Abstract
Nanofitins are small and hyperthermostable alternative protein scaffolds that display physicochemical properties making them suitable for the development of topical therapeutics, notably for the treatment of pulmonary infectious diseases. Local administration of biologics to the lungs involves a particularly stressful step of nebulization that is poorly tolerated by most antibodies, which limits their application by this delivery route. During the COVID-19 pandemic, we generated anti-SARS-CoV-2 monomeric Nanofitins of high specificity for the spike protein. Hit Nanofitin candidates were identified based on their binding properties with punctual spike mutants and assembled into a linear multimeric construction constituting of four different Nanofitins, allowing the generation of a highly potent anti-SARS-CoV-2 molecule. The therapeutic efficacy of the multimeric assembly was demonstrated both in in vitro and in vivo models. Interestingly, the neutralization mechanism of the multimeric construction seems to involve a particular conformation switch of the spike trimer. In addition, we reported the stability and the conserved activity of the tetrameric construction after nebulization. This advantageous developability feature for pulmonary administration associated with the ease of assembly, as well as the fast generation process position the Nanofitin technology as a potential therapeutic solution for emerging infectious diseases.
Keywords: protein scaffold, Nanofitin, nebulization, pulmonary delivery, antibody mimetic, infectious disease, inhalable
Graphical abstract

Viollet et al. describe the successful generation of potent and inhalable anti-SARS-CoV-2 Nanofitin and more generally the potential of the Nanofitin technology for the treatment of pulmonary infectious diseases by local administration.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has highlighted the dangers posed by novel infectious diseases and the need for rapid therapeutic responses to alleviate the burden on health care systems, both in the absence of and in addition to vaccines. While vaccines have been made available quickly due to global efforts, therapeutics are still necessary, not only to protect unvaccinated or poorly responding individuals, but also to face the rapid decline in vaccine effectiveness.
The symptoms of COVID-19 can vary greatly from no or mild symptoms to acute respiratory distress syndrome, potentially resulting in death from a concomitant uncontrolled inflammation.1,2,3 Multiple biologics have been developed or repurposed for the treatment of COVID-19 infection, focusing either on neutralizing inflammatory pathways or on neutralizing the infection by blocking the interaction between the viral spike protein and the host receptor angiotensin-converting enzyme 2 (ACE2). For the latter, most of the generated antibodies are directed against the spike protein and more precisely its receptor binding domain (RBD).4 However, the high mutation rate within the RBD has limited the isolation of pan-specific antibodies displaying a high neutralization potency against all the different variants of SARS-CoV-2 that have emerged since the beginning of the pandemic. Cocktails of antibodies have been developed to mitigate the risk of treatment ineffectiveness by broadening the range of therapeutic coverage against COVID-19 variants, with some level of efficacy demonstrated, but yet a poor effect on patients infected with the highly mutated Omicron variant.5,6
SARS-CoV-2 infection and replication begin in the upper respiratory tract and can progress over time to the deeper regions of the lungs in severe cases of the disease.7 Detection of respiratory viral infectious particles in the blood is relatively uncommon,8 yet all currently approved treatments involve administering antibodies through injection. This approach has limitations in clinical practice. Specifically, the slow distribution of antibodies from the blood to respiratory fluids requires high doses and delays in reaching therapeutic concentrations at the site of the infection of 1 or more days.9 This reduces the number of treatment courses available, narrows the therapeutic window for preventing severe COVID-19, and highlights the relevance of non-systemic treatments for this respiratory infectious disease. Nebulizer-assisted inhalation has the potential to overcome these limitations by quickly delivering a high drug load throughout the entire respiratory tract. Unfortunately, antibodies are highly sensitive to nebulization and aggregate during the process, which may be associated with a loss of efficiency and raise safety concern.10,11,12
In this context, we explored the use of the Nanofitin alternative scaffold for the development of an inhalable local treatment for SARS-CoV-2. Nanofitins are small and robust alternative protein scaffolds derived from Sac7d, a 66-residue protein found in the hyperthermophilic archaeon Sulfolobus acidocaldarius.13 Randomizing surface residues of Sac7d generates libraries of Nanofitins, from which highly specific binders can be selected while preserving their chemical and thermal stability.14 Additionally, their N- and C-termini end being on opposite faces of their variable domain, Nanofitins can be easily assembled into multi-specific/paratopic constructions using straightforward molecular approaches. In the case of COVID-19, multi-paratopic assembly based on alternative scaffolds can generate broadly neutralizing agent outperforming antibodies cocktails.15,16 Nanofitins’ extreme resistances to high temperature and extreme pH (pH 1–13) offer unique physico-chemical properties to streamline product development,17,18,19,20 notably in terms of manufacturing and formulation perspectives, positioning them as potential inhalable therapeutics in regard to the current therapeutic developments with other alternative scaffolds such as the inhalable anti-calin PRS-060.21
Here, anti-spike Nanofitins were generated and fused into a single chain multi-paratopic construct. We demonstrated its ability at reducing the viral load locally in vivo upon intranasal administration in huK18ACE2 mice challenged with SARS-CoV-2. Biodistribution in mice lung upon intratracheal administration and nebulization of the Nanofitin construct was investigated, showing a good distribution in the target organ as well as a full preservation of its functionality after aerosolization. Altogether, the data collected suggest that the Nanofitin scaffold is a promising modality for the development of local treatment against respiratory infectious diseases.
Results
Identification of anti-SARS-CoV-2 Nanofitins
Nanofitins are protein scaffolds that can be engineered for the specific molecular recognition of a given antigen through the randomization of 14 positions. The Nanofitins library was challenged by ribosome display through four consecutive rounds of selection to identify potential candidates of interest against the subunit 1 of the spike protein (Figure 1A). We isolated and sequenced 95 Nanofitin clones, leading to the selection of 31 unique Nanofitins compared for their ability to bind the spike S1 protein versus the RBD in a direct ELISA (Figure 1B). Of the 31 Nanofitins, 29 showed a positive signal at 1 μM against the S1 protein with 24 of them appearing to target the RBD (Figure 1C). Further characterizations were performed on four Nanofitin candidates: three Nanofitins binding the RBD (NF1, 2, and 3) and one Nanofitin interacting with another domain except the RBD (NF4). NF4’s absence of binding on the RBD domain was double confirmed by ELISA. The median effective concentrations (EC50) were determined for the three RBD binding Nanofitins with values of 0.6, 4.5, and 1.1 nM, respectively, for NF1, NF2, and NF3 (Figure 1D, left). Interestingly, NF4 was isolated from a panning campaign against the subunit 1 of the ancestral spike protein, but its affinity for the target seemed to be significantly improved in the context of the variant D614G. This was demonstrated in a direct ELISA, revealing an EC50 of 3 nM for the variant D614G (Figure 1D, left). The D614G mutation being known to have a strong impact on the structure of the trimeric spike protein, the observed increase of binding efficiency may reflect a better exhibition of the epitope of the Nanofitin 4 in the context of this mutation.22,23
Figure 1.
