Summary
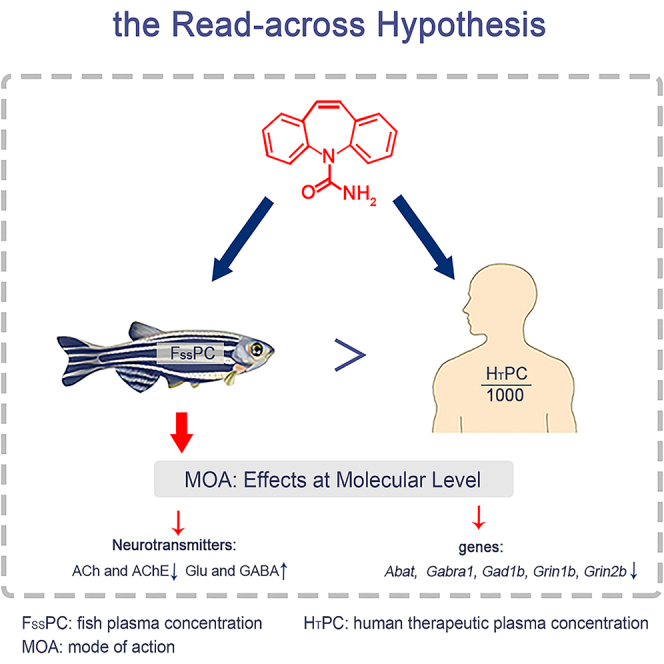
The fish plasma model (FPM) facilitated the environmental risk assessment of human drugs by using existing data on human therapeutic plasma concentrations (HTPCs) and predicted fish plasma concentrations (FPCs). However, studies on carbamazepine (CMZ) with both the mode of action (MOA) based biological effects at molecular level (such as neurotransmitter and gene level) and measured FPCs are lacking. Bioconcentration of CMZ in adult zebrafish demonstrated that the FPM underestimated the bioconcentration factors (BCFs) in plasma at environmental CMZ exposure concentrations (1–100 μg/L). CMZ significantly increased Glu and GABA, decreased ACh and AChE as well as inhibited the transcription levels of gabra1, grin1b, grin2b, gad1b, and abat when the actual FPCs were in the ranges of 1/1000 HTPC to HTPC. It is the first read-across study of CMZ integrating MOA-based biological effects at molecular level and FPCs. This study facilitates model performance against a range of different drug classes.
Subject areas: Biological sciences, Biochemistry, Physiology, Animal physiology
Graphical abstract
Highlights
-
•
The fish plasma model underestimated CMZ concentration at environmental levels
-
•
CMZ increased Glu and GABA, and decreased the levels of ACh and AChE
-
•
CMZ inhibited the transcription levels of gabra1, grin1b, grin2b, gad1b, and abat
-
•
CMZ showed a human-like biological effect on zebrafish at therapeutic plasma levels
Biological sciences; Biochemistry; Physiology; Animal physiology
Introduction
Active pharmaceutical ingredients (APIs) for human use, considered “pseudo-persistent” pollutants that continuously enter the aquatic environment through wastewater treatment plant effluent,1 expose aquatic organisms to large amounts of pharmaceutical residues in their habitat throughout their life cycle. Accumulation of several active human pharmaceuticals has been found in wild aquatic animals living in rivers and lakes.2 The biological effects of several human APIs, such as psychiatric medications, on fish have been found to be similar to those of their mode of action (MOA).3,4,5 The read-across hypothesis states that pharmacological effects of the drug on non-targeted organisms are observed when the concentration of the drug in non-targeted biological plasma is equal to the human therapeutic plasma concentration (HTPC), provided that the molecular target (usually a receptor or enzyme) is conserved.2,6 Several studies7,8,9 have demonstrated the evolutionary conservation of some protein targets of drugs that are structurally and functionally conserved in zebrafish (Danio rerio) and other aquatic species. Gunnarsson et al.7 examined 1318 human drug targets, 86% of which were conserved in zebrafish. The read-across hypothesis provides a reasonable idea for qualitatively predicting potential effects in non-target species, particularly fish, using data obtained during the drug discovery and development phases. The key to “reading across” the biological effects of drugs from humans to fish is to compare the fish plasma concentrations (FPCs) with the HTPCs.
Based on the read-across hypothesis, Huggett et al.10 developed the fish plasma model (FPM), which has been proposed as a toxicity screening technique to prioritize drugs with higher potential harm to wild fish.11,12 In this model, the theoretical partition coefficient of drugs between fish plasma and water (BCFplasma) can be calculated based on chemical lipophilicity, and then the FPCs of drugs can be predicted by combining them with the environmental concentrations in water.13,14 The FPM provides crucial FPC values for read-across of biological effects that can be compared with the HTPCs for environmental risk assessment. However, the FPM was developed using rainbow trout for hydrophobic organic compounds and, in its current formulation, does not account for interspecific or interindividual variability in pharmacokinetic and pharmacodynamic parameters.13,15 Huggett et al.10 suggested the use of a safety factor of 1000, which can be considered an initial assessment of uncertainty and should be further refined to account for the degree of uncertainty expressed in the model. In short, the reliability of FPC prediction by the FPM for polar drugs and certain fish species needs further verification. Our previous work16 on 31 polar endocrine disrupting compounds (EDCs) in the plasma of wild fish from Lake Taihu showed that the bioaccumulation of EDCs in fish plasma is not only dependent on hydrophobicity but also fish species-specific and compound-dependent. The classic FPM was appropriate for only a few EDCs (7 of 20), and the FPM underestimated the log BAFs (bioaccumulation factors) of the most less hydrophobic EDCs (log Kow: 1.5–3.5, 11 out of 20) while overestimating the log BAFs of several relatively higher hydrophobic EDCs (log Kow: 3.87–5.9) in fish plasma. Some studies have shown that FPM underestimates the FPC value of the active drug12,17 and thus may underestimate the environmental risk. The findings of oxazepam18 and tramadol19 about FPM suggested the differences between the predicted FPC and measured FPC are also due to drug types, external exposure concentrations, log Kow (octanol: water partition coefficient, a measure of lipophilicity) and log D (distribution coefficient). In most existing drug studies, the FPCs predicted by the FPM were used directly, and only a few studies used the measured FPC values to verify FPM.20 Therefore, it is necessary to validate the FPM of polar drugs combined with the measured FPCs.
To our knowledge, for neurological drugs (targeting at central nervous system), there are only four relevant studies18,19,21,22 that combine FPCs with the behavior of fish after exposure to neurological drugs as indicative endpoints, which can verify the read-across hypothesis to some extent. However, the previous studies of FPM on human drugs and biological effects for the read-across hypothesis lack a more direct correlation of FPCs with indicative endpoints at the molecular level based on MOA, that is, the behavioral results cannot confirm that the MOA of drugs is the same as that of humans. In a related article,23 mixtures of diclofenac and other nonsteroidal anti-inflammatory drugs were exposed to fish, and the read-across hypothesis was verified by examining the drug concentration, oxidative stress indicators, and corresponding genes in plasma. Nevertheless, the corresponding indicators are not specific enough due to the combination of multiple drugs. However, it is important to note that there are no established MOA relevant endpoints for antidepressants (in any species), except for serotonin transport inhibition at the synaptic level and behavioral modifications.22 Only ibuprofen24 and beclomethasone dipropionate (BDP)25 (immune system drugs) were studied on the read-across hypothesis at the genetic level. Comparing the effects of two different glucocorticoids, Margiotta-Casaluci et al.25 found gene expression was more sensitive than phenotypic responses at lower exposure concentrations. Patel et al.24 did not observe a relationship between gill cyclooxygenase (ptgs) gene expression and plasma ibuprofen, suggesting a need for understanding the sensitivity of genes as endpoints. Therefore, it is urgent to directly validate the read-across hypothesis of human drugs by combing the MOA-based biological effects at the molecular level and internal dosimetry of plasmas.
Carbamazepine (CMZ) can be used to treat psychomotor epilepsy and bipolar disorder,26 which can bring antiepileptic drug-induced encephalopathy (ADE) such as dizziness, ataxia, nystagmus, and tachycardia to varying degrees in patients with the increase of its dose27,28 by changing the antidiuretic hormone and norepinephrine mechanism.29 The concentrations of CMZ in the natural water environment were in the range of 1 ng/L to 12 μg/L.30 The reported concentration in municipal and urban wastewater was as high as 259 μg/L.31 CMZ modulates neurotransmitter release, uptake, and receptor binding, and acts as an agonist for human gamma-aminobutyric acid (GABA) receptors.32 One of the mechanisms of CMZ in humans is associated with the GABA neurotransmitter system. In mammals, CMZ can reduce impulse frequency during epileptic crises by regulating voltage-gated sodium channels, preventing action potential generation, and cell depolarization. As an agonist on GABA receptors, it also inhibits glutamic acid (Glu) release and chloride entry into the cell to achieve the same effect described previously. Gunnarsson et al.7 found the sequence similarity to human neuroactive drug targets (blocking voltage-gated sodium channels, one of the mechanisms of CMZ), was 62% in zebrafish. CMZ can also influence acetylcholine (ACh) secretion by regulating Ca2+ voltage gate channels.33 Studies have found that the expression of genes in the hypothalamus-pituitary-interrenal (HPI) (crha, actha, and ) axis and GABA (gad2 and abat) pathway were also altered by 100 μg/L of CMZ.34 Neurotransmitters such as GABA, Glu, ACh, and dopamine, which have been studied in zebrafish,35 mice,36 and humans37 by enzyme-linked immunosorbent assay (ELISA), can be used as specific MOA endpoints of CMZ. However, the previous studies on zebrafish did not focus on FPM, nor did they examine the relationship between internal exposure concentrations and biological effects. Studies on the biological effects of CMZ on zebrafish mainly focus on growth, development, oxidative stress, reproduction, etc.,32,38,39 which are not directly related to the target system of CMZ. The behavioral effects related to MOA are mostly based on external exposure concentration but not FPCs.40 Moreover, there has been little research on the expression of neurotransmitters and neurotransmitter-related genes based on FPCs and the FPM. CMZ, which has a clear and specific MOA, is a suitable model compound for the read-across hypothesis and the FPM research.20
The zebrafish is a small subtropical freshwater bony fish. Due to its similar function of the main neurotransmitter system to that of mammals, simple and clearly distinguished brain tissue, and about 75% of its sequenced genome similar to that of the human genome, it is popular as a preclinical screening in drug research.41 Zebrafish are frequently used as model animals in toxicological research because of their transparent bodies, which make it simple to examine morphological and physiological changes following treatment.42
FPM/read-across studies on antiepileptic/anticonvulsant drugs such as CMZ have been poorly studied. In addition, MOA-relevant endpoints of CMZ at the molecular level have not been used, especially neurotransmitters and neurotransmitter-related genes that are closely related to neural activity. It is helpful to facilitate model performance against a range of different drug classes by integrating FPCs and MOA-based biological effects at the molecular level. This study will directly address all facets of the read-across hypothesis by combining the MOA-based endpoints at the molecular level, internal exposure concentrations (zebrafish plasma concentrations), and external exposure concentrations (water concentrations). The objectives of our research were to (i) test the FPM of CMZ and (ii) investigate the MOA-based biological effects at molecular level using internal dosimetry. This study will directly verify the read-across hypothesis of CMZ in zebrafish and provide theoretical and technical support for the environmental risk assessment of drugs.
