Abstract

Ralstonia solanacearum is a phytopathogen causing bacterial wilt diseases of tomato and affecting its productivity, which leads to prominent economic losses annually. As an alternative to conventional pesticides, green synthesized nanoparticles are believed to possess strong antibacterial activities besides being cheap and ecofriendly. Here, we present the synthesis of silver nanoparticles (Sn-AgNPs) from medicinally important aqueous plant extracts of Salvia nubicola. Characterization of biologically synthesized nanoparticles was performed through UV–vis spectrophotometry, Fourier transform infrared spectroscopy (FTIR), energy-dispersive X-ray spectroscopy, X-ray diffraction, scanning electron microscopy, transmission electron microscopy (TEM), and thermogravimetric analysis. The antibacterial activity of the biosynthesized silver nanoparticles was tested against the phytopathogen R. solanacearum through in vitro experiments. Preliminary phytochemical analysis of the plant extracts revealed the presence of substantial amounts of flavonoids (57.08 mg GAE/g), phenolics (42.30 mg GAE/g), tannins, and terpenoids. The HPLC phenolic profile indicated the presence of 25 possible bioactive compounds. Results regarding green synthesized silver nanoparticles revealed the conformation of different functional groups through FTIR analysis, which could be responsible for the bioreduction and capping of Ag ions into silver NPs. TEM results revealed the spherical, crystalline shape of nanoparticles with the size in the range of 23–63 nm, which validates SEM results. Different concentrations of Sn-AgNPs (T1 (500 μg/mL) to T7 (78.1 μg/mL)) with a combination of plant extracts (PE-Sn-AgNPs) and plant extracts alone exhibited an efficient inhibition of R. solanacearum. These findings could be used as an effective alternative preparation against the bacterial wilt of tomato.
1. Introduction
Tomato is believed to be the most consumed and dominating vegetable all over the world, playing an important role in overcoming socioeconomic and food problems across the globe. Worldwide, China is the largest tomato-yielding country, with an annual yield of 65.15 million tons.1 Being low in calories, fat, and cholesterol, as well as being an excellent source of fiber and natural antioxidants, vitamins, carotenoids (Lycopene), and phenols, tomatoes are also regarded as being healthy for human health.2 The deep red color of many fruits and vegetables is mostly due to the brilliant red carotenoid component lycopene, which is present in abundance in tomatoes and tomato-based products.3 Agricultural productivity is critically influenced by plant pathogens causing various plant diseases, which lead to major economic losses worth billions of US dollars annually.4−6,7 The family members of solanaceae are greatly affected by the soil-borne phytopathogen R. solanacearum, a Gram-negative bacterium. More than 200 host plants of about 50 families are infected by R. solanacearum, which causes bacterial wilt disease in them.8 The bacteria inoculate injuries to plants’ roots through mechanical, nematode, or insect destruction. It was reported that R. solanacearum propagates in the plant xylem, hence obstructing its water transport system. It leads the plant toward wilting, the initial symptoms of which is chlorosis, dwarf growth, and ultimately its death and/or significant yield loss. Worldwide, it was given the second rank in phytopathogens. The disease is common in crops grown in tropical and subtropical regions.9−11
In spite of the economic importance of bacterial wilt, it is not well controlled.12 Previous literature showed that plants have their own defense mechanism because of the presence of significant compounds such as phenols and flavonoids due to which plants exhibit anticarcinogenic, antibacterial, antitumor, anti-inflammatory, antiviral, and antioxidant characteristics.13 Alternatively, other conventional methods such as mechanical, physical, crop rotation, use of healthy seeds, use of chemicals, resistant rootstocks, synthetic pesticides, and biological control are also in practice.14 The overuse of synthetic fertilizers is not ecofriendly, as it is hazardous to humans as well as to the environment, posing ecological and financial challenges in the world. Despite the fruitful results of conventional strategies, they have some drawbacks as well, and solanaceous plants are still greatly affected by bacterial wilt, which is why development of some new strategies to overcome this problem is needed.15
Nanotechnology is an emerging field that has promising results to control plant diseases caused by phytopathogens.16 Further, metal nanoparticles have a significant role in the control of plant diseases and are preferred to be used because of their easy perpetration and organic alterations at the nanoscale level. Metal nanoparticles can be synthesized by chemical, physical, and biological methods.7,17Among these, the biological method (green synthesis) is most favored for the synthesis of nanoparticles because it is safe, ecofriendly, nontoxic, efficient, economic, and biocompatible. Green chemistry is an advanced and alternate method for the synthesis of metal nanoparticles by employing biological agents such as bacteria, fungi, algae, plants, and human cells.18 Among all the agents, plants are given preference for nanoparticle synthesis because of the presence of phytochemicals such as proteins, vitamins, polyphenols, polysaccharides, terpenoids, and organic acids, which act as reducing and capping agents of the synthesized nanoparticles according to the desired shape and size. Due to its strong anti-inflammatory, antiangiogenetic, antifungal, and antibacterial activities, silver is given preference over other elements for the biological synthesis of nanoparticles.19 Currently, silver nanoparticles have fascinated people for their unusual biological activities such as food preservation, wound healing, drug delivery, cosmetics, biolabeling, water purification, and sensing. Furthermore, it has extraordinary applications such as in paints, electronics, textiles, and catalysis. Among plants, medicinal plants are more important for use because of the presence of secondary metabolites which act as stabilizing and reducing agents of nanoparticles at nanoscales.20
Salvia is the largest genus in the family Lamiaceae with nearly 1000 species of perennials, shrubs, and annuals. Salvare, the Latin name of Salvia, means “to heal”, so its medicinal importance is clear from its name. Since early times, Salvia species have been used for curing many diseases, including those of the central nervous system, dysentery, menstrual problems, boils, and digestive and hepatic problems.21−23,24 The biological activities of Salvia species may be attributed to the presence of various phytochemicals.
Salvia nubicola Wall. (Lamiaceae) is a green perennial herb, with a quadrangular stem, and a height range from 60 to 100 cm, whose leaves and stem have eglandular and glandular hairs and verticillaster inflorescence with a 2–6-flowered body, arranged on terminal spikes. The pale-yellow corolla has brownish markings, especially on the lower lip; the tube is curved ± included in or exserted from the calyx. Its fruiting and flowering seasons are from June to October. The plant is distributed in Nepal, Afghanistan, Bhutan, Tibet, Pakistan, Kashmir, N. India, and Sikkim.25 It contains flavonoids, diterpenes, nubiol, bisnubidiol, saponins, alkaloids, and phenyl ethanoic glycosides, due to which Salvia shows strong antimicrobial activities.26
1.1. Contributions
To the best of our knowledge, no study of the environmentally friendly synthesis of AgNPs utilizing a reducing agent derived from the medicinal plant S. nubicola has ever been reported. The current study therefore aimed to explore S. nubicola for antioxidant and phytochemical investigations. The study also aimed to biologically synthesize AgNPs using an aqueous plant extract of S. nubicola and evaluate its in vitro antibacterial activity.
2. Materials and Methods
2.1. Preparation of Plant Extract
The plant specimens were collected from various localities in Swat, Pakistan. The collected specimens were washed, shade-dried, and homogenized into fine powder. The powder material was stored and used for further analysis including phytochemical, antioxidant, and silver nanoparticle biosynthesis. For plant extract preparation, 1 g of fine powder of the plant was dissolved in 100 mL of distilled water (10 mg/mL). The solution was then heated at 60–70 °C for 20 min on a hot plate. Upon cooling, the aqueous solution was filtered three times using nylon fabric and a Whatman filter paper. The solution was stored in a refrigerator at 4 °C for further processing.27
2.2. Qualitative Screening
The qualitative analysis of the S. nubicola plant extracts was carried out by following the study of Arif et al.27with some minor modifications.
Flavonoid contents were assessed by taking 0.5 mg of plant crude extract in 5 mL of distilled water and keeping it for 5 min in a hot water bath. After filtration, 1 mL of the filtrate was separated into a vial. By the dropwise addition of 20% NaOH, a yellow color appeared, which showed the confirmation of the presence of flavonoids. For the measurement of glycoside contents, approximately 5 mg of crude plant extract was heated at 60 °C in distilled water for 15 min. Five milliliters of the extract was taken from the filtrate and was added to 2 mL of glacial acetic acid and 1 mL of concentrated sulfuric acid. With the dropwise addition of FeCl3, the blue-colored ring that appeared confirmed the presence of glycosides. For tannin investigation, 20 mL of distilled water was added to 2 mg of crude extract and heated for 5 min in a water bath. One milliliter of the filtrate was taken, and on dropwise addition of ferric chloride, a brownish color appeared to designate the presence of tannins. To estimate the terpenoid content, about 1 mg of the plant extract was added to 5 mL of distilled water; upon boiling the solution, it was filtered and mixed with 3 mL concentrated H2SO4 and 2 mL chloroform. The appearance of reddish-brown color showed that terpenoids are present.
