Abstract
Background
Condition mediums have a potential role in oocyte development. In this study, we evaluated the effects of different mediums on the developmental potential of vitrified immature human oocyte after IVM and parthenogenesis by ionomycin.
Methods
Immature oocytes were collected from 184 women after vitrification/thawing and maturation, in three types of IVM mediums separately. Finally, 151 IVM MΙΙ oocytes were obtained and randomly divided into six groups and underwent the following intervention. Fresh and vitrified-thawing MΙΙ oocytes were activated after IVM in three conditioned mediums by ionomycin. Mediums included 1) Minimum Essential Medium Alpha (α-MEM) (as control medium), 2) α-MEM supplemented with supernatants of Mesenchyme bone marrow (B.M), 3) α-MEM with ovarian growth factors (O.F). Then, scoring of parthenote embryos was undertaken in accordance with pertinent morphological properties. Moreover, the expression of Bax and Bcl2 were determined in the parthenote embryos.
Result
Percentage of the degenerated oocyte, 2–4 cells, 4–8 cells, and 16 cells, was different in the experimental groups. Also, cytoplasmic maturation and blastocyst formation rates were significantly different (p < 0.05) between the control and the other mediums. The highest mRNA expression levels of Bcl2 and Bax genes in parthenotes were observed in the fIVM O.F and vIVM α-MEM mediums, respectively. vIVM, α-MEM and fIVM O.F showed the lowest expression of Bcl2 and Bax genes, respectively.
Conclusion
Our findings indicate that the O.F. medium had more potent effects on oocyte growth and cytoplasmic maturation up to the blastocyst stage with the highest expression level of the BCL2 gene and the lowest relative amount of the BAX gene in this medium. The results of the present study have been verified only for parthenogenetically activated embryos, and any positive effect of the environment on the egg/embryo fertilized with sperm requires more extensive studies.
Keywords: IVM, Conditioned medium, Parthenogenesis, Developmental potential, Oocyte activation
Background
In vitro fertilization (IVF) is an effective method for the treatment of infertility, as part of which mature eggs are collected from ovaries and fertilized by sperm in a laboratory. Then, the fertilized eggs are transferred to a uterus [1, 2]. On the other hand, approximately 15% of retrieved oocytes are meiotic immature [3, 4].
Cryopreservation and vitrification of immature oocytes and then in vitro maturation (IVM) have been suggested for conservative IVF management. Since, most IVM conventions have been improved by gonadotropins such as LH or FSH, the side effects and costs of gonadotropin stimulants have to be minimized [5–7].
The crucial part of this technique includes the maturation process itself and the selection of the base IVM medium, which is very influential [8]. Nuclear maturation offers traceability in a polar body, due to the absence of a direct indicator, cytoplasmic maturing is challenging to detect. Oocyte parthenogenesis in vitro occurs by replicated activation without any sperm involvement [9]. In vitro parthenogenesis can be prompted by chemical, mechanical and electrical stimulations, it is a direct indicator for the assessment of oocyte developmental competency [9–12].
Supernatants of mesenchyme stem cells (MSCs) involve various components including cytokines and growth factors, e.g., fibroblast growth factor, epidermal growth factor, leukemia inhibitory factor, etc. [13, 14]. On the other hand, prolonging the culture time by producing reactive oxygen species (ROS) has destructive effects on future oocyte development. Important genes such as BCL2-associated X protein (BAX) stimulate cell death and act as pro-apoptotic factors, while the B-cell CLL/lymphoma 2 (BCL2) acts an anti-apoptotic gene which stimulates the cell survival [14]. Moreover, qualitative assessment of culture medium assists with the evaluation of the expression of these genes in cells cultured in that medium [15].
It is well-known that growth factors and cytokines stimulate meiotic development and the courses related to IVM. The aim of the present study was to define the cytoplasmic maturation of the cultured vitrified immature human oocytes in conditioned mediums after nuclear maturation and then to follow their ability of developmental potential through parthenogenesis by chemical stimulations with ionomycin. Additionally, the study aimed to assess the mRNA expression levels of Bax and Bcl2 genes in parthenotes embryo-derived activated matured oocytes.
