Abstract
Interest in vertebrate cardiac regeneration has exploded over the past two decades since the discovery that adult zebrafish are capable of complete heart regeneration, contrasting the limited regenerative potential typically observed in adult mammalian hearts. Undercovering the mechanisms that both support and limit cardiac regeneration across the animal kingdom may provide unique insights in how we may unlock this capacity in adult humans. In this review, we discuss key discoveries in the heart regeneration field over the last twenty years. Initially, seminal findings revealed that pre-existing cardiomyocytes are the major source of regenerated cardiac muscle, drawing interest into the intrinsic mechanisms regulating cardiomyocyte proliferation. Moreover, recent studies have identified the importance of intercellular interactions and physiological adaptations, which highlight the vast complexity of the cardiac regenerative process. Finally, we compare strategies that have been tested to increase the regenerative capacity of the adult mammalian heart.
Graphical Abstract

INTRODUCTION
In 2002, Kenneth Poss and his colleagues published a landmark paper showing that the adult zebrafish heart is fully capable of replenishing lost myocardial tissues without scars within two months after injury (Poss et al., 2002). Although previous researchers provided evidence of significant cardiomyocyte proliferation in adult newts, frogs, and lizards after myocardial injury (Mehdipour et al., 2023), this was the first compelling evidence of complete scarless heart regeneration in any adult vertebrate species. Being genetically tractable, zebrafish have since become a popular model to understand the cellular and molecular mechanisms underlying cardiac regeneration and repair.
There has been a desperate need to understand why our hearts can’t regenerate. It has been well established that the adult mammalian heart does not possess the ability to regenerate itself after injury. Therefore, after a heart attack, humans are often left with a permanent loss of heart muscle cells - cardiomyocytes - while a fibrotic scar eventually forms in place of the lost contractile tissue. This results in deterioration of cardiac contractility and a high risk of life-threatening cardiac arrhythmias. A recent heart disease and stroke statistical analysis reported that 36% of men and 47% of women (45 or older) will die within five years after a heart attack (Virani et al., 2020).
The discovery of the phenomena of heart regeneration in zebrafish has unequivocally kick-started the field of heart regenerative research. Another seminal report that neonatal mouse hearts can fully regenerate further propelled the field (Porrello et al., 2011). It has drawn more investigators to explore organ regeneration in a variety of species across the animal kingdom and at different developmental stages. Such an “evo devo” approach has uncovered conserved mechanisms of heart regeneration and may yield clues to unlock the ability to regenerate the mammalian heart. While early research efforts established the importance of pre-existing cardiomyocytes as a major source for newly regenerated tissue, recent studies revealed key intercellular and physiological control mechanisms required for natural heart regeneration to occur. In this review, we highlight the role of pre-existing cardiomyocytes as the source for newly regenerated cardiac muscle, as well as cell-to-cell interactions and physiological adaptations to oxygen and increased metabolism that regulate cardiomyocyte proliferation and heart regenerative capacity. Furthermore, we compare strategies that have been tested to increase the regenerative capacity of the adult mammalian heart.
PRE-EXISTING CARDIOMYOCYTES ARE THE MAJOR SOURCE OF NEW CARDIOMYOCYTES IN NATURAL HEART REGENERATION
Heart regeneration in fish and amphibians
The adult mammalian heart has limited regenerative capabilities and is generally considered a post-mitotic organ (Jewhurst & McLaughlin, 2015; Kikuchi & Poss, 2012; Rumyantsev, 1977). However, other vertebrates in the animal kingdom are able to robustly regenerate their hearts from birth to adulthood (Kikuchi & Poss, 2012; Vivien et al., 2016). For example, adult salamanders and newts are able to regenerate the myocardium after injury and, unlike their mammalian counterparts, their cardiomyocytes possess the ability to readily enter the cell cycle and successfully divide (Becker et al., 1974; Bettencourt-Dias et al., 2003; Oberpriller & Oberpriller, 1974). Similarly, the Western clawed frog Xenopus tropicalis possesses the ability to regenerate their hearts 30 days after apical resection (Liao et al., 2017). While early studies in amphibians demonstrated cardiomyocyte cell cycle re-entry in response to cardiac injury, the significance of pre-existing cardiomyocytes as the source of newly regenerated cardiac muscle was most notably validated and supported by seminal work using the zebrafish model.
Zebrafish (Danio rerio) are teleosts well known for their exceptional abilities to regenerate various tissues and organs (Gemberling et al., 2013; Jewhurst & McLaughlin, 2015) and are also genetically tractable and amenable to experimental manipulation. Like amphibians, zebrafish possess a remarkable capacity to regenerate their cardiac tissue (Poss et al., 2002). Where do newly generated cardiomyocytes come from? An early study exploited the differential folding rate and protein stability between green fluorescent proteins (GFP) and red fluorescent proteins (RFP) to track cardiomyocyte turnover and generation in zebrafish heart regeneration (Lepilina et al., 2006). This study provided evidence to support that new cardiomyocytes originate from cardiac progenitor cells (Lepilina et al., 2006). However, a technical flaw was found in this study (Kikuchi et al., 2010) and a more rigorous genetic lineage tracing approach was taken by two independent groups, yielding more compelling results that demonstrate pre-existing cardiomyocytes as a major source of regenerated cardiac muscle cells in regenerating zebrafish hearts (Jopling et al., 2010; Kikuchi et al., 2010). These studies established the requirement for the proliferation of pre-existing cardiomyocytes as a fundamental mechanism permitting natural heart regeneration.
Within the last decade, heart regenerative capacity in other fish species has also been evaluated. In a study done by Grivas et al., goldfish (Carassius auratus) with heart tissue damage induced by cauterization were observed to completely regenerate the lost tissue by 30 days post-injury (Grivas et al., 2014). The cardiac regenerative process in goldfish involves the inflammatory response, collagen deposition and neovascularization - all processes shown to occur during zebrafish heart regeneration (Frangogiannis, 2008; Grivas et al., 2014; Tahara et al., 2016). Interestingly, the teleost medaka (Oryzias latipes) exhibits an impaired response to apical resection compared to zebrafish (Ito et al., 2014). Much like the injury response observed in adult mammalian hearts, cardiac injury in medaka resolves with extensive fibrotic scarring without robust cardiomyocyte proliferation (Ito et al., 2014).
As an alternative to apical resection, which cleanly removes part of the heart and does not physiologically model cardiomyocyte death after myocardial infarction, cryoinjury has been developed as a more medically-relevant cardiac injury approach (Chablais et al., 2011; González-Rosa et al., 2011; Schnabel et al., 2011). In this model, a cryoprobe is used to induce necrotic heart lesions, which enables the study of mechanisms involved in clearance of damaged cardiac tissue. Using this approach, a comparative study between zebrafish and medaka cardiac injury responses noted transcriptional differences revealing that the innate immune response in medaka post-cardiac injury is delayed. Further characterization demonstrated blunted macrophage recruitment to the site of injury (Lai et al., 2017). The significance of macrophage recruitment in promoting cardiac regeneration was then further validated by impairing macrophage recruitment in zebrafish, which resulted in reduced regenerative capacity and decreased cardiomyocyte proliferation (Lai et al., 2017). These studies suggest a possible role for the innate immune response in reactivating cardiomyocyte proliferation in response to injury.
The Mexican tetra (Astyanax mexicanus) is another teleost species that exists in two distinct morphotypes: surface- and cave-dwelling (Potts et al., 2021; Stockdale et al., 2018). The two morphotypes are reported to exhibit different degrees of repair after apical resection. Cave-dwelling fish develop a fibrotic scar whereas 50% of surface fish are observed to regenerate their hearts by 64 days post-amputation (Stockdale et al., 2018). Interestingly, significant differences in cardiomyocyte cycling in these distinct populations after injury was not observed (Stockdale et al., 2018). Genomic analyses between these two morphotypes of Astyanax mexicanus further illustrate how environmental factors play a role in the differential gene expression and its effect on metabolism and physiology (Krishnan et al., 2020; Peuß et al., 2020). Therefore, future work in Astyanax mexicanus may provide unique insights as a comparative model to further elucidate how environmental conditions influence cardiac regenerative outcomes (Potts et al., 2021).
Heart regeneration in mammals
The seminal discovery that newborn mice possess a transient but robust capacity for heart regeneration ignited a surge of interest in this experimental model to understand the mechanisms that limit mammalian heart regeneration. Neonatal mice at postnatal day 1 can completely regenerate ventricular tissue after apical resection, while surgeries performed at postnatal day 7 resolve with fibrosis and scarring, similar to that seen in adult mice (Porrello et al., 2011). In response to apical resection, regenerative hearts exhibit widespread dedifferentiation and proliferation throughout the heart, with increased cardiomyocyte cycling at the site of injury (Porrello et al., 2011). It is possible that cardiomyocyte regeneration in this model arises through both compensatory chamber-wide cardiomyocyte proliferation as well as cardiomyocyte cell division at the injury border. It was further demonstrated that heart regeneration similarly occurs in neonates in response to left anterior descending artery (LAD) ligations, a more physiologically relevant injury model for myocardial infarction (Haubner et al., 2012). The observed loss of heart regenerative capacity in neonatal mice after birth suggests that physiological changes occurring during this developmental window inhibit mammalian heart regenerative potential. A similar developmental transition may also be observed in humans (Bergmann et al., 2009).
While the relevance of pre-existing cardiomyocytes as the source of newly regenerated cardiomyocytes had been established in fish and amphibian models of heart regeneration (Becker et al., 1974; Bettencourt-Dias et al., 2003; Jopling et al., 2010; Kikuchi et al., 2010), the progenitors of newly regenerated mammalian cardiomyocytes has historically been a highly debated topic. Progenitor cells from the epicardium have been proposed in the role of cardiomyocyte renewal in adult hearts (Smart et al., 2011; B. Zhou et al., 2008). The origin of de novo cardiomyocytes from nearby bone marrow stem cells has also been implicated (Orlic et al., 2001). Others have suggested the role of stem cell progenitor cells, such as the c-kit+ cardiac stem cells (Bearzi et al., 2007; Beltrami et al., 2003). However, the contribution of c-kit+ cardiac stem cells in cardiomyocyte renewal has been controversial (Bearzi et al., 2007; Bergmann et al., 2009; Elhelaly et al., 2019; Jesty et al., 2012; Khan & Koch, 2016; Kikuchi et al., 2010; Porrello et al., 2013; Senyo et al., 2013; Sultana et al., 2015; van Berlo et al., 2014).
In 2003, Beltrami and colleagues reported a set of cardiac stem cells (CSCs) expressing the stem cell marker c-kit as cardiomyocyte progenitor cells in 20–25 month-old rat hearts in vivo (Beltrami et al., 2003). Furthermore, these c-kit positive CSCs in humans were reported to be clonogenic and possess the ability to differentiate into a variety of cell types including cardiomyocytes in vitro and in vivo (Bearzi et al., 2007). Jesty et al. observed that EGFP-tagged c-kit positive cells could give rise to cardiomyocytes after cryoinjury, but this contribution was most noticeable in neonates with little to no contribution to new cardiomyocytes in adult mice (Jesty et al., 2012). However, more rigorous lineage tracing studies in neonatal mice labeling pre-existing cardiomyocytes through tamoxifen inducible Cre-lox recombination demonstrated that pre-existing cardiomyocytes were the primary source of new cardiomyocytes after injury after LAD ligation (Porrello et al., 2013). Additionally, using a combined stable isotope labeling and multi-isotope imaging mass spectrometry approach to study mammalian cardiomyocyte turnover, Senyo et al. further provided evidence that pre-existing cardiomyocytes are the primary source of new cardiomyocytes in mice (Senyo et al., 2013). Importantly, lineage tracing experiments performed by tamoxifen-inducible labeling of c-kit-positive cells revealed minimal contribution of this cell population to new cardiomyocytes after LAD ligation in mice of a variety of ages (van Berlo et al., 2014). It was then reported that c-kit positive cells do not express myocyte markers after embryonic day 13.5 when using transgenic reporter mice and may actually represent cardiac endothelial cells (Sultana et al., 2015). Therefore, at present, the field is in agreement that c-kit cells are irrelevant as the source of newly regenerated cardiac muscle cells in mammals.
CARDIOMYOCYTE POLYPLOIDIZATION: A BARRIER TO HEART REGENERATION
The cardiomyocytes of adult zebrafish are predominantly mononucleated and diploid (González-Rosa et al., 2018; Wills et al., 2008). Adult newts and salamanders, which retain cardiac regenerative potential, are also observed to have high mononucleated cardiomyocyte frequencies (Becker et al., 1974; Bettencourt-Dias et al., 2003). Additionally, the majority of cardiomyocytes in the newborn mouse heart are initially mononucleated and diploid but then undergo cell cycle arrest and binucleation within the first two weeks of postnatal development (Soonpaa et al., 1996). Therefore, elevated diploid cardiomyocyte frequencies are positively correlated with enhanced cardiac regenerative potential (Figure 1).
Figure 1. Cardiomyocyte polyploidization correlates with the loss of cardiomyocyte proliferative and heart regenerative capacity.

Adult zebrafish (A) and neonatal mice (B) retain a high frequency diploid cardiomyocytes that are capable of proliferation, correlates with their robust abilities to regenerate their hearts. Adult mice (C) retain much lower diploid cardiomyocyte frequency. Rather, their hearts contain mostly terminally differentiated polyploid binucleated cardiomyocytes that lack the ability to divide. This correlates with decreased heart regenerative potential.