2.3. Quantitative Analysis
Total flavonoid contents were determined following the methodology of Kim et al.28 with minor modifications. About 5 mg of S. nubicola crude extract was mixed with 10 mL of distilled water and was heated for 30 min. About 100 μL of the filtrate was taken into 500 μL of distilled water in a vial. The solution was then added to 1000 μL of (7%) sodium carbonate and 100 μL of Folin–Ciocalteu reagent and was reserved at room temperature for 90 min. The absorbance of blue color solution was recorded at 760 nm through a UV–visible spectrophotometer. Standard gallic acid was used for curve calibration of the total flavonoid content quantification and was expressed in mg GAE/g (gallic acid equivalent/g) of the dry sample.
Total phenol contents were measured using a spectrophotometer with Folin, sodium carbonate, and ethanol by following the methodology of Lubna et al.29 The antioxidant activity of the S. nubicola extract and its biosynthesized nanoparticles was determined by the DPPH scavenging assay, as discussed by Zangeneh30 with some minor modifications. Briefly, about 0.125 mg of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was dissolved in 20 mL of methanol, and 1 mg of plant powder was dissolved in 1 mL of methanol to prepare plant stock solutions (1 mg/mL). To investigate the free-radical scavenging activity of the S. nubicola extract and silver nanoparticles (Sn-AgNPs), 2 mL of DPPH was added with 1 mL of various concentrations (1000, 500, 250, 125 and 62.5 μg/mL) of plant extracts and AgNPs. Then, the samples were kept in the dark for 30 min at room temperature. The absorbance of the mixture was recorded at 517 nm using a UV–vis spectrophotometer (UV-1602, BMS). Standard ascorbic acid was used as the positive control.
2.4. Phenolic Profile of the Extract Using HPLC-UV
HPLC samples were prepared by mixing 1 g of powder of the sample with a mixture of methanol and water (20 mL; 1:1 v/v). Heating the sample at 40 °C in a water bath was followed by cooling and centrifugation at 4000 rpm for 10–15 min. The supernatant was collected and filtered by using a Whatman filter paper, and 2 mL of the sample was collected from the filtrate in a HPLC vial. An HPLC Agilent 1260 Infinity system was used to carry out the analysis of the bioactive compounds which comprised a C18 column, a UV detector, an autosampler, and a quaternary pump. The gradient system of the column consists of solution C (methanolic: acetic acid: deionized water 10:2:88 v/v) and solution D (methanolic: acetic acid: deionized water 90:2:8 v/v). The identification of possible compounds was carried out following the methodology of Fu et al.31 and Ovais et al.32
2.5. Biosynthesis of Sn-AgNPs
Plant-mediated synthesis of AgNPs was accomplished by following the methodology of Ahmad et al.16 with minor modifications. For the synthesis of silver nanoparticles, different concentrations of plant extracts and AgNO3 were used. S. nubicola plant extract was used as a source of biological reducing and stabilizing agent. Different experimental trials were conducted to investigate the influence of some parameters on the biosynthesis of nanoparticles, such as the concentration of plant extracts and silver nitrate and their different volumetric ratios. The stock solution (10 mg/mL) of the plant extract was serially diluted by twofold to prepare different concentrations (5 2.5, 1.25, 0.625, and 0.312 mg/mL). Similarly, the stock solution of AgNO3 (10 mM) was diluted four times to prepare different solutions (8, 6, 4, and 2 mM). The resultant 25 falcon tube mixtures were exposed to sunlight for 20 min to observe the color change. After finding the best combination of both reactants for nanoparticle synthesis, which were previously reacted in 1:1 v/v ratios, both the reactants were mixed in other volumetric ratios (1:9 to 9:1 v/v). Briefly, 900 μL of the plant extract was mixed with 100 μL of AgNO3 (9:1 ratio). The subsequent nine mixtures were prepared by decreasing the plant extract and increasing the AgNO3 volume by 100 μL until the final ratio of 1:9 was attained. The mixtures were prepared in an Eppendorf tube (1.5 mL) and were scaled up to falcon tubes (15 mL).
2.6. Characterization of Synthesized Particles
To separate the biosynthesized AgNPs, the solution was centrifuged (Eppendorf centrifuge, model 5425, Darmstadt, Germany) at 15,000 rpm for 10 min. The supernatant was disposed off, and the precipitate was collected in a distinct tube. The collected pellet of AgNPs was washed and dissolved in deionized water, followed by centrifugation again at 15,000 rpm for 10 min. The process was repeated four to five times, and the residual settled material was vacuum-dried and then used for characterization. The biosynthesized Sn-AgNPs were monitored by a UV–visible spectrophotometer (Multiskan Sky Model-1530) within the spectral range of 300–600 nm.
The existence of functional groups in the biosynthesized Sn-AgNPs was studied by analyzing the dried powder of the particles through Fourier transform infrared spectroscopy of 500–4000 cm–1, Spectrum Two 103385.33 The chemical nature of the biosynthesized nanoparticles was determined by EDX (INCA 200, Oxford, UK). XRD analysis was carried out to evaluate the morphology of the synthesized NPs by using a JDX-3532 system. The diffracted intensities were recorded from 10 to 80° at 2θ, while the average crystallite size was calculated from the width of XRD peaks by using the Scherrer equation34
where D is the average particle size of biogenic nanoparticles; K is the Sherrer constant (“0.9”); λ is the X-ray wavelength (typically 1.54 Å); β is the full width at half-maximum (fwhm); and θ is the Bragg diffraction angle.
Morphology and distribution of the biosynthesized nanoparticles were observed by a scanning electron microscope (JSM-5910, JEOL, Tokyo, Japan) at 200 kV under different magnifications (1000, 2500, 5000,10,000, 15000, 30,000, and 60,000). The size and shape of AgNPs synthesized by S. nubicola were determined by TEM. Electron micrographs were obtained using a JEOL JEM-1010 system at a magnification range of ×50–×600 K. TGA was used to analyze the surface weight loss and thermal stability of the biosynthesized AgNPs. A TGA instrument (Metler Toledo TGA/DSC1) was used to determine the thermal stability of the nanocomposites under a heating rate of 10 °C min–1 to 1000 °C.
2.7. Antibacterial Assay
In the present study, the antimicrobial potential of the biosynthesized silver nanoparticles was tested against Ralstonia solanacearum, a Gram-negative bacterium, by using 96-well microtiter plates by following the methodology of Tanase et al.35 with some modifications. For the growth of bacterial strains, nutrient broth (Basingstoke, Hampshire, UK) media was used. R. solanacearum was received from IAGS University of Punjab, Pakistan, with accession No. FCBP-PB-407. For the bacterial strain growth, 2.6 g of nutrient broth (Basingstoke, Hampshire, UK) media was dissolved in 200 mL of distilled water, followed by autoclaving to remove all possible pathogens in it. The media was inoculated with bacteria and was kept overnight in an incubator (FTC-90E Velp Scientifica) at 28 °C. For antibacterial activities, the bacterial strains were inoculated in the presence of silver nanoparticles with the concentrations of T1 = 500 μg/mL, T2 = 250 μg/mL, T3 = 125 μg/mL, T4 = 62.5 μg/mL, T5 = 31.25 μg/mL, T6 = 15.62 μg/mL, and T7 = 78.1 μg/mL of serially diluted plant extracts, and centrifuged AgNPs were added in the 96-well microtiter plate. The total volume of solution in the microtiter plate was 200 μL. Each type of experiment was carried out in triplicate. The microtiter plate was kept in a shaker incubator at 37 °C for shaking. For the observation of bacterial growth inhibition, the UV–visible spectrometer at the range of 600 nm was used to record the optical density at 0 and 24 h. Various experiments were performed for bacteria to check out the inhibitory effect of centrifuged nanoparticles, Sn-AgNPs, in combination with the plant extract (PE-Sn-AgNPs) and S. nubicola plant extracts alone.
2.8. Statistical Analysis
In the present work, the bacterial growth inhibitory effects were determined by using Origin Pro 8.5 and GraphPad Prism software (GraphPad Software, San Diego, CA, USA) and Statistix 8.1.
3. Results
3.1. Qualitative Screening
Methanolic plant extracts of S. nubicola indicated the presence of flavonoids, glycosides, tannins, and terpenoids. The TPC results of the methanolic and aqueous extracts of S. nubicola were evaluated by using the regression equation of the standard gallic acid. For TFC, a calibration curve was constructed by preparing the dilutions of 10, 20, 40, 60, 80, and 100 mg/mL and the regression equation for th estandard quercetin that was used for the determination of TFC (Table 1). Plants have antioxidant properties because of the presence of terpenoids, flavonoids, tannins, and glycosides.
Table 1. Qualitative Phytochemical Screening of Methanolic and Aqueous Extracts of S. nubicola.
| phytochemical groups | result |
|---|---|
| flavonoids | present |
| glycosides | present |
| tannins | present |
| terpenoids | present |
| TPC in aqueous extract | 42.30 (mg GAE/g) |
| TFC in methanolic extract | 57.08 (mg GAE/g) |
Methanolic plant extracts of S. nubicola were evaluated at different concentrations against DPPH, and the maximum inhibition was recorded (88.62%) for 1000 μg/mL of extract (Figure S1), while ascorbic acid as a control showed the optimum inhibition. A higher inhibition was displayed by ascorbic acid (97.62%), followed by Sn-AgNPs (91.56%), and the least inhibition was exhibited by S. nubicola extracts.
