Abstract

Cationic chitosan derivatives have been widely studied as potential antimicrobial agents. However, very little is known about their antiviral activity and mode of action against enveloped viruses. We investigated the ability of hydroxypropanoic acid-grafted chitosan (HPA-CS) and N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride (HTCC) to inactivate enveloped viruses like the human immunodeficiency virus (HIV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The membrane-disrupting potential of the chitosan derivatives was initially investigated in a hemolysis assay. At 1.0 mg/mL, about 80% hemolysis was observed for the cationic chitosan derivatives, which was significant when compared to almost no membrane-disrupting activity by the unmodified chitosan. Virus inhibition was evaluated using the luciferase-based antiviral assay against the HIV-1 NL4.3 virus (400 TCID). The IC50 of HPA-CS was 4.109 mg/mL, while the HTCC showed a higher antiviral activity at an IC50 = 0.225 mg/mL. For practical application, the antiviral efficacies of the HTCC-coated and uncoated nonmedical masks were evaluated for SARS- CoV-2 virus capture. The coated masks demonstrated an almost excellent performance with nearly 100% viral inhibition compared to less than 60% inhibition by the uncoated masks. Molecular docking predictions suggest that the HTCC polymers interact with the viral spike protein, blocking the coronavirus interaction with the target host cell’s angiotensin-converting enzyme 2 cellular receptors.
Introduction
The emergence of SARS-CoV-2 in December 2019 introduced a new respiratory disease, which was named by the World Health Organization (WHO) as the coronavirus disease 2019 (COVID-19).1 COVID-19 became the first major pandemic of the 21st century and the worst global outbreak of a respiratory disease since the Spanish Flu of 1918/19. Since the discovery of the human immunodeficiency (HIV) in 1981, it has been the most challenging pandemic for the public health system.2 According to UNAIDS data, there are more than 38 million people living with HIV, and 28.7 million people are receiving antiretroviral therapy (UNAIDS, 2022).
Viral pandemics are often caused by the emergence of new strains to which the population does not have immunity, protective vaccines have not been developed, and effective treatment does not exist. About the only public health measure option that is available at the outbreak of a new infectious disease is literally to physically stop or slow down the spread through regular hygienic measures like handwashing, restricting social contacts, and the wearing of appropriate personal protective equipment (PPE) like masks, condoms, etc. Masks have been used for centuries to protect against contacting respiratory and airborne infections. However, masks have largely remained passive physical barriers to the spread of infections despite enormous advancements in modern medical technologies.3
Regardless of the construction, most of the currently available masks work by almost identical physical filtration mechanisms that depend on the particulate size.4 Particles larger than a micrometer are mainly removed by simple mechanical filtration involving gravity and impaction with the fibers of the fabric, while particles on the other lower end of the size scale, i.e., <300 nm, are removed primarily by electrostatic attraction to the fibers. SARS-CoV-2 and most other mammalian viruses fall within the latter range and are therefore too small to be removed by gravity. Hence, it is reasonable to expect that the principle of electrostatic attraction would be how airborne viral particles will be removed. Interestingly, the particles retained by electrostatic charges aggregate into dendrites. For infectious virions, these dendrites can be the source of fomite transmission from contaminated mask surfaces. Innovations to mitigate this potentially major source of infection transmission would be a significant improvement in mask and PPE technologies. As this is a problem across most of the currently available masks, the commercial opportunities would be extensive. Furthermore, such a technology does not have to replace any mask types or significantly impact current manufacturing processes.
The effectiveness of pharmacological antiviral treatments is highly vulnerable to viral mutations that produce new variants and other strains.5 The development of broad-spectrum antiviral drugs and multivalent vaccines that could be used against several types of viruses offers some solution to the rapid emergence of new viral mutants.6,7 However, the drug discovery timeline is lengthy and lags behind the urgency of the need, especially during a new infection outbreak, due to the need to satisfy stringent regulatory safety and efficacy standards.8 A need thus exists for nonpharmaceutical materials that can effectively prevent the spread of infections.9−12
Chitosan is a biodegradable and biocompatible polysaccharide obtained by the N-deacetylation of chitin. It has been widely investigated for biomedical applications due to its very good safety profile and is approved by the United States Food and Drug Administration for tissue engineering and drug delivery.13−16 Structurally and chemically similar to cellulose, chitosan may be considered as the animal equivalent of the plant biopolymer. Unlike cellulose, though, chitosan possesses antimicrobial property, which is conferred on it by the substitution of the C-2 hydroxyl group with a readily protonated primary amine (pKa = ∼6.5). The antimicrobial potential of chitosan is therefore the strongest at acidic pH where it has a net cationic charge from the ammonium salts of the primary amine. At neutral, physiological (7.4), and higher pH conditions, chitosan loses this cationic charge and along with it its aqueous solubility and natural antimicrobial property. To overcome this limitation, chitosan has been synthetically modified, to give derivatives having increased activity and improved aqueous solubility.17,18
The conventional method of making water-soluble, antimicrobial chitosan derivatives involves the introduction of permanent positive charges on the polymer backbone using any of the three reactive centers (the two OH groups and the NH2 group).17,19,20 The HTCC is one of the most often preferred quaternary chitosan derivatives because of its ease of synthesis, permanent cationic charge, and enhanced microbicidal properties.21,22 It is used in various applications like the medical, paper-making, food, and textile industries as a potent antimicrobial inclusion.23 We have also previously reported the synthesis, characterization, and antibacterial evaluation of fluorinated HTCC derivatives, which were shown to be effective against various clinically isolated bacterial strains.24,25 Recently, using in vitro and ex vivo models of human airway epithelia, Milewska et al. (2021) reported that HTCC has antiviral activity against both MERS-CoV and SARS-CoV-2 infections.26 Recently, the synthesis, characterization, and antiviral activity of quaternized chitosan derivatives were reported by Teotia et al.25 They concluded that the inhibition of specific interactions between viral-host surface proteins and the destabilization of viral surface proteins, by interactions between viral capsid/envelope and the functional quaternary chitosan, is responsible for the overall activity.
In this work, we report on the antiviral activity of the cationic chitosan salt (HPA-CS) and HTCC. We investigated their membrane-disrupting potentials in a hemolytic assay and viral inhibition activity with pseudoviruses of HIV and SARS-CoV-2. We also investigated the ability of HTCC to prevent the persistence of SARS-CoV-2 viral particles on nonmedical face masks coated with the quaternized chitosan. A computational chemistry investigation was conducted to determine the interactions of HTCC with the viral spike proteins and the conformations adopted by the polymer.