In contrast, mammalian hearts generally have higher frequencies of polyploid cardiomyocytes (Kikuchi & Poss, 2012; Laflamme & Murry, 2011). The non-regenerating hearts of adult mice are composed of matured mostly polyploid cardiomyocytes that are terminally differentiated and considered postmitotic (Derks et al., 2020; Jewhurst & McLaughlin, 2015; Kikuchi & Poss, 2012; Rumyantsev, 1977; Soonpaa et al., 1996). Polyploidization in rodents occurs within the first two weeks after birth. A peak in cardiomyocyte DNA synthesis is observed around postnatal day 4 accompanied by a gradual rise in binucleated cardiomyocytes, which is largely complete by around postnatal day 10 (F. Li et al., 1996; Soonpaa et al., 1996). Interestingly, in sheep (Ovis aries), cardiomyocyte polyploidization occurs during gestational development (between 65–76 days after conception) and postnatal hearts have reduced proliferative capabilities with observed differences in inflammation and cardiomyocyte proliferation in response to myocardial damage (Herdrich et al., 2010; Jonker et al., 2015; Zgheib et al., 2014). Pigs can also regenerate their hearts within 2 to 3 days after birth and an increase in binucleated and multinucleated cardiomyocyte abundance is observed starting at 15 days after birth (Velayutham et al., 2020; Ye et al., 2018; Zhu et al., 2018). Finally, the human heart has been reported to contain a high frequency of mononucleated CMs, but most are tetraploid and exhibit impaired ability to re-enter and complete the cell cycle (Bergmann et al., 2009, 2015; Kikuchi & Poss, 2012; Laflamme & Murry, 2011; VYa et al., 1994).
The timing of polyploidization described above would suggest that cardiomyocyte endowment, the final number of cardiomyocytes in the heart, is set during the perinatal period. However, it was proposed that a thyroid hormone-dependent burst of cardiomyocyte proliferation after postnatal day 14 - a time when cardiomyocyte polyploidization is already complete - contributes to overall cardiomyocyte endowment (Naqvi et al., 2014). This finding has since been contested by other groups (Alkass et al., 2015; Soonpaa et al., 2015), highlighting the need for improved methods to quantify total cardiomyocyte numbers in the heart.
At the cellular level, cardiomyocyte polyploidization is a result of defective karyokinesis and/or cytokinesis, steps in the cell cycle required for nuclear and cytoplasmic division, respectively (Derks et al., 2020; Engel et al., 2006; Hesse et al., 2018; Leone et al., 2018; Van de Peer et al., 2017; Zebrowski & Engel, 2013). Aberrations and alterations to cytoskeletal components like the mitotic microtubule organizing center as well as formation of a defective cleavage furrow have been suggested to result in cytokinesis failure and give rise to binucleated cardiomyocytes (Leone et al., 2018). Midbody positioning during cytokinesis may also be a determinant of cardiomyocyte binucleation (Hesse et al., 2018). Cardiomyocytes undergoing binucleation exhibit asymmetrical midbody positioning which often resulted in daughter nuclei being in closer proximity to one another, in vitro and in vivo. A larger chromosomal bridge may hinder anaphase and eventual cytokinetic abscission (Engel et al., 2006; Hesse et al., 2018; Hu et al., 2012). Recent work also suggests that homotypic fusion may too be a source of binucleated cardiomyocytes in mice (Ali et al., 2020). However, in zebrafish, the occurrence of cellular fusion does not necessarily give rise to binucleated cells (Kirillova et al., 2021; Sawamiphak et al., 2017). Tetraploidy in human cardiomyocytes arises due to endomitosis, DNA replication in the absence of nuclear division (Edgar et al., 2014; Meckert et al., 2005).
These observations across multiple mammalian species have led to the hypothesis that cardiomyocyte polyploidization may be indicative of cell cycle withdrawal and loss of cardiac regenerative potential (Kikuchi & Poss, 2012; Kirillova et al., 2021; Patterson et al., 2017; Walsh et al., 2010). Supporting this hypothesis, induction of cardiomyocyte polyploidization in zebrafish hearts inhibits cardiac regeneration (González-Rosa et al., 2018) and binucleated cardiomyocytes in adult newts exhibit an impaired ability to properly divide (Matz et al., 1998). Additionally, the frequency of diploid cardiomyocytes in the heart depends on allelic variance (Gan et al., 2019, 2020; Patterson et al., 2017), and performing cardiac injuries in mice strains with higher natural frequencies of diploid cardiomyocytes positively correlates with enhanced regeneration and functional recovery (Patterson et al., 2017). A recent phylogenetic analysis profiling diploid cardiomyocyte frequency recently revealed mammalian species with considerably elevated diploid cardiomyocyte abundances (Hirose et al., 2019). It remains to be tested if these mammals possess enhanced cardiac regenerative potential. For example, the African spiny mouse is quickly gaining popularity as a mammalian model of tissue regeneration (Gaire et al., 2021) and possesses an approximately 2-fold higher frequency in diploid cardiomyocytes relative to that observed in adult mice. Recent reports observe enhanced cardiac repair and recovery in this model (Koopmans et al., 2021; Peng et al., 2021; Qi et al., 2021), but whether or not this involves increased cardiomyocyte cell cycle reactivation and proliferation is not clear.
Contrasting the hypothesis above, some evidence suggests that polyploid cardiomyocytes may retain at least some ability to divide. Postnatal binucleated rat cardiomyocytes stimulated with either serum or a combination of fibroblast growth factor 1 and p38 MAP kinase inhibitor have been observed to re-enter the cell cycle and proliferate through formation of pseudo bi-polar spindles in vitro (Leone & Engel, 2019). Adult binucleated mouse cardiomyocytes have also been reported to dedifferentiate, enter the cell cycle, and divide, when co-cultured with neonatal cardiomyocytes (W. E. Wang et al., 2017). Furthermore, other mammalian cell types are naturally polyploid yet retain the ability to proliferate (Pandit et al., 2013). Polyploid hepatocytes are able to complete their cell divisions through ploidy reversal (Duncan, 2013; M.-J. Wang et al., 2017) and a recent study monitoring the dynamics of cardiomyocyte ploidy through mouse postnatal development suggests that ploidy reversion in cardiomyocytes may be possible as well (Swift et al., 2023). Finally, it is likely that cardiomyocyte polyploidization is not the only barrier limiting cardiac regeneration. Though the hearts of medaka consist primarily of diploid cardiomyocytes (Chowdhury et al., 2022; Ito et al., 2014), heart regeneration still fails in these animals. This suggests that retaining high diploid cardiomyocyte frequency may not be sufficient to support a robust cardiac regenerative response. It is likely that extrinsic signals, possibly from recruited or resident macrophages, are additionally required to stimulate cardiomyocyte proliferation (Chowdhury et al., 2022; Ito et al., 2014; Lai et al., 2017).
CELL-TO-CELL INTERACTIONS REQUIRED FOR HEART REGENERATION
Lineage tracing experiments in both zebrafish and neonatal mice have provided a key insight: pre-existing cardiomyocytes are the main source of newly regenerated cardiac cardiac muscle. This discovery stresses the importance of understanding the intrinsic mechanisms that regulate cardiomyocyte cell cycle control, proliferation, and polyploidization. However, the lack of a robust cardiac regeneration response in other species that retain high frequencies of diploid cardiomyocytes (e.g., medaka), suggest that extrinsic regulators are also required for heart regeneration to occur. In this subsection, we provide an overview of recent work exploring the contributions of other cell types in the heart that may influence the cardiac regenerative response (Figure 2).
Figure 2. Cardiac regeneration requires intercellular communication between other cell types in the heart.

Though the proliferation of pre-existing cardiomyocytes to regenerate new cardiac muscle is required for cardiac regeneration, interactions between immune cells (A), the epicardium (B), and endothelium (C) are necessary to support the reparative process.
Immune cells
As discussed previously, the inability of medaka to regenerate its heart is, at least in part, due to a delayed innate immune response to injury (Ito et al., 2014; Lai et al., 2017). Specifically, the inefficient recruitment of macrophages at the site of injury results in an overall decrease in neutrophil clearance, cardiomyocyte proliferation, neovascularization, and scar resolution The importance of macrophage recruitment was further validated in zebrafish using clodronate liposome-mediated ablation, which resulted in a compromised reparative response. Furthermore, the recruitment of macrophages with Toll-like receptor agonist poly I:C enhanced heart regeneration in medaka (Lai et al., 2017). In adult axolotls, the depletion of macrophages with clodronate liposomes impairs cardiac regeneration after cryoinjury resulting in fibrosis and scarring (Godwin et al., 2013). Interestingly, activation of cardiomyocyte cell cycle markers after injury was still observed despite impaired macrophage recruitment. This may suggest that macrophages do not regulate cardiomyocyte cell cycle entry but rather may assist in cell cycle progression either directly or indirectly through interactions with fibroblasts or other components of the extracellular matrix (Godwin et al., 2013). Macrophage depletion with clodronate liposomes in neonatal mice also causes impaired heart regeneration resulting in fibrotic scars, decreased cardiac function, and decreased angiogenesis (Aurora et al., 2014).
It is important to note that postnatal day 1 and 14 mice exhibit unique age-dependent immune responses to myocardial infarction (Aurora et al., 2014). Activation of the Smad pathway has been shown to regulate phagocytosis and inflammation after myocardial infarction (B. Chen et al., 2019). Smad3 activation in macrophages expressing TGF-β enhanced reparative processes such as initiating phagocytosis of dead cells, regulating anti-inflammatory responses, and activating angiogenesis and fibrosis in the event of myocardial infarction protecting (B. Chen et al., 2019). Macrophages have also been categorized into two subsets. The M1 subset mediates the pro-inflammatory response while M2 populations mediates reparative processes (Nahrendorf et al., 2007). Lavine et al. has also demonstrated that there may be an embryonic-derived lineage of macrophages that promote cardiomyocyte proliferation and coronary angiogenesis while producing little inflammatory response while the more inflammatory monocyte-derived macrophage population exhibits enhanced immune cell recruitment with decreased cardiomyocyte proliferation and angiogenesis (Lavine et al., 2014).
Though evidence supports that macrophage recruitment to damaged heart tissue is generally beneficial for cardiac regeneration, the role of neutrophils in this process is less clear. Whether or not neutrophils promote or inhibit cardiac regeneration may be dependent on the timing and regulation of the inflammatory responses (Frangogiannis, 2008; K. Sun et al., 2021; Timmers et al., 2012). For example, the use of anti-inflammatory glucocorticoids in zebrafish resulted in impaired regenerative capacity of the heart that resulted in excessive collagen deposition after ventricular resection (W.-C. Huang et al., 2013). This provides evidence that the initial inflammatory response may be essential to enable the cardiac regenerative process. In addition, neutrophils have been observed to promote repair of the heart following infarction by helping to polarize macrophages towards the M2 reparative phenotype (Horckmans et al., 2017). Adverse effects associated with the neutrophils and a prolonged inflammatory response have additionally been described (Chia et al., 2009; Guasti et al., 2011; K. Sun et al., 2021; Vinten-Johansen, 2004).
Similar to macrophages and neutrophils, it has been suggested that T-cells may also help to secrete paracrine factors that induce cardiomyocyte proliferation (Dolejsi et al., 2022; J. Li et al., 2019). In fact, there may be age-dependent effects on T-cell mediation of the reparative processes in the heart. For example, adult T-cells producing IFN-5 transplanted into neonatal mice impaired cardiac regeneration after myocardial infarction, suggesting a possible trade-off with T-cell development and maturation with the regenerative capacity of the heart (Dolejsi et al., 2022). This may be correlated to the observed increase in T-helper 1 and 2 cells in patients with severe coronary artery disease and acute myocardial infarction (C. Li et al., 2019). However, it may also be possible that regulation of T helper cells can improve wound healing after myocardial infarction as observed with the knockout of the Dectin-2 receptor resulting in improved wound healing in mice (Yan et al., 2017).
Regulatory T cells (Tregs) also have been reported to aid in the regenerative processes of the heart after injury. Specific depletion of regulatory T cells in mice increased polarization of macrophages to the M1 pro-inflammatory phenotype and deterioration in cardiac function (Weirather et al., 2014). Activation of Tregs improves the healing processes with increased macrophage differentiation into the more reparative and anti-inflammatory M2 phenotype improving survivability of mice after LAD ligation. Additionally, Tregs may have the ability to modulate cardiomyocyte cytokine production and cell cycling. Tang et al. demonstrated Tregs can inhibit the production of inflammatory cytokines from cardiomyocytes effectively protecting the cardiomyocytes from inflammation and improving cardiac function (T.-T. Tang et al., 2011). Tregs have also been observed to decrease apoptosis of cardiomyocytes in a CD39-dependent manner while deficiency in CD39 Tregs resulted in an increase in apoptosis (Fung et al., 2020; Xia et al., 2015). In zebrafish, Tregs have been characterized to secrete growth factors stimulating cardiomyocytes to proliferate (Hui et al., 2017). Similarly, Treg-specific depletion in neonatal mice results in impaired regeneration of the heart after injury and is suggested to signal cardiomyocytes to proliferate through paracrine factors such as CCL24, GAS6 and AREG (J. Li et al., 2019).
Currently, the role of cardiac lymphatic vessels with respect to heart regeneration is currently not well characterized, but it has been suggested that these vessels play an important role in cell signaling and transport of macromolecules (Feng et al., 2021). Cardiac lymphatics have been reported to respond to cardiac injury and neo-lymphangiogenesis post-injury may aid in antigen clearance and the resolution of inflammation (Klotz et al., 2015; Vieira et al., 2018). In one study, lymphatic vasculature has also been observed to be fundamental for heart regeneration in zebrafish and mice (Gancz et al., 2019). However, the requirement of lymphatic vessels during heart regeneration isn’t yet substantiated. For example, zebrafish hearts can seemingly mount a complete regenerative response after cryoinjury in mutants that lack lymphatic vasculature, with exception of a small subset that may have enhanced cytokine signaling and inflammation after cryoinjury (Vivien et al., 2019). The cardiac lymphatic vessels may not be required for but may help modulate cardiac injury responses and facilitate the reparative processes of the heart (Feng et al., 2021; Harrison et al., 2019).