Selection and characterization of anti-spike Nanofitins
(A) Schematic representation of ribosome display selection for Nanofitin discovery. Nanofitin DNA libraries are designed and engaged into ribosome display cycle starting by their transcription in RNA and their translation to generate an RNA-ribosome-Nanofitin complex. Nanofitin complexes are selected against their target of interest and washed before their destabilization during the elution. Then, RNA is reverse transcribed and re-engaged for another round of selection or inserted into a final vector for screening and characterization. (B) Screening of Nanofitins in crude lysate after 4 rounds of ribosome display selection. (C) Evaluation of binding capacities to spike S1 and RBD. (D) Dose-response curves generated by ELISA for EC50 (left) and IC50 (right) determination. Inhibition was performed by measuring the interaction of spike protein to the human ACE2. Data are the average ± SEM of two or three experiments.
The ability of the Nanofitins 1–4 at neutralizing the spike-human ACE2 interaction was assessed by a competitive ELISA (Figure 1D, right). While the three RBD-targeting Nanofitins all displayed a neutralization potential to some extent, the Nanofitin 4 was unable to block the interaction between the spike protein and ACE2. Interestingly, the neutralization potency of the Nanofitins 1–3 (of 0.2, 127, and 47 nM, respectively) was highly correlated with their respective affinity (0.5, 380, and 31 nM, respectively) as measured by BLI (Figures 2A and 2B).
Figure 2.
Evaluation of tetrameric Nanofitin
(A) BLI binding analyses of monomeric and tetrameric Nanofitins to immobilized spike. (B) Binding kinetic parameters of Nanofitins on SARS-CoV-2 spike S1. (C) Neutralization of pseudotyped virus expressing D614G spike protein revealed by Luciferase assay in ACE2/TMPRSS2 expressing HEK293 cells. Data are the average ± SEM of two experiments containing three replicates each.
The binding efficiency of Nanofitins 1–3 has also been investigated by ELISA against several point mutants of the RBD (E484K, E484Q, L452R, and N439K) described in different SARS-CoV-2 variants (Figure S1). None of the three Nanofitins seemed to be impacted by the mutation N439K. The binding efficiency of both Nanofitins 1 and 3 was negatively impacted by the mutations of the residue E484, with a stronger impact observed for the mutation E484K as compared to the E484Q. Nanofitin 2 reacted differentially on these two mutants, with a decreased of its binding efficiency on the E484K, but an increase on the E484Q. A differential reactivity between the Nanofitins 1–3 was also observed with the mutation L452R, which totally abrogated the binding of the NF2 over the concentration range tested, decreased the binding efficiency of the NF3, and seemed to be relatively inert for Nanofitin 1. These data suggest that, while these three Nanofitins are targeting the same domain with an overlapping and neutralizing epitope, the topology of their complexes with the target most probably differs, making them differentially impacted by mutations within the RBD.
Multimeric Nanofitin demonstrates highly potent neutralization of SARS-CoV-2 infectivity in vitro
To strengthen the neutralization potency of the Nanofitin candidates and to mitigate the risk of detrimental mutations on the spike protein, we engineered a heterotetrameric structure by genetically fusing the RBD-binding modules NF1, NF2, and NF3 together with NF4, from the N- to C-terminus. This design aimed at providing an avidity effect by addressing the functional trimeric state of the spike protein, while generating a molecule of a higher molecular weight for a better retention in the pulmonary tract.11,24 We chose to incorporate three different RBD-binding modules instead of the repetition of three identical ones to mitigate the risk of a complete inhibition of the multimeric construct in case of a deleterious mutation on the RBD, as suggested by the diversity of binding property on the RBD single point mutants (Figure S1). Finally, NF4 was added because of its specificity for the commonly conserved D614G mutation to favor the anchorage of the tetramer through a distinct epitope.
Affinity of the tetrameric Nanofitin for the spike protein was evaluated by biolayer interferometry (BLI), revealing an increase in affinity with a dissociation constant (Kd) of 0.09 nM as compared with the respective monomers that are mainly driven by a slower dissociation rate (Figure 2B). Next, we compared the ability of the tetrameric Nanofitin at neutralizing the infectivity of SARS-CoV-2 pseudovirus in a cell-based assay to the most potent monomeric Nanofitin (NF1) and the therapeutic antibody casirivimab (Figure 2C).6 In this assay, the potency of the tetrameric Nanofitin was found to match that of the reference antibody (median inhibition concentration [IC50] of 61 and 85 pM, respectively), while NF1 displayed limited neutralization capacity (IC50 of 21 nM). These data are in agreement with early investigations performed on SARS-CoV-2 virus and showing a higher infectivity inhibition on A549 cell line of the heterodimer NF1-NF3 compared with its respective monomers. Viral RNA was collected in supernatant in absence or in presence of the test items and normalized with negative (PBS, 0%) and positive controls (remdesivir, 100%). While NF1 and NF3 demonstrated an infectivity inhibition effect only at the highest concentration tested of 1 μM, the dimer displayed a full dose response with a complete inhibition starting from 10 nM (Figure S2).
Despite our initial rationale, we observed that the tetrameric construct was not able to maintain its neutralization activity on the Omicron variant (Figure S3). However, we were able to apply our selection strategy again to identify in 3 months novel Nanofitin candidates with a rescued neutralization potential on this variant. This allowed the engineering of a second-generation tetramer displaying potent infectivity neutralization of Omicron pseudovirus (Figure S3).
