Abstract
The coordination of respiration and swallowing is a life-critical function in infants. Varying volume and rate of milk delivery changes swallowing frequency and bolus volume but any impact on swallow-respiration coordination is unknown. Five infant pigs were filmed with simultaneous high speed videofluoroscopy and plethysmography while feeding from an automatic system delivering milk across a range of volumes and frequencies. Swallow inspiration delay, respiratory cycle duration, and distribution of inspiratory and expiratory swallows were calculated. At constant volume, there were more inspiratory phase swallows when frequency increased. At high constant frequency, increasing volume changed swallow-respiration coordination patterns, with increased occurrence of inspiratory phase swallows. Respiratory cycle duration did not change in response to changes in oral milk delivery. These results suggest that the observed pattern of expiratory swallowing in infants is achieved primarily by regulation of milk intake, not modulation of respiratory patterns by oral sensation.
Keywords: coordination, dysphagia, infant, respiration, swallow
1 |. INTRODUCTION
The coordination of swallowing and respiration is a life critical function in infant mammals that protects the airway from aspiration of milk which could compromise lung function (German et al., 1998; Jadcherla, 2016). Coordination of respiration and swallowing occurs at multiple timescales. Direct connections between brainstem central pattern generators control reciprocal inhibition of swallowing and respiration (Dick et al., 1993; Huff et al., 2018) immediately around the swallow, resulting in swallow apnea (Paydarfar et al., 1995). At a larger timescale, soon after birth and through infancy, swallows occur preferentially during expiration in both humans (Vice & Gewolb, 2008) and pigs (Ballester et al., 2018). How this preferential association between swallow and expiration is regulated, and whether changes in feeding behavior are integrated into this control, is unknown.
Feeding and respiration share anatomical and neuromuscular pathways in the upper aerodigestive track (Pitts, 2014). Muscles of the pharynx, larynx, and posterior tongue are actively recruited in both swallowing and inspiration, requiring coordinated switching of motor neuron pools between behaviors (Horton et al., 2018). Feeding itself is made of a sequence of behaviors including oral processing (chewing) and pharyngeal swallow that move the bolus through a series of continuous anatomical spaces (Hiiemae & Crompton, 1985). The neurological control of oral and pharyngeal phases of feeding is distinct (Arce-McShane et al., 2020; Jean, 2001; Moore et al., 2014), yet sensorimotor information from the oral stage of feeding can modify characteristics of the pharyngeal swallow in several ways. Hyoid movements match expectations of motor learning (feedforward loops) only when food is delivered to the oral cavity (Humbert et al., 2012). Oral milk delivery with direct, independent control of frequency and aliquot volume, affects swallow rate and bolus volume (German et al., 1997, 2004). Furthermore, various forms of oral sensory stimulation improve function and coordination of sucking and swallowing in human infants (Barlow et al., 2014), indicating a broad impact of oral sensory stimulation on feeding coordination. However, whether oral stimuli are similarly important in modulating respiratory patterns to facilitate swallow-respiration coordination is unknown.
The goal of this study was to determine how changes in swallowing behavior driven by automatic milk delivery affect the coordination of respiration and swallowing in term infant pigs. The specific hypotheses were informed by previous work on the effects of varying frequency and volume on swallowing rate (German et al., 2004).
H1. increasing frequency of aliquot delivery while maintaining aliquot volume will decrease the duration of respiration cycles, decrease the delay between swallow and onset of subsequent inspiration, and decrease the number of breaths without a swallow.
H2. increasing aliquot volume while maintaining aliquot frequency constant will increase swallow-inspiration delay because of increased bolus volume, and lead to a reduction in inspiration phase swallows.
H3. Because volume flow rate affects swallowing, we further hypothesize that we will see interactions between volume and frequency in their effects on swallow-respiration coordination.
2 |. METHODS
2.1 |. Animals
Five term infant pigs (Yorkshire/Landrace cross, Shoup Investment Ltd.) were trained to feed on milk replacement solution (SoluStart, Land O’Lakes). Once pigs had mastered latching they were trained to feed using an automatic milk delivery system as described below. All procedures were approved by the NEOMED IACUC (protocol number #17–04-071).
