SUMMARY
The Duffy protein, a transmembrane molecule, acts as a receptor for various chemokines and facilitates binding between reticulocytes and the Plasmodium Duffy antigen binding protein. Duffy expression is associated with the Duffy chemokine receptor antigen genotype on chromosome 1 and exhibits variation across different geographic regions. Traditionally, the Duffy negative genotype and phenotype have been described to confer a certain level of protection against infection and symptom development. However, recent data suggest a shift in this behavior, with significantly higher prevalence observed in individuals with Duffy negative genotype or phenotype. Given that malaria is an endemic vector-borne disease in regions of Asia, Africa, and Latin America, posing a substantial global burden of disease and prioritizing public and global health, identifying evolutionary changes in infection and resistance patterns holds great importance for the design of strategies and reevaluation of conventional interventions. Hence, the aim of this review was to analyze the evolution of Plasmodium vivax and infection resistance patterns based on Duffy genotype and phenotype. The distribution of genotypes, phenotypes, and polymorphisms of P. vivax ligands and erythrocyte receptors varies geographically, notably resistance patterns of this microorganism in individuals with Duffy negative genotype and phenotype have significantly changed compared to studies conducted 30 years ago. The prevalence of vivax malaria in individuals with a Duffy negative status can reach up to 100%. Consequently, prioritizing research on this topic is essential for public health.
Keywords: Plasmodium, Plasmodium vivax, malaria, disease resistance, duffy blood-group system
INTRODUCTION
Malaria or paludism is a disease caused by the invasion of erythrocytes by parasites of the genus Plasmodium, resulting in alterations that can lead to the death of the affected individual. This parasitic infestation occurs subsequent to the bite of infected female Anopheles mosquitoes [1]. It is currently known that five species have the potential to induce pathology in humans, including P. falciparum, P. vivax, P. ovale wallikeri, P. ovale curtisi, and P. malariae. Additionally, there are other types of Plasmodium primarily affecting primates, which also possess the potential to cause disease in humans, as exemplified by P. knowlesi, associated with several cases of malaria in Southeast Asia [1].
The species that have gained the most relevance and prominence are P. falciparum and P. vivax, with the former causing a more severe disease and the latter being the most widely distributed species globally [2]. Other species capable of causing zoonosis include Plasmodium cynomolgi and Plasmodium inui, although they generally remain asymptomatic. Furthermore, cases of malaria resulting from Plasmodium simium and Plasmodium brasilianum have been described in South America, particularly in areas where P. vivax has been eradicated. Nonetheless, significant genetic and morphological similarities exist between P. brasilianum and P. malariae, leading to their occasional classification as the same family of parasites [3].
According to data provided by the World Health Organization (WHO), malaria stands as one of the most prevalent potentially deadly infectious diseases worldwide, with an estimated 229 million cases and 409,000 deaths reported globally in 2019 [2, 4]. The Centers for Disease Control and Prevention (CDC) reported 241 million cases worldwide and 627,000 deaths in 2020, with an approximate annual total of 2,000 new cases reported in the United States, predominantly resulting from imported cases by travelers and immigrants originating from countries with high transmission rates, notably those in sub-Saharan Africa and Southeast Asia [5].
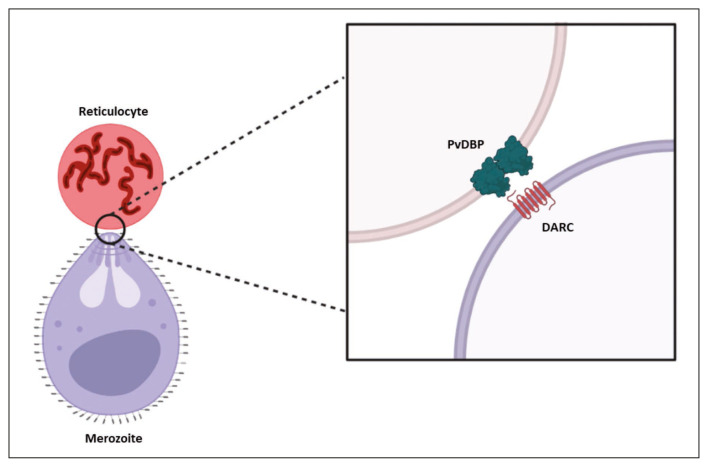
Despite the importance of P. vivax as the second most disease-causing species in humans, there remains much unknown about the invasion process due to the lack of a long-term parasite cultivation method, resulting in limitations in understanding the microorganism’s behavior [6, 7]. However, it has been noted that P. vivax demonstrates tropism for immature reticulocytes that express two surface receptors, with particular significance placed on the receptor for the Duffy antigen (Figure 1). This receptor engages with the Duffy binding protein 1 (DBP1), recognized as an essential step in the irreversible binding of P. vivax to the reticulocyte [8]. Traditional knowledge indicated that individuals lacking the expression of this receptor, possessing the Duffy genotype and phenotype, had a certain degree of protection against infestation and disease development [9]. However, recent data suggest a shift in this behavior, with significantly higher prevalence observed in individuals with Duffy negative genotype or phenotype [10].
