Abstract
Background
To protect healthcare workers (HCWs) from the consequences of disease due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is necessary to understand the risk factors that drive exposure and infection within hospitals. Insufficient consideration of key socioeconomic variables is a limitation of existing studies that can lead to bias and residual confounding of proposed risk factors for infection.
Methods
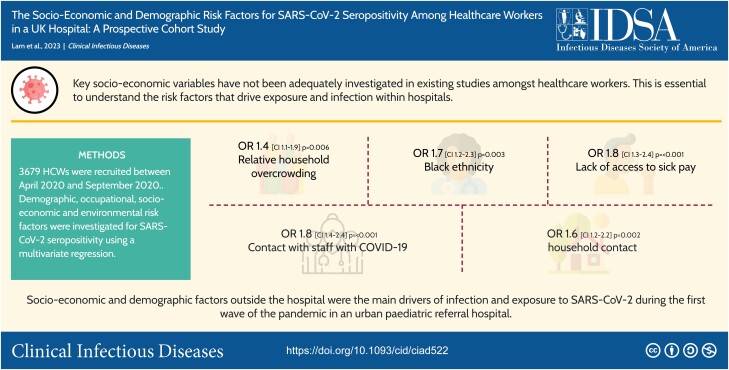
The Co-STARs study prospectively enrolled 3679 HCWs between April 2020 and September 2020. We used multivariate logistic regression to comprehensively characterize the demographic, occupational, socioeconomic, and environmental risk factors for SARS-CoV-2 seropositivity.
Results
After adjusting for key confounders, relative household overcrowding (odds ratio [OR], 1.4 [95% confidence interval {CI}, 1.1–1.9]; P = .006), Black, Black British, Caribbean, or African ethnicity (OR, 1.7 [95% CI, 1.2–2.3]; P = .003), increasing age (ages 50–60 years: OR, 1.8 [95% CI, 1.3–2.4]; P < .001), lack of access to sick pay (OR, 1.8 [95% CI, 1.3–2.4]; P < .001).
Conclusions
Socioeconomic and demographic factors outside the hospital were the main drivers of infection and exposure to SARS-CoV-2 during the first wave of the pandemic in an urban pediatric referral hospital. Overcrowding and out-of-hospital SARS-CoV-2 contact are less amenable to intervention. However, lack of access to sick pay among externally contracted staff is more easily rectifiable. Our findings suggest that providing easier access to sick pay would lead to a decrease in SARS-CoV-2 transmission and potentially that of other infectious diseases in hospital settings.
Clinical Trials Registration
Keywords: SARS-CoV-2, risk factors, healthcare workers, socioeconomic status, ethnicity
This London-based prospective cohort study demonstrated an increased risk of SARS-CoV-2 seropositivity in healthcare workers reporting difficulty accessing sick leave, living in overcrowded housing, and/or of Black ethnicity, highlighting the effect of structural and ethnic inequality during the COVID-19 pandemic.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/the-socio-economic-and-demographic-risk-factors-for-sars-cov-2-seropositivity-among-healthcare-workers-in-a-uk-hospital-a-prospective-cohort-study/update
The coronavirus disease 2019 (COVID-19) pandemic placed unprecedented pressure on healthcare systems globally. Healthcare workers (HCWs) remained at the forefront of the pandemic during the first wave of infections, while nonessential workers were placed under stringent lockdown measures. Infection rates of COVID-19 in HCWs were higher than in the general population during this period, both in the United Kingdom (UK) and internationally [1–4]. Numerous studies have attempted to identify risk factors for HCW acquisition of COVID-19 with a view to creating safer working environments for staff and limiting the spread of healthcare-associated COVID-19 [5, 6].
Existing published risk factors for HCW exposure and infection vary widely [5–10]. There is considerable heterogeneity in clinical, demographic, occupational, and environmental variables recorded between studies [5, 11], limiting our understanding of specific risks and key confounders. For example, several international studies have identified that cleaners and hospital porters are those most at risk of exposure and infection [6, 7, 12, 13], but most studies have failed to include potential socioeconomic confounders of this association. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been detected in air samples in hospital rooms, on surfaces, and on shared staff equipment [14, 15], potentially exposing non-patient-facing staff—particularly cleaners and porters—to SARS-CoV-2. However, the Office for National Statistics (ONS) does not classify cleaners and hospital porters among the highest-exposure occupations [1]. This divergence suggests that local variability and residual confounding for SARS-CoV-2 acquisition may explain the association rather than the occupation itself. There are several published studies of SARS-CoV-2 seroprevalence in pediatric hospitals, but none has investigated ethnicity or socioeconomic variables as risk factors for SARS-CoV-2 exposure and infection [2–4, 16–18].
To improve our understanding of the underlying socioeconomic and demographic determinants of SARS-CoV-2 infection and exposure, we undertook a prospective cohort study of risk factors for SARS-CoV-2 seropositivity in a tertiary London pediatric hospital during the first wave of the pandemic.
METHODS
Study Setting, Design, and Participants
The COVID-19 Staff Testing of Antibody Responses (Co-STARs) project was a single-center prospective cohort study evaluating antibody responses to COVID-19 in HCWs at Great Ormond Street Hospital (GOSH). It was conducted between April 2020 and September 2020 during the first wave of the COVID pandemic [19, 20]. Both clinical and nonclinical hospital staff ≥18 years were invited to participate. To ensure equity of access to the study, face-to-face active recruitment was used for hospital staff on external contracts without National Health Service (NHS) Trust email addresses, who were more difficult to contact. These staff members included cleaners, porters, and catering staff. Participants were excluded if they had significant immunosuppression, recent administration of blood products (including immunoglobulins or convalescent sera) since September 2019, and persistent symptoms of SARS-CoV-2 infection at the time or within 21 days of recruitment. All participants signed an informed consent form, and the study was approved by the UK NHS Health Research Authority and registered at ClinicalTrials.gov (NCT04380896) [20].