Nanofitins induce conformational modification on the spike trimer
Negative staining electron microscopy was performed with monomeric and tetrameric Nanofitins to better understand their interaction on the spike trimer. Conformational changes of the spike protein were monitored depending on the Nanofitins tested: while the majority of spike trimers was in three RBDs down conformation without any Nanofitin, the protein switched to a one RBD up conformation with NF1 and NF3 (Figures 3A and 3B). Interestingly, the conformation switch induced by NF1 is concentration dependent and exclusively led to the stabilization of the one RBD up conformation in presence of an excess of the monomeric Nanofitin 1 (Figure 3B). In addition, spike trimers tested with tetrameric Nanofitin adopted a two RBDs-up conformation (Figures 3A and 3B). Based on the binding properties observed in ELISA and the conformational changes in electron microscopy, we proposed a model for the Nanofitin tetramer in which NF3 and NF4 generated a clamp blocking the third RBD in a down position and locking the spike trimer in a two RBD-up conformation (Figure 3C).
Figure 3.
Conformational switch of trimeric spike by Nanofitins
(A) Two-dimensional structures of trimeric spike/Nanofitin mix obtained by negative staining. Spike protein was mixed with NF1, NF3, and tetramer (at ratios of 1:78, 1:85, and 1:78, respectively). Scale bar, 10 nm. (B) Distribution of trimeric spike conformations mixed with different Nanofitins. Analysis on spike trimers was based on the counting of at least 2,000 cleaned particles for each condition. (C) Proposed model for the clamp conformation on the third RBD down. NF4 (in blue) and NF3 (in orange) maintain the third RBD (in black) in a down position because of their fusion by the protein linker of 30 amino acids (in red). We also add in the representation another NF (NF2 in yellow) interacting to an RBD (in gray) in an up position.
Multimeric Nanofitins are stable to nebulization
Stability of the Nanofitin tetramer to nebulization was assessed at two concentrations (1 and 8 mg/mL) in a non-optimized formulation (PBS) using the Aeroneb solo vibrating mesh nebulizer (from Aerogen) by monitoring the protein recovery yield, protein fragmentation or aggregation by size exclusion chromatography, dynamic light scattering (DLS), flow cell microscopy (FCM), and visual inspection, before and after nebulization, as well as binding activity by ELISA.
A complete recovery of the protein material was achieved at the concentration of 8 mg/mL (102%) and nearly complete at 1 mg/mL (89%) (Figure 4A). No visible aggregates were detected in the nebulized samples, and a moderate increase of subvisible particles was observed by FCM. Moreover, no difference in the size exclusion chromatography ultra-high performance liquid chromatography (SEC-UPLC) profile of the samples at 1 and 8 mg/mL, before and after nebulization, could be observed (Figure 4B). Interestingly, the number of submicronic particles measured by DLS was unchanged after nebulization and the preparations conserved their monodispersion (Figure 4A). Finally, the binding activity of the samples was fully maintained upon nebulization, according to the overlapping binding curves observed by ELISA between the samples submitted or not to the nebulization step (Figure 4C).
Figure 4.
Stability of nebulized tetrameric Nanofitin
(A) Aggregates as measured by DLS and FCM in the reference and nebulized samples containing tetrameric Nanofitin at 1 or 8 mg/mL. (B) SEC-UPLC analysis of nebulized Nanofitin compared with the reference sample at 1 mg/mL (top) or 8 mg/mL (bottom). (C) Activity comparison using ELISA assay on spike protein between nebulized samples and reference sample at 1 mg/mL (Left) or 8 mg/mL (right). Data of nebulized samples are the average ± SEM of three replicates.
These data support the high tolerance of the Nanofitin tetramer to nebulization and suggest that nebulization can be a viable delivery method for Nanofitin-based therapeutics in the treatment of respiratory diseases.
Airways administration of the multimeric Nanofitin results in its efficient distribution in the lung
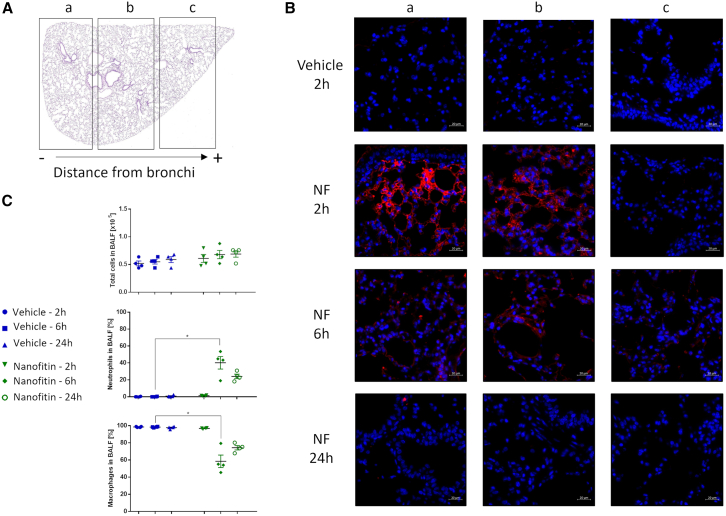
The Nanofitin tetramer was administrated intratracheally (10 mg/kg) in mice to evaluate the biodistribution of the Nanofitin in lung tissue over time. Immunofluorescence analyses were performed on one lung lobe that was divided into three longitudinal sections (a, b, and c), with section a being the closest to the bronchi (Figure 5A). At 2 h after administration, the Nanofitin tetramer was particularly observed near the bronchi until mid-lung (sections a and b, Figure 5B), with minimal fluorescence at the end of the longitudinal section (area c, Figure 5B). A similar biodistribution was observed 6 h after administration at lower fluorescence intensity. Finally, the tetrameric Nanofitin was barely detectable in the lung 24 h after its administration, indicative of its clearance.
Figure 5.
Distribution of tetrameric Nanofitin in lung
(A) Cross-section of mouse lung divided in different areas (a, b, c) depending on their distance from bronchi. (B) Detection of tetrameric Nanofitin (in red) at different timepoints and lung localizations. Scale bar, 20 µm. (C) Variation of total lymphocyte cells population (top), neutrophil population (middle), and macrophage (bottom) in bronchoalveolar lavage fluid (BALF) at 2, 6, and 24 h after Nanofitin administration. Statistical analysis based on one-way ANOVA test with p < 0.01 between the vehicle 6 h and Nanofitin 6 h groups (∗).