Epicardial cells
The epicardium is the layer of mesothelial tissue that envelops the heart, which has been observed to respond to cardiac injury. (Gemberling et al., 2015; González-Rosa et al., 2012; Lepilina et al., 2006; Tahara et al., 2016; Wills et al., 2008). The epicardium has been shown to regulate cardiomyocyte proliferation and heart repair (J. Cao & Poss, 2018; Sucov et al., 2009). It was proposed that epicardial progenitor cells may also contribute to regenerated cardiomyocytes (B. Zhou et al., 2008). A recent study using a Cre-lox reporter system in transgenic salamanders (Pleurodeles waltl) has suggested that epicardial cells can undergo an epicardial cell-to-cardiomyocyte conversion after cryoinjury (Eroglu et al., 2022). However, the fate mapping technique used in this study does not employ genetically encoded reporters to exclusively label epicardial cells. Rather, epicardial cells are targeted by injection of a cell-permeant Cre recombinase into the pericardium to activate a ubiquitously-expressed reporter. Therefore, it remains formally possible that non-epicardial cells may too be labeled with this approach at low frequency, especially in combination with a cardiac injury that would disrupt tissue integrity. The presence of epicardial derived CM progenitor cells has also been debated in the past with lineage tracing studies showing that epicardial cells in zebrafish can differentiate into other cell types, such as myofibroblasts and perivascular cells with no evidence of myocardial cell types (González-Rosa et al., 2012; Kikuchi, Gupta, et al., 2011). The epicardium may also assist in neutrophil recruitment and response after cardiac injury through C/EBP signaling (G. N. Huang et al., 2012). Regardless, after cryoinjury and ventricular resection of the zebrafish heart, the epicardium clearly responds to the injury and helps support cardiac regenerative processes including cardiomyocyte proliferation (González-Rosa et al., 2011; Lepilina et al., 2006; Schnabel et al., 2011).
It is possible that the epicardium serves as a cellular scaffold and an inducer of cardiomyocyte proliferation (González-Rosa et al., 2012; Lepilina et al., 2006; Marín-Juez et al., 2019). For example, the extracellular factor Neuregulin-1 (NRG1) from epicardial perivascular cells has been described as an activator of heart regeneration in zebrafish by acting as a mitogen for zebrafish cardiomyocytes (Gemberling et al., 2015). In addition, there is evidence that paracrine factors secreted from cells derived from the epicardium following cardiac injury promote angiogenesis (B. Zhou et al., 2011). In response to cardiac tissue damage, retinoic acid is another secreted factor of the epicardium that has been shown to stimulate cardiomyocyte proliferation (Kikuchi, Holdway, et al., 2011). Additionally, murine epicardium secreted extracellular vesicles and exosomes have also been reported to promote cardiomyocyte cell cycle activity and proliferation (del Campo et al., 2021).
With specific labeling of endothelial and epicardial cells, it has been observed that endothelial and epicardial cells exhibit a robust injury response after genetic ablation of cardiomyocytes using transgenic Cre-lox mice and diptheria toxin A (DTA) induced by 4-hydroxytamoxifen injection (J. Wang et al., 2011). This suggests that the epicardium has a role in heart regeneration and can facilitate the reparative processes of the heart post injury. Genetic ablation of the epicardium in transgenic zebrafish using nitroreductase to induce the conversion of the non-toxic metronidazole to a cytotoxin was also shown to significantly reduce the reparative processes of the heart after apical resection, suggesting that the epicardium has an important role in heart regeneration (J. Wang et al., 2015). In contrast, a recent report using a selective chemoptogenetic cardiomyocyte ablation strategy in zebrafish resulted in minimal activation of the epicardium and endocardium prior to successful heart regeneration (Missinato et al., 2021). However, epicardial and endocardium marker activation was only evaluated at 3 days post-chemoptogenetic ablation in this study. Therefore, it remains possible that activation of these cellular populations may still occur at later time points during the regenerative process.
The epicardium has also been described to consist of a heterogeneous population of cells distinguishable from one another by expression signatures (J. Cao et al., 2016). Through single cell transcriptome sequencing, it was discovered that all subsets of epicardial cells express the scaffolding protein caveolin 1 (cav1) and its expression facilitates the reparative processes of the heart after apical resection in zebrafish (J. Cao et al., 2016). It was also observed that epicardial cells expressing follistatin-like 1 (Fstl1) are the only epicardial cells capable of promoting regeneration acting as a cardiomyogenic factor after apical resection in mice (Y. Cao & Cao, 2018; Wei et al., 2015). On the other hand, another subset of epicardial cells expressing hapn1 has been reported to play a role in promoting cardiomyocyte expansion and its depletion inhibits regeneration in zebrafish (J. Sun et al., 2022). Though it is not entirely certain whether or not epicardial cells are required for heart regeneration and how the subsets of epicardial cells play a role in heart regeneration, these data further support the idea that there is crosstalk between cardiac tissue and the epicardium during the regenerative process of the heart.
Endothelial cells
Similar to the epicardium, endocardial endothelium also exhibits crosstalk with myocardial tissue after injury to heart tissue. Much like the epicardium, the endothelial cells of the heart have been observed to produce and secrete retinoic acid in response to heart injury which is seen to promote cardiomyocyte proliferation in zebrafish (Kikuchi, Holdway, et al., 2011). Crosstalk between the epicardium and endothelial cells may also indirectly mediate the reparative processes of the heart. For example, epicardial and endothelial cells seem to work together to develop a cellular scaffold that eventually enhances superficial and intra-ventricular coronary artery formation (Marín-Juez et al., 2019). These coronary vessels are suspected to effectively form scaffolds for cardiomyocytes during regeneration and revascularization is essential for cardiac repair (Marín-Juez et al., 2016, 2019). Research with single-cell RNA sequencing of neonatal mice hearts revealed that the expression of the ligand of many endothelial cells, R-spondin 1 (Rspo1), is restricted (Z. Wang et al., 2020). Rspo1 is an activator of the canonical Wnt pathway and suggested to be associated with angiogenesis (Binnerts et al., 2007; Z. Wang et al., 2020). It was observed that adenoviral mediated overexpression of Rspo1 resulted in an enhanced angiogenesis without significant effects on the cardiomyocyte themselves (Z. Wang et al., 2020).
Endothelial cells secrete angiocrines which can regulate cardiomyocyte compaction and proliferation (Rhee et al., 2021). The findings that secreted factors from endothelial cells can regulate cardiomyocyte proliferation and compaction supports the idea that these cells help with organization and maturation of cardiomyocytes in the heart. Endothelial-cardiomyocyte crosstalk through paracrine signaling may protect the myocardial tissue from injury such as pharmacological damage (Leucker et al., 2011). Interestingly, rat and mice cardiomyocytes overexpressing vascular endothelial growth factor B (VEGF-B) have also been observed to promote angiogenesis and enhance endothelial proliferation (Räsänen et al., 2021). These findings may suggest that though the epicardium and endocardium has been observed to enhance and aid in the reparative process of the heart, the myocardium may interact with these tissues to regulate cardiac regeneration as well.
METABOLIC ADAPTATIONS
Beyond our appreciation for the intercellular mechanisms that may be required to support vertebrate cardiac regeneration, recent findings provide evidence that physiological adaptations to support the increasing metabolic demands of an animal may negatively impact its ability to regenerate its heart (Figure 3).
Figure 3. The shift towards fatty acid oxidation to support increasing metabolic demand may inversely correlate with cardiac regenerative potential.

(A) After birth, the hearts of neonatal mice shift from utilizing glycolysis to fatty acid oxidation. (B) Animal metabolic needs increase both during mammalian postnatal development and during the evolution of endothermic mammals. (C) The shift to fatty acid metabolism and the increase in animal metabolic needs inversely correlate with the loss of heart regenerative capacity.
Lipid metabolism
One suggested barrier to mammalian heart regeneration is the maturation process of adult mammalian cardiomyocytes, which may be driven by a hypoxic-to-hyperoxic environmental transition after birth (Puente et al., 2014). The development of endothermy and the observed shift from glycolysis to fatty acid oxidation have also been hypothesized as “evolutionary trade-offs” that drive cardiomyocyte cell cycle arrest in adult mammals (Ross et al., 2022; Sakaguchi et al., 2020). The transition to the hyperoxic postnatal environment has been proposed to increase mitochondrial reactive oxygen species production, which activates the DNA damage response and inhibits cardiomyocyte cell cycle progression (Puente et al., 2014). Furthermore, the utilization of fatty acids in the mitochondria has been shown to increase chromatin oxidative stress in cardiomyocytes (Menendez-Montes et al., 2021). Inhibition of the DNA damage response increased the postnatal window of cardiomyocyte proliferation proliferation (Puente et al., 2014). This transition seems to be correlated with a metabolic shift in cardiomyocytes from glycolysis to fatty acid oxidation (Cardoso et al., 2020). Inhibition of pyruvate dehydrogenase kinase 4 (PDK4) enhances pyruvate dehydrogenase abundance and activity and shifts cardiomyocytes toward glycolytic metabolism. This resulted in increased cardiomyocyte proliferation and reduction in DNA damage response markers (Cardoso et al., 2020). Zebrafish embryos treated with long chain acyl carnitines, fatty acid intermediates, exhibited impairment to cardiac function and mitochondrial function (Park et al., 2021). On the other hand, the stimulation of glycolysis in zebrafish has also been observed to promote CM proliferation after injury (Fukuda et al., 2020). Furthermore, malonate has also been observed to induce CM proliferation and enhance cardiac regeneration through succinate dehydrogenase inhibition in turn decreasing production of reactive oxygen species (Bae et al., 2021). These findings indicate that metabolic substrate utilization, mitochondrial reactive oxygen species, the DNA damage response associated with the cardiac metabolic shift to fatty acid oxidation may play a key role in restricting mammalian heart regenerative capacity.
In contrast to the above work performed in the mouse model, the timing of the hypoxic-to-hyperoxic environmental shift in sheep does not correlate with the loss of cardiomyocyte proliferation and regenerative (Lock et al., 2019). Sheep cardiomyocytes can enter the cell cycle after injury during a proliferative window between 65–76 days of gestation but lose their proliferative potential after birth (Herdrich et al., 2010; Zgheib et al., 2014). Therefore, sheep seem to lose the ability to regenerate their hearts before birth, which could be due to the precocial nature of this animal where fetal maturation occurs rapidly within the intrauterine environment (Lock et al., 2019). Consistent with this, differences in growth and maturation have been observed between fetal sheep and rodent cardiomyocytes (Drake et al., 2022; Jonker et al., 2018).
Interestingly, in 3-month-old mice, it has also been suggested that hypoxic environments can induce cardiomyocyte proliferation. One study observed a decrease in ROS and oxidative DNA damage in hypoxic mice after 2 weeks of being in a hypoxic environment (7% O2) (Nakada et al., 2017). With chronic hypoxia, 3-month-old mice also were observed to exhibit a higher number of cardiomyocytes with a higher percentage of mononucleated cardiomyocytes and an increase to heart weight in comparison to normoxic mice with similar effects observed with mild hypoxia (10% O2) (Nakada et al., 2017). Similarly, one study using an EdU mini pump implanted in 3-month-old mice under hypoxic conditions also observed an increase in cardiomyocyte proliferation according to EdU incorporation further supporting the aforementioned study done by Nakada and colleagues (Johnson et al., 2023)
Acquisition of Endothermy
The acquisition of endothermy has also been proposed to be an evolutionary adaptation that is associated with the loss of regenerative capacity of the heart (Amram et al., 2021; Hirose et al., 2019; Ross et al., 2022). In support of this, there may be an inverse correlation with the development of endothermy and the regenerative capacity of the heart (Hirose et al., 2019). Many animals that possess robust heart regenerative capacity, such as fish, amphibians, and reptiles, also happen to be ectothermic (Hirose et al., 2019). Thyroid hormones have been hypothesized to drive the shift towards the development of endothermy. Consistent with this hypothesis, inhibition of thyroid hormone production during postnatal development decreases thermogenesis (Little & Seebacher, 2014; Payumo et al., 2021). Furthermore, it has been observed that exposure to thyroid hormones inhibits cardiomyocyte proliferation in fetal sheep and zebrafish in vitro while deficiency in thyroid hormone signaling in adult mice increases regenerative potential of the heart (Chattergoon et al., 2006; Hirose et al., 2019).
CARDIOMYOCYTE CELL-CYCLE REACTIVATION: CHALLENGES AND ALTERNATIVE STRATEGIES
While much progress has been made to understand the mechanisms that support or limit heart regeneration, how to best apply this knowledge to therapeutically reactivate a cardiac regenerative response in adult mammals is not clear. In this subsection, we compare existing approaches to reactivate the regenerative capacity of the mammalian heart (Figure 4).
Figure 4. Strategies to replace lost cardiomyocytes after injury.
