ABSTRACT
The prevalence of atopic diseases is increasing globally, particularly in children. Heritable genetics can partially explain risk of disease. Evidence also points to acquired genetic material, in the form of the microbiome, as an important factor in disease pathogenesis. The acquisition of the microbiome dynamically changes in response to differences in lifestyle and environmental factors. Also, in utero, maternal and environmental factors influence atopic risk for allergic rhinitis, eczema, asthma, and food allergy. Combining the analytical power of omics, we focus on how the microbiota mediates effects between mother, environment, immunity, and risk of atopic disease. In parallel, we stress that health care disparities impact asthma morbidity and mortality. Efforts to improve asthma outcomes must include multidisciplinary strategies.
INTRODUCTION
Maternal and fetal interactions, which are reflected in the microbiome, immune response, and transcriptome, are presumed to impact the onset of early life allergic disease (1-4). Given the high incidence of allergy, there is an unmet need to define biomarkers and predictive risk, as well as mechanisms of the development of allergic disease that may offer preventative and therapeutic strategies.
Importance of the Problem
Since the late 1970s, the incidence of allergic diseases in children has significantly increased in the United States and most developed countries, reaching epidemic levels. In the last 20 years, those rates have plateaued, or even decreased, while the incidence of allergic diseases in developing countries has increased (5-7). These trends (8,9) suggest that there are potentially modifiable factors, such as environmental exposures, that contribute to the risk of these diseases. Using allergic asthma as an example, lower rates of asthma occur in children who are not first born (10,11), attend day care in their first six months (10), are raised in close proximity to farm animals (3,12,13), or live with dogs or cats in their first year (14). Children born via vaginal delivery (15,16) and/or who have a diversity of microbiota early in life (17) also have lower rates of allergic disease. These factors are all manifestations of environmental exposure. Accumulating evidence supports a role for the microbiome in the development of allergic phenotypes (18,19), which stresses the importance of early exposures to diverse microbiota to modulate the risk of allergic diseases. We have demonstrated that allergen-specific responses in cord blood lymphocytes (20-26) and Proteobacteria DNA in cord blood are associated with decreased allergic disease at age 7 (1). Thus, the promotion of allergic responses may be triggered at birth, or even in utero by environmental exposures.
Childhood asthma is a disease of high prevalence (5,6) that eludes early diagnosis (27). In New York City, asthma rates among public school children aged 5–14 years have increased in all five New York City boroughs and remain highest in the Bronx (28). In the Bronx, Latino/a and Black children as well as those residing in high poverty neighborhoods have the highest rates of active asthma (29-31). The Bronx also has the highest percentage of homes with mice, rats, cockroaches, mold, and second-hand smoking exposure of the New York City boroughs, which are all manifestations of environmental/endotoxin exposure that can promote allergic disease and asthma (28). In rural settings, there is an inverse relationship between development of asthma and growing up on a farm where there is diverse microbial and endotoxin exposure (17). In our work, we strive to elucidate environmental endotoxin exposure modulation of allergic responses.
Diagnosis in young children is challenging due to the lack of specificity in the early clinical presentation of atopy and wheeze. While nearly half of the population will experience at least one wheezing episode in early childhood, most individuals will not go on to develop asthma (27,32). Spirometry, which is the gold standard for asthma diagnosis, is typically not utilized in children under the age of 6. Thus, characterization of early immune or microbial states that predispose toward clinical diagnosis of asthma may facilitate identification of individuals that are poised to develop disease.
The prototypic immune alteration in allergic asthma is that of dominant T-helper 2 cell (Th2) activation without adequate counter-regulation by T-helper 1 (Th1) and regulatory (Treg) cells (33). The process of allergic sensitization involves differentiation of these effector T-cell populations and reshaping of their cytokine profiles (34). Less is known regarding the differential basal immune states that predispose toward allergic sensitization and disease development. We have previously reported altered Th2 cytokine elaboration in response to common aeroallergens in the cord blood mononuclear cells of neonates with differing in utero microbial exposures (35). These differential immune signatures can be probed as early as birth.