Methods
Chemical Materials
Hyaluronidase, phosphate-buffered saline (PBS), sucrose, ionomycin, and 6-dimethylaminopurine (DMAP) were purchased from Sigma-Aldrich Company (St Louis, MO, USA), ethylene glycol (EG) and dimethyl sulfoxide (DMSO) were purchased from Merck Company (Germany); culture flasks, tubes, and dishes were obtained from Falcon (BD Biosciences, San Jose, CA); all reagents and chemicals used were of analytical grade.
Study Design
This study involved MΙΙ oocytes (n = 266) obtained after thawing of immature oocytes and IVM, randomly in three conditioned mediums from 184 women (18–46 years), who were admitted during one-year time frame from 2019 to 2020 in the Afzalipour Infertility Center in Kerman, Iran. The study was conducted in full accordance with the required ethical considerations by the authors’ ethics committee, and necessary ethical approvals were sought. After 48 h, the maturation stage of the oocytes was assessed under an inverted microscope; 151 matured oocytes (MII) were randomly divided into six groups (fresh IVM (fIVM) oocytes and vitrified IVM (vIVM) oocytes located in three mediums). They were evaluated for cytoplasmic maturity and first cleavage competence of the activated oocytes was measured by mRNA expiration of BAX and BCL2 genes.
Oocyte Collection
The oocyte collection was conducted 36 h after the injection of 10,000 IU of hCG (Switzerland, IBSA Co) through laparoscopy. The nuclear maturity of the collected oocytes was evaluated by a stereomicroscope (Japan, Olympus Co). After denudation by using 80 IU hyaluronidase (USA, Sigma Co) and mechanical dissection with pipetting, the denuded oocytes were considered for nuclear maturity; according to extrusion of the first polar body, oocytes were labeled MΙΙ or immature (GV or MΙ). Immature oocytes were collected and vitrified for this study [5].
Vitrification of Immature Oocytes
Considering the Al-Hasani’s vitrification method (2007), the immature oocytes were frozen. Firstly, immature oocytes were located in an equilibration solution containing 7.5% (EG) ethylene glycol (Germany, Merck Co) and 7.5% (DMSO) dimethyl sulfoxide (Germany, Merck Co) in Ham’s F10 medium complemented with 20% human serum albumin (HSA) (USA, Plasbumin Co) at room temperature, for 5 to15 minutes. Subsequently, oocytes were submerged in the vitrification solution containing 15% EG and 15% DMSO with 0.5 M sucrose (USA, Sigma Co) in Ham’s F10 medium complemented with 20% (HAS) for 50–60 Seconds at room temperature. Then, instantly the oocytes were placed inside cryotops that were rushed into fluid nitrogen. Subsequently, the top was located on the cryotop and put into the cane. These samples were moved into the liquid nitrogen storing tank for several months [16].
Thawing of Immature Oocytes
Thawing of the oocytes was carried out by immersion of the cryotop in thawing five-stage solution: thawing solution (1 M sucrose in Ham’s F10 medium complemented with 20%(HSA)) for 50–60 seconds, dilution solution 1 (0.5 M sucrose in Ham’s F10 medium complemented with 20%(HSA) for 3 min, dilution solution 2 (0.25 M sucrose in Ham’s F10 medium complemented with 20%(HSA) for 3 min, and 1 and 2 washing solutions (Ham’s F10 medium complemented with 20%(HAS) each for 3–5 min. After this step, the oocytes were located in the IVM medium in the incubator for 48 h. The viability of vitrified oocytes was assessed by stereomicroscope 2–3 h after culture, founded on the cytoplasmic morphology dark cytoplasm with fragmented membrane showing dead oocytes [17].
Oocyte Maturation Medium
In this study, three types of IVM mediums were used for the culture of immature human oocytes:
Medium I: Minimum Essential Medium Alpha (α-MEM) as control medium [18].
Medium 2: α-MEM supplemented with supernatants of Mesenchymal stem cells (MSCs) from bone marrow [19].
Medium 3: α-MEM supplemented with ovarian growth factors [estradiol 1 mg/ml, leptin 10 ng/ml, BDNF 3 ng/ml, GDNF 30 ng/ml, FGF 20.5 ng/ml, IGF-I 100 ng/ml] [20].