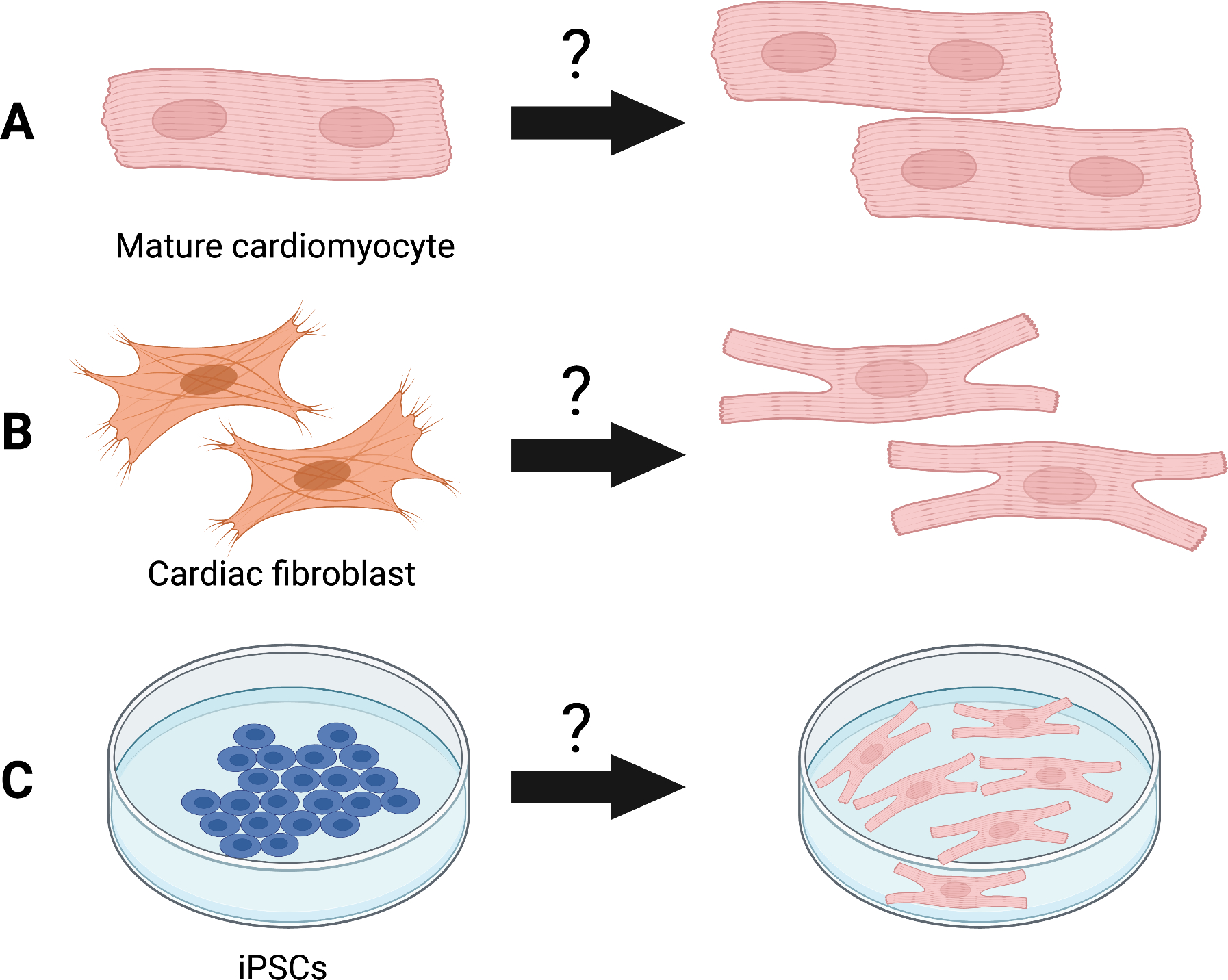
(A) Reactivation of cell cycle activity and proliferation of mature adult cardiomyocytes. (B) Heterocellular reprogramming and transdifferentiation of other cell types in the heart (e.g., cardiac fibroblasts) towards a cardiomyocyte-like fate. (C) Derivation of cardiomyocytes from patient-derived induced pluripotent stems (iPSCs).
Reactivation of cell cycle in adult cardiomyocytes
Genetic and epigenetic repression of embryonic cardiomyocyte gene expression during development and maturation may also contribute to the loss of cardiac regenerative capacity (Cui et al., 2018; Kraus, 2022). For example, the transcription factor Meis1 inhibits cardiomyocyte cycling during cardiogenesis by activating the expression of cyclin dependent kinase (CDK) inhibitors p15, p16, and p21 potentially contributing to cell cycle arrest in cardiomyocytes in mice (Mahmoud et al., 2013). The targeted expression of cyclin D2 and deregulation of D-cyclin-mediated Rb/E2f-signaling has also been suggested to affect cardiomyocyte proliferative potential (Hille et al., 2016; Pasumarthi et al., 2005). E2F-dependent genes have been reported to become silenced in adult mouse cardiomyocytes and results in epigenetic changes in heterochromatin associated with retinoblastoma (Rb) family and p130 recruiting heterochromatin protein 1 (HP1) proteins (Sdek et al., 2011). Depletion of both Rb and p130 increased proliferation in adult cardiomyocytes. Similarly, inhibition of HP1-γ resulted in an increase to genes associated with cardiomyocyte proliferation supporting that epigenetic changes have a role in cell cycle arrest (Sdek et al., 2011). Histone acetylation and methylation also regulates DNA synthesis and differentiation in cardiomyocytes (Kou et al., 2010). .
Key efforts in the field have focused on reactivating the cell cycle in terminally differentiated adult cardiomyocytes. For example, the glycogen synthase kinase-3 (GSK3) inhibitor 6-bromoindirubin-3’-oxime (BIO) has been observed to induce proliferation in both neonatal and adult rat CMs (Tseng et al., 2006). The use of GSK3 inhibitors to induce cell cycle entry in adult cardiomyocytes in vitro shows that it may be possible that terminally differentiated cardiomyocytes possess developmental plasticity and can be reprogrammed to divide. However, cardiomyocyte-specific deletion of GSK3 in vivo results in mitotic catastrophe through dysregulation of cell cycle checkpoints and severe DNA damage (J. Zhou et al., 2016).
Modulation of the Hippo signaling pathway has been another route researchers have investigated to improve cardiomyocyte proliferation and function (Heallen et al., 2011, 2013; Leach et al., 2017; Xin et al., 2013). Interestingly, the Hippo effector YAP has been characterized to play a role in crosstalk with canonical Wnt signaling by negatively regulating β-catenin by nuclear interactions and inhibiting CM proliferation (Heallen et al., 2011). This is generally supported by other work that shows deficiency or downregulation of Hippo signaling results in an increase in CM proliferation. There is additional evidence that Hippo signaling is upregulated in cases of human heart failure and deficiency in Hippo signaling can increase CM proliferation and may enhance the reparative process of the heart (Leach et al., 2017).
Similarly, stimulation of adult cardiomyocyte proliferation and cardiac regeneration has been observed with the modulation of NRG1 and its tyrosine kinase receptor ERBB2 (D’Uva et al., 2015). The study done by D’Uva et al., suggests that induction of ERBB2 can dedifferentiate adult cardiomyocytes and increase cardiomyocyte proliferation (D’Uva et al., 2015). These results further suggest that adult cardiomyocytes may be able to re-enter and potentially complete the cell cycle under permissive systemic conditions. Supporting the idea that cell cycle progression and completion could be regulated in adult cardiomyocytes, overexpression of cell-cycle regulators associated with stable cytokinesis cyclin-dependent kinase 1 (CDK1), CDK4, cyclin B1, and cyclin D1 was observed to induce cell-cycle re-entry, successful division, and proliferation (Mohamed et al., 2018). Other pathways and environmental factors have also been considered to play a role in regulating adult cardiomyocyte proliferation ranging from Notch signaling to regulation of the extracellular matrix (Gong et al., 2021).
Heterocellular reprogramming
The first known case of heterocellular reprogramming was done by Qian et al. using cardiac fibroblasts (Qian et al., 2012). In the study, retroviral administration of Gata4, Mef2x, and Tbx5 (GMT) was done by direct intramyocardial injection in murine cardiac fibroblasts. With the retroviral-mediated gene transfer of GMT into cardiac non-myocytes, transdifferentiation efficiency was estimated to be about 10–15% for both in vivo and in vitro induced cardiomyocytes (iCMs). iCMs in vivo were reported to resemble endogenous cardiomyocytes more than their in vitro counterparts which could indicate the microenvironment may enhance the transdifferentiation of cardiac non-myocytes to iCMs (Qian et al., 2012).
Since the development of GMT-derived iCMs, many have tried to further optimize the transdifferentiation protocol and conditions. The addition of other transcription factors associated with cardiomyocyte function and maturation has been a common approach to further optimize the transdifferentiation process (Addis et al., 2013; Singh et al., 2021; Song et al., 2012; Y. Tang et al., 2022). For example, the Hippo pathway effector transcriptional enhance associate domain 1 (Tead1) was observed to further increase transdifferentiation efficiency and can replace Tbx5 in the GMT cocktail (Singh et al., 2021). Though a general increase in cardiomyocyte associated structures and functionality was observed with cultures transdifferentiation by Gata4, Mef2c, and Tead1 (GMTd) compared to GMT treated cultures, human iCM cultures exhibited to increase slightly (~4% to ~8% cTnT positive cells, ~2% to ~6% ɑ-actinin positive cells at 2 weeks, and ~3% to ~12% ɑ-actinin positive cells at 4 weeks). Similar results are generally seen with the incorporation of other factors along with the GMT cocktail, such as Hand2, Nkx2.5, and TBX20, but there is general trend of these iCMs still being a small percentage of cells present (Addis et al., 2013; Muraoka et al., 2014; Y. Tang et al., 2022).
In addition to the use of lenti-viral transduction to overexpress specific transcription factors to aid in the transdifferentiation of cardiac non-myocytes into iCMs, some have investigated the potential of utilizing short hairpin RNA to silence specific factors to enhance the generation of iCMs (Patel et al., 2018; Pinnamaneni et al., 2022). Downregulation of p63 further enhances the generation efficiency and functionality of iCMs and epigenetically regulates iPSC reprogramming (Patel et al., 2018; Pinnamaneni et al., 2022). Others have reportedly enhanced the generation of iCMs with the addition of small-molecule cocktails with the GMT cocktail supplemented with other transcription factors and entirely with small-molecule cocktails alone (N. Cao et al., 2016; Singh et al., 2020). Optimization of the iCM culturing conditions by altering the microenvironments has also been done to yield higher transdifferentiation efficiency as well where the use of 3D microenvironments increase reprogramming efficiency (Jin et al., 2022.).
Though all of these instances represent progress within the field, as mentioned in Qian et al., the transdifferentiation of cardiac fibroblast behavior may also have unforeseen effects on heart function as fibroblasts and other cardiac non-myocytes contribute to the effects of scar formation and heart function (Qian et al., 2012).
iPS cell-derived cardiomyocytes
The discovery and development of induced pluripotent stem cells has been instrumental in developing the field of stem cell research as it stands today. With the discovery of the Yamanaka factors, Oct3/4, Sox2, c-Myc, and Klf4, somatic cells have been able to be reprogrammed into a state of pluripotency; referred to as induced pluripotent stem cells (iPSCs) today (Takahashi & Yamanaka, 2006). With the relative success of this technique, many have taken advantage of this discovery and investigated how to differentiate iPSCs into a variety of cell types such as CMs, developing multiple potential models for basic research and regenerative medicine (Yu et al., 2007; Zhang et al., 2009).
Though iPSCs can serve as useful models due to availability and protocol reproducibility, iPSC-derived cardiomyocytes are generally considered immature compared to those in adult mammals (Patterson et al., 2012; van den Berg et al., 2015). In particular, iPSC-derived cardiomyocytes (iPSC-CMs) are immature with respect to their cell morphology, transcriptome, electrophysiology, metabolic activity, and contractile force production (Gherghiceanu et al., 2011; Karbassi et al., 2020; van den Berg et al., 2015; Wickramasinghe et al., 2022). As a result, different conditions have been tested over the years to determine possible approaches to generate iPSC-CMs more comparable to mature cardiomyocytes including culturing long-term, stimulating cultures with electrical currents, utilizing mechanical stress, and the use of biochemical induction (Wu et al., 2021). Some have attempted to further optimize known differentiation protocols by investigating the effects of pathways associated with cardiomyocyte maturation such as the peroxisome-proliferator-associated receptor (PPAR) pathway due to its relation to fatty acid oxidation pathways (Wickramasinghe et al., 2022). With the PPAR pathway, the PPAR-delta (PPARd) isoform along with long-chain fatty acid supplementation was observed to increase binucleation of iPSC-CMs, enhance myofibril organization and contractility while also increasing mitochondrial content and activity along with gene regulatory networks associated with FAO (Wickramasinghe et al., 2022). This data suggests there are signaling pathways that may signal for maturation based on activation and or the downregulation of important pathways associated with maturing CMs such as the metabolic switch from glycolysis to fatty acid oxidation. These results also further support previous findings that fatty acid supplementation can enhance iPSC-CM maturation (Horikoshi et al., 2019; Yang et al., 2019). Though PPARd activation seems to enhance iPSC-CM maturation, much of the increases observed are still within several percentile of the control and differentiation efficiency may be seen as another problem being observed in less than 60% of the cells in culture.
Optimization and adjustments to culturing microenvironments have also been done. For example, with the critical role of the extracellular matrix, combinational polymer matrices were used to determine the potential effect on iPSC-CM maturation (Chun et al., 2015). Utilizing a variety of copolymer matrices, iPSC-CMs cultured with 4% polyethylene glycol (PEG) - 95% poly-ε-caprolacton (PCL) matrices ultimately exhibited increased expression of cardiac troponin I, integrin alpha-7, and increased levels of contractility along with mitochondrial activity (Chun et al., 2015). These results suggest that the microenvironment may play a vital role in iPSC-CM differentiation to which the substrate type can inadvertently affect mechanosensory transduction pathways that may play a role with intermediate filament regulation in iPS-CMs.