Investigation of genome-wide expression networks and gene regulatory networks prior to the development of asthma may provide clues of altered gene-gene interactions and epigenetic effects that underlie allergic predisposition. In a prior study, German cockroach extract (CR) stimulation of peripheral blood mononuclear cells (PBMCs) increased natural killer cell-type gene expression in two-year-olds who developed aeroallergen sensitization by age 3 and clinical asthma by the age of 7 (36). These differentially expressed genes were only found in children with both CR sensitization and asthma by the age of 7. Similar to our findings in a prior study, immune signatures correlated with home allergen levels (CR) (35). We posit that these differential transcriptomic responses represent early pathways of sensitization to CR. In order to identify non-allergen-specific biomarkers of asthma and to characterize underlying immune states of asthmatic predisposition, we analyzed the tetanus toxoid (TT)-stimulated PBMCs from the same study cohort using a network-centric approach. TT stimulation elicits an unbiased and broad immune recall response, since all the children received tetanus vaccination in infancy. It therefore provides a useful immune perturbation, allowing for characterization of more subtly altered immune networks in asthmatic poise prior to clinically diagnosable disease. We elucidate differences of gene expression networks and gene regulatory networks, and infer epigenetic changes, in children at the age of 2 who develop asthma by age 7 compared to those who do not.
MATERIALS AND METHODS
Prospective birth cohorts in Boston and Chicago were established and samples obtained. Samples were harvested for microbiome and transcriptomic analyses as previously described (1,2,35).
For the Boston cohort, procedures for obtaining umbilical cord blood serum have been describe previously (24). For the Chicago cohort, umbilical cord blood was obtained by venipuncture shortly after time of delivery. RNA extraction and sequencing were performed as previously described. All statistical analyses were performed in R (https://www.r-project.org/) unless otherwise specified. Outcomes were assessed in Project Viva Categorical and outcomes were modeled using logistic regression. Continuous outcomes were modeled using linear regression (1).
For transcriptomic analyses, expression data were downloaded from the Gene Expression Omnibus database (GSE96783) and consisted of RNA-seq data from children enrolled in the Urban Environment and Childhood Asthma (URECA) study, in which subjects have parental history of allergic disease and live in low-income urban areas (17,18). In this prior study, RNA sequencing was performed on PBMCs from children at the age of 2, incubated with either German CR or dust mite extracts, TT, or media alone (no stimulation). For our study, we utilize RNA-seq data from the TT-stimulated and unstimulated PBMCs, with in-depth analysis of gene expression networks and gene regulatory networks from TT-stimulated data. We compare TT-stimulated networks of children at the age of 2 who developed aeroallergen sensitizations (including CR, dust mite, or both) by the age of 3 and clinical asthma by the age of 7 (asthma, n=19 with TT data) versus matched subjects who did not have any aeroallergen sensitizations or asthma at age 7 (control, n=30). Asthma at seven years of age was defined by a prespecified algorithm including use of asthma medications in the previous year, spirometry with reversibility, and bronchial hyperresponsiveness assessed using a methacholine challenge (2).
Weighted gene correlation network analysis (WGCNA) was performed with a soft thresholding power of 12 to produce scale-free network topology, a signed network and topology overlap matrix, the default minimum module size of 30 genes, and a cut height of 0.15. Gene ontology (GO) enrichment analysis was performed using the PANTHER classification system, a comprehensive system that combines gene function, ontology, pathways, and statistical analysis tools to analyze large-scale genome-wide data (21,22) and molecular signatures database (MsigDB) (23,24) to characterize the biological processes captured by each gene expression module. Pairwise connectivity of WGCNA modules was calculated using Pearson correlations of module eigengenes, separately for controls and asthma. Statistically significant associations were assessed at p < 0.05 separately for each group with false discovery role (FDR) correction (2).
