ref No 57226) and was registered on the Australia New Zealand Clinical Trial registry (registration number: ACTRN12622000646741) before the end of follow‐up and before data analysis was conducted. The study protocol was amended before the first participant recruitment from 12‐month to 6‐month follow‐up due to COVID‐19 lockdowns. A statistical analysis plan was finalized before database lock and before data analysis was conducted.
Participants
Women aged 30 to 55 years, with a past diagnosis of HDP (gestational hypertension or preeclampsia), GD, or having had an SGA baby were eligible. Women from the community were approached via text message or email, using an obstetric database from each hospital site, identifying women having the aforementioned conditions who had given birth between January 1, 2013 and December 31, 2020. Women with known CVD, unstable medical conditions that would preclude diet and exercise participation, and cognitive impairment were excluded. Due to the influence of pregnancy and lactation on biochemical markers, women who were currently or recently (within 12 months) pregnant/breastfeeding or planning a pregnancy within the study period were excluded.
Intervention
Women were assessed and managed in person or via telehealth in the WHC by a cardiologist, a cardiovascular nurse, and a dietitian, where indicated. All women attended at least 1 visit, with further visits as clinically indicated. Women were provided education on the relationship between their specific pregnancy‐related condition and long‐term risk of CVD. Women underwent screening for, and management of, traditional risk factors, including T2D, hypertension, hyperlipidemia, smoking, overweight/obesity, and metabolic syndrome (elevated fasting glucose, insulin resistance, and elevated triglycerides). Verbal and written information on standard cardiovascular risk factors, diet, including limiting salt intake, and exercise were given, 37 , 38 as well as encouragement to adopt long‐term weight control strategies. Patients with elevated BP, fasting glucose levels, hemoglobin A1c, and dyslipidemia were prescribed lifestyle intervention by the WHC cardiovascular nurse, including verbal advice on components of a heart‐healthy diet (eg, a Mediterranean diet pattern), advice on dietary intake beneficial for hypertension (eg, the Dietary Approaches to Stop Hypertension diet) as well as advice on exercise tailored to the individual. Links to online resources were provided (Australian National Heart Foundation website with links to meal plans and walking groups). Further individualized dietary advice was provided through dietitian visits for those women who had a clinical indication (eg, obesity or diabetes). Medical therapy was initiated after a trial of lifestyle changes as clinically indicated with a discussion of plans for future pregnancy in the context of statins or antihypertensive therapy.
Outcomes
The co‐primary end points were the proportion of women who met guideline‐directed primary prevention CVD targets of (1) BP < 140/90 mm Hg (<130/80 mm Hg if known diabetes); and (2) total cholesterol to high‐density lipoprotein cholesterol ratio (TC:HDL‐C) <4.5. These were selected as they are both used in the primary prevention Australian CVD risk calculator (rather than low‐density lipoprotein cholesterol [LDL‐C] or triglycerides). 39 , 40 BP was measured as an average of 3 readings with an automatic BP cuff and the participant sitting in a quiet room. Fasting blood samples for lipids and diabetic markers were obtained at baseline and final follow‐up. Secondary end points included systolic and diastolic BP, TC, LDL‐C, HDL‐C, triglyceride levels, hemoglobin A1c, body mass index (BMI), weight, and waist circumference as well as diet and exercise levels. Diet and exercise levels were obtained from self‐report based on the preceding 7 days. Medication use and smoking information was self‐reported. Secondary end points also included a new diagnosis of hypertension, hypercholesterolemia, T2D, and impaired glucose tolerance/insulin resistance. New hypertension was defined as BP >140/90 mm Hg or BP >130/80 mm Hg for women with diabetes and confirmed with 24‐hour BP monitor where indicated. Hypercholesterolemia was defined as a fasting TC >5.5 mmol/L (212.7 mg/dL) or LDL‐C >3.5 mmol/L (135.3 mg/dL). T2D was defined as a hemoglobin A1c >6.4%. Impaired glucose tolerance or insulin resistance was defined as a fasting glucose of 5.6 to 6.8 mmol/L (102.6–124.2 mg/dL), hemoglobin A1c 5.7% to 6.4% or fasting insulin >20 mU/L.
Statistical Analysis
The study was designed to recruit 150 women to have 80% power to detect a 15% increase in the proportion meeting BP and lipid targets, using a type I error (alpha) of 0.025 for each co‐primary end point and allowing for 5% loss to follow‐up. Statistical significance for the co‐primary end points was defined as either or both end points having a P value <0.025, thus providing an overall alpha of 0.05. A McNemar test was used to assess the change from baseline to 6 months for the dichotomous outcomes and a paired t test was used for the continuous variables. All analyses were performed using R statistical software. 41
RESULTS
A total of 156 women were prospectively enrolled from May 2021 to April 2022 with follow‐up completed in October 2022. Women were on average 41.0 (±4.2) years old, with 23.1% having HDP only (including preeclampsia and gestational hypertension), 60.3% having GD only, 13.5% both HDP/GD, and 3.2% having an SGA baby. Women were on average 3.9 (±2.9) years postpartum from their last delivery. Women were seen at WHCs located in metropolitan Melbourne: Victorian Heart and Lung Clinic (43.6%), Alfred Hospital (26.9%), and Cabrini Hospital (29.5%). Our cohort identified as White (68.6%), East Asian (9.0%), and South Asian (11.5%), and most had a university education (80.5%). A high proportion were overweight/obese (58.3%, BMI > 25 kg/m2), and 23.7% reported a previous diagnosis of hypercholesterolemia, with a previous diagnosis of T2D or hypertension reported in 2.6% and 12.8%, respectively (Table 1).