Figure 1.
Interaction between the Duffy protein or DARC and the PvDBP of Plasmodium vivax merozoite. DARC: Duffy Antigen Receptor for Chemokines; PvDBP: P. vivax Reticulocyte Binding Protein; TfR: Transferrin receptor.
Source: authors.
This observation implies a notable evolution of this microorganism, along with shifts in human resistance patterns against the disease. Given the ongoing lack of knowledge regarding the molecular mechanisms associated with the infestation and reproduction of this microorganism in humans, conducting an evolutionary and transitional analysis of resistance patterns could enhance comprehension of the present disease dynamics and facilitate the identification of potential measures to control the global burden of malaria. Hence, the aim of this review was to analyze the evolution of P. vivax and infection resistance patterns based on Duffy genotype and phenotype.
MATERIALS AND METHODS
A literature search was performed in the PubMed, ScienceDirect, Web of Science, and MEDLINE databases, utilizing the terms “P. vivax,” “Duffy Blood-Group System,” and “Malaria,” along with their synonyms. Boolean operators were utilized to combine the terms. The inclusion criteria encompassed full-text articles that discussed historical aspects of P. vivax and evaluated infestation resistance patterns based on Duffy genotypes and phenotypes. Original research articles, systematic reviews, and meta-analyses were prioritized. The search included articles published until 2023, resulting in the identification of a total of 40 articles. Estimates and calculations derived from analytical studies were presented using original measures such as frequencies, percentages, confidence intervals (CI), mean differences (MD), relative risks (RR), odds ratios (OR), or hazard ratios (HR).
RESULTS
Plasmodium vivax evolution
The term “malaria” has its origins in the medieval Italian word “mal’aria,” meaning “bad air.” This belief arose from the notion that the disease was transmitted through the damp air of places such as swamps. Similarly, “paludismo” is derived from the Latin word “palus,” which means swamp or marsh, and the suffix “-ismo,” indicating a pathological process. Thus, “paludismo” refers to the disease of the swamp. The Greek term for this disease, “elonosia,” also translates to “the disease of the swamp” [4].
The Plasmodium genus, consisting of over 250 species, has ancient origins. Reports exist of Plasmodium dominicana sp. discovered in well-preserved amber samples dating back over 30 million years [4]. However, the precise origin of malaria in humans remains unclear. Theories propose that malaria evolved from P. falciparum infections in a common ancestor of humans and primates, gradually diverging as these organisms adapted to their environment and underwent changes [11]. Scientific literature also mentions the origin of P. vivax infestation hundreds of thousands of years ago in Southeast Asia, transmitted from macaques to humans [3, 11]. However, the credibility of these theories has diminished with the identification of closely related parasites to P. falciparum and P. vivax in wild chimpanzees, bonobos, and western gorillas. It is now considered plausible that both pathogens emerged more recently from parasites infecting African apes and were transmitted to humans [3, 11, 12]. The origin of P. ovale and P. malariae is even more uncertain and has been linked to transmission from primates to humans, albeit without further elaboration [4].
Malaria has been considered a driving force in shaping the current human genome according to numerous authors. Evolutionary mechanisms such as mutations in thalassemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, sickle cell disease, and the Duffy-negative genotype have emerged in various geographical locations following the migration of early hominids from Africa [13]. It is also believed that malaria, specifically caused by P. falciparum, spread from its African origins approximately 6,000 years ago. These theories are supported by the emergence of different malaria resistance alleles found worldwide. For example, analysis of G6PD haplotypes suggests that the African allele for P. falciparum resistance originated within the last 10,000 years, while HbE alleles (variants of the HBB gene) in Southeast Asia are estimated to have emerged around 5,000 years ago. These variants are associated with the development of thalassemia and sickle cell anemia, providing some level of protection against P. falciparum invasion [4, 13].