Experimental Section
Materials and Instruments
Chitosan (CS) with a degree of deacetylation (DDA) of 90.6% was purchased from DBFine chemicals (South Africa). Glycidyltrimethylammonium chloride (GTMAC), silver nitrate (AgNO3), reagent grades of glacial acetic acid (AcOH), acetone, and all other solvents were purchased from Sigma-Aldrich. NMR experiments were recorded on a 400 MHz Varian INOVA spectrometer at 30 °C. For sample preparation, unmodified chitosan was dissolved in an aqueous solution of deuterated hydrochloric acid (1% DCl/D2O v/v), while the cationic materials were dissolved in D2O. The BrightGlo luciferase reagent was purchased from Promega (United States). Fourier-transform infrared (FTIR) spectra of the chitosan derivatives were recorded on a PerkinElmer Spectrum 100 FT-IR spectrometer (USA). Malvern Zetaziser Nano ZS (Malvern, United Kingdom) instrument was used to obtain the hydrodynamic properties of the material. Small polymer particles for nonmedical mask coating were generated from the IME Technologies EC-DIG electrospinning unit (Geldrop, Netherlands). The surface and fracture morphology of coated masks were examined using an Auriga Zeiss scanning electron microscope (Germany) with a field emission gun (FE-SEM). A Victor Nivo multimode microplate reader at 540 nm (PerkinElmer, USA) was used to measure the luminescence of the particles.
Methods
Synthesis of HPA-CS
The HPA-CS was synthesized by following a previously reported method, with some minor modifications.27 Briefly, chitosan powder (200 mg) was first mixed with an aqueous solution of β-hydroxypropanoic acid (2% v/v, 40 mL), and the mixture was stirred overnight, using a magnetic stirrer to create a homogenous solution. The solution was placed in a polystyrene petri dish and maintained at 65 °C for 5 h for film formation. The resulting film was heated at 80 °C under high vacuum for 5 h, and the grafted copolymer was formed as a result of the dehydration of the chitosan hydroxypropanoate salts and the formation of the corresponding amide linkages. The grafted chitosan polymer was then washed with ethanol and acetone. The material was allowed to air dry overnight before submitting for testing. FT-IR (ATR): υ = 1655 (amide I) and 1325 (amide III), 1585 (amide II, NH ben.), 1377 (−CH3), 1591 (amide II, NH ben.), and 1700 cm–1 (acid, C=O). 1H NMR (400 MHz, D2O) δ 5–5.12 (br, H-1 of GlcN), 4.80–5.01 (q, −CH of lactyl unit), 4.23 (q, −CH of hydroxylated lactyl unit), 3.52–4.1 (m, H-3, H-4, H-5, and H-6), 2.91–3.21 (s,H-2), 2.06 ppm (s, NHAc), 1.43–1.52 (d, −CH3 of lactyl units), 1.32–1.42 (d, −CH3 of hydroxylated lactyl units).
Synthesis of HTCC and HTCC1
The HTTC polymers were synthesized by following a previously reported method with some modifications.28 Chitosan (2.0 g) was dissolved in 0.5% acetic acid (200 mL) and stirred for about 8 h at ambient temperature to obtain a clear solution. The derivatives were prepared under identical reaction temperature, time, and concentration of polymer, and the only difference was the mole ratio of GTMAC to sugar unit of chitosan. The mole ratio of GTMAC to sugar unit was used as 6:1, and 3:1, which led to two quaternary polymers HTCC and HTCC1, respectively.
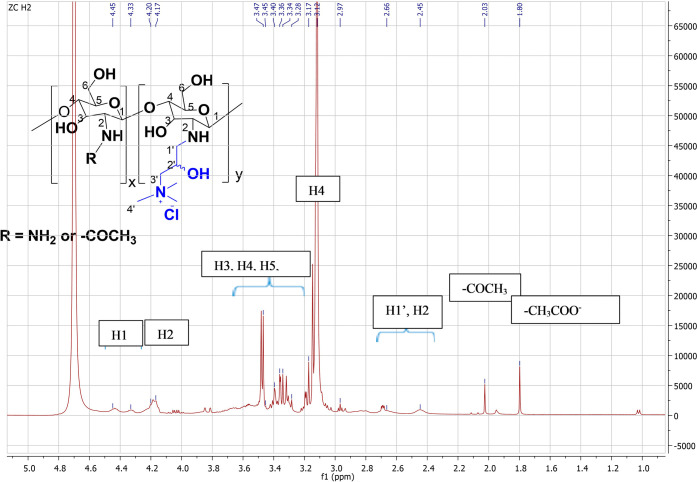
The reaction was then kept at 65 °C while stirring for 16 h after the addition of GTMAC. The reaction mixture was poured into excess acetone to obtain a white precipitate that was filtered through a sintered glass funnel and washed with the acetone–ethanol mixture (1:1). The precipitate was then freeze-dried to obtain a white powdered product that was characterized by 1H NMR and FT-IR spectroscopy (yield higher than 90%). FT-IR (ATR): υ = 3451–3102 (−OH and – NH2 or −NH−), 1650 (amide I, C=O str.), 1590 (amide II, NH ben.), and 1477 cm–1 (−N+ (CH3)3 ben.). 1H NMR (400 MHz, D2O, 30 °C): δ = 1.80 (s, −CH3COO−), 2.11 (s, −CH3CO−), 2.44–2.82 (m, NHCH2CH(OH)CH2– and C2H), 3.11 (s, −CH(OH)CH2N+(CH3)3), 3.30–3.91 (m, C3H–C5H, −CH(OH)CH2N + (CH3)3), 4.19 (s, −CH2CH(OH)CH2−), 4.44 (s, 1H).
Measurement of Zeta Potential
The zeta potential was used to measure the charge difference between the solution and the material dispersed in it. The chemically modified materials (HPA-CS and HTCC) were dissolved in phosphate-buffered saline (PBS), while the unmodified chitosan was dissolved in 0.1% acetic acid to aid dissolution and injected into a folded capillary cell. The materials were then analyzed using a refractive index of 1.330 for chitosan and an absorption value of 0.001 at room temperature.
Membrane Disruption Studies
Membrane disruption of the chitosan derivatives was investigated using a haemolysis assay as a model to assess the material’s capability of disrupting the lipid-bilayer membrane. This assay is typically used in nanomedicine to assess the red blood cell (RBC) toxicity of drug delivery systems.22 Briefly, whole blood was washed three times with PBS solution of pH 7.4 by centrifugation to obtained plasma free RBCs. A 2% RBC suspension was prepared using PBS pH 7.4. Serial dilutions in triplicates from 1 to 0.125 mg/mL of chitosan, HPA-CS, and HTCC were prepared in PBS pH 7.4. The samples were then added to the 2% RBC suspension in glass vials at a ratio of 4:1 and incubated at 37 °C for 4 h. RBCs incubated in PBS pH 7.4 served as the background control. Triton X-100 (1% v/v) was used as the 100% lipid-bilayer membrane disruptive agent. After incubation, the samples were centrifuged at room temperature (20–25 °C) and the supernatants withdrawn for hemoglobin content at 570 nm by UV/vis analysis.