In addition, Nanofitin administration had no effect on the total population of leukocytes in bronchoalveolar fluid (Figure 5C, top) at all time points explored. We noticed a transient increase in neutrophiles and reduction in macrophages for the groups used for the 6- and 24-h time points (Figure 5C, middle and bottom), reaching statistical significance only for the group at 6 h after administration. Overall, these data suggest that the administration of the tetrameric Nanofitin at 10 mg/kg was safe and well tolerated.
Nanofitin neutralization of SARS-CoV-2 in vivo in prophylactic or curative administration
The therapeutic activity of the Nanofitin tetramer upon intranasal administration was evaluated at 10 mg/kg using a prophylactic setup in huK18ACE2 mice challenged with a load of 104 plaque-forming units (PFU) of SARS-CoV-2, and compared with an irrelevant Nanofitin tetramer. The Nanofitins were administered daily for 4 consecutive days starting from the day before the infection. Lung viral load at day 5 (D5) after infection was monitored by qPCR and 50% tissue culture infectious dose (TCID50) measurement, showing a strong reduction of the viral load in the group treated with the Nanofitin tetramer as compared with the groups treated with the vehicle and the irrelevant tetramer (Figure 6A). Interestingly, the titer of infectious viral particles in the group treated with the Nanofitin tetramer was below the detection limit of the assay, demonstrating the high potential of the Nanofitin tetramer at neutralizing SARS-CoV-2 infection and amplification. Installation of the disease in the groups treated with the vehicle or the irrelevant tetramer was marked by a body weight loss of those mice along the study, which was not observed either for the uninfected group or for the group treated with the Nanofitin tetramer (Figure 6B). Finally, lung inflammation was assessed by measuring cell infiltration in tissues. Histological analysis associated with their inflammation scores reported evidence of inflammation in non-treated infected mice notably around the blood vessel and lung parenchyma, while the treated mice did not show any damage in lung tissue (Figures 6C and S4). Interestingly, a reduction of inflammation was also noticed in the irrelevant Nanofitin-treated group compared with the vehicle-treated group, suggesting a potential anti-inflammatory effect of the Nanofitin application.
Figure 6.
Intranasal administration of Nanofitin inhibits SARS-CoV-2 virus in animal models
(A) Variation of viral load and TCID50 in mice infected by SARS-CoV-2 virus treated by Nanofitin at 10 mg/kg. Five mice were included in each group. (∗) Statistical analysis was based on one-way ANOVA with p < 0.01 between infected mice treated by vehicle and non-relevant NF or tetrameric NF. ns, not significant. (B) Evolution of body weight in infected mice compared with the D0. (∗∗) Significance calculated by one-way ANOVA between the different mice groups at D5 p < 0.01. ns, not significant. (C) Lung histological pictures reflecting cell infiltration in the different setups. Black arrows indicate the cell infiltrations around blood vessels and bronchi. Scale bar, 500 μm. (D) Variation of viral load and TCID50 in mice infected by SARS-CoV-2 treated at 0.1, 1, or 10 mg/kg at D1, D2 after infection, and 2 h (−2) before infection for the prophylactic conditions. Five mice were included in each group. (∗∗∗) Statistical analysis was based on one-way ANOVA with p < 0.01 between infected mice group treated by vehicle and the infected mice groups treated by the tetrameric NF.
We completed the in vivo evaluation with a dose escalation study (0.1, 1, and 10 mg/kg), still using an intranasal prophylactic setup, but with an initial dosing of the Nanofitin tetramer 2 h before the infection instead of the day before. Reduction of the lung viral load was dose-dependent as shown by the qPCR analysis (Figure 6D, left). TCID50 measurements show a threshold with a clear wash out of the infectious viral particles in the group treated at 1 and 10 mg/kg, while a high titer was remaining in the group treated at 0.1 mg/kg (Figure 6D, right). In parallel of the dose escalation study using a prophylactic administration scheme, we investigated the curative potential of the Nanofitin tetramer with its administration starting the day after the infection. In this set up, the viral genome was reduced as compared with the untreated infected mice, and infectious viral particles were barely detectable (Figure 6D). Altogether, these data demonstrate that the tetrameric Nanofitin can prevent viral infection and achieve potent in vivo virus neutralization in both prophylactic and curative administration schemes.
Discussion
In this study, we aimed to assess the potential of the Nanofitin technology to address two major challenges in the treatment of pulmonary infectious diseases, specifically in relation to SARS-CoV-2. These challenges include (1) designing a technology platform that can quickly identify and develop highly effective therapeutic options against a variety of disease variants, reducing the risk of treatment failure during a pandemic outbreak of unknown infectious diseases and (2) delivering a high and rapid drug load to the site of infection in the airways.
Our strategy was to generate a Nanofitin assembly by using candidates isolated from the initial panning campaign without further optimization. The idea was to rely mostly on the avidity for the target spike protein, based on both its trimeric native structure and the targeting of two distant epitopes to create a molecular clamp and engineer a highly potent molecule. To this end, anti-spike Nanofitins were isolated from a naive synthetic library through four consecutive rounds of ribosome display, promptly revealing two main sets of Nanofitins. Our focus was on three neutralizing Nanofitins from the first set, which targeted the RBD with varying levels of reactivity toward SARS-CoV-2 variants (Nanofitins 1–3), as well as a fourth non-neutralizing Nanofitin from the second set that displayed preferential binding to the spike protein bearing the highly conserved mutation D614G (Nanofitin 4). By using this approach, we aimed to provide a therapeutic candidate that could display high potency in inhibiting SARS-CoV-2 infection both in vitro and in vivo by neutralizing viral homing locally in the airways against a broad range of variants using avidity to mitigate a potential loss of affinity for SARS-CoV-2 mutants. The assembly of three neutralizing Nanofitin modules targeting the RBD of the spike protein was found to be highly potent against the ancestral SARS-CoV-2 viral particle (Figure 2C), but ineffective in neutralizing the Omicron variant (Figure S3). This result differs from what has been reported with the DARPin-based therapeutic compound ensovibep, which relies on the assembly of three RBD-targeting DARPin modules.15 The difference in behavior may be due to the lower affinity (Figure 2B, from 0.5 to 380 nM) of the RBD-targeting Nanofitin modules engaged in the assembly as compared with the anti-spike DARPins constitutive of the ensovibep molecule from 30 to 90 pM,15 indicating the benefit of integrating an affinity maturation at an early stage in the development process on top of the avidity component. The multi-paratopic strategy aimed to provide a straightforward and time-efficient development stream for addressing emerging unknown infectious disease.19,26,27 In that way, we generated a new highly potent molecule against the Omicron variant in a time-efficient manner (approximately 100 days), using a similar approach as developed for the ancestral SARS-CoV-2 (Figure S3). This highlights that viable therapeutic solutions to address emerging infectious diseases can be generated within a constrained time frame, by leveraging a fast in vitro selection system together with the high modularity of the Nanofitin alternative scaffold platform. Future development will seek to optimize the manipulation of the different modules to be included in the assembly, both with regard to their epitope and affinity, to achieve a cross-reactivity against a broad spectrum of variants while keeping the rapidness of the development process.