Data Collection
Blood samples were taken at baseline and at each follow-up visit for anti-SARS-CoV-2 immunoglobulin G serology using previously published methods [19, 20]. At the recruitment visit, participants also undertook a comprehensive, standardized online questionnaire (Supplementary Appendix C). This included sociodemographic factors including self-assigned ethnicity [21]; details of previous exposure to SARS-CoV-2; symptomatic episodes consistent with COVID-19 with any subsequent complications; previous SARS-CoV-2 diagnostic test results; occupation and medical history; and a comprehensive assessment of risk factors for exposure, susceptibility to infection, and severe disease [20].
Follow-up Appointments
All seropositive participants attended monthly follow-up visits for repeat antibody testing up to 250 days after the date of infection. Seronegative participants were followed up every 6 months. At each follow-up appointment, participants completed a shortened version of the baseline questionnaire, focusing on any significant changes since the last visit, including recurrent SARS-CoV-2 exposure and/or COVID-19 symptoms.
Statistical Analysis
The risk of SARS-CoV-2 exposure or infection was estimated by fitting a logistic regression model using seropositivity for SARS-CoV-2 as the binary dependent outcome variable. Demographic (age, sex, and ethnicity), occupational (occupation, income, and working conditions), socioeconomic, environmental, and SARS-CoV-2 exposure factors were included as model predictors. Variables were chosen due to clinical and epidemiological relevance. As no statistical difference in seropositivity was estimated between White British, White Other, and White Irish ethnicities, these variables were merged into a single variable “White.” Univariate and multivariate logistic regression models were used to estimate odds ratios (ORs). Univariate models were fitted for each variable independently, while a multivariate regression was performed excluding variables that had a proportion of missing values >30%. Similarly, univariate and multivariate logistic regression models were performed to estimate the relationship between SARS-CoV-2 seropositivity and self-reported symptoms. Collinearity was assessed by calculating the variance inflation factor (VIF) for all variables selected in the multivariate model. All VIF values ranged between 1 and 2, suggesting that no collinearity was detected in our model, and therefore no variable was removed.
The impact of socioeconomic deprivation on the risk of SARS-CoV-2 seropositivity was included in the model by linking the postal code metadata to area deprivation using the index of multiple deprivation as reported by the UK Ministry of Housing, Communities and Local Government [22].
All analyses were performed using R software [23].
The Supplementary Material includes the study protocol (for study flowcharts see Supplementary Appendix A, for schedule of procedures see Supplementary Appendix B), power calculations (Supplementary Figures 1 and 2), detailed laboratory methodology, the questionnaires used for data collection (Supplementary Appendix C), and sites of international collaboration (Supplementary Appendix D).
RESULTS
A total of 3646 staff members were recruited out of a total 5755 employees at GOSH (63.3%). Of the total number of staff approached for recruitment, <1% declined to participate. There were 53 confirmed inpatient cases with COVID-19 diagnosed by polymerase chain reaction on nasopharyngeal swabs during the study period. As shown in Table 1, 24% (712/3646) of the participants were categorized as seropositive, defined as presenting a SARS-CoV-2–positive test at any point during the study period. The majority of the participants were female (77% [2801/3646]) and White (54% [1951/3646]). Most participants self-reported symptoms (64%), but 1.3% (48/3646) sought medical attention and only 11 of them (0.3%) required hospitalization (Table 1).
Table 1.
Demographics of Study Participants
| Characteristic | Total | Seropositive |
|---|---|---|
| Total participants | 3646 (100) | 712 (24.26) |
| Demographic characteristics | ||
| Age, y | ||
| 17–30 | 1062 (29.12) | 172 (16.19) |
| 30–40 | 1183 (32.44) | 213 (18.00) |
| 40–50 | 731 (20.05) | 170 (23.25) |
| 50–60 | 513 (14.07) | 122 (23.78) |
| 60–80 | 156 (4.27) | 35 (22.43) |
| Sex | ||
| Male | 808 (22.16) | 167 (20.67) |
| Female | 2801 (76.82) | 543 (19.38) |
| Undetermined | 30 (0.82) | 0 (0) |
| Ethnicity | ||
| White | 1951 (53.51) | 410 (21.01) |
| Any other | 64 (1.75) | 15 (23.44) |
| Arab | 31 (0.85) | 8 (25.80) |
| South Asian | 416 (11.41) | 110 (26.44) |
| Black | 259 (7.10) | 95 (36.68) |
| Chinese | 49 (1.34) | 11 (22.45) |
| Mixed | 95 (2.6) | 24 (25.26) |
| Occupation | ||
| AHPs | 814 (22.32) | 155 (19.04) |
| Cleaning/Catering/Porters | 190 (5.21) | 71 (37.36) |
| Doctor | 588 (16.12) | 96 (16.32) |
| ICT | 44 (1.21) | 13 (29.55) |
| Manager | 213 (5.8) | 44 (20.66) |
| Nurse | 1258 (34.50) | 233 (18.52) |
| Other | 237 (6.50) | 56 (23.63) |
| Scientist | 299 (8.20) | 44 (14.72) |
| Symptoms and severity | ||
| Asymptomatic | 1316 (36.09) | 266 (20.21) |
| Abnormal smell sensation | 425 (11.66) | 257 (60.47) |
| Abnormal taste sensation | 477 (13.08) | 276 (57.86) |
| Admitted to hospital | 11 (0.3) | 5 (45.45) |
| Altered conscious state | 11 (0.30) | 5 (45.45) |
| Attended hospital | 48 (1.32) | 16 (33.33) |
| Chills | 162 (4.44) | 56 (34.57) |
| Conjunctivitis | 21 (0.58) | 8 (38.1) |
| Cough | 1003 (27.51) | 313 (31.21) |
| Diarrhea | 301 (8.26) | 113 (37.54) |
| Extreme fatigue | 700 (19.20) | 267 (38.14) |
| Fever (temperature >38°C) | 649 (17.80) | 244 (37.6) |
| Headache | 254 (6.97) | 91 (35.83) |
| Loss of appetite | 148 (4.06) | 58 (39.19) |
| Muscle pain | 796 (21.83) | 300 (37.69) |
| Nose bleed | 19 (0.52) | 8 (42.11) |
| Runny nose | 597 (16.37) | 176 (29.48) |
| Shortness of breath | 555 (15.22) | 174 (31.35) |
| Vomiting | 80 (2.19) | 27 (33.75) |
| Wheeze | 318 (8.72) | 96 (30.19) |
Data are presented as No. (%).
