SUMMARY
Introduction
Toxocariasis is an infection caused in canines, felines, humans, and other vertebrates by species of the genus Toxocara, such as T. canis and T. cati. The embryonated eggs of these parasites are the main form of acquisition of the infection both for definitive hosts, such as the dog and the cat, respectively and for paratenic hosts, such as humans and other vertebrates. Toxocariasis infection in humans causes visceral larva migrans syndrome. When deposited on park soils, environmental contamination becomes a risk for environmental, human, and animal health.
Objective
To systemically estimate the prevalence of Toxocara spp. eggs in park soils in Latin America.
Methods
A systematic review and meta-analysis were performed to evaluate the prevalence of Toxocara eggs in park soils in Latin America, defined by copro-parasitological, molecular and immunological techniques. We searched PubMed, Scopus, Web of Sciences, Embase, LILACS and SciELO for studies published from 1900 through 28 January 2023. A meta-analysis was performed using a random-effects model to calculate the pooled prevalence and 95% confidence intervals (95% CI). Heterogeneity was measured through I2 statistics.
Results
Forty-nine studies (2,508 parks and 12,833 samples) were included, of whom 44 had a low risk of bias. The pooled prevalence of Toxocara eggs in parks in Latin America was 50.0% (95% CI: 40.0%–60.0%). Argentina had the highest prevalence of Toxocara eggs in parks (100%), followed by Brazil (66%) and Venezuela (63%). The pooled prevalence of Toxocara eggs in soil samples was 20.0% (95% CI: 14.0%–26.0%); in faecal samples, it was 13.0% (95% CI: 6.0%–23.0%).
Conclusion
The presence of Toxocara canis eggs in public parks in Latin America is a zoonotic and public health threat for the people who go to these places, especially if children play on the ground with dirt or contaminated objects; since many pet owners and general public are not adequately informed about the mode of transmission of this parasite.
Keywords: Toxocara, prevalence, park, Latin America, systematic review, meta-analysis
INTRODUCTION
Zoonotic diseases still represent a significant disease burden globally, especially in low and middle-income countries. Despite that, their prevention and control have decreased due to the increase in globalization, population, migration, internal and external displacement of animals and humans, and the recent COVID-19 pandemic [1]. It is estimated that 20% of zoonoses worldwide are of parasitic origin, with companion animals and humans being the most vulnerable [2]. Parasites associated with zoonoses include helminths such as Toxocara canis and Toxocara cati, which are globally distributed, infect dogs, cats and accidentally other hosts, including humans [3]. Indeed, the usual localization of Toxocara canis is in the small intestine. However, migrating larvae can be found in the intestinal cavity and numerous organs (lungs, eyes, heart, and liver, among others) [4].
Toxocara canis parasites in pregnant canines can grow up to 10 centimetres and invade puppies before birth. Dog puppies not dewormed at around two weeks old excrete Toxocara spp. eggs, equivalent to 10,000 per gram of faeces [5, 6]. These eggs can survive for about three years in soil, which increases the chances of infesting humans [7]. In its biological cycle, this nematode is also a large soil reservoir. Consequently, contaminated soils in open public parks are a health risk for people, especially children, due to their playing habits, which involve handling the soil and putting their hands in their mouths, leading to geophagy [7, 8]. In Latin America, there are very few studies on the prevalence of Toxocara eggs in soils in public areas [2]. A study conducted on a university campus in Venezuela evaluated soil samples, finding that 43.8% had eggs and larvae of helminths, highlighting Toxocara spp. as the most frequent. In another study carried out in public parks in La Plata, Argentina, a prevalence of 70% of nematode eggs was detected, of which 33% corresponded to Toxocara spp. In Colombia, there are few studies, one of them in public parks in Tunja, where it was found that 60.7% of the parks were positive for nematodes in samples of canine faecal matter and 100% on land [9]. The nematodes found were eggs and larvae of Toxocara spp, Ancylostoma spp, Trichuris vulpis, and Strongyloides spp. In Bogotá, they established the presence of gastrointestinal nematodes in the soils of public parks, finding Toxocara spp. eggs 5.4%, Strongyloides spp. 3.3%, among others [9]. Another study in three public parks in Duitama, Boyacá, found larval eggs of Toxocara canis in 25 samples, representing 34.7% of the total analyzed [10]. Although the prevalence of these parasites differs, their presence is substantial and merits further assessment. All in all, the cases of Toxocara in humans represent a threat to public health. Although they are not subjected to epidemiological surveillance, in 2018, a study was carried out in which the mobility of Toxocara eggs mediated as a risk factor was identified by walking dogs in contaminated parks from there to the home [11, 12]. In another study conducted in a low-income community from Bogotá, positive titers were found in 47.5% of inhabitants, and 46.3% of the puppies were positive for Toxocara [13]. Over time, different studies have identified contaminated parks as a source of Toxocara infection, recognising that human disease can be fatal [10, 14, 15]. Therefore, the present meta-analysis aimed to estimate the prevalence of Toxocara in park soils in Latin America.
METHODS
Registration and reporting
All procedures used in this study were consistent with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [16]. A short protocol version was uploaded to the International Prospective Register of Systematic Reviews (PROSPERO) with code CRD42023404643 (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=404643).
Data sources and searches
The search strategy followed the Peer Review of Electronic Search Strategies (PRESS) checklist [17]. It was built using MeSH, Emtree, and free terms for ‘Toxocara park’, ‘Toxocara egg’, and ‘Toxocara soil’, searching by the countries of Latin America. In addition, the following databases were searched without language restriction from 1900 through 28 January 2023: PubMed, Scopus, Web of Sciences, Embase, LILACS and SciELO. The complete search strategy is in Table 1.
Table 1.
Search strategies.
| Source | PubMed |
|---|---|
| Search | Formula |
| #1 | Ascaridae [MH] OR Toxocara [MH] OR “Toxocar*” [TIAB] OR (“park” [TIAB] AND “soil*” [TIAB]) OR “garden*” [TIAB] |
| Source | Scopus |
| Search | Formula |
| #1 | TITLE-ABS-KEY (“Toxocar*” OR (“park” W/3 “soil*”) OR “soils*”) |
| Source | Web of Science |
| Search | Formula |
| #1 | TI=(“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”) OR AB=(“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”) OR AK=(“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”) OR KP=(“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”) OR TS=(“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”) |
| Source | Embase |
| Search | Formula |
| #1 | ‘Rickettsia’/exp OR (“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”):ti OR (“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”):ab OR (“Toxocar*” OR (“park” NEAR/3 “soils”) OR “parks”):kw |
| Source | LILACS |
| Search | Formula |
| #1 | (Toxocar* OR (park adj3 soils) OR parks).ti. OR (Toxocar* OR (park adj3 soils) OR parks).ab. OR (Toxocar* OR (park adj3 soils) OR parks).kw. |
| Source | Scielo |
| Search | Formula |
| #1 | (Toxocara) AND (parks) OR (soil) |
Study selection and data extraction
Peer-reviewed published articles reporting contamination by Toxocara spp. eggs in parks, with either parasitological or molecular confirmation, were included. We considered egg detection for parasitological tests and PCR for tests based on molecular biology. On the other hand, case reports, case series, editorials, commentaries, and review articles were excluded.
