Key Points
Question
Is interval cytoreductive surgery (ICS) with hyperthermic intraperitoneal chemotherapy (HIPEC) associated with improved outcomes in advanced-stage ovarian cancer?
Findings
In this comparative effectiveness cohort study of 196 patients with advanced-stage ovarian cancer, ICS with HIPEC was associated with a statistically significant improvement in progression-free survival and overall survival compared with ICS without HIPEC, with no increase in the toxicity profile. The frequency of peritoneal recurrence was lower in the ICS with HIPEC group than that in the ICS without HIPEC group.
Meaning
This study suggests that ICS with HIPEC was associated with improved survival outcomes for patients with advanced-stage ovarian cancer; the lower incidence of peritoneal recurrence after ICS with HIPEC may have a significant association with the survival rate.
Abstract
Importance
Hyperthermic intraperitoneal chemotherapy (HIPEC) followed by interval cytoreductive surgery (ICS) has shown survival benefits for patients with advanced-stage ovarian cancer. However, there is still a lack of consensus regarding the integration of HIPEC into clinical practice.
Objective
To evaluate the safety and effectiveness of ICS with HIPEC compared with ICS alone in clinical practice for patients with advanced-stage ovarian cancer.
Design, Setting, and Participants
This prospective, multicenter, comparative effectiveness cohort study enrolled 205 patients with stage III or IV ovarian cancer who had received at least 3 cycles of neoadjuvant chemotherapy followed by ICS with HIPEC or ICS without HIPEC at 7 Korean Gynecologic Oncology Group institutions between September 1, 2017, and April 22, 2022. Nine patients were excluded because they did not meet the inclusion criteria.
Exposures
Neoadjuvant chemotherapy followed by ICS with HIPEC or ICS without HIPEC.
Main Outcomes and Measures
The primary end point was progression-free survival (PFS). Overall survival (OS) and the safety profile were the key secondary end points.
Results
This study included 196 patients (median age, 58.0 years [range, 38-82 years]), of whom 109 underwent ICS with HIPEC and 87 underwent ICS without HIPEC. The median duration of follow-up was 28.2 months (range, 3.5-58.6 months). Disease recurrence occurred in 128 patients (65.3%), and 30 patients (15.3%) died. Interval cytoreductive surgery with HIPEC was associated with a significant improvement in median PFS compared with ICS without HIPEC (22.9 months [95% CI, 3.5-58.6 months] vs 14.2 months [95% CI, 4.0-56.2 months]; P = .005) and median OS (not reached [95% CI, 3.5 months to not reached] vs 53.0 [95% CI, 4.6-56.2 months]; P = .002). The frequency of grade 3 or 4 postoperative complications was similar in both groups (ICS with HIPEC, 3 of 109 [2.8%] vs ICS without HIPEC, 3 of 87 [3.4%]; P > .99). Among patients with recurrence, the frequency of peritoneal recurrence was lower in the ICS with HIPEC group than in the ICS without HIPEC group (21 of 64 [32.8%] vs 41 of 64 [64.1%]; P = .001).
Conclusions and Relevance
This study suggests that ICS in conjunction with HIPEC was associated with longer PFS and OS than ICS without HIPEC for patients with advanced-stage ovarian cancer and was not associated with higher rates of postoperative complications. The lower rate of peritoneal recurrence after HIPEC may be associated with improved OS.
This comparative effectiveness cohort study evaluates the safety and effectiveness of interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) compared with interval cytoreductive surgery alone in clinical practice for patients with advanced-stage ovarian cancer.
Introduction
Ovarian cancer is one of the most lethal malignant gynecologic tumors,1 and most patients with ovarian cancer develop peritoneal carcinomatosis. Maximal cytoreductive surgery with platinum-based chemotherapy is the standard treatment modality for advanced-stage ovarian cancer.2,3 However, despite achieving complete remission after primary treatment, 60% to 80% of patients with advanced-stage ovarian cancer experience relapse.4,5 Several new combinations with platinum-based chemotherapy, including targeted agents, antiangiogenic agents, immunotherapy, and intraperitoneal chemotherapy, have been developed to improve survival outcomes.6
Hyperthermic intraperitoneal chemotherapy (HIPEC) combines intraperitoneal chemotherapy with hyperthermia and maintains a high chemotherapeutic drug concentration in tumor cells and reduces the adverse effects of intraperitoneal catheter–related complications. Moreover, hyperthermia enhances the penetration of chemotherapeutic agents at the peritoneal surface and increases its cytotoxic effect.7 Several randomized clinical trials8,9 have demonstrated survival benefits for patients with advanced-stage ovarian cancer conferred by HIPEC followed by interval cytoreductive surgery (ICS) compared with ICS alone, with no significant association with higher rates of adverse effects.
Regarding the OVHIPEC (Interval Debulking Surgery +/− Hyperthermic Intraperitoneal Chemotherapy in Stage III Ovarian Cancer) study,8 the benefit of HIPEC for patients with initial stage IV ovarian cancer, the effect of maintenance therapy such as bevacizumab or poly (ADP-ribose) polymerase (PARP) inhibitors after HIPEC, and the pattern of recurrence after HIPEC that may affect survival outcomes need to be investigated further. Despite randomized clinical trials demonstrating the survival benefits of HIPEC, the application of ICS with HIPEC remains clinically limited. The potential barriers to incorporating HIPEC in clinical practice include prolonged operative time, hospitalization, and treatment-related complications.10 Moreover, to our knowledge, no data exist for ICS with HIPEC for maintenance therapy. Hence, the clinical application of HIPEC varies significantly across institutions and clinicians in South Korea, warranting the need for comparative effectiveness studies using observational data acquired from clinical practice. In this study, we compared the safety and effectiveness of ICS with HIPEC with that of ICS without HIPEC and reported the outcomes associated with ICS with HIPEC, including treatment-related toxic effects, site of first recurrence, and survival among patients with stage III or IV ovarian cancer.