Antiviral Activity against Pseudo-HIV Virus (Luciferase-Based Assay)
The antiviral assay was performed following a documented protocol.29 The TZM-bl cell lines were maintained at 37 °C and 5% CO2 in Dulbecco’s modified Eagle medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 50 μg/mL gentamycin, and 25 mM Hepes buffer. Briefly, 10 μL of each of the unmodified chitosan and cationic chitosan derivatives (HPA-CS, HTCC, and HTCC-1) were diluted 10-fold from the different concentrations of each of the materials in DMEM in a 96-well plate to achieve varying concentrations. HIV-1 NL4.3 (50 μL) (400 TCID) was added to all wells except the cell control wells and incubated for 1 h at 37 °C, 5% CO2. Then, TZM-bl cell suspension prepared at a density of 10,000 cells/mL in DMEM containing dextran was seeded (10,000 cells/well) in a 96-well plate and incubated at 37 °C, 5% CO2 for 48–72 h. A positive control was set up using a known reverse transcriptase inhibitor, azidothymidine (AZT), at a 300 μg/mL starting concentration, including the negative control, which was set up using uninfected cells. Cells infected with the virus without treatment were also included in the experiment (virus control). Subsequently, the DMEM medium (as previously prepared) was replaced, and 100 μL of BrightGlo luciferase reagent was added to each well under dark conditions followed by incubating it at room temperature for 2 min to allow complete cell lysis. All the contents were transferred to a corresponding 96-well bottom flat black plate. Luminescence was read immediately in a Victor Nivo multimode microplate reader at 540 nm. The level of viral replication was expressed as a percentage of the HIV-1 inhibition following this equation:
The half-maximal inhibitory concentration (IC50) was calculated using GraphPad Prism Software (v.5.00.288).
Electrospraying Method
Nonmedical masks were purchased from a local manufacturer, Aerox Industries, South Africa, and coated with the polycationic chitosan formulations. Uncoated masks were used as controls. Chitosan derivatives were dissolved in 0.1% acetic acid, and 10 mL of solution was drawn into a syringe equipped with metallic capillary. A rotating metal drum collector attached with a foil and mask was set to collect the sprayed polymer particles, at approximately 15 cm from the capillary tip. The polymer solutions were extruded for the needle point at a constant flow rate of 10 μL/min using a pump. The applied voltage was set at 15 kV. Following deposition, the films were first dried at ambient temperature and then at a vacuum oven set at 50 °C for 24 h. The coated mask yielded a uniform coating of the ±198 cm2 surface area. A total of 20 mg of the cationic chitosan was coated onto a mask of surface area ± 198 cm2.
Demonstration of SARS-CoV-2 Inhibition by Nonmedical Mask Coated with Polycationic Chitosan
The antiviral testing was done by following a method that has been previously reported with minor modifications.30 HTCC and HPA-CS polymers were evaluated for their potential to inhibit the SARS-Cov-2 virus using a 96 well plate, and tests were done in triplicates. This was performed using cell control and virus control. To the cell control wells, 150 μL of growth media was added and to the other wells, 100 μL of media was added. The masks were incubated with 400 μL of SARS-CoV-2 for 1 h at room temperature. Afterward, 50 μL of the flow through was added in triplicates to the 96-well plate. This was followed by the addition of 30,000 cells/100 μL/well of Vero cells and centrifugation at 3500 rpm for 3.5 h. After centrifugation, the experiment was incubated at 37 °C, 5% CO2, and 95% humidity for 72 h. Afterward, 150 μL of media was removed from the plate and 100 μL of Bright-Glo luciferase substrate was added to all wells. This was followed by 2 min of incubation at room temperature. Then, 150 μL was transferred to a black plate and luminescence was read.
Retention of Mask Quality after Coating
To demonstrate that the basic quality standards of the mask was not adversely affected after coating with the antiviral cationic chitosan formulation, samples of coated and uncoated masks were submitted to Protechnik Laboratories, a division of Armscor SOC Ltd., which is accredited to conduct evaluations according to SANS 1866:2008 and SANS 1866 – 1:2018. For these tests, locally manufactured nonmedical masks were purchased from Aerox Industries (Pty) Ltd. and Kunene Health Care (Pty) Ltd. Aerox Industries is a local manufacturer from whom we purchased nonmedical grade masks, while Kunene Health Care is a local distributor of imported medical-grade masks. One set of masks from each supplier/brand was coated with the antiviral formulation, while another set of masks was submitted as purchased to be used as controls. The tests included sodium chloride penetration, absorption, breathability, and flammability.
Mechanistic Investigation (Preparation of Ligands and the Spike Protein)
The native chitosan and HTCC were prepared in GausView6, and geometry optimized at the B3LYP/6-31 + G(d) level of theory using the Gaussian16W program.31 Consequently, the cryo-electron microscopic (EM) spike protein deposited in the protein data bank with an accession code PDB:7JWY was retrieved.32 The protein was prepared by removing all the crystal water and ions. Following that, the missing residues in the loop regions were modeled for each chain using MODELER module in UCSF Chimera.33,34 The binding site detection and druggability assessment were conducted on the refined structure using the SiteMap in Schrodinger [Schrödinger 2021-4, LLC, NY, USA].
Molecular Docking of Chitosan and HTCC onto SARS-CoV-2 Spike Protein
Molecular docking is a valuable tool in drug design used to determine the most probable ligand conformation and binding affinity toward the target active site. This technique is critical in predicting the interactions between the inhibitor molecule and the target protein. Herein, molecular docking of chitosan and HTCC was performed against spike SARS-CoV-2 to shed light on the binding affinity profiles of these ligands. The AutoDock Vina implemented in Chimera1.15 was used to dock the materials.33,35 The grid box was created around the identified active site with dimensions of 16.45, 16.76, and 16.19 for X, Y, and Z, respectively. The center coordinates for X, Y, and Z were 186.17, 185.99, and 257.68, respectively. The docking process was performed with an exhaustiveness set to 8 at an energy range of 3. The binding poses were sorted according to their docking scores upon docking completion, and the best pose was selected based on visual inspection and the root-mean square deviation (RMSD) values less than 3 Å.
Molecular Dynamics
The docked complexes were further subjected to 20 ns molecular dynamics (MD) simulations to evaluate their dynamic behavior and thermodynamic properties using Desmond implemented in Schrodinger (D E Shaw Research (DESRES)).36 The chitosan and HTCC ionization states were determined using Epik at an appropriate pH of 7.0 ± 2.0.37 The complexes’ protonation states were determined using PROPKA embedded in Maestro.38 Following that, the restrained energy minimization of the complexes was performed using the OPLS4 force field.39 Maestro’s “System Setup” was used to prepare the complexes for MD simulations, placed in an orthorhombic box with a 10 Å buffer distance. A TIP3Psolvation model with a 9 Å cut-off for van der Waals was used with a time step of 2.0 fs and the initial temperature set to 300 K.40 The pressure of the systems was set to 1.01325 bar and neutralized with a 0.15 M NaCl buffer. Moreover, the sampling interval during the simulation was set to 20 ps, and the MD simulations were performed under the NPT ensemble for 1 μs.