In addition, we observed that incubation of the trimer of the spike protein with the tetrameric Nanofitin triggers a conformational shift from all RBD down to two RBDs up (Figure 3B). This behavior differs from what has been reported for neutralizing antibody or other alternative scaffold proteins interacting with the RBD, which all allow a conformational switch with a potential sequential opening of the structure from a closed trimer to a three RBDs-up conformation as occurring in the ACE2-S1 interaction.15,25,26 We proposed a model where Nanofitin 3 and Nanofitin 4 blocked the three RDBs up conformation by generating a clamp on the third RBD and locking it in a down position (Figure 3C). As the linker length between NF3 and NF4 should significantly impact the ability to generate this clamp, it would be interesting to evaluate how the conformational shift of the spike protein is altered by modifying the number of amino acids contained in the linker.
Beside the question of its broad neutralization, we also investigated a way to bolster the potential of Nanofitin technology by using inhalation. Indeed, the literature suggests that intravenous administration of antibodies results in poor distribution in the lungs, limiting the therapeutic window. Meanwhile, most anti-infective treatments for pulmonary diseases are still in parenteral form, including all approved COVID-19 treatments. One main challenge restricting the development of antibody-based inhaled treatments is the nebulization process, which can alter the structural stability and function of the biomolecules. Accordingly with the intrinsic high stability of the Nanofitin scaffold to both chemical and physical stresses,14 we showed here that the anti-spike tetrameric Nanofitin fully retains its functionality and is relatively stable during nebulization with the Aeroneb solo vibrating mesh nebulizer, demonstrating that Nanofitins are amenable to nebulized formulation. Although we did not fully characterize the aerodynamic properties of the Nanofitin aerosol, our results obtained by laser diffraction showed that the volume median diameter of the aerosol 4.3 μm (Figure 4A), in agreement with the performances of the Aerogen solo, is compatible with efficient lung deposition.27 Early lung biodistribution study upon intratracheal administration performed in mice demonstrated the deposition of the Nanofitin tetramer in most of the regions of the lung. Kinetic of clearance of the Nanofitin from the lung seemed to match what was described for another alternative scaffold (PRS-060) that reaches a clinical stage as an inhaled formulation with a dosing twice a day.21 Similarly, Koussoroplis et al.24 reported the elimination of an antibody from the pulmonary tract within 24 h with one-half of its elimination occurring in the first hours after administration. Systemic exposure after intratracheal administration was not explored at this stage and would be interesting to document to evaluate the fate of the molecule after disappearance from the lung. It is anticipated that the systemic bioavailability will be very limited, as described for medium and large proteins upon intratracheal administration.28 As an example, Guilleminault et al.29 demonstrated that cetuximab administrated by inhalation was efficient to target epidermal growth factor receptor-positive tumor in lung with a limited transfer into the blood. Similarly, only 1% of PRS-220, a 20-kDa anticalin directed against CTGF, was demonstrated to reach the systemic compartment after lung delivery.30
Ultimately, anti-spike tetrameric Nanofitin was shown to be efficient at neutralizing the infection with SARS-CoV-2 in vivo in the huK18ACE2 mouse model. This demonstrated the ability of the Nanofitins at engaging their target locally after lung delivery in an in vivo context. The demonstration was performed both using a prophylactic and curative set-up, suggesting that the Nanofitin compound can also be efficient to treat the disease in its acute phase. In summary, we describe here the use of anti-spike Nanofitins as potent neutralization therapeutics for local application in the respiratory tract. With their stability, small size, and ease for high-yield production in bacteria, Nanofitins are suitable scaffolds to develop inhalable multivalent molecules in a fast-track process and may contribute to the global response in case of new emerging diseases.
Materials and methods
Nanofitin generation
Nanofitins were generated by ribosome display selections against SARS-CoV-2 spike proteins (Sino Biological Inc.) from naive libraries using four rounds of selection as previously described.17,31 After their initial screening, the Nanofitins were produced in Escherichia coli and purified by their His-tag at their N-terminal using affinity chromatography.
Multimeric construct
Multimer protein was obtained by the combination of monomeric Nanofitin modules selected for their individual properties. Construct was cloned into pET21a vector between NdeI and HindIII restriction sites with the addition of an HA-tag at the N-terminus and a His-tag at the C-terminus. The three RBD binding Nanofitin modules were genetically fused with spacers of 15 amino acids between modules, followed by non-RBD-binding module at the C-terminus linked by a 30-amino acid spacer.
Anti-spike ELISA
The EC50 of each Nanofitin module was evaluated by ELISA. Briefly, recombinant proteins at 5 μg/mL were coated into 96-well plates for 1 h at room temperature and blocked with TBS BSA 0.5% (w/v) for 1 h. Then, serial dilutions of Nanofitins in TBS Tween 0.1% (v/v) were loaded on the plate for 1 h, followed by the addition of RGS-His horseradish peroxidase conjugate antibody (Qiagen) diluted 1:4,000 into TBS tween 0.1% for 1 h. The revelation was carried out with a solution of 0.1 mg/mL TMB (Merck), H2O2 at 0.012% in a citrate/acetate buffer 0.1 M, pH 6. Absorbance at 450 nm was recorded using a Varioskan ELISA plate reader (Thermo Fisher Scientific). The anti-spike Nanofitins were compared with an irrelevant Nanofitin selected against chicken lysozyme.32 EC50 values were calculated by fitting the absorbance data to a sigmoidal dose response using GraphPad Prism software.