Methods
Study Design and Patients
This multicenter, prospective, comparative effectiveness cohort study was conducted at 7 institutions in South Korea (Yonsei University College of Medicine, Ajou University School of Medicine, CHA Bundang Medical Center, Seoul National University Bundang Hospital, Kyungpook National University Chilgok Hospital, Ewha Womans University College of Medicine, and Korea University Medical Center) between September 1, 2017, and April 22, 2022. This study was approved by the institutional review board of each participating institution and conducted in accordance with the Declaration of Helsinki.11 All patients provided written informed consent before participation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients aged 19 years or older with a new diagnosis of advanced-stage (International Federation of Gynecology and Obstetrics stage III or IV) epithelial ovarian, fallopian tube, and/or primary peritoneal carcinoma were included. Patients were treated with neoadjuvant chemotherapy (NAC) on the basis of their performance status, medical comorbidities, radiologic imaging findings, and diagnostic laparoscopy results.12 The complete eligibility criteria are provided in eTable 1 in Supplement 1.
We recommended that all patients receive 3 cycles of NAC with carboplatin (area under the curve of 5-6 mg/mL/min) and paclitaxel (175 mg/m2 of body surface area), ICS with or without HIPEC, and 3 cycles of postoperative adjuvant chemotherapy (POAC). The clinician determined the total number of cycles of NAC or POAC and maintenance therapy after POAC. All patients underwent cytoreductive surgery to remove all visible tumors and achieve complete cytoreduction (R0). In addition, to minimize potential bias in surgery, this study was conducted at 7 institutions by clinicians experienced in administering HIPEC to patients with advanced-stage ovarian cancer. The Korean Gynecologic Oncology Group institutions participating in our study minimized the differences in surgical quality by performing standardized surgical procedures according to the surgical manual.13
During surgery, patients who achieved optimal cytoreduction, defined as less than 1 cm of residual disease, were registered in the study at the clinician’s discretion. Hyperthermic intraperitoneal chemotherapy was performed after ICS using an open or closed technique. Cisplatin (100 mg/m2) or paclitaxel (175 mg/m2) was perfused, and chemotherapeutic agents were diluted in 3 L of 1.5% dextrose solution for peritoneal dialysis or in normal saline. Initially, 3 L of a heated perfusion solution was infused into the abdominal cavity at a rate of 800 to 1000 mL/min through the inflow tube using the Belmont Hyperthermic Pump (Belmont Instrument Corp). Three intra-abdominal thermometers (1 positioned in the pelvis and 2 in the diaphragm area) were used to monitor the temperature within the peritoneal cavity during infusion, which was constantly maintained at 42 °C. The HIPEC procedure lasted 90 minutes, the perfusion solution was completely drained, and bowel anastomosis was performed if needed. To prevent nephrotoxicity in patients who underwent HIPEC with cisplatin, sodium thiosulfate was administered intravenously at a bolus dose of 9 g/m2 in 200 mL at the start of HIPEC perfusion, followed by continuous infusion (12 g/m2 in 1000 mL) over 6 hours during and after HIPEC.14 Patients received additional POAC after surgery. During follow-up, serum cancer antigen 125 (CA-125) measurements and computed tomography were performed 1 month after surgery and every 3 months for 1 year and every 6 months thereafter until 5 years after chemotherapy completion.
Outcomes
The primary end point was progression-free survival (PFS), defined as the time interval between the date of diagnosis and the date of disease progression. The secondary end points included overall survival (OS), defined as the time interval from diagnosis until death due to any cause, and perioperative morbidity. Progression was defined according to the Response Evaluation Criteria in Solid Tumors, version 1.115 or the biochemical assessment of the Gynecologic Cancer InterGroup CA-125 criteria,16 whichever was performed first. At the time of data cutoff, PFS and OS data were censored at the last follow-up date for patients who remained alive without distant metastasis or locoregional relapse and who were unavailable for follow-up. No loss to follow-up occurred in our study. Perioperative complications (within 30 days postoperatively) were graded according to the Memorial Sloan Kettering Cancer Center Surgical Secondary Events grading system,17 and other adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.18
Statistical Analysis
We determined that 205 patients with sufficient follow-up for observation of 102 events of disease progression or death would provide 80% power to detect a 50% longer median PFS (14 months vs 10 months; hazard ratio [HR] of 0.67 for disease progression or death)8 in the ICS with HIPEC group compared with the ICS alone group, with an overall 2-sided type I error rate of .05.