With the variety of developments made over the years further unraveling the mysteries of iPSC-CM maturation, it is also important to appreciate the heterogeneity of cardiomyocytes in the heart. In fact, a variety of cardiomyocyte subtype-specific differentiation protocols exist (Grassam-Rowe et al., 2020; Lyra-Leite et al., 2022). Additionally, it is worth mentioning that with PPARd activation, iPSC-CM transcriptomes were still observed to vary from one another depending on the culture formats from 2D monolayers to 3D engineered heart tissues; yet another reminder of the complexities and caveats associated with different model systems (Wickramasinghe et al., 2022).
CONCLUSION
The work and progress in the field of heart regeneration over the last two decades has been immense. From the discovery (and debate) of the origin of newly regenerated cardiomyocytes to the appreciation of intercellular interactions and the significance of metabolic adaptations, it is clear that cardiac regeneration is complex. The proliferation of pre-existing cardiomyocytes alone is likely not sufficient to support the cardiac regenerative response. Therefore, efforts in determining the extrinsic mechanisms that permit cardiomyocytes to divide will likely be a major area of research in future. How loss of cardiac regenerative potential in mammals may be linked to the development of endothermy and the subsequent metabolic shift from glycolysis to fatty acid metabolism may help us further understand the evolutionary framework for how heart regenerative capacity is lost in mammals.
However, with the plethora of knowledge obtained over the years regarding the mechanisms that both support and limit heart regeneration, there is still much left to be desired from a therapeutic standpoint. Some have tested modulation of different signaling pathways to initiate cell cycle re-entry and stimulate cardiomyocyte proliferation. Others have approached the issue with attempts at derivation of cardiomyocytes from sources other than terminally differentiated cardiomyocytes. For example, iPSCs have been a popular model and potential source of new cells in many different fields. Heterocellular reprogramming is another approach where surrounding fibroblasts are modified with the modulation of different molecular effectors to induce cellular reprogramming to generate new cardiomyocytes in vivo. Unfortunately, as of the time of writing, these two general approaches to generate new cardiomyocytes are still burdened with the difficulties of generating developmentally mature cardiomyocytes at high enough efficiencies to be therapeutically relevant. The use of surrounding cardiac fibroblasts to generate new cardiomyocytes in vivo could be a viable therapeutic system, but like many other techniques, it is worth noting that this approach may alter the composition of the heart tissue itself. All existing strategies have their own limitations and caveats. Regardless, the work in the field over the past two decades has given hope that there is a possibility to enhance the regenerative potential of the adult human heart.
ACKNOWLEDGEMENTS
We thank the members of Guo N. Huang’s lab at the University of California, San Francisco and Alexander Y. Payumo’s lab at San Jose State University for thoughtful conversations and input. All figures were generated using BioRender.com.
FUNDING INFORMATION
This work is supported by an NIH SuRE-First award (R16GM146643) Dr. Payumo, and NIH awards (R01HL138456 and R01HL157280), American Heart Association Transformation Award and Established Investigator Award, and Tobacco-Related Disease Research Program Award P0558275 to Dr. Huang.
Footnotes
CONFLICT OF INTERESTS
The authors have no conflicts of interest to disclose.
REFERENCES
- Addis RC, Ifkovits JL, Pinto F, Kellam LD, Esteso P, Rentschler S, Christoforou N, Epstein JA, & Gearhart JD (2013). Optimization of Direct Fibroblast Reprogramming to Cardiomyocytes Using Calcium Activity as a Functional Measure of Success. Journal of Molecular and Cellular Cardiology, 60, 97–106. 10.1016/j.yjmcc.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SR, Menendez-Montes I, Warshaw J, Xiao F, & Sadek HA (2020). Homotypic Fusion Generates Multinucleated Cardiomyocytes in the Murine Heart. Circulation, 141(23), 1940–1942. 10.1161/CIRCULATIONAHA.119.043530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkass K, Panula J, Westman M, Wu T-D, Guerquin-Kern J-L, & Bergmann O (2015). No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell, 163(4), 1026–1036. 10.1016/j.cell.2015.10.035 [DOI] [PubMed] [Google Scholar]
- Amram AV, Cutie S, & Huang GN (2021). Hormonal control of cardiac regenerative potential. Endocrine Connections, 10(1), R25–R35. 10.1530/EC-20-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, & Olson EN (2014). Macrophages are required for neonatal heart regeneration. The Journal of Clinical Investigation, 124(3), 1382–1392. 10.1172/JCI72181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Salamon RJ, Brandt EB, Paltzer WG, Zhang Z, Britt EC, Hacker TA, Fan J, & Mahmoud AI (2021). Malonate Promotes Adult Cardiomyocyte Proliferation and Heart Regeneration. Circulation, 143(20), 1973–1986. 10.1161/CIRCULATIONAHA.120.049952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, LeCapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, … Anversa P (2007). Human cardiac stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104(35), 14068–14073. 10.1073/pnas.0706760104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RO, Chapin S, & Sherry R (1974). Regeneration of the ventricular myocardium in amphibians. Nature, 248(5444), 145–147. 10.1038/248145a0 [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, & Anversa P (2003). Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell, 114(6), 763–776. 10.1016/S0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, & Frisén J (2009). Evidence for cardiomyocyte renewal in humans. Science (New York, N.Y.), 324(5923), 98–102. 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, & Frisén J (2015). Dynamics of Cell Generation and Turnover in the Human Heart. Cell, 161(7), 1566–1575. 10.1016/j.cell.2015.05.026 [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Mittnacht S, & Brockes JP (2003). Heterogeneous proliferative potential in regenerative adult newt cardiomyocytes. Journal of Cell Science, 116(19), 4001–4009. 10.1242/jcs.00698 [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim K-A, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, Dixon M, Hazell SA, Wagle M, Nie W-S, Tomasevic N, Williams J, Zhan X, Levy MD, Funk WD, & Abo A (2007). R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14700–14705. 10.1073/pnas.0702305104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Navis A, Cox BD, Dickson AL, Gemberling M, Karra R, Bagnat M, & Poss KD (2016). Single epicardial cell transcriptome sequencing identifies Caveolin 1 as an essential factor in zebrafish heart regeneration. Development (Cambridge, England), 143(2), 232–243. 10.1242/dev.130534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, & Poss KD (2018). The epicardium as a hub for heart regeneration (review). Nature Reviews. Cardiology, 15(10), 631–647. 10.1038/s41569-018-0046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N, Huang Y, Zheng J, Spencer CI, Zhang Y, Fu J-D, Nie B, Xie M, Zhang M, Wang H, Ma T, Xu T, Shi G, Srivastava D, & Ding S (2016). Conversion of human fibroblasts into functional cardiomyocytes by small molecules. Science, 352(6290), 1216–1220. 10.1126/science.aaf1502 [DOI] [PubMed] [Google Scholar]
- Cao Y, & Cao J (2018). Covering and Re-Covering the Heart: Development and Regeneration of the Epicardium. Journal of Cardiovascular Development and Disease, 6(1), 3. 10.3390/jcdd6010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso AC, Lam NT, Savla JJ, Nakada Y, Pereira AHM, Elnwasany A, Menendez-Montes I, Ensley EL, Petric UB, Sharma G, Sherry AD, Malloy CR, Khemtong C, Kinter MT, Tan WLW, Anene-Nzelu CG, Foo RS-Y, Nguyen NUN, Li S, … Sadek HA (2020). Mitochondrial Substrate Utilization Regulates Cardiomyocyte Cell Cycle Progression. Nature Metabolism, 2(2), 167–178. [PMC free article] [PubMed] [Google Scholar]
- Chablais F, Veit J, Rainer G, & Jaźwińska A (2011). The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Developmental Biology, 11, 21. 10.1186/1471-213X-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattergoon NN, Giraud GD, & Thornburg KL (2006). Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. Journal of Endocrinology, 192(2), R1–R8. 10.1677/JOE-06-0114 [DOI] [PubMed] [Google Scholar]
- Chen B, Huang S, Su Y, Wu Y-J, Hanna A, Brickshawana A, Graff J, & Frangogiannis NG (2019). Macrophage Smad3 Protects the Infarcted Heart, Stimulating Phagocytosis and Regulating Inflammation. Circulation Research, 125(1), 55–70. 10.1161/CIRCRESAHA.119.315069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, & Wang D-Z (2012). MicroRNAs in cardiovascular development. Journal of Molecular and Cellular Cardiology, 52(5), 949–957. 10.1016/j.yjmcc.2012.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S, Nagurney JT, Brown DFM, Raffel OC, Bamberg F, Senatore F, Wackers FJT, & Jang I-K (2009). Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. The American Journal of Cardiology, 103(3), 333–337. 10.1016/j.amjcard.2008.09.085 [DOI] [PubMed] [Google Scholar]
- Chowdhury K, Lin S, & Lai S-L (2022). Comparative Study in Zebrafish and Medaka Unravels the Mechanisms of Tissue Regeneration. Frontiers in Ecology and Evolution, 10. 10.3389/fevo.2022.783818 [DOI] [Google Scholar]
- Chun YW, Balikov DA, Feaster TK, Williams CH, Sheng CC, Lee J-B, Boire TC, Neely MD, Bellan LM, Ess KC, Bowman AB, Sung H-J, & Hong CC (2015). Combinatorial Polymer Matrices Enhance In Vitro Maturation of Human Induced Pluripotent Cell Cell-Derived Cardiomyocytes. Biomaterials, 67, 52–64. 10.1016/j.biomaterials.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Wang Z, Bassel-Duby R, & Olson EN (2018). Genetic and epigenetic regulation of cardiomyocytes in development, regeneration and disease. Development (Cambridge, England), 145(24), dev171983. 10.1242/dev.171983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo CV, Liaw NY, Gunadasa-Rohling M, Matthaei M, Braga L, Kennedy T, Salinas G, Voigt N, Giacca M, Zimmermann W-H, & Riley PR (2021). Regenerative potential of epicardium-derived extracellular vesicles mediated by conserved miRNA transfer. Cardiovascular Research, 118(2), 597–611. 10.1093/cvr/cvab054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks W, Murganti F, & Bergmann O (2020). Cardiomyocyte renewal in the failing heart: Lessons from the neonate? Biophysical Reviews, 12(4), 785–787. 10.1007/s12551-020-00739-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolejsi T, Delgobo M, Schuetz T, Tortola L, Heinze KG, Hofmann U, Frantz S, Bauer A, Ruschitzka F, Penninger JM, Campos Ramos G, & Haubner BJ (2022). Adult T-cells impair neonatal cardiac regeneration. European Heart Journal, 43(28), 2698–2709. 10.1093/eurheartj/ehac153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RR, Louey S, & Thornburg KL (2022). Intrauterine growth restriction elevates circulating acylcarnitines and suppresses fatty acid metabolism genes in the fetal sheep heart. The Journal of Physiology, 600(3), 655–670. 10.1113/JP281415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW (2013). Aneuploidy, polyploidy and ploidy reversal in the liver. Seminars in Cell & Developmental Biology, 24(4), 347–356. 10.1016/j.semcdb.2013.01.003 [DOI] [PubMed] [Google Scholar]
- D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, & Tzahor E (2015). ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nature Cell Biology, 17(5), Article 5. 10.1038/ncb3149 [DOI] [PubMed] [Google Scholar]
- Edgar BA, Zielke N, & Gutierrez C (2014). Endocycles: A recurrent evolutionary innovation for post-mitotic cell growth. Nature Reviews. Molecular Cell Biology, 15(3), 197–210. 10.1038/nrm3756 [DOI] [PubMed] [Google Scholar]