Abbreviations: AHPs, allied health professionals; ICT, Information Computing Technology.
SARS-CoV-2 Risk Factors for HCWs
Demographic Risks of SARS-CoV-2 Infection or Exposure
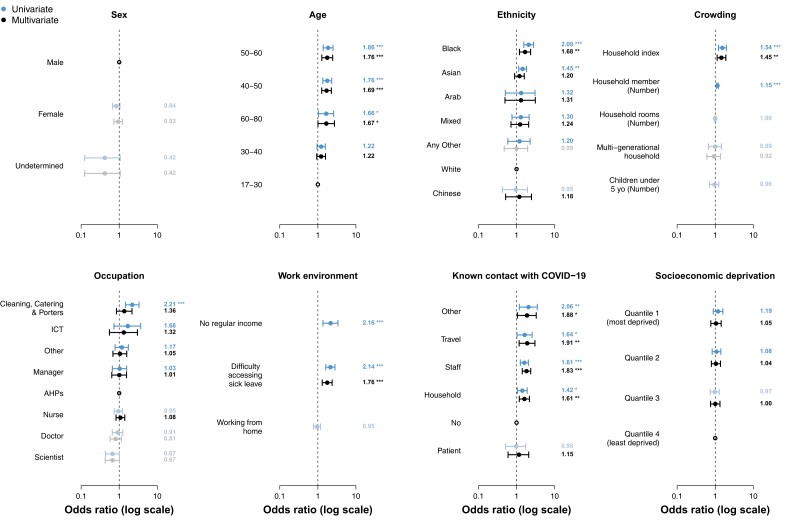
No difference in SARS-CoV-2 seropositivity was observed between male or female staff. Higher rates of seropositivity were seen in those aged 40–60 years, which remained significant in multivariate analysis. On univariate analysis, Black and South Asian ethnicity were associated with higher rates of seropositivity (40.0% and 26.4%, respectively). However, on multivariate analysis, South Asian ethnicity was no longer significant, whereas Black ethnicity remained significant (OR, 1.7 [95% confidence interval {CI}, 1.2–2.3]; P = .003) (Figure 1 and Table 2)
Figure 1.
Risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seropositivity among healthcare workers (HCWs). Risk of SARS-CoV-2 seropositivity among HCWs was estimated using univariate and multivariate logistic regression model. Points show the best estimate of the odds ratio (OR), while error bars represent the 95% confidence interval (CI) for the estimate OR. Bold indicates whether the OR is higher than the reference group (bold) or lower (gray). Statistical significance of the estimated ORs is presented next to the CI bars. *P < .05; **p<0.01; ***P < .001. Abbreviations: AHPs, allied health professionals; COVID-19, coronavirus disease 2019; ICT, Information Computing Technology.
Table 2.
Association of Risk Factors With COVID-19 Seropositivity
| Characteristic | Total, No. | Seropositive, No. | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Demographic characteristics | ||||||
| Age, y | ||||||
| 17–30 | 719 | 142 | 1.0 (reference) | … | 1.0 (reference) | … |
| 30–40 | 752 | 174 | 1.2 (1.0–1.6) | .114 | 1.2 (.9–1.6) | .146 |
| 40–50 | 469 | 142 | 1.8 (1.4–2.3) | <.001*** | 1.7 (1.3–2.3) | <.001*** |
| 50–60 | 331 | 104 | 1.9 (1.4–2.5) | <.001*** | 1.8 (1.3–2.4) | <.001*** |
| 60–80 | 107 | 31 | 1.7 (1.0–2.6) | .03* | 1.7 (1.0–2.7) | .043* |
| Sex | ||||||
| Male | 496 | 138 | 1.0 (reference) | … | 1.0 (reference) | … |
| Female | 1865 | 455 | 0.8 (.7–1.1) | .118 | 0.9 (.7–1.2) | .6 |
| Undetermined | 17 | 0 | … | … | … | … |
| Ethnicity | ||||||
| White | 1639 | 373 | 1.0 (reference) | … | 1.0 (reference) | … |
| Any other | 46 | 12 | 1.2 (.6–2.3) | .596 | 1.0 (.5–2.0) | .987 |
| Arab | 25 | 7 | 1.3 (.5–3.1) | .537 | 1.3 (.5–3.1) | .556 |
| South Asian | 333 | 88 | 1.4 (1.0–1.8) | .015* | 1.2 (.9–1.6) | .23 |
| Black | 218 | 83 | 2.1 (1.6–2.8) | <.001*** | 1.7 (1.2–2.3) | .003** |
| Chinese | 41 | 9 | 1.0 (.4–1.9) | .903 | 1.2 (.5–2.5) | .667 |
| Mixed | 76 | 21 | 1.3 (.8–2.1) | .325 | 1.2 (.7–2.1) | .427 |
| Occupational characteristics | ||||||
| Occupation | ||||||
| AHPs | 548 | 136 | 1.0 (reference) | … | 1.0 (reference) | … |
| Cleaning/Catering/Porters | 121 | 51 | 2.2 (1.5–3.3) | <.001*** | 1.4 (.8–2.2) | .196 |
| Doctor | 342 | 79 | 0.9 (.7–1.2) | .56 | 0.8 (.6–1.1) | .232 |
| ICT | 28 | 10 | 1.7 (.7–3.7) | .2 | 1.3 (.6–3.0) | .512 |
| Manager | 138 | 35 | 1.0 (.7–1.6) | .895 | 1.0 (.6–1.6) | .979 |
| Nurse | 859 | 205 | 1.0 (.7–1.2) | .684 | 1.1 (.8–1.4) | .592 |
| Other | 154 | 43 | 1.2 (.8–1.7) | .435 | 1.0 (.7–1.6) | .832 |
| Scientist | 188 | 34 | 0.7 (.4–1.0) | .06 | 0.7 (.4–1.0) | .064 |
| Working from home | ||||||
| No | 1748 | 440 | 1.0 (reference) | … | 1.0 (reference) | … |
| Yes | 630 | 153 | 1.0 (.8–1.2) | .659 | 0.9 (.7–1.2) | .486 |
| Regular income | ||||||
| Yes | 835 | 155 | 1.0 (reference) | … | … | … |
| No | 100 | 33 | 2.2 (1.4–3.4) | .001** | … | … |
| Difficulty accessing sick leavea | ||||||
| No | 2134 | 497 | 1.0 (reference) | … | … | … |
| Yes | 244 | 96 | 2.1 (1.6–2.8) | <.001*** | 1.8 (1.3–2.4) | <.001*** |
| Environmental characteristics | ||||||
| Public transport | ||||||
| No | 473 | 123 | 1.0 (reference) | … | … | … |
| Yes | 637 | 128 | 0.7 (.5–1.0) | .02* | … | … |
| Known contact with COVID-19 | ||||||
| No | 1496 | 328 | 1.0 (reference) | … | 1.0 (reference) | … |
| Yes: household | 263 | 75 | 1.4 (1.1–1.9) | .019* | 1.6 (1.2–2.2) | .002** |
| Yes: other | 60 | 22 | 2.1 (1.2–3.5) | .009** | 1.9 (1.1–3.3) | .029* |
| Yes: patient | 65 | 14 | 1.0 (.5–1.7) | .941 | 1.2 (.6–2.1) | .651 |
| Yes: staff | 402 | 125 | 1.6 (1.3–2.0) | <.001*** | 1.8 (1.4–2.4) | <.001*** |
| Yes: travel | 92 | 29 | 1.6 (1.0–2.6) | .034* | 1.9 (1.2–3.0) | .008** |
| Crowding | ||||||
| Multigenerational household | ||||||