The references retrieved from the search strategy were independently screened by titles and abstracts by four researchers (JR U-B, MD M-R, EA H-B and EA A-B). Then, the remaining full-text references were screened by the same researchers. As mentioned above, observational studies that reported the contamination of parks with parasitological or molecular confirmation of Toxocara spp. were included for quantitative synthesis (meta-analysis). Two researchers (LV M-G and OD) independently completed the data extraction form for each included study. The extracted information was: publication type, country, publication date, type of samples (soil or faecal samples) obtained in the parks, total parks evaluated and the number of infected parks or samples evaluated by serological or molecular tests. A third researcher (A A-C) checked the list of articles and data extractions to ensure that duplicate reports or information from the same study would not be included. Discrepancies in the study selection or data extraction processes were resolved through discussion or by a third party (AJ R-M).
Risk of bias assessment
Two researchers (JR U-B and MD M-R) independently assessed the risk of bias through the Newcastle Ottawa Scale adapted for cross-sectional studies (NOS-CS) [18]. A survey with seven or more stars was deemed low risk of bias. Contrarily, a study with less than seven stars was considered a high risk of bias.
The assessment of publication bias is not recommended for proportional meta-analysis due to the following reasons:
conventional funnel plots and Egger’s test are inaccurate for these analyses,
there is no evidence that proportions adjust correctly to funnel plots or Egger’s tests.
Therefore, we did not perform a publication bias assessment due to the above [19, 20].
Data analyses
The statistical analysis was performed in Stata 17.0© (Stata Corporation, College Station, TX, USA). A random-effects model (DerSimonian and Laird method) was performed to estimate pooled prevalences with their corresponding 95% Confidence Intervals (95% CI). We approached heterogeneity by Cochran’s Q statistic and I2 index. Values equal to or greater than 60% were defined as high heterogeneity for the I2 statistic, and p-values <0.05 were considered indicators of heterogeneity in Cochran’s Q test. In addition, we performed subgroup analyses by methods and countries. Also, a sensitivity analysis included only those studies at low risk of bias.
RESULTS
Selection of studies
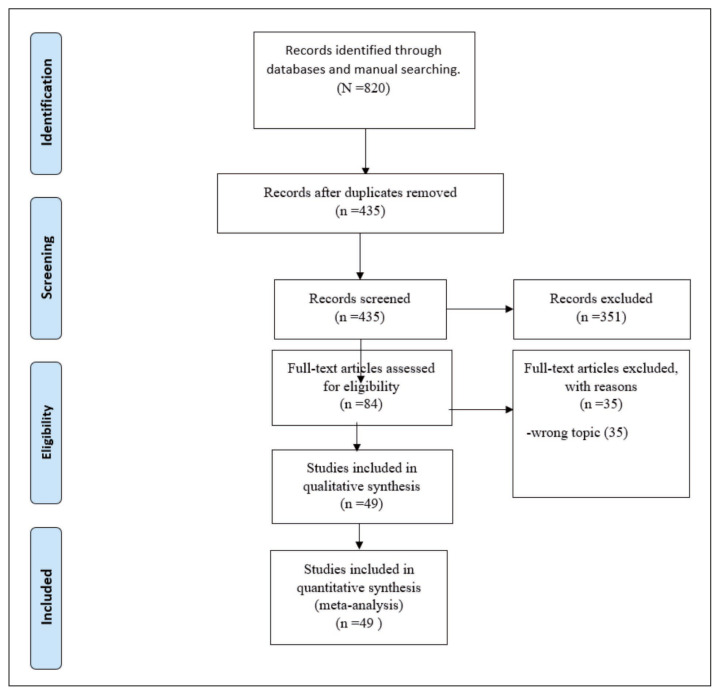
Our search strategy yielded 820 articles. After removing duplicates and screening for titles and abstracts, 84 articles underwent full-text review. Finally, 49 articles were included in the systemic review and meta-analysis [21–69]. The PRISMA flow chart is shown in Figure 1.
Figure 1.
PRISMA Flow Diagram.
Characteristics of included studies
The characteristics of the included articles are summarized in Table 2. A total of 49 articles were included, in which 2,508 parks were evaluated, with 12,833 samples obtained from the parks (2,487 faecal samples and 10,346 soil samples). The studies ranged from 1989 to 2021, but 2019 there were 7 (14%) (Table 2). The studies were distributed as follows: Peru (11 studies), Mexico (9 studies), Brazil (7 studies), Colombia (4 studies), Venezuela (4 studies), Argentina (3 studies), Chile (3 studies), Bolivia (1 study), Costa Rica (1 study), Cuba (1 study), Ecuador (1 study), Paraguay (1 study) and Uruguay (1 study). All faecal samples were evaluated by centrifugation-flotation, searching for Toxocara eggs. The presence of Toxocara eggs in soil samples was assessed by the following methods: Double W, centrifugation-flotation, and sedimentation.
Table 2.
Characteristics of the included studies.
| Author | Year-Publication | Year of sample collection | Country | Total number of evaluated parks | Total number of parks contaminated with Toxocara | Sample obtained from the parks | Methods used to assess soil samples | Methods used to evaluate fecal samples | Total number of samples evaluated | Total number of samples contaminated with Toxocara | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chavez A et al. | 2002 | 1998–1999 | Peru | 558 | 235 | Soil | Double “W” | NR | NR | NR | [32] |
| Vargas-Nava A et al. | 2020 | 2013 | Mexico | 236 | 18 | Soil | Centrifugation-flotation | NR | 1180 | 115 | [65] |
| Lettieri-Texeira M et al. | 2008 | 2007 | Brazil | NR | NR | Soil | Sedimentation | NR | 25 | 7 | [45] |
| Paquet-Durand I et al. | 2007 | 2005–2006 | Costa Rica | NR | NR | Soil | Sedimentation | NR | 44 | 6 | [55] |
| Fecal | NR | Centrifugation-flotation | 69 | 5 | |||||||
| Farfan-Pajuelo D et al. | 2019 | 2018 | Peru | 10 | 7 | Soil | Double “W” | NR | NR | NR | [54] |
| Devera R et al. (A) | 2008 | 2004 | Venezuela | 20 | 11 | Soil | Sedimentation | NR | 80 | 23 | [33] |
| Devera R et al. (B) | 2020 | 2019 | Venezuela | 10 | 8 | Soil | Sedimentation | NR | 40 | 13 | [24] |
| Santarem V et al. | 1998 | 1995–1996 | Brazil | 10 | 6 | Soil | Centrifugation-flotation | NR | 120 | 21 | [63] |
| Guzmán-Quinche F et al. | 2019 | 2014 | Ecuador | 35 | 16 | Fecal | NR | Centrifugation-flotation | 245 | 31 | [58] |
| Cáceres-Pinto C et al. | 2016 | 2012 | Peru | 21 | 14 | Soil | Double “W” | NR | 276 | 74 | [27] |
| Malca C et al. | 2019 | 2014–2016 | Peru | 131 | 1 | Soil | Double “W” | NR | NR | NR | [47] |
| Lopez F et al. | 2005 | 1999 | Peru | 123 | 78 | Soil | Double “W” | NR | NR | NR | [46] |
| Polo-Teran et al. | 2007 | 2005–2006 | Colombia | 52 | 49 | Soil | Sedimentation | NR | 1560 | 84 | [56] |
| La Rosa V et al. | 2001 | 1999 | Peru | 108 | 37 | Soil | Double “W” | NR | NR | NR | [68] |
| Marques JP et al. | 2012 | 2010 | Brazil | 120 | 82 | Soil | Centrifugation-flotation | NR | NR | NR | [48] |
| Eisen A et al. | 2019 | 2018 | Brazil | NR | NR | Soil | Centrifugation-flotation | NR | 162 | 1 | [35] |
| Sedimentation | NR | 216 | 0 | ||||||||
| Martínez-Barbabosa I et al. | 1998 | 1996 | Mexico | 82 | 7 | Soil | Centrifugation-flotation | NR | NR | NR | [49] |
| Cazorla-Perfetti D et al. | 2007 | 2004 | Venezuela | 38 | 24 | Soil | Centrifugation-flotation | NR | NR | NR | [31] |
| Romero-Núñez C et al. | 2009 | 2005 | Mexico | NR | NR | Soil | Centrifugation-flotation | NR | 310 | 186 | [60] |
| Faecal | NR | Centrifugation-flotation | 200 | 135 | |||||||
| Benavides-Melo C et al. | 2020 | 2019 | Colombia | 31 | 17 | Soil | Centrifugation-flotation | NR | 155 | 19 | [26] |
| Menocal-Heredia L et al. | 2018 | 2013–2014 | Cuba | 23 | 3 | Faecal | Centrifugation-flotation | Centrifugation-flotation | 28 | 1 | [44] |
| Armstrong W et al. | 2011 | 2003 | Chile | 87 | 6 | Soil | Centrifugation-flotation | NR | 193 | 70 | [25] |
| Díaz-Anaya A et al. | 2015 | 2013 | Colombia | 28 | 28 | Soil | Centrifugation-flotation | NR | 124 | 53 | [34] |
| Faecal | NR | Centrifugation-flotation | 124 | 5 | |||||||
| Medina-Pinto R et al. | 2018 | 2015 | Mexico | 20 | 1 | Faecal | NR | Centrifugation-flotation | 100 | 1 | [50] |
| Lee D et al. | 2021 | 2018–2020 | Brazil | 20 | 11 | Soil | Centrifugation-flotation | NR | 83 | 13 | [43] |
| Ramírez-Rubio L et al. | 2019 | 2016–2017 | Mexico | 56 | 26 | Soil | Centrifugation-flotation | NR | 560 | 66 | [59] |
