Abstract
Facial palsy (FP) is a known consequence of head trauma, manifesting either immediately at the time of injury or with delayed onset, typically occurring 2 days or more post-trauma. Unilateral FP is the more common presentation and is often attributed to partial or complete transection of facial nerves or delayed onset edema. Conversely, bilateral facial palsy is a rare occurrence, reported in only a small number of cases, accounting for approximately 3% of patients presenting with bilateral weakness. In this report, we present the case of a previously healthy 28-year-old female who suffered a closed head injury during the Beirut Port Blast. Four days following the incident, the patient exhibited right-sided peripheral FP, which was consistent with a right temporal bone fracture. Subsequently, on the fifth day, the right-sided FP worsened, accompanied by the development of new FP on the left side, characterized by sparing of the frontal region, indicating a central origin for the left-sided FP. Laboratory investigations revealed severe hypovolemic hyponatremia with a sodium level of 105 mmol/L. As isotonic saline fluid replacement was initiated, there was progressive improvement in the left-sided FP. The right-sided palsy also resolved gradually with the implementation of facial rehabilitation therapy. It is important to note that severe head trauma, particularly with a concussive injury, can lead to facial paralysis through various mechanisms. Furthermore, severe hyponatremia should be considered a potential cause of central facial palsy, particularly in the presence of bilateral facial involvement. A thorough evaluation is encompassing assessment of palsy patterns, comprehensive imaging studies, and metabolic investigations is crucial for accurate diagnosis and timely intervention, resulting in successful treatment.
Keywords: Facial, Palsy, Hyponatremia, Neurosurgery, Sequential symptoms, Isotonic saline
Introduction
Acute facial paralysis (FP) is an infrequent condition that significantly impacts the patient's quality of life and overall well-being. Bell's palsy is the most common cause, often attributed to the reactivation of latent herpes virus infection, with an incidence ranging from 13 to 34 per 100,000 individuals. In contrast, facial paralysis resulting from trauma is less prevalent, and its onset can be either acute or delayed, with the latter being more commonly observed, typically manifesting 2 days or more after head trauma [[1], [2]–3]. Bilateral acute facial palsy is an exceedingly rare occurrence and is typically associated with various central conditions, including tumors, infections (such as tuberculosis or meningitis), metabolic and inflammatory disorders (eg, diabetes, sarcoidosis, lupus), or other central diseases like Guillain-Barré syndrome. Temporal bone fracture accounts for merely 3% of all cases presenting with bilateral FP [4,5]. We present a noteworthy case of a young female patient who experienced sequential bilateral facial paralysis following head trauma. Remarkably, each side of the face exhibited distinct etiologies, contributing to this unusual presentation.
Case presentation
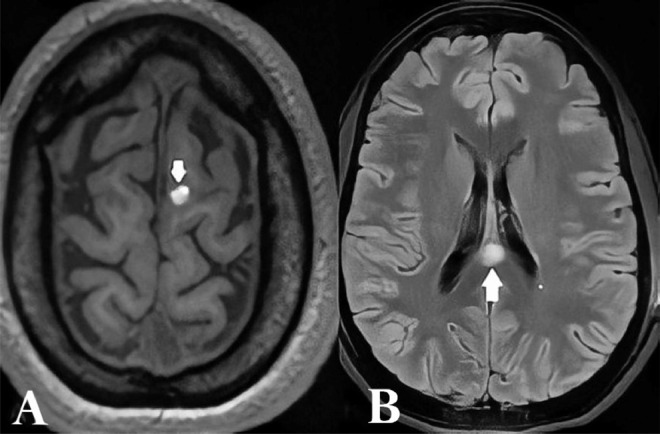
We present the case of a 28-year-old previously healthy female who sustained a closed head injury during the Beirut Port Blast on August 4, 2020. The injury resulted in a superficial scalp cut wound in the left parietal area, which was promptly sutured in the emergency department. She was admitted for a 24-hour surveillance period. Four days following the incident, the patient experienced progressive weakness in the right upper and lower regions of her face, eventually stabilizing at House-Brackmann grade IV. No other neurological deficits were observed. Magnetic resonance imaging (MRI) of the brain revealed a 1.5 cm area of high Fluid-attenuated inversion recovery (T2/FLAIR) signal with diffusion restriction in the posterior aspect of the corpus callosum, indicating axonal injury. Another small focus of diffusion restriction was noted in the left centrum semiovale, along with a 1 cm hemorrhagic contusion in the left superior frontal gyrus and at the right cingulate gyrus (Fig. 1).
Fig. 1.
Cranial MRI on First Presentation. (A) left superior frontal gyrus 1 cm hemorrhagic focus, (B) high FLAIR T2 signal involving the posterior aspect of the corpus callosum.