- Elhelaly WM, Cardoso AC, Pereira AHM, Elnawasany A, Ebrahimi S, Nakada Y, & Sadek HA (2019). C-Kit Cells Do Not Significantly Contribute To Cardiomyogenesis During Neonatal Heart Regeneration. Circulation, 139(4), 559–561. 10.1161/CIRCULATIONAHA.117.033150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, & Keating MT (2006). Anillin localization defect in cardiomyocyte binucleation. Journal of Molecular and Cellular Cardiology, 41(4), 601–612. 10.1016/j.yjmcc.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Eroglu E, Yen CYT, Tsoi Y-L, Witman N, Elewa A, Joven Araus A, Wang H, Szattler T, Umeano CH, Sohlmér J, Goedel A, Simon A, & Chien KR (2022). Epicardium-derived cells organize through tight junctions to replenish cardiac muscle in salamanders. Nature Cell Biology, 24(5), 645–658. 10.1038/s41556-022-00902-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Travisano S, Pearson CA, Lien C-L, & Harrison MRM (2021). The Lymphatic System in Zebrafish Heart Development, Regeneration and Disease Modeling. Journal of Cardiovascular Development and Disease, 8(2), 21. 10.3390/jcdd8020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG (2008). The immune system and cardiac repair (review). Pharmacological Research : The Official Journal of the Italian Pharmacological Society, 58(2), 88–111. 10.1016/j.phrs.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R, Marín-Juez R, El-Sammak H, Beisaw A, Ramadass R, Kuenne C, Guenther S, Konzer A, Bhagwat AM, Graumann J, & Stainier DY (2020). Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish. EMBO Reports, 21(8), e49752. 10.15252/embr.201949752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung THW, Yang KY, & Lui KO (2020). An emerging role of regulatory T-cells in cardiovascular repair and regeneration. Theranostics, 10(20), 8924–8938. 10.7150/thno.47118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire J, Varholick JA, Rana S, Sunshine MD, Doré S, Barbazuk WB, Fuller DD, Maden M, & Simmons CS (2021). Spiny mouse (Acomys): An emerging research organism for regenerative medicine with applications beyond the skin. NPJ Regenerative Medicine, 6(1), 1. 10.1038/s41536-020-00111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan P, Patterson M, Velasquez A, Wang K, Tian D, Windle JJ, Tao G, Judge DP, Makita T, Park TJ, & Sucov HM (2019). Tnni3k alleles influence ventricular mononuclear diploid cardiomyocyte frequency. PLoS Genetics, 15(10), e1008354. 10.1371/journal.pgen.1008354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan P, Patterson M, Watanabe H, Wang K, Edmonds RA, Reinholdt LG, & Sucov HM (2020). Allelic variants between mouse substrains BALB/cJ and BALB/cByJ influence mononuclear cardiomyocyte composition and cardiomyocyte nuclear ploidy. Scientific Reports, 10, 7605. 10.1038/s41598-020-64621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancz D, Raftrey BC, Perlmoter G, Marín-Juez R, Semo J, Matsuoka RL, Karra R, Raviv H, Moshe N, Addadi Y, Golani O, Poss KD, Red-Horse K, Stainier DY, & Yaniv K (2019). Distinct origins and molecular mechanisms contribute to lymphatic formation during cardiac growth and regeneration. ELife, 8, e44153. 10.7554/eLife.44153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemberling M, Bailey TJ, Hyde DR, & Poss KD (2013). The zebrafish as a model for complex tissue regeneration. Trends in Genetics : TIG, 29(11), 10.1016/j.tig.2013.07.003. 10.1016/j.tig.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemberling M, Karra R, Dickson AL, & Poss KD (2015). Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. ELife, 4, e05871. 10.7554/eLife.05871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M, Barad L, Novak A, Reiter I, Itskovitz-Eldor J, Binah O, & Popescu L (2011). Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: Comparative ultrastructure. Journal of Cellular and Molecular Medicine, 15(11), 2539–2551. 10.1111/j.1582-4934.2011.01417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, & Rosenthal NA (2013). Macrophages are required for adult salamander limb regeneration. Proceedings of the National Academy of Sciences of the United States of America, 110(23), 9415–9420. 10.1073/pnas.1300290110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Jiang Z, Zagidullin N, Liu T, & Cai B (2021). Regulation of cardiomyocyte fate plasticity: A key strategy for cardiac regeneration. Signal Transduction and Targeted Therapy, 6, 31. 10.1038/s41392-020-00413-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Rosa JM, Martín V, Peralta M, Torres M, & Mercader N (2011). Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development, 138(9), 1663–1674. 10.1242/dev.060897 [DOI] [PubMed] [Google Scholar]
- González-Rosa JM, Peralta M, & Mercader N (2012). Pan-epicardial lineage tracing reveals that epicardium derived cells give rise to myofibroblasts and perivascular cells during zebrafish heart regeneration. Developmental Biology, 370(2), 173–186. 10.1016/j.ydbio.2012.07.007 [DOI] [PubMed] [Google Scholar]
- González-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, & Burns CG (2018). Myocardial polyploidization creates a barrier to heart regeneration in zebrafish. Developmental Cell, 44(4), 433–446.e7. 10.1016/j.devcel.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassam-Rowe A, Ou X, & Lei M (2020). Novel cardiac cell subpopulations: Pnmt-derived cardiomyocytes. Open Biology, 10(8), 200095. 10.1098/rsob.200095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas J, Haag M, Johnson A, Manalo T, Roell J, Das TL, Brown E, Burns AR, & Lafontant PJ (2014). Cardiac repair and regenerative potential in the goldfish (Carassius auratus) heart. Comparative Biochemistry and Physiology. Toxicology & Pharmacology : CBP, 163, 14–23. 10.1016/j.cbpc.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, Cosentino M, & Venco A (2011). Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thrombosis and Haemostasis, 106(4), 591–599. 10.1160/TH11-02-0096 [DOI] [PubMed] [Google Scholar]
- Guo Y, & Pu W (2020). Cardiomyocyte Maturation: New Phase in Development (review). Circulation Research, 126(8), 1086–1106. 10.1161/CIRCRESAHA.119.315862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MR, Feng X, Mo G, Aguayo A, Villafuerte J, Yoshida T, Pearson CA, Schulte-Merker S, & Lien C-L (2019). Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. ELife, 8, e42762. 10.7554/eLife.42762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, & Penninger JM (2012). Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY), 4(12), 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, & Martin JF (2013). Hippo signaling impedes adult heart regeneration. Development (Cambridge, England), 140(23), 4683–4690. 10.1242/dev.102798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, & Martin JF (2011). Hippo Pathway Inhibits Wnt Signaling to Restrain Cardiomyocyte Proliferation and Heart Size. Science (New York, N.Y.), 332(6028), 458–461. 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdrich BJ, Danzer E, Davey MG, Allukian M, Englefield V, Gorman JH, Gorman RC, & Liechty KW (2010). Regenerative healing following fetal myocardial infarction. European Journal of Cardio-Thoracic Surgery : Official Journal of the European Association for Cardio-Thoracic Surgery, 38(6), 691–698. 10.1016/j.ejcts.2010.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Doengi M, Becker A, Kimura K, Voeltz N, Stein V, & Fleischmann BK (2018). Midbody Positioning and Distance Between Daughter Nuclei Enable Unequivocal Identification of Cardiomyocyte Cell Division in Mice. Circulation Research, 123(9), 1039–1052. 10.1161/CIRCRESAHA.118.312792 [DOI] [PubMed] [Google Scholar]
- Hille S, Dierck F, Kühl C, Sosna J, Adam-Klages S, Adam D, Lüllmann-Rauch R, Frey N, & Kuhn C (2016). Dyrk1a regulates the cardiomyocyte cell cycle via D-cyclin-dependent Rb/E2f-signalling. Cardiovascular Research, 110(3), 381–394. 10.1093/cvr/cvw074 [DOI] [PubMed] [Google Scholar]
- Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J, Smith M, Gillett E, Muroy SE, Schmid T, Wilson E, Field KA, Reeder DM, Maden M, Yartsev MM, … Huang GN (2019). Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science (New York, N.Y.), 364(6436), 184–188. 10.1126/science.aar2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, & Steffens S (2017). Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. European Heart Journal, 38(3), 187–197. 10.1093/eurheartj/ehw002 [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S, Bosnjak ZJ, & Bai X (2019). Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells, 8(9), 1095. 10.3390/cells8091095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-K, Coughlin M, & Mitchison TJ (2012). Midbody assembly and its regulation during cytokinesis. Molecular Biology of the Cell, 23(6), 1024–1034. 10.1091/mbc.E11-08-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Thatcher JE, McAnally J, Kong Y, Qi X, Tan W, DiMaio JM, Amatruda JF, Gerard RD, Hill JA, Bassel-Duby R, & Olson EN (2012). C/EBP Transcription Factors Mediate Epicardial Activation During Heart Development and Injury. Science (New York, N.Y.), 338(6114), 1599–1603. 10.1126/science.1229765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W-C, Yang C-C, Chen I-H, Liu Y-ML, Chang S-J, & Chuang Y-J (2013). Treatment of Glucocorticoids Inhibited Early Immune Responses and Impaired Cardiac Repair in Adult Zebrafish. PLoS ONE, 8(6), e66613. 10.1371/journal.pone.0066613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui SP, Sheng DZ, Sugimoto K, Gonzalez-Rajal A, Nakagawa S, Hesselson D, & Kikuchi K (2017). Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Developmental Cell, 43(6), 659–672.e5. 10.1016/j.devcel.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Ito K, Morioka M, Kimura S, Tasaki M, Inohaya K, & Kudo A (2014). Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Developmental Dynamics, 243(9), 1106–1115. 10.1002/dvdy.24154 [DOI] [PubMed] [Google Scholar]
- Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin A. Yu., Fleischmann BK, & Kotlikoff MI (2012). C-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proceedings of the National Academy of Sciences of the United States of America, 109(33), 13380–13385. 10.1073/pnas.1208114109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewhurst K, & McLaughlin KA (2015). Beyond the Mammalian Heart: Fish and Amphibians as a Model for Cardiac Repair and Regeneration (review). Journal of Developmental Biology, 4(1), 1. 10.3390/jdb4010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kim H, Min S, Choi YS, Seo SJ, Jeong E, Kim SK, Lee H-A, Jo S-H, Park J-H, Park B-W, Sim W-S, Kim J-J, Ban K, Kim Y-G, Park H-J, & Cho S-W (n.d.). Three-dimensional heart extracellular matrix enhances chemically induced direct cardiac reprogramming. Science Advances, 8(50), eabn5768. 10.1126/sciadv.abn5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Yang Y, Bian Z, Schena G, Li Y, Zhang X, Eaton DM, Gross P, Angheloiu A, Shaik A, Foster M, Berretta R, Kubo H, Mohsin S, Tian Y, & Houser SR (2023). Systemic Hypoxemia Induces Cardiomyocyte Hypertrophy and Right Ventricular Specific Induction of Proliferation. Circulation Research, 132(6), 723–740. 10.1161/CIRCRESAHA.122.321604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Kamna D, Loturco D, Kailey J, & Brown LD (2018). IUGR impairs cardiomyocyte growth and maturation in fetal sheep. The Journal of Endocrinology, 239(2), 253–265. 10.1530/JOE-18-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Louey S, Giraud GD, Thornburg KL, & Faber JJ (2015). Timing of cardiomyocyte growth, maturation, and attrition in perinatal sheep. The FASEB Journal, 29(10), 4346–4357. 10.1096/fj.15-272013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Martí M, Raya A, & Belmonte JCI (2010). Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature, 464(7288), 606–609. 10.1038/nature08899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, & Murry CE (2020). Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine (review). Nature Reviews. Cardiology, 17(6), 341–359. 10.1038/s41569-019-0331-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, & Koch WJ (2016). c-kit+ Cardiac stem cells: Spontaneous creation or a perplexing reality. Circulation Research, 118(5), 783–785. 10.1161/CIRCRESAHA.115.308103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, & Poss KD (2011). Tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development (Cambridge, England), 138(14), 2895–2902. 10.1242/dev.067041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, & Poss KD (2011). Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Developmental Cell, 20(3), 397–404. 10.1016/j.devcel.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DYR, & Poss KD (2010). Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature, 464(7288), 601–605. 10.1038/nature08804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, & Poss KD (2012). Cardiac Regenerative Capacity and Mechanisms (review). Annual Review of Cell and Developmental Biology, 28, 719–741. 10.1146/annurev-cellbio-101011-155739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirillova A, Han L, Liu H, & Kühn B (2021). Polyploid cardiomyocytes: Implications for heart regeneration. Development (Cambridge, England), 148(14), dev199401. 10.1242/dev.199401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA, & Riley PR (2015). Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature, 522(7554), 62–67. 10.1038/nature14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans T, van Beijnum H, Roovers EF, Tomasso A, Malhotra D, Boeter J, Psathaki OE, Versteeg D, van Rooij E, & Bartscherer K (2021). Ischemic tolerance and cardiac repair in the spiny mouse (Acomys). NPJ Regenerative Medicine, 6, 78. 10.1038/s41536-021-00188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou CY-C, Lau SL-Y, Au K-W, Leung P-Y, Chim SS-C, Fung K-P, Waye MM-Y, & Tsui SK-W (2010). Epigenetic regulation of neonatal cardiomyocytes differentiation. Biochemical and Biophysical Research Communications, 400(2), 278–283. 10.1016/j.bbrc.2010.08.064 [DOI] [PubMed] [Google Scholar]
- Kraus L (2022). Targeting Epigenetic Regulation of Cardiomyocytes through Development for Therapeutic Cardiac Regeneration after Heart Failure. International Journal of Molecular Sciences, 23(19), 11878. 10.3390/ijms231911878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan J, Persons JL, Peuß R, Hassan H, Kenzior A, Xiong S, Olsen L, Maldonado E, Kowalko JE, & Rohner N (2020). Comparative transcriptome analysis of wild and lab populations of Astyanax mexicanus uncovers differential effects of environment and morphotype on gene expression. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 334(7–8), 530–539. 10.1002/jez.b.22933 [DOI] [PubMed] [Google Scholar]
- Laflamme MA, & Murry CE (2011). Heart Regeneration. Nature, 473(7347), 326–335. 10.1038/nature10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S-L, Marín-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, & Stainier DY (2017). Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. ELife, 6, e25605. 10.7554/eLife.25605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, & Mann DL (2014). Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proceedings of the National Academy of Sciences of the United States of America, 111(45), 16029–16034. 10.1073/pnas.1406508111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, Segura A, Willerson JT, & Martin JF (2017). Hippo Pathway Deficiency Reverses Systolic Heart Failure Post-Infarction. Nature, 550(7675), 260–264. 10.1038/nature24045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M, & Engel FB (2019). Pseudo-bipolar spindle formation and cell division in postnatal binucleated cardiomyocytes. Journal of Molecular and Cellular Cardiology, 134, 69–73. 10.1016/j.yjmcc.2019.07.005 [DOI] [PubMed] [Google Scholar]