| Alonso J et al. (A) | 2001 | 1998 | Argentina | 5 | 1 | Soil | Centrifugation-flotation | NR | 475 | 6 | [22] |
| Alonso J et al. (B) | 2006 | 2003–2004 | Argentina | 44 | 10 | Soil | Centrifugation-flotation | NR | 612 | 26 | [23] |
| Melín-Coloma M et al. | 2016 | 2014 | Chile | 43 | 0 | Soil | Double “W” | NR | NR | NR | [51] |
| Alcantara N et al. | 1989 | NR | Brazil | 96 | 31 | Soil | Centrifugation-flotation | NR | 298 | 74 | [21] |
| Faecal | NR | Centrifugation-flotation | 277 | 51 | |||||||
| Vidal M et al. | 2019 | NR | Brazil | NR | NR | Faecal | NR | Centrifugation-flotation | 92 | 23 | [67] |
| Garcia-Blasquez D et al. | 2017 | 2016 | Peru | 10 | 9 | Soil | Double “W” | NR | NR | NR | [38] |
| Gallardo J et al. | 2015 | NR | Venezuela | 32 | 20 | Soil | Double “W” | NR | NR | NR | [37] |
| Montalvo-Sabino E et al. | 2014 | 2014 | Peru | 11 | 10 | Soil | Double “W” | NR | NR | NR | [53] |
| Iannacone J et al. | 2012 | 2007–2008 | Peru | NR | NR | Soil | Centrifugation-flotation | NR | 117 | 81 | [41] |
| Young-Candia C et al. | 2011 | 2010 | Peru | 25 | 12 | Soil | Double “W” | NR | 200 | 14 | [69] |
| Guarín-Patarroyo C et al. | 2016 | NR | Colombia | 3 | 3 | Soil | Centrifugation-flotation | NR | 72 | 25 | [39] |
| Castillo Y et al. | 2001 | 1998–1999 | Peru | 17 | 12 | Soil | Double “W” | NR | NR | NR | [30] |
| Canese A et al. | 2003 | NR | Paraguay | 51 | 27 | Soil | Centrifugation-flotation | NR | NR | NR | [28] |
| Lara-Reyes E et al. | 2019 | NR | Mexico | 27 | 22 | Soil | Centrifugation-flotation | NR | NR | NR | [42] |
| Poma R et al. | 2018 | 2016 | Bolivia | 10 | 8 | Faecal | NR | Centrifugation-flotation | 300 | 19 | [57] |
| Mendoza-Meza D et al. | 2015 | 2013 | Colombia | NR | NR | Soil | Centrifugation-flotation | NR | 13 | 5 | [52] |
| Fonrouge R et al. | 2000 | NR | Argentina | 22 | 15 | Soil | Centrifugation-flotation | NR | 242 | 32 | [36] |
| Tsuji O et al. | 1996 | 1995 | Mexico | 156 | 17 | Soil | Centrifugation-flotation | NR | 281 | 36 | [66] |
| Romero-Núñez C et al. (C) | 2013 | NR | Mexico | 7 | 7 | Soil | Centrifugation-flotation | NR | 2374 | 587 | [61] |
| Salinas P et al. | 2001 | 1996–1998 | Chile | 110 | 25 | Soil | Centrifugation-flotation | NR | 159 | 29 | [62] |
| Castillo D et al. | 2000 | 1999 | Chile | 96 | 36 | Faecal | NR | Centrifugation-flotation | 288 | 39 | [29] |
| Tiyo R et al. | 2008 | 2003–2004 | Brazil | 17 | 17 | Soil | Centrifugation-flotation | NR | 375 | 195 | [64] |
| Hernandez S et al. | 2003 | 2000 | Uruguay | 70 | 37 | Faecal | NR | Centrifugation-flotation | 764 | 99 | [40] |
NR: Not reported.
Risk of bias assessment
In the risk of bias assessment, five studies were at high risk, while the remaining 44 were at low risk of bias (Table 3).
Table 3.
Quality assessment of included studies.
| Study | Newcastle - Ottawa quality assessment scale for cross-sectional studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | |||||||
| Representativeness of the sample | Sample size | Non-respondents | Ascertainment of the exposure (risk factor) | The subjects in different outcome groups are comparable based on the study design or analysis. Confounding factors are controlled. Maximum: ☆☆ | Assessment of outcome | Statistical test | SCORE | Evidence quality (bias) | |
| Chavez A et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Vargas-Nava A et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Lettieri-Texeira M et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Paquet-Durand I et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Farfan-Pajuelo D et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | High risk | |
| Devera R et al. (A) | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Devera R et al. (B) | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Santarem V et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Guzmán-Quinche F et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Cáceres-Pinto C et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Malca C et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Lopez F et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Polo-Teran et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| La Rosa V et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Marques JP et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Eisen A et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Martínez-Barbabosa I et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | High risk | |
| Cazorla-Perfetti D et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | High risk | |
| Romero-Núñez C et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Benavides-Melo C et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Menocal-Heredia L et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Armstrong W et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Díaz-Anaya A et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Medina-Pinto R et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | High risk | |
| Lee D et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Ramírez-Rubio L et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Alonso J et al. (A) | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Alonso J et al. (B) | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Melín-Coloma M et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Alcantara N et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Vidal M et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Garcia-Blasquez D et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Gallardo J et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | High risk | |
| Montalvo-Sabino E et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Iannacone J et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Young-Candia C et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Guarín-Patarroyo C et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Castillo Y et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Canese A et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Lara-Reyes E et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Poma R et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Mendoza-Meza D et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Fonrouge R et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Tsuji O et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Romero-Núñez C et al. (C) | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Salinas P et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
| Castillo D et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Tiyo R et al. | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 8 | Low risk |
| Hernandez S et al. | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | Low risk |
Prevalence of Toxocara in parks
The pooled prevalence of Toxocara eggs in parks in Latin America was 50.0% (95.0% CI: 40.0%–60.0%) with high heterogeneity (I2=95.88%) (Figure 2). In the subgroup analysis according to country (Figure 3), Colombia had the highest prevalence of Toxocara eggs in parks (92%; 95% CI 64.0%–100.0%), followed by Brazil (66.0%; 95% CI 40.0%–88.0%), and Venezuela (63.0%; 95% CI 53.0%–73.0%). In the sensitivity analysis, after removing the articles at high risk of bias, heterogeneity was decreased (I2=96.09%) (Figure 4).