| No | 2233 | 557 | 1.0 (reference) | … | 1.0 (reference) | … |
| Yes | 145 | 36 | 1.0 (.7–1.4) | .975 | 0.9 (.6–1.4) | .689 |
| Household rooms | ||||||
| No. of rooms | 2378 | … | 1.0 (1.0–1.1) | .98 | … | … |
| Household members | ||||||
| No. of members | 2378 | … | 1.1 (1.1–1.2) | <.001*** | … | … |
| Children | ||||||
| No. of children <5 y | 864 | … | 1.0 (.7–1.2) | .774 | … | … |
| Household index | ||||||
| Household members/rooms | 2378 | … | 1.5 (1.2–2.0) | <.001*** | 1.4 (1.1–1.9) | .006** |
| Socioeconomic characteristics | ||||||
| Deprivation index quantileb | ||||||
| 4 (least deprived) | 501 | 120 | 1.0 (reference) | … | 1.0 (reference) | … |
| 3 | 635 | 149 | 1.0 (.7–1.3) | .848 | 1.0 (.8–1.3) | .978 |
| 2 | 768 | 195 | 1.1 (.8–1.4) | .562 | 1.0 (.8–1.4) | .767 |
| 1 (most deprived) | 474 | 129 | 1.2 (.9–1.6) | .243 | 1.1 (.8–1.4) | .76 |
Statistical significance of the logistic regression presented next to the P values.
Abbreviations: AHPs, allied health professionals; CI, confidence interval; COVID-19, coronavirus disease 2019; ICT, Information Computing Technology; OR, odds ratio.
aDo any working people in your household have difficulty accessing sick leave/pay?
bIndex of multiple deprivation as reported by the United Kingdom Ministry of Housing, Communities and Local Government (www.gov.uk/government/statistics/english-indices-of-deprivation-2019).
* P < .05.
** P < .01.
*** P < .001.
Occupational Risks of SARS-CoV-2 Infection or Exposure
Seropositivity varied by specialty; however, occupations with known exposure to aerosolizing procedures (anesthetics, pediatric intensive care unit [ICU], cardiac ICU) did not have increased risk of seropositivity relative to staff working on other inpatient or outpatient wards.
Univariate analysis identified cleaners, porters, and catering staff as having the highest risk of seropositivity at 42.1% (OR, 2.2 [95% CI, 1.5–3.3]; P < .001). The second highest occupational rate was in the Information Computing Technology (ICT) Department at 35.7%, though this was not statistically significant on univariate analysis (OR, 1.7 [95% CI, .7–3.7]; P = .512). One potential explanation for this finding is that ICT is a small department.
Overall, on multivariate analysis, no occupation was found to have a statistically significant risk of seropositivity (Figure 1). Clinical staff did not have higher rates of seropositivity than nonclinical staff, and working from home did not impact rates of seropositivity.
Contact With COVID-19/Symptoms of COVID-19
In total, 34.4% of staff had a known contact with COVID-19 at the time of the survey. Compared to those that did not have a known contact with COVID-19, those with a known contact had an increased risk of seropositivity (30% compared to 21.9%), except if the known contact was a patient. Contact involving travel to Italy, China, Iran, or South Korea between the months of December 2019 and February 2020 (OR, 1.9 [95% CI, 1.2–3.0]; P = .008), other staff members (OR, 1.8 [95% CI, 1.4–2.4]; P < .001), household members (OR, 1.6 [95% CI, 1.2–2.2]; P = .002), and other SARS-CoV-2 contact (OR, 1.9 [95% CI, 1.1–3.3]; P = .029) all remained statistically significant on the multivariate analysis. Thirty-eight percent (347/911) of those staff who reported symptoms consistent with SARS-CoV-2 infection were seropositive, compared to 13% (365/2735) of asymptomatic staff.
Socioeconomic Risks of SARS-CoV-2 Infection or Exposure
Staff that lived in households in which 1 or more household members did not have access to adequate sick leave had a statistically significant increased risk of COVID-19 (OR, 1.8 [95% CI, 1.3–2.4]; P < .001) (Figure 1). Staff who self-reported that their income was not always enough to cover basic needs of housing, transport, and food had higher rates of COVID-19: 33% compared with 15.7% in those who did not (OR, 2.2 [95% CI, 1.4–3.4]; P = .001). However, this was not included in the multivariate analysis as >30% of the entries contained missing values. Moreover, entries with missing data regarding income were not missing at random, with seropositive individuals characterized by a higher OR of income missing values and some ethnicities such as Black or Asian presenting lower ORs of income missing values when compared to White (Table 3). Areas of social deprivation based on postal codes were analyzed for COVID-19 risk. The quartiles of lowest to highest rates of deprivation did not show significant increased risk on multivariate analysis.