Table 1.
Baseline Characteristics
| No.=156 | |
|---|---|
| Age, y, mean (SD) | 41.0 (4.2) |
| Time since last delivery, y, mean (SD) | 3.9 (2.9) |
| Race or ethnicity, No. (%) | |
| White | 107 (68.6%) |
| East Asian | 14 (9.0%) |
| Aboriginal or Torres Strait Islander | 1 (0.6%) |
| South Asian* | 18 (11.5%) |
| Other* | 16 (10.3%) |
| Traditional risk factors | |
| BMI, kg/m2, mean (SD) | 28.1 (6.4) |
| BMI groups, No. (%) | |
| Underweight | 1 (0.6%) |
| Healthy weight | 64 (41.0%) |
| Overweight | 39 (25.0%) |
| Obese | 52 (33.3%) |
| Smoking, No. (%) | |
| Current smoker | 7 (4.5%) |
| Ex‐smoker, <12 mo | 5 (3.2%) |
| Ex‐smoker, >12 mo | 43 (27.6%) |
| Never | 101 (64.7%) |
| Preexisting diagnosis of type 2 diabetes, No. (%) | 4 (2.6%) |
| Preexisting diagnosis of hypercholesterolemia, No. (%) | 37 (23.7%) |
| Preexisting diagnosis of hypertension, No. (%) | 20 (12.8%) |
| Polycystic ovarian syndrome, No. (%) | 28 (17.9%) |
| Pregnancy conditions | |
| Gestational hypertension, No. (%) | 41 (26.3%) |
| Gestational diabetes, No. (%) | 115 (73.7%) |
| Preeclampsia, No. (%) | 42 (26.9%) |
| Placental abruption, No. (%) | 7 (4.5%) |
| Placenta previa, No. (%) | 11 (7.1%) |
| Preterm, No. (%), missing=1 | 14 (9.0%) |
| Medical history | |
| Chronic inflammatory condition, No. (%) | 11 (7.1%) |
| Impaired glucose tolerance, No. (%) | 17 (10.9%) |
| Obstructive sleep apnea, No. (%) | 6 (3.8%) |
| Highest level of education, No. (%), missing=2 | |
| Primary or high school | 16 (10.4%) |
| Technical qualification | 14 (9.1%) |
| Undergraduate university degree | 39 (25.3%) |
| Postgraduate university degree | 85 (55.2%) |
BMI indicates body mass index.
South Asian ethnicity included Indian, Sir Lankan, Pakistani, and Bangladeshi. Other included African, Black, Middle Eastern, Native Hawaiian or Other Pacific Islander, New Zealand First Nations, American Indian or Native Alaskan, Latino, and not disclosed.
Primary and Secondary Outcomes
All women attended a WHC at least once, with 69.5% additionally reviewed by a dietitian. No women were lost to follow‐up; however, 1.3% and 5.8% declined attending a final in‐person follow‐up examination and repeat laboratory tests, respectively. At baseline, 69.2% of women met the specified BP target and increasing to 80.5% at 6 months (P=0.004). A sensitivity analysis assuming the 2 women with no 6‐month BP measures did not meet this BP target showed similar significant results for meeting BP targets (P=0.012). An improvement to meeting the 6‐month BP target occurred in 26 women, 98 and 22 remained stable (meeting and not meeting the BP target, respectively), and 8 women regressed.
At baseline 80.6% of women met the TC:HDL‐C ratio target, and there was a small but statistically insignificant increase to 83.7% at 6 months (P=0.182). Assuming the 1 woman with missing lipids at baseline and the 9 with missing lipids at 6 months did not meet the lipid target at their respective visit, our sensitivity analysis had similar nonsignificant results for the target lipid ratio (P=0.803). An improvement to meeting the 6‐month lipid target occurred in 7 women, 116 and 22 remained stable meeting and not meeting the lipid target, respectively, and 2 women regressed.
Specified secondary outcomes were significantly improved, including reductions in mean systolic BP (−6.9 mm Hg [95% CI, −9.1 to −4.7], P<0.001), diastolic BP (−3.1 mm Hg [95% CI, −4.4 to −1.8], P<0.001), total cholesterol (−4.6 mg/dL [95% CI, −9.1 to −0.2], P=0.042), LDL‐C (−4.2 mg/dL [95% CI, −8.2 to −0.2], P=0.042), BMI (−0.6 kg/m2 [95% CI, −0.8 to −0.3], P<0.001), and waist circumference (−2.3 cm [95% CI, −3.3 to −1.3], P<0.001; Table 2 and Figure).
Table 2.