Demographic data were summarized as median and range or frequency and percentage. Study variables were compared using the Mann-Whitney test, the χ2 test, and the Fisher exact test. Cases with missing data were removed using pairwise deletion. Progression-free survival and OS curves were estimated using the Kaplan-Meier method and the log-rank test, and PFS and associated 95% CIs were estimated using the Kaplan-Meier method. Cox proportional hazards regression analysis was used to investigate the associations of the prognostic factors with survival, expressed as HRs with 95% CIs. The results of additional subgroup analyses were reported as HRs with 95% CIs using a forest plot. All analyses were performed using SPSS, version 21.0 (IBM Corp). All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
Patients
In total, 205 patients from 7 South Korean institutions were enrolled; 9 patients were excluded for not meeting inclusion criteria. The study included 196 patients (median age, 58.0 years [range, 38-82 years]), of whom 109 were assigned to undergo ICS with HIPEC and 87 were assigned to undergo ICS without HIPEC (Figure 1). The patients’ baseline characteristics and demographic characteristics were similar in both the groups (Table 1). The surgical outcomes are presented in Table 2. Of the 109 patients undergoing HIPEC, 75 (68.8%) received HIPEC with cisplatin and 34 (31.2%) received HIPEC with paclitaxel. Surgical procedures showed no significant difference (P = .25) between the ICS without HIPEC group and the ICS with HIPEC group with respect to bowel resection (17 of 87 [19.5%] vs 27 of 109 [24.8%]), parietal peritonectomy (74 of 87 [85.1%] vs 87 of 109 [79.8%]), and lymphadenectomy (51 of 87 [58.6%] vs 76 of 109 [69.7%]). The median operative time was longer in the ICS with HIPEC group than in the ICS without HIPEC group (485 minutes [range, 131-825 minutes] vs 319 minutes [range, 57-875 minutes]; P < .001) because of the additional 90 minutes required for HIPEC perfusion and massive irrigation of the abdominal cavity. The median time interval between surgery and the start of adjuvant chemotherapy was significantly longer in the ICS with HIPEC group than in the ICS without HIPEC group (23 days [range, 10-52 days] vs 20 days [range, 2-57 days]; P < .001).
Figure 1. Flow Diagram of Patients.
HIPEC indicates hyperthermic intraperitoneal chemotherapy; ICS, interval cytoreductive surgery; and NAC, neoadjuvant chemotherapy.
Table 1. Baseline Characteristics of Patients.
| Characteristic | ICS without HIPEC (n = 87) | ICS with HIPEC (n = 109) | P value |
|---|---|---|---|
| Age, median (range), y | 59 (39-82) | 58 (38-79) | .16 |
| Histologic type, No. (%) | |||
| High-grade serous | 77 (88.5) | 102 (93.6) | .09 |
| Othera | 10 (11.5) | 7 (6.4) | |
| FIGO stage, No. (%) | |||
| III | 40 (46.0) | 54 (49.5) | .10 |
| IV | 47 (54.0) | 55 (50.5) | |
| Grade, No. (%) | |||
| 2 | 2 (2.3) | 3 (2.8) | .64 |
| 3 | 75 (86.2) | 101 (92.7) | |
| Unknown | 10 (11.5) | 5 (4.6) | |
| ASA score before NAC, No. (%) | |||
| 1-2 | 53 (60.9) | 78 (71.6) | .36 |
| 3 | 25 (28.7) | 31 (28.4) | |
| Unknown | 9 (10.3) | 0 | |
| BRCA status, No. (%) | |||
| Wild-type | 63 (72.4) | 80 (73.4) | .23 |
| BRCA1/2 mutation | 18 (20.7) | 26 (23.9) | |
| Unknown | 6 (6.9) | 3 (2.8) | |
| Maintenance therapy, No. (%) | |||
| No | 66 (75.9) | 81 (74.3) | .22 |
| Bevacizumab | 12 (13.8) | 15 (13.8) | |
| PARP inhibitors | 9 (10.3) | 13 (11.9) | |
| CA-125 level, median (range), U/mL | 1271.8 (75.9-24 304.0) | 1386.4 (13.3-32 172.0) | .61 |
| HE4 level, median (range) U/mL | 511.3 (61.9-3035.5) | 509.1 (30.7-3126.0) | .48 |
| Total No. of cycles of chemotherapy, median (range) | 6 (5-11) | 7 (4-12) | .62 |
Abbreviations: ASA, American Society of Anesthesiologists; CA-125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; HE4, human epididymis protein 4; HIPEC, hyperthermic intraperitoneal chemotherapy; ICS, interval cytoreductive surgery; NAC, neoadjuvant chemotherapy; PARP, poly (ADP-ribose) polymerase.
Clear-cell, mucinous, endometrioid, carcinosarcoma, poorly differentiated carcinoma.
Table 2. Surgical Characteristics.
| Characteristic | ICS without HIPEC (n = 87) | ICS with HIPEC (n = 109) | P value |
|---|---|---|---|
| HIPEC regimen, No. (%) | |||
| Cisplatin | NA | 75 (68.8) | NA |
| Paclitaxel | NA | 34 (31.2) | NA |
| HIPEC technique, No. (%) | |||
| Open | NA | 62 (56.9) | NA |
| Closed | NA | 47 (43.1) | NA |
| Residual disease, No. (%) | |||
| No gross tumor | 55 (63.2) | 74 (67.9) | .30 |
| ≤1.0 cm | 32 (36.8) | 35 (32.1) | |
| Surgery procedures, No. (%) | |||
| Bowel resection | 17 (19.5) | 27 (24.8) | .25 |
| Parietal peritonectomy | 74 (85.1) | 87 (79.8) | |
| Lymphadenectomy | 51 (58.6) | 76 (69.7) | |
| Estimated blood loss, median (range), mL | 400 (10-2700) | 550 (10-5050) | .008 |
| Operative time, median (range), min | 319 (57-875) | 485 (131-825) | <.001 |
| Duration of hospitalization, median (range), d | 9 (4-68) | 13 (5-87) | <.001 |
| Time interval between surgery and start of adjuvant chemotherapy, median (range), d | 20 (2-57) | 23 (10-52) | <.001 |
Abbreviations: HIPEC, hyperthermic intraperitoneal chemotherapy; ICS, interval cytoreductive surgery; NA, not applicable.