In ancient times, around the 4th and 5th centuries BC, malaria was mentioned in texts such as the “Corpus Hippocraticum,” which referred to benign tertian fever and quartan fever, likely corresponding to pathological processes caused by P. vivax and P. malariae, respectively. Hippocrates not only described the fever’s characteristics but also linked it to stagnant waters, emanating vapors, and seasonal patterns [4]. Subsequently, during the 1st and 2nd centuries AD, multiple episodes of severe fevers were described, likely associated with malaria caused by widespread circulation of P. falciparum during that era [14, 15]. The Middle Ages were also marked by malaria epidemics that spread across Europe, reaching England. Much of the available data suggests that England, Italy, and France were endemic areas for malaria between the 6th and 9th centuries AD [16].
The term “malaria” became known in England around 1740 through the letters of Horace Walpole, the 4th Earl of Oxford. However, it was not until 1827 that the term was introduced in a British scientific publication [4]. Seven years prior, in 1820, the alkaloids of quinine were successfully isolated by the pharmacist Joseph Caventou and the toxicologist Pierre Joseph Pelletier, who demonstrated their efficacy in treating intermittent fevers [17].
Before that time, malaria was recognized, but its cause remained unclear, with a prevailing belief that it was a disease associated with swamps. It was during the late 19th century that the expansion of empires sparked a particular interest in this disease to safeguard settlers and newly acquired territories from its lethal impact. In 1878, Alphonse Laveran, a French surgeon, made the initial observation of Plasmodium gametocytes in a blood sample from an Algerian patient using a microscope. Subsequently, in 1880, he witnessed gametocyte exflagellation in the fresh blood of a febrile young soldier. This pivotal discovery definitively established malaria as a parasitic disease, dispelling earlier suspicions of it being bacterial in nature [4].
In later years, the proposition of mosquito transmission of malaria was made by the Scottish physician Patrick Manson, based on his research on filariasis. However, the confirmation that the Anopheles mosquito could transmit this parasite came in 1897, when Ronald Ross established this fact. Two years later, Ross further asserted that only the female mosquito served as the transmission vector. This discovery prompted the implementation of mosquito elimination measures, including fumigation, which gained significant attention in 1930 with the introduction of insecticides like pyrethrum and 1,1,1-trichloro-2,2-di(4-chlorophenyl)ethane (DDT) [1, 4]. Prior to these developments, during the First World War, infectious diseases such as Bartonella quintana and malaria posed significant challenges to military forces, affecting over 1.5 million soldiers. The conditions were exacerbated by extended stays in the trenches, particularly in endemic regions like Italy, Macedonia, Mesopotamia, Palestine, and England [18]. The end of the war saw over 70,000 malaria cases reported among British military personnel, and the disease spread among the civilian population as soldiers returned to their homes [4, 18, 19].
During the Second World War, Benito Mussolini attempted to drain the Pontine Marshes to prevent mosquito breeding, but German sabotage undermined this effort, leading to malaria outbreaks. Extensive use of DDT was employed during the war to control vectors and prevent new cases of the disease [4].
The Italian and Yugoslav prisoners of war who escaped after the Second World War and sought treatment at the Swiss Tropical and Public Health Institute (Swiss TPH), one of the current centers dedicated to the study of this pathology, are among the first cases of malaria treated at this institution [20]. Meanwhile, in 1947, the presence of pre-erythrocytic schizonts of Plasmodium in the liver of a monkey was observed by Cyril Garnham and Henry Shortt. Formal research on malaria by the Swiss TPH began in 1950 and has continued since then [20].
Drawing on this experience, the eradication of malaria through antimalarial treatment with chloroquine and vector control using DDT was proposed by the WHO in 1955 during the World Health Assembly in Mexico. However, this strategy faced criticism, as some doubted the achievement of the proposed goals, particularly in Africa, where financial and social conditions would impede the proper implementation of the program. As anticipated, the desired results were not attained. While 18 countries managed to eradicate malaria by 1970, and eight additional countries achieved it in subsequent years, the resistance of the vectors and the inadequate infrastructure in economically disadvantaged countries were underestimated [1, 4, 20].
In 1975, Europe was declared malaria-free in a significant accomplishment. However, a resurgence of the disease occurred in Central America, South America, and Southeast Asia. Tropical regions still harbored a substantial reservoir of endemic malaria, posing challenges to the eradication program. Consequently, international support for disease control experienced a decline throughout the 1970s and 1980s [21]. In 1982, Krotosky successfully deciphered the latent exoerythrocytic hypnozoites of P. vivax, shedding light on the cause of malaria relapses. The emergence of chloroquine-resistant parasites, spreading across sub-Saharan Africa, and the rise of DDT-resistant Anopheles mosquitoes were also observed [1]. Dr. Manuel Elkin Patarroyo introduced the first malaria vaccine attempt in 1988. However, due to concerns regarding good clinical practices and limited effectiveness in children under 5 years old, the vaccine’s use was not extended as it was deemed ineffective for public health purposes [22].