Figure 2.
Toxocara eggs prevalence in parks.
Figure 3.
Subgroup analysis by country when evaluating the prevalence of parks with Toxocara eggs.
Figure 4.
Sensitivity analysis according to the risk of bias when evaluating the prevalence of parks with Toxocara eggs.
Prevalence of Toxocara in parks according to sample source
The pooled overall prevalence of Toxocara eggs in soil samples collected from parks was 20.0% (95% CI: 14.0%–26.0%) (Figure 5). However, in the subgroup analysis according to methods (Figure 6), the prevalence of Toxocara eggs in soil was 22.0% (95% CI: 15.0%–30.0%) for Centrifugal-flotation, 14.0% (95% CI: 4.0%–27.0%) for sedimentation, and 17.0% (95% CI: 14.0%–21.0%) for Double W method. On the other hand, the pooled prevalence of Toxocara in faecal samples collected from parks was 13.0% (95% CI: 6.0%–23.0%) (Figure 7). No studies reported molecular findings, then were omitted.
Figure 5.
Prevalence of Toxocara eggs in samples from park soils.
Figure 6.
Subgroup analysis by the method of the prevalence of Toxocara eggs in soil parks sampling.
Figure 7.
Prevalence of Toxocara in faecal samples obtained in parks.
DISCUSSION
Toxocariasis is a highly prevalent parasitic disease, especially in Latin America [70]. Although its clinical importance, which may lead even to fatal cases, is not a condition under surveillance in most countries of the region [71–73].
Based on the search of six databases, this systematic review and meta-analysis estimated the pooled prevalence of Toxocara eggs in parks from Latin America. As different reports have suggested, there is significant variability in the prevalence of this parasite among countries [74]. The global prevalence in Latin American parks land in the present study, using the search for studies published in databases of studies in different Latin American countries between 1989 and 2023, allowed the development of a meta-analysis. Additional studies have reported the existence of Toxocara eggs in soil samples in parks in public areas in Latin America, in Colombia 2.5%, Puerto Rico 6.5%, Costa Rica 7%, Ecuador 8.5%, Mexico 12.5%, Uruguay 16.1%, Cuba 17.9%, Bolivia 27%, Peru 27.7%, Brazil 29.7, Argentina 35.1%, Paraguay 53%, Venezuela 63.16%, and Chile 66.7%. That shows that soils are critical for Toxocara transmission and need intervention to prevent and control disease in animals and humans [75–78]. Additionally and consistently, studies in Italy have shown similar results. In a survey in the Central region of the country, evaluating Toxocara prevalence in the soil of 22 public playgrounds of Ancona, it found that parasites were detected in the soil samples of 95.5% of playgrounds and that the most prevalent helminth found was Toxocara canis, in the soil samples from 54.5% of playgrounds [79].
The parasitological methods used in the studies mainly were Double w, with a prevalence of 44.1%, centrifugation at 10%, sedimentation at 13.7%, and flotation at 23.9%. Currently, the serological diagnosis of toxocariasis is carried out using the ELISA immune-enzymatic technique in some places to detect the presence of antigens secreted by the Toxocara larvae, allowing the diagnosis of the different clinical presentations of the disease [80–82]. Nevertheless, there is a lack of studies using molecular tools, as standardized PCR for Toxocara in the region’s laboratories is primarily unavailable.
Variations according to multiple factors, as expected, were observed. The variation per year can be due to the socioeconomic level, age, pollution, culture, and the varied climate of the region where the study was carried out influencing factors. Other factors may be the presence of garbage deposits, not picking up the excrement by pet owners and the community’s commitment to cleanliness improvement and implementation of public parks [76]. Additionally, a certain margin of inaccuracy remains due to bias that cannot be undone when conducting analyses involving the sampling of the soil and the faeces of animals. Nevertheless, the results obtained in this study indicate that the soils of public parks in Latin America are sites of risk of infection with Toxocara, considerably [83].
It is very striking that in Latin American countries such as Costa Rica, Colombia, and Venezuela, there are very few studies carried out on Toxocara prevalence in park soils, especially Venezuela, according to other studies carried out, and with the present study, it is one of the most countries with the highest prevalence in Latin America [2, 6, 55, 74].
We can observe that this zoonosis is widely distributed worldwide in low-, middle-and high-income countries due to the habit of having companion dogs that are frequently infected or acquire the parasite from the environment. Becoming a great source of infection for human beings. For Toxocara, the soil is a great reservoir where the eggs evolve to the larvae stage (L2), which allows them to remain stable for one to three years; this raises the possibility of a human being becoming infected, which can not only become infected, but can end up having severe clinical consequences, and even death when associated with the development of visceral larva migrans syndrome [80, 84, 85].
Toxocara is an intestinal nematode that parasitises dogs (T. canis) and cats (T. cati), mainly those that have a wandering habit and are not subject to health plans, which are disseminators of this parasite, playing an essential role in the Toxocara cycle and its transmission to humans [76]. Dogs and cats are the definitive hosts of T. canis and T. catis, which lodges in the intestinal lumen of these animals and is excreted in the form of eggs, larvae, and adults in the faeces [78]. These faeces are deposited in public spaces such as parks and other green areas, representing a potential risk to public health [78]. Exposing the human population to the risk of infection, especially children interacting with pets and the soil in these spaces [4]. That is associated with geophagy habits.
Toxocariasis is a zoonotic disease of great importance in terms of morbidity and, in some cases, mortality that it can cause in humans because of how complex its control can be for public health. Regarding its association with other pathologies, this Toxocara infection can be considered forgotten and neglected due to the scarcity of solutions and national and Latino studies [86]. Toxocariasis is a pathology that is not regularly reported in Latin America. Its diagnosis is infrequent because it is not even considered clinical suspicion and also due to the lack of reagents since the diagnosis is made by serology [87–89].
There are no clearly defined risk groups since the infection does not respect age, sex, occupation, or social condition. However, it is recognized that children under ten may be at the most significant risk for contracting the infection due to the habits of playful activity and lack of care in hygiene, which facilitate the transmission of the disease [90, 91].
These results make us see that the population of stray animals increases considerably every day, and many, due to poor pet ownership, are abandoned on the streets. Consequently, they present unfavorable health conditions, bringing them diseases, including toxocariasis [4]. Furthermore, the number of dogs without an owner and a leash is higher compared to the number of dogs with an owner and on a leash and of dogs with an owner and without a leash, which shows that good breeding of dogs and population control of stray dogs is not carried out. However, the results suggest no correlation between the average number of dogs and the number of Toxocara eggs [80, 86, 92].
Unlike studies in Latin America, in countries such as France, Switzerland, South Korea, and Spain, a low prevalence of the disease is observed because they control infections in domestic and stray dogs, reducing the percentage of contamination towards the human being. However, it does not eradicate the risk since the parasite is found naturally in the soil and the environment [93, 94].