Results
Uptake and bioconcentration of CMZ in zebrafish
Uptake in fish muscle and plasma
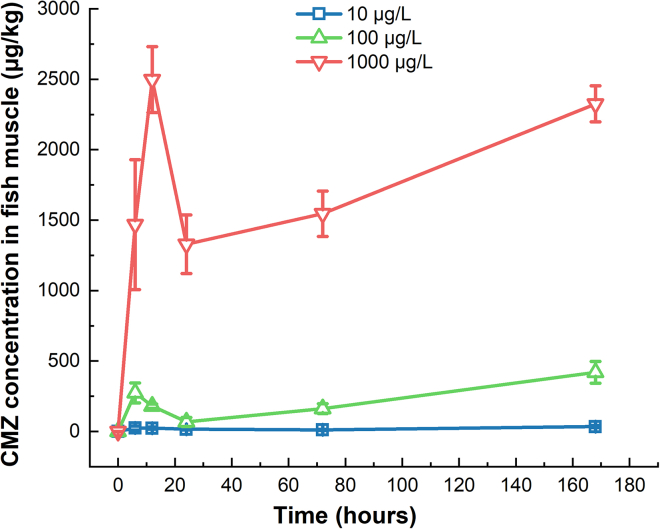
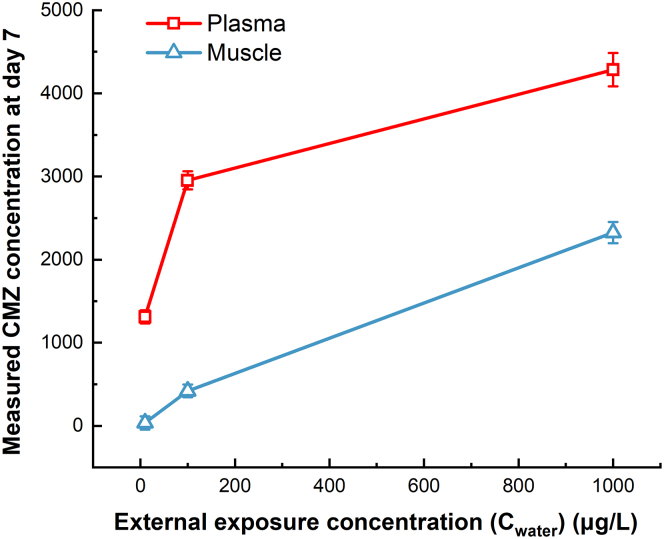
The uptake kinetics of CMZ in the muscle of zebrafish at different levels of external exposure (10, 100, 1000 μg/L) were shown in Figure 1. The external concentration (EC) of CMZ at 1 μg/L was inherently low, the changes in CMZ concentration in fish muscle were not as significant as those at the three high levels (10, 100, 1000 μg/L). Therefore, exposure settings with higher CMZ concentrations (10, 100, 1000 μg/L) were preferred to be used to explore the uptake and bioconcentration of CMZ in zebrafish. After reaching the first peak at 6–12 h, the concentration of CMZ decreased significantly at 12–24 h. After that, the concentration rised again, probably due to a second peak. After seven days of exposure, the CMZ concentrations in muscle were 35.36 ± 7.90, 420.32 ± 77.55, and 2325.40 ± 127.13 μg/kg (mean ± SD, n = 3) at external exposure concentrations of 10, 100, and 1000 μg/L, respectively. The CMZ concentrations in the muscles increased with the corresponding measured plasma concentrations, but were significantly lower than those in plasma (Figure 2). The spearman rank correlation tests revealed that on day 7, there was a good correlation between the concentration of CMZ in muscle and plasma (R2 = 0.72, , n = 3). Nichols et al.43 proposed that the tissue-plasma partition coefficient value might be a good indicator for estimating drug distribution in fish. Moreover, a higher tissue-plasma partition coefficient indicates higher transportability from blood to tissue.44 Muscle-plasma partition coefficient (L/kg) was calculated on day 7 of this study (Table 1). The mean muscle-plasma partition coefficients of the drug were 0.14 ± 0.03 to 0.54 ± 0.03 (mean ± SD, n = 3) at different levels, lower than that of CMZ in wild fish (1.1 L/kg) measured by Tanoue et al.,44 indicating that the transport capacity of CMZ from zebrafish plasma to muscle was relatively low, which may partly explain the discrepancy between the values predicted by the FPM and those measured in our study.
Figure 1.
Fish-muscle uptake of CMZ in 7-day exposure at various concentrations of CMZ
All the CMZ concentrations were detected by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). After reaching the first peak at 6–12 h, the concentration of CMZ decreased at 12–24 h. After that, the concentration rised again, probably to a second peak. Error bars represent as mean ± SD (n = 3).
Figure 2.
Bioconcentration of CMZ in fish plasma (μg/L) and muscle (μg/kg) after 7-day exposure with various external concentrations (10, 100, and 1000 μg/L)
All the CMZ concentrations were detected by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). The CMZ concentrations in the muscle increased with the corresponding measured plasma concentrations, but were significantly lower than those in plasma. Error bars represent as mean ± SD (n = 3).
Table 1.
Muscle-plasma partition coefficient of CMZ at day 7
| Concentration of CMZ (μg/L) | 10 | 100 | 1000 |
|---|---|---|---|
| Muscle-plasma partition coefficient | 0.26 ± 0.06 | 0.14 ± 0.03 | 0.54 ± 0.03 |
The mean muscle-plasma partition coefficients of CMZ at different levels were less than 0.55. Plus or minus represent as mean ± SD (n = 3).
Predicted versus measured fish plasma concentrations
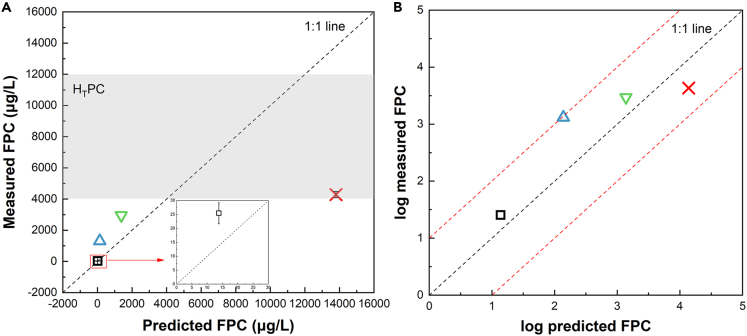
The predicted FPCs by Equation 2 of the FPM as well as the actual measured FPCs were listed in Table 2 and Figure 3. The predicted FPCs of the three low-concentration exposed groups (1, 10, and 100 μg/L), were significantly lower than the actual measured FPCs; however, the situation was opposite in the high concentration exposure group (1000 μg/L). Similar results were observed by Weil et al.,45 that the actual FPCs of salbutamol in Pimephales promelas were underestimated at low concentrations and overestimated at high concentrations by the FPM. Discrepancies between predicted FPCs by the FPM and measured FPCs were especially pronounced at 10 and 1000 μg/L in our study. When the external exposure concentration was 10 μg/L, the difference was the largest, and the measured FPC value was about 9.5 times the predicted value. At the highest exposure concentration of 1000 μg/L, the measured FPC value was only 31.05% of the predicted value. The bioconcentration of CMZ in zebrafish under different external exposure concentrations was quite different. When exposed to 10 μg/L CMZ, the measured BCFplasma was one order of magnitude higher than the predicted BCFplasma. In contrast to the aforementioned, the measured BCFplasma were approximately one-third of the predicted BCFplasma at 1000 μg/L CMZ. However, as shown in Figure 3B, the bioconcentration factors (BCFs) of zebrafish were still within the predicted range (the measured BCFs were between 0.1 and 10 times the theoretical BCFs). Comparison of FPM from Coote natural Marsh in Canada showed that the measured BCFs of phenytoin, N,N-diethyl-m-toluamide, fluoxetine, valsartan, and triclocarban in carp (log KOW values ranged from 2.2 to 4.9) were within 3 times of the predicted BCFs.46 In addition, the lower the effect ratio (ER) values, the higher the probability of pharmacological response. In particular, drugs with an ER < 1000 are of concern (1000 is a safety factor that takes into account a 10-fold variation from humans to animals, a 10-fold variation in species sensitivity, and a 10-fold variation from mammals to non-mammals). In our study, the ER values, based on the ratio of measured HTPC and FSSPC calculated from Equation 3, were < 1000, indicating that pharmacological responses associated with therapeutic activity would be observed in exposed zebrafish.
Table 2.
Predicted and measured values of fish plasma concentrations (FPCs), effect ratio (ER) and bioconcentration factors in plasma (BCFplasma) at 7-day exposure at various external water concentrations
| External exposure concentrationa (Cwater) (μg/L) | 1 | 10 | 100 | 1000 |
|---|---|---|---|---|
| Predicted FPCb (μg/L) | 13.80 | 138.00 | 1380.00 | 13800.00 |
| Measured FPCb (μg/L) | 25.47 ± 3.78 | 1311.10 ± 80.26 | 2953.30 ± 109.20 | 4284.33 ± 200.52 |
| Measured FPC/Predicted FPC | 1.84 ± 0.27 | 9.50 ± 0.58 | 2.14 ± 0.08 | 0.31 ± 0.01 |
| Predicted ERc | 289.86e - 869.57d | 28.99e - 86.96d | 2.90e - 8.70d | 0.29e - 0.87d |
| Measured ERc | 157.07e - 471.20d | 3.05e - 9.15d | 1.35e - 4.06d | 0.93e - 2.80d |
| Predicted BCFplasmaf | 13.80 | |||
| Measured BCFplasmag | 25.47 ± 3.78 | 131.11 ± 8.03 | 29.53 ± 1.09 | 4.28 ± 0.20 |
The predicted FPCs were lower than the actual measured FPCs at environmentally relevant concentrations (1, 10 and 100 μg/L); the predicted FPCs were higher than the measured FPCs at 1000 μg/L. The predicted BCFplasma were lower than the actual measured BCFplasma at environmentally relevant concentrations. The ER values were <1000, indicating that pharmacological responses associated with therapeutic activity would be observed in exposed zebrafish. Plus or minus represent as mean ± SD (n = 3).
Nominal concentration.
Cwater × Pblood: water.
HTPC/FSSPC.
Minimum HTPC: 4000 μg/L.
Maximum HTPC: 12000 μg/L.
Pblood: water.
FssPC/Cwater.
Figure 3.
Relationship between predicted and measured fish plasma concentrations (FPCs) of CMZ in zebrafish following a 7-day study
The predicted plasma concentration is based on Equations 1 and 2. All the plasma concentrations were detected by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS).
(A) Relationship between predicted and measured FPC. The predicted FPCs at 1, 10, and 100 μg/L were lower than the actual measured FPCs. The measured FPCs were less than the maximum human therapeutic concentration (HTPC, 12000 μg/L). The gray area in figure (A) indicates the HTPC range: 4000–12000 μg/L.
(B) Relationship between log predicted FPC and log measured FPC. The measured FPCs were in the range of 10 times the predicted FPCs. The red dashed line denotes log unit deviations. The black dashed line is 1:1 line. External exposure concentration for each plasma concentration: □: 1 μg/L;  : 10 μg/L;
: 10 μg/L;  : 100 μg/L;
: 100 μg/L;  : 1000 μg/L. Error bars represent as mean ± SD (n = 3).
: 1000 μg/L. Error bars represent as mean ± SD (n = 3).