Clinical Outcomes
At the time of data cutoff (July 1, 2022), the duration of median follow-up to data cutoff or death was 28.2 months (range, 3.5-58.6 months). Disease recurrence occurred in 128 patients (65.3%) in the overall cohort (64 of 109 patients [58.7%] in the ICS with HIPEC group and 64 of 87 patients [73.6%] in the ICS without HIPEC group). The median PFS was 22.9 months (95% CI, 3.5-58.6 months) in the ICS with HIPEC group and 14.2 months (95% CI, 4.0-56.2 months) in the ICS without HIPEC group (HR, 0.61; 95% CI, 0.43-0.87; P = .005) (Figure 2A).
Figure 2. Kaplan-Meier Curves of Progression-Free Survival (PFS) and Overall Survival (OS) According to Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
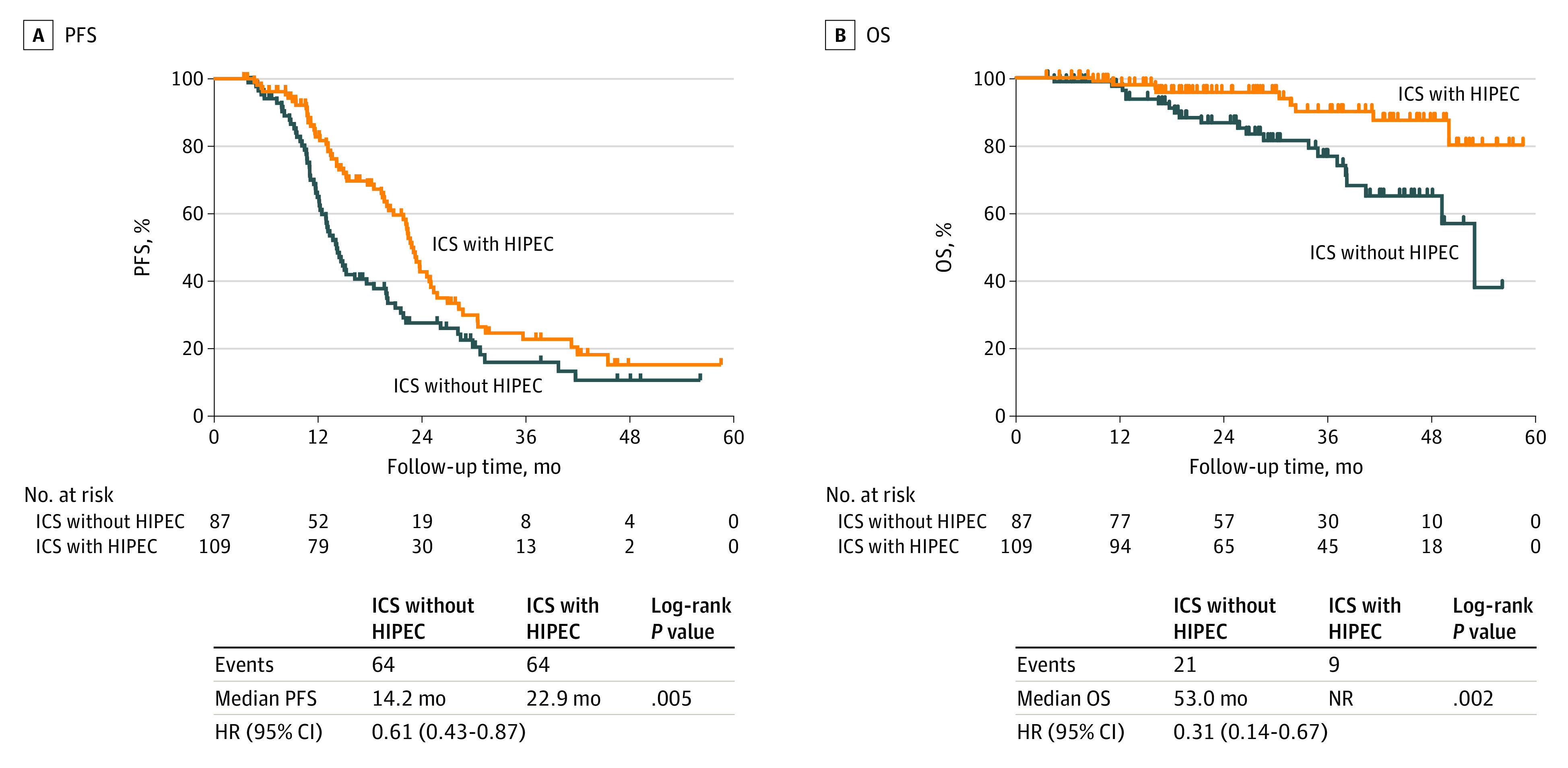
HR indicates hazard ratio; ICS, interval cytoreductive surgery; and NR, not reached.