In 1992, the WHO proposed a new strategy for early diagnosis and treatment of malaria, which demonstrated effectiveness in several nations. However, African countries faced complex challenges that hindered the implementation of this strategy. Consequently, malaria control became a pivotal focus on the WHO’s agenda, particularly with the appointment of a new Director [21].
Following the onset of the new millennium, efforts were made to develop a new eradication strategy primarily centered on aggressive disease control in endemic countries and the elimination of endemic pockets. The objective was to reduce the prevalence of malaria and foster research into vaccines, medications, and insecticides to address vulnerable populations [23]. From 2015 onwards, the global technical strategy for achieving a malaria-free world was embraced, with a target of eliminating malaria in a minimum of 35 countries by 2030. This agenda emphasizes the management of P. falciparum resistance to antimalarial drugs, Anopheles resistance to insecticides, asymptomatic malaria infections, and community-based involvement in healthcare. By the conclusion of 2020, approximately 24 countries reported the interruption of endemic malaria transmission for a duration of at least three years, and among them, 11 received certification from the WHO as malaria-free nations [17, 20].
Duffy genotype and phenotype as mechanisms of resistance to Plasmodium vivax malaria
The Duffy glycoprotein functions as a non-selective receptor, interacting with various CXC chemokines such as IL-8 and melanoma growth-stimulating activity (MGSA), as well as CC chemokines like monocyte chemotactic protein-1 (MCP-1) and CCL5, which attract monocytes, memory CD4+ T lymphocytes, and eosinophils [24]. The Duffy protein is involved in the homeostatic control of circulating chemokines and the modulation of chemokine gradients from the blood to the tissues, regulating the entry of monocytes and neutrophils during immune responses [24, 25].
Located on chromosome 1, specifically at 1q23.2, the ACKR1 gene, also known as DARC or FY, encodes the Duffy antigen receptor for chemokines (Duffy protein). It produces two mRNA variants, resulting in isoforms A and B. Isoform B is recognized as the canonical or reference sequence and both isoforms generate the antigens expressed on erythroid cells [24, 26].
A common DARC polymorphism, the G125A polymorphism (rs12075), presents co-dominant alleles FYA (G125) and FYB (125). This polymorphism leads to three positive genotypes: FYA/FYA, FYA/FYB, and FYB/FYB, corresponding to the Fy(a+b−), Fy(a+b+), and Fy(a−b+) phenotypes, respectively. Additionally, a mutation at position T-33C (Table 1) suppresses the expression of the Duffy antigen, resulting in the Fy(a−b−) phenotype. Other mutations can also silence the expression of the Duffy antigen, and various polymorphisms weaken the expression of the FYB and FYA alleles, often denoted as FYBW and FYAW, with ‘W’ indicating weakness [26, 27].
Table 1.
Reported Duffy Phenotypes and Genotypes.
| Description | Phenotype | Genotype |
|---|---|---|
| Heterozygous Duffy positive | Fy(a+b+) | Fy(a+b+) |
| Fy(a+b−) | FY*A/*BES | |
| FY*A/*A | ||
| Homozygous Duffy positive | Fy(a−b+) | FY*B/*B |
| FY*B/*BES | ||
| Weak Duffy expression | Fy(a + w) | FY*A/*AW |
| Negative Duffy (ES: erythrocyte silent) | Fy(a−b−) | FY*BES/*BES |
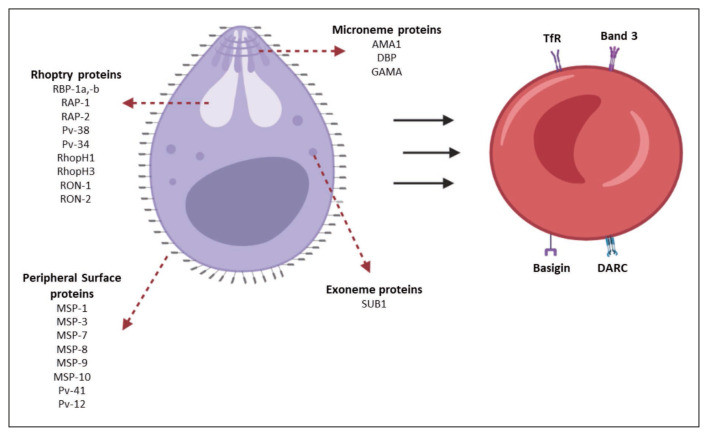
The interaction with DARC is known to be essential for the invasion of Plasmodium vivax and Plasmodium knowlesi (Figure 2). Therefore, the absence of this protein has been considered a factor that prevents the invasion of these merozoites into red blood cells/reticulocytes [24, 25]. African populations exhibit lower expression of the Duffy antigen, which has been associated with a decreased proportion of P. vivax malaria in this region. Furthermore, heterozygous phenotypes Fy(a+b−) and Fy(a−b+) (Duffy−positive heterozygotes) have shown some degree of resistance to parasite infestation compared to the Fy(a+b+) phenotype [24, 27].