Competitive ELISA
Human ACE2 recombinant protein (Sino Biological Inc.) diluted at 2.5 μg/mL in TBS solution was coated for 1 h in a 96-well plate before a blocking in TBS BSA 0.5%. Then, serial dilutions of Nanofitins pre-incubated for 30 min with 1 nM SARS-CoV-2 spike S1-Fc recombinant protein (Sino Biological Inc.) were added on the wells for 1 h before the addition of the peroxidase-AffiniPure donkey anti-human IgG, Fc fragment (1:10000 in TBS tween 0.1%). Revelation was performed with TMB solution. IC50 values were calculated using GraphPad Prism software.
BLI analyses
Determination of Nanofitin affinity was performed by interferometry with an Octet RED96 system (Sartorius). First, Protein A biosensors (Sartorius) were functionalized with SARS-CoV-2 spike S1-Fc recombinant protein (Sino Biological Inc.) at 5 μg/mL in a TBS solution containing BSA 0.01% and Tween 20 0.002% until reaching a threshold of 1 nm. After an equilibration step of 60 s, Nanofitin association was evaluated by exposing biosensors to a Nanofitin concentration range from 1 to 0 μM for 180 s. The association and dissociation steps were measured for 180 s. Biosensor regeneration was performed by three cycles containing a first step of wash in glycine pH 2 for 10 s followed by a second one in TBS buffer. Biosensor only exposed to TBS BSA Tween 20 solution was used as a background reference and subtracted to the other biosensors.
Negative staining electron microscopy
Nanofitin binding occupancy on spike trimer was determined by negative staining electron microscopy. Nanofitins were incubated with recombinant spike trimer (ACROBiosystems) on negative staining grid and three-dimensional map were reconstructed. Spike protein (0.019 μM final) was mixed with Nanofitins and incubated for 15 min at 4°C. The mix was then dispensed on 400 mesh 12-nm carbon grid before recording.
Cell culture
HEK293T ACE2/TMPRSS2 cells (Virongy Biosciences Inc.) were maintained at 37°C, 5% CO2 in DMEM supplemented with 10% FBS, Glutamax, and 1% penicillin/streptomycin.
Lentivirus neutralization assay
The different Nanofitins were evaluated for their anti-viral activity in neutralization assay using SARS-CoV-2 spike D614G pseudovirus (BPS Bioscience). HEK 293T ACE2/TMPRSS2 cells were seeded at 10,000 cells/well into a white walled 96-well plate 24 h before infection. Then, Nanofitins and the casirivimab were pre-incubated in DMEM from 500 nM to 0.005 pM with lentivirus at multiplicity of infection of (MOI) 0.03 for 30 min at 37°C. We added 10 μL of the Nanofitin-virus mixture to cells. Plates were incubated at 37°C and 5% CO2 for 48 h. Final luminescence was recorded by adding 50 μL of One step Luciferase reagent (BPS Bioscience) to each well, agitated 15 min and read on a Glomax Bioluminometer (Promega).
SARS-CoV-2 virus neutralization assay
Lung cancer A549 cells stably transfected with human ACE2 receptor were infected with SARS-CoV-2 virus (strain BetaCoV/France/IDF0372/2020) at MOI 0.1. Cells were pre-incubated with Nanofitins at different concentrations or with remdesivir at 10 μM as positive controls for 2 h before the addition of virus. After 1 h of infection, the supernatant was removed and fresh medium containing Nanofitins or remdesivir was added. Then, samples were collected after 72 h of incubation and a quantitative RT-PCR was performed to measure the viral replication in the different conditions using the Luna Universal One-Step RT-qPCR kit (New England Biolabs) in an Applied Biosystems QuantStudio 7 thermocycler (Thermo Fisher Scientific). The primers 5′-TAATCAGACAAGGAACTGATTA-3′ and 5′-CGAAGGTGTGACTTCCATG-3′ were used for the amplification. Raw data were normalized using negative controls (PBS, 0%) and positive controls (remdesivir. 100%) and expressed in percentage of viral replication inhibition.
Nebulization assay
Tetrameric Nanofitin was prepared at 1 mg/mL and 8 mg/mL in PBS and nebulized using the vibrating mesh nebulizer Aeroneb solo, from Aerogen. Droplet size and flow rate of the aerosol spray were measured by laser diffraction, using the Spraytec (Malvern Panalytical) before droplet collection, at the aerosol outflow by condensation into a 15-mL tube. Collected nebulizates were visually controlled to detect visible aggregates (>50 μm), tested by FCM (FC200-IPAC, Occhio) and DLS (DynaPro NanoStar, Wyatt Technology) to analyze subvisible and submicronic aggregates, respectively. Total protein concentration was measured by BCA assay (Thermo Fisher Scientific) and binding activity of the Nanofitin was checked by ELISA. Nanofitin integrity was evaluated by SDS-PAGE and SEC-UPLC. For the latter, Nanofitin was prepared at 150 μg/mL in sodium phosphate buffer at 100 mM supplemented with 200 mM NaCl at pH 7. Then, 10 μL of the solution were injected on the column Acquity UPLC BEH SEC column 125 Å, 1.7 μM, 4.6 mm × 150 mm (Waters Corporation) at 0.3 mL/min for a 10-min run.
Pharmacokinetics assay
Adult female C57BL/6 mice at 8 weeks of age were supplied by Janvier Labs for the Artimmune/CNRS Orleans facility. All experiments are performed using the guideline of the French Ministry of Agriculture for experiments with laboratory animals (reference ApaFis 25609). Tetrameric Nanofitin at 10 mg/kg in PBS was intratracheally administrated in C57BL/6 female mice to assess the Nanofitin biodistribution after 2, 6, and 24 h. Each group is composed of four animals. At each time point, mice were sacrificed and the tissue collected for the different readouts: first, bronchoalveolar lavage fluids were collected by washing four times lungs with 0.5 mL saline solution and centrifuged at 400×g for 10 min at 4°C. Cell pellets were analyzed after a staining with Turk’s solution. Second, immunohistochemistry was carried out with lung lobe fixed in 4% buffered formaldehyde before being embedded in paraffin. Then, 3-μm sections were cut and staining with the C29F4 HA-Tag antibody (Cell Signaling Technology) diluted at 1:1,000 followed by an incubation with a anti-rabbit PE donkey IgG (Biolegend) at 1:800. Microscopy analysis was performed on a Leica microscope.