Biological effects based on FPCs and external exposure concentrations of CMZ
Neurotransmitters and AChE activity
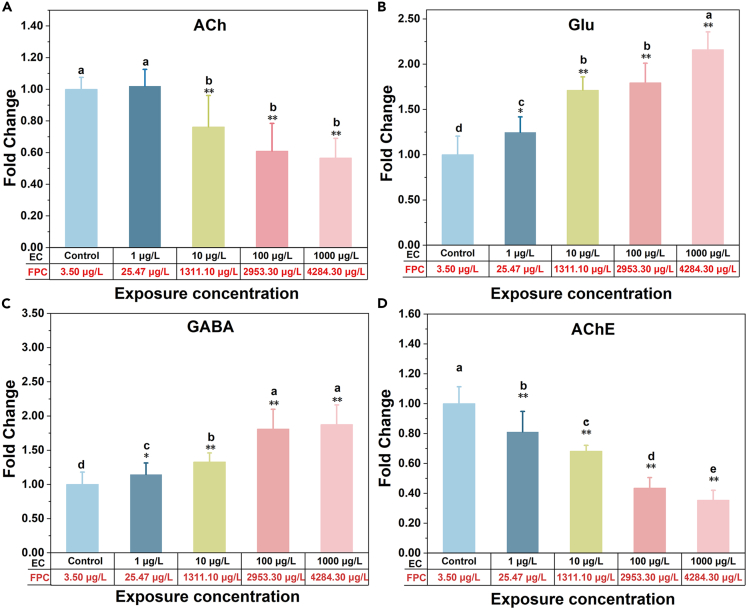
CMZ regulates neurotransmitter release, uptake, and receptor binding, and can be used as an agonist for human GABA receptors.32 In this study, combing FPCs as the internal dosimetry, specific markers of CMZ at the molecular level, including neurotransmitters and related enzyme, as well as the expression of related receptor genes and behavior, were selected as toxic endpoints, to directly investigate the read-across hypothesis of CMZ. Three neurotransmitters and AChE were detected in the zebrafish of all the treated groups under different concentrations (1, 10, 100, 1000 μg/L) and corresponding plasma concentrations of CMZ (25.47 ± 3.78, 1311.10 ± 80.26, 2953.30 ± 109.20, 4284.33 ± 200.52 μg/L, mean ± SD, n = 3) (Figure 4). With the increase of CMZ exposure concentration, the levels of ACh and AChE in zebrafish were gradually decreased compared with the control group (). In particular, AChE was significantly different among exposed groups (Figure 4D). ACh was rapidly hydrolyzed into choline and acetic acid under the action of AChE, and the excitatory effect was terminated.47 Nkoom et al.48 found that the AChE activity of zebrafish was significantly inhibited in a concentration-dependent and time-dependent manner when exposed to CMZ. The inhibitory effect became more and more obvious with the increase of CMZ concentration and less distinct with the extension of time. These results were inconsistent with previous work,49 where zebrafish were exposed to 10 μg/L and 10000 μg/L CMZ. It was found that the AChE activity considerably increased in muscle (10000 μg/L) and head (10 and 10000 μg/L) after exposure of 63 days (). Glu is the most common excitatory neurotransmitter. Under normal physiological conditions, Glu is mainly involved in synaptic plasticity, learning, and memory.50 As shown in Figures 4B and 4C, Glu and GABA levels appreciably increased after seven days of CMZ exposure compared with the control group (), which was the most significant in the 1000 μg/L exposure group (). There is a dose-dependent effect between Glu and GABA levels and CMZ concentration. Chen et al.34 found that exposure to CMZ (1, 10, 100 μg/L) increased GABA concentration, reduced Glu concentration in zebrafish embryos, and also altered the expression of Glu receptors and Glu decarboxylase-related genes. These four indexes are related to the conduction of nerve signals. The GABA receptor gene family in fishes is similar to that in mammals, suggesting that CMZ is likely to play a role in fish similar to that in humans, in a similar MOA.51
Figure 4.
Neurotransmitters and AChE activity in zebrafish were affected after 7-day exposure in four concentration levels of CMZ
Neurotransmitters and AChE activity in zebrafish were affected after 7-day exposure in four concentration levels of CMZ. Neurotransmitters (A–C) and AChE activity (D) were evaluated via enzyme-linked immunosorbent assay (ELISA). CMZ significantly decreased ACh (A) and AChE activity (D), increased Glu (B) and GABA(C). External concentration: EC; the corresponding fish plasma concentration: FPC. Error bars represent as mean ± SD. (n = 3; Duncan test: ∗: p < 0.05, ∗∗: p < 0.01).
Even though the external exposure concentration is the same or comparable, the outcomes might be very different. The probable reasons are that the absorption, distribution, and metabolism of the target pollutants in the organism vary depending on the experimental conditions (such as exposure time, light, temperature, oxygen content, etc.). In our present work, using FSSPCs as internal concentrations, the neuronal effects of CMZ on zebrafish were precisely evaluated. On the other hand, comparing the measured FSSPCs with HTPC is an important step in determining whether drugs will show pharmacological effects on fish by the FPM and the read-across hypothesis. To ensure that the exposure groups had the same experimental conditions and period as the control, the data of the control group after seven days were used as the control group. The spearman correlation analysis found that ACh (R2 = −0.73, ), Glu (R2 = 0.85, ), GABA (R2 = 0.82, ), and AChE (R2 = 0.88, ) were significantly correlated with plasma CMZ concentration. When the FSSPC (25.47 ± 3.78, μg/L at 1 μg/L external water exposure, mean ± SD, n = 3) of CMZ was in the same order of magnitude of HTPC/1000 (4–12 μg/L, considering the safety factor of 1000), significant changes were observed in all indicators except for ACh. When the FSSPCs of CMZ were much higher than HTPC/1000 (4–12 μg/L), the four indicators related to nerve signaling showed dose-dependent effects. It suggested that zebrafish do show an associated pharmacological response based on MOA at different concentrations of CMZ exposure.
Expression of neurotransmitter-related genes
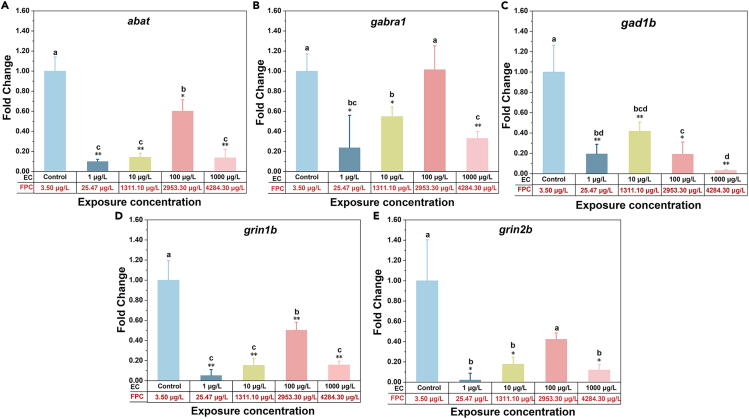
Abat and gad1b are involved in regulating the glutamate-GABA metabolic pathway. gabra1 and gad1b are also related to glutamic acid metabolism. Grin1b and grin2b can encode neurotransmitter receptors. The trend of these neurotransmitter-related genes in our study is similar to previous studies.34,52 The ordinate of Figure 5 is fold change, which is the relative gene expression level relative to the control group. As can be seen in Figure 5A, the expression of the abat gene in zebrafish was significantly down-regulated compared with the control group after seven days of exposure to CMZ with four concentrations (). The down-regulation degree of this relative gene expression at 1 μg/L was markedly higher than that in the other three groups (), but there was no significant difference among the other three groups (). For gabra1, except for 100 μg/L, the relative gene expression of zebrafish was significantly down-regulated compared with the control group after seven days (). The downregulation at 1 μg/L and 1000 μg/L was greater than that of the 10 μg/L exposure group () (Figure 5B). For gad1b and grin1b, the relative gene expression levels of zebrafish at four concentrations were significantly down-regulated compared with the control group () (Figures 5C and 5D). The down-regulation degree of grin1b at 100 μg/L was significantly lower than that in the other three exposure groups (), and there was no significant difference in the expression of this gene in the other three exposure groups () (Figure 5D). Compared with the control group, the expression of grin2b was down-regulated, and the 1, 100, and 1000 μg/L groups were significantly down-regulated (). The down-regulation degree of the 100 μg/L exposure group was significantly lower than that of the other three exposure groups () (Figure 5E).
Figure 5.
CMZ inhibited the expression of neurotransmitter-related genes when the actual FPCs were in the ranges of 1/1000 HTPC to HTPC
CMZ inhibited the expression of neurotransmitter-related genes when the actual FPCs were in the ranges of 1/1000 HTPC to HTPC. The expression of abat (A), gabra1 (B), gad1b (C), grin1b (D), and grin2b (E) genes in zebrafish after 7-day exposure at four concentration levels of CMZ were determined by real-time PCR. External concentrations: ECs; the corresponding fish plasma concentrations: FPCs. Error bars represent as mean ± SD. (n = 3; Duncan test ∗: p < 0.05, ∗∗: p < 0.01).
The results indicated that except for gabra1 at 100 μg/L, the relative gene expressions of abat, gabra1, gad1b, grin1b, and grin2b concerned were down-regulated compared with those in the control group after exposure to different concentrations of CMZ leading to the FSSPCs of CMZ (25.47 ± 3.78 to 4284.30 ± 200.52 μg/L, mean ± SD, n = 3) were higher than HTPC/1000 (4–12 μg/L). In addition, the expression of the five genes was significantly down-regulated at 1 μg/L, where FSSPC of CMZ (25.47 μg/L) was in the same order of magnitude as of HTPC/1000 (4–12 μg/L). The expression levels of gabra1, gad1b, grin1b, and grin2b genes in the 10 μg/L exposure group were all higher than those in the 1000 μg/L exposure group (only for gabra1 gene, the difference was significant, ). Combined with the BCF of CMZ based on the internal exposure concentration of zebrafish in section 2.1.1, it was found that the BCF of the 10 μg/L exposure group (131.11 ± 8.03, mean ± SD, n = 3) was much higher than that of the 1000 μg/L exposure group (4.28 ± 0.20, mean ± SD, n = 3). That may explain the difference to some extent and further indicate the importance and necessity of studying biological effects based on internal exposure concentration. A previous study evaluating DNA strand breaks reported that exposure to 0.31 μg/L CMZ for three days induced DNA breaks.53 It indicated that CMZ had certain genotoxicity, which can reduce the expression of neurotransmitter-related genes. In a word, when the FSSPCs of CMZ were of the same order of magnitude with or much higher than the corresponding HTPC, except for gabra1 and grin2b at 2953.30 μg/L internal exposure groups, the remaining results were significantly reduced compared to the blank group and showed a trend of first increasing and then decreasing. This proved that CMZ shows a human-like mode of effects on zebrafish, and the read-across hypothesis of CMZ is valid directly at the molecular level.
Larva behavior
AChE activity plays a crucial role in the development and maintenance of axial muscle devices and sensory neurons in fish. Changes in its activity may cause muscle contractions in fish, resulting in abnormal repetitive or excessive motor behaviors.54 Changes in behavior can indicate that the pollutant has directly or indirectly interfered with the nervous system.55 After light adaptation, the fundamental motor ability of zebrafish larvae was observed by alternating light and dark stimulation. Figure S2 showed the results of the total distance traveled by zebrafish larvae in the control group and the groups exposed to CMZ at different concentrations. In the three exposure groups with relatively low concentration levels (1, 10, 100 μg/L), the total distance traveled by larvae was slightly lower than that of the control group, but there was no significant difference (). In the 1000 μg/L exposure group (measured FPC = 4284.30 μg/L), the total movement distance of the larvae was significantly higher than that of the control group (), suggesting that CMZ exposure may interfere with the nervous system of zebrafish when the PFC concentration was higher than the maximum HTPC (12 mg/L). Although CMZ causes changes in neurotransmitters and AChE at the molecular level, the organism is self-regulating, and no effect will likely be observed at the macro level if the exposure concentration is not very high. This may partly explain the molecular level and behavioral inconsistencies. However, previous researchers had observed that the movement and rest percentage of zebrafish embryos and larvae were significantly affected at 1 μg/L of CMZ.32 That may be due to differences in the duration of light exposure and the change of exposure solution. Li et al.56 revealed that higher concentrations of CMZ (2.0 and 20 mg/L) could significantly reduce the percentage of spermatozoa motile and velocity. In summary, the study suggested that the effects of CMZ on the behavior of zebrafish larvae were neither linear nor dose-dependent.