2.2 |. Automatic milk delivery system
The custom-designed automatic milk delivery system (Neurovation LLC) consisted of a high-pressure syringe pump, high frequency valve, and control hardware. The system was programmed to deliver milk in aliquots of known volume at set frequencies, with independent frequency and volume settings.
2.3 |. Experimental design
We developed a matrix of settings that varied both parameters (frequency and volume) (Table 1). Animals received frequency-volume combinations randomly. Owing to restrictions in place during the COVID-19 pandemic, not all animals received all possible combinations of frequency and volume. All animals were recorded feeding on a bottle as a control for automated milk delivery.
TABLE 1.
Table of volume and frequency settings.
| 2 Hz | 3 Hz | 4 Hz | 5 Hz | |
|---|---|---|---|---|
| 0.2 mL | ||||
| 0.225 mL | ||||
| 0.24 mL | ||||
| 0.3 mL | ||||
| 0.375 mL | ||||
| 0.4 mL | ||||
| 0.5 mL | ||||
| 0.6 mL | ||||
Note: White cells are combinations not used in the analysis. Grey cells are combinations used only in testing the effect of varying volume at fixed frequency. Black cells are cells used both in testing varying volume at fixed frequencies (columns), and in testing varying frequency at a fixed volume (rows).
2.4 |. Recording
Animals were recorded between 6 and 12 days of age as they fed on milk mixed with radio opaque barium from either a bottle or the automated milk delivery system. High speed (100 fps) digital videofluoroscopy was recorded with simultaneous plethysmography from a band placed around the thorax (Ballester et al., 2018). Videos and plethysmography traces were synchronized with a manually placed square wave pulse.
2.5 |. Swallow-respiration parameters
Swallow times were identified from high speed video recordings as the frame before posterior movement of the bolus out of the valleculae (Gould et al., 2017). Inter and intra rater reliability in swallow identification was above 95% (Ballester et al., 2018). From plethysmography traces, the beginning of inspiration and expiration phases were manually identified. Inter rater identifications were all within less than 0.01 s of each other for respiratory phase identification. Respiratory cycles were classified as containing a swallow or not (Figure 1).
FIGURE 1.
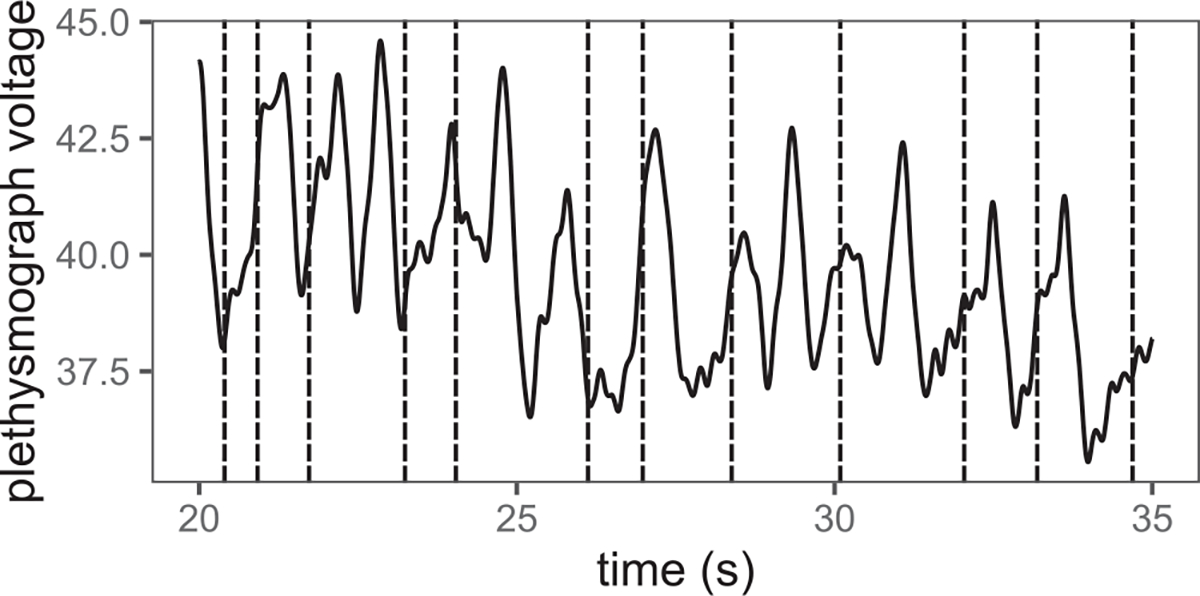
Representative trace of respiration measures from a plethysmograph (solid line) obtained during infant pig milk bottle feeding. Increases in voltage represent chest expansion (inspiration), decreases in voltage represent contraction (expiration). Vertical dashed lines indicate occurrence of swallows. Note presence of respiratory cycles without swallows, as well as both inspiratory and expiratory phase swallows.