Figure 2.
Reticulocyte binding proteins and ligands of Plasmodium vivax merozoite. DARC: Duffy Antigen Receptor for Chemokines; PvDBP: P. vivax Reticulocyte Binding Protein. Source: authors.
Changes in resistance patterns over time for P. vivax infection based on the Duffy status
In a concise manner, notable changes have been observed in the tropism and resistance patterns of P. vivax infestation capacity over the past 50 years [28–30]. The importance of the Duffy status in the presentation of this disease is acknowledged, as it is a crucial step [31]. Thus, variations in findings since the 1970s indicate the rapid evolution of the reticulocyte invasion mechanism for replication. These observations are backed by both basic and clinical studies [32, 33].
Some of the earliest studies found in the literature began to be published in the 1970s. In 1977, the difference between reticulocyte surface receptors and their impact on the development of Plasmodium disease was described by Miller et al. [32]. The effect of trypsin and neuraminidase on enzymatic susceptibility was tested. Approximately 10 years later, results on the role of the Duffy blood group in the invasion of P. vivax merozoites were published by authors such as Perkins ME and Barnwell et al., confirming the higher susceptibility to the disease in individuals with this receptor compared to those without it [33, 34]
During the 1990s, the results of an experimental study in basic sciences were published by Chitnis et al. [35]. They described the binding domain of the Duffy antigen to the surface of erythrocytes. It was stated that the interaction between the P. vivax ligand and the erythrocyte was mediated by a peptide-peptide interaction, and the potential to block this ligand-receptor binding was attributed to the glycosylation of the Duffy antigen [35]. Based on these findings, other authors chose to map regions in order to identify binding residues of functional domains between P. vivax and erythrocyte surface receptors [36]. They demonstrated the expression of chimeric domains containing sequences of P. vivax regions in human erythrocytes, providing an explanation for the ligand-receptor binding involved in invasion [36]. It was inferred that this step occurred exclusively in individuals expressing a positive genotype and phenotype. Kar et al. confirmed this hypothesis through an epidemiological study involving 708 individuals (n=324 malaria cases vs. n=384 control cases) in Ao Nagas, India. They found no cases among individuals who were Duffy negative, thereby attributing resistance to vivax malaria to those with a negative phenotype [37].
During the early 2000s, the emergence of studies described the high polymorphism of ligand and receptor binding proteins. Although the function of these proteins was not necessarily altered by this polymorphism, it had the potential to affect susceptibility [38]. It is important to note that this variability was more pronounced in certain regions, such as Papua New Guinea, compared to minimal variability observed in Korea [38, 39]. Concurrently, Cole-Tobian et al. from the Division of Geographic Medicine at Case Western Reserve University described these polymorphisms as mechanisms employed by P. vivax to evade immunity [40]. However, they also highlighted the counteracting effect of age-related enhanced immunity, which specifically targeted certain regions of the antigen. Thus, over time, the confirmation of variability in susceptibility patterns to the disease depended on the expression of erythrocyte ligands and receptors [40]. Until this point in time, the Duffy negative genotype and phenotype continued to be associated with protection against vivax malaria, as no cases of the disease were observed in individuals with this characteristic [41].
A decade later, in the early 2010s, a larger body of studies with varied outcomes emerged, dependent on the geographic region. In Colombia, the distribution of Duffy genotype and malaria infection was analyzed based on ethnicity by Gonzalez et al. [42]. An association between genotype and ethnicity was identified (p=0.003), and individuals with a negative phenotype showed no cases of vivax malaria, indicating a potential “natural resistance” against the disease [42]. However, isolated cases of vivax malaria in Duffy negative individuals were already being reported in Ethiopia and Cameroon, with a higher prevalence observed in children [43, 44]. Additionally, molecular epidemiology studies conducted in the same decade revealed over 10 cases of vivax malaria in Duffy negative individuals from the Republic of Congo, even in regions previously reported to exhibit resistance [45].