Discussion
Uptake and bioconcentration of CMZ in zebrafish
Internal concentration plays an important role in risk assessment. The internal concentration can bypass complex and confounding variables and directly correlate the concentration of the target substance in the organism with the biological effect. Another advantage of internal concentration is that risk assessments for different environmental areas can be linked. These can provide a direct link between laboratory and field studies. The effect concentration is usually reported as the concentration of water or the concentration of sediment or soil (external exposure). These numbers are hardly comparable, but toxicity data based on the internal concentration can also link these aspects.57 The importance of measuring internal exposure also lies in the fact that certain drugs can be bioconcentrated, potentially resulting in internal concentration hundreds of times higher than EC. If the FPC is predicted based on FPM alone, without understanding the true plasma concentration, the read-across hypothesis cannot be validated by comparing it with the human therapeutic concentration. Moreover, it is possible to draw incorrect conclusions about the exposure concentration at which the drug causes a reaction, thus incorrectly estimating the threat of the drug’s presence in the environment to non-target organisms.6 In this study, it was found that FPM underestimated the internal concentration of CMZ in fish (i.e., FPC) at environmentally relevant concentrations (1, 10, 100 μg/L), which may lead to bias in the environmental risk assessment of CMZ. Ignoring dietary chemical exposure in this study, CMZ may be absorbed by the organism from the environment through fish respiration and dermal surface. CMZ uptake is the end result of competition between chemical uptake at the respiratory surface (e.g., gills in fish) and chemical elimination, including respiratory exchange, fecal excretion, metabolic bioconversion of parent compounds, and growth dilution.58 However, Chang et al.59 found that the possibility of CMZ (pKa = 15.96) to enter gill cells through the ionic state was very low through the in vitro primary gill cell culture system (FIGCS). After a single administration, blood concentration decreases from the peak and then rises again to form the second or even multiple peaks, which is the multiple-peak phenomenon of the drug time curve. The multiple-peak phenomenon of CMZ has been observed only in humans60 and rabbits.61 Our study shows that the absorption of CMZ in zebrafish may also have a multi-peak phenomenon. Therefore, the specific uptake mechanism of CMZ needs to be further explored. In the future, a comprehensive and systematic study of the multi-peak phenomenon of CMZ absorption should be conducted to deeply analyze the absorption, distribution, metabolism and excretion processes, and establish a systematic pharmacokinetic multi-peak model.
This study demonstrates that when the FPM is used as a screening tool for the risk assessment of CMZ, the overestimated prediction is not severe from the point of view of the precautionary principle, as the maximum exposure concentration designed for the experiment (1000 μg/L) far exceeds the environmental level. In contrast, predicted theoretical BCF values lower than actual BCFplasma values may lead to underestimating toxicological risks in wild fish. By comparing the muscle-plasma concentration ratios determined in this study with those (0.59–2 L/kg) in literature,44 it can be shown that the transport of CMZ from plasma into muscle is lower in zebrafish than in humans. At 1000 μg/L, muscle-plasma partition coefficient was 0.54 ± 0.03 (mean ± SD, n = 3), which was more than two times higher than the ratios of the other two concentrations, indicating that CMZ entered the muscle from the blood. It may partly explain why the measured value is much lower than predicted at 1000 μg/L exposure level. It has been suggested that the correlation of membrane-based transporters and their binding to plasma proteins can affect the distribution of APIs between water and fish plasma.4 CMZ binding to plasma proteins is not conducive to its transport to muscle. Alexishvili et al.60 found that the pharmacokinetics of CMZ was S-like kinetic curves, and the concentration-time curve had multiple peaks. It can be inferred that the amount of CMZ absorbed by zebrafish may not linearly increase with the increase of environmental concentrations, that is, even if zebrafish inhale a higher concentration of CMZ, it may not be fully absorbed or utilized by plasma. However, the predicted value based on FPM increases linearly with the increase of the EC. The transport of CMZ in zebrafish and its binding to hemoglobin may be responsible for the irregular changes in the ratio of measured to predicted values. It is worth noting that one of the most common sources of multiple peaks is biliary secretion, followed by intestinal reabsorption of drugs, a process known as “enterohepatic circulation”, and a hallmark of its involvement in drug disposal is the presence of multiple peaks in the plasma concentration-time distribution. Anticonvulsants provide enterohepatic circulation62 and CMZ is also a class of anticonvulsants. Besides, CMZ is a substrate and inducer for cytochrome (CYP) 3A4 and CYP2B6. CMZ can induce the metabolism of many drugs; on the other hand, its metabolism can be affected by various CYP inhibitors and inducers.63 It was found that CYP2AD2, CYP3A65, CYPIA, CYP2P9, and CYP2Y3 were the prime CYP genes expressed in the liver of adult zebrafish. In zebrafish, CYP3A65 is 54% the same as human CYP3A4, and CYP2Y3 shares synsyne with the CYP2 gene family of human drug metabolizing enzymes, such as CYP2B6.64 It is reasonable to infer that CMZ is metabolized in zebrafish, resulting in the difference in predicted values of the measured values. In the future, the distribution of drugs in aquatic organisms should be better studied in conjunction with their pharmacokinetics.
Many different human drugs (including neurologic, cardiovascular, reproductive, and immune system drugs) with target systems, species, external exposure concentrations, FPCs, MOA-relevant endpoints, and whether measured FPCs compared to HTPCs are listed in Table 3. In the study of levonorgestrel, it was found that the predicted FPC was lower than the measured FPC based on log D (3.32, ).65 Patel et al.24 found that the relationship between predicted FPC and measured FPC was related to log D () or log Kow and ibuprofen exposure concentrations (Table 3). In a study of wild fish plasma in Texas, Du et al.17 found that the theoretical FPM underestimated the actual FPCs of diphenhydramine by a factor of 10. Interestingly, in the FPM verification for sertraline (log D7.4 = 3.77),22 levonorgestrel (log Kow = 3.72)65 and BDP (log Kow = 3.69),25 the predicted and measured concentrations of these three substances in fathead minnow are very close. The FPM is likely more suitable for compounds with log Kow or log D close to 3.7. Anyway, the relationship between the predicted and measured concentrations was found to be related to drug type, log Kow, log D, external exposure concentrations, etc. The FPM was developed with rainbow trout for hydrophobic organic compounds,10 and its reliability in FPCs prediction of polar drugs and specified fish species needs to be further verified. There may be several reasons for the difference between predicted BCFs and measured BCFs in fish plasma. A study from Coote Natural Marsh in Canada found that the measured BCFs of sertraline in plasma of goldfish were correlated with the predicted BCFs, but not in carp.66 It showed that the applicability of the FPM in different fishes was also varied. Even with the same fish, the binding of environmental pollutants to plasma was influenced by biological factors such as plasma pH and rainbow trout strain/quality.67 Most of other drugs whose predicted value was significantly different from the measured value were polar ionizable substances. They had weak hydrophobicity, which underestimated their enrichment ability in plasma. The study of Fu et al.68 showed that ionizable substances have reduced BCF values due to easy ionization, and ionic substances could not easily pass through the cell membrane, so they were not easy to accumulate in organisms. The physicochemical properties of drugs, especially hydrophobicity and ionization, are the key factors affecting the prediction value of the FPM. In the future improvement of the model, the effect of pH on drug ionization should be taken into account.17,69 Other major reasons are differences in branchial uptake/elimination mechanisms, plasma protein binding, and/or hepatic clearance.45
Table 3.
Examples of read-across hypothesis studies based on fish plasma model about human drugs
| Drug | Target System | Species | EEC | FPC (μg/L) |
MOA-relevant Endpoints | Measured FPC compared to HTPC | Reference | |
|---|---|---|---|---|---|---|---|---|
| Predicted FPC | Measured FPC | |||||||
| Sertraline (log D7.4 = 3.77) | Central nervous System (antidepressant) |
Fathead minnow (Pimephalespromelas) | 3, 10, 30 μg/L | Predicted FPC ≈ Measured FPC |
|
> HTPC | Valenti et al.22 | |
| Fluoxetine (log D7.4 = 1.99) | Central nervous System (antidepressant) |
Fathead minnow (Pimephalespromelas) | 0.1, 1, 10, 20, 38, 72 mg/L | When EEC >10 mg/L: Predicted FPC < Measured FPC |
|
0.1, 1, 10 mg/L: < HTPC 20 mg/L: ≈ HTPC 38, 72 mg/L: > HTPC |
Margiotta et al.21 | |
| Oxazepam (log D7.4 = 2.63) | Central nervous System (Anti-anxiety) |
Fathead minnow (Pimephalespromelas) | 0.8, 4.7, 30.6 μg/L | Female: Predicted FPC > Measured FPC |
|
30.6 μg/L: ≤ HTPC | Huerta et al.18 | |
| Tramadol (log Kow, log Dow, log Dlipw) | Central nervous System (analgesic) |
Fathead minnow (Pimephalespromelas) | 1, 10, 100 μg/L | Predicted FPC > Measured FPC |
|
< HTPC | Tanoue et al.19 | |
| 25 compounds | Central nervous system | Zebrafish (Danio rerio) | 0.625, 1.25, 2.5, 0, 0.3125, 5 mM | – | – |
|
– | Winter et al.70 |
| 57 compounds | Central nervous system | Zebrafish (Danio rerio) | 1.25, 2.5, 5 mM | – | – |
|
– | Winter et al.71 |
| 100 compounds | Cardiovascular system | Zebrafish (Danio rerio) | 10, 100, 1000 μg/mL | – | – |
|
– | Milan et al.72 |
| Adrenaline Cisapride Haloperidol Terfenadine Theophylline Verapamil |
Cardiovascular system | Zebrafish (Danio rerio) | 10, 20,40; 0.05, 0.075, 0.10; 0.0025, 0.05, 0.010; 0.4, 1.6, 1.8; 0.0001, 0.001, 0.10; 0.25, 0.50, 1; 0.05, 0.075, 010 mmol/L |
– | – |
|
– | Parker et al.73 |
| Gesogestrel Drospirenone Gestodene Levonorgestrel |
Reproductive system | Fathead minnow (Pimephalespromelas) | 100 ng/L; 100 ng/L; 1, 10, 100 ng/L; 0.1, 1, and 10 μg/L |
– | – |
|
– | Runnalls et al.74 |
| Dutasteride | Reproductive system | Fathead minnow (Pimephalespromelas) | 10, 32, and 100 μg/L | – | – |
|
– | Margiotta-Casaluci et al.75 |
| Ethinylestradiol Levonorgestrel (log Kow = 3.72; log D7.4 = 3.32) |
Reproductive system | Fathead minnow (Pimephalespromelas) | Ethinylestradiol: 0.25, 2.5, 12.5 ng/L(mixture exposure) Levonorgestrel: 0.25, 2.5, 12.5 ng/L(mixture exposure, measured: 5.2 and 15.8 ng/L) |
Levonorgestrel (log Kow = 3.72): Predicted FPC ≈ Measured FPC Levonorgestrel (log D7.4 = 3.32): Predicted FPC < Measured FPC |
|
Ethinylestradiol except for 0.25 ng/L(single): Predicted FPC ≤ HTPC(0.04–0.16 μg/L) Levonorgestrel: except for 100 ng/L(single): Predicted FPC < HTPC |
Runnalls et al.65 | |
| Dexamethasone | Reproductive system Respiratory system Development |
Fathead minnow (Pimephalespromelas) | 0.1, 1, 10, 100, and 1,000 μg/L | – | – |
|
– | LaLone et al.76 |
| 10 synthetic GCs (in vitro) Beclomethasone dipropionate Prednisolone |
Immune system Metabolism |
Fathead minnow (Pimephalespromelas) Rainbow trout (Oncorhynchusmykiss) |
100 ng/L, 1, 10 μg/L |
– | – |
|
– | Kugathas and Sumpter77 |
| Beclomethasone dipropionate | Immune system Metabolism HPI axis |
Fathead minnow (Pimephalespromelas) | 0.1, 1, 10 μg/L | – | – |
|
– | Kugathas et al.78 |
| Beclomethasone Dipropionate (log Kow = 3.69; log D7.4 = 4.07) |
Immune system Metabolism Secondary pharmacology |
Fathead minnow (Pimephalespromelas) | 10, 100, 1000 ng/L | Predicted FPC ≈ Measured FPC |
|
10 ng/L: within HTPC 1000 ng/L: > HTPC |
Margiotta-Casaluci et al.25 | |
| Diclofenac | Immune system Renal system GI system |
Rainbow trout (Oncorhynchusmykiss) | 0.5, 1, 5, 25 μg/L | – | – |
|
– | Mehinto et al.79 |
| Ibuprofen (log Kow = 3.58; log D7.4 = 4.07) | Immune system | Fathead minnow (Pimephalespromelas) | 105, 278, 409, 502 μg/L | log Kow = 3.58:105, 278 μg/L: Predicted FPC > Measured FPC; 409, 502 μg/L: Predicted FPC < Measured FPC. log D7.4 = 4.07: Predicted FPC > Measured FPC |
|
105 μg/L: < HTPC 78, 409 μg/L: ≈ HTPC 502 μg/L: > HTPC |
Patel et al.24 | |
EEC, external exposure concentration; FPC, fish plasma concentration; MOA, mode of action; HTPC, human therapeutic plasma concentration; SERT, serotonin transporter; SSCs, secondary sexual characteristics; E2, estradiol; T, testosterone; 11-KT, 11 keto-testosterone; GC, glucocorticoid; HPI, hypothalamic–pituitary–interrenal; PEPCK, phosphoenolpyruvate carboxykinase; GR, glucocorticoid receptor; AR, androgen receptor; GI, gastrointestinal; ptgs, prostaglandin-endoperoxide synthase; Predicted FPC ≈ Measured FPC, fish plasma model has a good accuracy; Dow, octanol –water distribution coefficient; Dlipw, liposome-water distribution coefficient.