Twenty-one of 87 patients (24.1%) in the ICS without HIPEC group and 9 of 109 patients (8.3%) in the ICS with HIPEC group died. The median OS was not reached (95% CI, 3.5 months to not reached) in the ICS with HIPEC group and was 53.0 months (95% CI, 4.6-56.2 months) in the ICS without HIPEC group (HR, 0.31; 95% CI, 0.14-0.67; P = .002) (Figure 2B). Exploratory subgroup analyses for PFS (eFigure 1 in Supplement 1) and OS (eFigure 2 in Supplement 1) revealed that the association of HIPEC with survival was consistent across the post hoc subgroups, including age, histologic type, stage, American Society of Anesthesiologists score, BRCA status, maintenance therapy, and residual disease. The PFS benefit associated with HIPEC persisted for patients 59 years of age or older, those with high-grade serous carcinoma, those with stage IV disease, those with wild-type BRCA, those undergoing maintenance therapy after HIPEC, and those who underwent optimal surgery.
We compared the survival outcomes according to HIPEC drugs among patients who underwent ICS with HIPEC (eFigure 3 in Supplement 1) and found no significant difference in PFS and OS between the cisplatin and paclitaxel groups. In addition, we analyzed the association of HIPEC with survival among patients with stage IV disease; 102 patients had stage IV disease, 47 underwent ICS without HIPEC, and 55 underwent ICS with HIPEC (eTable 3 in Supplement 1). Furthermore, ICS with HIPEC was associated with a significant difference in PFS and OS (eFigure 4 in Supplement 1). Kaplan-Meier curves for PFS and OS stratified by stage IV disease among patients who underwent ICS with HIPEC are shown in eFigure 5 in Supplement 1. Patients with stage IVB disease who underwent ICS with HIPEC showed a significant difference in OS.
Safety and Site of First Disease Recurrence
The comparison of postoperative complications is presented in eTable 2 in Supplement 1. Most postoperative grade 2 complications occurred among patients with anemia who underwent transfusion, and most were corrected within 3 days of transfusion. The duration of treatment for patients with grade 2 complications was the longest for those treated with intravenous and oral medications for postoperative pulmonary thromboembolism. The frequency of grade 3 or 4 postoperative complications was similar in both groups (ICS with HIPEC, 3 of 109 [2.8%]; ICS without HIPEC, 3 of 87 [3.4%]; P ≥ .99). The most common grade 3 adverse events in the ICS with HIPEC group were postoperative hemorrhage (2 of 109 [1.8%]) and hydronephrosis (1 of 109 [0.9%]). No patient died within the 30-day postoperative period (grade 5 adverse events). There was no significant difference in postoperative complications between the 2 groups. The site of disease recurrence was the peritoneum in 21 of 64 cases (32.8%) in the ICS with HIPEC group vs 41 of 64 cases (64.1%) in the ICS without HIPEC group (Table 3; eFigure 6 in Supplement 1). Median estimated blood loss was higher in the ICS with HIPEC group compared with the ICS without HIPEC group (550 mL [range, 10-5050 mL] vs 400 mL [range, 10-2700]) (Table 3).
Table 3. Sites of Recurrencea.
| Variable | ICS without HIPEC (n = 87) | ICS with HIPEC (n = 109) | P value |
|---|---|---|---|
| Patients with recurrence, No. (%) | 64 (73.6) | 64 (58.7) | <.001 |
| Recurrence site, No./total No. (%) | |||
| Intraperitoneal | 41/64 (64.1) | 21/64 (32.8) | .001 |
| Extraperitoneal | 5/64 (7.8) | 10/64 (15.6) | |
| Visceral metastasis | 6/64 (9.4) | 13/64 (20.3) | |
| Lymph node | 16/64 (25.0) | 30/64 (46.9) |
Abbreviations: HIPEC, hyperthermic intraperitoneal chemotherapy; ICS, interval cytoreductive surgery.
Because some patients had more than 1 site of recurrence, the total number of recurrence sites can exceed 64.
Discussion
In this study, we compared the safety and effectiveness of ICS with HIPEC and ICS without HIPEC for patients with advanced-stage ovarian cancer. We observed a significantly superior survival benefit associated with ICS with HIPEC, without higher rates of postoperative complications. In addition, the survival benefit remained consistent, irrespective of maintenance therapy. The lower frequency of peritoneal recurrence in the ICS with HIPEC group suggests that the recurrence pattern could be associated with the improvement in OS.
To our knowledge, this is the first prospective, multicenter, comparative effectiveness cohort study to report that ICS with HIPEC was associated with significantly improved survival outcomes among patients with optimally debulked stage III and IV ovarian cancer outside of a clinical trial. These results are consistent with the survival benefit reported in previous randomized clinical trials.8,9 Although previous studies found that ICS with HIPEC was associated with a survival benefit for patients with stage III and IV ovarian cancer, the clinical application of ICS with HIPEC remains limited. The OVHIPEC trial showed that ICS with HIPEC resulted in longer recurrence-free survival and OS compared with ICS alone, with acceptable perioperative morbidity.8 However, that trial did not identify a survival benefit of ICS with HIPEC for patients with stage IV cancer and for those who underwent frontline maintenance therapy. Moreover, the survival duration of both groups in the OVHIPEC cohort was shorter than that in the Gynecologic Oncology Group 172 study.19 Lim et al9 reported the survival benefit of adding HIPEC to ICS for patients undergoing ICS after NAC using a prespecified subgroup analysis. Although that trial demonstrated a survival benefit associated with HIPEC for patients with stage IV disease, a subgroup analysis of patients who underwent NAC was unplanned, the sample size of each patient group was relatively small (43 patients in the control group and 34 in the HIPEC group), and those who underwent frontline maintenance were not included. Furthermore, neither of the 2 randomized clinical trials analyzed the recurrence pattern after ICS with HIPEC.