MSC Isolation and Culture
Bone marrow MSCs were provided from the cell culture laboratory of the Afzalipour Medical University (Kerman, Iran). These cells were then incubated in an MSC growth medium containing (α-MEM) supplemented with 10% FBS and 2% penicillin–streptomycin (100 U/mL and 100 mg/mL). After 3 days, no adherent cells were detached by 2–3 washes with 1X PBS and adherent cells were cultured further in the complete medium until 90% confluency Devotee cells were trypsinized and planted at a concentration of 3 × 105 cells/mL for more expansion. Therefore, the medium was transformed once, the supernatant of these cells was collected after 48 h, signified CM of MSCs, and filtered by a 0.2-μm membrane for immediate use [21].
Artificial Oocyte Activation
Matured oocytes in IVM medium were parthenogenetically stimulated using ionomycin (USA, Sigma co) and 6 (DMAP) 6-dimethylaminopurine (Sigma co, St Louis). Then, α-MEM medium acting as a control medium supplemented with 10 uM of ionomycin at the temperature of 37 °C for 6 min. Later on, the oocytes were removed to IVF culture medium (Italy, Vitrolife co) supplemented with 2 mM of 6-dimethylaminopurine (DMAP) and kept at 6% CO2, and 37 °C for 3 h. Parthenogenesis embryos had to be evaluated after 24–72 h in terms of development, and then transferred to G1 or cleavage medium to be monitored for the growth and morphology of Parthenote blastocysts. The arrival of the inner cell mass (ICM) was also evaluated. We could then follow the developmental potential of stimulated oocyte even 6 days after activation [22].
Scoring of Parthenote Embryos
The morphology of parthenote embryos was scored by size, blastomere number, and cytoplasmic fragmentation, within the categorized scores of A, B, and C. Score A was associated with the best quality with equal symmetrical blastomeres that had minor fragmentation; B had moderate fragmentation in equal blastomeres, while scale C parthenote embryos had unequal size with severe cytoplasmic fragmentation [14].
Evaluation of BAX and BCL2 Gene Expression
RNA Extraction and cDNA Synthesis
The extraction of total RNA and DNA (cDNA) synthesis from IVM matured oocytes was performed considering the real-time PCR analysis protocol outlined by Zuccotti et al. (2002). Oocytes were then transferred to the Eppendorf tube having lysis buffers (1.5 μl). cDNA was synthesized by addition of Taq polymerase (1.25 μl), specific primers (2 μl) and Master Mix (20.75 μl) to each 2 μl cDNA used for PCR mixture. Subsequently, samples were located in a thermocycler (Bio-Rad) at 75 °C (5 min). Consequently, for the reverse transcription (RT): 5 μl from 5 × RT buffer, 0.25 μl from10 u RNase inhibitor, 1 μl from 200 u RT enzyme, and 3 μl from 10 mM dNTP were additional to the reaction. RT reaction was done at 25 °C (10 min), 37 °C (15 min), 42 °C (45 min), and 72 °C (10 min). Subsequently, the samples were preserved at 4 °C overnight (14).
Quantitative Real-time Polymerase Chain Reaction (qPCR)
Real-time PCR primers were projected for target genes mRNAs after alignment of these regions between all of them in EBML-EBI. For internal control or housekeeping gene GAPDH was used, it was purchased from Metabion Company (Germany). Real-time PCR was performed by a RotorGene6000 thermo cycler (Corbett Research, Inc.). Transcript abundance of Bax and Bcl2 were examined by real-time PCR (qPCR). Raw data were then investigated within the Relative Quantification Software (REST) version 2.2.3 (Qiagen, Inc.) to examine the automatic cycle threshold (Ct) setting for transmission baseline and threshold for Ct determination. For the analysis, Delta Ct values were used, and relative expression (RE) of the sample gene was considered using the ΔΔCT method [14].
Statistical Analysis
Data analysis was conducted using the SPSS statistical software version 21 for windows. The differences were studied using Chi-square test, and P values of less than or equal to 0.05 was considered to be significant.