- Leone M, Musa G, & Engel FB (2018). Cardiomyocyte binucleation is associated with aberrant mitotic microtubule distribution, mislocalization of RhoA and IQGAP3, as well as defective actomyosin ring anchorage and cleavage furrow ingression. Cardiovascular Research, 114(8), 1115–1131. 10.1093/cvr/cvy056 [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, & Poss KD (2006). A Dynamic Epicardial Injury Response Supports Progenitor Cell Activity during Zebrafish Heart Regeneration. Cell, 127(3), 607–619. 10.1016/j.cell.2006.08.052 [DOI] [PubMed] [Google Scholar]
- Leucker TM, Bienengraeber M, Muravyeva M, Baotic I, Weihrauch D, Brzezinska AK, Warltier DC, Kersten JR, & Pratt PF (2011). Endothelial–cardiomyocyte crosstalk enhances pharmacological cardioprotection. Journal of Molecular and Cellular Cardiology, 51(5), 803–811. 10.1016/j.yjmcc.2011.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zong W, Zhang M, Tu Y, Zhou Q, Ni M, Li Z, Liu H, & Zhang J (2019). Increased Ratio of Circulating T-Helper 1 to T-Helper 2 Cells and Severity of Coronary Artery Disease in Patients with Acute Myocardial Infarction: A Prospective Observational Study. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research, 25, 6034–6042. 10.12659/MSM.913891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, & Gerdes AM (1996). Rapid Transition of Cardiac Myocytes from Hyperplasia to Hypertrophy During Postnatal Development. Journal of Molecular and Cellular Cardiology, 28(8), 1737–1746. 10.1006/jmcc.1996.0163 [DOI] [PubMed] [Google Scholar]
- Li J, Yang KY, Tam RCY, Chan VW, Lan HY, Hori S, Zhou B, & Lui KO (2019). Regulatory T-cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner. Theranostics, 9(15), 4324–4341. 10.7150/thno.32734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Dong W, Lv L, Guo H, Yang J, Zhao H, Huang R, Yuan Z, Chen Y, Feng S, Zheng X, Huang J, Huang W, Qi X, & Cai D (2017). Heart regeneration in adult Xenopus tropicalis after apical resection. Cell & Bioscience, 7, 70. 10.1186/s13578-017-0199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AG, & Seebacher F (2014). The evolution of endothermy is explained by thyroid hormone-mediated responses to cold in early vertebrates. Journal of Experimental Biology, 217(10), 1642–1648. 10.1242/jeb.088880 [DOI] [PubMed] [Google Scholar]
- Lock MC, Darby JRT, Soo JY, Brooks DA, Perumal SR, Selvanayagam JB, Seed M, Macgowan CK, Porrello ER, Tellam RL, & Morrison JL (2019). Differential Response to Injury in Fetal and Adolescent Sheep Hearts in the Immediate Post-myocardial Infarction Period. Frontiers in Physiology, 10, 208. 10.3389/fphys.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyra-Leite DM, Gutiérrez-Gutiérrez Ó, Wang M, Zhou Y, Cyganek L, & Burridge PW (2022). A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protocols, 3(3), 101560. 10.1016/j.xpro.2022.101560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, & Sadek HA (2013). Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature, 497(7448), 249–253. 10.1038/nature12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Juez R, El-Sammak H, Helker CSM, Kamezaki A, Mullapuli ST, Bibli S-I, Foglia MJ, Fleming I, Poss KD, & Stainier DYR (2019). Coronary Revascularization During Heart Regeneration Is Regulated by Epicardial and Endocardial Cues and Forms a Scaffold for Cardiomyocyte Repopulation. Developmental Cell, 51(4), 503–515.e4. 10.1016/j.devcel.2019.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Juez R, Marass M, Gauvrit S, Rossi A, Lai S-L, Materna SC, Black BL, & Stainier DYR (2016). Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proceedings of the National Academy of Sciences of the United States of America, 113(40), 11237–11242. 10.1073/pnas.1605431113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz D. g., Oberpriller J. o., & Oberpriller J. c. (1998). Comparison of mitosis in binucleated and mononucleated newt cardiac myocytes. The Anatomical Record, 251(2), 245–255. [DOI] [PubMed] [Google Scholar]
- Meckert PC, Rivello HG, Vigliano C, González P, Favaloro R, & Laguens R (2005). Endomitosis and polyploidization of myocardial cells in the periphery of human acute myocardial infarction. Cardiovascular Research, 67(1), 116–123. 10.1016/j.cardiores.2005.02.017 [DOI] [PubMed] [Google Scholar]
- Mehdipour M, Park S, & Huang GN (2023). Unlocking cardiomyocyte renewal potential for myocardial regeneration therapy. Journal of Molecular and Cellular Cardiology, 177, 9–20. 10.1016/j.yjmcc.2023.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Montes I, Abdisalaam S, Xiao F, Lam NT, Mukherjee S, Szweda LI, Asaithamby A, & Sadek HA (2021). Mitochondrial fatty acid utilization increases chromatin oxidative stress in cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America, 118(34), e2101674118. 10.1073/pnas.2101674118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missinato MA, Zuppo DA, Watkins SC, Bruchez MP, & Tsang M (2021). Zebrafish heart regenerates after chemoptogenetic cardiomyocyte depletion. Developmental Dynamics : An Official Publication of the American Association of Anatomists, 250(7), 986–1000. 10.1002/dvdy.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed TMA, Ang Y-S, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, & Srivastava D (2018). Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell, 173(1), 104–116.e12. 10.1016/j.cell.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, Nakashima H, Akiyama M, Wada R, Inagawa K, Nishiyama T, Kaneda R, Fukuda T, Takeda S, Tohyama S, Hashimoto H, Kawamura Y, Goshima N, Aeba R, … Ieda M (2014). MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. The EMBO Journal, 33(14), 1565–1581. 10.15252/embj.201387605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, & Pittet MJ (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of Experimental Medicine, 204(12), 3037–3047. 10.1084/jem.20070885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, … Sadek HA (2017). Hypoxia induces heart regeneration in adult mice. Nature, 541(7636), Article 7636. 10.1038/nature20173 [DOI] [PubMed] [Google Scholar]
- Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DIK, Lefer DJ, Graham RM, & Husain A (2014). A Proliferative Burst During Preadolescence Establishes the Final Cardiomyocyte Number. Cell, 157(4), 795–807. 10.1016/j.cell.2014.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberpriller JO, & Oberpriller JC (1974). Response of the adult newt ventricle to injury. Journal of Experimental Zoology, 187(2), Article 2. 10.1002/jez.1401870208 [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, & Anversa P (2001). Bone marrow cells regenerate infarcted myocardium. Nature, 410(6829), Article 6829. 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- Pandit SK, Westendorp B, & de Bruin A (2013). Physiological significance of polyploidization in mammalian cells. Trends in Cell Biology, 23(11), 556–566. 10.1016/j.tcb.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Park D-D, Gahr BM, Krause J, Rottbauer W, Zeller T, & Just S (2021). Long-Chain Acyl-Carnitines Interfere with Mitochondrial ATP Production Leading to Cardiac Dysfunction in Zebrafish. International Journal of Molecular Sciences, 22(16), 8468. 10.3390/ijms22168468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi KBS, Nakajima H, Nakajima HO, Soonpaa MH, & Field LJ (2005). Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circulation Research, 96(1), 110–118. 10.1161/01.RES.0000152326.91223.4F [DOI] [PubMed] [Google Scholar]
- Patel V, Singh VP, Pinnamaneni JP, Sanagasetti D, Olive J, Mathison M, Cooney A, Flores ER, Crystal RG, Yang J, & Rosengart TK (2018). P63 Silencing Induces Reprogramming of Cardiac Fibroblasts into Cardiomyocyte –Like Cells. The Journal of Thoracic and Cardiovascular Surgery, 156(2), 556–565.e1. 10.1016/j.jtcvs.2018.03.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien C-L, Makita T, Lusis AJ, Kumar SR, & Sucov HM (2017). Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nature Genetics, 49(9), 1346–1353. 10.1038/ng.3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Chan DN, Ha I, Case D, Cui Y, Handel BV, Mikkola HK, & Lowry WE (2012). Defining the nature of human pluripotent stem cell progeny. Cell Research, 22(1), 178–193. 10.1038/cr.2011.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payumo AY, Chen X, Hirose K, Chen X, Hoang A, Khyeam S, Yu H, Wang J, Chen Q, Powers N, Chen L, Bigley RB, Lovas J, Hu G, & Huang GN (2021). Adrenergic-thyroid hormone interactions drive postnatal thermogenesis and loss of mammalian heart regenerative capacity. Circulation, 144(12), 1000–1003. 10.1161/CIRCULATIONAHA.121.054846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Shindo K, Donahue RR, Gao E, Ahern BM, Levitan BM, Tripathi H, Powell D, Noor A, Elmore GA, Satin J, Seifert AW, & Abdel-Latif A (2021). Adult spiny mice (Acomys) exhibit endogenous cardiac recovery in response to myocardial infarction. Npj Regenerative Medicine, 6(1), Article 1. 10.1038/s41536-021-00186-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuß R, Box AC, Chen S, Wang Y, Tsuchiya D, Persons JL, Kenzior A, Maldonado E, Krishnan J, Scharsack JP, Slaughter BD, & Rohner N (2020). Adaptation to low parasite abundance affects immune investment and immunopathological responses of cavefish. Nature Ecology & Evolution, 4(10), 1416–1430. 10.1038/s41559-020-1234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnamaneni JP, Singh VP, Kim MB, Ryan CT, Pugazenthi A, Sanagasetti D, Mathison M, Yang J, & Rosengart TK (2022). P63 silencing induces epigenetic modulation to enhance human cardiac fibroblast to cardiomyocyte-like differentiation. Scientific Reports, 12, 11416. 10.1038/s41598-022-15559-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzotti BD, Ganapathy B, Haubner B, Penninger J, & Kühn B (2016). A cryoinjury model in neonatal mice for cardiac translational and regeneration research. Nature Protocols, 11(3), 542–552. 10.1038/nprot.2016.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, & Sadek HA (2011). Transient Regenerative Potential of the Neonatal Mouse Heart. Science (New York, N.Y.), 331(6020), 1078–1080. 10.1126/science.1200708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, & Sadek HA (2013). Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proceedings of the National Academy of Sciences of the United States of America, 110(1), 187–192. 10.1073/pnas.1208863110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, & Keating MT (2002). Heart regeneration in zebrafish. Science (New York, N.Y.), 298(5601), 2188–2190. 10.1126/science.1077857 [DOI] [PubMed] [Google Scholar]
- Potts HG, Stockdale WT, & Mommersteeg MTM (2021). Unlocking the Secrets of the Regenerating Fish Heart: Comparing Regenerative Models to Shed Light on Successful Regeneration. Journal of Cardiovascular Development and Disease, 8(1), 4. 10.3390/jcdd8010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, … Sadek HA (2014). The Oxygen Rich Postnatal Environment Induces Cardiomyocyte Cell Cycle Arrest Through DNA Damage Response. Cell, 157(3), 565–579. 10.1016/j.cell.2014.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Dasa O, Maden M, Vohra R, Batra A, Walter G, Yarrow JF, Aranda JM, Raizada MK, & Pepine CJ (2021). Functional heart recovery in an adult mammal, the spiny mouse. International Journal of Cardiology, 338, 196–203. 10.1016/j.ijcard.2021.06.015 [DOI] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu J, & Srivastava D (2012). In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature, 485(7400), 593–598. 10.1038/nature11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räsänen M, Sultan I, Paech J, Hemanthakumar KA, Yu W, He L, Tang J, Sun Y, Hlushchuk R, Huan X, Armstrong E, Khoma O-Z, Mervaala E, Djonov V, Betsholtz C, Zhou B, Kivelä R, & Alitalo K (2021). VEGF-B Promotes Endocardium-Derived Coronary Vessel Development and Cardiac Regeneration. Circulation, 143(1), 65–77. 10.1161/CIRCULATIONAHA.120.050635 [DOI] [PubMed] [Google Scholar]
- Rhee S, Paik DT, Yang JY, Nagelberg D, Williams I, Tian L, Roth R, Chandy M, Ban J, Belbachir N, Kim S, Zhang H, Phansalkar R, Wong KM, King DA, Valdez C, Winn VD, Morrison AJ, Wu JC, & Red-Horse K (2021). Endocardial/endothelial angiocrines regulate cardiomyocyte development and maturation and induce features of ventricular non-compaction. European Heart Journal, 42(41), 4264–4276. 10.1093/eurheartj/ehab298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross I, Omengan DB, Huang GN, & Payumo AY (2022). Thyroid hormone-dependent regulation of metabolism and heart regeneration. The Journal of Endocrinology, 252(3), R73–R84. 10.1530/JOE-21-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumyantsev PP (1977). Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. International Review of Cytology, 51, 186–273. [PubMed] [Google Scholar]
- Sakaguchi A, Nishiyama C, & Kimura W (2020). Cardiac regeneration as an environmental adaptation (review). Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1867(4), 118623. 10.1016/j.bbamcr.2019.118623 [DOI] [PubMed] [Google Scholar]