S. nubicola methanolic plant extracts tested against DPPH proved the significance of the free-radical scavenging activity of the methanol plant extract. S. nubicola AgNPs showed a high scavenging activity compared to only plant extracts (Table S1).
3.2. HPLC-UV Analysis
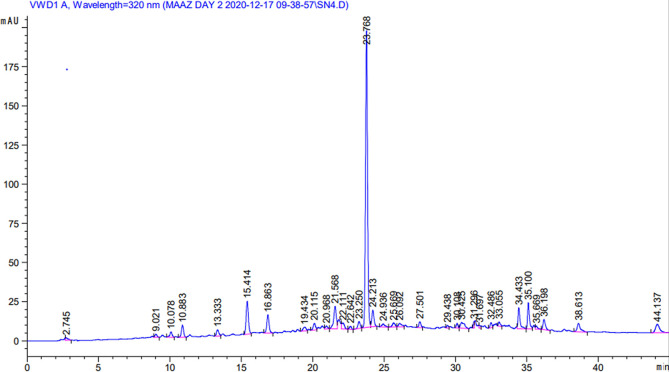
Crude extracts of S. nubicola were subjected to HPLC analysis to identify the possible bioactive compounds. The peak area and retention time of these compounds were compared with those of the standard chromatogram and the available literature.32 The HPLC chromatogram of the S. nubicola plant is displayed in Figure 1, which reveals the presence of 25 possible phytochemicals (Table S2). The most prominent among these were quercetin 3,7-di-O-glucoside, hydroxy benzoic acid derivative, phloroglucinol, quercetin-3-malonylglucoside, ellagic acid, and quercetin-7-O-sophoroside.
Figure 1.
HPLC chromatogram of the methanolic extract of S. nubicola.
3.3. Spectral Analysis
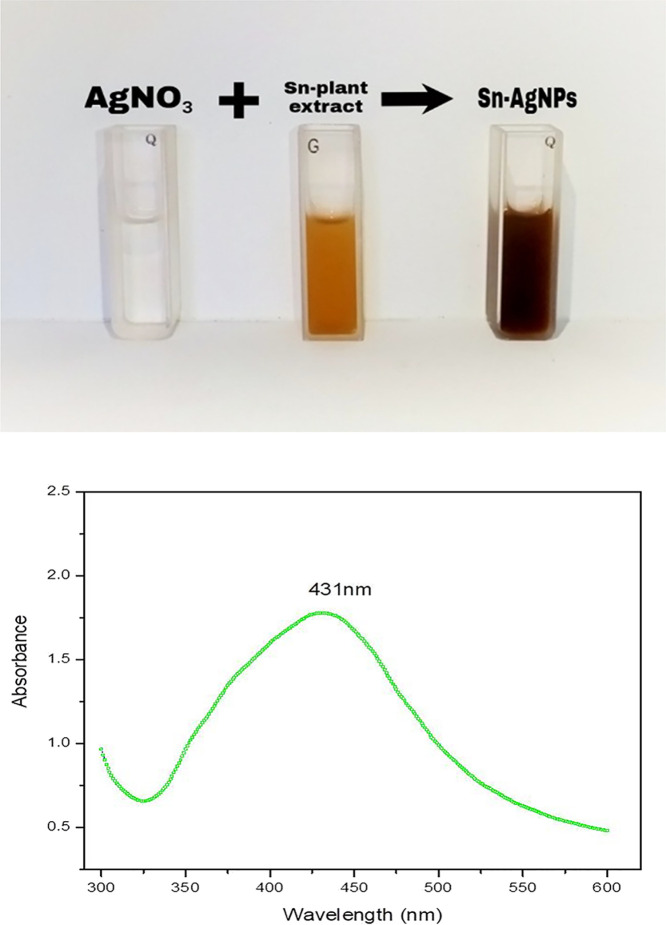
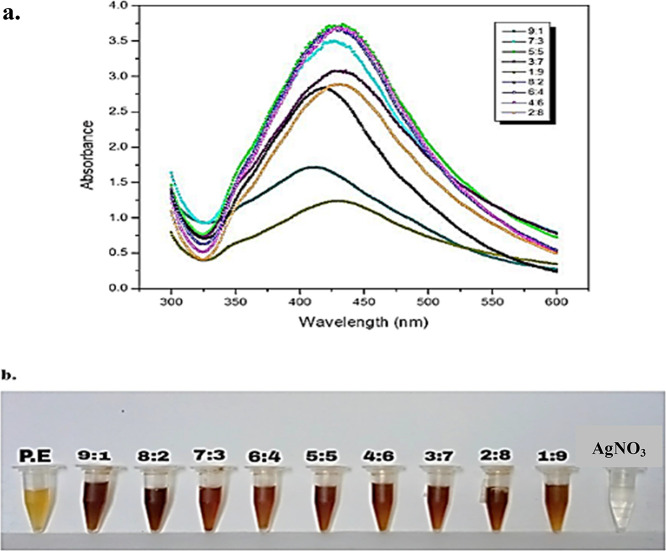
After mixing AgNO3 (4 mM) and the aqueous plant extract (1.25 mg/mL) in equal volumes (1:1) at room temperature, the color of the reaction mixture started turning brown upon exposure to sunlight for 20 min. UV–visible light is an important tool for the verification of NPs in colloidal solutions. After 24 h, the resultant solution of Sn-AgNPs was investigated through UV–vis spectroscopy for the surface plasmon resonance profiles of Sn-AgNPs. Results showed the highest peak at 431 nm in the UV–vis spectrum, which indicated the formation of NPs (Figure 2). Nine different volumetric ratios were obtained by mixing AgNO3 (4 Mm) and Salvia nubicola (1.25 mg/mL). By increasing the plant extract (1.25 mg/mL) from 10 to 60%, the absorbance spectra intensity increased, while it decreased on further increasing the concentration of plant extracts from 70 to 90%. The highest and narrowest absorbance peak with consistent characteristics was observed when 60% plant extract (1.25 mg/mL) and 40% silver nitrate (4 mM) were mixed (6:4 v/v). However, 9:1 and 1:9 did not show characteristic silver NP peaks (Figure 3). The Sn-AgNP mixtures were analyzed through a UV–vis spectrophotometer at different time intervals, where specific changes in the absorption spectrum intensity were observed from 0 to 48 h only, which further confirmed the synthesis of stable Sn-AgNPs (Figure S2).
Figure 2.
Studying the effect of AgNO3 salt on the biosynthesis of S. nubicola silver nanoparticles and UV–vis absorption spectra of the Sn-AgNPs by using S. nubicola.
Figure 3.
(a) Absorption spectra of Sn-AgNPs at various concentrations of S. nubicola plant extract and silver salt (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9). (b) Different volumetric ratios of Sn-AgNPs showing color changes.
3.4. Fourier Transform Infrared Spectroscopy
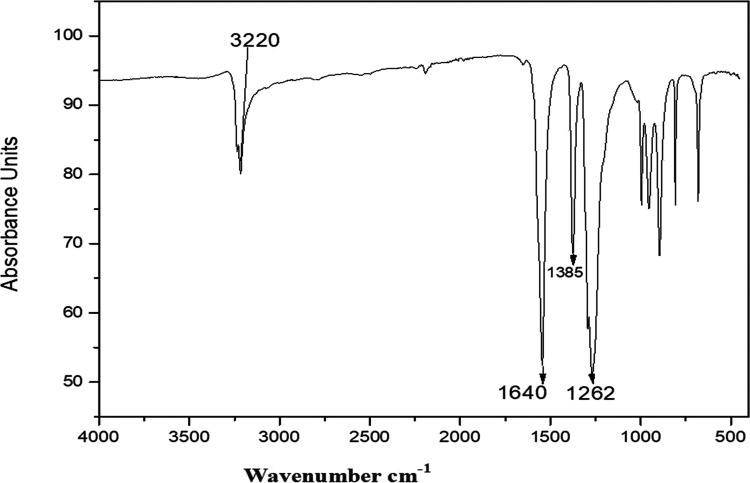
FTIR spectroscopy was used to explore various functional groups that could be involved in the formation of stable AgNPs. The FTIR pattern of the different absorption bands at particular wavelengths showed the confirmation of different functional groups. The spectra of the dried centrifuged powder of Sn-AgNPs are shown in Figure 4. A broad absorption band at 3220 cm–1 was observed, which corresponded to the stretching vibration of the O–H group due to alcohol. Another strong and sharp peak observed at 1640 cm–1 was assigned to the stretching vibration of the N–O group of the nitro compound. The bending vibration of the phenol was attributed to the bands at 1385 cm–1. The C–O stretching mode in the alkyl aryl ether compounds was assigned to the bands at 1260 cm–1 (Table S3). The FTIR pattern indicated the presence of capping and reducing biological groups in the extracts of S. nubicola, with their potential involvement in the bioreduction and stability of the synthesized nanoparticles. The FTIR pattern confirmed those agents in the S. nubicola extract which could be responsible for the bioreduction and stabilization of silver ions into silver NPs. The obtained bands could be attributed mainly to flavonoids, phenols, and terpenoids present in the plant extracts.