Post-MD Trajectory Analysis
Post-MD analyses were performed using Maestro of Schrodinger. Several matrices, such as root-mean-square deviation (RMSD) and root-mean-square fluctuation (RMSF), were evaluated from the MD trajectories using the in-built scripts from Schrodinger. The binding free energies were examined using the thermal_mmgbsa.py script available from Schrodinger.com, and the calculations were set at an interval of 1 ns (step size = 10).
Results and Discussion
Synthesis of Cationic Chitosan Derivatives
The chitosan derivatives were synthesized by grafting β-hydroxypropanoic acid onto amino groups in chitosan without using a catalyst to form water soluble HPA-CS. The HTCC synthesis was carried out by introducing the quaternary ammonium group with GTMAC as a reagent. The FT-IR spectra in Figure 1 indicated wide bands around 3200–3400 and 2800–2950 cm–1, for all the polymers, which correspond to amide A (NH/OH stretching) and amide B, the asymmetric stretching of CH and NH3+, respectively.24 The distinctive double peaks around 1655 cm–1 on chitosan for primary amine (N–H) bending shifted to 1591 cm–1 on the HPA-CS polymer, indicating the overlapping of the peaks from the free amino band of chitosan and the amide that coupled chitosan and lactic acid oligomers.41 Also, HPA-CS showed a sharp peak around 1700 cm–1 indicating the presence of a carbonyl band from the reaction of the −COOH group of the lactic acid with the chitosan primary amine.
Figure 1.
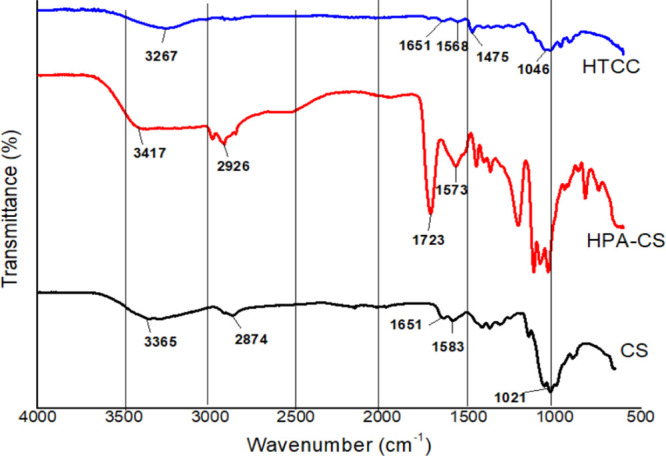
FTIR spectra of chitosan and chitosan derivatives.
The degree of grafting LA (the number of lactyl units) side chain onto the chitosan was calculated using the 1H NMR, since two peaks corresponding to lactyl units bonded to the chitosan and the hydroxylated lactyl units are well separated. The number of lactyl units for CS-HPA shown in the SI was evaluated to be 1.53. When the number of methyl protons in hydroxylated lactyl unit was set to 3, the number of lactyl units multiplied by the repeat unit weight of 72 g mole–1 yielded the length of LA side chain 110.1 g mole–1.
A noticeable change in HTCC from CS is the peak around 1480 cm–1, verifying the presence of the quaternary ammonium salt. Additionally, the primary amine bands at around 1655 cm–1 for CS were weakened and a small band at 1640 cm–1 for HTCC was recorded. It was also observed that the characteristic peaks of primary and secondary alcohols between 1102 and 1082 cm–1 did not change in HTCC, as compared to the normal chitosan. This could indicate that the introduction of quaternary amino groups only occurs at the NH2 sites on chitosan. The HTCC1 showed a similar FTIR pattern with the HTCC.
The introduction of the quaternary ammonium groups onto chitosan and the selective substitution at the primary amine groups was further confirmed by 1H NMR (Figure 2) as the spectra showed the presence of the −N+Me3 group in the polymer chain with an intense peak at 3.12 ppm. However, due to the overlapping of the signals from the protons of the chitosan backbone and substituent group in the 1H NMR, the degree of quaternization (DQ) was not calculated from the proton spectra. The DQ of the HTCC and HTCC1 polymers was determined by conductometric titration of Cl– ions with AgNO3 solution.
Figure 2.
1H NMR spectrum of cationic chitosan derivative (HTCC) in D2O performed at 30 °C.
The DLS measurements of chitosan derivatives presented in Table 1 show zeta potentials ranging from 10.9 to 27.5 mV, and these were independent of the weight ratio. HTCC showed the highest positive zeta potential of 27.5 mV, indicating the presence of a quaternary ammonium group in the polymer backbone, followed by 19.6 mV of HPA-CS from the grafting β-hydroxypropanoic acid increasing polymers’ zeta potential. The unmodified chitosan also showed positive zeta potential; however, polymer modification significantly improved the polymer surface charges.
Table 1. Average Zeta Potential (mV) of Chitosan, HTCC, and HPA-CS in Aqueous Medium.
| sample | average zeta potential ± SD (mV) |
|---|---|
| chitosan | 10.9 ± 0.60 |
| HTCC | 27.5 ± 11.5 |
| HPA-CS | 19.6 + 3.06 |
Membrane Disruption Studies
The chemically modified cationic biopolymers were investigated for their lipid-bilayer membrane disruption capability using RBCs as a model. Enveloped viruses like SARS-CoV-2 either share or have the same lipid bilayer membrane construct as human mammalian cells such as RBCs. The cationic chitosan salt HPA-CS-and the quaternary chitosan HTCC showed almost 80% membrane disruption of RBCs, compared to almost no membrane disruptive activity by unmodified chitosan at 1.0 mg/mL (Figure 3). This was taken as confirmation of the veracity of the concept that cationic chitosan are effective membrane disruptors.
Figure 3.
Membrane disruption of unmodified chitosan and cationic chitosan derivatives.
Luciferase-Based Antiviral Activity Assay against HIV-1
In general, polymers exert their antiviral properties by using their high molecular weight and multivalent binding capability through steric shielding of the viral surface or competitive inhibition of the interactions.42 The blocking of host cell receptor from viral entry can be achieved either via polymer binding to the host cell or the virus, both of which leads to a reduction in viral entry.43
Unlike antibacterial drugs and materials, which may cover a wide spectrum of pathogens, antivirals are used to treat a narrower range of organisms. Hence, broad spectrum antiviral materials that can reduce the spread of viruses without developing resistance are needed to combat other epidemic viral infections. The similarities between HIV-1 and SARS-CoV-2 provide novel strategies for developing antiretroviral drugs or materials that can inhibit both viruses. During the early days of COVID-19, it was treated with drugs that were previously used in HIV-1 treatment such as lopinavir and remdesivir. However, studies showed a lack or limited efficacy.44,45 Here, the antiviral activity results indicate that the cationic chitosan (HTCC and HPA-CS) may inhibit the HIV-1 viral replication (Figure 4).
Figure 4.