Result
In this study, we used 266 human oocytes in the MII phase matured by IVM protocols in three mediums from patients undergoing infertility treatment in the Afzalipour Infertility Center Kerman, Iran. The vitrified immature human oocytes were thawed and randomly cultured in these conditioned mediums for IVM. Afterward, nuclear oocytes maturation was monitored by detailed microscopic valuation for its morphology and was then classified into the oocyte stages of GV, MI, and MII, followed by the assessment of the developmental competence of MII oocytes (matured in conditioned IVM mediums) in these different mediums. The oocyte cytoplasmic maturation was examined by the developmental competence of in vitro matured oocytes through chemical activation and following culture to the blastocyst stage.
Embryo formation of activated oocytes was evaluated 2–7 days after parthenogenesis using ionomycin. In the conditioned medium group, the percentage of degenerated oocytes was 2–4 cells, 4–8 cells and 16 cells. Blastocyst development was evaluated and compared in these groups.
Subsequently 151 IVM matured human oocytes (MII) were divided into one of three mediums. They were then parthenogenetically stimulated using ionomycin and 6 (DMAP) in the culture medium and the oocyte development was monitored every day: (1) 50 oocytes in Minimum Essential Medium Alpha (α-MEM) (as control medium); (2) 51 oocytes in α-MEM supplemented with supernatants of mesenchymal stem cells (MSCs) from bone marrow; and (3) 50 oocytes in α-MEM supplemented with ovarian growth factors (Table 1).
Table 1.
Developmental competence of in vitro matured oocytes
| Developmental parameter | fIVM α-MEM | vIVM α-MEM | fIVM B.M | vIVM B.M | fIVM O.F | vIVM O.F | p value |
|---|---|---|---|---|---|---|---|
| MII oocyte(n) | 45 | 45 | 45 | 46 | 42 | 43 | 0.170 |
| Activated oocyte(n) | 25 | 25 | 25 | 26 | 25 | 25 | 0.701 |
| Degenerated after activation | 6(24%) | 11(44%) | 1(4%) | 5(19.2%) | 2(8%) | 3(12%) | 0.031 |
| 2PN (%) | 76% | 56% | 96% | 80.76% | 92% | 88% | 0.211 |
| 2–4 cell arrest | 63.1% | 78.5% | 37.5% | 85.7% | 13.04% | 45.45% | 0.048 |
| 4–8 cell arrest | 31.5% | 21.4% | 54.1% | 14.2% | 39.13% | 50% | 0.029 |
| Morula | 5.2% | – | 8.3% | – | 34.78% | 4.54% | 0.364 |
| Blastocyst | – | – | – | – | 13.04% | – | 0.024 |
Blastocyst formation rates were significantly different (p < 0.001) between control and the other mediums shown in Table 1.
The embryo was categorized by A, B, and C score according to intensity of fragmentation and condition of the blastomeres.
Score A: equal symmetrical blastomeres with minor fragmentation.
Score B: equal blastomeres with moderate fragmentation.
Score C: unequal blastomeres with severe fragmentation.
The differences between the number of arrests in 4–8 cells (p = 0/041), score A (4–8) (p = 0/045) were statistically significant (Table2).
Table 2.
Parthenote embryo score after activated in vitro matured oocytes
| Developmental parameter | fIVM α-mem | vIVM α-mem | fIVM B.M | vIVM B.M | fIVM O.F | vIVM O.F | p value |
|---|---|---|---|---|---|---|---|
| Arrest in 2–4 cell(n) | 12 | 11 | 9 | 18 | 3 | 10 | 0.072 |
| Score A (2–4) | 7 | 5 | 4 | 10 | 2 | 4 | 0.287 |
| Score B (2–4) | 3 | 4 | 1 | 6 | 1 | 4 | 0.212 |
| Score C (2–4) | 2 | 2 | 4 | 2 | – | 2 | 0.591 |
| Arrest in 4–8 cell(n) | 6 | 3 | 13 | 3 | 9 | 11 | 0.041 |
| Score A (4–8) | 3 | – | 5 | 3 | 4 | 6 | 0.045 |
| Score B (4–8) | 3 | 2 | 4 | – | 2 | 4 | 0.310 |
| Score C (4–8) | – | 1 | 4 | – | 3 | 1 | 0.510 |
Primer sequences used for real-time PCR (qPCR) in embryos are listed in Table 3.
Table 3.