Figure 4.
FTIR analysis of the biosynthesized Sn-AgNPs.
3.5. Energy-Dispersive X-ray Analysis
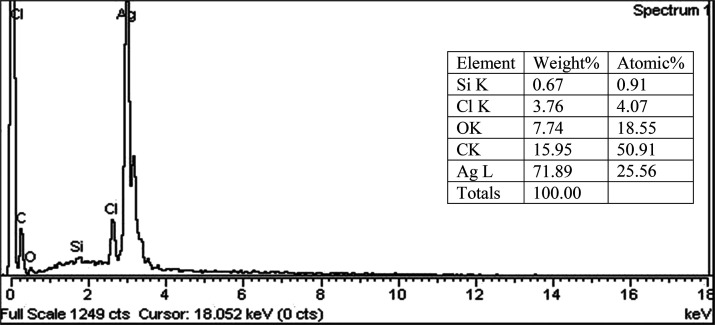
The estimation of elemental composition on the surface of fabricated NPs as well as the percent weight of each element was carried out by EDX analysis. Various peaks were recorded in S. nubicola-induced NPs by using the EDX spectrum (Figure 5). The highest energy peak of EDX analysis confirmed the presence of silver (Ag) elements in the synthesized NPs. Silver, Cl, Al, O, and C elements were confirmed by the major energy peaks. The Ag element was observed with the highest weight of 71.8%, followed by C (15.95%), O (7.74%), Cl (3.76%), and Si (0.67%). C and O confirmed the presence of phytochemicals on the surface of the biosynthesized AgNPs. The optical spectra of S. nubicola-induced synthesized NPs were found to be between 3 and 4 Kev in the EDX analysis.
Figure 5.
EDX analysis of the biosynthesized nanoparticles by S. nubicola.
3.6. X-ray Diffraction of Biosynthesized AgNPs
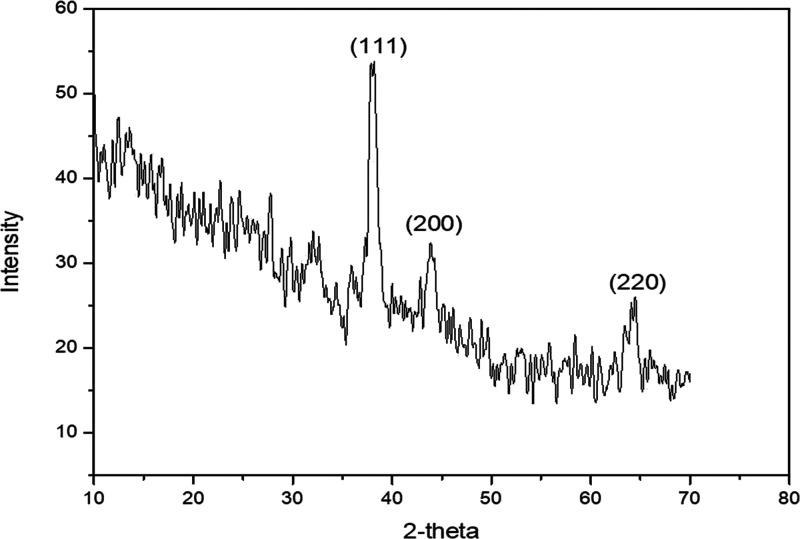
X-ray diffraction patterns elucidate the crystalline nature of the biosynthesized silver NPs. The detailed XRD analysis of S. nubicola showed Bragg’s reflections at three different angles, and it was performed over the scanning range of 2θ diffraction angle from 10 to 80°. The XRD analysis of S. nubicola silver NPs indicated three intense peaks in the whole spectrum of 2θ values ranging from 10 to 80° located at 38.16, 44, and 64.24°, corresponding to the Ag crystal planes of 111, 200, and 220, respectively (JCPDS file No. 04-078). The calculated particle size at 38.16 (2θ) was ∼33 nm, at 44.10 (2θ) was ∼36 nm, while at 64.24 (2θ) was ∼27 nm. The K value was taken from the shape of the nanoparticle using the TEM images reported elsewhere.34 Accordingly, a spherical morphology for silver was depicted, which was further confirmed by the TEM images (Figure 6).
Figure 6.
XRD patterns of the synthesized silver NPs of S. nubicola.
3.7. Scanning Electron Microscopy Analysis
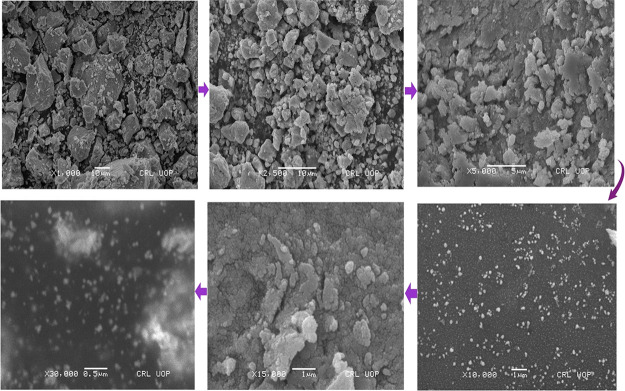
The morphology of the biosynthesized Sn-AgNPs is illustrated in Figure 7. SEM images showed that the synthesized NPs were in the size range of 25–47 nm. However, the average size of the synthesized Sn-AgNPs particles was found to be 36 nm. The characteristic morphology of the NPs displayed a uniform spherical-shaped surface and was crystalline. Visual observations showed well-dispersed NPs in the solution without aggregation.
Figure 7.
Scanning electron micrographs of S. nubicola-mediated silver NPs (Sn-AgNPs).
3.8. TEM Analysis of AgNPs
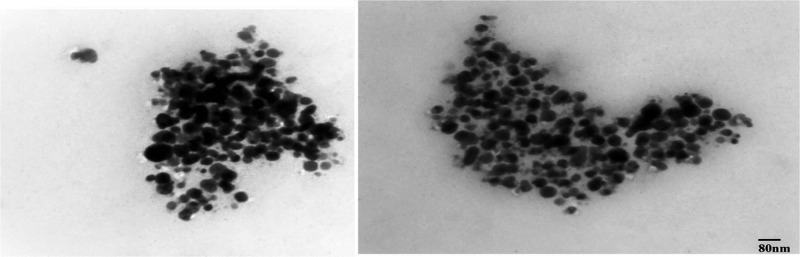
The size and morphology distribution of Sn-AgNPs under a transmission electron microscope confirmed that the average particle size was in the range of 23–63 nm with a spherical shape, further validating the SEM results. The NPs were mostly found in the form of small particles with negligible agglomeration. TEM results revealed the synthesized silver NPs with diverse morphological features. Overall, polydispersed AgNPs of varying sizes were observed at different magnifications. The TEM micrographs showed particles with variability in interparticle spaces, sizes, and disparity, as some NPs were found in the form of agglomerates, while some were found as individual particles. The particle size can be clearly found by drawing a line on the nanometer bar, as shown in Figure 8. It is clear from our TEM images that the majority of Ag particle sizes are in the range of 23–63 nm, which is consistent with our XRD results.
Figure 8.
TEM images of green synthesized silver nanoparticles from S. nubicola plant extract at 100× magnification.
3.9. Thermogravimetric Analysis
The thermal stability of biosynthesized NPs was determined using a thermogravimetric analyzer between 100 and 700 °C (Figure S3). The weight losses of Sn nanocomposites took place in three curve degradation steps: the first weight loss of 25% revealed by the first peak corresponded to the removal of water below 250 °C; the second peak was observed between 250 and 450 °C, which referred to the second weight loss because of the decomposition of various functional groups (which not only acted as reducing agents but also as capping agents); the third and last peak curve was obtained between the temperature range of 450 and 600 °C, which was due to the conversion of silver ions into the oxide form.
3.10. In Vitro Antibacterial Activity
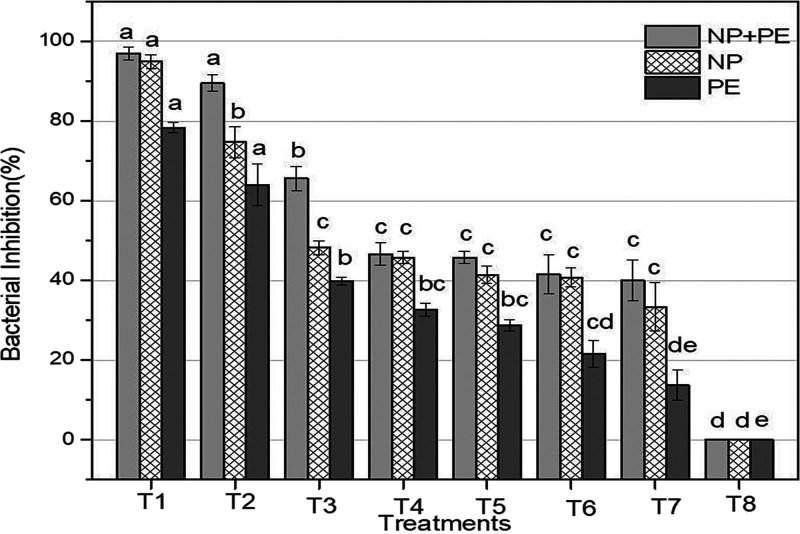
In the present study, the antibacterial activity of Sn-AgNPs (T1 (500 μg/mL) to T7 (78.1 μg/mL)) alone and in combination with S. nubicola aqueous extracts represented as PE-Sn-AgNPs was determined. Furthermore, the same concentration of the corresponding plant extract was also tested for its antibacterial activity. All assays were performed by using the 96-well microtiter plate method against the plant pathogenic bacteria, i.e., R. solanacearum (G negative) (Figure 9).