Biological effects based on FPCs
In this study, MOA-based biological effects at the molecule level and FPCs were integrated to facilitate the read-across hypothesis. Although the molecular level may not directly reflect the macroscopic effect, the molecular level is more sensitive and is an early warning mode or indicator. Exposing zebrafish to progesterone, Zucchi et al.80 observed significant changes in the expression of genes associated with ovarian physiology when concentrations were about 90 times lower than those that induced visible histopathological effects. Margiotta-Casaluci et al.25 compared dose-response curves regarding the codependent endpoint (gene expression/phenotype) of the synthetic glucocorticoid BDP and found that under certain circumstances, changes in gene expression were more sensitive than phenotypic responses, but not in other contexts (androgen receptor, AR; interleukin-6, IL6; tumor necrosis factor alpha, TNFα). The authors emphasize that these results need to be interpreted with some caution, as gene expression and phenotypic responses are measured at different sites. Patel et al.24 did not observe a relationship between gill cyclooxygenase (ptgs) gene expression and plasma ibuprofen, suggesting a need for more research on the factors that influence gene expression and their sensitivity as endpoints. We directly used external exposure concentration and FPM models to predict the FPC of larval fish and compared this FPC with HTPC. Zebrafish larvae appear to be more commonly used as a screening tool in epilepsy studies.32,81,82 However, larval fish have some limitations due to their immature central nervous system. It is important to note that behavioral studies of the same antiseizure drugs may have different results in adult and larva zebrafish. For example, Siebel et al.83 reported that phenytoin (PHT) can suppress seizures in adult zebrafish. In contrast, Afrikanova et al.84 did not observe an anticonvulsant effect of PHT on larvae. In this study, the neurotransmitter and neurotransmitter-related genes of adult zebrafish were significantly changed under exposure to various CMZ concentrations. The behavior of larvae was affected only at 1000 μg/L. The difference in results may be due to the difference in the development of the nervous system between adult and larva. Another possible reason is that molecular indicators are inherently more sensitive than behavioral indicators. Margiotta-Casaluci et al.25 found that BDP has a much greater effect on phosphoenolpyruvate carboxykinase (PEPCK) gene expression than blood glucose in fathead minnows. Therefore, molecular indicators cannot be ignored in the early warning of ecological risks.
CMZ is an antiepileptic drug that is also used in the treatment of bipolar disorder. It is a GABA receptor agonist, and it has also been shown to enhance GABA receptors composed of α1, β2, and γ2 subunits. Glu and GABA are the major neurotics in the brain. Inhibitory GABA and excitatory Glu together control many processes, including the overall level of arousal in the brain. Balanced interactions are needed to maintain physiological balance. So, as our study turned out, the same changes in GABA and Glu are likely to occur. CMZ weakly blocks calcium inflow through N-methyl-D-aspartate (NMDA) subtype glutamate receptor when Glu is increased, and also exerts anti-glutamatergic effects by inhibiting calcium inflow while relatively reducing the postsynaptic efficacy of glutamate.85,86 The key factor that causes the quantum release of ACh is the Ca2+ influx caused by the depolarization of nerve endings. When the nerve impulse is transmitted to the endplate, the membrane potential decreases, resulting in the opening of voltage gate channels that allow Ca2+ to pass through, allowing Ca2+ to enter the endplate, thus stimulating the endplate to secrete ACh. In other words, CMZ may inhibit calcium influx in zebrafish, thereby causing a decrease in ACh.33 In our study, CMZ significantly increased Glu and GABA, decreased ACh and AChE as well as inhibited the transcription levels of gabra1, grin1b, grin2b, gad1b, and abat when the actual FPCs were in the ranges of 1/1000 HTPC to HTPC. It suggests that when FPCs were in the ranges of 1/1000 HTPC to HTPC, CMZ would have a human-like effect on zebrafish. Valenti et al.22 observed statistically significant reductions in therapeutic target binding (SERT) and shelter-seeking behavior when the FPC of sertraline exceeded the human therapeutic threshold. A statistically significant behavioral effect was observed in fish exposed to 4.7 μg/L oxazepam in the novel tank diving test.18 The synthetic estrogen ethinylestradiol (EE2) significantly inhibited egg production only at plasma concentrations 3–11 times higher than the higher value of HTPCs (0.04–0.16 ng/mL).65 Patel et al.24 demonstrated a mode-related response to prostaglandin E metabolite (PGEM) levels in ibuprofen-exposed fish at plasma concentrations similar to those in humans. The relationship between plasma concentration and HTPC and whether they produce the same MOA endpoints as humans are different for drugs with different mechanisms of action. Even if the target system is the same, whether drugs with different therapeutic effects will produce the same MOA endpoints as humans varies depending on the nature of the drug itself and the external exposure concentration. Therefore, more data on plasma concentrations and HTPC for different classes of drugs are needed to study the read-across hypothesis.
In conclusion, the FPM and the read-across hypothesis of drugs were examined in conjunction with neuro-correlated outcomes and internal dosimetry with FPCs. The FPCs might be utilized to estimate the concentrations of CMZ in muscle since it can be assumed that CMZ in muscle derives from plasma. The low muscle-plasma ratios (0.14–0.54) suggested that zebrafish showed a relatively poor ability for CMZ transfer from plasma to muscle. Bioconcentration of CMZ in muscle and plasma of zebrafish demonstrated that the FPM underestimated the BCFs in plasma at environmental CMZ exposure concentrations (1–100 μg/L). At low ambient exposure (1 and 10 μg/L, whose corresponding FPCs were 25.47–1311.10 μg/L and greater than the equal HTPC), CMZ dramatically raised Glu and GABA, decreased ACh and AChE, and inhibited the transcription of gabra1, grin1b, grin2b, gad1b, and abat in zebrafish. These revealed that zebrafish could experience MOA-based neurological effects similar to those of humans from psychotropic medications like CMZ when the FPCs exceeded the equal HTPC. These findings demonstrated the benefits of combining FPC and MOA-based neural central-system-related effects at molecular levels of antiepileptic drugs in read-across and ecological risk assessment. FPCs could advance knowledge of the ecological effects of CMZ exposure on aquatic vertebrates and offer theoretical and practical support for evaluating the environmental risk associated with human drugs. Acute toxicological thresholds for aquatic organisms are usually obtained by measuring exposure concentrations in the external water environment rather than internal exposure concentrations in the blood and/or target organs. From the read-across hypothesis combined measured plasma concentrations with HTPCs, we can infer whether residual drugs in water will have effects similar to those in humans in aquatic organisms at the molecular level, even if we see no adverse effects macroscopically. Depending on the internal concentrations of drugs such as FPCs, it is possible to determine whether the observed effects are due to the high concentration of the drugs in the organism or whether the organism is more sensitive than other species. That is, even if exposure duration or external/environmental exposure doses differ in different studies, the same indicator will be comparable based on internal exposure concentrations. Therefore, there is a great impetus to test the internal exposure concentrations to further analyze the toxic effects on different/same species. Last, but not least, it is recommended that the biological effect indicators based on MOA in this paper should be further enhanced and that more associated genes can be selected out based on transcriptomics in the future.
Limitations of the study
Our study revealed that zebrafish could experience MOA-based neurological effects similar to those of humans from psychotropic medications like CMZ when the FPCs exceeded the equal HTPC. However, individual differences in plasma concentration should be considered to better control the distribution of high, medium, and poor CMZ within the samples. Another limitation of this study was difficulty in acquiring zebrafish brains samples to evaluate the neurotransmitter levels. However, this could be resolved by using more zebrafish and more sophisticated experimental operation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Carbamazepine | Dr. Ehrenstorfer GmbH | CAS: 298-46-4 |
| Methanol | Merck | CAS: 67-56-1 |
| Acetonitrile | Merck | CAS: 75-05-8 |
| Formic acid | Anpel | CAS: CAEQ-4-014784-0500 |
| Critical commercial assays | ||
| ACh ELISA kits | Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China | Cat. #ml022717 |
| GABA ELISA kits | Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China | Cat. #ml092740 |
| Glu ELISA kits | Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China | Cat. #ml076518 |
| AChE ELISA kits | Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China | Cat. #ml022715 |
| Experimental models: Organisms/strains | ||
| Zebrafish: AB strain | Shanghai Feri Biotechnology Co., LTD, China | N/A |
| Oligonucleotides | ||
| Primers for qPCR, see Table S1 | This paper | N/A |
| Software and algorithms | ||
| SPSS version 20.0 | IBM | https://www.ibm.com/cn-zh/spss |
| Zebrabox VideoTrack 3.5 | Viewpoint, France | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xialin Hu (xlhu@tongji.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Test organisms
The experimental zebrafish were type AB adult males, purchased from Shanghai Feri Biotechnology Co., LTD. The fish were without obvious disease or visible deformity, with average weight of 0.26 g (0.22 g - 0.30 g). The zebrafish were cultured in an automatic recirculating aquaculture system and acclimated in a laboratory fish house (light: dark = 14:10, temperature: 28 ± 1°C, dissolved oxygen > 5 mg/L) for two weeks. Newly hatched brine shrimp were fed during the exposure period at 2 % of zebrafish body weight, and food remnants and faces were removed daily. All experiments involving the use of animals were conducted in strict accordance with the guideline of the Animal Ethics Committee of Tongji University.
Exposure
The adult male zebrafish were randomly divided into five groups, including a control group and four treatment groups of CMZ, covering environmentally-relevant concentrations (1, 10 and 100 μg/L) and pharmacologically relevant ones (1000 μg/L). The concentrations of CMZ in natural water environment were in the range of 1 ng/L to 12 μg/L.30 The reported concentration in municipal and urban wastewater was as high as 259 μg/L.31 Therefore, we set three environmentally relevant concentrations of of CMZ (1, 10, and 100 μg/L) in this study. According to Equations 1 and 2, the lowest exposure concentration (1 ug/L) could produce a plasma concentration equal to 0.138 mg/L, considering a log Kow of 2.77 (pH = 7.4). On the other hand, the highest exposure concentration (1000 ug/L) is predicted to produce a plasma concentration of 13.8 mg/L, close to 12 mg/L, which is on the higher side of the HTPC range (4 - 12 mg/L).87 Considering the above, exposure concentrations were used to be able to produce plasma concentrations in fish ranging from HTPC/1000 to HTPC, which makes it possible to explore the read-across hypothesis based on different FPC concentration gradients. The blank group without CMZ was used as control group. In the experiment, the proportion of zebrafish growing environment was 1.5 g fish/L water. The study lasted for seven days and aquatic culture water in glass vessels was half-renewed every day. The measured water concentration in day 7 when we collected the fish samples was 0.75, 8.04, 89.97 and 877.26 μg/L in exposure with nominal concentration of 1, 10, 100, and 1000 μg/L, respectively. Therefore, we use nominal exposure concentration as the abscissa for convenience of expression. The zebrafish were taken from each exposure concentration (1, 10, 100 and 1000 μg/L) at the time point of 0 h, 6 h, 12 h, 24 h, 72 h, and 168 h to detect the CMZ concentration in fish muscles. At 0 d and 7 d of exposure, the CMZ concentration in fish plasma, acetylcholine (ACh), glutamic acid (Glu), gamma-aminobutyric acid (GABA), acetylcholinesterase (AChE), and neurotransmitter-related genes were examined.