Furthermore, there is no consensus regarding the role of HIPEC in the era of maintenance treatment. Walker et al20 demonstrated that intraperitoneal chemotherapy did not make a difference in outcomes when maintenance treatment with bevacizumab was used. Possibly, maintenance treatment may offset the benefit associated with HIPEC; this may have occurred with intraperitoneal chemotherapy in the study by Walker et al.20 Hyperthermia depletes BRCA1/2 protein function and upregulates heat-shock proteins (HSPs).21,22 The heat-induced HSP90 inhibition impairs DNA repair in tumor cells and induces further BRCA1/2 protein degradation, while increasing the sensitivity to platinum chemotherapy and PARP inhibitors.23 Koole et al24 reported that in the OVHIPEC trial, patients with homologous recombination-deficient tumors without pathogenic BRCA1/2 mutation derived maximal benefit from ICS with HIPEC. In our study, there was no difference in BRCA mutation status or maintenance treatment (bevacizumab, PARP inhibitor) between the 2 patient groups. Our post hoc subgroup analysis revealed that ICS with HIPEC was associated with improvement in PFS in the maintenance therapy group. However, the number of patients receiving maintenance therapy was limited in our study, and further studies are needed to evaluate the association of HIPEC with survival outcomes in the presence of maintenance therapy. The optimal maintenance strategy in the frontline setting after HIPEC is under active investigation in the OVHIPEC-225 and CHIPPI-1808 (Chimiothérapie Hyperthérmique Intra-Péritonéale au cours d’une chirurgie Première ou Intervallaire)26 studies.
Residual disease after cytoreductive surgery is the most important prognostic factor for improved survival for patients with advanced-stage ovarian cancer.17,27,28 Furthermore, minimizing intraperitoneal residual tumors through optimal debulking surgery was associated with improved survival for patients with stage IV cancer.29,30,31 Application of HIPEC and optimal cytoreductive surgery to the residual tumor–containing cells intrinsically resistant to chemotherapy after NAC may be beneficial in prolonging survival.32 Therefore, ICS with HIPEC may be associated with improved intraperitoneal disease control and locoregional treatment in the abdomen with enhancement of activity and penetration depth of hyperthermic cytotoxic agents to the peritoneal surfaces,33 resulting in a significant reduction in peritoneal recurrence.
As described previously, HIPEC may alter the recurrence pattern in ovarian cancer and was associated with a lower rate of peritoneal recurrence, which was consistent with our findings.34,35 Ceresoli et al34 reported that recurrence in the peritoneum was associated with poor OS among patients who underwent ICS with HIPEC. Koole et al35 conducted a central blinded revision of all imaging studies performed in OVHIPEC and confirmed whether HIPEC targets the peritoneal surface by analyzing the site of recurrence. Interval cytoreductive surgery with HIPEC improves survival outcomes by reducing the incidence of peritoneal recurrence. In our study, the rate of peritoneal recurrence was lower in the ICS with HIPEC group, possibly indicating that reduction of the incidence of peritoneal recurrence was associated with improved survival outcomes.
The ICS with HIPEC group had a longer time to initiate chemotherapy, longer hospital stays, and higher estimated blood loss compared with the ICS without HIPEC group; however, these differences seem to have limited clinical significance. The time to return to chemotherapy was significantly longer for the HIPEC group; however, this difference may not be significantly associated with survival. The median time to chemotherapy was 23 days for patients with ICS with HIPEC and 20 days for the ICS without HIPEC group, which were shorter compared with that in the OVHIPEC study (33 days vs 30 days),8 which may not be a clinically meaningful difference. Median estimated blood loss was higher in the ICS with HIPEC group compared with the ICS without HIPEC group (550 mL [range, 10-5050 mL] vs 400 mL [range, 10-2700 mL]). No criterion standard for estimating blood loss exists currently; the methods are often complex, vague, or difficult to incorporate in the intraoperative period. Moreover, HIPEC can cause deranged hemostasis and coagulation, thereby affecting intraoperative blood loss.36,37 Insensible loss occurs because of increased operation time due to HIPEC.38 The duration of hospitalization was longer in ICS with HIPEC group. However, these differences have limited clinical significance. In the Korean insurance system, the mean cost of hospital stay is relatively lower than that in Western countries. Therefore, patients who underwent ICS with HIPEC had extended hospitalizations according to the patient’s request or careful discharge planning.
Limitations
Our study has some limitations. First, the patients were not randomly assigned to the ICS with HIPEC or ICS without HIPEC groups, and the decision to proceed with HIPEC to ICS was at the primary clinician’s discretion, introducing the possibility of selection and treatment bias. Second, different types of drugs (cisplatin and paclitaxel) used in HIPEC may result in bias in data interpretation. Third, the median follow-up duration was 28.2 months, warranting the need for long-term follow-up to fully understand the association of ICS with HIPEC with outcomes of advanced-stage ovarian cancer.