Table 3.
Association Between Variables and Missing “Regular Income” Data
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Seropositivity | 1.8 (1.5–2.2) | <.001*** |
| Age, y | ||
| 17–30 | 1.0 (reference) | … |
| 30–40 | 1.2 (.9–1.5) | .14 |
| 40–50 | 1.3 (1–1.7) | .057 |
| 50–60 | 1.6 (1.2–2.1) | .004** |
| 60–80 | 1 (.6–1.6) | .94 |
| Sex | ||
| Male | 1.0 (reference) | … |
| Female | 1 (.8–1.2) | .9 |
| Undetermined | 1.5 (.5–4.9) | .45 |
| Ethnicity | ||
| White | 1.0 (reference) | … |
| Any other | .5 (.3–.9) | .031 |
| Arab | .5 (.2–1.2) | .12 |
| South Asian | .7 (.5–.9) | .003** |
| Black | .6 (.4–.8) | <.001*** |
| Chinese | 1.2 (.6–2.5) | .55 |
| Mixed | 1 (.6–1.7) | .86 |
| Occupation | ||
| AHPs | 1.0 (reference) | … |
| Cleaning/Catering/Porters | .3 (.2–.5) | <.001*** |
| Doctor | 1.1 (.8–1.5) | .65 |
| ICT | 1 (.4–2.4) | .99 |
| Manager | 1.1 (.7–1.7) | .62 |
| Nurse | 1.2 (.9–1.5) | .13 |
| Other | 0.7 (.5–1) | .03* |
| Scientist | 1.7 (1.2–2.5) | .004** |
| Working from home | ||
| No | 1.0 (reference) | … |
| Yes | 1.1 (.9–1.4) | .25 |
| Difficulty accessing sick leave | ||
| No | … | … |
| Yes | .6 (.5–.8) | .0013** |
| Known contact with COVID-19 | ||
| No | 1.0 (reference) | … |
| Yes: household | 1.5 (1.2–2.1) | .003** |
| Yes: other | 1.1 (.6–2) | .7 |
| Yes: patient | 2 (1.1–3.6) | .021* |
| Yes: staff | 1.5 (1.2–1.9) | .0012** |
| Yes: travel | 1.3 (.8–2) | .29 |
| Multigenerational household | ||
| No | 1.0 (reference) | … |
| Yes | .8 (.5–1.1) | .17 |
| Household index | ||
| Household members/rooms | .9 (.7–1.2) | .52 |
| Deprivation index quartile | ||
| 4 (least deprived) | 1.0 (reference) | … |
| 3 | 1 (.8–1.3) | .82 |
| 2 | 1.1 (.9–1.4) | .45 |
| 1 (most deprived) | 1.2 (.9–1.6) | .26 |
Statistical significance of the logistic regression is presented next to the P values.
Abbreviations: AHPs, allied health professionals; CI, confidence interval; COVID-19, coronavirus disease 2019; ICT, Information Computing Technology; OR, odds ratio.
* P < .05.
** P < .01.
*** P < 0.001.
Environmental Risks of SARS-CoV-2 Infection or Exposure
Presence of children under the age of 5 in the household did not change seropositivity rates of COVID-19. Neither did households that had >2 generations of family members. An increasing number of household members marginally increased the risk of COVID-19 on univariate analysis (OR, 1.1 [95% CI, 1.1–1.2]; P < .001). More notable was that an increased ratio of household members relative to rooms in the house increased the risk of COVID-19, which remained significant on multivariate analysis (OR, 1.4 [95% CI, 1.1–1.9]; P = .006).
DISCUSSION
This large prospective study of HCWs in a pediatric tertiary referral hospital demonstrated that lack of access to sick pay, relative household overcrowding, Black ethnicity, and increasing age were independently associated with SARS-CoV-2 seropositivity.
GOSH is a large tertiary pediatric hospital in central London. The hospital faced unprecedented demand as pediatric units across London were closed to increase adult bed capacity. However, thankfully, relative to adult centers, very few inpatients were severely infected with acute respiratory COVID-19 during the first wave of the pandemic. At GOSH, all inpatients were tested for COVID-19 on admission and were admitted to side rooms pending test results. Only 53 inpatients were diagnosed with COVID-19 during the period when this study was conducted. It is therefore unsurprising that contact with a patient with COVID-19 was not shown to be a risk factor for seropositivity in this study, whereas other types of contact with COVID-19 were. This is supported by findings by Goldblatt et al demonstrating low in-hospital transmission rates among HCWs in pediatric facilities in 8 European countries [24]. Moreover, clinical staff did not have higher rates of seropositivity than nonclinical staff. This suggests that seropositive staff members primarily acquired COVID-19 either from other staff members at work or outside work [25–27].
Structural inequalities related to socioeconomic status (SES) and ethnicity have been directly linked with COVID-19 [28–30]. HCWs from a lower socioeconomic background are more likely to live in overcrowded housing, which is a risk factor for respiratory illnesses [31]. Many studies use occupation as a surrogate for SES but have not investigated how income, job security, household environment, and living in an area of social deprivation may impact SARS-CoV-2 seropositivity [5, 6, 32]. Studies of HCWs in other UK hospitals found that HCWs from minority ethnic groups had a significantly increased risk of seropositivity (OR, 1.92 [95% CI, 1.14–3.23]; P = .01) [7].
Staff in nonclinical roles such as cleaners, porters, and catering staff had the highest risk of acquiring SARS-CoV-2 prior to consideration of confounders in a multivariate analysis. This observed higher risk among nonclinical support staff compared to clinical staff has also been reported by other studies from the UK, Norway, and the United States [12, 13, 33]. The fact that occupation did not remain a significant risk factor after controlling for confounders suggests that the postulated association between occupational risk and SARS-CoV-2 seropositivity is actually due to underlying demographic and socioeconomic factors.
