Abstract
Introduction and importance:
While 14-day triple therapy with clarithromycin is a common approach for eradicating Helicobacter pylori infection, it is essential to note that this treatment does not come without potential side effects.
Case presentation:
We present the case of a 34-year-old male who presented to the emergency department with severe vomiting and abdominal pain. Subsequent evaluation revealed that the patient had developed drug-induced liver injury (DILI).
Clinical discussion:
DILI can cause acute hepatocellular or cholestatic damage, and chronic injury can lead to hepatocellular, cholestatic, vascular, or neoplastic manifestations.
Conclusion:
Clinicians should exercise caution and be alert to the potential hepatotoxic effects of medications, especially when initiating triple therapy for H. pylori infection.
Keyword: clarithromycin, drug-induced liver injury, Helicobacter pylori
Introduction
Highlights
Triple therapy with clarithromycin for Helicobacter pylori infection has potential side effects that need to be considered.
Drug-induced liver injury can cause various forms of liver damage, both acute and chronic.
Healthcare providers should be cautious and vigilant about the hepatotoxic effects of medications, particularly when prescribing triple therapy for H. pylori infection.
Helicobacter pylori (H. pylori) is estimated to impact ~4.4 billion individuals worldwide, with varying prevalence rates ranging from 28 to 84% across different populations1. Triple therapy has dramatically improved H. pylori infection management. The standard triple therapies for empirically eradicating H. pylori consist of a twice-daily (BD) dose of a proton pump inhibitor, amoxicillin 1000 mg, and clarithromycin 500 mg2,3. These regimens are typically administered for a duration of 7–14 days and are considered the standard of care in such cases3. The optimal duration and first-line treatment for eradicating H. pylori infection are subjects of ongoing debate due to regional variations in antibiotic resistance and individual susceptibility to adverse reactions2. Resistance to clarithromycin cannot be overcome by increasing the dose or duration of treatment3. When clarithromycin resistance exceeds 15%, a 14-day clarithromycin-containing triple therapy becomes less than 90% effective1,3. Nonetheless, it is essential to acknowledge the potential risks associated with this therapy, including hepatotoxicity and cholestasis. Clarithromycin has been associated with causing mild, asymptomatic elevations in serum alanine aminotransferase (ALT) levels in around 1–2% of individuals within the population4.
Drug-induced liver injury (DILI) accounts for ~10% of acute hepatitis cases in the United States5. While hepatotoxicity related to clarithromycin use is well-documented, its specific role in triple therapy-induced liver injury requires further investigation.
In this case report, we describe the presentation of a 34-year-old male who sought emergency medical care due to severe vomiting and abdominal pain. These symptoms occurred 3 days after initiating triple therapy to eradicate H. pylori infection. This case has been reported per the SCARE (Surgical CAse REport) 2020 criteria6.
Case presentation
A 34-year-old male presented to our emergency room with troubling abdominal pain. He had been grappling with intermittent abdominal pain for the past 3 days, accompanied by multiple episodes of vomiting. The abdominal pain was acute on the onset, dull in nature, rated to be nine out of ten in intensity, predominantly localized in the right upper abdomen, diffuse in nature with a peculiar pattern worsening a few hours after taking clarithromycin. As per the patient’s account, the symptoms began after taking the fifth dose of the triple therapy. Typically, these symptoms last for about 1–2 h after medication intake and subside on their own during the initial phase. However, in the current case, the pain experienced was severe and persistent. Abdominal pain was followed by vomiting in multiple episodes. Vomiting, particularly prominent after meals, added to the distressing symptoms experienced by the patient. Vomitus did not contain blood. Notably, there were no reports of jaundice or fever. His bladder and bowel habits were regular.