Figure 9.

Microtiter plate showing in vitro activity.
The effects of different treatments on the in vitro inhibition of R. solanacearum are shown in Figure 10. The highest concentration (T1 = 500 μg/mL) of Sn-AgNPs and PE-Sn-AgNPs was equally effective against the tested pathogen, while the same concentration of plant extract exhibited a significantly lower antibacterial activity. Moreover, T1 (500 μg/mL)–T3 (125 μg/mL) of PE-Sn-AgNPs exhibited more than 60% antibacterial activity, which shows the superiority of uncentrifuged nanoparticles (PE-Sn-AgNPs) over the centrifuged (Sn-AgNPs) and the plant extract alone in the same concentrations. However, almost a significantly comparable activity was observed in response to T4 (62.5 μg/mL)–T7 (7.81 μg/mL) of Sn-AgNPs and PE-Sn-AgNPs in contrast to the same concentrations of the plant extract alone. Overall, the activity against R. solanacearum of all treatments followed a concentration-dependent pattern, i.e., the activity decreased with a decrease in the treatment concentration, suggesting the efficacy of the lowest possible concentration for the significant inhibition of the tested pathogen.
Figure 10.
Effect of different concentrations (twofold dilution) of PE + NPs, NPs, and PE against R. solanacearum. Plant extract refers to an aqueous extract of the aerial parts of S. nubicola. Columns of the same treatments sharing the same alphabets are not significantly different. The HSD test was applied for each treatment, separately.
The study illustrated that Sn-AgNPs biosynthesized by using 1.25 mg/mL plant extracts (S. nubicola) and AgNO3 (4 mM) possessed antibacterial activity. Overall, the results showed that the highest antibacterial activities were displayed by the Sn-AgNPs synthesized (4 mM of silver nitrate and 1.25 mg/mL of plant extract). Furthermore, the highest activity was exhibited by uncentrifuged PE-Sn-AgNPs, followed by centrifuged Sn-AgNPs, and then plant extract.
4. Discussion
Plants often show high biological activity due to the presence of various phytochemicals; hence, they are used for the biosynthesis of nanoparticles because of their good reducing and capping properties.36,37Salvia species have antimalarial, antifeedant, antifungal, insecticide, and antileishmanial phenomena due to the presence of various phytochemicals.38
In DPPH assay, the color of the mixture changed from purple to yellow, which is generally ascribed to the action of antioxidants that reduce DPPH to its nonradical form.39 The interaction between DPPH and the aqueous plant extract (S. nubicola) might have occurred by the transfer of hydrogen ions and electrons. Further, the highest activity of Ag nanoparticles might be due to the presence of various types of functional groups (confirmed by FTIR and HPLC), which were responsible for the capping and stabilizing properties of silver NPs. Biologically green synthesized AgNPs from medicinal plants reveal a high scavenging effect against the free radical formation in the living cell.40 They have strong redox character to deactivate free radicals.. Several reports have shown high antioxidant activity of the biosynthesized AgNPs. The free radicals can also be neutralized by the application of silver NPs as antioxidants in the case of those diseases caused by the free radicals. The present study results are in positive correlation with the study of the previous literature.41−43
The obtained results of HPLC were supported by the findings of Arif et al.,27 where, using the same methodology, some of these compounds were identified in the leaf extracts of Euphorbia wallichii. These results were also similar to that of Ahmad et al.,16 in which the leaf extracts of Polygonatum geminiflorum were investigated. The color change was due to the bioreduction of Ag ions in the reaction mixture, which revealed the biosynthesis of Sn-AgNPs. UV–vis light is an important tool for the verification of NPs in colloidal solutions. Probably, it might be due to the presence of some secondary metabolites, which still can reduce the remaining metals up to 48 h. The effect of different time intervals on the absorption spectra of Sn-AgNPs is in agreement with the findings of Sumitha et al.44 Smaller particle sizes represent peaks at shorter wavelengths, while those of a larger particle size indicates a longer wavelength peak.45 Previously, Shankar et al.46 also revealed the importance of terpenoids in Geranium plant extracts for the synthesis of AgNPs. The present study is also in accordance with previous studies, where the secondary metabolite, phenolic, and flavonoid compounds in S. nubicola plant extracts not only act as capping agents but also act as reducing agents for the biosynthesis of Sn-AgNPs. The present study results coincide with that of the previously reported studies.47,48 Similar results of almost the same range were obtained in the previously reported green synthesis of AgNPs from different plants extracts such as Prosopis juliflora, Benincasa hispida, Allium cepa, Salvia hispanica, pomegranate leaves, and Parkia speciose.49−51
The FTIR patterns confirmed those agents in S. nubicola extract which could be responsible for the bioreduction and stabilization of silver ions into silver NPs. The obtained bands could be attributed mainly to flavonoids, phenols, and terpenoids present in plant extracts. As consistent with previous reports, the FTIR results of the present work confirmed the formation of Sn-AgNPs.52 Moreover, the results of the present study agree with those found in the reported literature.53 Important linkages were formed during the biosynthesis of Sn-AgNPs, which agree with the findings investigated previously,46,54 which show the frequent involvement of the above mentioned biological groups in the synthesis of nanoparticles using different plant species.
Different elements were observed through EDX which revealed the presence of various phytochemicals on the surface of the Sn-AgNPs, which act not only as capping agents but also as reducing agents for AgNPs. Normally, the silver absorption peaks of EDX appear at 3 keV.55 EDX pattern exhibited a good signal for silver particles, which may be attributed to the phytochemicals of the S. nubicola extract that bound to the surface of Sn-AgNPs, revealing the reduction of the silver element. These phytochemicals upon adsorption onto the surface of AgNPs may have reduced Ag+ and led to the formation of metallic silver (Ag°). There may be a cluster of AgNPs, as can be seen in the TEM image, which eventually leads to the formation of metallic colloidal silver particles, as discussed previously.56 Similar results of the EDX spectra for biosynthesized AgNPs were reported earlier with an optical absorption range of 2.7–3.4 keV.57 The EDX analysis of the present study is in accordance with the earlier reported studies.58
Since the nanosize is dependent on the intensity of the peaks and broadening, in our result, the intensity of the Ag XRD peaks was low compared to other reports. Similarly, the broadness of the peaks further confirmed the smaller nanosized Ag particles. Previously, Krishnaraj et al.59 reported that in the XRD spectra, the two high peaks at 32.51 and 44.50° could be due to the presence of phytochemical compounds in the aqueous extract of Salvia coating the surface of the synthesized Sn-AgNPs and capping it. Our results agree with other studies on AgNPs formed from other plant extracts.60−62 The sharpening of the peak indicates that the Salvia NPs are in the nano-range. Overall, the sharpness of the peaks varies due to the variations in the particle size. The results confirmed the crystalline nature of the biosynthesized AgNPs. The findings obtained from the present study are in accordance with those previously reported.63,64
The size of the synthesized NPs was in positive correlation with the findings of the previously reported studies of SEM.65 However, the lower magnification images showed some form of aggregation, as shown in Figure 10, which may be considered the common characteristics for silver NPs. Some organic layers around the NPs in the higher magnification images indicated the presence of secondary metabolites. The findings of the present study are in congruence with the previous literature.57,66