RESULTS
Differential Presence of Bacteria Associated with Clinical Phenotype
We previously reported that allergic responses can be identified in perinatal cord blood lymphocytes (20,21,24), an observation that has been confirmed by other groups (20,37-39). Specifically, we demonstrated that cord blood mononuclear cells (CBMC) stimulated with allergens [e.g., dust mite (Der f1) and cockroach (Bla g2)] demonstrated allergen-specific proliferation and IL13 secretion. Based on these results, we postulated that cord blood (CB) contained stimuli to modulate allergic immune responses. Therefore, we analyzed CB from a Boston birth cohort, termed VIVA, with 16S sequencing and identified bacterial DNA (bDNA) from diverse bacteria (35). We identified compositional differences in the CB microbiome that produced IL-13. Increases in IL-13 levels inversely correlated with Proteobacteria (35). Interestingly, Proteobacteria has a potent innate immune-stimulating response by lipopolysaccharide (LPS) via TLR4 that could shift the immune response toward TH1 rather than TH2 (40). Also, we asked if the level of Proteobacteria correlated with the development of an asthma phenotype in children defined as the incidence of wheeze at age 7.
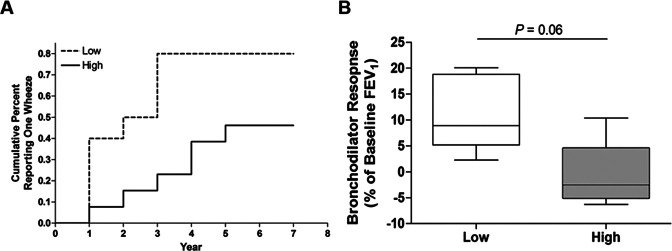
Two lines of evidence suggest that abundance of Proteobacterial circulating free bacterial DNA (cfbDNA) is related to risk of asthma (1); lower abundance is associated with offspring who have increased likelihood of Th2 responses to common aeroallergens (35) and atopic mothers, shown in this study. Children who had at least one response to an annual questionnaire assessing wheezing were analyzed for Proteobacteria abundance. Proteobacteria was dichotomized by greater or less than the median and its association with wheezing within the first seven years after birth using Cox proportional hazards models (Figure 1A). Lower exposure to Proteobacteria was associated with an increased risk of wheezing in a univariate model [HR (95%CI) = 3.60 (1.70-7.64), p = 0.001]. To determine if this association was independent of maternal atopic status, we adjusted for maternal atopy. Lower exposure to Proteobacteria was associated with an increased risk of wheezing in the multivariate model [aHR (95%CI) = 2.80 (1.46-5.36), p = 0.002]. Children were evaluated by pulmonary function tests at mid-childhood follow-up (median age 8.0 years, n = 9). Exposure to lower Proteobacteria trended toward an association with increased bronchodilator responses at mid--childhood (p = 0.060) (Figure 1B).
Fig. 1.
Lower Proteobacteria exposure is associated with increased risk of wheezing. (A) Kaplan-Meier curves for median of imputed cumulative percent parental reported wheezing at each year post-birth for children with high (solid, n=13) and low (dashed, n=10) exposure to Proteobacteria cfbDNA. (B) Children who had pulmonary function tests at mid-childhood follow-up (median age 8.0 years) were dichotomized for abundance of Proteobacteria, high (gray, n=4) and low (white, n=5). The groups were assessed for associations with changes in pulmonary function tests at mid-childhood. Bronchodilator response displayed as box and whisker plots for children with high or low (greater or less than the median, respectively) Proteobacteria exposure. P < 0.05 was considered statistically significant, Mann-Whitney U-test. From reference (1).