During the late 2010s and early 2020s, there was a disproportionate and unexpected rise in the prevalence of vivax malaria among individuals with a negative genotype and phenotype, leading to a paradigm shift in our understanding of this disease. In Senegal, a study conducted on 48 asymptomatic children over two consecutive years analyzed samples on four occasions. It revealed 15 P. vivax-infected samples, all of which exhibited Duffy negativity [46]. Although some regions still exhibit very low or no prevalence of the disease in populations with characteristics of “resistance”, the Republic of Congo reported an approximate 8% prevalence of vivax malaria in Duffy negative individuals in 2021 [47, 48]. Significant variants continue to be reported in Brazil, which may have a stronger association with disease severity rather than the infection itself [49]. Nevertheless, according to Wilairatana et al., who conducted a meta-analysis to determine the current prevalence of vivax malaria in Duffy negative individuals, studies (n=11) reported up to 100% of vivax malaria cases in Duffy negative individuals, primarily from Africa, Asia, and Latin America. Therefore, a prevalence of 25% was estimated in this subgroup [10].
These findings emphasize that changes in resistance patterns based on Duffy status have been demonstrated in epidemiological studies over a period of around 30 years. These changes should be taken into account when formulating future research guidelines on Plasmodium and malaria in order to develop more accurate control strategies customized for specific geographic populations. It can be noted that the previously observed “natural resistance” in individuals with a Duffy negative genotype and phenotype has been compromised, potentially due to an unknown alternative invasion pathway or the emergence of polymorphisms that confer affinity regardless of the presence of specific erythrocyte surface receptors.
CONCLUSIONS
The distribution of genotypes, phenotypes, and polymorphisms of P. vivax ligands and erythrocyte receptors varies geographically, notably resistance patterns of this microorganism in individuals with Duffy negative genotype and phenotype have significantly changed compared to studies conducted 30 years ago. The prevalence of vivax malaria in individuals with a Duffy negative status can reach up to 100%. Consequently, prioritizing research on this topic is essential for public health.
Footnotes
Conflicts of interest
The authors declare no conflict of interest in regard to this work.
Contributions
All authors contributed to the design, research, analysis and interpretation of the data, writing of the original draft, review and editing, and approval of the final version of the manuscript.
Funding
None to declare.
REFERENCES
- 1. Douglas NM, Burkot TR, Price RN. Malaria eradication revisited. Int J Epidemiol. 2022;51(2):382–392. doi: 10.1093/ije/dyab259. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Guidelines for malaria [Internet] [Consulted 04 Jun 2023]. Available in: https://www.who.int/publications/i/item/guidelines-for-malaria.
- 3. Sharp PM, Plenderleith LJ, Hahn BH. Ape Origins of Human Malaria. Annu Rev Microbiol. 2020;74:39–63. doi: 10.1146/annurev-micro-020518-115628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boualam MA, Pradines B, Drancourt M, Barbieri R. Malaria in Europe: A Historical Perspective. Front Med (Lausanne) 2021;8:691095. doi: 10.3389/fmed.2021.691095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Malaria [Internet] [Consulted 04 Jun 2023]. Available in: https://www.cdc.gov/parasites/malaria/index.html.
- 6. Patarroyo MA, Molina-Franky J, Gómez M, Arévalo-Pinzón G, Patarroyo ME. Hotspots in Plasmodium and RBC Receptor-Ligand Interactions: Key Pieces for Inhibiting Malarial Parasite Invasion. Int J Mol Sci. 2020;21(13):4729. doi: 10.3390/ijms21134729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molina-Franky J, Reyes C, Picón Jaimes YA, Kalkum M, Patarroyo MA. The Black Box of Cellular and Molecular Events of Plasmodium vivax Merozoite Invasion into Reticulocytes. Int J Mol Sci. 2022;23(23):14528. doi: 10.3390/ijms232314528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan LJ, Dietrich MH, Nguitragool W, Tham WH. Plasmodium vivax Reticulocyte Binding Proteins for invasion into reticulocytes. Cell Microbiol. 2020;22(1):e13110. doi: 10.1111/cmi.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langhi DM, Jr, Bordin JO. Duffy blood group and malaria. Hematology. 2006;11(5):389–398. doi: 10.1080/10245330500469841. [DOI] [PubMed] [Google Scholar]