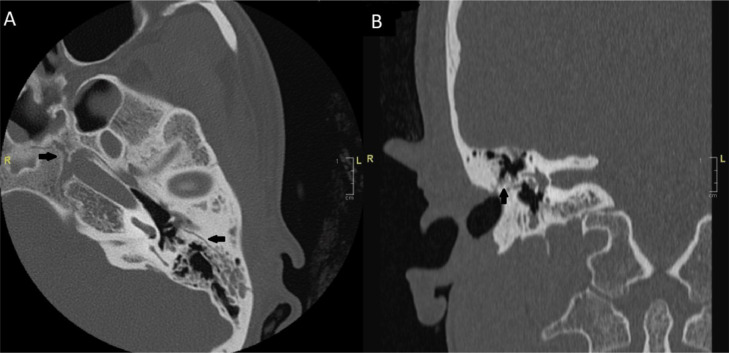
Furthermore, a computed tomography (CT) scan of the temporal bone revealed bilateral fractures. The right-sided fracture involved the geniculate ganglion without bony spicules, while the left longitudinal fracture spared the otic capsule and facial nerve (Fig. 2).
Fig. 2.
Cranial CT scan on First Presentation. Black arrow: fractured temporal bone. (A) axial cut, left longitudinal temporal bone fracture, sparing the geniculate ganglion, and facial nerve, (B) coronal cut, right geniculate ganglion involved in the fracture.
Steroid treatment was initiated at a dosage of 1 mg/kg/d. Five days later, the patient returned to the emergency department, presenting with restlessness, mild drowsiness, and delayed responses to verbal commands. She exhibited signs of dehydration, confusion, generalized hypotonia, and developed new-onset facial paralysis on the left side, progressing the previous right-sided paralysis to House-Brackmann grade VI. Notably, there was frontal sparing on the left side, suggesting a central origin for the left-sided facial paralysis. Consequently, she was admitted to the intensive care unit for appropriate management. Lacrimation was defective on the right side but normal on the left side. A subsequent MRI of the brain demonstrated more extensive diffusion restriction with a low Apparent diffusion coefficient (ADC) signal, involving the corpus callosum and bilateral cerebral white matter at the corona radiata (Fig. 3). Laboratory investigations unveiled severe hyponatremia with a sodium level of 105 mmol/L.
Fig. 3.
Cranial MRI on Second Presentation. Axial DWI sequences (A, B) and axial DWI (C, D) show extensive diffusion restriction with low ADC signal involving the corpus callosum and the cerebral white matter at the corona radiata bilaterally.
Clinical assessments of urine and serum osmolarities indicated evidence of hypovolemic hyponatremia, with no signs of the syndrome of inappropriate secretion of antidiuretic hormone. To address the hyponatremia, isotonic saline fluid replacement was initiated, gradually correcting the sodium level by a rise of 6-8 mmol/L/d. Over the course of 10 days, the patient exhibited progressive improvement, and ultimately complete recovery (House-Brackmann grade I) of the left facial paralysis was achieved following the correction of natremia. The right-sided facial paralysis returned to its baseline of House-Brackmann grade IV. Subsequently, the patient was discharged and commenced facial physiotherapy and rehabilitation, including electric stimulation. At the 6-week follow-up, the patient demonstrated complete recovery of bilateral facial paralysis.
Discussion
Temporal bone fractures can be categorized as either longitudinal or transverse fractures, based on the relationship between the fracture line and the long axis of the petrous bone. This classification system was initially established by Ulrich in 1926 [6]. Longitudinal fractures account for approximately 70%-90% of all temporal bone fractures, while transverse fractures constitute around 10%-30% of cases [7]. Facial palsy (FP) is more commonly associated with patients who have suffered a transverse fracture and may manifest immediately after the injury, progressing to complete paralysis [8]. Furthermore, patients with fractures that violate the otic capsule have a higher prevalence of developing FP, cerebrospinal fluid (CSF) leak, profound hearing loss, and intracranial complications such as epidural or subarachnoid hematomas [6]. The majority of injuries occur within the labyrinthine segment at the geniculate ganglion, resulting in nerve contusion, nerve sheath hematoma, or partial/complete nerve transection [9]. Nerve transection or compression by bone fragments typically leads to immediate post-traumatic paralysis, whereas edema, swelling, and hematoma can cause delayed onset of paralysis by compressing the nerve without transection [10,11].