Mass deworming measures should be implemented for dogs without owners, diagnosis and deworming in dogs with owners, immediate elimination of dog faeces, since at that moment the eggs are not yet infective, application of ovicidal substances on surfaces and elements that may be contaminated with faecal matter from canines and educational campaigns directed at the community on the risks of acquiring toxocariasis [90, 95, 96].
The dog has always been and will be one more component of the human family; in the rural environment, to take care of the house, and in the urban environment, mainly as a companion animal, we have an increasing canine population, and good management is not carried out mainly of excreta [97]. Prevention of infections in humans depends on the treatment and prevention of Toxocara spp. diseases in animals [98]. Contamination can be reduced in public areas by establishing restrictions on stray animals, collecting faeces by pet owners, and preventing animal access in areas such as children’s playgrounds [90].
In order to reduce human exposure, dogs and cats should be dewormed. Pups from 3 weeks to 3 months of age shed large numbers of Toxocara canis eggs. Cats excrete Toxocara cati, especially between 2 and 6 months of life [99].
Good hygiene can help prevent infection or severe illness. Hands and raw food should be washed thoroughly before eating. Children should be taught not to eat dirt and to wash their hands after playing with pets or participating in outdoor activities. Children should not play in areas where animal faeces have been found [99].
Adequate control of this parasitic zoonosis would substantially reduce public health risks, mainly in the risk of infection in children under ten, who are more susceptible to contagion and more susceptible to acquiring more pathogenic forms of toxocariasis [96]. Therefore, it is necessary to promote collaboration between researchers related to toxocariasis to achieve superior research worldwide, especially in developing countries where it is more critical due to its high incidence, as well as the epidemiological, clinical, ecological, molecular, and treatment associated with toxocariasis [100].
CONCLUSIONS
The presence of Toxocara canis eggs in public parks in Latin America is a risk of zoonosis and public health for the people who go to these places, especially if children play on the ground, with the earth, or with contaminated objects, since many of them pet owners and the general public are not adequately informed about the mode of transmission of this parasitosis.
Awareness should be created in the population about implementing adequate health plans, such as deworming, vaccination, hygiene practices, and prevention in children. Furthermore, the findings show us the role of stray dogs with a high prevalence of Toxocara canis in the transmission of this zoonotic infectious disease; those that, due to defecation habits, in the streets and public squares, eventually lead to environmental contamination, which is why, at the government level, programs to control the ownerless canine population should be strengthened, promoting the responsibility of pets, trade, and sanitation of public parks and recreation areas.
Footnotes
Declaration of competing interest
The authors declare no conflicts of interest.
Funding
None.
REFERENCES
- 1. Mellado-Sola I, Rodríguez-Molino P, Armas EA, et al. Impact of coronavirus pandemic on tuberculosis and other imported diseases screening among migrant minors in Spain. Trop Med Infect Dis. 2022;8(1) doi: 10.3390/tropicalmed8010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodriguez-Morales AJ, Bonilla-Aldana DK, Gallego-Valencia V, et al. Toxocariasis in Colombia: More Than Neglected. Curr Trop Med Rep. 2020;7(1):17–24. [Google Scholar]
- 3. Rodríguez-Morales AJ, Echeverri-Cataño LF, Delgado O. Need for a consensus in the diagnosis of human toxocariasis: implications for the latin american public health. Rev Per Med Exper Salud Pub. 2011;28(1):161–162. doi: 10.1590/s1726-46342011000100029. [DOI] [PubMed] [Google Scholar]
- 4. Aghaei S, Riahi SM, Rostami A, et al. Toxocara spp. infection and risk of childhood asthma: a systematic review and meta-analysis. Acta Trop . 2018;182:298–304. doi: 10.1016/j.actatropica.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 5. Merigueti Y, Santarém VA, Ramires LM, et al. Protective and risk factors associated with the presence of toxocara spp. eggs in dog hair. vet Parasitol . 2017;244:39–43. doi: 10.1016/j.vetpar.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 6. Delgado OM, Rosas-Bustamante J, Ortegoza J, et al. Acute Cases of Toxocariasis Classified by Igg Antibodies Avidity in Venezuela. J Egypt Soc Parasitol. 2011;41(3):611–614. [PubMed] [Google Scholar]
- 7. Maurelli MP, Santaniello A, Fioretti A, Cringoli G, Rinaldi L, Menna LF. The presence of toxocara eggs on dog’s fur as potential zoonotic risk in animal-assisted interventions: a systematic review. Animals (Basel) 2019;9(10) doi: 10.3390/ani9100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winders WT, Menkin-Smith L. Toxocara Canis (Visceral Larva Migrans, Toxocariasis) In Statpearls Treasure Island (FL), 2020. [Google Scholar]
- 9. Díaz-Anaya AM, Pulido-Medellín MO, Giraldo-Forero JC. Nematodes with zoonotic potential in parks of the city of Tunja, Colombia] Salud Publica de Mexico . 2015;57(2):170–176. [PubMed] [Google Scholar]
- 10. Lopez-Osorio S, Penagos-Tabares F, Chaparro-Gutierrez JJ. Prevalence of Toxocara spp. in dogs and cats in South America (Excluding Brazil) Adv Parasitol. 2020;109:743–778. doi: 10.1016/bs.apar.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 11. Bojar H, Klapec T. Contamination of selected recreational areas in Lublin Province, Eastern Poland, by eggs of Toxocara spp., Ancylostoma spp. and Trichuris spp. Ann Agric Environ Med. 2018;25(3):460–463. doi: 10.26444/aaem/92252. [DOI] [PubMed] [Google Scholar]
- 12. Panova OA, Khrustalev AV. Dog walking brings toxocara eggs to people’s homes. Vet Parasitol. 2018;262:16–19. doi: 10.1016/j.vetpar.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 13. Agudelo C, Villareal E, Cáceres E, et al. Human and dogs toxocara canis infection in a poor neighborhood in Bogota. Mem Inst Oswaldo Cruz. 1990;85(1):75–78. doi: 10.1590/s0074-02761990000100012. [DOI] [PubMed] [Google Scholar]