Differential Response to Vaccination in Children with Allergic Disease
Immune perturbations in translational studies of humans, particularly young children, are limited by ethical considerations. Therefore, to investigate dysregulation of the immune response in allergic subjects, we analyzed the molecular response to a standard of care vaccination (e.g., DTaP). We analyzed the dysregulated networks in polyallergen-sensitized children measured to elucidate the molecular basis of immune dysregulation in allergic disease.
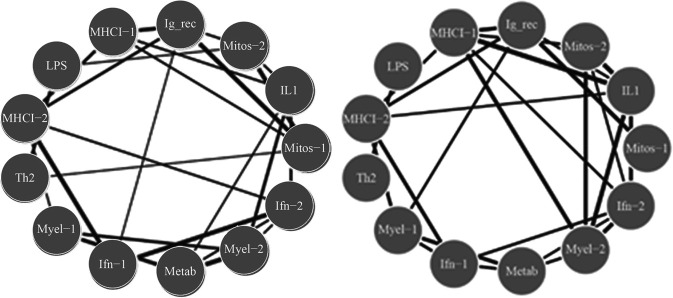
We analyzed RNA-seq data of in vitro TT-stimulated PBMCs collected from children at the age of 2 that were previously immunized with DTaP at least one month prior to TT stimulation in vitro. By age 7, 19 were diagnosed with asthma and 30 were not (controls) (GEO GSE96783 dataset). DESeq2 was used to analyze TT-stimulated gene expression in PBMCs compared to unstimulated expression levels. Differentially expressed genes (5737 in total) were analyzed using WGCNA to define 10 modules of co-regulated genes (not shown). Biological functions in each module were analyzed by GO enrichment analysis that included modules related to IFN-γ, TH1 and TH2 adaptive responses, as well as additional innate immune response modules. Networks of WGCNA modules were constructed for each cohort by calculating Pearson correlations between asthma and controls (at age 7) for each module, and significant differences between cohorts were assessed using the Fisher Z-transform (p < 0.01). Interestingly, we found that the TT-stimulated expression networks demonstrated significant alterations between asthma and controls, with altered connectivity involving the interferon gamma (IFN-γ), TH1 and myeloid modules differentiation (Figure 2). In response to the same antigen (TT), our analysis showed that the children who developed asthma utilized different modules of genes compared to the children who did not develop asthma.
Fig. 2.
Extensive differences of network connectivity in tetanus toxoid-stimulated PBMCs in two-year-olds who are subsequently diagnosed with asthma. Pearson correlations between gene expression modules are displayed for the control (left) and asthmatic cohort (right), demonstrating utilization of different modules of genes in the two groups. Only statistically significant different edges are shown [p < 0.05 from Fisher's exact test comparing correlation coefficient (control and asthma), with edge thickness displayed as a function of strength of interaction]. From reference (2).
In addition, the regulatory networks demonstrated extensive alterations in transcription factors previously associated with allergic responses. Examples include several transcription factors that demonstrate different regulatory strength in allergy versus controls. For example, GATA3, which is a master transcription factor for TH2 cell differentiation, shows increased regulatory strength (p < 0.001), whereas TBX21 (TBET), which is a master transcription factor for TH1 cell differentiation, does not show increased regulatory strength, as expected. Our results indicate that specific transcription factors exhibit dysregulation of gene targets, and that the direction of regulatory shift depends systematically on WGCNA module membership of the target gene.
The previous networks were constructed from RNA-seq data at age 2, whereas the diagnosis of asthma occurred at age 7. Therefore, we asked if the response at age 2 could predict the diagnosis of asthma at age 7 using a random forest algorithm (R package) based on ratios of gene expression. Our analysis showed an accuracy of 72% predictive value. We anticipate that the analysis of a larger cohort with increased power and inclusion of additional variables may further increase the predictive accuracy.
Our analysis demonstrates the power of network-level systems analyses. Specifically, analyses of the spectrum of memory immune responses to standard of care vaccinations uncovers molecular patterns that predict wheeze by age 7 based on omics data at age 2.