In 1971, De Villiers proposed that a longitudinal fracture in the petrous part of the temporal bone may produce a mirrored fracture image on the opposite side within the temporal bone, along with backward displacement of the petrous apex and coronal splitting in the sphenoidal bone, resulting in bilateral facial palsy [12]. However, transverse fractures in the petrous bone do not involve bilateral facial nerves. Although there is limited information linking hyponatremia with facial palsy, a case report described symptoms such as new-onset facial drooping, slurred speech, generalized weakness, and stroke-like features in a patient with a sodium level of 99 mmol/L [13]. Patients with post-traumatic head injury leading to pituitary dysfunction are prone to developing disorders that disrupt homeostasis, including hyponatremia. Severe hyponatremia, without an adequate rise in other osmoles required to balance extracellular fluid osmolality, can result in increased water retention within cells and ultimately lead to fatal cerebral edema [14]. In such cases of serum hypotonicity, various homeostatic mechanisms, collectively termed "regulatory volume decrease," are employed to maintain cell volume [14,15].
Conclusions
While central facial palsy is a highly uncommon occurrence, it is important to consider severe hyponatremia as a potential cause, particularly when no other known explanations exist, regardless of the underlying mechanism. Urgent identification and treatment of hyponatremia in cases of facial palsy are crucial for emergency physicians, neurologists, and neurosurgeons, not only to prevent the complications associated with unrecognized severe hyponatremia but also to improve the recovery from facial palsy. This particular case underscores the necessity of further investigations aimed at identifying the associations and understanding the pathophysiology of facial palsy induced by hyponatremia.
Patient consent
The patient signed an informed consent and was granted approval for the publication. The IRB at the hospital does not require further documentation if the data will be shared anonymously.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Eliçora SŞ, Dinç AE, Bişkin S, Damar M, Bilgin E. Bilateral facial paralysis caused by bilateral temporal bone fracture. A case report and a literature review. Case Rep Otolaryngol. 2015;2015 doi: 10.1155/2015/306950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holla SP, Smith RR, Sanford RA. Bilateral traumatic facial paralysis. Neurosurgery. 1980;6:290–292. [PubMed] [Google Scholar]
- 3.Roth J, Toaff JS, Margalit N, Salame K. Traumatic facial Diplegia and Horner Syndrome: case report. Eur J Trauma Emerg Surg. 2007;33:425–429. doi: 10.1007/s00068-007-6913-z. [DOI] [PubMed] [Google Scholar]
- 4.Ghiasi S, Banaei M. Bilateral facial paralysis caused by temporal bone fracture: a case report. Arch Trauma Res. 2016;6:26892. doi: 10.5812/atr.26892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keane JR. Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature. Neurology. 1994;44:1198–1202. doi: 10.1212/wnl.44.7.1198. [DOI] [PubMed] [Google Scholar]
- 6.Zayas JO, Feliciano YZ, Hadley CR, Gomez AA, Vidal JA. Temporal bone trauma and the role of multidetector CT in the emergency department. Radiographics. 2011;31:1741–1755. doi: 10.1148/rg.316115506. [DOI] [PubMed] [Google Scholar]
- 7.Swartz JD, Harnsberger HR, Mukherji SK. The temporal bone. Contemporary diagnostic dilemmas. Radiol Clin North Am. 1998;36:819–853. doi: 10.1016/s0033-8389(05)70066-x. [DOI] [PubMed] [Google Scholar]
- 8.Fisch U. Facial paralysis in fractures of the petrous bone. Laryngoscope. 1974;84:2141–2154. doi: 10.1288/00005537-197412000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fisch U. Management of intratemporal facial nerve injuries. J Laryngol Otol. 1980;94:129–134. doi: 10.1017/s0022215100088575. [DOI] [PubMed] [Google Scholar]
- 10.Little SC, Kesser BW. Radiographic classification of temporal bone fractures: clinical predictability using a new system. Arch Otolaryngol Head Neck Surg. 2006;132:1300–1304. doi: 10.1001/archotol.132.12.1300. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Mittal RS. Post-traumatic delayed bilateral facial nerve palsy (FNP): diagnostic dilemma of expressionless face. J Clin Diagn Res. 2015;9:15–16. doi: 10.7860/JCDR/2015/11255.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Villiers JC. Fracture dislocation of the petrous bone. J Neurol Neurosurg Psychiatry. 1971;34:105–106. doi: 10.1136/jnnp.34.1.105-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holloman LN, Kolade VO, Zapko DR, Youngblood LB. Reversible stroke-like symptoms with severe hyponatremia. Tenn Med. 2013;106:35–37. doi: 10.1007/s00063-012-0120-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang CH, Liao JJ, Chuang CH, Lee CT. Recurrent hyponatremia after traumatic brain injury. Am J Med Sci. 2008;335:390–393. doi: 10.1097/MAJ.0b013e318149e6f1. [DOI] [PubMed] [Google Scholar]
- 15.Varol S, Ozdemir HH, Akil E, Arslan D, Aluclu MU, Demir CF, et al. Facial diplegia: etiology, clinical manifestations, and diagnostic evaluation. Arq Neuropsiquiatr. 2015;73(12):998–1001. doi: 10.1590/0004-282x20150174. PMID: 26677119. [DOI] [PubMed] [Google Scholar]