Dose–response curves showing the antiviral activity of unmodified chitosan C5 and its cationic derivative HPA-CS, HTCC, and HTCC-1 against HIV-1. AZT (red) was used as positive control, and uninfected and untreated TZM-bl cell lines were used as negative control. Inhibitory concentration at 50% inhibition (mg/mL) of C5 is 0.056 mg/mL, HTCC is 0.23 mg/mL, HTCC-1 is 8.03 mg/mL, and HPA is 4.11 mg/mL.
The antiviral efficacy of the cationic chitosan derivatives was evaluated using the luciferase-based antiviral assay against the HIV-1 NL4.3 virus (400 TCID) and by using azidothymidine (AZT) as the positive control and uninfected cells as the negative control.46 The unmodified chitosan (C5) did not cause any morphological changes in the virus, as it failed to reduce at least 50% of RLU at any dilution (Table 2). The cationic chitosan HTCC1 and HPA-CS showed weak antiviral activity against HIV-1 with IC50 = 8.028 mg/mL, and IC50 = 4.109 mg/mL, respectively (Figure 4). The HTCC chitosan derivative showed 100% inhibition of HIV-1 at the highest concentration of 280 mg/mL while the C5 showed 53% of HIV-1 inhibition at 120 mg/mL. The percentage of HIV inhibition HTCC-1 was 78% at 90 mg/mL, and HPA-CS showed 92% at 230 mg/mL concentration. It should be noted that homopolymers HTCC and HTCC1 have the same chemical structures, but the HTCC displayed lower IC50 values. This is probably due to the higher degree of quaternization (DQ) of HTCC (58%) compared to HTCC1 (29%).
Table 2. Summary of IC50 and Percentage of HIV-1 Inhibition at the Highest Concentration.
| material | IC50 (mg/mL) | % of inhibition at the highest concentration |
|---|---|---|
| C5 | 0.055 | 53 |
| HTCC | 0.23 | 100 |
| HTCC1 | 8.03 | 78 |
| HPA-CS | 4.11 | 92 |
These findings correspond with the hemolytic study, where the HTCC showed higher activity when compared to HPA-CS and unmodified chitosan. The significant factor for hemolytic and antiviral activity toward HIV-1 is attributed to the presence of the quaternary ammonium group in the polymer chain. This might be due to the permanent positive charges on the surface of HTCC, which, when bound to viral proteins, could form a complex with the virus and hence neutralize its ability to infect the cells. This study showed the possibility of having a drug that can inhibit the replication of both HIV-1 and SARS-CoV-2. The mechanism of how HTCC and HPA-CS inhibit HIV-1 replication requires further investigation.
Electrospraying of Chitosan Derivatives on Masks
The electrodynamic spraying technique was used to fabricate the nonsurgical masks with small particles of modified cationic chitosan.47 This form of surface coating forms a barrier that serves as a protective layer by improving the antiviral activity of the surface. In this case, the coating was implemented to hinder or inactivate SARS-CoV-2 without disrupting the fiber structure. To confirm the successful coating of polymers on masks, surface morphology was visualized by scanning electron microscopy (SEM) as shown in Figure 5. The distribution of polymer droplets for both chitosan and HTCC on the mask surface was obtained using the same parameters of concentration, flow rate, and applied voltage. As a result, the polymeric nanoparticles were less hetero-dispersed with rounded spheres. The polymer entanglements observed on the unmodified chitosan (Figure 5 B) could be attributed to strong Coulombic fission, compared to modified chitosan derivatives. The relatively small particles on HTCC (Figure 5D) are associated with the fast droplet drying rate during the electrospraying process, as the attraction force is weakened by the quaternary ammonium group. The particle size of electrosprayed nanoparticles is also influenced by the rapid shrinkage of particles during the solvent evaporation step. When comparing the pristine and the electrosprayed mask, it is evident that the original mask fibers were not damaged during the coating process, and the polymer particles were fairly distributed on the surface, while keeping the masks’ integrity.
Figure 5.
SEM images (high and low magnifications) of (A) uncoated mask, (B) CS electrosprayed mask, (C) HPA-CS electrosprayed mask, and (D) HTCC electrosprayed mask.
SARS-Cov-2 Antiviral Activity of Coated Fabrics Testing
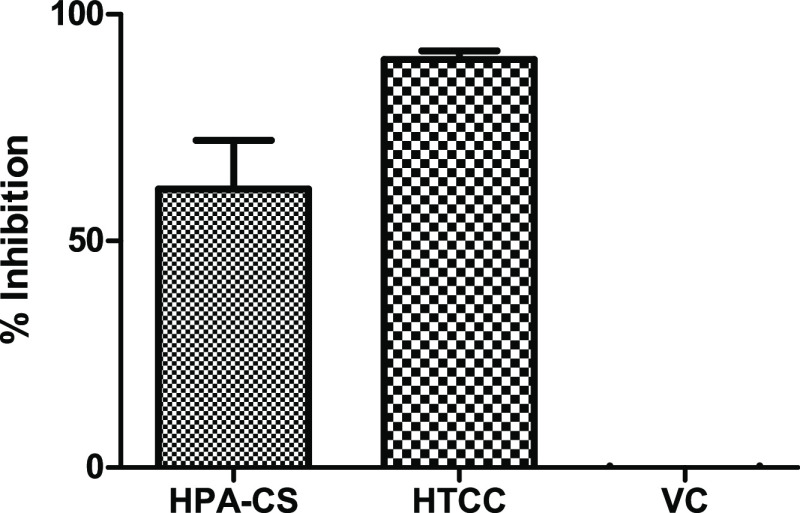
The SARS-CoV-2 was used to demonstrate the antiviral activity of HPA-CS and HTCC after coating onto polypropylene as described in the Methods section. The result presented in Figure 6 shows that the HTCC was superior and inhibited almost 100% of active viral particles from penetrating the coated polypropylene fabric. The HPA-CS (A1) material inhibited about 23% of the virus, while viral control (VC) is uninhibited SARS-CoV-2 virus.
Figure 6.
Antiviral activity of cationic chitosan material HPA-CS (A1) and quaternary chitosan HTCC (C1) coated onto polypropylene. The viral control (VC) is an uninhibited SARS-CoV-2 virus.
The virus capture efficacies of the HTCC coated (AC) and uncoated (A) AE300 masks were evaluated for SARS-CoV-2 virus capture, and comparative performance data is shown in Figure 7. The coated masks demonstrated an exceptionally high viral particle filtration with nearly 100% inhibition, compared to less than 60% inhibition by the uncoated masks. The results show that the virus-capture efficacy of commercial nonmedical masks was significantly improved by the cationic chitosan coating.
Figure 7.
SARS-CoV-2 virus capture onto uncoated (A1-3) and HTCC-coated (AC1-3) masks.
Finally, the coated masks from both commercial suppliers passed all the test criteria to demonstrate the retention of mask quality after coating. It should be noted that the same masks that passed these experiments from Aerox Industries were used in the SARS-CoV-2 inhibition demonstration described above (3.5). Therefore, the antiviral coating technology used for masks inhibited the penetration of active viral particles through the fabric without adversely impacting the quality standards of the manufactured mask.