Method details
Determination of CMZ in biological samples
Three fish were randomly selected and combined into one sample to test the CMZ concentration in fish muscles and there are three parallels. Combined with the method of orbital plasma collection, zebrafish were cut off from the posterior orbit with scissors after euthanizing by an ice bath, and 5 - 20 μL capillary tubes were used for blood collection of zebrafish. The collected blood was quickly added to the 1.5 mL eppendorf (EP) tube washed with heparin in advance. The blood of all 45 zebrafish was collected for each group and each blood sample was not less than 100 μL. The collected blood is placed in a centrifuge tube containing 0.5 mL of heparin sodium solution. In order to avoid hemolysis, a low speed (3500 r/min) was centrifuged at room temperature for 10 min to remove blood cells, and the supernatant was absorbed to obtain orange plasma, which was stored in a glass container at -20°C for later use. Add 5 mL acetonitrile into the centrifuge tube, shake for 30 s, swirl for 1 min, centrifuge at 3500 r/min for 10 min, and take the supernatant into another clean glass centrifuge tube. Blow out acetonitrile with nitrogen gas. Transfer the above residual liquid into a 50 mL brown glass bottle, rinse the centrifuge tube with 15 mL 0.2% formic acid water, and combine the solution. Add 0.05g Na2EDTA and shake. The HLB column (500 mg, 6 cc, Waters Milford, MA, USA) was fully activated with a solution of 6 mL methanol and 6 mL reagent water (pure water or 0.2% formic acid water), and the extraction liquid passed through the HLB solid phase extraction column at a rate of 2-2.5 mL/min. Rinse with 10 mL pure water solution and drain the water in the column. Eluent was eluted with 9 mL methanol solution, collected in 10 mL glass centrifuge tube, dried under weak nitrogen, and re-dissolved in 1 mL methanol: water = 20:80 (v:v) solution, and then tested with 0.22 μm nylon filter membrane.
All the samples were detected by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS, Xevo TQ, Waters, MA, USA), outfitted with a triple-stage quadrupole mass spectrometer (Xevo TQ, Waters, MA, USA) and connected to an ACQUITY UPLC automated injector (Waters, MA, USA). Injection volume was 5 μL. A Waters ACQUITY UPLCTM BEH C18 column (2.1 mm 150 mm, 1.7 m (id) (Waters, Switzerland) was used for the chromatographic separation. LOD and LOQ were defined as the concentration of S/N=3 and 10, respectively. For fish muscle samples, the recoveries were 98.5% and the relative standard deviations were 2.7 % (n = 6). The obtained LOD and LOQ were 0.35 μg/kg and 1.17 μg/kg. For fish plasma samples, the recoveries were 92.3 % and the relative standard deviations were 6.2 %. The LOD and LOQ were 0.46 μg/L and 1.08 μg/L. All meet the requirements of quantitative analysis. The column temperature was maintained at 40°C. The mobile phases were analyzed by adding 0.1% formic acid in Milli-Q water for Solvent A and chromatographic grade methanol for solvent B with the following gradients:
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0 | 80 | 20 |
| 10.5 | 10 | 90 |
| 12.1 | 80 | 20 |
| 15 | 80 | 20 |
Mass determination was performed with an ESI source in the positive (ESI+) ion mode. Nitrogen was used as desolvation gas at the flow rate of 550 L/h. And argon was applied as the collision gas at rate of 0.13 mL/min. The optimum MS parameters were: ionization voltage, 3.20 kV; cone voltage, 35 V; desolvation temperature, 150°C; and ion source temperature, 120°C. CMZ were analyzed in the multiple reaction monitoring (MRM) mode.
Neurotransmitters and AChE activity in zebrafish
The levels of three neurotransmitters, including acetylcholine (ACh), glutamic acid (Glu) and gamma-aminobutyric acid (GABA), and acetylcholinesterase (AChE), were evaluated via enzyme-linked immunosorbent assay (ELISA). According to the test of Tufi et al.,88 the collected whole zebrafish were placed in a frozen storage tube, flash-frozen in liquid nitrogen, and put in a -80°C refrigerator for testing. Three zebrafish for one sample were minced into small pieces and homogenized in ice-cold phosphate-buffered saline (PBS) with a glass homogenizer on ice. To further break the cells, the suspension was subjected to freeze-thaw cycles. The homogenates are then centrifugated for 5 min at 5000 rpm. ELISA was performed following the manufacturer's instruction of the ELISA Kit (Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China). The results of the 0 day as the control group (Figure S1).
Real-time PCR for neurotransmitter-related genes
At the pre-exposure and 7-d exposure time points, total RNA was extracted from 15 whole zebrafish (15 for each concentration) following the protocols of TransZol Up Plus RNA Kit (Cat#ER501-01, Beijing TransGen Biotech Co., Ltd, Beijing, China). The extracted total RNA was qualified by Agilent Bioanalyzer 2100 (Agilent Technologies, SantaClara, CA, US), and then was purified by RNAClean XP Kit (Beijing TransGen Biotech Co., Ltd, Beijing, China) and RNase-Free DNase Set kit (Beijing TransGen Biotech Co., Ltd, Beijing, China). The concentration and purity of RNA were assessed by NanoDrop ND-2000 spectrophotometer (Thermo Fisher, USA). The synthesis of first-strand complementary cDNA was achieved via reverse transcription using ReverTra Ace qPCR Kit (TOYOBO Life Science, Shanghai, China) following the manufacturer's protocol. Quantitative real-time PCR was executed with Power SYBR Green PCR Master Mix (Applied Biosystems Co., Ltd, USA), which was performed using a QuantStudio 5 Real-Time PCR System (Applied Biosystems Co., Ltd, USA) and run with the Software QuantStudio™ Design &Analysis Software. 9 μL of the gene-specific qPCR reaction solution and 1 μL of the sample were added to the 384-well plate. The procedure was as follows: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Five genes were screened in this study, including gabra1, grin1b, grin2b, abat, and gad1b. The primers were designed by Primer Express 3.0.1 software and synthesized by Sangon Biotech (Shanghai) Co., Ltd. The primer sequences and related information were shown in Table S1. Actin was used as the reference gene for qPCR quantification.
Zebrafish larval motor behavior
AChE may cause muscle contraction in fish, leading to abnormal repetitive or excessive motor behavior.54 Therefore, 24 larvae were randomly chosen after being exposed to CMZ for 7 days for each group to study the behavior. Larvae were moved to a 96-well plate and allowed to acclimate to the observation room at 28°C for 10 min before the monitoring. The Zebrabox (ViewPoint Life Science, France) was used to record the locomotor sum distance during 60-minute light-dark periods (10 min of light alternating with 10 min of darkness). The software Video Track 3.5 (Viewpoint, France) was used for the analysis. Healthy adult wild-type zebrafish that reached sexual maturity were mated with males and females the night before exposure, and eggs were collected the following morning when the fish house was lit for 1 hour. Zebrafish embryos were exposed in glass dishes and transferred to porous plates on the seven days to test zebrafish behavior. A control group and four treatment groups with CMZ exposure concentrations of 1, 10, 100 and 1000 μg/L were set. The solution was updated every 24 hours during exposure. The solution in the blank control group was 10% Hanks solution (containing 0.1% DMSO). The solution of the exposed group was diluted step by step by the mother liquor of the blank control group. Each group contained 50 zebrafish embryos that had developed to the blastocyst stage (3-5 hpf). Embryos were cultured and exposed to light in an incubator. The light time and temperature of the incubator were consistent with the conditions of the fish house. Zebrafish larvae were placed in 96-well plates, and 1mL exposure solution and one larva were added to each well. There were 24 larvae in each group. Let it acclimate in Zebrabox (viewpoint, France) for ten minutes. The total test time was 60 minutes. After 10 minutes of light adaptation, alternate between darkness and light every 10 minutes. The Zebrabox system recorded the swimming movement of larvae during the test cycle, and indirectly evaluated the neural growth and development of zebrafish embryos.
The fish plasma model
The original fish plasma model (FPM) was proposed by Huggett et al.10 The predicted bioconcentration factor (BCF or P Blood: Water), is the distribution coefficient of pollutants in plasma and water, which can be calculated by Equation 1 with log KOW. Tanoue et al.19 used Dow and Dlipw to refine the fish plasma model. It was found that the Kow (2.77), Dow (2.77), and Dlipw (2.92) of CMZ are very close at pH 7.4, so we finally choose to calculate with Kow. Data of Kow, Dow, and Dlipw are from http://www.chemspider.com/Chemical. The steady-state plasma concentration in fish (FSSPC) by the measured or predicted environmental concentration (EC) and P Blood: Water of the target pollutants in the water body was calculated using Equation 2 for a specific calculation. The max human therapeutic plasma concentration (HTPC) was defined as 1200 μg/mL,87 which was used to calculate effect ratio (ER) values (Equation 3).
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
Bioconcentration (BCF) is the process by which a chemical substance is absorbed by an organism from the ambient environment only through its respiratory and dermal surfaces (e.g., gills in fish), i.e., chemical exposure in the diet is not included.58 In our experiment, dietary intake of CMZ was not considered. If respiratory and dermal surfaces are assumed to be the primary pathway of exposure to CMZ, the measured bioconcentration factor in plasma (BCFplasma) corresponds to the chemical distribution of aqueous phases and arterial plasma by contacting an aqueous solution, and it can be calculated by:
| (Equation 4) |
Quantification and statistical analysis
The Mean and standard deviation (SD) were calculated by Excel. The blank group was used as the control group for unified normalized data processing. SPSS (version 20, SPSS Inc., USA) was used to perform statistical analyses. The data of neurotransmitters and neurotransmitter-related genes were analyzed for normality (Quantile-Quantile plot test) and heteroscedasticity (Rank correlation coefficient test). The Duncan test was used to compare the treatment and control means in cases where the homogeneity and heteroscedasticity criteria were satisfied. On each diagram of the figures, the p value is indicated, and asterisk depicted the degree of significance (∗p < 0.05; ∗∗ p < 0.01). Figures were plotted using Origin 2018 software (OriginLab Corp., MA, USA).
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 22076141, 21777122, 21577103].
Author contributions
Writing—Original Draft, W.Y.; Investigation, Y.B. and J.H.; Conceptualization and Methodology, X.H.; Writing—Review & Editing, X.H., W.Y., T.X., D.Y.
Declaration of interests
The authors declare no competing interests.
Published: August 23, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107688.