DISCUSSION
We observed that lower circulating Proteobacteria DNA in umbilical cord blood serum is associated with maternal atopy, greater hazard of wheezing, and greater bronchodilator response within the first seven years after birth. These findings are limited in their generalizability due to the small sample size; however, cfbDNA merits evaluation in a larger population as a novel biomarker for asthma risk independent of common risk factors.
We previously reported that reduced Proteobacteria is associated with IL-13 responses to common allergens (35). In this study, we identified associations between Proteobacteria DNA in cord blood, maternal atopy (a strong risk factor for asthma), and common features of asthma (e.g., early-life wheezing and airflow reversibility). Proteobacteria are a diverse phyla of Gram negative bacteria and, as compared to Bacteroidetes (also Gram negative), lipopolysaccharide (LPS) derived from Proteobacteria may have additional immunomodulatory properties (41). LPS in mattress dust has previously been negatively correlated with peripheral blood mononuclear cell IL-13 responses to allergen challenge in childhood (42). In concordance, administration of an LPS mimetic in murine models of asthma skews immune response toward IFN-γ (Th1 cytokine) production and suppresses airway hyper-reactivity (22). Together, these data suggest that LPS exposure, both endogenous and exogenous, could suppress allergic responses implicated in the pathogenesis of childhood asthma. These findings support the hypothesis that early bacterial exposure patterns immune development which may drive later pulmonary disease.
We characterize asthmatic immune interaction by probing gene-gene interactions and identifying significantly altered interactions in our gene expression networks. Gene network investigation powered by associations of stimulated gene expression across subjects allows us to uncover immune imbalances that precede clinical diagnosis of asthma.
The differential gene networks elucidated with TT stimulation suggest that broad immune imbalances prime allergic sensitization. Prior transcriptomic studies of childhood asthma have investigated subjects who already have a disease diagnosis and have primarily investigated differential gene expression as opposed to differential gene interactions. Significant differential gene expression in response to German CR, but not TT, was previously reported in this cohort (36). However, this was only found in children with both CR sensitization and asthma by the age of 7, and not in children without CR sensitization. We posit that the differential response to CR, which perturbs a much more limited set of genes (∼800 for asthma and ∼300 for controls, versus ∼5000 for TT stimulation in both groups), represents an allergic response to CR. In contrast, TT elicits more widespread non-allergen-specific stimulation of immune responses in both groups, allowing us to uncover network states poised for allergic sensitization. Since allergic asthma can be triggered by diverse allergens depending on individual exposure, it is valuable to identify non-exposure-dependent differential immune responses (i.e., to TT) that characterize allergic predisposition.
Our present transcriptomic network analyses allow us to identify group-level network differences. However, it would be challenging to perform individual diagnosis based upon this framework, since construction of subject-level networks would require several datasets per subject or would rely on unstable statistical inference methods. In the future, it will be interesting to determine whether altered expression and regulatory networks can be discerned even earlier in life (e.g., by studying CBMCs), as epigenetic influences begin in utero.
In conclusion, we have described a novel framework to characterize transcriptomic network alterations, shown that gene network dysregulation can be detected in atopically predisposed individuals long before clinical asthma diagnosis, and provided evidence that these atopically primed networks are a result of widespread alterations of the epigenetic landscape. Our approach indicates the potential to identify development of allergic disease including asthma prior to clinical diagnosis.
REFLECTIONS
Analyses of the impact of microbiota and immunity on early asthma are warranted. In parallel, we stress that health care disparities impact asthma mortality and morbidity. For example, African Americans are three times more likely to die from asthma than non-Hispanic whites (43). Also, as noted, asthma incidence is increasing in children in underserved neighborhoods in the Bronx. Indeed, persistent analysis of social determinants of health with a focus on social justice are powerful strategies to address health care needs. As Albert Einstein said, “Striving for social justice is the most valuable thing to do in life” (Figure 3).