Primary and Secondary Outcomes Before and After the WHC Intervention
| Outcomes | Baseline N=156 | 6‐mo Follow‐up N=156 | Change | P value† |
|---|---|---|---|---|
| Co‐primary outcomes | n/N (%) | n/N (%) | Proportion difference | |
| BP target* | 108/156 (69.2%) | 124/154 (80.5%) | 11.3% | 0.004 |
| Lipid target‡ | 125/155 (80.6%) | 123/147 (83.7%) | 3.1% | 0.182 |
| Secondary outcomes | Mean (SD) | Mean (SD) | Mean difference (95% CI) | P value§ |
|---|---|---|---|---|
| Systolic BP, mm Hg | 126.3 (16.2) | 119.4 (10.1) | −6.9 (−9.1 to −4.7) | <0.001 |
| Diastolic BP, mm Hg | 82.6 (10.6) | 79.4 (8.2) | −3.1 (−4.4 to −1.8) | <0.001 |
| TC:HDL‐C ratio | 3.6 (1.0) | 3.5 (1.0) | −0.1 (−0.2 to 0) | 0.042 |
| TC, mg/dL | 199.7 (33.5) | 195.1 (34.4) | −4.6 (−9.1 to −0.2) | 0.042 |
| Low‐density lipoprotein cholesterol, mg/dL | 119.0 (30.0) | 115.0 (31.9) | −4.2 (−8.2 to −0.2) | 0.042 |
| HDL‐C, mg/dL | 58.5 (15.2) | 58.2 (14.0) | 0 (−1.4 to 1.4) | 0.962 |
| Triglycerides, mg/dL | 108.4 (50.5) | 106.0 (53.6) | −2.9 (−9.0 to −3.2) | 0.353 |
| Hemoglobin A1c, % | 5.4 (0.5) | 5.3 (0.4) | −0.1 (−0.1 to 0) | <0.001 |
| Fasting insulin, mU/L | 10.0 (6.9) | 9.7 (6.8) | −0.2 (−0.8 to 1.3) | 0.656 |
| Fasting glucose, mg/dL | 93.6 (12.6) | 93.6 (12.6) | 0.0 (−1.8 to 1.8) | 0.685 |
| Body mass index, kg/m2 | 28.1 (6.4) | 27.5 (5.9) | −0.6 (−0.8 to −0.3) | <0.001 |
| Waist circumference, cm | 94.3 (14.7) | 91.8 (14.2) | −2.3 (−3.3 to −1.3) | <0.001 |
BP indicates blood pressure; HDL‐C, high‐density lipoprotein cholesterol; TC, total cholesterol; and WHC, Women's Heart Clinic.
Blood pressure target is <140/90 mm Hg or <130/80 mm Hg if individual has diabetes.
McNemar test.
Lipid target is TC:HDL‐C<4.5.
Paired t test.
Figure . Visual summary of the women's health study: design and results.
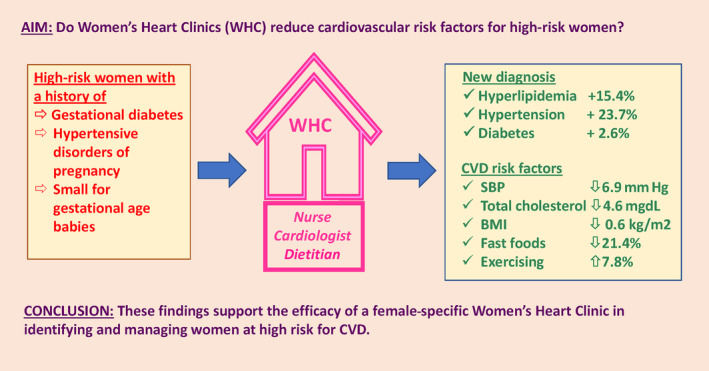
BMI indicates body mass index; CVD, cardiovascular disease; SBP, systolic blood pressure; and WHC, Women's Heart Clinic.
Hypertension Changes
During the first WHC visit, 12.8% (20/156) women reported a preexisting diagnosis of hypertension and an additional 37 women (total 57/156, 36.5%) were diagnosed with hypertension. Antihypertensive medications use increased from 18/156 (11.5%) to 39/156 (25.0%) at 6 months, comprising angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (84.6%, 33/39), calcium‐channel blockers (7/39, 17.9%), diuretics (5/39, 12.8%), and beta blockers (4/39, 10.3%). A further 5 women, on preexisting therapy, had uptitration of their antihypertension medications.
Cholesterol Changes
During the first WHC visit, 37/156 (23.7%) women reported a preexisting high cholesterol diagnosis with 4/156 (2.6%) on statins. An additional 24 women met diagnostic criteria for hypercholesterolemia (total 61/156, 39.1%), and by 6‐month follow‐up, an additional 11/156 (7.1%) had a statin initiated, comprising rosuvastatin (73.3%, 11/15), atorvastatin (20.0%, 3/15), and pravastatin (6.7%, 1/15).
Type 2 Diabetes Changes
During the first WHC visit, 2.6% (4/156) of women reported a preexisting diagnosis of T2D and an additional 4 women (total 8/156, 5.1%) were diagnosed with T2D, all with a history of GD. Diabetic therapy was taken by 4/156 (2.6%) at baseline, increasing to 8/156 (5.1%) at 6 months, with a further 2 women on metformin for impaired glucose tolerance/polycystic ovarian syndrome (Table 3).