The patient denied concurrent use of any herbal medications, except for the prescribed postoperative medication, which includes (cefixime 200 mg twice a day for 5 days and paracetamol 1000 mg three times a day for 2 days). Of significance, he had a previous history of subjective jaundice during periods of stress like fever, but his bilirubin level was not documented as he did not seek medical attention. He never had surgery in the past. He denies any drug or food allergies. He is non-alcoholic and a non-smoker.
Three weeks before the current presentation, he underwent an uneventful laparoscopic cholecystectomy for chronic cholecystitis. Stool antigen testing for H. pylori was positive, confirming the presence of H. pylori infection. Gastric endoscopic biopsy results obtained before the surgery also confirmed the presence of H. pylori, which prompted the initiation of triple therapy comprising esomeprazole 20 mg BD, clarithromycin 500 mg BD, and amoxicillin 1 g BD.
Upon examination, the patient’s vital signs revealed a blood pressure of 90/60 mmHg, a pulse rate of 98 beats per minute, and a respiratory rate of 17 cycles per minute. The oral mucosa appeared dry. Tenderness was noted in the right upper quadrant of the abdomen, although no rigidity or rebound tenderness existed. Neurologically, the patient was alert and oriented to time, place, and person. Cranial nerve examination revealed no abnormalities and cerebellar testing yielded normal results without signs of asterixis or focal neurological deficits.
Initial liver function tests demonstrated elevated levels of aspartate aminotransferase (AST) at 296 U/l, alanine aminotransferase (ALT) at 282 U/l, and lactate dehydrogenase (LDH) at 588 U/l. Additionally, alkaline phosphatase (ALP) levels were markedly elevated at 1020 U/l, and gamma-glutamyl transferase (GGT) levels were also elevated. Total bilirubin levels were 3.2 mg/dl, with direct bilirubin levels measuring 2.4 mg/dl. Creatine kinase levels were within the normal range at 70 U/l. The patient’s international normalized ratio (INR) on admission was 1.7, subsequently reducing throughout the hospitalization. Complete blood count, random blood sugar, and renal function test results were all within normal limits. Viral markers for hepatitis A, B, C, and E were negative. Antinuclear antibodies, anti-mitochondrial antibodies and anti-smooth muscle antibodies were negative. Initial lab finding are shown in Table 1. On ultrasound evaluation, the liver appeared normal in size, but the parenchyma exhibited minimal hypoechoic, granular, and heterogenic features. A dilated common bile duct (CBD) measuring 8.5 mm was also observed, as shown in Figure 1. Remarkably, after 1 week of observation, the CBD size reduced to 5 mm, as in Figure 2.
Table 1.
Initial laboratory value
| Test | Result | Reference range |
|---|---|---|
| Serum amylase | 130 U/l | 25–125 U/l |
| Serum lipase | 64 U/l | 10–140 U/l |
| Blood urea | 19.8 mg/dl | 7–20 mg/dl |
| Blood creatinine | 1.1 mg/dl | 0.7–1.3 mg/dl |
| Sodium | 145 mmol/l | 135–145 mmol/l |
| Potassium | 4.9 mmol/l | 3.5–5.1 mmol/l |
| Total bilirubin | 3.2 mg/dl | 0.3–1.0 mg/dl |
| Direct bilirubin | 2.4 mg/dl | 0.1–0.3 mg/dl |
| Alkaline phosphatase | 1020 U/l | 80–306 U/l |
| AST (aspartate aminotransferase) | 296 U/l | 5–40 U/l |
| ALT (alanine aminotransferase) | 282 U/l | <31 U/l |
| INR (international normalized ratio) | 1.4 | 0.8–1.2 |
| Gamma-glutamyl transferase (GGT) | 300 U/l | 0–55 U/l |
| Random blood glucose | 74.2 mg/dl | 70–100 mg/dl |
| Serum calcium | 8.7 mg/dl | 8.5–10.5 mg/dl |
| Serum creatinine | 1.3 mg/dl | 0.7–1.3 mg/dl |
| Vitamin B12 | 1262 pg/ml | 200–900 pg/ml |
| 25-hydroxy vitamin D | 11.4 ng/ml | 20–50 ng/ml |
Figure 1.