In vivo efficacy
HuK19ACE2 C57BL/6J female mice (8–12 weeks age old) were obtained from Jackson Laboratories and housed in a CIPHE BLS3 facility. All mice are maintained according the standard procedures of the French and European guideline for animal care (Reference ApaFis 26484). HuK18ACE2 mice were infected with 104 PFU SARS-CoV-2 intranasally. We divided 20 mice into 4 groups: (1) uninfected mice, (2) infected mice treated with vehicle, and infected mice treated with (3) tetrameric or (4) irrelevant NF at 10 mg/kg. Treatment was intranasally delivered once per day: the day before infection, the day of infection and the 2 days after the infection. Sacrifice was performed at D5 to assess viral load and lung inflammation by qRT-PCR, TCID, and histopathological analyses.
Dose escalation study
HuK18ACE2 mice were infected with 1.1 × 104 PFU SARS-CoV-2 intranasally and treated with tetrameric Nanofitin at 0.1, 1, or 10 mg/kg. Prophylactic and curative approaches were assessed by administrating intranasally the Nanofitin once per day respectively 2 h before the infection and the 2 days after the infection (D1 and D2) or only D1 and D2. Mice were sacrificed 5 days after infection for the evaluation of lung viral load by qRT-PCR and TCID50 and the tissue inflammation by histopathological analysis.
Histological analyses
Lung tissues were directly fixed in 4% buffered formaldehyde solution for 24–72 h before being embedded in paraffin. Hematoxylin and Eosin staining was performed in 3-μm sections to evaluate inflammation using Leica microscope.
Acknowledgments
We thank N. Segueni, B. Ryffel, and A. Zarubica for their assistance in the in vivo assays and the access to the Artimmune and CIPHE facilities. We also thank L. Maršálek from Eyen SE for the cryo-EM services and Michel Perez from Institut Pasteur for virus neutralization assay. We are grateful to Nadege Prel and Anne Chevrel for the helpful discussions. Part of the activities were sponsored by the French Government in the framework of the "Programme d'Investissements d'Avenir" (Investment for the Future Program, PIA) and the program "France Relance" via BPI France (DOS0162668/00-DOS0162669/00) under the project "Respitude." Part of the activities were sponsored by the European Union through a grant from the European Regional Development Fund (ERDF/FEDER) via the Region Pays de Loire (PL0031807), in the framework of «React EU » – the European Union addressing the COVID-19 pandemic - under the project "PulmoVia." Part of this work was supported by C-VALO, the French National Research Agency, Region Centre-Val de Loire and the European Union through the European Regional Development Fund (ERDF/FEDER) (ANR-17-SATE-0003, Infinhitim Program).
Author contributions
S.V., O.K. and M.C. designed research; E.E., J.P., L.N., D.L., T.S., N.H.V., S.H., S.V., O.K., and M.C. performed research; E.E., J.P., L.N., D.L.P., T.S., N.H.V., S.H., S.V., O.K., and M.C. analyzed data; and S.V., E.E., and M.C., wrote the paper.
Declaration of interests
The Nanofitin technology described in this study, commercialized by Affilogic, uses the patent application owned by Institut Pasteur and Center National de la Recherche Scientifique (CNRS): “OB-fold used as scaffold for engineering new specific binders”; PCT/IB2007/004388. Affilogic SAS, Nantes, France, provided support for the study and participated in study design, conducted the study, and provided data collection, management and interpretation. S.V., E.E, J.P., L.N., M.C., S.H., are employees of Affilogic SAS. O.K. is the CEO and the owner of Affilogic SAS.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.08.010.
Supplemental information
Data and code availability
The data that support the findings of this study are available from the corresponding author, S.V., upon reasonable request.
References
- 1.Liu J., Li Y., Liu Q., Yao Q., Wang X., Zhang H., Chen R., Ren L., Min J., Deng F., et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov. 2021;7:17–24. doi: 10.1038/s41421-021-00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krynytska I., Marushchak M., Birchenko I., Dovgalyuk A., Tokarskyy O. COVID-19-associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review) Iran. J. Microbiol. 2021;13:737–747. doi: 10.18502/ijm.v13i6.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R., Lan Z., Ye J., Pang L., Liu Y., Wu W., Qin X., Guo Y., Zhang P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021;12:589095. doi: 10.3389/fimmu.2021.589095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumari M., Lu R.-M., Li M.-C., Huang J.-L., Hsu F.-F., Ko S.-H., Ke F.-Y., Su S.-C., Liang K.-H., Yuan J.P.-Y., et al. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. J. Biomed. Sci. 2022;29:68. doi: 10.1186/s12929-022-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moench T.R., Botta L., Farrer B., Lickliter J.D., Kang H., Park Y., Kim C., Hoke M., Brennan M., McSweeney M.D., et al. 2022. A Randomized, Double-Blind, Phase 1 Study of IN-006, an Inhaled Antibody Treatment for COVID-19 (Infectious Diseases (Except HIV/AIDS)) [DOI] [Google Scholar]
- 9.Tabrizi M., Bornstein G.G., Suria H. Biodistribution Mechanisms of Therapeutic Monoclonal Antibodies in Health and Disease. AAPS J. 2010;12:33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayor A., Thibert B., Huille S., Respaud R., Audat H., Heuzé-Vourc'h N. Inhaled antibodies: formulations require specific development to overcome instability due to nebulization. Drug Deliv. Transl. Res. 2021;11:1625–1633. doi: 10.1007/s13346-021-00967-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Respaud R., Vecellio L., Diot P., Heuzé-Vourc'h N. Nebulization as a delivery method for mAbs in respiratory diseases. Expert Opin. Drug Deliv. 2015;12:1027–1039. doi: 10.1517/17425247.2015.999039. [DOI] [PubMed] [Google Scholar]