Table 3.
New Diagnoses and Medication Changes After the WHC Intervention
| Preexisting/at baseline* N=156 | After WHC intervention† N=156 | Change in proportion | |
|---|---|---|---|
| Diagnosis of high cholesterol‡ | 37/156 (23.7%) | 61/156 (39.1%) | 15.4% |
| On statin therapy | 4 /156 (2.6%) | 15/156 (9.6%) | 5.8% |
| Diagnosis of hypertension§ | 20/156 (12.8%) | 57/156 (36.5%) | 23.7% |
| On antihypertensive medication | 18/156 (11.5%) | 39/156 (25.0%) | 10.3% |
| Diagnosis of diabetes | 4/156 (2.6%) | 8/156 (5.1%) | 2.6% |
| Diagnosis of impaired glucose tolerance /insulin resistanceǁ | 17/156 (10.9%) | 53/156 (34.0%) | 23.1% |
| On diabetes medication | 4/156 (2.6%) | 10/156 (6.4%) | 3.8% |
BP indicates blood pressure; and WHC, Women's Heart Clinic.
Preexisting based on self‐reported physician diagnosis.
After WHC based on historical diagnosis as well as detection of new diagnosis from fasting pathology and clinical examination.
High cholesterol defined as total cholesterol >5.5 mmol/L (212.7 mg/dL) or low‐density lipoprotein cholesterol >3.5 mmol/L (135.3 mg/dL).
Hypertension defined as BP >140/90 mm Hg or BP >130/80 mm Hg for 8 women with diabetes (historical and new diagnosis).
Impaired glucose tolerance/insulin resistance defined as hemoglobin A1c 5.7% to 6.5% or fasting blood glucose 5.7 to 6.9 mmol/L (102.6–124.2 mg/dL) or fasting insulin >20 mU/L.
Diet and Exercise Changes
At 6 months, there were significant increases in healthy fats (weekly intake of ≥2 servings per week of fish/olive oil from 36.5% to 51.0%, P=0.012) and nuts (≥2 servings per week from 57.7% to 71.6%, P=0.013), with a reduction in fast food intake (≥3 times per week from 33.8% to 11.0%, P<0.001). Physical activity significantly increased from 84.0% to 92.9% (P=0.025) meeting recommendations of ≥2.5 hours of moderate physical activity or ≥1.25 hours of vigorous physical activity per week. There was no increase in vegetable and fruit intake (Table 4).
Table 4.
Adherence to a Heart‐Healthy Lifestyle
| Outcome | Baseline N=156 | 6‐mo N=155 | Change N=155 | P value† |
|---|---|---|---|---|
| Vegetables ≥3 servings/d | 97/155 (62.6%) | 97/155 (62.6%) | 0/155 (0.0%) | 1.000 |
| Fruit ≥2 servings/d | 89/156 (57.1%) | 96/155 (61.9%) | 7/155 (4.5%) | 0.420 |
| Processed meat ≥2 servings/wk | 46/156 (29.5%) | 32/155 (20.6%) | 14/155 (9.0%) | 0.089 |
| Fish/olive oil ≥2 servings/wk | 57/156 (36.5%) | 79/155 (51.0%) | 22/155 (14.2%) | 0.012 |
| Nuts ≥2 servings/wk | 90/156 (57.7%) | 111/155 (71.6%) | 21/155 (13.5%) | 0.013 |
| Sugar‐sweetened beverages ≤1 serving/wk | 124/156 (79.5%) | 125/155 (80.6%) | 1/155 (0.6%) | 0.890 |
| Fast food ≥3 times/wk | 52/154 (33.8%) | 17/155 (11.0%) | 33/154 (21.4%) | <0.001 |
| Exercise at guideline recommendations* | 131/156 (84.0%) | 143/154 (92.9%) | 12/154 (7.8%) | 0.025 |
Guideline recommendations for exercise defined as ≥2.5 h moderate physical activity or ≥1.25 h vigorous physical activity per week.
McNemar test.
Patient Experience
Of 131 patients completing the surveys, 87.8% (95% CI, 82.2%–93.4%) felt motivated to change their lifestyle after attending the WHC, 95.4% (95% CI, 91.3%–99.0%) felt that they understood more about their risk for heart disease, and 91.6% (95% CI, 86.9%–96.4%) felt that the WHC was beneficial in addition to their usual general practice visits to treat their heart disease risk.
DISCUSSION
A multidisciplinary, female‐specific, health care service improved cardiovascular risk factor control in women at high CVD risk based on past pregnancy‐related conditions. We found a large proportion of women with past HDP and GD had undetected and undertreated hypertension and hypercholesterolemia. Following a WHC intervention, women at high CVD risk had clinically meaningful reductions in BP, lipids, BMI, and waist circumference, with significantly higher adherence to a heart‐healthy lifestyle.