From these data we calculated swallow-inspiration delay, the time between a swallow and the closest onset of inspiration (Ballester et al., 2018). We calculated this delay as a relative measure: when positive, inspiration onset occurs after the swallow; when negative, before (Figure 2). We used the manually identified phase of respiration to calculated duration of respiratory cycles. We also classified each swallow as inspiratory or expiratory based on the synchronized respiratory trace.
FIGURE 2.

Representative inspiratory and expiratory swallows. Plethysmograph trace is indicated by the solid line. The shaded region is inspiration. The grey dot marks the transition between expiration and inspiration. The vertical dashed line marks occurrence of the swallow. The two headed arrow indicates the swallow respiration delay.
2.6 |. Statistics
To test the impact of varying volume or frequency on swallow inspiration delay (H1, H2), we used linear mixed models with either volume or frequency as a fixed factor and individual as a random factor. The swallow was the unit of analysis. Post hoc tests were used to identify significant differences between groups.
To test the impact of varying volume or frequency on respiratory cycle duration (H1, H2), we used linear mixed models with either volume or frequency, as well as presence or absence of swallows in a respiratory cycle and their interaction as fixed factors, and individual as a random factor. The unit of analysis was the respirataory cycle. We used post hoc tests to identify significant differences among groups (H3).
We used chi squared test of independence to determine whether the distribution of respiratory cycles with inspiratory, expiratory, both, or no swallows changed among treatment variables (H2, H3). Where appropriate we calculated effect sizes (Cohen’s D). All statistics were performed in R (Core R., 2022), using the packages lmer4 and emmeans.
3 |. RESULTS
Increasing frequency at 0.3 mL aliquot volume changes swallow respiration coordination but not respiration cycle duration.
There is a significant main effect of frequency on swallow inspiration delay (analysis of variance [ANOVA], F(3, 89.93) = 4.28, p = 0.007). The average swallow inspiration delay for 5 Hz, the highest frequency, is negative and significantly different from the lowest frequency (p = 0.022, Cohen’s D = 0.72), indicating that swallows are occurring in early inspiration rather than late expirations (Figure 3a, Table 2a). Respiration cycle length is significantly longer for cycles which include at least one swallow (ANOVA, F(1, 306.75) = 33.72, p < 0.001, Cohen’s D = 0.75), but cycle length does not differ among frequencies (Figure 3b, Table 2b). The proportion of respiratory cycles without swallows decreases with increasing frequency, and the proportion of swallows occurring during inspiration increases (χ2 (6) = 29.995, p < 0.001, Table 2c).
FIGURE 3.