- 12.Sécher T., Bodier-Montagutelli E., Parent C., Bouvart L., Cortes M., Ferreira M., MacLoughlin R., Ilango G., Schmid O., Respaud R., Heuzé-Vourc'h N. Aggregates Associated with Instability of Antibodies during Aerosolization Induce Adverse Immunological Effects. Pharmaceutics. 2022;14:671. doi: 10.3390/pharmaceutics14030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmondson S.P., Shriver J.W. DNA-Binding Proteins Sac7d and Sso7d from Sulfolobus. Methods Enzymol. 2001;334:129–145. doi: 10.1016/S0076-6879(01)34463-4. [DOI] [PubMed] [Google Scholar]
- 14.Mouratou B., Schaeffer F., Guilvout I., Tello-Manigne D., Pugsley A.P., Alzari P.M., Pecorari F. Remodeling a DNA-binding protein as a specific in vivo inhibitor of bacterial secretin PulD. Proc. Natl. Acad. Sci. USA. 2007;104:17983–17988. doi: 10.1073/pnas.0702963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothenberger S., Hurdiss D.L., Walser M., Malvezzi F., Mayor J., Ryter S., Moreno H., Liechti N., Bosshart A., Iss C., et al. Ensovibep, a Novel Trispecific DARPin Candidate that Protects against 2 SARS-CoV-2 Variants. bioRvix. 2022 doi: 10.1101/2021.02.03.429164. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothenberger S., Hurdiss D.L., Walser M., Malvezzi F., Mayor J., Ryter S., Moreno H., Liechti N., Bosshart A., Iss C., et al. The trispecific DARPin ensovibep inhibits diverse SARS-CoV-2 variants. Nat. Biotechnol. 2022;40:1845–1854. doi: 10.1038/s41587-022-01382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garlich J., Cinier M., Chevrel A., Perrocheau A., Eyerman D.J., Orme M., Kitten O., Scheibler L. Discovery of APL-1030, a Novel, High-Affinity Nanofitin Inhibitor of C3-Mediated Complement Activation. Biomolecules. 2022;12:432. doi: 10.3390/biom12030432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcion G., Hermetet F., Neiers F., Uyanik B., Dondaine L., Dias A.M.M., Da Costa L., Moreau M., Bellaye P.S., Collin B., et al. Nanofitins targeting heat shock protein 110: An innovative immunotherapeutic modality in cancer. Int. J. Cancer. 2021;148:3019–3031. doi: 10.1002/ijc.33485. [DOI] [PubMed] [Google Scholar]
- 19.Michot N., Guyochin A., Cinier M., Savignard C., Kitten O., Pascual M.-H., Pouzieux S., Ozoux M.-L., Verdier P., Vicat P., Dumas J. Albumin binding Nanofitins, a new scaffold to extend half-life of biologics – a case study with exenatide peptide. Peptides. 2022;152 doi: 10.1016/j.peptides.2022.170760. [DOI] [PubMed] [Google Scholar]
- 20.Goux M., Becker G., Gorré H., Dammicco S., Desselle A., Egrise D., Leroi N., Lallemand F., Bahri M.A., Doumont G., et al. Nanofitin as a New Molecular-Imaging Agent for the Diagnosis of Epidermal Growth Factor Receptor Over-Expressing Tumors. Bioconjug. Chem. 2017;28:2361–2371. doi: 10.1021/acs.bioconjchem.7b00374. [DOI] [PubMed] [Google Scholar]
- 21.Matschiner G., Fitzgerald M.F., Moebius U., Hohlbaum A.M., Gille H., Jensen K., Kirchfeld K., Rattenstetter B., Laforge A., Bel Aiba R.S., et al. Elarekibep (PRS-060/AZD1402): a new class of inhaled Anticalin medicine targeting IL-4Ra for T2 endotype asthma. J. Allergy Clin. Immunol. 2023;151:966–975. doi: 10.1016/j.jaci.2022.12.815. [DOI] [PubMed] [Google Scholar]
- 22.Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T.P., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C., et al. Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell. 2020;183:739–751.e8. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Zhang J., Chen J., Lin M., Gong T., Cheng B., Ji C., Rits-Volloch S., Zhu H., Woosley A.N., et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science. 2021;62:525–527. doi: 10.1126/science.abf230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koussoroplis S.J., Paulissen G., Tyteca D., Goldansaz H., Todoroff J., Barilly C., Uyttenhove C., Van Snick J., Cataldo D., Vanbever R. PEGylation of antibody fragments greatly increases their local residence time following delivery to the respiratory tract. J. Control Release. 2014;187:91–100. doi: 10.1016/j.jconrel.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Walls A.C., Xiong X., Park Y.-J., Tortorici M.A., Snijder J., Quispe J., Cameroni E., Gopal R., Dai M., Lanzavecchia A., et al. Unexpected Receptor Functional Mimicry Elucidates Activation of Coronavirus Fusion. Cell. 2019;176:1026–1039.e15. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benton D.J., Wrobel A.G., Xu P., Roustan C., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020;588:327–330. doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todoroff J., Vanbever R. Fate of nanomedicines in the lungs. Curr. Opin. Colloid Interf. Sci. 2011;16:246–254. doi: 10.1016/j.cocis.2011.03.001. [DOI] [Google Scholar]
- 28.Patton J.S., Fishburn C.S., Weers J.G. The Lungs as a Portal of Entry for Systemic Drug Delivery. Proc. Am. Thorac. Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- 29.Guilleminault L., Azzopardi N., Arnoult C., Sobilo J., Hervé V., Montharu J., Guillon A., Andres C., Herault O., Le Pape A., et al. Fate of inhaled monoclonal antibodies after the deposition of aerosolized particles in the respiratory system. J. Control Release. 2014;196:344–354. doi: 10.1016/j.jconrel.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Welk V., Pavlidou M., Wurzenberger C., Hansbauer E.-M., Wurzenberger C., Grüner S., Peper-Gabriel J., Jaquin T., Konitsiotis A., Morgenstern J., et al. Mechanisms of Lung Injury and Repair. European Respiratory Society; 2021. Development of PRS-220, a potential best-in-class, inhaled CTGF/CCN2 inhibitor for the treatment of IPF; p. 732. [DOI] [Google Scholar]
- 31.Huet S., Gorre H., Perrocheau A., Picot J., Cinier M. Use of the Nanofitin Alternative Scaffold as a GFP-Ready Fusion Tag. PLoS One. 2015;10:e0142304. doi: 10.1371/journal.pone.0142304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correa A., Pacheco S., Mechaly A.E., Obal G., Béhar G., Mouratou B., Oppezzo P., Alzari P.M., Pecorari F. Potent and Specific Inhibition of Glycosidases by Small Artificial Binding Proteins (Affitins) PLoS One. 2014;9:e97438. doi: 10.1371/journal.pone.0097438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, S.V., upon reasonable request.