- 14. Eisen AKA, Demoliner M, Oliveira KG, et al. Soil contamination of a public park by human and canine mastadenovirus, as well as hookworms and toxocara spp eggs. Rev Inst Med Trop Sao Paulo. 2019;61:e60. doi: 10.1590/S1678-9946201961060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazhab-Jafari K, Zibaei M, Maraghi S, et al. Prevalence of Toxocara eggs in the soil of public parks of Khorramshahr City, Southwest Iran. Ann Parasitol. 2019;65(4):351–356. [PubMed] [Google Scholar]
- 16. Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. Press Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 18. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 19. Barker TH, Migliavaca CB, Stein C, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21(1) doi: 10.1186/s12874-021-01381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hunter J, Saratzis A, Sutton A, Boucher R, Sayers R, Bown M. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67(8):897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 21. Alcântara N, Bavia E, Silvão RM, Carvalho E. Environmental contamination by Toxocara spp eggs in public areas of Salvador, Bahia State, Brazil. Rev Soc Brasil Med Trop. 1989;22:187–190. doi: 10.1590/s0037-86821989000400005. [DOI] [PubMed] [Google Scholar]
- 22. Alonso J, Stein M, Chamorro M, Bojanich M. Contamination of soils with eggs of Toxocara in a subtropical city in Argentina. J Helminthol. 2001;75(2):165–168. [PubMed] [Google Scholar]
- 23. Alonso JM, Luna AC, Fernández GJ, Bojanich MV, Alonso ME. Huevos de toxocara en suelos destinados a la recreación en una ciudad Argentina. Acta Bioquím Clin Latin Am. 2006;40(2):219–222. [Google Scholar]
- 24. Antonio DR, Daniel A-LV, José V-RF, Dario A-RI, Yanitza B-MY. Toxocara Spp. y otros helmintos en muestras de suelo de plazas y parques de Puerto Ordaz, Municipio Caroní, Estado Bolívar, Venezuela. Kasmera . 2020;48(2):e48231618. [Google Scholar]
- 25. Armstrong W, Oberg C, Orellana J. Presencia de huevos de parásitos con potencial zoonótico en parques y plazas públicas de la ciudad De Temuco, Región De La Araucanía, Chile. Archivos de medicina veterinaria . 2011;43(2):127–134. [Google Scholar]
- 26. Benavides Melo CJ, Vallejo Timarán DA, Astaiza Martínez JM, Bastidas Coral YS, Portilla Armero JA. Identificación De Huevos De Toxocara Spp. En Zonas Verdes De Conjuntos Cerrados Del Municipio De Pasto - Colombia. Biosalud. 2017;16(2):44– 52. [Google Scholar]
- 27. Cáceres Pinto CM, Bustinza Cárdenas RH, Valderrama Pomé AA. Contaminación con huevos de toxocara sp y evaluación sanitaria de parques en la ciudad De Abancay, Perú. Rev Invest Vet Perú. 2017;28:376–386. [Google Scholar]
- 28. Canese A, Domínguez R, Otto C, Ocampos C, Mendonca E. Huevos Infectivos de toxocara, en arenas de plazas y parques de Asunción, Paraguay. Rev Chil Ped. 2003;74(6):611–616. [Google Scholar]
- 29. Castillo D, Paredes C, Zañartu C, et al. Contaminación ambiental por huevos de Toxocara spp. en algunas plazas y parques públicos de Santiago De Chile, 1999. Bol Chil Parasitol. 2000;55(3–4):86–91. [PubMed] [Google Scholar]
- 30. Castillo Y, Bazan H, Alvarado D, Saez G. Estudio epidemiológico de toxocara canis en parques recreacionales del distrito de San Juan De Lurigancho, Lima-Perú. Parasitol al Día . 2001;25:109–114. [Google Scholar]
- 31. Cazorla Perfetti DJ, Morales Moreno P, Acosta Quintero ME. Contaminación de suelos con huevos de Toxocara spp.(Nematoda, Ascaridida) en parques públicos de la ciudad de Coro, Estado Falcón, Venezuela. Rev Científ. 2007;17(2):117–122. [Google Scholar]
- 32. Chávez A, Casas E, Serrano M, et al. Riesgo De Contraer Enfermedades Parasitarias En Los Parques Públicos De Lima Y Callao. Rev Investig Vet Perú . 2002;13(2):84–91. [Google Scholar]
- 33. Devera R, Blanco Y, Hernández H, Simoes D. Toxocara Spp. Y Otros helmintos en plazas y parques de ciudad Bolívar, Estado Bolívar (Venezuela) Enferm Infec Microbiol Clín. 2008;26(1):23–26. doi: 10.1157/13114391. [DOI] [PubMed] [Google Scholar]
- 34. Díaz-Anaya AM, Pulido-Medellín MO, Giraldo-Forero JC. Nematodos con potencial zoonótico en parques públicos de la ciudad De Tunja, Colombia. Salud Pub Mexico . 2015;57(2):170–176. [PubMed] [Google Scholar]
- 35. Eisen AKA, Demoliner M, Oliveira KGd, et al. Soil Contamination of a Public Park by Human and Canine Mastadenovirus, as Well as Hookworms and Toxocara Spp Eggs. Rev Inst Med Trop São Paulo . 2019;61 doi: 10.1590/S1678-9946201961060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fonrouge R, Guardis MdV, Radman NE, Archelli SM. Contaminación de suelos con huevos de toxocara sp. en plazas y parques públicos de la ciudad de La Plata. Buenos Aires, Argentina. Bol Chil Parasitol. 2000;55(3–4):83–85. [PubMed] [Google Scholar]
- 37. Gallardo J, Forlano M. Diagnóstico de huevos de toxocara spp. del suelo en parques y plazas públicas de la ciudad de Barquisimeto, Estado Lara, Venezuela. Gac Cien Vet. 2015;20(1):4–9. [Google Scholar]
- 38. García Blásquez DP. Presencia De Huevos De Toxocara Spp. En Parques Públicos Del Distrito De Jesús De Nazareno En La Región Ayacucho. 2017 [Google Scholar]
- 39. Guarín-Patarroyo CE, Serrato MJ, Sánchez-Cuerv FR. Determinación de huevos de Toxocara canis en suelo de tres parques públicos de Duitama (Boyacá) Cien Agricul. 2016;13(1):59–66. [Google Scholar]
- 40. Hernández S, Contera M, Acufia A, Elhordoy D, Vignolo J. Toxocara spp en muestras de suelo y heces de plazas de la ciudad de Monte Video. J Trop Pathol. 2003;32(1) [Google Scholar]
- 41. Iannacone J, Flores LA, Cárdenas-Callirgos J. Contaminación de los suelos con huevos de Toxocara canis en parques públicos de Santiago De Surco, Lima, Perú 2007–2008. Neotrop Helminthol. 2012;6(1):97–108. [Google Scholar]
- 42. Lara-Reyes E, Figueroa-Ochoa J, Quijano-Hernández I, et al. Frecuencia de parásitos gastrointestinales de perros en parques públicos de dos municipios vecinos del Estado De México. Nova . 2019;17(32):75–81. [Google Scholar]
- 43. Lee DAB, de Oliveira ELC, Lee GAS, da Silva PL, Santos POM, Lima VFS. Potentially zoonotic parasites in the soil of public squares in the city of Aracaju (Sergipe, Northeastern Brazil) Vet Parasitol Reg Studies Reports . 2021;26:100619. doi: 10.1016/j.vprsr.2021.100619. [DOI] [PubMed] [Google Scholar]
- 44.Lenina Tamara MH.Prevalencia Huevos De Toxocara Canis Y Otros Helmintos En Parques De La Habana. Paper presented at the Cuba Salud; 2018; 2018. [Google Scholar]
- 45. Lettieri Teixeira M, Rossi Lp, De Freitas L, Gasparin N, Piva S, And Meneghello Fuentefria A. Prevalence of Toxocara canis infection in public squares of the Concórdia City, Santa Catarina, Brazil. Parasitol Latinoamer. 2008;63:69–71. [Google Scholar]