Among our cohort of young women, mean age 41 years and an average of 3.9 years since affected pregnancy, almost a third had undiagnosed or undertreated hypertension. After a 6‐month WHC intervention, we found a significant and clinically meaningful drop in systolic BP of 6.9 mm Hg. This degree of BP lowering correlates with a long‐term reduction in cardiovascular events of more than 10%. 42 Our findings are consistent with past literature whereby women with a HDP diagnosis are at 3‐fold higher risk of hypertension (risk ratio [RR], 3.46 [95% CI, 2.67–4.49]), and this risk is highest in the first 5 years postpartum (RR, 5.34 [95% CI, 2.74–10.39]). 4 A large Danish study (N=482 972) 43 estimated that 14% of women who had HDP in their 20s would develop hypertension within a decade and 32% within 2 decades of their affected pregnancy. Due to low awareness, many women with past pregnancy‐related conditions are not referred or do not attend screening for hypertension and T2D. 21 , 44 A WHC may, therefore, have a large impact by detecting and treating hypertension in women who would otherwise be missed and advocating for better BP screening in the primary care setting. In addition, it was not just women who met diagnostic criteria for hypertension who benefited from the WHC. Average BP was reduced in the entire cohort of women, reflecting significant improvements in healthy lifestyle, likely from sex‐specific education about women's risk for CVD and preventative strategies.
The proportion of women who met the recommended TC:HDL ratio did not significantly alter following our WHC intervention. This is likely due to a high proportion of women already being at primary prevention targets (79.5% TC:HDL‐C <4.5). Importantly, despite most women meeting lipid targets, we still found significant reductions in mean LDL‐C and TC following the WHC intervention. These improvements in lipids were largely lifestyle driven, as the minority of women (8%) met criteria for statin therapy due to being low or very low CVD risk on the Australian CVD risk calculator. This is similar to our management of hypertension where, despite 36.5% of women being diagnosed with hypertension, only 25% received medications, with the remainder effectively managed with lifestyle interventions. Of note, the current study excluded women who were planning a pregnancy, breastfeeding, or were pregnant. Discussion with the patient of the possible teratogenicity of statins and certain types of antihypertensive medications (eg, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers) is important in women of child‐bearing age.
Meta‐analyses have shown that women with GD have an 8‐ to 10‐fold risk of developing T2D. 45 , 46 , 47 , 48 Genetic studies discovered links between GD and future T2D, especially in South Asian women, 49 emphasizing the importance of monitoring. Correspondingly, suboptimal screening for T2D in women who have had GD is evident. 19 Our data suggest that WHCs could play a key role in improving management for these high‐risk women. Despite the young cohort, 4 women were newly diagnosed with T2D, resulting in a total of 8/156 women with T2D. Importantly, over a third were found to have impaired glucose tolerance/insulin resistance, with two thirds undiagnosed before their WHC assessment. Women received education regarding their future risk for T2D and CVD, as well as lifestyle changes. The significant reductions in weight, BMI, and waist circumference from the WHC intervention could be expected to lower rate of progression to T2D in women with insulin resistance and their risk for future CVD.
Although WHCs are common in North America in particular, 32 there has been only a single study analyzing their effectiveness. 36 This study (n=100), conducted in Singapore, developed a heart‐health program for women aged 21 to 99 with a broad range of cardiovascular conditions. It demonstrated benefit in diabetic control and BMI but no difference in BP or lipids. 36 A small number of studies assessed interventions for women with past pregnancy‐related risk factors, using maternal health care services. One Canadian study of a nurse‐led maternal health clinic, with cardiologists assessing high‐risk patients, 50 diagnosed metabolic syndrome in 17.4% (n=92) following a pregnancy complication, compared with healthy cohort rates of 6.8%. 51 Other studies found lifestyle‐focused interventions improved weight 31 and T2D diagnosis as effectively as medication 30 for women with past GD. The HH4M (Heart Health 4 Moms) US study of women with preeclampsia found a lifestyle and education intervention improved CVD risk factor knowledge, healthy eating, and physical activity. 52 Our study is the first to support a female‐specific cardiovascular health care service, run by cardiologists and cardiac nurses, to mitigate CVD risk in women with past pregnancy‐related conditions. The majority of our participants positively rated the clinic, stating it was beneficial in addition to general practice and felt they better understand their heart disease risk. The impetus for a WHC is clear: to help overcome the marked disparities facing female patients in CVD screening and treatment, compared with men. 2 , 3
Previous research has shown that women do not perceive themselves to be at risk for CVD, even in the presence of multiple risk factors. 36 , 50 Despite our cohort being highly educated, we found significant rates of undetected hypertension and dyslipidemia. We delivered education on a woman's risk, including their risk related to pregnancy‐related risk factors, and motivated many to make significant healthy lifestyle changes. Daily fruit and vegetable servings did not change; however, their benefit in a healthy diet is well known so perhaps specific education regarding this had less impact. We also saw a significant increase in the proportion of women meeting exercise recommendations. These results suggest that a WHC is effective in addressing risk of CVD among women.