Supplemental information
References
- 1.dos Santos C.R., Arcanjo G.S., de Souza Santos L.V., Koch K., Amaral M.C.S. Aquatic concentration and risk assessment of pharmaceutically active compounds in the environment. Environ. Pollut. 2021;290 doi: 10.1016/j.envpol.2021.118049. [DOI] [PubMed] [Google Scholar]
- 2.Cerveny D., Grabic R., Grabicová K., Randák T., Larsson D.G.J., Johnson A.C., Jürgens M.D., Tysklind M., Lindberg R.H., Fick J. Neuroactive drugs and other pharmaceuticals found in blood plasma of wild European fish. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106188. [DOI] [PubMed] [Google Scholar]
- 3.Brodin T., Fick J., Jonsson M., Klaminder J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science. 2013;339:814–815. doi: 10.1126/science.1226850. [DOI] [PubMed] [Google Scholar]
- 4.Barreto A., Santos J., Capitão A., Eusébio R., Pinheiro Damasceno É., Luísa Machado A., Rocha L.S., Calisto V., Amorim M.J.B., Maria V.L. Assessment of diphenhydramine toxicity-Is its mode of action conserved between human and zebrafish? Environ. Int. 2022;164 doi: 10.1016/j.envint.2022.107263. [DOI] [PubMed] [Google Scholar]
- 5.Leuthold D., Klüver N., Altenburger R., Busch W. Can Environmentally Relevant Neuroactive Chemicals Specifically Be Detected with the Locomotor Response Test in Zebrafish Embryos? Environ. Sci. Technol. 2019;53:482–493. doi: 10.1021/acs.est.8b04327. [DOI] [PubMed] [Google Scholar]
- 6.Rand-Weaver M., Margiotta-Casaluci L., Patel A., Panter G.H., Owen S.F., Sumpter J.P. The Read-Across Hypothesis and Environmental Risk Assessment of Pharmaceuticals. Environ. Sci. Technol. 2013;47:11384–11395. doi: 10.1021/es402065a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunnarsson L., Jauhiainen A., Kristiansson E., Nerman O., Larsson D.G.J. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 2008;42:5807–5813. doi: 10.1021/es8005173. [DOI] [PubMed] [Google Scholar]
- 8.Brown A.R., Gunnarsson L., Kristiansson E., Tyler C.R. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunnarsson L., Snape J.R., Verbruggen B., Owen S.F., Kristiansson E., Margiotta-Casaluci L., Österlund T., Hutchinson K., Leverett D., Marks B., Tyler C.R. Pharmacology beyond the patient - The environmental risks of human drugs. Environ. Int. 2019;129:320–332. doi: 10.1016/j.envint.2019.04.075. [DOI] [PubMed] [Google Scholar]
- 10.Huggett D.B., Cook J.C., Ericson J.F., Williams R.T. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Human and Ecological Risk Assessment. 2003;9:1789–1799. doi: 10.1080/714044797. [DOI] [Google Scholar]
- 11.Nozaki K., Tanoue R., Kunisue T., Tue N.M., Fujii S., Sudo N., Isobe T., Nakayama K., Sudaryanto A., Subramanian A., et al. Pharmaceuticals and personal care products (PPCPs) in surface water and fish from three Asian countries: Species-specific bioaccumulation and potential ecological risks. Sci. Total Environ. 2023;866 doi: 10.1016/j.scitotenv.2022.161258. [DOI] [PubMed] [Google Scholar]
- 12.Henneberger L., Klüver N., Mühlenbrink M., Escher B. Trout and Human Plasma Protein Binding of Selected Pharmaceuticals Informs the Fish Plasma Model. Environ. Toxicol. Chem. 2022;41:559–568. doi: 10.1002/etc.4934. [DOI] [PubMed] [Google Scholar]
- 13.Fitzsimmons P.N., Fernandez J.D., Hoffman A.D., Butterworth B.C., Nichols J.W. Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss) Aquat. Toxicol. 2001;55:23–34. doi: 10.1016/s0166-445x(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 14.Grabicová K., Randák T., Cerveny D., Turek J., Kolářová J., Brooks B.W., Grabic R. Influence of time-dependent sampling on fish plasma levels of select pharmaceuticals and per- and polyfluoroalkyl substances (PFASs) Environ. Pollut. 2022;315 doi: 10.1016/j.envpol.2022.120338. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell D.J., Mastrocco F., Margiotta-Casaluci L., Brooks B.W. An integrated approach for prioritizing pharmaceuticals found in the environment for risk assessmerit, monitoring and advanced research. Chemosphere. 2014;115:4–12. doi: 10.1016/j.chemosphere.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Bao Y., Huang W., Hu X., Yin D. Distribution of 31 endocrine-disrupting compounds in the Taihu Lake and application of the fish plasma model. Environ. Sci. Eur. 2020;32:80. doi: 10.1186/s12302-020-00347-0. [DOI] [Google Scholar]
- 17.Du B., Haddad S.P., Luek A., Scott W.C., Saari G.N., Kristofco L.A., Connors K.A., Rash C., Rasmussen J.B., Chambliss C.K., Brooks B.W. Bioaccumulation and trophic dilution of human pharmaceuticals across trophic positions of an effluent-dependent wadeable stream. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2014.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huerta B., Margiotta-Casaluci L., Rodríguez-Mozaz S., Scholze M., Winter M.J., Barceló D., Sumpter J.P. Anti-anxiety drugs and fish behavior: establishing the link between internal concentrations of oxazepam and behavioral effects. Environ. Toxicol. Chem. 2016;35:2782–2790. doi: 10.1002/etc.3448. [DOI] [PubMed] [Google Scholar]
- 19.Tanoue R., Margiotta-Casaluci L., Huerta B., Runnalls T.J., Nomiyama K., Kunisue T., Tanabe S., Sumpter J.P. Uptake and Metabolism of Human Pharmaceuticals by Fish: A Case Study with the Opioid Analgesic Tramadol. Environ. Sci. Technol. 2017;51:12825–12835. doi: 10.1021/acs.est.7b03441. [DOI] [PubMed] [Google Scholar]
- 20.Duarte I.A., Fick J., Cabral H.N., Fonseca V.F. Bioconcentration of neuroactive pharmaceuticals in fish: Relation to lipophilicity, experimental design and toxicity in the aquatic environment. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.152543. [DOI] [PubMed] [Google Scholar]
- 21.Margiotta-Casaluci L., Owen S.F., Cumming R.I., de Polo A., Winter M.J., Panter G.H., Rand-Weaver M., Sumpter J.P. Quantitative cross-species extrapolation between humans and fish: The case of the anti-depressant fluoxetine. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenti T.W., Jr., Gould G.G., Berninger J.P., Connors K.A., Keele N.B., Prosser K.N., Brooks B.W. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol. 2012;46:2427–2435. doi: 10.1021/es204164b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmon P., Owen S.F., Margiotta-Casaluci L. Pharmacology-informed prediction of the risk posed to fish by mixtures of non-steroidal anti-inflammatory drugs (NSAIDs) in the environment. Environ. Int. 2021;146 doi: 10.1016/j.envint.2020.106222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel A., Panter G.H., Trollope H.T., Glennon Y.C., Owen S.F., Sumpter J.P., Rand-Weaver M. Testing the "read-across hypothesis" by investigating the effects of ibuprofen on fish. Chemosphere. 2016;163:592–600. doi: 10.1016/j.chemosphere.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margiotta-Casaluci L., Owen S.F., Huerta B., Rodríguez-Mozaz S., Kugathas S., Barceló D., Rand-Weaver M., Sumpter J.P. Internal exposure dynamics drive the Adverse Outcome Pathways of synthetic glucocorticoids in. Sci. Rep. 2016;6 doi: 10.1038/srep28122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunze A., Amann B.L., Grunze H. Efficacy of Carbamazepine and Its Derivatives in the Treatment of Bipolar Disorder. Med. Lith. 2021;57:433. doi: 10.3390/medicina57050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anca Oana D., Predoi V., Calina D., Arsene A. Reference Module in Biomedical Sciences. 2023. Carbamazepine. [DOI] [Google Scholar]
- 28.Delcker A., Wilhelm H., Timmann D., Diener H.C. Side effects from increased doses of carbamazepine on neuropsychological and posturographic parameters of humans. Eur. Neuropsychopharmacol. 1997;7:213–218. doi: 10.1016/s0924-977x(97)00406-9. [DOI] [PubMed] [Google Scholar]
- 29.Ubaid A., Waheed F., Waheed S. Carbamazepine-Induced Uncontrolled Hypertension. Am. J. Ther. 2020;27:E696–E698. doi: 10.1097/mjt.0000000000001055. [DOI] [PubMed] [Google Scholar]
- 30.Adeleye A.S., Xue J., Zhao Y., Taylor A.A., Zenobio J.E., Sun Y., Han Z., Salawu O.A., Zhu Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127284. [DOI] [PubMed] [Google Scholar]
- 31.Feijoo S., Kamali M., Dewil R. A review of wastewater treatment technologies for the degradation of pharmaceutically active compounds: Carbamazepine as a case study. Chem. Eng. J. 2023;455 doi: 10.1016/j.cej.2022.140589. [DOI] [Google Scholar]
- 32.Qiang L., Cheng J., Yi J., Rotchell J.M., Zhu X., Zhou J. Environmental concentration of carbamazepine accelerates fish embryonic development and disturbs larvae behavior. Ecotoxicology. 2016;25:1426–1437. doi: 10.1007/s10646-016-1694-y. [DOI] [PubMed] [Google Scholar]
- 33.Özerman-Edis B., Nurten A., Kara İ. Blockage of Voltage-Dependent Calcium Channels Affects Twitch Response of Rat Skeletal Muscle. Neurochem. J. 2021;15:154–158. doi: 10.1134/s1819712421020136. [DOI] [Google Scholar]
- 34.Chen H., Yang H., Zhao Y., Gu X., Martyniuk C.J. Development and Molecular Investigation into the Effects of Carbamazepine Exposure in the Zebrafish (Danio rerio) Int. J. Environ. Res. Public Health. 2020;17:8882. doi: 10.3390/ijerph17238882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosa J.G.S., Lima C., Lopes-Ferreira M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022;23:6647. doi: 10.3390/ijms23126647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing L., Huttner W.B. Neurotransmitters as Modulators of Neural Progenitor Cell Proliferation During Mammalian Neocortex Development. Front. Cell Dev. Biol. 2020;8:391. doi: 10.3389/fcell.2020.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayachandran P., Battaglin F., Strelez C., Lenz A., Algaze S., Soni S., Lo J.H., Yang Y., Millstein J., Zhang W., et al. Breast cancer and neurotransmitters: emerging insights on mechanisms and therapeutic directions. Oncogene. 2023;42:627–637. doi: 10.1038/s41388-022-02584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraz S., Lee A.H., Pollard S., Srinivasan K., Vermani A., David E., Wilson J.Y. Paternal Exposure to Carbamazepine Impacts Zebrafish Offspring Reproduction Over Multiple Generations. Environ. Sci. Technol. 2019;53:12734–12743. doi: 10.1021/acs.est.9b03393. [DOI] [PubMed] [Google Scholar]
- 39.Liang X., Csenki Z., Ivánovics B., Bock I., Csorbai B., Molnár J., Vásárhelyi E., Griffitts J., Ferincz Á., Urbányi B., Ács A. Biochemical Marker Assessment of Chronic Carbamazepine Exposure at Environmentally Relevant Concentrations in Juvenile Common Carp (Cyprinus carpio) Antioxidants. 2022;11:1136. doi: 10.3390/antiox11061136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calcagno E., Durando P., Valdés M.E., Franchioni L., Bistoni M.d.l.Á. Effects of carbamazepine on cortisol levels and behavioral responses to stress in the fish Jenynsia multidentata. Physiol. Behav. 2016;158:68–75. doi: 10.1016/j.physbeh.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia H., Luo K.Q. Fluorescence resonance energy transfer -based sensor zebrafish for detecting toxic agents with single -cell sensitivity. J. Hazard Mater. 2021;408 doi: 10.1016/j.jhazmat.2020.124826. [DOI] [PubMed] [Google Scholar]
- 43.Nichols J.W., Du B., Berninger J.P., Connors K.A., Chambliss C.K., Erickson R.J., Hoffman A.D., Brooks B.W. Observed and modeled effects of pH on bioconcentration of diphenhydramine, a weakly basic pharmaceutical, in fathead minnows. Environ. Toxicol. Chem. 2015;34:1425–1435. doi: 10.1002/etc.2948. [DOI] [PubMed] [Google Scholar]
- 44.Tanoue R., Nomiyama K., Nakamura H., Kim J.W., Isobe T., Shinohara R., Kunisue T., Tanabe S. Uptake and Tissue Distribution of Pharmaceuticals and Personal Care Products in Wild Fish from Treated-Wastewater-Impacted Streams. Environ. Sci. Technol. 2015;49:11649–11658. doi: 10.1021/acs.est.5b02478. [DOI] [PubMed] [Google Scholar]