- 46. López F, Chávez A, Casas E. Contaminación de los parques públicos de los distritos de Lima oeste con huevos de Toxocara sp. Rev Invest Vet Perú. 2005;16(1):76–81. [Google Scholar]
- 47. Malca C, Chávez VA, Pinedo VR, Abad-Ameri D. Contaminación con huevos de toxocara spp en parques públicos del distrito de La Molina, Lima, Y Su Relación Con El programa de vigilancia sanitaria de parques y jardines. Rev Invest Vet Perú. 2019;30:848–855. [Google Scholar]
- 48. Marques JP, Guimarães CdR, Boas AV, Carnaúba PU, Moraes Jd. Contamination of Public parks and squares from guarulhos (são paulo state, brazil) by Toxocara spp. and and Anchylostoma spp. Rev Inst Med Trop São Paulo. 2012;54:267–271. doi: 10.1590/s0036-46652012000500006. [DOI] [PubMed] [Google Scholar]
- 49. Martínez-Barbabosa I, Presas AMF, Tsuji ÓV, Hernández AR. Frecuencia de toxocara canis en perros y áreas verdes del sur de la ciudad de México, distrito federal. Vet México. 1998;29(3):239–244. [Google Scholar]
- 50. Medina-Pinto RA, Rodríguez-Vivas RI, Bolio-González ME. Nematodos intestinales de perros en parques públicos de Yucatán, México. Biomédica. 2018;38(1):105–110. doi: 10.7705/biomedica.v38i0.3595. [DOI] [PubMed] [Google Scholar]
- 51. Melín-Coloma M, Villaguala-Pacheco C, Lisboa-Navarro R, Landaeta-Aqueveque C. Estudio De La Presencia De Huevos De Toxocara Sp. En Suelos De Áreas Públicas De La Ciudad De Chillán, Chile. Rev Chil Infectol. 2016;33(4):428–432. doi: 10.4067/S0716-10182016000400007. [DOI] [PubMed] [Google Scholar]
- 52. Mendoza Meza DL, Maldonado Santana H. Optimización de una metodología para el aislamiento y detección molecular de huevos de Toxocara canis en Muestras De Suelo. 2015 [Google Scholar]
- 53. Montalvo-Sabino E, Cipriano-Fonseca F, Marcelo-Andrade E, et al. Factors associated with contamination of public parks (Huánuco, Perú) by Toxocara canis eggs and other endoparasites of zoonotic importance. Neotrop Helm. 2014;8(2):259–268. [Google Scholar]
- 54. Pajuelo DF, Quispe RQ, Prado AR, Lozano LL. Prevalencia de huevos de toxocara spp. en áreas recreacionales del distrito Gregorio Albarrací Lanchipa Y El Nivel De Contaminación (Ligero, Moderado, Alto) Ciencia & Desarrollo. 2019;(24):58–65. [Google Scholar]
- 55. Paquet-Durand I, Hernández J, Dolz G, Zuñiga JJ, Schnieder T, Epe C. Prevalence of Toxocara spp., Toxascaris leonina and Ancylostomidae in public parks and beaches in different climate zones of Costa Rica. Acta Trop. 2007;104(1):30–37. doi: 10.1016/j.actatropica.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 56. Polo-Terán LJ, Cortés-Vecino JA, Villamil-Jiménez LC, Prieto E. Contaminación de los parques públicos de la localidad de suba, bogotá con nematodos zoonóticos. Rev Salud Pub. 2007;9:550–557. doi: 10.1590/s0124-00642007000400007. [DOI] [PubMed] [Google Scholar]
- 57. Poma R, Alvarado A, Bernal N, Pallarico N, Alanes L. Presencia de huevos de toxócara spp. en plazas con parques de la ciudad de El Alto, Bolivia. Rev Estud Agro-Vet. 2018;2(2):234–241. [Google Scholar]
- 58. Quinche FSG, Marcillo RLG, Carrasco RUC. Contaminación ambiental con huevos de toxocara spp, en los parques públicos de la ciudad De Loja. Rev Ecuat Ciencia Animal. 2019;3(1):80–92. [Google Scholar]
- 59. Ramírez-Rubio L, García-Cueto OR, Tinoco-Gracia L, Quintero-Núñez M, Cueto-González SA, Trasviña-Muñoz E. Frecuencia De Huevos De Toxocara Canis En Parques Públicos De Mexicali, Baja California, México. Revista internacional de contaminación ambiental. 2019;35(3):589–595. [Google Scholar]
- 60. Romero Núñez C, García Contreras AdC, Mendoza Martínez GD, Torres Corona NC, Ramírez Durán N. Contaminación por Toxocara spp. en parques de Tulyehualco, México. Rev Científica. 2009;19(3):253–256. [Google Scholar]
- 61. Romero-Núñez C, Yañez-Arteaga S, Mendoza-Martínez GD, Bustamante-Montes LP, Ramírez-Durán N. Contaminación y viabilidad de huevos de Toxocara spp. en suelo y heces colectadas en parques públicos, Calles Y Perros En Toluca, México. Rev Científica. 2013;23(6):475–479. [Google Scholar]
- 62. Salinas P, Matamala M, Schenone H. Prevalencia de hallazgo de huevos de Toxocara canis en plazas de la región metropolitana de la ciudad de Santiago, Chile. Bol Chil Parasitol. 2001;56(3–4):102–105. [Google Scholar]
- 63. Santarém VA, Sartor IF, Bergamo FMM. Contaminação, Por ovos de Toxocara spp, de parques e praças públicas de Botucatu, São Paulo, Brasil. Rev Soc Bras Med Trop. 1998;31:529–532. [PubMed] [Google Scholar]
- 64. Tiyo R, Guedes T, Falavigna D, Falavigna-Guilherme A. Seasonal Contamination of public squares and lawns by parasites with zoonotic potential in Southern Brazil. J Helminthol. 2008;82(1):1–6. doi: 10.1017/S0022149X07870829. [DOI] [PubMed] [Google Scholar]
- 65. Vargas Nava AI, Castro Del Campo N, EnrÍquez Verdugo I, Portillo Loera JJ, Barraza Tizoc CL, Gaxiola Camacho SM. Prevalence and Viability of Toxocara Spp. Eggs in Soil of Public Parks in Northwestern Mexico. Iran J Parasitol. 2020;15(2):196–203. [PMC free article] [PubMed] [Google Scholar]
- 66. Vásquez Tsuji O, Ruiz Hernández A, Martínez Barbarosa I, Merlín Marín PN, Tay Zavala J, Pérez Torres A. Contaminación de suelos por huevos de toxocara sp en parques públicos y jardines de casas-habitación de la Ciudad De México. Bol Chil Parasitol . 1996:54–58. [PubMed] [Google Scholar]
- 67. Vidal MLB, Azevedo J, Novaes MT, Martins IVF. Diagnostic of gastrointestinal helminths in sand and canine feces from public locations in Alegre City, Espírito Santo–Brazil. Braz J Vet Med. 2019;41:e104619. [Google Scholar]
- 68. Virgilio La Rosa V, Chávez A, Casas E. Contaminación De Parques Públicos Del Cono Norte Con Huevos De Toxocara Spp. Rev Invest Vet Perú. 2001;12(1):116–121. [Google Scholar]
- 69. Young-Candia C, Yauri-Lazo R, Yance-Contreras S, et al. Frecuencia De Toxocara Sp. En Los Parques Del Distrito De Breña. Lima-Perú, 2010. Rev Per Epidemiol. 2011;15(3):1–4. [Google Scholar]
- 70. Bolívar-Mejía A, Rodríguez-Morales AJ, Paniz-Mondolfi AE, Delgado O. Cardiovascular manifestations of human toxocariasis. Arch Cardiol Mexico. 2013;83(2):120–129. doi: 10.1016/j.acmx.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 71. Gómez-Marín JE, Londoño ÁL, Cabeza-Acevedo N, et al. Ocular Toxocariasis in parasitology consultation in Quindío, Colombia: description of cases and contact studies. J Trop Ped. 2021;67(1) doi: 10.1093/tropej/fmaa096. [DOI] [PubMed] [Google Scholar]