The interaction of NPs in a suspension due to a higher surface area and multiple reactive active sites in the NPs may result in their agglomeration. Furthermore, secondary metabolites and different phytochemicals present in plants may also lead to agglomeration.67 In this study, as evidenced by the presence of organic layers around the NPs in the TEM images, the agglomeration may have been strongly dependent on the secondary metabolites, such as phenolics, flavonoids, alkaloids, and so forth. The results showed that some biosynthesized secondary metabolites present in the plant extract were stably attached to NPs, despite the consistent centrifugation as in the preliminary step. The results obtained by TEM were in positive correlation with the findings by XRD. The TEM micrographs of Sn-AgNPs showed the presence of individual NPs with a spherical shape, which is a distinctive character of silver NPs.68 According to the study of Zhao et al.,69 the biosynthesized NPs with a spherical shape are more reactive than rod-shaped or hexagonal particles. Similar to our findings, spherical Ag-NPs with an average particle size of less than 40 nm were synthesized using the extract of Lepidium draba root.70 The TGA results of the present study are similar to the findings previously reported.71,72
The surface area and size of the silver NPs have a great influence on the NPs’ biological activities.73 Due to the smaller size at the nanoscale, nanoparticles have a larger surface area and have many active reactive sites, which make them ideal for their interaction with bacterial cell walls, enhance the permeability of bacterial cell walls, and subsequently stimulate their antibacterial activity.68 It is also evident from the in vitro experiment performed that silver nanoparticles have high inhibition activity against the tested pathogen compared to the plant extract alone. As shown in previous studies, although silver nanoparticles can be considered efficient antibacterial agents, the exact mechanism of their action has yet to be explored. The bacterial cell membrane contains some negatively charged organic and inorganic compounds which provide an easy platform for the positively charged silver NPs to interact and adsorb to the bacterial cell wall.74,75 After the nanoparticles enter the bacterial cell, they affect the structure and biological compounds such as carbohydrates, DNA, proteins, and lipids, compromising the physiological functions of bacteria and interfering with their viability. A possible mechanism of the antibacterial action of AgNPs includes the abnormal growth and altered respiratory function of bacteria in response.73,76 The higher activity of uncentrifuged NPs may be due to the synergism of NPs and unreacted plant secondary metabolites present in the aqueous extract. This conclusion is supported by the plant extract alone, which also showed lower activity than all other treatments. The results of our study are in positive correlation with previous research.77,61,64,53 Same results were also obtained by Dilbar et al.,78 where they used Stachys emodi to synthesize nanoparticles, and their uncentrifuged NPs showed effective results against Erwinia carotovora. Chen et al.79 have found similar positive results of silver nanoparticles against Ralstonia solanacearum. According to the study of Cheng et al.,80 green synthesized silver nanoparticles, by using the flower extracts of Canna indica L., Cosmos bipinnata Cav., and Lantana camara L., showed strong antibacterial effects against the plant pathogen Ralstonia solanacearum. Silver nanoparticles fabricated by using the Stachys parviflora plant as a reducing agent also showed strong antibacterial effects against the phytopathogen Xanthomonas campesti.81
5. Conclusions
The phytochemical analysis in the present study revealed the presence of important phytochemicals in S. nubicola, which were the finest reducing agents for the biogenic synthesis of Sn-AgNPs. The DPPH scavenging assay confirmed the antioxidant quality of S. nubicola, and the presence of 25 biologically active compounds was confirmed by HPLC. The biologically synthesized silver nanoparticles had a spherical morphology, and the particle size in the range of 23–63 nm was confirmed by XRD, SEM, and TEM. The presence of silver and other elements in the nanoparticles was confirmed by EDX analysis. Most of the functional groups were identified through FTIR. TGA was used for the investigation of the thermal stability of Sn-AgNPs. The fabricated nanoparticles exhibited strong antibacterial activity against the Gram-negative bacterium R. solanacearum in vitro. The effect of uncentrifuged particles (PE-Sn-AgNPs) was superior to that of centrifuged (Sn-AgNPs) and plant extract alone in vitro. Further studies need to evaluate the in-planta activity and to study the antimicrobial effects of Sn-AgNPs for the control of other diseases in plants to allow the ecofriendly and less costly commercialization of nanopesticides.
Acknowledgments
The authors thank the researchers, supporting project number (RSP2023R110), at King Saud University Riyadh Saudi Arabia, for the financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03164.
Antioxidant activity of S. nubicola plant extract against DPPH radicals; scavenging activity of S. nubicola AgNPs and plant extracts; identification of different phytochemical compounds in S. nubicola; UV–vis spectra-indicated absorbance intensities of Sn-AgNPs at various time intervals; vibration bands of different groups at various peaks shown by FTIR spectra; and TGA of Sn-AgNPs (PDF)
This research work was supported by the researchers, supporting project number (RSP2023R110), King Saud University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
Supplementary Material
References
- Yuan W.; Jiang H.; Sun M.; Zhou Y.; Zhang C.; Zhou H. Geographical origin identification of chinese tomatoes using long-wave Fourier-Transform Near-Infrared Spectroscopy combined with deep learning methods. Food Anal. Method. 2023, 16, 664–676. 10.1007/s12161-023-02444-1. [DOI] [Google Scholar]
- Paolo D.; Bianchi G.; Scalzo R. L.; Morelli C. F.; Rabuffetti M.; Speranza G. The chemistry behind tomato quality. Nat. Prod. Commun. 2018, 13, 1934578X1801300. 10.1177/1934578x1801300927. [DOI] [Google Scholar]
- Przybylska S.; Tokarczyk G. Lycopene in the prevention of cardiovascular diseases. Int. J. Mol. Sci. 2022, 23, 1957. 10.3390/ijms23041957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafique K.; Rauf C. A.; Gul A.; Bux H.; Memon R. A.; Ali A.; Farrakh S. Evaluation of d-genome synthetic hexaploid wheats and advanced derivatives for powdery mildew resistance. Pak. J. Bot. 2017, 49, 735–743. [Google Scholar]
- Sher H.; Aldosari A.; Ali A.; de Boer H. J. Indigenous knowledge of folk medicines among tribal minorities in Khyber Pakhtunkhwa, northwestern Pakistan. J. Ethnopharmacol. 2015, 166, 157–167. 10.1016/j.jep.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Syed-Ab-Rahman S. F.; Singh E.; Pieterse C. M. J.; Schenk P. M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. 10.1016/j.plantsci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Ali M.; Ahmed T.; Wu W.; Hossain A.; Hafeez R.; Islam Masum M.; Wang Y.; An Q.; Sun G.; Li B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials. 2020, 10, 1146. 10.3390/nano10061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W.; Yang J.; Tan Y.; Munir S.; Liu Q.; Zhang J.; Ji G.; Zhao Z. Ralstonia solanacearum, a deadly pathogen: Revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere 2022, 21, 100479 10.1016/j.rhisph.2022.100479. [DOI] [Google Scholar]
- Abebe A. M.; Choi J.; Kim Y.; Oh C.-S.; Yeam I.; Nou I.-S.; Lee J. M. Development of diagnostic molecular markers for marker-assisted breeding against bacterial wilt in tomato. Breed. Sci. 2020, 70, 462–473. 10.1270/jsbbs.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlin L.; Escourrou A.; Cassan C.; Macia F. M.; Peeters N.; Genin S.; Baroukh C. Unravelling physiological signatures of tomato bacterial wilt and xylem metabolites exploited by Ralstonia solanacearum. Environ. Microbiol. 2021, 23, 5962–5978. 10.1111/1462-2920.15535. [DOI] [PubMed] [Google Scholar]
- Gashaw T.; Sitotaw B.; Yilma S. Evaluation of rhizosphere bacterial antagonists against Ralstonia solanacearum causing tomato (Lycopersicon esculentum) wilt in Central Ethiopia. Int. J. Agron. 2020, 2022, 6341555 10.1155/2022/6341555. [DOI] [Google Scholar]
- Magar T. R.; Lee S. Y.; Kim H. J.; Lee S. W. Biocontrol of bacterial wilt in tomato with a cocktail of lytic bacteriophages. Appl. Microbiol. Biotechnol. 2022, 106, 3837–3848. 10.1007/s00253-022-11962-7. [DOI] [PubMed] [Google Scholar]
- Zreen Z.; Hameed A.; Kiran S.; Farooq T.; Zaroog M. S. A comparative study of Diospyros malabarica (Gaub) extracts in various polarity-dependent solvents for evaluation of phytoconstituents and biological activities. BioMed. Res. Int. 2022, 2022, 1–16. 10.1155/2022/4746223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada K.; Sakai S.; Kajihara H.; Tanaka S.; Ito S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 2016, 65, 551–560. 10.1111/ppa.12443. [DOI] [Google Scholar]