There are several approaches that have been proposed to explain the antiviral mode of action employed by cationic polymers.43,48 In this study, we investigated the interactions between chitosan-based materials with SARS-Cov-2 outer membrane proteins using computational models. The complexes were evaluated through molecular dynamics to assess the stability and the binding free energy profiles of the investigated materials.
Binding Site Detection and Druggability Assessment Using SiteMap
Understanding the protein or enzyme binding site properties during the design of new inhibitors is paramount. The identified binding site for spike protein is druggable, and its pocket volume accommodates the compounds during docking. Five binding sites were detected and are druggable, as shown by their SiteScore and Dscore (Table 3).
Table 3. Binding Sites Identified via SiteMap and Their SiteScores, Dscore, and Occupancy Volumes.
| SiteMap entries | SiteScore | Dscore | volume |
|---|---|---|---|
| site 1 | 1.076 | 1.043 | 2525.166 |
| site 2 | 1.046 | 1.022 | 3425.884 |
| site 3 | 1.044 | 1.035 | 2536.485 |
| site 4 | 1.042 | 1.073 | 2936.080 |
| site 5 | 1.030 | 0.991 | 1465.639 |
Molecular Docking Studies
The unmodified chitosan and HTCC materials were evaluated against the SARS-CoV-2 spike protein. Notably, the compounds bind favorably in the S1 domain (docking score of −6.7 and −6.5, respectively), corresponding to site 2, as shown in Figure 8. The active site (site 1) corresponds to the S2 domain and is responsible for binding to other proteins. Targeting this active site 2, responsible for attachment to ACE2 receptors, will disrupt the binding process and thus impair the function of SARS-CoV-2. The unmodified chitosan interacted with the spike protein via PHE880, ASN881, GLY884, VAL885, THR886, ARG1065, GLU1066, and PHE1095 from chains A & B, as shown in Figure 9a. Likewise, the HTCC displayed the same interactions with minor discrepancies as the side chain formed additional interactions with the active site residues (Figure 9b).
Figure 8.

3D representation of the SARS-CoV-2 spike protein showing the binding sites validation using SiteMap. Site 1 is in the S2 domain, while site 2 is in the S1 domain.
Figure 9.
3D structures of the complexes showing the docked structures of (a) chitosan (b) HTCC into SARS-CoV-2 spike protein.
Binding Free Energy Assessment
This study evaluated the dynamic behavior of the SARS-CoV-2 spike protein upon binding of chitosan and HTCC materials and further elucidated their thermodynamic properties to provide insights into the interactions between the active site residues and the investigated materials after a 20 ns MD simulation run. The findings indicated that chitosan and HTCC are potent toward SARS-CoV-2 spike protein, as shown by their binding free energies. The interactions from the docked structures show Glu1066, as shown in Figure 9, which plays a crucial role in the binding events of materials. The post-MD simulation interaction also shows similar interaction with that of the docking results.
Based on the docking score (Table 3) of the chitosan and HTCC, the materials were allowed to interact with RBD of S protein and ACE2 receptor, which opened the scope for further studies as a futuristic scope for its development as a potent biopolymer against SARS-CoV-2. The binding affinity exhibited by the HTCC toward S protein was found to be higher than that of unmodified chitosan, with binding energies ΔGbind of −54.41 and −27.80 kcal/mol respectively (Table 4).
Table 4. Binding Free Energies of Chitosan and HTCC Complexed with SARS-CoV-2 Spike Protein.
| complexes | docking scores (kcal/mol) | ΔGbind (kcal/mol) |
|---|---|---|
| chitosan | –6.7 | –27.80 |
| HTCC | –6.5 | –54.41 |
The root-mean-square deviation (RMSD) of backbone atoms (C, C-α, and N) for protein in complex with HTCC and chitosan were performed to assess the stability of the complexes under investigation over the simulation time. The RMSD plots (see SI) show that the systems are stable and deviate below 4 Å. Furthermore, the root-mean-square fluctuations (RMSF) were performed to establish the amino acid residues, prone to fluctuations during the simulation. Comparing the chitosan and HTCC, the RMSD shows that the protein stabilizes approximately at 3 Å for both complexes. At the same time, noticeable dynamic changes are observed for chitosan and HTCC, which is to be expected as they slightly differ in chemical structure, thus influencing their mobility within the active site. Several amino acids interacted with the materials through different interactions, as shown in the SI. The interaction fraction diagrams indicate that most interactions are hydrogen bonds, water bridges, and minor hydrophobic and ionic contributions.
The molecular docking studies reveal that HTCC possesses efficient binding affinity against the SARS-CoV-2 spike protein, and it can be considered a potent material with a low cytotoxic effect. These findings are in agreement with the in vitro antiviral activity data, which validates the potential of biocompatible chitosan derivatives as preventive intervention against Covid-19.
Conclusions
To ensure efficient virus capture for improved protection against viral infections, it is typically required that the materials have structural features at the nanoscale similar to the size of the virus. Spray coating offers a convenient way to fabricate nanofibers with well-controlled structures and properties. Two cationic chitosan-based material formulations intended to promote antiviral activity were coated onto nonmedical masks. The cationic chitosan derivatives were synthesized and characterized for their physiochemical properties. The HTCC membrane disruption and anti-HIV activity results were better than HPA-CS. The cationic chitosan materials were formulated into a solution for application to the surface of a mask fabric through spray coating, followed by characterization with SEM. The antiviral inhibitory activity of the HTCC coated commercially available mask was demonstrated against the pseudo-SARS-CoV-2. To highlight the mechanism of action, computational molecular dynamics simulations showed that the cationic side chain of the HTCC interacts with the amino acid residues of the spike protein of SARS-CoV-2 when bound to the virus envelope. The findings reported herein demonstrate the potential of these cationic chitosan derivatives as antiviral agents for healthcare applications.
Acknowledgments
This work was supported by the South African Medical Research Council Self-Initiated Research Grant (SAMRC-SAR) programme and the CSIR.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c02143.