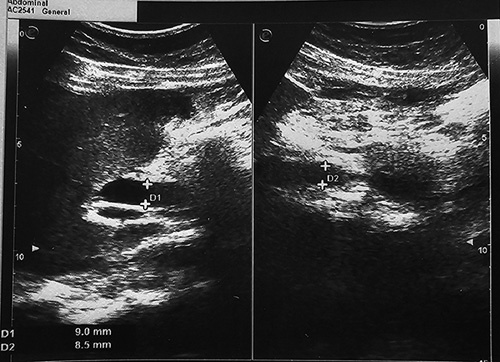
Ultrasound during admission showing dilated common bile duct measuring 8.5 mm.
Figure 2.
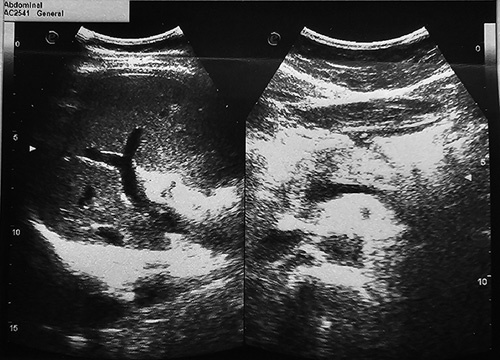
Ultrasound after 1 week of admission showing common bile duct measuring 5 mm.
During the patient’s hospitalization, intravenous fluid therapy was initiated, and close monitoring of liver function tests and coagulation profiles was performed. The triple therapy was immediately discontinued. The patient’s liver function gradually improved with meticulous care and adherence to medical recommendations. After 1 week of hospitalization, the CBD size decreased to 5 mm. Before discharge, the patient received clear instructions regarding drug avoidance, including Tylenol, alcohol, and other hepatotoxic drugs. Additionally, he was advised to seek immediate medical attention in the event of bleeding, abdominal pain, or any signs of confusion reported by his caregiver. A follow-up appointment was scheduled 1 week later to assess liver function, which ultimately returned to baseline levels after 3 weeks, as shown in Figure 3.
Figure 3.
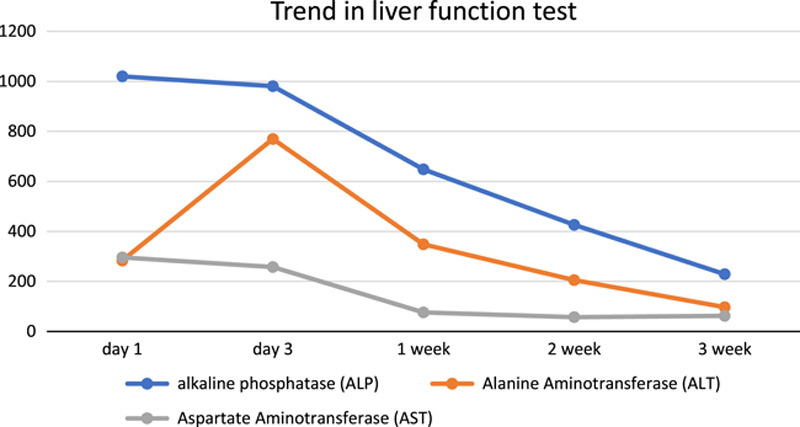
Trend in liver function test.
Discussion
H. pylori infection is known to be linked to various gastroduodenal pathologies, such as peptic ulcer disease, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma7. A complex interplay of bacterial virulence factors, host genetics, and environmental factors influences the development of these conditions. The selection of an appropriate H. pylori eradication regimen should consider the local prevalence of clarithromycin resistance and the patient’s prior use of macrolides8. Quadruple therapies, such as bismuth quadruple and concomitant regimens, are recommended as first-line treatments. In areas with low clarithromycin resistance and patients without previous macrolide use, a 14-day clarithromycin-containing triple therapy can be considered3,9.