Lack of access to sick pay was independently associated with higher rates of SARS-CoV-2 seropositivity. In the UK, 27% of NHS Estates and Facilities workers (which includes cleaners, porters, catering, security, engineering, capital delivery, and maintenance staff) are outsourced to service delivery partners, 7% are employed by NHS wholly owned subsidiaries, and 66% are directly employed by the NHS [34]. The ONS reports that as of April–June 2022, 20.2% of workers in the field of health and social care are on zero-hours contracts, and a Freedom of Information request submitted by the Financial Times found that in 2013, NHS hospitals used almost 100 000 zero-hours contracts [35, 36]. While zero-hours contracts are legal in the UK [37], lower-paid staff members who cannot afford to lose income are more likely to work when they are unwell or have had a COVID-19 contact [38]. This economic vulnerability may make self-isolation challenging and could contribute to the spread of COVID-19 and other infectious diseases among nonclinical healthcare staff. The US Centers for Disease Control and Prevention has highlighted the importance of avoiding incentives that encourage people to come to work when symptomatic [39].
Overcrowded housing is a recognized risk factor for respiratory and other infectious diseases, and overcrowding affects 3% of households in England, with ethnic minorities disproportionately affected [40, 41]. It is therefore unsurprising that there was an increased risk of seropositivity with a higher ratio of household members to rooms (OR, 1.4 [95% CI, 1.1–1.9]; P = .006). Overcrowded housing is also considered a measure of poverty and further highlights the impact that SES has on COVID-19 infection among HCWs [42].
The role that ethnicity plays in SARS-CoV-2 seropositivity has been discussed by many preceding studies [43–45]. We found a significant association between seropositivity and both South Asian and Black ethnicity on the univariate analysis. This correlates with findings from other studies in the UK [6, 28]. Studies in the United States have shown that Black and South Asian workers are more likely to be employed in healthcare, social assistance, and other essential industries [46, 47]. ONS data have shown that while minority ethnic groups have higher rates of death from COVID-19, much of this difference is attributable to socioeconomic factors, living conditions, and occupational exposure [48, 49]. In the UK, poverty rates are the highest in the Bangladeshi (65%) community, followed by Pakistani (55%), Black African (45%), Black Caribbean (30%), Indian (25%), and White British (20%) [31]. On multivariate analysis, only Black ethnicity remained significant, whereas South Asian ethnicity did not. There may be other unmeasured confounding factors that contribute to the Black ethnic risk of SARS-CoV-2 infection that we have not identified in our analysis.
This study benefited from a large, diverse, engaged cohort of recruited HCWs. Other strengths included the collection of data on a wide range of demographic, occupational, and socioeconomic factors, as well as data on exposure to COVID-19. This allowed a detailed consideration of the influence of socioeconomic and environmental variables, which are often overlooked. Our study design ensured that our cohort was truly representative of the entirety of HCWs in the hospital by actively recruiting cleaners, porters, and catering staff, who were harder to reach due to lack of access to NHS Trust emails (these staff members at the time were employed by external organizations and so may not have had NHS email accounts). This enabled us to achieve similar recruitment levels for all staff groups. Additionally, our data were gathered over a relatively short time period (April–September 2020), and we used the MSD assay to test antibodies against SARS-CoV-2 [19, 50], meaning that we would not expect antibodies to have waned during this time period. Consequently, seropositivity rates are more likely to be a true reflection of exposure to COVID-19.
There are, however, some important limitations. Data including some socioeconomic factors such as difficulty accessing sick leave were self-reported and could be subject to reporting bias. These data could be useful to elucidate whether this behavior was driven primarily by a lack of contractual sick leave, or by perceptions about the effects of taking sick leave on their employment—which may be driven by wider socioeconomic and workplace factors. Staff on lower incomes may feel unable to isolate even if they do have access to statutory sick leave, influenced by their overall economic precarity, concern about repercussions, or a sense of duty to their work. There was also a small proportion of staff who declined to participate in the study (36 participants or 0.99% of those recruited), and the lack of their demographic and occupational data makes it difficult to determine the extent that this may have influenced transmission.
The COVID-19 pandemic highlighted the effect of structural and ethnic inequality on communicable disease in the UK [48]. This is corroborated in our data demonstrating an increased risk of SARS-CoV-2 seropositivity in staff reporting difficulty accessing sick leave, those living in overcrowded housing, and those of Black ethnicity. These data emphasize the importance of taking economic, ethnic, and social factors into account when forming public health policy, and underscore the impacts of social determinants of health in the UK. Since the pandemic, cleaning staff at GOSH have been brought in house, under full hospital employment, and several NHS trusts have done the same [51]. This should be considered around the country to ensure that all staff have equitable access to information, participation in the life of the hospital, and a safe place to work.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Tanya Lam, Department of Infectious Diseases, Great Ormond Street Hospital, London, United Kingdom.
Anja Saso, Department of Infectious Diseases, Great Ormond Street Hospital, London, United Kingdom; Department of Tropical and Infectious Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom; Medical Research Council Gambia at London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Arturo Torres Ortiz, Department of Infectious Diseases, Imperial College London, London, United Kingdom; Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
James Hatcher, Department of Microbiology, Great Ormond Street Hospital, London, United Kingdom.
Marc Woodman, Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
Shruthi Chandran, Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
Rosie Thistlethwayte, Management, Great Ormond Street Hospital, London, United Kingdom.
Timothy Best, Department of Microbiology, Great Ormond Street Hospital, London, United Kingdom.
Marina Johnson, Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
Helen Wagstaffe, Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
Annabelle Mai, Clinical Immunology, Camelia Botnar Laboratories, Great Ormond Street Hospital, London, United Kingdom.
Matthew Buckland, Clinical Immunology, Camelia Botnar Laboratories, Great Ormond Street Hospital, London, United Kingdom.
Kimberly Gilmour, Clinical Immunology, Camelia Botnar Laboratories, Great Ormond Street Hospital, London, United Kingdom.
David Goldblatt, Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
Louis Grandjean, Department of Infection, Immunity and Inflammation, Institute of Child Health, University College London, London, United Kingdom.