Primers used in the qRT-PCR
| Gene | Primer sequence | Length(bp) | Code number | Tm |
|---|---|---|---|---|
| GAPDH | FOR:5´-GACTTCAACAGCAACTCCCAC-3´ REV:5´- TCCACCACCCTGTTGCTGTA-3´ | 125 | NM_001289726.1 | 80 |
| Bax | FOR:5´- CGGCGAATTGGAGATGAACTG-3´ REV:5´-GCAAAGTAGAAGAGGGCAA-3´ | 161 | XM_006540584.1 | 83.5 |
| Bcl2 | FOR:5´- ACCGTCGTGACTTCGCAGAG -3´ REV:5´- GGTGTGCAGATGCCGGTTCA -3´ | 239 | NM_009741.1 | 84 |
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
BCL2-associated X protein (BAX)
B-cell CLL/lymphoma 2 (BCL2)
In our study, the expression mRNA levels of BAX and BCL2 (which acts as an apoptotic gene in embryo-derived fresh and vitrified IVM human oocyte) was significantly different (p ˂ 0.05) in the three-maturation media. The highest expression of BAX gene and the lowest expression levels of BCL2 were observed in vIVM (in α-MEM), while the highest expression levels of BCL2 gene and the lowest relative amounts of BAX were observed in fIVM in O.F groups (Table 3) (Figs. 1, 2).
Fig. 1.
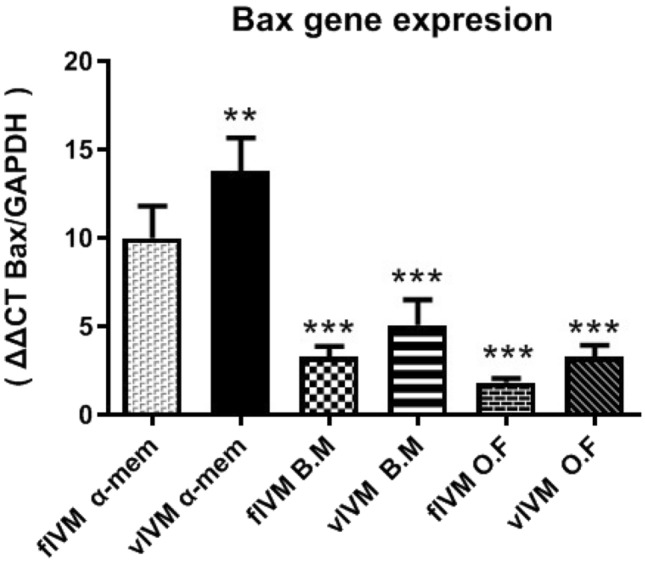
Expression rate of Bax gene in different groups
Fig. 2.
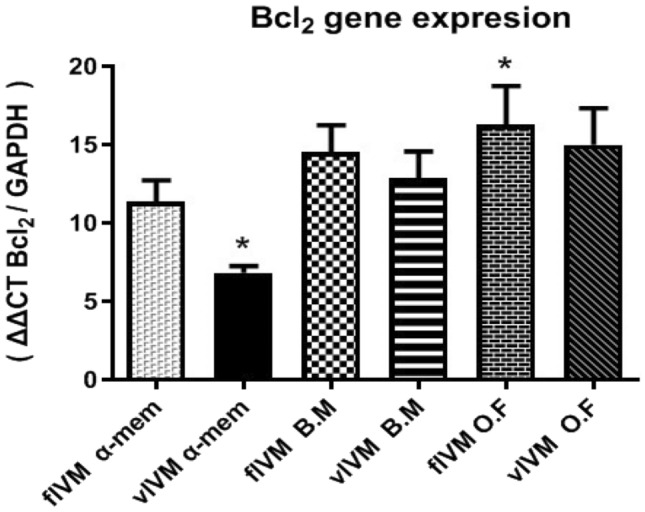
Expression rate of Bcl2 gene in different groups
Discussion
Oocyte cryopreservation and in vitro maturation of immature oocytes is an important technique and promising method pointed to preserve female fertility in ART [23]. Supplementation or additions to the typical oocyte culture medium seems to be essential for improving the maturation of immature oocytes. In order to attain better results, different variations like culture conditions mediums, hormonal treatment, and monitoring must be checked. Several studies demonstrate that growth factors, cytokines, amino acids, FSH, LH, PMSG, HMG, transferrin, insulin, and estradiol are useful for oocyte maturation in the IVM medium [24, 25].