Further insights on MD trajectory analyses, root-mean-square deviation, root-mean-square fluctuations, interaction contacts between the ligand and the protein, ligand interaction diagram after 20 ns MD simulations, and calculations for the materials degree of substitution (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bogoch I. I.; Watts A.; Thomas-Bachli A.; Huber C.; Kraemer M. U. G.; Khan K. Pneumonia of Unknown Aetiology in Wuhan, China: Potential for International Spread via Commercial Air Travel. J. Travel Med. 2020, 27, taaa008. 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justiz Vaillant A. A.; Gulick P. G.. HIV Disease Current Practice; StatPearls Publishing: Treasure Island (FL), 2022. [Google Scholar]
- Huang H.; Fan C.; Li M.; Nie H.-L.; Wang F.-B.; Wang H.; Wang R.; Xia J.; Zheng X.; Zuo X.; Huang J. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747–3754. 10.1021/acsnano.0c02618. [DOI] [PubMed] [Google Scholar]
- Maduna L.; Patnaik A. Textiles in Air Filtration. Text. Prog. 2017, 49, 173–247. 10.1080/00405167.2018.1461921. [DOI] [Google Scholar]
- Strasfeld L.; Chou S. Antiviral Drug Resistance: Mechanisms and Clinical Implications. Infect. Dis. Clin. North Am. 2010, 24, 413–437. 10.1016/j.idc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman J. A.; Lee J.; Cho N.-J. Nanomedicine for Infectious Disease Applications: Innovation towards Broad-Spectrum Treatment of Viral Infections. Small 2016, 12, 1133–1139. 10.1002/smll.201500854. [DOI] [PubMed] [Google Scholar]
- Halfmann P. J.; Frey S. J.; Loeffler K.; Kuroda M.; Maemura T.; Armbrust T.; Yang J. E.; Hou Y. J.; Baric R.; Wright E. R.; Kawaoka Y.; Kane R. S. Multivalent S2-Based Vaccines Provide Broad Protection against SARS-CoV-2 Variants of Concern and Pangolin Coronaviruses. EBioMedicine 2022, 86, 104341 10.1016/j.ebiom.2022.104341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-H.; Strych U.; Hotez P. J.; Bottazzi M. E. The SARS-CoV-2 Vaccine Pipeline: An Overview. Curr. Trop. Med. Rep. 2020, 7, 61–64. 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuelta-Herrero J. L.; Chamorro-de-Vega E.; Rodríguez-González C. G.; Alonso R.; Herranz-Alonso A.; Sanjurjo-Sáez M. Effectiveness, Safety, and Costs of a Treatment Switch to Dolutegravir Plus Rilpivirine Dual Therapy in Treatment-Experienced HIV Patients. Ann. Pharmacother. 2018, 52, 11. 10.1177/1060028017728294. [DOI] [PubMed] [Google Scholar]
- Mohammadi Pour P.; Fakhri S.; Asgary S.; Farzaei M. H.; Echeverría J. The Signaling Pathways, and Therapeutic Targets of Antiviral Agents: Focusing on the Antiviral Approaches and Clinical Perspectives of Anthocyanins in the Management of Viral Diseases. Front. Pharmacol. 2019, 10, 1207. 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szunerits S.; Barras A.; Khanal M.; Pagneux Q.; Boukherroub R. Nanostructures for the Inhibition of Viral Infections. Molecules 2015, 20, 14051–14081. 10.3390/molecules200814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos E. V. R.; Pereira A. E. S.; de Oliveira J. L.; Carvalho L. B.; Guilger-Casagrande M.; de Lima R.; Fraceto L. F. How Can Nanotechnology Help to Combat COVID-19? Opportunities and Urgent Need. J. Nanobiotechnol. 2020, 18, 125. 10.1186/s12951-020-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya V. K.; Inamdar N. N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. 10.1016/j.reactfunctpolym.2008.03.002. [DOI] [Google Scholar]
- Ruiz G. A. M.; Corrales H. F.. Chitosan, Chitosan Derivatives and Their Biomedical Applications. In Biological Activities and Application of Marine Polysaccharides; Shalaby E. A., Ed.; IntechOpen: Rijeka, 2017; p Ch. 5. 10.5772/66527. [DOI] [Google Scholar]
- Li J.; Cai C.; Li J.; Li J.; Li J.; Sun T.; Wang L.; Wu H.; Yu G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. 10.3390/molecules23102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahariah P.; Másson M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. 10.1021/acs.biomac.7b01058. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Liu X.; Chen Q. Synthesis and Characterization of Water-Soluble Chitosan Derivate and Its Antibacterial Activity. Carbohydr. Polym. 2007, 69, 142–147. 10.1016/j.carbpol.2006.09.010. [DOI] [Google Scholar]
- Lal S.; Arora S.; Rani S.; Kumar P.; Dabas P.; Malik J. Synthesis and Characterization of Water-Soluble Chitosan Derivatives: Spectral, Thermal and Biological Studies. J. Macromol. Sci., Part A: Pure Appl.Chem. 2020, 57, 791–799. 10.1080/10601325.2020.1784756. [DOI] [Google Scholar]
- Jia Z.; Shen D.; Xu W. Synthesis and Antibacterial Activities of Quaternary Ammonium Salt of Chitosan. Carbohydr. Res. 2001, 333, 1–6. 10.1016/S0008-6215(01)00112-4. [DOI] [PubMed] [Google Scholar]
- Andreica B.-I.; Cheng X.; Marin L. Quaternary Ammonium Salts of Chitosan. A Critical Overview on the Synthesis and Properties Generated by Quaternization. Eur. Polym. J. 2020, 139, 110016 10.1016/j.eurpolymj.2020.110016. [DOI] [Google Scholar]
- Xu T.; Xin M.; Li M.; Huang H.; Zhou S.; Liu J. Synthesis, Characterization, and Antibacterial Activity of N O-Quaternary Ammonium Chitosan. Carbohydr. Res. 2011, 346, 2445–2450. 10.1016/j.carres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Eldar-Boock A.; Miller K.; Sanchis J.; Lupu R.; Vicent M. J.; Satchi-Fainaro R. Integrin-Assisted Drug Delivery of Nano-Scaled Polymer Therapeutics Bearing Paclitaxel. Biomaterials 2011, 32, 3862–3874. 10.1016/j.biomaterials.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Du Y.; Huang R.; Gao L. Preparation and Modification of N-(2-Hydroxyl) Propyl-3-Trimethyl Ammonium Chitosan Chloride Nanoparticle as a Protein Carrier. Biomaterials 2003, 24, 5015–5022. 10.1016/S0142-9612(03)00408-3. [DOI] [PubMed] [Google Scholar]
- Cele Z. E. D.; Somboro A. M.; Amoako D. G.; Ndlandla L. F.; Balogun M. O. Fluorinated Quaternary Chitosan Derivatives: Synthesis, Characterization, Antibacterial Activity, and Killing Kinetics. ACS Omega 2020, 5, 29657–29666. 10.1021/acsomega.0c01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotia A.; Laurén I.; Borandeh S.; Seppälä J. Quaternized Chitosan Derivatives as Viable Antiviral Agents: Structure–Activity Correlations and Mechanisms of Action. ACS Appl. Mater. Interfaces 2023, 15, 18707–18719. 10.1021/acsami.3c01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewska A.; Chi Y.; Szczepanski A.; Barreto-Duran E.; Dabrowska A.; Botwina P.; Obloza M.; Liu K.; Liu D.; Guo X.; Ge Y.; Li J.; Cui L.; Ochman M.; Urlik M.; Rodziewicz-Motowidlo S.; Zhu F.; Szczubialka K.; Nowakowska M.; Pyrc K. HTCC as a Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622–e01620. 10.1128/JVI.01622-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai N.; Ramay H. R.; Chou S.-H.; Zhang M. Chitosan and Lactic Acid-Grafted Chitosan Nanoparticles as Carriers for Prolonged Drug Delivery. Int. J. Nanomed. 2006, 1, 181–187. 10.2147/nano.2006.1.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque J.; Adhikary U.; Yadav V.; Samaddar S.; Konai M. M.; Prakash R. G.; Paramanandham K.; Shome B. R.; Sanyal K.; Haldar J. Chitosan Derivatives Active against Multidrug-Resistant Bacteria and Pathogenic Fungi: In Vivo Evaluation as Topical Antimicrobial. Mol. Pharm. 2016, 13, 3578–3589. 10.1021/acs.molpharmaceut.6b00764. [DOI] [PubMed] [Google Scholar]
- Sarzotti-Kelsoe M.; Bailer R. T.; Turk E.; Lin C.; Bilska M.; Greene K. M.; Gao H.; Todd C. A.; Ozaki D. A.; Seaman M. S.; Mascola J. R.; Montefiori D. C. Optimization and Validation of the TZM-Bl Assay for Standardized Assessments of Neutralizing Antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre K. B.; Gray E. S.; Lambson B. E.; Moore P. L.; Choge I. A.; Mlisana K.; Karim S. S. A.; McMahon J.; O’Keefe B.; Chikwamba R.; Morris L. Mannose-Rich Glycosylation Patterns on HIV-1 Subtype C Gp120 and Sensitivity to the Lectins, Griffithsin Cyanovirin-N and Scytovirin. Virology 2010, 402, 187–196. 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch M. J.; et al. Gaussian; Gaussian, Inc.: Wallingford CT, 2016.