- Kumari R.; Singh D. P. Nano-biofertilizer: An emerging eco-friendly approach for sustainable agriculture. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2020, 90, 733–741. 10.1007/s40011-019-01133-6. [DOI] [Google Scholar]
- Ahmad M.; Ali A.; Ullah Z.; Sher H.; Dai D.-Q.; Ali M.; Iqbal J.; Zahoor M.; Ali I. Biosynthesized silver nanoparticles using Polygonatum geminiflorum efficiently control fusarium wilt disease of tomato. Front. Bioeng. Biotechnol. 2022, 10, 1679. 10.3389/fbioe.2022.988607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndaba B.; Roopnarain A.; Rama H.; Maaza M. Biosynthesized metallic nanoparticles as fertilizers: An emerging precision agriculture strategy. J. Integr. Agric. 2022, 21, 1225–1242. 10.1016/S2095-3119(21)63751-6. [DOI] [Google Scholar]
- Alfuraydi A. A.; Devanesan S.; Al-Ansari M.; AlSalhi M. S.; Ranjitsingh A. J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2019, 192, 83–89. 10.1016/j.jphotobiol.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Kocak Y.; Oto G.; Meydan I.; Seckin H.; Gur T.; Aygun A.; Sen F. Assessment of therapeutic potential of silver nanoparticles synthesized by Ferula pseudalliacea Rech F. plant. Inorg. Chem. Commun. 2022, 140, 109417 10.1016/j.inoche.2022.109417. [DOI] [Google Scholar]
- Mohammadzadeh V.; Barani M.; Amiri M. S.; Yazdi M. E. T.; Hassanisaadi M.; Rahdar A.; Varma R. S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606 10.1016/j.scp.2022.100606. [DOI] [Google Scholar]
- Ullah Z.; Ali U.; Ali S.; Ali A.; Alam N.; Sher H.. Medicinal flora and cultural values of Arkot-Biakand Valley Hindu Kush Region Swat, Pakistan. In ethnobilogy of mountain communities in Asia; Springer: Berlin/Heidelberg, Germany, 2021, pp. 327–380. [Google Scholar]
- Ortiz-Mendoza N.; Aguirre-Hernandez E.; Fragoso-Martínez I.; González-Trujano M.; Basurto-Peña F.; Mar-tínez-Gordillo M. A review on the ethnopharmacology and phytochemistry of the Neotropical sages (Salvia Subgenus Calosphace; Lamiaceae) emphasizing Mexican species. Front. Pharmacol. 2022, 13, 867892 10.3389/fphar.2022.867892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S. U.; Ullah Z.; Ali A.; Aziz M. A.; Alam N.; Sher H.; Ali I. Traditional knowledge of medicinal flora among tribal communities of Buner Pakistan. Phytomed. Plus. 2022, 2, 100277 10.1016/j.phyplu.2022.100277. [DOI] [Google Scholar]
- Sher H.; Ali A.; Ullah Z.; Sher H. Alleviation of poverty through sustainable management and market promotion of medicinal and aromatic plants in Swat, Pakistan. Ethnobot. Res. Appl. 2022, 23, 1–19. 10.32859/era.23.16.1-19. [DOI] [Google Scholar]
- Hedge I. C.Labiatae in Flora of Pakistan. University of Karachi: Karachi, Pakistan, 1990, 192, 310p. [Google Scholar]
- Haq F. U.; Ali A.; Akhtar N.; Aziz N.; Khan M. N.; Ahmad M.; Musharraf S. G. A high-throughput method for dereplication and assessment of metabolite distribution in Salvia species using LC-MS/MS. J. Adv. Res. 2020, 24, 79–90. 10.1016/j.jare.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif M.; Ullah R.; Ahmad M.; Ali A.; Ullah Z.; Ali M.; Al-Joufi F. A.; Zahoor M.; Sher H. Green synthesis of silver nanoparticles using Euphorbia wallichii leaf extract: its antibacterial action against citrus canker causal agent and antioxidant potential. Molecules. 2022, 27, 3525. 10.3390/molecules27113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-O.; Jeong S. W.; Lee C. Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- Lubna Asaf S.; Hamayun M.; Gul H.; Lee I. J.; Hussain A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. 10.1080/17429145.2018.1436199. [DOI] [Google Scholar]
- Zangeneh M. M. Green synthesis and formulation a modern chemotherapeutic drug of Spinacia oleracea L. leaf aqueous extract conjugated silver nanoparticles; Chemical characterization and analysis of their cytotoxicity, antioxidant, and anti-acute myeloid leukemia properties in comparison to doxorubicin in a leukemic mouse model. Appl. Organomet. Chem. 2020, 34, e5295 10.1002/aoc.5295. [DOI] [Google Scholar]
- Fu Y.; Luo J.; Qin J.; Yang M. Screening techniques for the identification of bioactive compounds in natural products. J. Pharm. Biomed. Anal. 2019, 168, 189–200. 10.1016/j.jpba.2019.02.027. [DOI] [PubMed] [Google Scholar]
- Ovais M.; Ayaz M.; Khalil A. T.; Shah S. A.; Jan M. S.; Raza A.; Shahid M.; Shinwari Z. K. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complement. Altern. Med. 2018, 18, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbrink K.; Ni G.; Sönnergren H.; Schmidtchen A.; Pang C.; Bajpai R.; Car J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. 10.1186/s13643-016-0400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford J. I.; Wilson A. J. C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. 10.1107/S0021889878012844. [DOI] [Google Scholar]
- Tanase C.; Berta L.; Mare A.; Man A.; Talmaciu A. I.; Roşca I.; Mircia E.; Volf I.; Popa V. I. Biosynthesis of silver nanoparticles using aqueous bark extract of Picea abies L. and their antibacterial activity. Holz. Roh.-Werkst. 2020, 78, 281–291. 10.1007/s00107-020-01502-3. [DOI] [Google Scholar]
- Sher H.; Aldosari A.; Ali A.; de Boer H. J. Economic benefits of high value medicinal plants to Pakistani communities: an analysis of current practice and potential. J. Ethnobiol. Ethnomed. 2014, 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq A.; Shah G. M.; Zada A.; Ali A.; Shah A. Z.; Fatima I. Phytochemical analysis and in-vitro anti-bacterial and anti-fungal activity of Verbascum arianthum (Benth). Pure Appl. Biol. 2021, 10, 797–806. [Google Scholar]
- Jassbi A. R.; Zare S.; Firuzi O.; Xiao J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2016, 15, 829–867. 10.1007/s11101-015-9427-z. [DOI] [Google Scholar]
- Singh J.; Kumar S.; Dhaliwal A. S. Green synthesis of silver nanoparticles using Ocimum tenuiflorum leaf extract: Characterization, antioxidant and catalytic activity. AIP Conf. Proc. 2021, 2352, 40017. 10.1063/5.0052349. [DOI] [Google Scholar]
- Zhaleh M.; Zangeneh A.; Goorani S.; Seydi N.; Zangeneh M. M.; Tahvilian R.; Pirabbasi E. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl. Organomet. Chem. 2019, 33, e5015 10.1002/aoc.5015. [DOI] [Google Scholar]
- Alahmad A.; Feldhoff A.; Bigall N.; Rusch P.; Scheper T.; Walter J.-G. Hypericum perforatum L.-Mediated Green Synthesis of Silver Nanoparticles Exhibiting Antioxidant and Anticancer Activities. Nanomaterials 2021, 11, 487. 10.3390/nano11020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donga S.; Chanda S. Facile green synthesis of silver nanoparticles using Mangifera indica seed aqueous extract and its antimicrobial, antioxidant and cytotoxic potential (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2021, 49, 292–302. 10.1080/21691401.2021.1899193. [DOI] [PubMed] [Google Scholar]
- Aygun A.; Ozdemir S.; Gulcan M.; Yalçın M. S.; Uçar M.; Şen F. Characterization and antioxidant-antimicrobial activity of silver nanoparticles synthesized using Punica granatum extract. Int. J. Environ. Sci. Technol. 2022, 19, 2781–2788. 10.1007/s13762-021-03246-w. [DOI] [Google Scholar]
- Sumitha S.; Vasanthi S.; Shalini S.; Chinni S. V.; Gopinath S. C.; Anbu P.; Bahari M. B.; Harish R.; Kathiresan S.; Ravichandran V. Phyto-mediated photo catalysed green synthesis of silver nanoparticles using Durio zibethinus seed extract: Antimicrobial and cytotoxic activity and photocatalytic applications. Molecules 2018, 23, 3311. 10.3390/molecules23123311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Yao L.; Cao W.; Yang Y.; Cui Y.; Yang D.; Qian G. Stable and wide-wavelength tunable luminescence of CsPbX3 nanocrystals encapsulated in metal–organic frameworks. J. Mater. Chem. C 2022, 10, 5550–5558. 10.1039/D2TC00075J. [DOI] [Google Scholar]
- Shankar S. S.; Ahmad A.; Sastry M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- Morales-Lozoya V.; Espinoza-Gómez H.; Flores-López L. Z.; Sotelo-Barrera E. L.; Nuĭñez-Rivera A.; Cadena-Nava R. D.; Alonso-Nuñez G.; Rivero I. A. Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855 10.1016/j.apsusc.2020.147855. [DOI] [Google Scholar]
- Khan S.; Bibi G.; Dilbar S.; Iqbal A.; Ahmad M.; Ali A.; Ullah Z.; Jaremko M.; Iqbal J.; Ali M.; Haq I.; Ali I. Biosynthesis and characterization of iron oxide nanoparticles from Mentha spicata and screening its combating potential against Phytophthora infestans. Front. Plant Sci. 2022, 13, 1001499 10.3389/fpls.2022.1001499. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Arya G.; Kumari R. M.; Sharma N.; Gupta N.; Kumar A.; Chatterjee S.; Nimesh S. Catalytic, antibacterial and antibiofilm efficacy of biosynthesized silver nanoparticles using Prosopis juliflora leaf extract along with their wound healing potential. J. Photochem. Photobiol. B Biol. 2019, 190, 50–58. 10.1016/j.jphotobiol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Hernandez-Morales L.; Espinoza-Gómez H.; Flores-López L. Z.; Sotelo-Barrera E. L.; Nuĭñez-Rivera A.; Cadena-Nava R. D.; Alonso-Nuĭñez G.; Espinoza K. A. Study of the green synthesis of silver nanoparticles using a natural extract of dark or white Salvia hispanica L. seeds and their antibacterial application. Appl. Surf. Sci. 2019, 489, 952–961. 10.1016/j.apsusc.2019.06.031. [DOI] [Google Scholar]