Clarithromycin, an erythromycin derivative, has been synthesized by replacing the C-6 hydroxyl group with a methoxy group9. This modification enhances its oral bioavailability and gastrointestinal tolerance. It is currently considered the most potent antibiotic against H. pylori, with the lowest minimum inhibitory concentrations compared to other drugs9. Clarithromycin exerts its bacteriostatic effect by inhibiting protein synthesis, achieved by binding to the 50S subunit of bacterial ribosomes10,11.
Drug-induced liver injury is a complex phenomenon that can arise unexpectedly as an idiosyncratic reaction to a typically nontoxic drug or as a predictable consequence of the intrinsic toxicity of a drug taken in high doses5. The range of liver injuries caused by drugs includes acute and chronic conditions. An acute injury can be hepatocellular (cytotoxic), cholestatic, or mixed, while chronic injury may manifest as hepatocellular, cholestatic, vascular, or neoplastic damage12. Drug-induced liver injury can present solely as liver-related symptoms or be accompanied by harm to other organs or systemic manifestations. Drugs and their reactive metabolites have the potential to bind covalently to mitochondria, leading to direct hepatotoxicity13. This hepatotoxicity primarily arises from the build-up of various oxidative stress and mitochondrial dysfunction, ultimately resulting in cellular death13. Additionally, the immune system, encompassing innate and adaptive responses, plays a crucial role in developing idiosyncratic immunological reactions triggered by these drugs. DILI is a diagnosis that should be considered after ruling out other potential causes of liver disease14. It is essential to conduct a thorough evaluation to exclude alternative etiologies before attributing the liver injury to drug-induced causes. Although the exact mechanism of how clarithromycin causes liver injury is still unclear, its toxicity primarily arises from the inhibition of the mitochondrial electron transport chain, whereas erythromycin (another macrolide) toxicity is mainly attributed to the accumulation of bile acids4. Old age, concomitant use of hepatotoxic drugs like acetaminophen and certain antibiotics, as well as other stressors like surgery and anesthetic agents, can exacerbate the hepatic injury caused by clarithromycin3,4.
Our patient has no history of previous exposure to macrolides, so he was started on triple therapy with esomeprazole 20 mg BD, clarithromycin 500 mg BD, and amoxicillin 1 g BD. Our patient presented with the onset of abdominal pain and vomiting 3 days after initiating triple therapy. There was no prior history of a similar illness following amoxicillin administration for other medical conditions. The patient had a history of uncomplicated use of proton pump inhibitors for gastritis. Testing for viral hepatitis and autoimmune hepatitis yielded negative results. Clinical assessment and laboratory investigations ruled out Wilson’s disease and hemochromatosis. The patient demonstrated drastic symptomatic and liver function test improvement following the discontinuation of antibiotics during the hospital stay, suggesting DILI. The absence of fever, leukocytosis, and sepsis helps exclude cholangitis as the cause of our patient’s symptoms15. Our patient’s observed bile duct enlargement can be due to post-cholecystectomy, but the exact reason is unknown16. Elevated bilirubin levels in our patient may be attributed to liver injury and a physiological, genetic defect. Additionally, the use of postoperative paracetamol for pain management might have contributed to the liver injury in our patient. Our patient shows the presence of both cytotoxic as well cholestatic patterns of hepatic injury.
Clinicians should remain vigilant for potential hepatotoxic effects of medications, particularly when initiating triple therapy for H. pylori infection, and promptly discontinue treatment if DILI is suspected. We recognize certain limitations in our case report, which include its retrospective nature and lack of liver biopsy. Further research is warranted to enhance our understanding of the underlying mechanisms and risk factors associated with drug-induced hepatotoxicity in such cases.