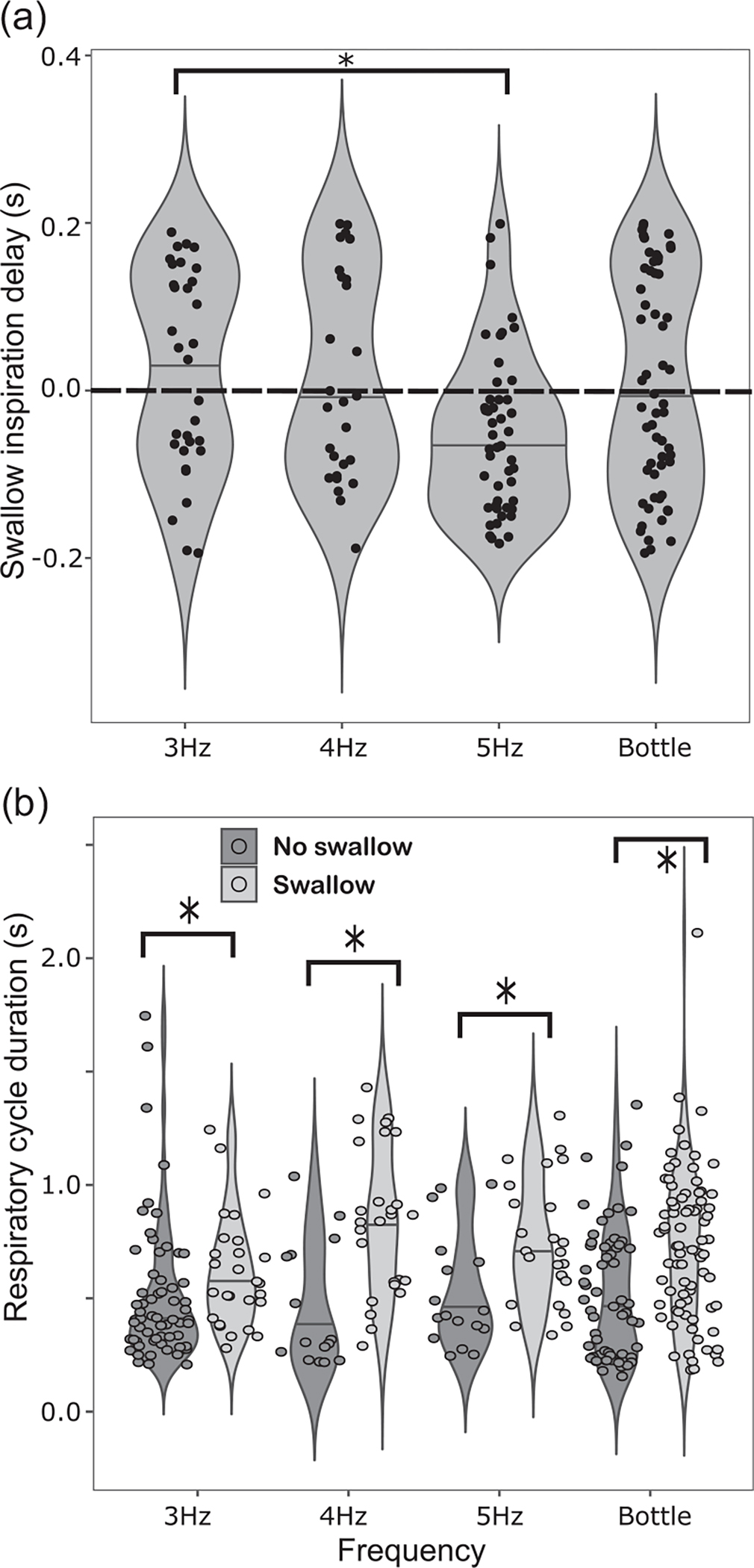
Violin plot with median and overlain scatterplot of each swallow inspiration delay (a) and respiration cycle duration measurement (b) for delivery of 0.3 mL aliquots of milk at three different frequencies. Brackets with asterisks indicate significant pairwise differences between groups. Dotted line in (a) indicates time of swallow.
TABLE 2.