Insulin growth factor, epidermal growth factor, GDNF, and FGF2 promote oocyte maturation, the rate of meiotic progression, and embryonic developmental competence [26, 27]. While oxidative stress is one of the greatest disturbing factors in the culture medium, concentrations of substrates and metabolites could also affect the unnecessary emergence of reactive oxygen species (ROS) [28]. Mature oocytes have several proteins and appropriate embryonic mRNA that protect their own developmental process, yet developmental arrest might occur at any stage of this course, during in vitro culture, from zygote to blast [29].
Several investigations described that the Bcl-2 high expression indicates good quality of oocytes, whereas all forms of oocytes establish Bax expression, yet reveal in denuded oocytes, denoting the highest level of Bax expression [30]. Embryo development could be affected by the functional balance between apoptosis and cellular proliferation. Moreover, the BCL-2 gene family is the main factor in the apoptotic pathway [31]. Guo-Min Zhang et al. defined that when the maturation was prolonged, the ratio of BAX/BCL2 was significantly increased. Consequently, BCL2 and BAX gene is key to evaluating the oocyte aging process. In this study, the highest increase in the ratio of BAX/BCL2 was observed in the α-MEM vIVM group [32, 33].
Complete maturation of oocyte is vital for the developing competence of embryos. Loss of developing competence is related to the lack of specific proteins in oocytes cultured in vitro to metaphase II [34]. On the other hand, parthenogenesis in vitro is achieved by oocyte artificial activation, without any sperm involvement. Parthenogenesis is a suitable practical assessment of oocyte developmental competence which can be induced in vitro by chemical, mechanical and electrical stimulations [9]. In our experiments, the induced elevation of intracellular Ca2 levels in oocyte, with ionomycin (as ionophore) and 6-DMAP (as protein synthesis inhibitor) promotes oocyte activation and parthenote development upto the blastocyst phase [35].
The mechanism of oocyte artificial activation is almost similar to that which occurs after IVF or ICSI for oocyte cytoplasm; therefore, the intracellular calcium level is suddenly elevated, followed by the activation of a sequence of molecular changes, leading to the maturation-promoting factor (MPF) that triggers high initiation nuclear maturation and in vitro development, achievement of meiosis, DNA synthesis, and pronuclear founding [36]. The cause of oocyte maturation arrest is not fully clear; however, it might be induced by an abnormal response to hyper-stimulation of the ovary [37].
Conclusion
Our findings indicate that the O.F. medium had more potent effects on oocyte growth and cytoplasmic maturation up to the blastocyst stage with the highest expression level of the BCL2 gene and the lowest relative amount of the BAX gene in this medium. The results of the present study have been verified only for parthenogenetically activated embryos, any positive effect of the environment on the egg/embryo fertilized with sperm requires more and more extensive studies.
Acknowledgements
The authors would like to thank the infertility center of Afzalipour Hospital in Kerman.
Abbreviations
- OF
Ovarian growth factors
- IVF
In vitro fertilization
- IVM
In vitro maturation
- ART
Reproductive technology
- MSCs
Mesenchyme stem cells
- BAX
BCL2-associated X protein
- BCL2
B-cell CLL/lymphoma 2
Funding
Funding for this research was provided by deputy of research affairs of Gerash University of medical sciences [98000005]. Deputy of research affairs of Gerash University of medical sciences had no role in the design of the study and collection, analysis, and interpretation of data and in writing of the manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethics approval was received from the ethics committee of the deputy of research affairs of Gerash university of medical sciences. Reference Number: IR.GERUMS.REC.1400.001. All methods were in accordance with the guidelines and regulations of the Declaration of Helsinki.
Informed Consent
Informed consent was also obtained in writing from all study participants.