Limitations
Although this study was prospective, it was nonrandomized with the comparator being the same cohort of women, from baseline to 6 months. With no control comparator it is not possible to measure the extent women would have improved without the intervention. However, as we anticipate CVD risk to worsen with time, this would have been expected to attenuate rather than exaggerate the results. Due to the COVID‐19 pandemic occurring just before first participant recruitment, the follow‐up, originally planned for 12 months, was reduced to 6 months, limiting assessment of long‐term durability of the findings. A large proportion of women were consequently assessed via telehealth, although BP and laboratory tests were performed in person. Telehealth may have reduced the impact of the intervention; however, the ability to attend a clinic remotely may also have improved compliance given our cohort consisted of mothers with young children. A high proportion had university degrees, the majority identified as White, and recruitment was from a hospital‐based obstetric list, limiting generalizability. However, 31.4% of women identified as South Asian, East Asian, Aboriginal or Torres Strait Islander, and other ethnic groups, higher than that seen in similar studies. One might anticipate even higher rates of undiagnosed risk factors and room for improvement among women from ethnic minority groups and those with a lower level of education.
CONCLUSIONS
Women's heart health programs are thought to be useful in CVD prevention, yet little research had been conducted to confirm their effectiveness. Our findings strongly support the benefit of female‐specific cardiovascular health care services in risk factor control and healthy lifestyle adherence in women with past pregnancy‐related conditions. These improvements in cardiovascular risk factors are expected to correlate with long‐term cardiovascular outcome benefits for women and require further evaluation.
Sources of Funding
This project was supported by the Australian Government's Medical Research Future Fund as part of the Rapid Applied Research Translation program through Monash Partners. S.Z. was supported by a Heart Foundation Future Leader Fellowship (ID 102627).
Disclosures
S.Z. has received speaking honoraria from Novartis, consulting fees for an advisory committee for Medtronic, and a research grant to their institution from Abbott Vascular (Australia), none of which relate to the content of this manuscript. The remaining authors have no disclosures to report.
This article was sent to Kerry‐Anne Rye, PhD, Senior Guest Editor, for review by expert referees, editorial decision, and final disposition.
See Editorial by Chan and Cheung.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Cardiovascular diseases. World Health Organization. Accessed January 23, 2023. https://www.who.int/health‐topics/cardiovascular‐diseases#tab=tab_1
- 2. Khamis RY, Ammari T, Mikhail GW. Gender differences in coronary heart disease. Heart. 2016;102:1142–1149. doi: 10.1136/heartjnl-2014-306463 [DOI] [PubMed] [Google Scholar]
- 3. Woodward M. Cardiovascular disease and the female disadvantage. Int J Environ Res Public Health. 2019;16:1165. doi: 10.3390/ijerph16071165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sukmanee J, Liabsuetrakul T. Risk of future cardiovascular diseases in different years postpartum after hypertensive disorders of pregnancy: a systematic review and meta‐analysis. Medicine (Baltimore). 2022;101:e29646. doi: 10.1097/MD.0000000000029646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lo CCW, Lo ACQ, Leow SH, Fisher G, Corker B, Batho O, Morris B, Chowaniec M, Vladutiu CJ, Fraser A, et al. Future cardiovascular disease risk for women with gestational hypertension: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9:e013991. doi: 10.1161/JAHA.119.013991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marschner S, von Huben A, Zaman S, Reynolds HR, Lee V, Choudhary P, Mehta LS, Chow CK. Pregnancy‐related cardiovascular conditions and outcomes in a United States Medicaid population. Heart. 2022;108:1524–1529. doi: 10.1136/heartjnl-2021-320684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pathirana MM, Lassi Z, Ali A, Arstall M, Roberts CT, Andraweera PH. Cardiovascular risk factors in women with previous gestational diabetes mellitus: a systematic review and meta‐analysis. Rev Endocr Metab Disord. 2020;22:729–761. doi: 10.1007/s11154-020-09587-0 [DOI] [PubMed] [Google Scholar]
- 8. Tranidou A, Dagklis T, Tsakiridis I, Siargkas A, Apostolopoulou A, Mamopoulos A, Goulis DG, Chourdakis M. Risk of developing metabolic syndrome after gestational diabetes mellitus—a systematic review and meta‐analysis. J Endocrinol Investig. 2020;44:1139–1149. doi: 10.1007/s40618-020-01464-6 [DOI] [PubMed] [Google Scholar]