- Swilam N.; Nematallah K. A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. 10.1038/s41598-020-71847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudimalla A.; Jose J.; Varghese R. J.; Thomas S. Green synthesis of silver nanoparticles using Nymphaea odorata extract incorporated films and antimicrobial activity. J. Polym. Environ. 2021, 29, 1412–1423. 10.1007/s10924-020-01959-6. [DOI] [Google Scholar]
- Veeraraghavan V. P.; Periadurai N. D.; Karunakaran T.; Hussain S.; Surapaneni K. M.; Jiao X. Green synthesis of silver nanoparticles from aqueous extract of Scutellaria barbata and coating on the cotton fabric for antimicrobial applications and wound healing activity in fibroblast cells (L929). Saudi J. Biol. Sci. 2021, 28, 3633–3640. 10.1016/j.sjbs.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P. K.; Tyagi S.; Gola D.; Arya A.; Ayatollahi S. A.; Alshehri M. M.; Sharifi-Rad J. Ascorbic acid and pol-yphenols mediated green synthesis of silver nanoparticles from Tagetes erecta L. aqueous leaf extract and studied their anti-oxidant properties. J. Nanomater. 2021, 2021, 6515419 10.1155/2021/6515419. [DOI] [Google Scholar]
- Singh H.; Du J.; Singh P.; Yi T. H. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1163–1170. 10.1080/21691401.2017.1362417. [DOI] [PubMed] [Google Scholar]
- Nicolae-Maranciuc A.; Chicea D.; Chicea L. M. Ag nanoparticles for biomedical applications-synthesis and characterization-a review. Int. J. Mol. Sci. 2022, 23, 5778. 10.3390/ijms23105778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyliev G.; Vorobyova V.; Skiba M.; Khrokalo L. Green synthesis of silver nanoparticles using waste products (apricot and black currant pomace) aqueous extracts and their characterization. Adv. Mater. Sci. Eng. 2020, 2020, 1–11. 10.1155/2020/4505787. [DOI] [Google Scholar]
- Ali A.; Sattar M.; Hussain F.; Tareen M.; Militky J.; Noman M. Single-step green synthesis of highly concentrated and stable colloidal dispersion of core-shell silver nanoparticles and their antimicrobial and ultra-high catalytic properties. Nanomaterials 2021, 11, 1007. 10.3390/nano11041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraj C.; Jagan E. G.; Rajasekar S.; Selvakumar P.; Kalaichelvan P. T.; Mohan N. Synthesis of silver nano-particles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces. 2010, 76, 50–56. 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Chand K.; Jiao C.; Lakhan M. N.; Shah A. H.; Kumar V.; Fouad D. E.; Chandio M. B.; Maitlo A. A.; Ahmed M.; Cao D. Green synthesis, characterization and photocatalytic activity of silver nanoparticles synthesized with Nigella sativa seed extract. Chem. Phys. Lett. 2021, 763, 138218 10.1016/j.cplett.2020.138218. [DOI] [Google Scholar]
- Csakvari A. C.; Moisa C.; Radu D. G.; Olariu L. M.; Lupitu A. I.; Panda A. O.; Pop G.; Chambre D.; Socoliuc V.; Copolovici D. M. Green synthesis, characterization, and antibacterial properties of silver nanoparticles obtained by using diverse varieties of Cannabis sativa leaf extracts. Molecules 2021, 26, 4041. 10.3390/molecules26134041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi-Derazkola S.; Yousefinia A.; Naghizadeh A.; Lashkari S.; Hosseinzadeh M. Green synthesis and characterization of silver nanoparticles using Elaeagnus angustifolia bark extract and study of its antibacterial effect. J. Polym. Environ. 2021, 29, 3539–3547. 10.1007/s10924-021-02122-5. [DOI] [Google Scholar]
- Alsubki R.; Tabassum H.; Abudawood M.; Rabaan A. A.; Alsobaie S. F.; Ansar S. Green synthesis, characterization, enhanced functionality and biological evaluation of silver nanoparticles based on Coriander sativum. Saudi J. Biol. Sci. 2021, 28, 2102–2108. 10.1016/j.sjbs.2020.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salayova A.; Bedlovicova Z.; Daneu N.; Balaz M.; Lukacova Bujnakova Z.; Balazova L.; Tkacikova L. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. 10.3390/nano11041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuggam S.; Chinni S. V.; Mutusamy P.; Gopinath S. C.; Anbu P.; Venugopal V.; Enugutti B. Green synthesis and characterization of silver nanoparticles using Spondias mombin extract and their antimicrobial activity against biofilm-producing bacteria. Molecules 2021, 26, 2681. 10.3390/molecules26092681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin S.; Nouren S.; Bhatti H. N.; Iqbal D. N.; Iftikhar S.; Majeed J.; Mustafa R.; Nisar N.; Nisar J.; Nazir A. Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus. Green Process. Synth. 2020, 9, 87–96. 10.1515/gps-2020-0010. [DOI] [Google Scholar]
- Awwad A. M.; Salem N. M. Green synthesis of silver nanoparticles by mulberry leavesextract. Nanosci. Nanotechnol. 2012, 2, 125–128. 10.5923/j.nn.20120204.06. [DOI] [Google Scholar]
- Kambale E. K.; Nkanga C. I.; Mutonkole B.-P. I.; Bapolisi A. M.; Tassa D. O.; Liesse J.-M. I.; Krause R. W.; Memvanga P. B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493 10.1016/j.heliyon.2020.e04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Wang Y.; Ran F.; Cui Y.; Liu C.; Zhao Q.; Gao Y.; Wang D.; Wang S. A comparison between sphere and rod nanopar-ticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. 10.1038/s41598-017-03834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benakashani F.; Allafchian A.; Jalali S. A. H. Green synthesis, characterization and antibacterial activity of silver nanoparticles from root extract of Lepidium draba weed. Green Chem. Lett. Rev. 2017, 10, 324–330. 10.1080/17518253.2017.1363297. [DOI] [Google Scholar]
- Moteriya P.; Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1556–1567. 10.1080/21691401.2016.1261871. [DOI] [PubMed] [Google Scholar]
- Pallavi S. S.; Rudayni H. A.; Bepari A.; Niazi S. K.; Nayaka S. Green synthesis of silver nanoparticles using Streptomyces hirsutus strain SNPGA-8 and their characterization, antimicrobial activity, and anticancer activity against human lung carcinoma cell line A549. Saudi J. Biol. Sci. 2022, 29, 228–238. 10.1016/j.sjbs.2021.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin I. X.; Zhang J.; Zhao I. S.; Mei M. L.; Li Q.; Chu C. H. The Antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. 10.2147/IJN.S246764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A.; Verma R.; Kumari S.; Sharma A.; Shandilya P.; Li X.; Batoo K. M.; Imran A.; Kulshrestha S.; Kumar R. Photocatalytic dye degradation and antimicrobial activities of Pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 2020, 10, 7881. 10.1038/s41598-020-64419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Radadi N. S.; Hussain T.; Faisal S.; Shah S. A. R. Novel biosynthesis, characterization and bio-catalytic potential of green algae (Spirogyra hyalina) mediated silver nanomaterials. Saudi J. Biol. Sci. 2022, 29 (1), 411–419. 10.1016/j.sjbs.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oves M.; Rauf M. A.; Aslam M.; Qari H. A.; Sonbol H.; Ahmad I.; Zaman G. S.; Saeed M. Green synthesis of silver nanoparticles by Conocarpus lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29 (1), 460–471. 10.1016/j.sjbs.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H.; Jun B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilbar S.; Sher H.; Binjawhar D. N.; Ali A.; Ali I. A. Novel Based Synthesis of Silver/Silver Chloride Nanoparticles from Stachys emodi Efficiently Controls Erwinia carotovora, the Causal Agent of Blackleg and Soft Rot of Potato. Molecules 2023, 28 (6), 2500. 10.3390/molecules28062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Li S.; Luo J.; Wang R.; Ding W. Enhancement of the antibacterial activity of silver nanoparticles against phytopathogenic bacterium Ralstonia solanacearum by stabilization. J. Nanomater. 2016, 2016, 1–15. 10.1155/2016/7135852. [DOI] [Google Scholar]
- Cheng H. J.; Wang H.; Zhang J. Z. Phytofabrication of silver nanoparticles using three flower extracts and their antibacterial activities against pathogen Ralstonia solanacearum strain YY06 of bacterial wilt. Front. Microbiol. 2020, 11, 2110. 10.3389/fmicb.2020.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilbar S.; Sher H.; Ali A.; Ullah Z.; Ali I. Biological synthesis of Ag-nanoparticles using Stachys parviflora and its inhibitory potential against Xanthomonas campestris. South Afr. J. Botany 2023, 157, 409–422. 10.1016/j.sajb.2023.04.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.