Footnotes
Hakimeh Akbari is an Assistant Professor, Hossein Foruozandeh is an Assistant Professor, Masoud Mohammadi is a Lecturer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khalili MA, Shahedi A, Ashourzadeh S, et al. Vitrification of human immature oocytes before and after in vitro maturation: a review. J Assist Reprod Genet. 2017;34(11):1413–1426. doi: 10.1007/s10815-017-1005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbari H, Vaghefi SHE, Shahedi A, et al. Conditioned mediums and human oocytes in vitro maturation. Int J Pharm Phytopharm Res (eIJPPR) 2018;8(2):64–71. [Google Scholar]
- 3.Hatırnaz Ş, Ata B, Hatırnaz ES, et al. Oocyte in vitro maturation: a sytematic review. Turk J Obstet Gynecol. 2018;15(2):112. doi: 10.4274/tjod.23911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbari H. The effect of conditioned media on mouse oocytes ultrastructure following in vitro maturation. Gene Reports. 2020;20:100732. doi: 10.1016/j.genrep.2020.100732. [DOI] [Google Scholar]
- 5.Akbari H, Vaghefi SHE, Shahedi A, et al. The effect of conditioned media on human oocyte maturation and developmental competence. Pharmacophore. 2017;8(6s):e1173457. [Google Scholar]
- 6.Tukur HA, Aljumaah RS, Swelum AA-A, et al. The making of a competent oocyte–a review of oocyte development and its regulation. J Anim Reprod Biotechnol. 2020;35(1):2–11. doi: 10.12750/JARB.35.1.2. [DOI] [Google Scholar]
- 7.Kharche S, Goel P, Kumar Jha B, et al. Factors influencing in-vitro embryo production efficiency of caprine oocytes: a review. Indian J Anim Sci. 2011;81(4):344. [Google Scholar]
- 8.Fernández-Hernández P, Sánchez-Calabuig MJ, García-Marín LJ, et al. Study of the metabolomics of equine preovulatory follicular fluid: a way to improve current in vitro maturation media. Animals. 2020;10(5):883. doi: 10.3390/ani10050883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharche SD, Birade HS. Parthenogenesis and activation of mammalian oocytes for in vitro embryo production: a review. Adv Biosci Biotechnol. 2013;4(2):170–182. doi: 10.4236/abb.2013.42025. [DOI] [Google Scholar]
- 10.Jia Z, Zhang J, Wu Z, et al. Leptin enhances maturation and development of calf oocytes in vitro. Reprod Domest Anim. 2012;47(5):718–723. doi: 10.1111/j.1439-0531.2011.01949.x. [DOI] [PubMed] [Google Scholar]
- 11.Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2018;24(1):1–14. doi: 10.1093/humupd/dmx029. [DOI] [PubMed] [Google Scholar]
- 12.Heo JS, Choi Y, Kim H-S, et al. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37(1):115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samsonraj RM, Raghunath M, Nurcombe V, et al. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbari H, Eftekhar Vaghefi SH, Shahedi A, et al. Mesenchymal stem cell-conditioned medium modulates apoptotic and stress-related gene expression, ameliorates maturation and allows for the development of immature human oocytes after artificial activation. Genes. 2017;8(12):371. doi: 10.3390/genes8120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anchamparuthy V, Pearson R, Gwazdauskas F. Expression pattern of apoptotic genes in vitrified-thawed bovine oocytes. Reprod Domest Anim. 2010;45(5):e83–90. doi: 10.1111/j.1439-0531.2009.01527.x. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hasani S, Ozmen B, Koutlaki N, et al. Three years of routine vitrification of human zygotes: is it still fair to advocate slow-rate freezing? Reprod Biomed Online. 2007;14(3):288–293. doi: 10.1016/S1472-6483(10)60869-3. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y-X, Chian R-C, editors. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med, 27(6) Newyork: Thieme Medical Publishers; 2009 456–64. [DOI] [PubMed]
- 18.Mota GB, eSilva IO, de Souza DK, et al. Insulin influences developmental competence of bovine oocytes cultured in α-MEM plus follicle-simulating hormone. Zygote. 2015;23(4):563–72. doi: 10.1017/S0967199414000239. [DOI] [PubMed] [Google Scholar]
- 19.Nikoozad Z, Ghorbanian MT, Rezaei A. Comparison of the liver function and hepatic specific genes expression in cultured mesenchymal stem cells and hepatocytes. Iran J Basic Med Sci. 2014;17(1):27. [PMC free article] [PubMed] [Google Scholar]