- 72. de-la-Torre A, López-Castillo CA, Rueda JC, Mantilla RD, Gómez-Marín JE, Anaya JM. Clinical patterns of uveitis in two ophthalmology centres in Bogota, Colombia. Clin Exp Ophthalmol. 2009;37(5):458–466. doi: 10.1111/j.1442-9071.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- 73. Wygant CM, Cohle SD. Fatal Visceral Larva Migrans from Toxocara catis infection of the heart and liver in a child. Cardiovasc Pathol. 2023;63:107496. doi: 10.1016/j.carpath.2022.107496. [DOI] [PubMed] [Google Scholar]
- 74. Delgado O, Rodríguez-Morales AJ. Aspectos clínico-epidemiológicos de la toxocariasis: una enfermedad desatendida en Venezuela y América Latina. Bol Malar Salud Ambiental. 2009;49:1–33. [Google Scholar]
- 75. Fahrion AS, Schnyder M, Wichert B, Deplazes P. Toxocara eggs shed by dogs and cats and their molecular and morphometric species-specific identification: is the finding of t. cati eggs shed by dogs of epidemiological relevance? Vet Parasitol. 2011;177(1–2):186–189. doi: 10.1016/j.vetpar.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 76. Ikotun K, Sowemimo O, Chou CM, et al. High seroprevalence of Toxocara antibodies in pregnant women attending an antenatal clinic at a university hospital in Ile-Ife, Nigeria. Trans R Soc Trop Med Hyg. 2020;114(4):301–307. doi: 10.1093/trstmh/trz116. [DOI] [PubMed] [Google Scholar]
- 77. Khazan H, Khazaei M, Tabaee SS, Mehrabi A. Prevalence of Toxocara spp. eggs in public parks in Tehran City, Iran. Iran J Parasitol. 2012;7(3):38–42. [PMC free article] [PubMed] [Google Scholar]
- 78. Walsh MG. Toxocara infection and diminished lung function in a nationally representative sample from the United States Population. Int J Parasitol. 2011;41(2):243–247. doi: 10.1016/j.ijpara.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 79. Giacometti A, Cerioni O, Fortuna M, et al. Prevalence of Toxocara spp in public playgrounds in a town of Central Italy. Infez Med. 1999;7(3):163–167. [PubMed] [Google Scholar]
- 80. Allahdin S, Khademvatan S, Rafiei A, Momen A, Rafiei R. Frequency of Toxoplasma and Toxocara spp. antibodies in epileptic patients, in South Western Iran. Iran J Child Neurol. 2015;9(4):32–40. [PMC free article] [PubMed] [Google Scholar]
- 81. Eslahi AV, Badri M, Khorshidi A, et al. Prevalence of Toxocara and Toxascaris infection among human and animals in Iran with meta-analysis approach. BMC Infect Dis. 2020;20(1):20. doi: 10.1186/s12879-020-4759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Borecka A, Gawor J. Prevalence of Toxocara canis infection in dogs in the Warszawa Area. Wiad Parazytol. 2000;46(4):459–462. [PubMed] [Google Scholar]
- 83. Nicoletti A, Gomez-Puerta LA, Arroyo G, et al. Toxocara brain infection in pigs is not associated with visible lesions on brain magnetic resonance imaging. Am J Trop Med Hyg. 2020;103(1):273–275. doi: 10.4269/ajtmh.19-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Arpino C, Gattinara GC, Piergili D, Curatolo P. Toxocara Infection and epilepsy in children: a case-control study. Epilepsia. 1990;31(1):33–36. doi: 10.1111/j.1528-1157.1990.tb05356.x. [DOI] [PubMed] [Google Scholar]
- 85. Giudice PAF, Lescano SAZ, Gonzales WHR, et al. Serosurvey and associated risk factors of anti-toxocara spp. antibodies in bovines from slaughterhouses of Southeastern Brazil. Parasit Vectors. 2021;14(1):250. doi: 10.1186/s13071-021-04755-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abdel Aziz AR, Hassan AA, Elmahallawy EK, Elshahawy IS, Almuzaini AM. Prevalence and Associated Risk Factors of Toxocara Infection in Dogs in Northern and Southern Egypt. Vet Parasitol Reg Stud Reports. 2019;17:100305. doi: 10.1016/j.vprsr.2019.100305. [DOI] [PubMed] [Google Scholar]
- 87. Alvarado-Esquivel C. Toxocara infection in psychiatric inpatients: a case control seroprevalence study. PLoS One. 2013;8(4):e62606. doi: 10.1371/journal.pone.0062606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bradbury RS, Hobbs CV. Toxocara seroprevalence in the USA and its impact for individuals and society. Adv Parasitol. 2020;109:317–339. doi: 10.1016/bs.apar.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 89. Toxocara Canis. J Small Anim Pract. 1997;38(11):531–534. [PubMed] [Google Scholar]
- 90. Alvarado-Esquivel C, Alvarado-Felix AO, Alvarado-Felix GA. Low toxocara seroprevalence in people in rural Durango, Mexico. Eur J Microbiol Immunol (Bp) 2019;9(3):91–93. doi: 10.1556/1886.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holland C, Smith HV. Toxocara: The Enigmatic Parasite. Wallingford, UK: Cambridge, MA, CABI Pub; 2006. [Google Scholar]
- 92. Dumenigo B, Lau N, Bravo JR. Prevalence of Toxocara canis in dogs in the city of Havana. Rev Cubana Med Trop. 1994;46(2):99–102. [PubMed] [Google Scholar]
- 93. Alvarado-Esquivel C, Hernandez-Tinoco J, Sanchez-Anguiano LF. Toxocara infection in gardeners: a case control seroprevalence study. Asian Pac J Trop Med. 2014;7S1:S79–81. doi: 10.1016/S1995-7645(14)60207-8. [DOI] [PubMed] [Google Scholar]
- 94. Alvarado-Esquivel C, Hernandez-Tinoco J, Sanchez-Anguiano LF. Seroepidemiology of Toxocara infection in patients with vision impairment and blindness in Durango, Mexico. J Clin Med Res. 2015;7(3):176–181. doi: 10.14740/jocmr2032w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Abe N, Yasukawa A. Prevalence of Toxocara Sspp. eggs in sandpits of parks in Osaka City, Japan, with notes on the prevention of egg contamination by fence construction. J Vet Med Sci. 1997;59(1):79–80. doi: 10.1292/jvms.59.79. [DOI] [PubMed] [Google Scholar]
- 96. Abedi B, Akbari M, KkodaShenas S, et al. The global prevalence of toxocara spp. in pediatrics: a systematic review and meta-analysis. Clin Exp Pediatr. 2021;64(11):575–581. doi: 10.3345/cep.2020.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Carden SM, Meusemann R, Walker J, et al. Toxocara canis: egg presence in melbourne parks and disease incidence in Victoria. Clin Exp Ophthalmol. 2003;31(2):143–146. doi: 10.1046/j.1442-9071.2003.00622.x. [DOI] [PubMed] [Google Scholar]
- 98. De Andrade Lima Coelho R, De Carvalho LB, Jr, Perez EP, et al. Prevalence of Toxocariasis in northeastern brazil based on serology using recombinant toxocara canis antigen. Am J Trop Med Hyg. 2005;72(1):103–107. [PubMed] [Google Scholar]
- 99. Fragoso RP, Monteiro MB, Lemos EM, Pereira FE. Anti-toxocara antibodies detected in children attending elementary school in Vitoria, State of Espirito Santo, Brazil: Prevalence and Associated Factors. Rev Soc Bras Med Trop. 2011;44(4):461–466. doi: 10.1590/s0037-86822011000400012. [DOI] [PubMed] [Google Scholar]
- 100. Gothe R, Reichler I. Toxocara canis: frequency of detection and extent of infection in bitches of various breeds and husbandry and their litters in South Germany. Tierarztl Prax. 1990;18(3):293–300. [PubMed] [Google Scholar]