Sample size, mean, and standard deviation by relevant group for (a) swallow inspiration delay, (b) respiratory cycle duration, and (c) respiratory phase occurrences of swallows for 0.3 mL aliquots of milk delivered at 3, 4, and 5 Hz.
| (a) | ||||
|---|---|---|---|---|
|
| ||||
| Volume (mL) | Frequency (Hz) | Number of swallows | Mean (s) | Standard deviation |
| 0.3 | 3 | 32 | 0.0246 | 0.1200 |
| 0.3 | 4 | 27 | 0.0122 | 0.1232 |
| 0.3 | 5 | 48 | −0.0530 | 0.0961 |
| (b) | |||||
|---|---|---|---|---|---|
|
| |||||
| Volume (mL) | frequency (Hz) | Swallow | Number of cycles | Mean (s) | Standard deviation |
| 0.3 | 3 | Absent | 66 | 0.5001 | 0.3049 |
| 0.3 | 3 | Present | 27 | 0.6165 | 0.2458 |
| 0.3 | 4 | Absent | 15 | 0.4591 | 0.2748 |
| 0.3 | 4 | Present | 26 | 0.8427 | 0.3237 |
| 0.3 | 5 | Absent | 17 | 0.5264 | 0.2535 |
| 0.3 | 5 | Present | 25 | 0.7413 | 0.2726 |
| (c) | |||
|---|---|---|---|
|
| |||
| Respiratory cycle swallow class | 3 Hz | 4 Hz | 5 Hz |
| Inspiratory and expiratory swallows | 7 | 7 | 11 |
| Expiratory swallow | 8 | 11 | 2 |
| Inspiratory swallow | 12 | 8 | 12 |
| No swallow | 66 | 15 | 17 |
Only large aliquot volumes or combinations of high frequency and elevated volume affect swallow respiration coordination.
At 2 Hz, both 0.6 mL (p = 0.024, Cohen’s d = 0.43) and 0.5 mL (p = 0.026, Cohen’s d = 0.35) aliquot volumes resulted in mean negative swallow inspiration delays significantly different to bottle feeding (ANOVA, F(2, 184.82) = 4.55, p = 0.012, Figure 4a, Table 3a), indicating a trend to more swallows occurring in early inspiration. There was no difference among aliquot volume in swallow inspiration delay at 3 or 4 Hz. At 5 Hz, swallow inspiration delay for the largest aliquot volume (0.3 mL) was negative and significantly different from both bottle feeding (p = 0.009, Cohen’s d = 0.51, Figure 4b, Table 3b) and 0.24 mL aliquot volume (p = 0.048, Cohen’s D = 0.46) (main effect ANOVA, F(2, 169.61) = 4.9, p = 0.009), indicating a trend for more swallows occurring in inspiration. For all conditions respiration cycles containing swallows were longer than those without. At 2 Hz, respiration cycle duration when a swallow occurred was longer for the largest volume (0.6 mL) than on a bottle (p = 0.023, Cohen’s d = 0.38, Figure 4c, Table 3b). At 4 Hz, larger volumes were associated with longer respiration cycle duration (ANOVA, F(3, 321.88) = 6.96, p < 0.001, cohen’s d = 0.76, Figure 4d, Table 3b). At 3 Hz, there are fewer breaths without swallows for the larger volume (χ2 (3) = 17.726, p < 0.001, Table 3c). There are no significant distribution changes when varying volume at the other frequencies.
FIGURE 4.

Violin plots with median and overlain scatterplot of each swallow-inspiration delay (a, b) and respiration cycle duration (c, d) measurement for delivery of aliquots of milk of varying volumes at 2 Hz (a, c), 4 Hz (d), and 5 Hz (a). Brackets with asterisks indicate significant pairwise differences between groups. Horizontal dashed lines in (a) and (b) indicate time of occurrence of the swallow.
TABLE 3.