- 20.McElroy SL, Byrne JA, Chavez SL, et al. Parthenogenic blastocysts derived from cumulus-free in vitro matured human oocytes. PLoS ONE. 2010;5(6):e10979. doi: 10.1371/journal.pone.0010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Lee YW, Rui YF, et al. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013;4(3):1–15. doi: 10.1186/scrt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Fried EP, Ross P, Zang G, et al. Human parthenogenetic blastocysts derived from noninseminated cryopreserved human oocytes. Fertil Steril. 2008;89(4):943–947. doi: 10.1016/j.fertnstert.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Shirasawa H, Terada Y. In vitro maturation of human immature oocytes for fertility preservation and research material. Reprod Med Biol. 2017;16(3):258–267. doi: 10.1002/rmb2.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guimarães A, Pereira S, Diógenes M, et al. Effect of insulin–transferrin–selenium (ITS) and L-ascorbic acid (AA) during in vitro maturation on in vitro bovine embryo development. Zygote. 2016;24(6):890–899. doi: 10.1017/S0967199416000228. [DOI] [PubMed] [Google Scholar]
- 25.Nogueira D, Sadeu JC, Montagut J, editors. In vitro oocyte maturation: current status. Seminars in reproductive medicine, 30(3). Newyork: Thieme Medical Publishers; 2012. 199–213. [DOI] [PubMed]
- 26.Hammami S, Morató R, Romaguera R, et al. Developmental competence and embryo quality of small oocytes from pre-pubertal goats cultured in IVM medium supplemented with low level of hormones, insulin–transferrin–selenium and ascorbic acid. Reprod Domest Anim. 2013;48(2):339–344. doi: 10.1111/j.1439-0531.2012.02160.x. [DOI] [PubMed] [Google Scholar]
- 27.Niu B, Li B, Wu C, et al. Melatonin promotes goat spermatogonia stem cells (SSCs) proliferation by stimulating glial cell line-derived neurotrophic factor (GDNF) production in Sertoli cells. Oncotarget. 2016;7(47):77532. doi: 10.18632/oncotarget.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan K-C, Lin Y-C, Chang Y-C, et al. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J Assist Reprod Genet. 2019;36(2):325–334. doi: 10.1007/s10815-018-1363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sha Q-Q, Zheng W, Wu Y-W, et al. Dynamics and clinical relevance of maternal mRNA clearance during the oocyte-to-embryo transition in humans. Nat Commun. 2020;11(1):1–16. doi: 10.1038/s41467-020-18680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang MY, Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci. 2002;70(3–4):159–169. doi: 10.1016/S0378-4320(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 31.Haouzi D, Hamamah S. Pertinence of apoptosis markers for the improvement of in vitro fertilization (IVF) Curr Med Chem. 2009;16(15):1905–1916. doi: 10.2174/092986709788186075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang G-M, Gu C-H, Zhang Y-L, et al. Age-associated changes in gene expression of goat oocytes. Theriogenology. 2013;80(4):328–336. doi: 10.1016/j.theriogenology.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Izadi M, Vaghefi SHE, Akbari H, et al. Assessment of mouse oocytes ultrastructure following vitrification before and after in vitro maturation. Int J Morphol. 2018;36(1):180–188. doi: 10.4067/S0717-95022018000100180. [DOI] [Google Scholar]
- 34.Richani D, Dunning KR, Thompson JG, et al. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27(1):27–47. doi: 10.1093/humupd/dmaa043. [DOI] [PubMed] [Google Scholar]
- 35.Liu S, Cui K, Li HL, et al. Comparison of chemical, electrical, and combined activation methods for in vitro matured porcine oocytes. In Vitro Cellular Dev Biol Anim. 2015;51(2):103–112. doi: 10.1007/s11626-014-9819-1. [DOI] [PubMed] [Google Scholar]
- 36.Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol. 2014;382(1):480–487. doi: 10.1016/j.mce.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Luong XG, Daldello EM, Rajkovic G, et al. Genome-wide analysis reveals a switch in the translational program upon oocyte meiotic resumption. Nucleic Acids Res. 2020;48(6):3257–3276. doi: 10.1093/nar/gkaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]