Fig. 3.

Albert Einstein riding a bicycle in front of Ben Meyer's house in Santa Barbara, California, Leo Baeck Institute, F 5314A.
ACKNOWLEDGMENTS AND FINANCIAL SUPPORT
We would like to thank contributors, including Benjamin Turturice, Yi-Shin Chang, Cody Schott, Diane Gold, Augusto Litonjua, Emily Oken, and Sheryl Rifas-Shiman. We would also like to thank those who supported my membership in the ACCA, including John Carethers and Ellen Gravallese.
Footnotes
Potential Conflicts of Interest: None Disclosed.
REFERENCES
- 1.Turturice BA, et al. Lower perinatal exposure to Proteobacteria is an independent predictor of early childhood wheezing. J Allergy Clin Immunol. 2018 doi: 10.1016/j.jaci.2018.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang YS, et al. Immune network dysregulation precedes clinical diagnosis of asthma. Sci Rep. 2020;10(1):12784. doi: 10.1038/s41598-020-69494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein MM, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375(5):411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang HJ, et al. The Cohort for Childhood Origin of Asthma and allergic diseases (COCOA) study: design, rationale and methods. BMC Pulm Med. 2014;14:109. doi: 10.1186/1471-2466-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akinbami LJ, Simon AE, Rossen LM. Changing trends in asthma prevalence among children. Pediatrics. 2016;137(1) doi: 10.1542/peds.2015-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher MI, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 7.Subbarao P, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70(10):998–1000. doi: 10.1136/thoraxjnl-2015-207246. [DOI] [PubMed] [Google Scholar]
- 8.Eldeirawi K, et al. Associations of doctor-diagnosed asthma with immigration -status, age at immigration, and length of residence in the United States in a sample of -Mexican American school children in Chicago. J Asthma. 2009;46(8):796–802. [PubMed] [Google Scholar]
- 9.Eldeirawi K, et al. Associations of place of birth with asthma and wheezing in -Mexican American children. J Allergy Clin Immunol. 2005;116(1):42–8. doi: 10.1016/j.jaci.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 10.Ball TM, et al. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343(8):538–43. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 11.Kragh M, et al. Divergent response profile in activated cord blood T cells from first-born child implies birth-order-associated in utero immune programming. Allergy. 2016;71(3):323–32. doi: 10.1111/all.12799. [DOI] [PubMed] [Google Scholar]
- 12.Wennergren G, et al. Asthma in late adolescence—farm childhood is protective and the prevalence increase has levelled off. Pediatr Allergy Immunol. 2010;21(5):806–13. doi: 10.1111/j.1399-3038.2010.01057.x. [DOI] [PubMed] [Google Scholar]
- 13.von Mutius E. The microbial environment and its influence on asthma prevention in early life. J Allergy Clin Immunol. 2016;137(3):680–9. doi: 10.1016/j.jaci.2015.12.1301. [DOI] [PubMed] [Google Scholar]
- 14.Ownby D, Johnson CC. Recent understandings of pet allergies. F1000Res. 2016;5 doi: 10.12688/f1000research.7044.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-Rodriguez JA, et al. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. 2016;4(6):1111–22. doi: 10.1016/j.jaip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokholm J, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138(3):881–9 e2. doi: 10.1016/j.jaci.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Ege MJ, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 18.Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell. 2018;172(6):1173–7. doi: 10.1016/j.cell.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Stokholm J, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9(1):141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold DR, et al. Associations of cord blood fatty acids with lymphocyte proliferation, IL-13, and IFN-gamma. J Allergy Clin Immunol. 2006;117(4):931–8. doi: 10.1016/j.jaci.2005.12.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ly NP, et al. Cord blood cytokines and acute lower respiratory illnesses in the first year of life. Pediatrics. 