COVID-19 Staff Testing of Antibody Responses (Co-STARs) Study Team:
Dorcas Mirambe-Korsah, Fernanda Fenn Torrente, Jakub Wyszynski, Victoria Gander, Amy Leonard, Louise Myers, Aimee Vallot, Camille Paillas, Rose Fitzgerald, Adam Twigg, Rabia Manaf, Lois Gibbons, Hollie Powell, Richard Nar-Dorh, Ally Gray, Elias Fernandez, Aline Minja, Emily Beech, Waffa Girshab, Pei Shi Chia, Kate Webb, Malti Nakrani, Kim Gardiner, Valerija Karaluka, Karen Ryan, Dorothy Lee, Katie Groves, Hamad Khan, Shamime Nsubuga, Olivia Rosie-Wilkinson, Julia Spires, Nuria Sanchez-Clemente, Sapriya Kaur, Natasha Carroll, Jemma Efford, Gabriel Bredin, Celma Marisa Dos Santos Domingues, Sophie Foxall, Helen Ashton, Abbey Afzal, Sally Mainland, Kate Crumpler, Lucinda Dawson, Claire Smith, Maria Tabbu, Laura Chiverton, Jade Sugars, Jordan Mooney, Dorothy Chikusu, Fariba Tahami, Baratth Samy, Shomona Begum, Dhimple Patel, Philippa Wiltshire, Annie Susay, Anna Ryan, Luke Lancaster, Kavita Thind, Kate Speller, Rachel Sterling, Connor Tugulu, Sandhya Ghurburrun, Steffi Gray, Joy Mugas, Moe Kishma, Kathleen Akpokomua, Sophie White, Eleana Pieri, Sabina Shamsad, Demi Alexandrou, Odera Aguele, Katherine Miles, Anamika Jain, Subishma Gautam, Oliver Simms, Rachel Goff, Zarif Shams, Tinya Chirinda, Aaliya Nur, and Tarekur Rahman
Notes
Acknowledgments. The authors dedicate this article to the staff members at Great Ormond Street Hospital (GOSH) who died of coronavirus disease 2019 (COVID-19) during the first wave of the pandemic. The authors also thank all of the staff at GOSH who took part in the study. In addition, the authors are grateful for all the hard work undertaken by the Great Ormond Street laboratory staff and the staff in the immunology laboratories, both in the Camelia Botnar Laboratory and the Great Ormond Street Institute of Child Health, who ensured that the polymerase chain reaction tests and serological assays were completed in a timely manner. Finally, the authors acknowledge the support of the GOSH Research and Development, Governance, Finance, Management, Estates, Operations, and Communications departments.
Co-STARs Study Team. Dorcas Mirambe-Korsah, Fernanda Fenn Torrente, Jakub Wyszynski, Victoria Gander, Amy Leonard, Louise Myers, Aimee Vallot, Camille Paillas, Rose Fitzgerald, Adam Twigg, Rabia Manaf, Lois Gibbons, Hollie Powell, Richard Nar-Dorh, Ally Gray, Elias Fernandez, Aline Minja, Emily Beech, Waffa Girshab, Pei Shi Chia, Kate Webb, Malti Nakrani, Kim Gardiner, Valerija Karaluka, Karen Ryan, Dorothy Lee, Katie Groves, Hamad Khan, Shamime Nsubuga, Olivia Rosie-Wilkinson, Julia Spires, Nuria Sanchez-Clemente, Sapriya Kaur, Natasha Carroll, Jemma Efford, Gabriel Bredin, Celma Marisa Dos Santos Domingues, Sophie Foxall, Helen Ashton, Abbey Afzal, Sally Mainland, Kate Crumpler, Lucinda Dawson, Claire Smith, Maria Tabbu, Laura Chiverton, Jade Sugars, Jordan Mooney, Dorothy Chikusu, Fariba Tahami, Baratth Samy, Shomona Begum, Dhimple Patel, Philippa Wiltshire, Annie Susay, Anna Ryan, Luke Lancaster, Kavita Thind, Kate Speller, Rachel Sterling, Connor Tugulu, Sandhya Ghurburrun, Steffi Gray, Joy Mugas, Moe Kishma, Kathleen Akpokomua, Sophie White, Eleana Pieri, Sabina Shamsad, Demi Alexandrou, Odera Aguele, Katherine Miles, Anamika Jain, Subishma Gautam, Oliver Simms, Rachel Goff, Zarif Shams, Tinya Chirinda, Aaliya Nur, and Tarekur Rahman.
Disclaimer. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. L. G. was supported by the Wellcome Trust (award numbers 201470/Z/16/Z and 226007/Z/22/Z); the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award number 1R01AI146338); the GOSH Charity (award number VC0921); and the GOSH/Institute of Child Health Biomedical Research Centre (www.nihr.ac.uk). A. S. was supported by the Wellcome Trust (award number 220565/Z/20/Z).
References
- 1. United Kingdom Office for National Statistics . Which occupations have the highest potential exposure to the coronavirus (COVID-19)? 2020. Available at: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/articles/whichoccupationshavethehighestpotentialexposuretothecoronaviruscovid19/2020-05-11. Accessed 28 October 2022.
- 2. Oygar PD, Büyükçam A, ZŞ B, et al. SARS-CoV-2 seropositivity among pediatric health care personnel after the first peak of the pandemic: nationwide surveillance in Turkey. Int J Infect Dis 2021; 113:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guarnieri V, Moriondo M, Giovannini M, et al. Surveillance on healthcare workers during the first wave of SARS-CoV-2 pandemic in Italy: the experience of a tertiary care pediatric hospital. Front Public Health 2021; 9:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tatsi EB, Dellis C, Petridou E, et al. SARS-CoV-2 seroepidemiological study in healthcare workers and discordant results using seven different diagnostic methods. Infection 2022; 50:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect 2021; 108:120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife 2020; 9:1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax 2020; 75:1089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA 2020; 324:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Self WH, Tenforde MW, Stubblefield WB, et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020; 20:1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gómez-Ochoa SA, Franco OH, Rojas LZ, et al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2021; 190:161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Molvik M, Danielsen AS, Grøsland M, Telle KE, Kacelnik O, Eriksen-Volle HM. SARS-CoV-2 in health and care staff in Norway, 2020. Tidsskr Nor Laegeforen 2021; 141. [DOI] [PubMed] [Google Scholar]
- 13. Barrett ES, Horton DB, Roy J, et al. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in hospital workers: results from a screening study in New Jersey, United States in spring 2020. Open Forum Infect Dis 2020; 7:ofaa534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore G, Rickard H, Stevenson D, et al. Detection of SARS-CoV-2 within the healthcare environment: a multi-centre study conducted during the first wave of the COVID-19 outbreak in England. J Hosp Infect 2021; 108:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020; 323:1610–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris CR, Sullivan P, Mantus G, et al. Prevalence of SARS-CoV-2 antibodies in pediatric healthcare workers. Int J Infect Dis 2021; 105:474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ceruelo EE, Ruíz MAE, López-Peláez MO, Garoz BF, Antón JA, García RJ. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a pediatric monographic hospital in Madrid (Spain). Enferm Infecc Microbiol Clin (Engl Ed) 2022; 40:326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heyming TW, Sanger T, Tongol A, Schomberg J, Bacon K, Lara B. Provider antibody serology study of virus in the emergency room (PASSOVER) study: special population COVID-19 seroprevalence. West J Emerg Med 2021; 22:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grandjean L, Saso A, Torres Ortiz A, et al. Long-term persistence of spike protein antibody and predictive modeling of antibody dynamics after infection with severe acute respiratory syndrome coronavirus 2. Clin Infect Dis 2022; 74:1220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ClinicalTrials.gov . COVID-19 staff testing of antibody responses study (Co-STARs). Available at: https://clinicaltrials.gov/ct2/show/NCT04380896. Accessed 28 October 2022.