Sample size, mean, and standard deviation for relevant groups for (a) swallow inspiration delay, (b) respiratory cycle duration, and (c) respiratory phase occurrences of swallows for different aliquot volumes at 2, 3, 4, and 5 Hz milk delivery.
| (a) | ||||
|---|---|---|---|---|
|
| ||||
| Frequency (Hz) | Volume (mL) | Number of swallows | Mean (s) | Standard deviation |
| 2 | 0.5 | 59 | −0.0349 | 0.0996 |
| 2 | 0.6 | 74 | −0.0440 | 0.1005 |
| 3 | 0.3 | 32 | 0.0246 | 0.1200 |
| 3 | 0.4 | 43 | 0.0064 | 0.1255 |
| 4 | 0.225 | 39 | 0.0147 | 0.1105 |
| 4 | 0.3 | 27 | 0.0122 | 0.1232 |
| 4 | 0.375 | 61 | 0.0098 | 0.1125 |
| 5 | 0.24 | 72 | −0.0072 | 0.1030 |
| 5 | 0.3 | 48 | −0.0530 | 0.0961 |
| NA | bottle | 58 | 0.0060 | 0.1293 |
| (b) | |||||
|---|---|---|---|---|---|
|
| |||||
| Volume (mL) | Frequency (Hz) | Swallow | Number of cycles | Mean (s) | Standard deviation |
| 0.5 | 2 | Absent | 46 | 0.6018 | 0.2660 |
| 0.5 | 2 | Present | 71 | 0.8211 | 0.2526 |
| 0.6 | 2 | Absent | 35 | 0.5831 | 0.3197 |
| 0.6 | 2 | Present | 74 | 0.8535 | 0.3181 |
| 0.3 | 3 | Absent | 66 | 0.5001 | 0.3049 |
| 0.3 | 3 | Present | 27 | 0.6165 | 0.2458 |
| 0.4 | 3 | Absent | 50 | 0.5711 | 0.3834 |
| 0.4 | 3 | Present | 67 | 0.8167 | 0.3477 |
| 0.225 | 4 | Absent | 52 | 0.5564 | 0.2485 |
| 0.225 | 4 | Present | 54 | 0.6749 | 0.2132 |
| 0.3 | 4 | Absent | 15 | 0.4591 | 0.2748 |
| 0.3 | 4 | Present | 26 | 0.8427 | 0.3237 |
| 0.375 | 4 | Absent | 26 | 0.6822 | 0.3396 |
| 0.375 | 4 | Present | 50 | 0.9004 | 0.2633 |
| 0.24 | 5 | Absent | 37 | 0.5992 | 0.2378 |
| 0.24 | 5 | Present | 54 | 0.8397 | 0.3364 |
| 0.3 | 5 | Absent | 17 | 0.5264 | 0.2535 |
| 0.3 | 5 | Present | 25 | 0.7413 | 0.2726 |
| Bottle | NA | Absent | 62 | 0.5159 | 0.2899 |
| Bottle | NA | Present | 79 | 0.7284 | 0.3351 |
| (c) | |||
|---|---|---|---|
| 2 Hz | |||
| Respiratory cycle swallow class | 0.5 mL | 0.6 mL | |
| Inspiratory and expiratory swallows | 16 | 19 | |
| Expiratory swallow | 30 | 17 | |
| Inspiratory swallow | 25 | 38 | |
| No swallow | 46 | 35 | |
| 3 Hz | |||
| Respiratory cycle swallow class | 0.3 mL | 0.4 mL | |
| Inspiratory and expiratory swallows | 7 | 12 | |
| Expiratory swallow | 8 | 27 | |
| Inspiratory swallow | 12 | 28 | |
| No swallow | 66 | 50 | |
| 4 Hz | |||
| Respiratory cycle swallow class | 0.225 mL | 0.3 mL | 0.375 mL |
| Inspiratory and expiratory swallows | 8 | 7 | 15 |
| Expiratory swallow | 19 | 11 | 19 |
| Inspiratory swallow | 27 | 8 | 16 |
| No swallow | 52 | 15 | 26 |
| 5 Hz | |||
| Respiratory cycle swallow class | 0.24 mL | 0.3 mL | |
| Inspiratory and expiratory swallows | 14 | 11 | |
| Expiratory swallow | 16 | 2 | |
| Inspiratory swallow | 24 | 12 | |
| No swallow | 37 | 17 |
4 |. DISCUSSION
There is a limited overall effect of automated milk delivery on respiration parameters. Respiration cycle duration is largely unaffected by changes in frequency or volume of milk delivery. The most consistent changes relate to when in a respiration cycle swallows occur. At high frequency and at certain volume-frequency combinations more swallows occur during inspiration rather than in late expiration as is seen in bottle-feeding pigs throughout infancy (Ballester et al., 2018; Bond et al., 2020). There is also a reduction in the number of respiratory cycles without swallows in them. Taken together, this indicates that swallow rate drives swallow-respiration coordination, and that regulating milk intake is key to maintaining the preferred swallow-expiration association. Sensory feedback from oral milk delivery does not appear to modify respiratory patterns.