2007;119(1):e171–8. doi: 10.1542/peds.2006-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaub B, et al. TLR2 and TLR4 stimulation differentially induce cytokine secretion in human neonatal, adult, and murine mononuclear cells. J Interferon Cytokine Res. 2004;24(9):543–52. doi: 10.1089/jir.2004.24.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaub B, et al. Neonatal immune responses to TLR2 stimulation: influence of maternal atopy on Foxp3 and IL-10 expression. Respir Res. 2006;7:40. doi: 10.1186/1465-9921-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaub B, et al. Fetal cord blood: aspects of heightened immune responses. J Clin Immunol. 2005;25(4):329–37. doi: 10.1007/s10875-005-4180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schroeter CH, et al. Nuclear factor kappa B activation in human cord blood mononuclear cells. Pediatr Res. 2004;56(2):212–8. doi: 10.1203/01.PDR.0000132850.33375.D0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willwerth BM, et al. Prenatal, perinatal, and heritable influences on cord blood immune responses. Ann Allergy Asthma Immunol. 2006;96(3):445–53. doi: 10.1016/S1081-1206(10)60912-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of -preschool wheeze. Lancet. 2014;383(9928):1593–604. doi: 10.1016/S0140-6736(14)60615-2. [DOI] [PubMed] [Google Scholar]
- 28.Health, M.O.o.C.P. Bronx Community Health Dashboard: Asthma. Montefiore Medical Center.; 2019. [Google Scholar]
- 29.Wright RJ, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health. 2004;94(4):625–32. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck AF, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574–80. doi: 10.1016/j.jpeds.2013.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters SW, Konty K, Day S, Agerton T, Olson C. Epi Data Brief. New York City: Dpeartment of Health and Mental Hygiene; 2021. Disparities Among Children with Asthma in New York City. [Google Scholar]
- 32.Martinez FD, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 33.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary -disease. J Clin Invest. 2008;118(11):3546–56. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smale ST, Fisher AG. Chromatin structure and gene regulation in the immune -system. Annu Rev Immunol. 2002;20:427–62. doi: 10.1146/annurev.immunol.20.100301.064739. [DOI] [PubMed] [Google Scholar]
- 35.Turturice BA, et al. Perinatal bacterial exposure contributes to IL-13 aeroallergen response. Am J Respir Cell Mol Biol. 2017;57(4):419–27. doi: 10.1165/rcmb.2017-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman MC, et al. Allergen-induced activation of natural killer cells represents an early-life immune response in the development of allergic asthma. J Allergy Clin Immunol. 2018;142(6):1856–66. doi: 10.1016/j.jaci.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan-Yeung M, et al. Umbilical cord blood mononuclear cell proliferative response to house dust mite does not predict the development of allergic rhinitis and asthma. J Allergy Clin Immunol. 1999;104(2 Pt 1):317–21. doi: 10.1016/s0091-6749(99)70373-8. [DOI] [PubMed] [Google Scholar]
- 38.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32(1):43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 39.Hansen LG, et al. Prediction of allergy from family history and cord blood IgE -levels. A follow-up at the age of 5 years. Cord blood IgE. IV. Pediatr Allergy Immunol. 1993;4(1):34–40. doi: 10.1111/j.1399-3038.1993.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 40.Backhed F, et al. Structural requirements for TLR4-mediated LPS signalling: a -biological role for LPS modifications. Microbes Infect. 2003;5(12):1057–63. doi: 10.1016/s1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- 41.Vatanen T, et al. Variation in microbiome LPS immunogenicity contributes to -autoimmunity in humans. Cell. 2016;165(6):1551. doi: 10.1016/j.cell.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 42.Abraham JH, et al. Infant home endotoxin is associated with reduced allergen--stimulated lymphocyte proliferation and IL-13 production in childhood. J Allergy Clin Immunol. 2005;116(2):431–7. doi: 10.1016/j.jaci.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 43. CDC WONDER Online Database. Available at: https://wonder.cdc.gov. Accessed -January 21, 2021.