- 9. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta‐analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 10. Xie W, Wang Y, Xiao S, Qiu L, Yu Y, Zhang Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: systematic review and meta‐analysis. BMJ. 2022;378:e070244. doi: 10.1136/bmj-2022-070244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edstedt Bonamy A‐K, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124:2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884 [DOI] [PubMed] [Google Scholar]
- 12. Pariente G, Sheiner E, Kessous R, Michael S, Shoham‐Vardi I. Association between delivery of a small‐for‐gestational‐age neonate and long‐term maternal cardiovascular morbidity. Int J Gynecol Obstet. 2013;123:68–71. doi: 10.1016/j.ijgo.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 13. Ngo AD, Roberts CL, Chen JS, Figtree G. Delivery of a small‐for‐gestational‐age infant and risk of maternal cardiovascular disease—a population‐based record linkage study. Heart Lung Circ. 2015;24:696–704. doi: 10.1016/j.hlc.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 14. Bukowski R, Davis KE, Wilson PWF. Delivery of a small for gestational age infant and greater maternal risk of ischemic heart disease. PLoS One. 2012;7:e33047. doi: 10.1371/journal.pone.0033047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grieger JA, Hutchesson MJ, Cooray SD, Bahri Khomami M, Zaman S, Segan L, Teede H, Moran LJ. A review of maternal overweight and obesity and its impact on cardiometabolic outcomes during pregnancy and postpartum. Ther Adv Reprod Health. 2021;15:2633494120986544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucas HR, Williams RC, Hollar LN, Johnson‐Javois B, Miller HB, Stoermer A, Colditz GA, James AS, Herrick CJ. Understanding gestational diabetes, future diabetes risk, and diabetes prevention: a qualitative study of patient, provider, and staff perspectives. Clin Diabetes. 2022;40:39–50. doi: 10.2337/cd21-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roth H, Homer CSE, Arnott C, Roberts L, Brown M, Henry A. Assessing knowledge of healthcare providers concerning cardiovascular risk after hypertensive disorders of pregnancy: an Australian national survey. BMC Pregnancy Childbirth. 2020;20:717. doi: 10.1186/s12884-020-03418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aslam A, Perera S, Watts M, Kaye D, Layland J, Nicholls SJ, Cameron J, Zaman S. Previous pre‐eclampsia, gestational diabetes and hypertension place women at high cardiovascular risk: but do we ask? Heart Lung Circ. 2021;30:154–157. doi: 10.1016/j.hlc.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 19. Moses RG, Suthers R, Gemert TE, Martin MB, Webb AJ. Gestational diabetes—major problems with post‐partum testing. Aust N Z J Obstet Gynaecol. 2021;61:536–539. doi: 10.1111/ajo.13312 [DOI] [PubMed] [Google Scholar]
- 20. Campbell A, Stanhope KK, Platner M, Joseph NT, Jamieson DJ, Boulet SL. Demographic and clinical predictors of postpartum blood pressure screening attendance. J Women's Health. 2022;31:347–355. doi: 10.1089/jwh.2021.0161 [DOI] [PubMed] [Google Scholar]
- 21. Jones EJ, Hernandez TL, Edmonds JK, Ferranti EP. Continued disparities in postpartum follow‐up and screening among women with gestational diabetes and hypertensive disorders of pregnancy: a systematic review. J Perinat Neonatal Nurs. 2019;33:136–148. doi: 10.1097/JPN.0000000000000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cushman M, Shay CM, Howard VJ, Jiménez MC, Lewey J, McSweeney JC, Newby LK, Poudel R, Reynolds HR, Rexrode KM, et al. Ten‐year differences in women's awareness related to coronary heart disease: results of the 2019 American Heart Association national survey: a special report from the American Heart Association. Circulation (New York, NY). 2020;143:e239–e248. doi: 10.1161/CIR.0000000000000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mosca L, Hammond G, Mochari‐Greenberger H, Towfighi A, Albert MA. Fifteen‐year trends in awareness of heart disease in women results of a 2012 American Heart Association national survey. Circulation. 2013;127:1254–1263. doi: 10.1161/CIR.0b013e318287cf2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guideline for assessing and managing CVD risk and Australian CVD risk calculator. Heart Foundation. Accessed January 23, 2023. https://www.heartfoundation.org.au/health‐professional‐tools‐(2)/Guideline‐for‐managing‐CVD.
- 26. Majmundar M, Doshi R, Patel KN, Zala H, Kumar A, Kalra A. Prevalence, trends and outcomes of cardiovascular diseases in pregnant patients in the United States: 2010 to 2019. Eur Heart J. 2022;146:726–737. doi: 10.1161/circ.146.suppl_1.13230 [DOI] [PubMed] [Google Scholar]
- 27. Diabetes: Australian facts. Australian Institute of Health and Welfare. Accessed March 5, 2023. https://www.aihw.gov.au/reports/diabetes/diabetes/contents/summary.
- 28. Australia's mothers and babies. Australian Institute of Health and Welfare. Accessed March 5, 2023. https://www.aihw.gov.au/reports/mothers‐babies/australias‐mothers‐babies/contents/antenatal‐period/maternal‐medical‐conditions.