Overall, volume effects are more subtle than frequency effects and are restricted to either very high aliquot volumes at low frequencies, or increases in aliquot volume at high frequencies. This is consistent with previous results indicating that volume flow rate is a key driver of swallowing frequency (German et al., 2004). However, very large aliquot volumes, or very high volume flow rates, may also butt against the anatomical and physiological limitations of the infant feeding system.
Our result suggest that regulation of swallow-respiration coordination at the level of preferential expiratory phase swallowing is driven primarily by management of liquid food intake, which allows infants to maintain manageable bolus sizes and expiratory phase swallowing (Mayerl et al., 2020). This is unlike the well documented regulation of short timescale swallow apnea, which is controlled by reciprocal inhibition of brainstem networks and occurs regardless of where in a respiration cycle a swallow occurs (Huff et al., 2018; Pitts et al., 2015). The coordination of respiration and swallowing is important for airway protection in preventing aspiration, but also to maintain regular airflow and thus provide the growing infant with adequate oxygen. These results suggest this latter function is guaranteed by behavioral regulation of food intake, not direct modulation of the respiratory drive to match respiratory rate to swallowing rate. In infant pigs, sucking rate and swallowing rate remain fairly consistent throughout infancy despite increasing body size (Mayerl et al., 2019), but the size of individual bolus sizes increases (Mayerl et al., 2020). The preferential occurrence of swallows in expiration is maintained through infancy (Ballester et al., 2018) and even post weaning (Bond et al., 2020). This tight regulation of food intake rates both orally and at swallowing may be crucial for the maintenance of respiratory performance during feeding in infants in the absence of direct modulation of respiratory drive. Thus in conditions, such as prematurity, where coordination of suck and swallow is impaired (Barlow, 2009; Mayerl et al., 2019), a failure to adequately regulate food intake may significantly affect respiration performance during feeding.
This study had several limitations. The parameter space of frequency/volume combinations was not completely sampled. Some of this undersampling is due to hardware limitations that make certain volume/frequency combinations unachievable. Others are due to the COVID-19 pandemic interfering with the intensive animal care schedule necessary for these experiments. Further experiments will evaluate the volume/frequency parameter space more fully to test the impact of volume flow rate directly.
ACKNOWLEDGMENTS
The authors would like to thank Dr Stanley Dannemiller and the staff at the NEOMED CMU for their assistance with animal care during a pandemic. Funding from the Eunice Kennedy Schriver National Institute of Child Health and Human Development to Rebecca German (R01HD096881) supported this work.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
All data relating to this study are available from the first author on request.