- 21. United Kingdom Office for National Statistics . Ethnic group, national identity and religion. 2021. Available at: https://www.ons.gov.uk/methodology/classificationsandstandards/measuringequality/ethnicgroupnationalidentityandreligion. Accessed 19 July 2023.
- 22. Government of the United Kingdom . English indices of deprivation 2019. 2019. Available at: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019. Accessed 6 November 2022.
- 23. R Core Team . The R project for statistical computing. Available at: https://www.r-project.org/. Accessed 28 October 2022.
- 24. Goldblatt D, Johnson M, Falup-Pecurariu O, et al. Cross-sectional prevalence of SARS-CoV-2 antibodies in healthcare workers in paediatric facilities in eight countries. J Hosp Infect 2021; 110:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia-Basteiro AL, Moncunill G, Tortajada M, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun 2020; 11:3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kluytmans-Van Den Bergh MFQ, Buiting AGM, Pas SD, et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Network Open 2020; 3:e209673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020; 20:1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathur R, Rentsch CT, Morton CE, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet 2021; 397:1711–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel JA, Nielsen FBH, Badiani AA, et al. Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health 2020; 183:110–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abuelgasim E, Saw LJ, Shirke M, Zeinah M, Harky A. COVID-19: unique public health issues facing Black, Asian and minority ethnic communities. Curr Probl Cardiol 2020; 45:100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joseph Rowntree Foundation . Poverty rates among ethnic groups in Great Britain. 2007. Available at: https://www.jrf.org.uk/report/poverty-rates-among-ethnic-groups-great-britain. Accessed 28 October 2022.
- 32. Alishaq M, Jeremijenko A, Al-Kanaani Z, et al. Prevalence and risk factors for SARS-CoV-2 infection and seroprevalence among clinical and non-clinical staff in a national healthcare system. PLoS One 2021; 16:e0257845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA 2020; 324:893–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Health Service . Estates and facilities workforce action plan: building, developing and engaging our people. 2022. Available at: https://www.england.nhs.uk/wp-content/uploads/2022/06/B0292-NHS-estates-and-facilities-workforce-action-plan.pdf. Accessed 1 September 2023.
- 35. Financial Times . Employers increase zero hours contracts. 2013. Available at: https://www.ft.com/content/04a86a6c-9f8a-11e2-b4b6-00144feabdc0. Accessed 28 October 2022.
- 36. Office for National Statistics . EMP17: people in employment on zero hours contracts. 2023. Available at: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/datasets/emp17peopleinemploymentonzerohourscontracts. Accessed 28 October 2022.
- 37. Government of the United Kingdom . Zero hours contracts: guidance for employers. 2015. Available at: https://www.gov.uk/government/publications/zero-hours-contracts-guidance-for-employers/zero-hours-contracts-guidance-for-employers. Accessed 19 July 2023.
- 38. Himmelstein DU, Woolhandler S. Health insurance status and risk factors for poor outcomes with COVID-19 among U.S. health care workers: a cross-sectional study. Ann Intern Med 2020; 173:410–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dyal JW, Grant MP, Broadwater K, et al. COVID-19 among workers in meat and poultry processing facilities—19 states, April 2020. MMWR Morb Mortal Wkly Rep 2020; 69. [DOI] [PubMed] [Google Scholar]
- 40. Government of the United Kingdom . Ethnicity facts and figures: overcrowded households. 2023. Available at: https://www.ethnicity-facts-figures.service.gov.uk/housing/housing-conditions/overcrowded-households/latest. Accessed 28 October 2022.
- 41. Krieger J, Higgins DL. Housing and health: time again for public health action. Am J Public Health 2002; 92:758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Social Metrics Commission . A new measure of poverty for the UK. 2018. Available at: https://socialmetricscommission.org.uk/MEASURING-POVERTY-summary-report.pdf. Accessed 1 September 2023.
- 43. Apea VJ, Wan YI, Dhairyawan R, et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open 2021; 11:e042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med 2021; 174:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis 2021; 72:703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med 2020; 63:817–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roberts JD, Dickinson KL, Koebele E, et al. Clinicians, cooks, and cashiers: examining health equity and the COVID-19 risks to essential workers. Toxicol Ind Health 2020; 36:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marmot M, Allen J. COVID-19: exposing and amplifying inequalities. J Epidemiol Community Health 2020; 74:681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. United Kingdom Office for National Statistics . Coronavirus (COVID-19) related deaths by ethnic group, England and Wales. 2020. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/coronavirusrelateddeathsbyethnicgroupenglandandwales/2march2020to10april2020. Accessed 28 October 2022.
- 50. Torres Ortiz A, Fenn Torrente F, Twigg A, et al. The influence of time on the sensitivity of SARS-CoV-2 serological testing. Sci Rep 2022; 12:10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Financial Times . NHS trust brings workers in-house after industrial action. 2020. Available at: https://www.ft.com/content/d2cabb4a-4373-11ea-a43a-c4b328d9061c. Accessed 28 October 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.