- 29. Lui NA, Jeyaram G, Henry A. Postpartum interventions to reduce long‐term cardiovascular disease risk in women after hypertensive disorders of pregnancy: a systematic review. Front Cardiovasc Med. 2019;6:160. doi: 10.3389/fcvm.2019.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi‐Sunyer X, Fowler S, Kahn SE. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ferrara A, Hedderson MM, Brown SD, Albright CL, Ehrlich SF, Tsai A‐L, Caan BJ, Sternfeld B, Gordon NP, Schmittdiel JA, et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the Gestational Diabetes' Effects on Moms (GEM) cluster randomized controlled trial. Diabetes Care. 2016;39:65–74. doi: 10.2337/dc15-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundberg GP, Mehta LS, Sanghani RM, Patel HN, Aggarwal NR, Aggarwal NT, Braun LT, Lewis SJ, Mieres JH, Wood MJ, et al. Heart centers for women: historical perspective on formation and future strategies to reduce cardiovascular disease. Circulation. 2018;138:1155–1165. doi: 10.1161/CIRCULATIONAHA.118.035351 [DOI] [PubMed] [Google Scholar]
- 33. Garcia M, Miller VM, Gulati M, Hayes SN, Manson JE, Wenger NK, Bairey Merz CN, Mankad R, Pollak AW, Mieres J, et al. Focused cardiovascular care for women: the need and role in clinical practice. Mayo Clin Proc. 2016;91:226–240. doi: 10.1016/j.mayocp.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 34. Edwards KS, Hekler AC, Baum J, Nejedly M, Tsai S, Khandelwal A, Naderi S, Hoover V, Tremmel JA. Psychological distress among female cardiac patients presenting to a women's heart health clinic. Am J Cardiol. 2019;123:2026–2030. doi: 10.1016/j.amjcard.2019.03.029 [DOI] [PubMed] [Google Scholar]
- 35. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, Guerrero M, Kunadian V, Lam CSP, Maas AHEM, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. 2021;397:2385–2438. doi: 10.1016/S0140-6736(21)00684-X [DOI] [PubMed] [Google Scholar]
- 36. Low TT, Chan SP, Wai SH, Ang Z, Kyu K, Lee KY, Ching A, Comer S, Tan NQP, Thong EGHE, et al. The women's heart health programme: a pilot trial of sex‐specific cardiovascular management. BMC Women's Health. 2018;18:56. doi: 10.1186/s12905-018-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blood cholesterol. Heart Foundation. Accessed February 5, 2023. https://www.heartfoundation.org.au/Heart‐health‐education/High‐blood‐cholesterol
- 38. Five ways to lower cholesterol. Heart Foundation. Accessed February 5, 2023. https://www.heartfoundation.org.au/Blog/Five‐ways‐to‐lower‐cholesterol
- 39. ASCVD Risk Estimator Plus. American College of Cardiology. Accessed December 12, 2022. https://tools.acc.org/ascvd‐risk‐estimator‐plus/#!/calculate/estimate/
- 40. CVD check: calculator. Australian Chronic Disease Prevention Alliance. Accessed December 12, 2022. https://www.cvdcheck.org.au/calculator.
- 41. R software, Version 4.2.2; The R Project for Statistical Computing, Released: Oct 2022. Accessed July 14, 2023. https://www.r‐project.org/
- 42. Rahimi K, Bidel Z, Nazarzadeh M, Copland E, Canoy D, Ramakrishnan R, Pinho‐Gomes A‐C, Woodward M, Adler A, Agodoa L, et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant‐level data meta‐analysis. Lancet. 2021;397:1625–1636. doi: 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Behrens I, Basit S, Melbye M, Lykke JA, Wohlfahrt J, Bundgaard H, Thilaganathan B, Boyd HA. Risk of post‐pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. doi: 10.1136/bmj.j3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Linnenkamp U, Greiner GG, Haastert B, Adamczewski H, Kaltheuner M, Weber D, Icks A. Postpartum screening of women with GDM in specialised practices: data from 12,991 women in the GestDiab register. Diabetic Med. 2022;39:e14861. doi: 10.1111/dme.14861 [DOI] [PubMed] [Google Scholar]
- 45. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta‐analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. You H, Hu J, Liu Y, Luo B, Lei A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review & meta‐analysis. Indian J Med Res. 2021;154:62–77. doi: 10.4103/ijmr.IJMR_852_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dennison RA, Chen ES, Green ME, Legard C, Kotecha D, Farmer G, Sharp SJ, Ward RJ, Usher‐Smith JA, Griffin SJ. The absolute and relative risk of type 2 diabetes after gestational diabetes: a systematic review and meta‐analysis of 129 studies. Diabetes Res Clin Pract. 2021;171:108625. doi: 10.1016/j.diabres.2020.108625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cheung N, Byth K. Population health significance of gestational diabetes. Diabetes Care. 2003;26:2005–2009. doi: 10.2337/diacare.26.7.2005 [DOI] [PubMed] [Google Scholar]
- 49. Lamri A, Limbachia J, Schulze KM, Desai D, Kelly B, de Souza RJ, Paré G, Lawlor DA, Wright J, Anand SS. The genetic risk of gestational diabetes in South Asian women. Elife. 2022;11:11. doi: 10.7554/eLife.81498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith GN. The maternal health clinic: improving women's cardiovascular health. Semin Perinatol. 2015;39:316–319. doi: 10.1053/j.semperi.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 51. Cusimano MCB, Pudwell JMPH, Roddy MRNB, Cho C‐KJP, Smith GNMDP. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy‐related complications. Am J Obstet Gynecol. 2014;210:438.e1–438.e9. doi: 10.1016/j.ajog.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 52. Rich‐Edwards JW, Stuart JJ, Skurnik G, Roche AT, Tsigas E, Fitzmaurice GM, Wilkins‐Haug LE, Levkoff SE, Seely EW. Randomized trial to reduce cardiovascular risk in women with recent preeclampsia. J Women's Health (Larchmt). 2019;28:1493–1504. doi: 10.1089/jwh.2018.7523 [DOI] [PubMed] [Google Scholar]