- 10. Wilairatana P, Masangkay FR, Kotepui KU, De Jesus Milanez G, Kotepui M. Prevalence and risk of Plasmodium vivax infection among Duffy-negative individuals: a systematic review and meta-analysis. Sci Rep. 2022;12(1):1–13. doi: 10.1038/s41598-022-07711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loy DE, Liu W, Li Y, et al. Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. Int J Parasitol. 2017;47(2–3):87–97. doi: 10.1016/j.ijpara.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rayner JC, Liu W, Peeters M, Sharp PM, Hahn BH. A plethora of Plasmodium species in wild apes: a source of human infection? Trends Parasitol. 2011;27(5):222–229. doi: 10.1016/j.pt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gelabert P, Olalde I, de-Dios T, Civit S, Lalueza-Fox C. Malaria was a weak selective force in ancient Europeans. Sci Rep. 2017;7:1377. doi: 10.1038/s41598-017-01534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kousoulis AA, Chatzigeorgiou KS, Danis K, et al. Malaria in Laconia, Greece, then and now: a 2500-yearold pattern. Int J Infect Dis. 2013;17(1):e8–e11. doi: 10.1016/j.ijid.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 15. Marciniak S, Prowse TL, Herring DA, et al. Plasmodium falciparum malaria in 1st–2nd century CE southern Italy. Curr Biol. 2016;26(23):R1220–R1222. doi: 10.1016/j.cub.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 16. Newfield TP. Malaria and malaria-like disease in the early Middle Ages. Early Medieval Europe. 2017;25(3):251–300. [Google Scholar]
- 17. Brabin B. Analysing malaria events from 1840 to 2020: the narrative told through postage stamps. Malar J. 2021;20(1):399. doi: 10.1186/s12936-021-03932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rickard Christophers S. Malaria in war. Trans R Soc Trop Med Hyg. 1939;33(3):277–292. [Google Scholar]
- 19. Wigglesworth VB. Malaria in War. Nature. 1941;147(3728):436–439. [Google Scholar]
- 20. Meier L, Casagrande G, Abdulla S, Masanja H. A brief history of selected malaria vaccine and medical interventions pursued by the Swiss Tropical and Public Health Institute and partners, 1943–2021. Acta Trop. 2022;225:106115. doi: 10.1016/j.actatropica.2021.106115. [DOI] [PubMed] [Google Scholar]
- 21. Sachs JD. A new global effort to control malaria. Science. 2002;298(5591):122–124. doi: 10.1126/science.1077900. [DOI] [PubMed] [Google Scholar]
- 22. Patarroyo ME, Amador R, Clavijo P, et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332(6160):158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- 23. Li XH, Kondrashin A, Greenwood B, Lindblade K, Loku Galappaththy G, Alonso P. A Historical review of WHO certification of malaria elimination. Trends Parasitol. 2019;35(2):163–171. doi: 10.1016/j.pt.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 24. Höher G, Fiegenbaum M, Almeida S. Molecular basis of the Duffy blood group system. Blood Transfus. 2018;16(1):93–100. doi: 10.2450/2017.0119-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaur H, Sehgal R, Rani S. Duffy antigen receptor for chemokines (DARC) and susceptibility to Plasmodium vivax malaria. Parasitol Int. 2019;71:73–75. doi: 10.1016/j.parint.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 26. Gruszczyk J, Lim NTY, Arnott A, et al. Structurally conserved erythrocyte-binding domain in Plasmodium provides a versatile scaffold for alternate receptor engagement. Proc Natl Acad Sci USA. 2016;113(2):E191–200. doi: 10.1073/pnas.1516512113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gai PP, van Loon W, Siegert K, et al. Duffy antigen receptor for chemokines gene polymorphisms and malaria in Mangaluru, India. Malar J. 2019;18:328. doi: 10.1186/s12936-019-2966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rougeron V, Daron J, Fontaine MC, Prugnolle F. Evolutionary history of Plasmodium vivax and Plasmodium simium in the Americas. Malar J. 2022;21(1):141. doi: 10.1186/s12936-022-04132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cornejo OE, Escalante AA. The origin and age of Plasmodium vivax. Trends Parasitol. 2006;22(12):558–563. doi: 10.1016/j.pt.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wiscovitch-Russo R, Narganes-Stordes Y, Cano RJ, Toranzos GA. Origin of the New World Plasmodium vivax: Facts and New Approaches. Int Microbiol. 2019;22(3):337–342. doi: 10.1007/s10123-018-00053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camargo-Ayala PA, Garzón-Ospina D, Moreno-Pérez DA, Ricaurte-Contreras LA, Noya O, Patarroyo MA. On the Evolution and Function of Plasmodium vivax Reticulocyte Binding Surface Antigen (pvrbsa) Front Genet. 2018;9:372. doi: 10.3389/fgene.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller LH, Haynes JD, McAuliffe FM, Shiroishi T, Durocher JR, McGinniss MH. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977;146(1):277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perkins ME. Erythrocyte invasion by the malarial merozoite: recent advances. Exp Parasitol. 1989;69(1):94–99. doi: 10.1016/0014-4894(89)90175-6. [DOI] [PubMed] [Google Scholar]