- Zhou T.; Tsybovsky Y.; Gorman J.; Rapp M.; Cerutti G.; Chuang G.-Y.; Katsamba P. S.; Sampson J. M.; Schön A.; Bimela J.; Boyington J. C.; Nazzari A.; Olia A. S.; Shi W.; Sastry M.; Stephens T.; Stuckey J.; Teng I.-T.; Wang P.; Wang S.; Zhang B.; Friesner R. A.; Ho D. D.; Mascola J. R.; Shapiro L.; Kwong P. D. Cryo-EM Structures of SARS-CoV-2 Spike without and with ACE2 Reveal a PH-Dependent Switch to Mediate Endosomal Positioning of Receptor-Binding Domains. Cell Host Microbe 2020, 28, 867–879.e5. 10.1016/j.chom.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Lasker K.; Schneidman-Duhovny D.; Webb B.; Huang C. C.; Pettersen E. F.; Goddard T. D.; Meng E. C.; Sali A.; Ferrin T. E. UCSF Chimera, MODELLER, and IMP: An Integrated Modeling System. J. Struct. Biol. 2012, 179, 269–278. 10.1016/j.jsb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O.; Olson A. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger Release 3: Desmond Molecular Dynamics System; DE Shaw Research: New York: 2017. [Google Scholar]
- Shelley J. C.; Cholleti A.; Frye L. L.; Greenwood J. R.; Timlin M. R.; Uchimaya M. Epik: A Software Program for PKa prediction and Protonation State Generation for Drug-like Molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Li H.; Robertson A. D.; Jensen J. H. Very Fast Empirical Prediction and Rationalization of Protein PKa Values. Proteins: Struct., Funct., Bioinf. 2005, 61, 704–721. 10.1002/prot.20660. [DOI] [PubMed] [Google Scholar]
- Lu C.; Wu C.; Ghoreishi D.; Chen W.; Wang L.; Damm W.; Ross G. A.; Dahlgren M. K.; Russell E.; von Bargen C. D.; Abel R.; Friesner R. A.; Harder E. D. OPLS4: Improving Force Field Accuracy on Challenging Regimes of Chemical Space. J. Chem. Theory Comput. 2021, 17, 4291–4300. 10.1021/acs.jctc.1c00302. [DOI] [PubMed] [Google Scholar]
- Price D. J.; Brooks C. L. III A Modified TIP3P Water Potential for Simulation with Ewald Summation. J. Chem. Phys. 2004, 121, 10096–10103. 10.1063/1.1808117. [DOI] [PubMed] [Google Scholar]
- Kowalczyk D.; Kordowska-Wiater M.; Nowak J.; Baraniak B. Characterization of Films Based on Chitosan Lactate and Its Blends with Oxidized Starch and Gelatin. Int. J. Biol. Macromol. 2015, 77, 350–359. 10.1016/j.ijbiomac.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Li J.; Yu F.; Chen Y.; Oupický D. Polymeric Drugs: Advances in the Development of Pharmacologically Active Polymers. J. Controlled Release 2015, 219, 369–382. 10.1016/j.jconrel.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar Y.; Al-Batty S.; Rahman H.; Ashwaq O.; Sarief A.; Sadique Z.; Sreekumar P. A.; Haque S. K. M. Polymeric Materials as Potential Inhibitors Against SARS-CoV-2. J. Polym. Environ. 2022, 30, 1244–1263. 10.1007/s10924-021-02272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Zhang D.; Du G.; Du R.; Zhao J.; Jin Y.; Fu S.; Gao L.; Cheng Z.; Lu Q.; Hu Y.; Luo G.; Wang K.; Lu Y.; Li H.; Wang S.; Ruan S.; Yang C.; Mei C.; Wang Y.; Ding D.; Wu F.; Tang X.; Ye X.; Ye Y.; Liu B.; Yang J.; Yin W.; Wang A.; Fan G.; Zhou F.; Liu Z.; Gu X.; Xu J.; Shang L.; Zhang Y.; Cao L.; Guo T.; Wan Y.; Qin H.; Jiang Y.; Jaki T.; Hayden F. G.; Horby P. W.; Cao B.; Wang C. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled Multicentre Trial. Lancet 2020, 395, 1569–1578. 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J. H.; Tomashek K. M.; Dodd L. E. Remdesivir for the Treatment of Covid-19 - Preliminary Report Reply. N. Engl. J. Med. 2020, 383, 994. 10.1056/nejmc2022236. [DOI] [PubMed] [Google Scholar]
- Cho J.; Lee Y. J.; Kim J. H.; Kim S. i.; Kim S. S.; Choi B.-S.; Choi J.-H. Antiviral Activity of Digoxin and Ouabain against SARS-CoV-2 Infection and Its Implication for COVID-19. Sci. Rep. 2020, 10, 16200. 10.1038/s41598-020-72879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. A. S.; Mendes A. C.; Stephansen K.; Engwer C.; Goycoolea F. M.; Boisen A.; Nielsen L. H.; Chronakis I. S. Development of Electrosprayed Mucoadhesive Chitosan Microparticles. Carbohydr. Polym. 2018, 190, 240–247. 10.1016/j.carbpol.2018.02.062. [DOI] [PubMed] [Google Scholar]
- Rakowska P. D.; Tiddia M.; Faruqui N.; Bankier C.; Pei Y.; Pollard A. J.; Zhang J.; Gilmore I. S. Antiviral Surfaces and Coatings and Their Mechanisms of Action. Commun. Mater. 2021, 2, 53. 10.1038/s43246-021-00153-y. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.