Conclusion
In conclusion, this case report highlights the event of an acute liver injury induced by triple therapy involving clarithromycin. Vigilance is crucial when administering this treatment for H. pylori infection due to the potential hepatotoxicity. Clinicians should consider alternative options and closely monitor patients for adverse reactions. This case underscores the need for further research and awareness regarding drug-induced liver injury to ensure patient safety and optimize treatment outcomes.
Ethical approval
None.
Consent
Written informed consent was obtained from the patient to publish this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
None.
Author contribution
N.R.S., A.W., M.B., B.P., R.P., R.R., R.S., and M.P.: conceptualization, design and preparation of the manuscript; M.P. and N.R.S.: finalization of the manuscript.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
This is not an original research project involving human participants in an interventional or observational study, so it does not apply to this case report.
Guarantor
Dr Nava Raj Sharma.
Data availability statement
Available upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 7 August 2023
Contributor Information
Nava Raj Sharma, Email: navarajsharma19900227@gmail.com.
Abhishesh Wagle, Email: Wagle.abhishesh@gmail.com.
Manoj Bist, Email: Manojbistamanisha18@gmail.com.
Bishal Panthi, Email: Panthibishal2050@gmail.com.
Ritu Pokhrel Dahal, Email: ritupokhrel5@gmail.com.
Rajesh Rokaya, Email: Dr.rajeshrokayaofficial@gmail.com.
Rayana Shrestha, Email: Rayanashrestha20@gmail.com.
Madalasa Pokhrel, Email: Pokhrel.madalasa92@gmail.com.
References
- 1. Saleem N, Howden CW. Update on the management of Helicobacter pylori infection. Curr Treat Options Gastroenterol 2020;18:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papastergiou V. Treatment of Helicobacter pylori infection: meeting the challenge of antimicrobial resistance. World J Gastroenterol 2014;20:9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graham DY, Lee Y, Wu M. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014;12:177–186.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodhead JL, Yang K, Oldach D, et al. Analyzing the mechanisms behind macrolide antibiotic-induced liver injury using quantitative systems toxicology modeling. Pharm Res 2019;36:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edhi AI, Hakim S, Shams C, et al. Clarithromycin-associated acute liver failure leading to fatal, massive upper gastrointestinal hemorrhage from profound coagulopathy: case report and systematic literature review. Case Reports Hepatol 2020;2020:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agha RA, Franchi T, Sohrabi C, et al. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg 2020;84:226–230. [DOI] [PubMed] [Google Scholar]
- 7. Suerbaum S, Michetti P. Helicobacter pylori Infection. New Engl J Med 2002;347:1175–1186. [DOI] [PubMed] [Google Scholar]
- 8. Zagari RM, Frazzoni L, Marasco G, et al. Treatment of Helicobacter pylori infection: a clinical practice update. Minerva Med 2021;112:281–287. [DOI] [PubMed] [Google Scholar]
- 9. Rimbara E, Noguchi N, Kawai T, et al. Novel mutation in 23S rRNA that confers low-level resistance to clarithromycin in Helicobacter pylori . Antimicrob Agents Chemother 2008;52:3465–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007;20:280–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Versalovic J, Shortridge D, Kibler K, et al. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori . Antimicrob Agents Chemother 1996;40:477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleiner DE. Histopathological challenges in suspected drug-induced liver injury. Liver Int 2018;38:198–209. [DOI] [PubMed] [Google Scholar]
- 13. Ye H, Nelson LJ, Moral MG, et al. Dissecting the molecular pathophysiology of drug-induced liver injury. World J Gastroenterol 2018;24:1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalasani NP, Maddur H, Russo MW, et al. ACG Clinical Guideline: diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2021;116:878–898. [DOI] [PubMed] [Google Scholar]
- 15. Wada K, Takada T, Kawarada Y, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007;14:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park SM, Kim WS, Bae IH, et al. Common bile duct dilatation after cholecystectomy: a one-year prospective study. J Korean Surg Soc 2012;83:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon reasonable request.