Conclusions
This comparative effectiveness cohort study suggests that HIPEC after ICS improved PFS and OS among patients with stage III and IV epithelial ovarian cancer and can be safely performed without the risk of serious adverse events. The lower incidence of peritoneal recurrence after ICS with HIPEC may have a significant association with the OS rate.
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Postoperative Complications According to the Memorial Sloan-Kettering Cancer Center Surgical Secondary Events Grading System From Days 0 to 30
eTable 3. ICS Without HIPEC and ICS With HIPEC Patients in Stage IV
eFigure 1. Exploratory Subgroup Analyses of Progression-Free Survival
eFigure 2. Exploratory Subgroup Analyses of Overall Survival
eFigure 3. Kaplan-Meier Curves of Progression-Free Survival and Overall Survival According to Drug of HIPEC (A,B) in Patients Who Underwent ICS With HIPEC
eFigure 4. Kaplan-Meier Curves of Progression-Free Survival and Overall Survival According to HIPEC (A,B) in Patients With Stage IV
eFigure 5. Kaplan-Meier Curves of Progression-Free Survival and Overall Survival Stratified by Stage IV and HIPEC
eFigure 6. Comparison of Recurrence Patterns After ICS With HIPEC and ICS Without HIPEC
Data Sharing Statement
References
- 1.Sant M, Chirlaque Lopez MD, Agresti R, et al. ; EUROCARE-5 Working Group . Survival of women with cancers of breast and genital organs in Europe 1999-2007: results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2191-2205. doi: 10.1016/j.ejca.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 2.Kyrgiou M, Salanti G, Pavlidis N, Paraskevaidis E, Ioannidis JP. Survival benefits with diverse chemotherapy regimens for ovarian cancer: meta-analysis of multiple treatments. J Natl Cancer Inst. 2006;98(22):1655-1663. doi: 10.1093/jnci/djj443 [DOI] [PubMed] [Google Scholar]
- 3.Lee YJ, Lee JY, Nam EJ, Kim SW, Kim S, Kim YT. Rethinking radical surgery in interval debulking surgery for advanced-stage ovarian cancer patients undergoing neoadjuvant chemotherapy. J Clin Med. 2020;9(4):1235. doi: 10.3390/jcm9041235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RJ Jr, Alvarez RD, Armstrong DK, et al. ; National Comprehensive Cancer Networks . Ovarian cancer, version 2.2013. J Natl Compr Canc Netw. 2013;11(10):1199-1209. doi: 10.6004/jnccn.2013.0142 [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Woo HY, Kim YN, et al. Dynamics of the tumor immune microenvironment during neoadjuvant chemotherapy of high-grade serous ovarian cancer. Cancers (Basel). 2022;14(9):2308. doi: 10.3390/cancers14092308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortez AJ, Tudrej P, Kujawa KA, Lisowska KM. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018;81(1):17-38. doi: 10.1007/s00280-017-3501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27(6):365-374. doi: 10.1053/ctrv.2001.0232 [DOI] [PubMed] [Google Scholar]
- 8.van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230-240. doi: 10.1056/NEJMoa1708618 [DOI] [PubMed] [Google Scholar]
- 9.Lim MC, Chang SJ, Park B, et al. ; HIPEC for Ovarian Cancer Collaborators . Survival after hyperthermic intraperitoneal chemotherapy and primary or interval cytoreductive surgery in ovarian cancer: a randomized clinical trial. JAMA Surg. 2022;157(5):374-383. doi: 10.1001/jamasurg.2022.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charo LM, Jou J, Binder P, et al. Current status of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer in the United States. Gynecol Oncol. 2020;159(3):681-686. doi: 10.1016/j.ygyno.2020.09.022 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Lee YJ, Lee JY, Cho MS, et al. Incorporation of paclitaxel-based hyperthermic intraperitoneal chemotherapy in patients with advanced-stage ovarian cancer treated with neoadjuvant chemotherapy followed by interval debulking surgery: a protocol-based pilot study. J Gynecol Oncol. 2019;30(1):e3. doi: 10.3802/jgo.2019.30.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon S, Lee SJ, Lim MC, et al. Surgical manual of the Korean Gynecologic Oncology Group: ovarian, tubal, and peritoneal cancers. J Gynecol Oncol. 2017;28(1):e6. doi: 10.3802/jgo.2017.28.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YJ, Seon KE, Jung DC, et al. Interval debulking surgery with or without hyperthermic intraperitoneal chemotherapy in advanced-stage ovarian cancer: single-institution cohort study. Front Oncol. 2022;12:936099. doi: 10.3389/fonc.2022.936099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19(20):4054-4057. doi: 10.1200/JCO.2001.19.20.4054 [DOI] [PubMed] [Google Scholar]
- 17.Chi DS, Franklin CC, Levine DA, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: a change in surgical approach. Gynecol Oncol. 2004;94(3):650-654. doi: 10.1016/j.ygyno.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute, National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Published November 27, 2017. Accessed July 27, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- 19.Armstrong DK, Bundy B, Wenzel L, et al. ; Gynecologic Oncology Group . Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34-43. doi: 10.1056/NEJMoa052985 [DOI] [PubMed] [Google Scholar]