REFERENCES
- Arce-McShane FI, Sessle BJ, Ram Y, Balcer CA, Ross CF, & Hatsopoulos NG (2020). Multiple regions of primate orofacial sensorimotor cortex encode bite force and gape. 10.1101/2020.08.16.252817v2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester A, Gould F, Bond L, Stricklen B, Ohlemacher J, Gross A, DeLozier K, Buddington R, Buddington K, Danos N, & German R (2018). Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model. Dysphagia, 33, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM (2009). Oral and respiratory control for preterm feeding. Current Opinion in Otolaryngology & Head and Neck Surgery, 17, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow SM, Lee J, Wang J, Oder A, Hall S, Knox K, Weatherstone K, & Thompson D (2014). Frequency-modulated orocutaneous stimulation promotes non-nutritive suck development in preterm infants with respiratory distress syndrome or chronic lung disease. Journal of Perinatology, 34, 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond LE, Mayerl CJ, Stricklen BM, German RZ, & Gould FDH (2020). Changes in the coordination between respiration and swallowing from suckling through weaning. Biology Letters, 16, 20190942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core R (2022). TEAM, 2017. R: A language and environment for statistical computing, R Foundation for Statistical Computing. http://www.R.Proj.Org [Google Scholar]
- Dick TE, Oku Y, Romaniuk JR, & Cherniack NS (1993). Interaction between central pattern generators for breathing and swallowing in the cat. The Journal of Physiology, 465, 715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German R, Crompton A, Owerkowicz T, & Thexton A (2004). Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia). Dysphagia, 19, 147–154. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, Hertweck DW, & Thexton AJ (1997). Determinants of rhythm and rate in suckling. The Journal of Experimental Zoology, 278, 1–8. [DOI] [PubMed] [Google Scholar]
- German RZ, Crompton AW, & Thexton AJ (1998). The coordination and interaction between respiration and deglutition in young pigs. Journal of Comparative Physiology A: Sensory, Neural, and Behavioral Physiology, 182, 539–547. [DOI] [PubMed] [Google Scholar]
- Gould FDH, Yglesias B, Ohlemacher J, & German RZ (2017). Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig model. Dysphagia, 32, 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiiemae KM, & Crompton AW (1985). Mastication, food transport and swallowing. In Hildebrand M, Bramble D, Liem K & Wake D, (Eds.), Functional Vertebrate Morphology (pp. 262–290). Harvard University Press. [Google Scholar]
- Horton K-K, Segers LS, Nuding SC, O’Connor R, Alencar PA, Davenport PW, Bolser DC, Pitts T, Lindsey BG, Morris KF, & Gestreau C (2018). Central respiration and mechanical ventilation in the gating of swallow with breathing. Frontiers in Physiology, 9,785. 10.3389/fphys.2018.00785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff A, Reed MD, Smith BK, Brown EH, Ovechkin AV, & Pitts T (2018). Strategies for the integration of cough and swallow to maintain airway protection in humans. Lung, 196, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert IA, Lokhande A, Christopherson H, German R, & Stone A (2012). Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral vs. pharyngeal sensory processing. Journal of Applied Physiology, 112, 1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadcherla S (2016). Dysphagia in the high-risk infant: Potential factors and mechanisms–. The American Journal of Clinical Nutrition, 103, 622S–628S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A (2001). Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiological Reviews, 81, 929–969. [DOI] [PubMed] [Google Scholar]
- Mayerl CJ, Gould FDH, Bond LE, Stricklen BM, Buddington RK, & German RZ (2019). Preterm birth disrupts the development of feeding and breathing coordination. Journal of Applied Physiology, 126, 1681–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl CJ, Myrla AM, Bond LE, Stricklen BM, German RZ, & Gould FDH (2020). Premature birth impacts bolus size and shape through nursing in infant pigs. Pediatric Research, 87, 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JD, Kleinfeld D, & Wang F (2014). How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends in Neurosciences, 37, 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydarfar D, Gilbert RJ, Poppel CS, & Nassab PF (1995). Respiratory phase resetting and airflow changes induced by swallowing in humans. The Journal of Physiology, 483, 273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T (2014). Airway protective mechanisms. Lung, 192, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts T, Gayagoy AG, Rose MJ, Poliacek I, Condrey JA, Musslewhite MN, Shen TY, Davenport PW, & Bolser DC (2015). Suppression of abdominal motor activity during swallowing in cats and humans. PLoS One, 10, e0128245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vice FL, & Gewolb IH (2008). Respiratory patterns and strategies during feeding in preterm infants. Developmental Medicine & Child Neurology, 50, 467–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relating to this study are available from the first author on request.