- 45.Weil M., Falkenhain A.-M., Scheurer M., Ryan J.J., Coors A. Uptake and Effects of the Beta-Adrenergic Agonist Salbutamol in Fish: Supporting Evidence for the Fish Plasma Model. Environ. Toxicol. Chem. 2019;38:2509–2519. doi: 10.1002/etc.4543. [DOI] [PubMed] [Google Scholar]
- 46.Barber L.B., Brown G.K., Nettesheim T.G., Murphy E.W., Bartell S.E., Schoenfuss H.L. Effects of biologically-active chemical mixtures on fish in a wastewater-impacted urban stream. Sci. Total Environ. 2011;409:4720–4728. doi: 10.1016/j.scitotenv.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 47.Bertrand C., Chatonnet A., Takke C., Yan Y.L., Postlethwait J., Toutant J.P., Cousin X. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7 - Gene structure and polymorphism; Molecular forms and expression pattern during development. J. Biol. Chem. 2001;276:464–474. doi: 10.1074/jbc.M006308200. [DOI] [PubMed] [Google Scholar]
- 48.Nkoom M., Lu G., Liu J., Dong H. Biological uptake, depuration and biochemical effects of diclofenac and carbamazepine in Carassius carassius. Ecotoxicol. Environ. Saf. 2020;205 doi: 10.1016/j.ecoenv.2020.111106. [DOI] [PubMed] [Google Scholar]
- 49.da Silva Santos N., Oliveira R., Lisboa C.A., Mona e Pinto J., Sousa-Moura D., Camargo N.S., Perillo V., Oliveira M., Grisolia C.K., Domingues I. Chronic effects of carbamazepine on zebrafish: Behavioral, reproductive and biochemical endpoints. Ecotoxicol. Environ. Saf. 2018;164:297–304. doi: 10.1016/j.ecoenv.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Long R., DU J. Metabotropic glutamate receptors and related nervous diseases. Chin. Pharmacol. Bull. 2009;25:998–1001. [Google Scholar]
- 51.Monesson-Olson B., McClain J.J., Case A.E., Dorman H.E., Turkewitz D.R., Steiner A.B., Downes G.B. Expression of the eight GABAA receptor a subunits in the developing zebrafish central nervous system. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gawel K., Kukula-Koch W., Banono N.S., Nieoczym D., Targowska-Duda K.M., Czernicka L., Parada-Turska J., Esguerra C.V. 6-Gingerol, a Major Constituent of Zingiber officinale Rhizoma, Exerts Anticonvulsant Activity in the Pentylenetetrazole-Induced Seizure Model in Larval Zebrafish. Int. J. Mol. Sci. 2021;22:7745. doi: 10.3390/ijms22147745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuccaro A., Frenzilli G., Del Barga I., Costagliola D. Genotoxic effects in zebrafish (Danio rerio) erythrocytes of two pharmacological compounds in an impacted Italian river. Mar. Environ. Res. 2006;62:S310–S311. [Google Scholar]
- 54.Umar A.M., Aisami A. Acetylcholinesterase enzyme (AChE) as a biosensor and biomarker for pesticides: A mini review. Bull. Environ. Sci. Sust. Manage. 2020;4:7–12. [Google Scholar]
- 55.McDonald T.A. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46:745–755. doi: 10.1016/s0045-6535(01)00239-9. Pii s0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- 56.Li Z.H., Li P., Rodina M., Randak T. Effect of human pharmaceutical Carbamazepine on the quality parameters and oxidative stress in common carp (Cyprinus carpio L.) spermatozoa. Chemosphere. 2010;80:530–534. doi: 10.1016/j.chemosphere.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 57.Escher B.I., Hermens J.L.M. Internal exposure: Linking bioavailability to effects. Environ. Sci. Technol. 2004;38:455A–462A. doi: 10.1021/es0406740. [DOI] [PubMed] [Google Scholar]
- 58.Arnot J.A., Gobas F.A. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006;14:257–297. [Google Scholar]
- 59.Chang E.D., Hogstrand C., Miller T.H., Owen S.F., Bury N.R. The Use of Molecular Descriptors To Model Pharmaceutical Uptake by a Fish Primary Gill Cell Culture Epithelium. Environ. Sci. Technol. 2019;53:1576–1584. doi: 10.1021/acs.est.8b04394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexishvili M.M., Rukhadze M.D., Okujava V.M. Simultaneous determination of carbamazepine and carbamazepine 10,11-epoxide by using microcolumn HPLC: Study of pharmacokinetics of carbamazepine in a volunteer. Biomed. Chromatogr. 1997;11:36–41. doi: 10.1002/(sici)1099-0801(199701)11:1<36::Aid-bmc622>3.3.Co;2-t. [DOI] [PubMed] [Google Scholar]
- 61.Rukhadze M.D., Alexishvili M.M., Gonashvili M.V., Okujava N.V., Sebiskveradze M.V. Interaction of carbamazepine and promethazine in rabbits. Biomed. Chromatogr. 2003;17:62–69. doi: 10.1002/bmc.209. [DOI] [PubMed] [Google Scholar]
- 62.Davies N.M., Takemoto J.K., Brocks D.R., Yáñez J.A. Multiple Peaking Phenomena in Pharmacokinetic Disposition. Clin. Pharmacokinet. 2010;49:351–377. doi: 10.2165/11319320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Fuhr L.M., Marok F.Z., Hanke N., Selzer D., Lehr T. Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug-Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics. 2021;13:270. doi: 10.3390/pharmaceutics13020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goldstone J.V., McArthur A.G., Kubota A., Zanette J., Parente T., Jönsson M.E., Nelson D.R., Stegeman J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Runnalls T.J., Beresford N., Kugathas S., Margiotta-Casaluci L., Scholze M., Scott A.P., Sumpter J.P. From single chemicals to mixtures-Reproductive effects of levonorgestrel and ethinylestradiol on the fathead minnow. Aquat. Toxicol. 2015;169:152–167. doi: 10.1016/j.aquatox.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Muir D., Simmons D., Wang X., Peart T., Villella M., Miller J., Sherry J. Bioaccumulation of pharmaceuticals and personal care product chemicals in fish exposed to wastewater effluent in an urban wetland. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glover C.N., Klaczek C.E., Goss G.G., Saari G.N. Factors Affecting the Binding of Diltiazem to Rainbow Trout Plasma: Implications for the Risk Assessment of Pharmaceuticals in Aquatic Systems. Environ. Toxicol. Chem. 2022;41:3125–3133. doi: 10.1002/etc.5493. [DOI] [PubMed] [Google Scholar]
- 68.Fu W., Franco A., Trapp S. Methods for estimating the bioconcentration factor of ionizable organic chemicals. Environ. Toxicol. Chem. 2009;28:1372–1379. doi: 10.1897/08-233.1. [DOI] [PubMed] [Google Scholar]
- 69.Zhu M., Chen J., Peijnenburg W.J.G.M., Xie H., Wang Z., Zhang S. Controlling factors and toxicokinetic modeling of antibiotics bioaccumulation in aquatic organisms: A review. Crit. Rev. Environ. Sci. Technol. 2022;53:1431–1451. doi: 10.1080/10643389.2022.2142033. [DOI] [Google Scholar]
- 70.Winter M.J., Redfern W.S., Hayfield A.J., Owen S.F., Valentin J.-P., Hutchinson T.H. Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early-stage development drugs. J. Pharmacol. Toxicol. Methods. 2008;57:176–187. doi: 10.1016/j.vascn.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Winter M.J., Pinion J., Tochwin A., Takesono A., Ball J.S., Grabowski P., Metz J., Trznadel M., Tse K., Redfern W.S., et al. Functional brain imaging in larval zebrafish for characterising the effects of seizurogenic compounds acting via a range of pharmacological mechanisms. Br. J. Pharmacol. 2021;178:2671–2689. doi: 10.1111/bph.15458. [DOI] [PubMed] [Google Scholar]
- 72.Milan D.J., Peterson T.A., Ruskin J.N., Peterson R.T., MacRae C.A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.Cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 73.Parker T., Libourel P.-A., Hetheridge M.J., Cumming R.I., Sutcliffe T.P., Goonesinghe A.C., Ball J.S., Owen S.F., Chomis Y., Winter M.J. A multi-endpoint in vivo larval zebrafish (Danio rerio) model for the assessment of integrated cardiovascular function. J. Pharmacol. Toxicol. Methods. 2014;69:30–38. doi: 10.1016/j.vascn.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Runnalls T.J., Beresford N., Losty E., Scott A.P., Sumpter J.P. Several Synthetic Progestins with Different Potencies Adversely Affect Reproduction of Fish. Environ. Sci. Technol. 2013;47:2077–2084. doi: 10.1021/es3048834. [DOI] [PubMed] [Google Scholar]
- 75.Margiotta-Casaluci L., Hannah R.E., Sumpter J.P. Mode of action of human pharmaceuticals in fish: The effects of the 5-alpha-reductase inhibitor, dutasteride, on reproduction as a case study. Aquat. Toxicol. 2013;128–129:113–123. doi: 10.1016/j.aquatox.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 76.LaLone C.A., Villeneuve D.L., Olmstead A.W., Medlock E.K., Kahl M.D., Jensen K.M., Durhan E.J., Makynen E.A., Blanksma C.A., Cavallin J.E., et al. Effects of a glucocorticoid receptor agonist, dexamethasone, on fathead minnow reproduction, growth, and development. Environ. Toxicol. Chem. 2012;31:611–622. doi: 10.1002/etc.1729. [DOI] [PubMed] [Google Scholar]
- 77.Kugathas S., Sumpter J.P. Synthetic Glucocorticoids in the Environment: First Results on Their Potential Impacts on Fish. Environ. Sci. Technol. 2011;45:2377–2383. doi: 10.1021/es104105e. [DOI] [PubMed] [Google Scholar]
- 78.Kugathas S., Runnalls T.J., Sumpter J.P. Metabolic and Reproductive Effects of Relatively Low Concentrations of Beclomethasone Dipropionate, a Synthetic Glucocorticoid, on Fathead Minnows. Environ. Sci. Technol. 2013;47:9487–9495. doi: 10.1021/es4019332. [DOI] [PubMed] [Google Scholar]
- 79.Mehinto A.C., Hill E.M., Tyler C.R. Uptake and Biological Effects of Environmentally Relevant Concentrations of the Nonsteroidal Anti-inflammatory Pharmaceutical Diclofenac in Rainbow Trout (Oncorhynchus mykiss) Environ. Sci. Technol. 2010;44:2176–2182. doi: 10.1021/es903702m. [DOI] [PubMed] [Google Scholar]
- 80.Zucchi S., Castiglioni S., Fent K. Progesterone Alters Global Transcription Profiles at Environmental Concentrations in Brain and Ovary of Female Zebrafish (Danio rerio) Environ. Sci. Technol. 2013;47:12548–12556. doi: 10.1021/es403800y. [DOI] [PubMed] [Google Scholar]
- 81.Nieoczym D., Socała K., Gawel K., Esguerra C.V., Wyska E., Wlaź P. Anticonvulsant Activity of Pterostilbene in Zebrafish and Mouse Acute Seizure Tests. Neurochem. Res. 2019;44:1043–1055. doi: 10.1007/s11064-019-02735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siebel A.M., Menezes F.P., da Costa Schaefer I., Petersen B.D., Bonan C.D. Rapamycin suppresses PTZ-induced seizures at different developmental stages of zebrafish. Pharmacol. Biochem. Behav. 2015;139 Pt B:163–168. doi: 10.1016/j.pbb.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 83.Siebel A.M., Piato A.L., Schaefer I.C., Nery L.R., Bogo M.R., Bonan C.D. Antiepileptic drugs prevent changes in adenosine deamination during acute seizure episodes in adult zebrafish. Pharmacol. Biochem. Behav. 2013;104:20–26. doi: 10.1016/j.pbb.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 84.Afrikanova T., Serruys A.-S.K., Buenafe O.E.M., Clinckers R., Smolders I., de Witte P.A.M., Crawford A.D., Esguerra C.V. Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs. PLoS One. 2013;8:e54166. doi: 10.1371/journal.pone.0054166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ayano G. 2016. Bipolar Disorders and Carbamazepine: Pharmacokinetics,Pharmacodynamics, Therapeutic Effects and Indications of Carbamazepine: Review of Articles. [Google Scholar]
- 86.Iannaccone T., Sellitto C., Manzo V., Colucci F., Giudice V., Stefanelli B., Iuliano A., Corrivetti G., Filippelli A. Pharmacogenetics of Carbamazepine and Valproate: Focus on Polymorphisms of Drug Metabolizing Enzymes and Transporters. Pharmaceuticals. 2021;14:204. doi: 10.3390/ph14030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu-zhen L.I., Ri-lai C., Heng-mei L.I., Xin-yu L.I.U., Cheng L.I., Fan-tao Z., Man-lin W. Analysis of serum concentration of carbamazepine in 381 cases. Chin. J. Hosp. Pharm. 2009;29:1109–1111. 1001-5213(2009)29:13<1109:3lckmx>2.0.Tx;2-l. [Google Scholar]
- 88.Tufi S., Leonards P., Lamoree M., de Boer J., Legler J., Legradi J. Changes in Neurotransmitter Profiles during Early Zebrafish (Danio rerio) Development and after Pesticide Exposure. Environ. Sci. Technol. 2016;50:3222–3230. doi: 10.1021/acs.est.5b05665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.