- Sawamiphak S, Kontarakis Z, Filosa A, Reischauer S, & Stainier DYR (2017). Transient cardiomyocyte fusion regulates cardiac development in zebrafish. Nature Communications, 8, 1525. 10.1038/s41467-017-01555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel K, Wu C-C, Kurth T, & Weidinger G (2011). Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PloS One, 6(4), e18503. 10.1371/journal.pone.0018503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdek P, Zhao P, Wang Y, Huang C, Ko CY, Butler PC, Weiss JN, & MacLellan WR (2011). Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. The Journal of Cell Biology, 194(3), 407–423. 10.1083/jcb.201012049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-D, Guerquin-Kern J-L, Lechene CP, & Lee RT (2013). Mammalian Heart Renewal by Preexisting Cardiomyocytes. Nature, 493(7432), 433–436. 10.1038/nature11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Pinnamaneni JP, Pugazenthi A, Sanagasetti D, Mathison M, Martin JF, Yang J, & Rosengart TK (2021). Hippo Pathway Effector Tead1 Induces Cardiac Fibroblast to Cardiomyocyte Reprogramming. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 10(24), e022659. 10.1161/JAHA.121.022659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Pinnamaneni JP, Pugazenthi A, Sanagasetti D, Mathison M, Wang K, Yang J, & Rosengart TK (2020). Enhanced Generation of Induced Cardiomyocytes Using a Small-Molecule Cocktail to Overcome Barriers to Cardiac Cellular Reprogramming. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease, 9(12), e015686. 10.1161/JAHA.119.015686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, & Riley PR (2011). De novo cardiomyocytes from within the activated adult heart after injury. Nature, 474(7353), 640–644. 10.1038/nature10188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, & Olson EN (2012). Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature, 485(7400), 599–604. 10.1038/nature11139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soonpaa MH, Kim KK, Pajak L, Franklin M, & Field LJ (1996). Cardiomyocyte DNA synthesis and binucleation during murine development. The American Journal of Physiology, 271(5 Pt 2), H2183–2189. 10.1152/ajpheart.1996.271.5.H2183 [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, Zebrowski DC, Platt C, Rosenzweig A, Engel FB, & Field LJ (2015). Cardiomyocyte Cell-Cycle Activity during Preadolescence. Cell, 163(4), 781–782. 10.1016/j.cell.2015.10.037 [DOI] [PubMed] [Google Scholar]
- Stockdale WT, Lemieux ME, Killen AC, Zhao J, Hu Z, Riepsaame J, Hamilton N, Kudoh T, Riley PR, van Aerle R, Yamamoto Y, & Mommersteeg MTM (2018). Heart Regeneration in the Mexican Cavefish. Cell Reports, 25(8), 1997–2007.e7. 10.1016/j.celrep.2018.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov HM, Gu Y, Thomas S, Li P, & Pashmforoush M (2009). Epicardial Control of Myocardial Proliferation and Morphogenesis. Pediatric Cardiology, 30(5), 617–625. 10.1007/s00246-009-9391-8 [DOI] [PubMed] [Google Scholar]
- Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang G-Y, Hajjar RJ, Zhou B, Moon A, & Cai C-L (2015). Resident c-kit+ cells in the heart are not cardiac stem cells. Nature Communications, 6, 8701. 10.1038/ncomms9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Peterson EA, Wang AZ, Ou J, Smith KE, Poss KD, & Wang J (2022). Hapln1 defines an epicardial cell subpopulation required for cardiomyocyte expansion during heart morphogenesis and regeneration. Circulation, 146(1), 48–63. 10.1161/CIRCULATIONAHA.121.055468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Li Y, & Jin J (2021). A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduction and Targeted Therapy, 6, 79. 10.1038/s41392-020-00455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift SK, Purdy AL, Kolell ME, Andresen KG, Lahue C, Buddell T, Akins KA, Rau CD, O’Meara CC, & Patterson M (2023). Cardiomyocyte ploidy is dynamic during postnatal development and varies across genetic backgrounds. Development, 150(7), dev201318. 10.1242/dev.201318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara N, Brush M, & Kawakami Y (2016). Cell Migration During Heart Regeneration in Zebrafish. Developmental Dynamics : An Official Publication of the American Association of Anatomists, 245(7), 774–787. 10.1002/dvdy.24411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, & Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Tang T-T, Yuan J, Zhu Z-F, Zhang W-C, Xiao H, Xia N, Yan X-X, Nie S-F, Liu J, Zhou S-F, Li J-J, Yao R, Liao M-Y, Tu X, Liao Y-H, & Cheng X (2011). Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Research in Cardiology, 107(1), 232. 10.1007/s00395-011-0232-6 [DOI] [PubMed] [Google Scholar]
- Tang Y, Aryal S, Geng X, Zhou X, Fast VG, Zhang J, Lu R, & Zhou Y (2022). TBX20 Improves Contractility and Mitochondrial Function During Direct Human Cardiac Reprogramming. Circulation, 146(20), 1518–1536. 10.1161/CIRCULATIONAHA.122.059713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, & de Kleijn DPV (2012). The innate immune response in reperfused myocardium (review). Cardiovascular Research, 94(2), 276–283. 10.1093/cvr/cvs018 [DOI] [PubMed] [Google Scholar]
- Tseng A-S, Engel FB, & Keating MT (2006). The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chemistry & Biology, 13(9), 957–963. 10.1016/j.chembiol.2006.08.004 [DOI] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin S-CJ, Middleton RC, Marbán E, & Molkentin JD (2014). C-kit+ Cells Minimally Contribute Cardiomyocytes to the Heart. Nature, 509(7500), 337–341. 10.1038/nature13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer Y, Mizrachi E, & Marchal K (2017). The evolutionary significance of polyploidy. Nature Reviews Genetics, 18(7), Article 7. 10.1038/nrg.2017.26 [DOI] [PubMed] [Google Scholar]
- van den Berg CW, Okawa S, Chuva de Sousa Lopes SM, van Iperen L, Passier R, Braam SR, Tertoolen LG, del Sol A, Davis RP, & Mummery CL (2015). Transcriptome of human foetal heart compared with cardiomyocytes from pluripotent stem cells. Development, 142(18), 3231–3238. 10.1242/dev.123810 [DOI] [PubMed] [Google Scholar]
- Velayutham N, Alfieri CM, Agnew EJ, Riggs KW, Baker RS, Ponny SR, Zafar F, & Yutzey KE (2020). Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine. Journal of Molecular and Cellular Cardiology, 146, 95–108. 10.1016/j.yjmcc.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira JM, Norman S, Villa del Campo C, Cahill TJ, Barnette DN, Gunadasa-Rohling M, Johnson LA, Greaves DR, Carr CA, Jackson DG, & Riley PR (2018). The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. The Journal of Clinical Investigation, 128(8), 3402–3412. 10.1172/JCI97192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinten-Johansen J (2004). Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury (review). Cardiovascular Research, 61(3), 481–497. 10.1016/j.cardiores.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, … null, null. (2020). Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation, 141(9), e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Vivien CJ, Hudson JE, & Porrello ER (2016). Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regenerative Medicine, 1, 16012. 10.1038/npjregenmed.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivien CJ, Pichol-Thievend C, Sim CB, Smith JB, Bower NI, Hogan BM, Hudson JE, Francois M, & Porrello ER (2019). Vegfc/d-dependent regulation of the lymphatic vasculature during cardiac regeneration is influenced by injury context. NPJ Regenerative Medicine, 4, 18. 10.1038/s41536-019-0079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VYa B, Sarkisov DS, Arefyeva AM, Panova NW, & Gvasava IG (1994). Polyploidy in cardiac myocytes of normal and hypertrophic human hearts; range of values. Virchows Archiv: An International Journal of Pathology, 424(4), 429–435. 10.1007/BF00190566 [DOI] [PubMed] [Google Scholar]
- Walsh S, Pontén A, Fleischmann BK, & Jovinge S (2010). Cardiomyocyte cell cycle control and growth estimation in vivo—An analysis based on cardiomyocyte nuclei. Cardiovascular Research, 86(3), 365–373. 10.1093/cvr/cvq005 [DOI] [PubMed] [Google Scholar]
- Wang J, Cao J, Dickson AL, & Poss KD (2015). Epicardial regeneration is guided by cardiac outflow tract and Hh signaling. Nature, 522(7555), 226–230. 10.1038/nature14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Panáková D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin Y-F, Sabeh MK, Werdich AA, Yelon D, MacRae CA, & Poss KD (2011). The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development (Cambridge, England), 138(16), 3421–3430. 10.1242/dev.068601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-J, Chen F, Lau JTY, & Hu Y-P (2017). Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death & Disease, 8(5), e2805. 10.1038/cddis.2017.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng CS, Zhou L, Zhou B, Duan DD, Chen X, Houser SR, & Zeng C (2017). Dedifferentiation, Proliferation and Redifferentiation of Adult Mammalian Cardiomyocytes after Ischemic Injury. Circulation, 136(9), 834–848. 10.1161/CIRCULATIONAHA.116.024307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cui M, Shah AM, Tan W, Liu N, Bassel-Duby R, & Olson EN (2020). Cell-Type-Specific Gene Regulatory Networks Underlying Murine Neonatal Heart Regeneration at Single-Cell Resolution. Cell Reports, 33(10), 108472. 10.1016/j.celrep.2020.108472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, Tian X, Liu Q, Wang A, Matsuura Y, Bushway P, Cai W, Savchenko A, Mahmoudi M, Schneider MD, … Ruiz-Lozano P (2015). Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature, 525(7570), 479–485. 10.1038/nature15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe NM, Sachs D, Shewale B, Gonzalez DM, Dhanan-Krishnan P, Torre D, LaMarca E, Raimo S, Dariolli R, Serasinghe MN, Mayourian J, Sebra R, Beaumont K, Iyengar S, French DL, Hansen A, Eschenhagen T, Chipuk JE, Sobie EA, … Dubois NC (2022). PPARdelta activation induces metabolic and contractile maturation of human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell, 29(4), 559–576.e7. 10.1016/j.stem.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills AA, Holdway JE, Major RJ, & Poss KD (2008). Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development, 135(1), 183–192. 10.1242/dev.010363 [DOI] [PubMed] [Google Scholar]
- Wu P, Deng G, Sai X, Guo H, Huang H, & Zhu P (2021). Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Bioscience Reports, 41(6), BSR20200833. 10.1042/BSR20200833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N, Jiao J, Tang T-T, Lv B-J, Lu Y-Z, Wang K-J, Zhu Z-F, Mao X-B, Nie S-F, Wang Q, Tu X, Xiao H, Liao Y-H, Shi G-P, & Cheng X (2015). Activated regulatory T-cells attenuate myocardial ischaemia/reperfusion injury through a CD39-dependent mechanism. Clinical Science (London, England: 1979), 128(10), 679–693. 10.1042/CS20140672 [DOI] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, & Olson EN (2013). Hippo pathway effector Yap promotes cardiac regeneration. Proceedings of the National Academy of Sciences of the United States of America, 110(34), 13839–13844. 10.1073/pnas.1313192110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Zhang H, Fan Q, Hu J, Tao R, Chen Q, Iwakura Y, Shen W, Lu L, Zhang Q, & Zhang R (2017). Dectin-2 Deficiency Modulates Th1 Differentiation and Improves Wound Healing After Myocardial Infarction. Circulation Research, 120(7), 1116–1129. 10.1161/CIRCRESAHA.116.310260 [DOI] [PubMed] [Google Scholar]
- Yang X, Rodriguez ML, Leonard A, Sun L, Fischer KA, Wang Y, Ritterhoff J, Zhao L, Kolwicz SC, Pabon L, Reinecke H, Sniadecki NJ, Tian R, Ruohola-Baker H, Xu H, & Murry CE (2019). Fatty Acids Enhance the Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cell Reports, 13(4), 657–668. 10.1016/j.stemcr.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, D’Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, Tee GZ, Pua CJ, Pena EM, Cheng RB, Chen WC, Abdurrachim D, Lalic J, Tan RS, Lee TH, Zhang J, & Cook SA (2018). Early Regenerative Capacity in the Porcine Heart. Circulation, 138(24), 2798–2808. 10.1161/CIRCULATIONAHA.117.031542 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, & Thomson JA (2007). Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science, 318(5858), 1917–1920. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- Zebrowski DC, & Engel FB (2013). The Cardiomyocyte Cell Cycle in Hypertrophy, Tissue Homeostasis, and Regeneration. In Nilius B, Amara SG, Gudermann T, Jahn R, Lill R, Offermanns S, & Petersen OH (Eds.), Reviews of Physiology, Biochemistry and Pharmacology, Vol. 165 (pp. 67–96). Springer International Publishing. 10.1007/112_2013_12 [DOI] [PubMed] [Google Scholar]
- Zgheib C, Allukian MW, Xu J, Morris MW, Caskey RC, Herdrich BJ, Hu J, Gorman JH, Gorman RC, & Liechty KW (2014). Mammalian Fetal Cardiac Regeneration Following Myocardial Infarction is Associated with Differential Gene Expression Compared to the Adult. The Annals of Thoracic Surgery, 97(5), 1643–1650. 10.1016/j.athoracsur.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, & Kamp TJ (2009). Functional Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Circulation Research, 104(4), e30–e41. 10.1161/CIRCRESAHA.108.192237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, He H, Ma Q, Oh J-H, Butterfield C, Lin R-Z, Melero-Martin JM, Dolmatova E, Duffy HS, von Gise A, Zhou P, Hu YW, Wang G, Zhang B, Wang L, Hall JL, Moses MA, McGowan FX, & Pu WT (2011). Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. The Journal of Clinical Investigation, 121(5), 1894–1904. 10.1172/JCI45529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, & Pu WT (2008). Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature, 454(7200), 109–113. 10.1038/nature07060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, Song J, Yuan A, Shanmughapriya S, Guo Y, Gao E, Koch W, Woodgett JR, Muniswamy M, Kishore R, Lal H, & Force T (2016). Loss of Adult Cardiac Myocyte GSK-3 Leads to Mitotic Catastrophe Resulting in Fatal Dilated Cardiomyopathy. Circulation Research, 118(8), 1208–1222. 10.1161/CIRCRESAHA.116.308544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, Hunter JD, Borovjagin AV, Walcott GP, Chen JY, Qin G, & Zhang J (2018). Regenerative Potential of Neonatal Porcine Hearts. Circulation, 138(24), 2809–2816. 10.1161/CIRCULATIONAHA.118.034886 [DOI] [PMC free article] [PubMed] [Google Scholar]