- 20.Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2019;37(16):1380-1390. doi: 10.1200/JCO.18.01568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunne M, Dou YN, Drake DM, et al. Hyperthermia-mediated drug delivery induces biological effects at the tumor and molecular levels that improve cisplatin efficacy in triple negative breast cancer. J Control Release. 2018;282:35-45. doi: 10.1016/j.jconrel.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 22.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla K, Giles FJ. Targeting HSP90 for cancer therapy. Br J Cancer. 2009;100(10):1523-1529. doi: 10.1038/sj.bjc.6605066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krawczyk PM, Eppink B, Essers J, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A. 2011;108(24):9851-9856. doi: 10.1073/pnas.1101053108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koole SN, Schouten PC, Hauke J, et al. Effect of HIPEC according to HRD/BRCAwt genomic profile in stage III ovarian cancer: results from the phase III OVHIPEC trial. Int J Cancer. 2022;151(8):1394-1404. doi: 10.1002/ijc.34124 [DOI] [PubMed] [Google Scholar]
- 25.Koole S, van Stein R, Sikorska K, et al. ; OVHIPEC-2 Steering Committee and the Dutch OVHIPEC group . Primary cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (HIPEC) for FIGO stage III epithelial ovarian cancer: OVHIPEC-2, a phase III randomized clinical trial. Int J Gynecol Cancer. 2020;30(6):888-892. doi: 10.1136/ijgc-2020-001231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Hajj H, Vanseymortier M, Hudry D, et al. Rationale and study design of the CHIPPI-1808 trial: a phase III randomized clinical trial evaluating hyperthermic intraperitoneal chemotherapy (HIPEC) for stage III ovarian cancer patients treated with primary or interval cytoreductive surgery. ESMO Open. 2021;6(2):100098. doi: 10.1016/j.esmoop.2021.100098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vergote I, Tropé CG, Amant F, et al. ; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Group; NCIC Clinical Trials Group . Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943-953. doi: 10.1056/NEJMoa0908806 [DOI] [PubMed] [Google Scholar]
- 28.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248-1259. doi: 10.1200/JCO.2002.20.5.1248 [DOI] [PubMed] [Google Scholar]
- 29.Winter WE III, Maxwell GL, Tian C, et al. ; Gynecologic Oncology Group . Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2008;26(1):83-89. doi: 10.1200/JCO.2007.13.1953 [DOI] [PubMed] [Google Scholar]
- 30.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103(3):1083-1090. doi: 10.1016/j.ygyno.2006.06.028 [DOI] [PubMed] [Google Scholar]
- 31.Lee YJ, Kim HS, Rim JH, et al. Germline BRCA, chemotherapy response scores, and survival in the neoadjuvant treatment of ovarian cancer. BMC Cancer. 2020;20(1):185. doi: 10.1186/s12885-020-6688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YJ, Kim D, Shim JE, et al. Genomic profiling of the residual disease of advanced high-grade serous ovarian cancer after neoadjuvant chemotherapy. Int J Cancer. 2020;146(7):1851-1861. doi: 10.1002/ijc.32729 [DOI] [PubMed] [Google Scholar]
- 33.Goodman MD, McPartland S, Detelich D, Saif MW. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J Gastrointest Oncol. 2016;7(1):45-57. doi: 10.3978/j.issn.2078-6891.2015.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceresoli M, Verrengia A, Montori G, et al. Effect of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on relapse pattern in primary epithelial ovarian cancer: a propensity score based case-control study. J Gynecol Oncol. 2018;29(3):e53. doi: 10.3802/jgo.2018.29.e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koole SN, Bruijs L, Fabris C, et al. Central radiology assessment of the randomized phase III open-label OVHIPEC-1 trial in ovarian cancer. Int J Gynecol Cancer. 2020;30(12):1928-1934. doi: 10.1136/ijgc-2020-001825 [DOI] [PubMed] [Google Scholar]
- 36.Bell JC, Rylah BG, Chambers RW, Peet H, Mohamed F, Moran BJ. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol. 2012;19(13):4244-4251. doi: 10.1245/s10434-012-2496-y [DOI] [PubMed] [Google Scholar]
- 37.Preti V, Chang D, Sugarbaker PH. Pulmonary complications following cytoreductive surgery and perioperative chemotherapy in 147 consecutive patients. Gastroenterol Res Pract. 2012;2012:635314. doi: 10.1155/2012/635314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamavonian R, McLachlan R, Fisher OM, et al. The effect of intraoperative fluid administration on outcomes of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Oncol. 2019;10(2):235-243. doi: 10.21037/jgo.2018.12.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Postoperative Complications According to the Memorial Sloan-Kettering Cancer Center Surgical Secondary Events Grading System From Days 0 to 30
eTable 3. ICS Without HIPEC and ICS With HIPEC Patients in Stage IV
eFigure 1. Exploratory Subgroup Analyses of Progression-Free Survival
eFigure 2. Exploratory Subgroup Analyses of Overall Survival
eFigure 3. Kaplan-Meier Curves of Progression-Free Survival and Overall Survival According to Drug of HIPEC (A,B) in Patients Who Underwent ICS With HIPEC
eFigure 4. Kaplan-Meier Curves of Progression-Free Survival and Overall Survival According to HIPEC (A,B) in Patients With Stage IV
eFigure 5. Kaplan-Meier Curves of Progression-Free Survival and Overall Survival Stratified by Stage IV and HIPEC
eFigure 6. Comparison of Recurrence Patterns After ICS With HIPEC and ICS Without HIPEC
Data Sharing Statement