- 34. Barnwell JW, Nichols ME, Rubinstein P. In vitro evaluation of the role of the Duffy blood group in erythrocyte invasion by Plasmodium vivax. J Exp Med. 1989;169(5):1795–1802. doi: 10.1084/jem.169.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chitnis CE, Chaudhuri A, Horuk R, Pogo AO, Miller LH. The domain on the Duffy blood group antigen for binding Plasmodium vivax and P. knowlesi malarial parasites to erythrocytes. J Exp Med. 1996;184(4):1531–1536. doi: 10.1084/jem.184.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ranjan A, Chitnis CE. Mapping regions containing binding residues within functional domains of Plasmodium vivax and Plasmodium knowlesi erythrocyte-binding proteins. Proc Natl Acad Sci USA. 1999;96(24):14067–1472. doi: 10.1073/pnas.96.24.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kar S, Seth S, Seth PK. Duffy blood groups and malaria in the Ao Nagas in Nagaland, India. Hum Hered. 1991;41(4):231–235. doi: 10.1159/000154007. [DOI] [PubMed] [Google Scholar]
- 38. Xainli J, Adams JH, King CL. The erythrocyte binding motif of Plasmodium vivax duffy binding protein is highly polymorphic and functionally conserved in isolates from Papua New Guinea. Mol Biochem Parasitol. 2000;111(2):253–260. doi: 10.1016/s0166-6851(00)00315-7. [DOI] [PubMed] [Google Scholar]
- 39. Suh IB, Hoffman KJ, Kim SH, et al. The analysis of Plasmodium vivax Duffy receptor binding domain gene sequence from resurgent Korea isolates. Parasitol Res. 2001;87(12):1007–1010. doi: 10.1007/s004360100478. [DOI] [PubMed] [Google Scholar]
- 40. Cole-Tobian JL, Cortés A, Baisor M, et al. Age-acquired immunity to a Plasmodium vivax invasion ligand, the duffy binding protein. J Infect Dis. 2002;186(4):531–539. doi: 10.1086/341776. [DOI] [PubMed] [Google Scholar]
- 41. Cavasini CE, Tarelho Pereira FJ, Ribeiro WL, Wunderlich G, Ferreira MU. Duffy blood group genotypes among malaria patients in Rondônia, Western Brazilian Amazon. Rev Soc Bras Med Trop. 2001;34(6):591–595. doi: 10.1590/s0037-86822001000600016. [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez L, Vega J, Ramirez JL, Bedoya G, Carmona-Fonseca J, Maestre A. Relationship between genotypes of the Duffy blood groups and malarial infection in different ethnic groups of Choco, Colombia. Colomb Med (Cali) 2012;43(3):189–195. [PMC free article] [PubMed] [Google Scholar]
- 43. Fru-Cho J, Bumah VV, Safeukui I, Nkuo-Akenji T, Titanji VP, Haldar K. Molecular typing reveals substantial Plasmodium vivax infection in asymptomatic adults in a rural area of Cameroon. Malar J. 2014;13:170. doi: 10.1186/1475-2875-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lo E, Yewhalaw D, Zhong D, et al. Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malar J. 2015;14:84. doi: 10.1186/s12936-015-0596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brazeau NF, Whitesell AN, Doctor SM, et al. Plasmodium vivax Infections in Duffy-Negative Individuals in the Democratic Republic of the Congo. Am J Trop Med Hyg. 2018;99(5):1128–1133. doi: 10.4269/ajtmh.18-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Niang M, Sane R, Sow A, et al. Asymptomatic Plasmodium vivax infections among Duffy-negative population in Kedougou, Senegal. Trop Med Health. 2018;46:45. doi: 10.1186/s41182-018-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roesch C, Popovici J, Bin S, et al. Genetic diversity in two Plasmodium vivax protein ligands for reticulocyte invasion. PLoS Negl Trop Dis. 2018;12(10):e0006555. doi: 10.1371/journal.pntd.0006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brazeau NF, Mitchell CL, Morgan AP, et al. The epidemiology of Plasmodium vivax among adults in the Democratic Republic of the Congo. Nat Commun. 2021;12(1):4169. doi: 10.1038/s41467-021-24216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferreira NS, Mathias JLS, Albuquerque SRL, et al. Duffy blood system and G6PD genetic variants in vivax malaria patients from Manaus, Amazonas, Brazil. Malar J. 2022;21(1):144. doi: 10.1186/s12936-022-04165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]