Abstract
Garcinia livingstonei is a traditional herbal medicine that showed beneficial health effects and bioactivities. Four compounds have been isolated from the plant leaves and were elucidated as lupeol, betulin, podocarpusflavone A, and amentoflavone. The inhibitory activities of G. livingstonei extract and isolated metabolites against fatty acid synthase (FAS), α-glucosidase, and xanthine oxidase (XO) were investigated in vitro. The affinity of the compounds toward the studied enzymes was investigated in silico. The plant extract inhibited FAS, α-glucosidase, and XO with IC50 values of 26.34, 67.88, and 33.05 µg/mL, respectively. Among the isolated metabolites, betulin exhibited the most inhibitory activity against α-glucosidase and XO with IC50 values of 38.96 and 30.94 µg/mL, respectively. Podocarpusflavone A and betulin were the most potent inhibitors of FAS with IC50 values of 24.08 and 27.96 µg/mL, respectively. Computational studies corroborated these results highlighting the interactions between metabolites and the enzymes. In conclusion, G. livingstonei and its constituents possess the potential to modulate enzymes involved in metabolism and oxidative stress.
Keywords: Garcinia livingstonei, Fatty acid synthase, Xanthine oxidase, Alpha-glucosidase
1. Introduction
Herbal agents such as Garcinia cambogia have been traditionally used all over the world to reduce body weight (Golzarand et al., 2020). Garcinia (=Rheedia), a plant genus of Guttiferae, has many important species with biflavonoids of important physiological activities (Ogunwa 2018). Many species have edible fruits and multiple phytochemicals were reported in this genus such as xanthones (Delle Monache et al., 1984, Sordat-Diserens et al., 1992, Mbwambo et al., 2006), benzophenones (Gustafson et al., 1992, Baggett et al., 2005, Acuna et al., 2012) and biflavonoids (Acuna et al., 2012). Garcinia species have many pharmacological uses as antifungal (Sordat-Diserens et al., 1992), antioxidant (Merza et al., 2004), antiviral against HIV (Gustafson et al., 1992) and anti-inflammatory (Khanum et al., 2004). Garcinia livingstonei trees are indigenous to Africa and traditionally used in South Africa for the treatment of respiratory problems and tuberculosis (Kaikabo and Eloff 2011). This plant is a rich source of xanthones, benzophenones, flavonoids and phenolic acids (Muriithi et al., 2016). Studies on the beneficial effects of G. livingstonei revealed the anti-bacterial, antioxidant and antiparasitic activities of the leaves and root bark, effects that were attributed to the contained active constituents (Mbwambo et al., 2006, Kaikabo and Eloff, 2011, Lyles et al., 2014, Tabit et al., 2016).
Obesity is a current prevalent problem among populations in both developing and developed countries. It exacerbates numerous health complications and in most cases it is associated with diseases like type 2 diabetes mellitus (DM) and cardiovascular diseases (Kopelman 2000). Fatty acid synthase (FAS) is considered a potential target for the management of obesity since it performs a crucial function in de novo lipogenesis. Previous researchers have proved that the inhibition of FAS can lead to feeding inhibition and dramatic decrease in body weight (Tian et al., 2004). Another significant approach in controlling obesity is to inhibit digestive enzymes such as α-glucosidase and hence delaying carbohydrates absorption and extending the digestion time (Mahboubi 2019). α-glucosidase is an intestinal enzyme that breaks down polysaccharides into glucose that is absorbed into the blood (Zhang et al., 2012). Acarbose, an inhibitor of α-glucosidase, is currently used in combination with other treatments and diet to manage blood glucose levels. It reduces body weight efficiently in communities that have high carbohydrate consumption rates (Nakhaee and Sanjari 2013).
Xanthine oxidase (XO) activity and levels of uric acid have been found to be high with metabolic disorders like obesity and DM. XO is responsible for uric acid production from purine nucleotides (Battelli et al. 2016). It catalyzes the oxidation of hypoxanthine to xanthine then to uric acid and generates the reactive oxygen species (ROS) superoxide radicals. Nakamura et al. reported a strong XO inhibitor that reduced weight gain and they explained this by induction of the salvage pathway via increasing hepatic hypoxanthine levels leading to tendency of the body to catabolism (Nakamura et al., 2021).
In molecular docking analysis, the stability of drug-enzyme complex is strongly influenced by polar and hydrophobic interactions between the drug and protein's active site amino acid residues (Antar et al., 2022). Particularly, the polar interactions are important for the binding of the drugs into the enzyme active site (Kamel and Lamsabhi 2021). Consequently, these polar interactions contribute significantly to molecular recognition, drug affinity and configuration (Abukhalil et al., 2020). Another leading factor contributing to binding energy is the hydrophobic interaction between the drug lipophilic surface and the enzyme active site hydrophobic regions (Elsayed et al., 2020). Thus, for a thermodynamically favorable drug-enzyme interaction, a suitable geometrical coincidence between the drug and the active site is essential.
In this study, FAS, α-glucosidase, and XO inhibitory activities of G. livingstonei extract and the isolated compounds have been evaluated and binding interactions between the isolated metabolites and the target enzymes have been investigated using molecular docking.
2. Materials and methods
2.1. General experimental procedures
Spectral data used for identification of the isolated compounds were obtained using Bruker Avance III 400 MHz NMR machine (Bruker AG, Switzerland) with Smart Probe and Topspin 3.1 Software. CDCl3, acetone‑d6 were used as NMR solvents (Cambridge Isotope Laboratories, Inc., (Andover, MA). For chromatography, silica gel for column chromatography technical grade, pore size 60 Å, 230–400 mesh particle size, 40–63 μm particle size (E. Merck) was used. Thin layer chromatography (TLC) was performed using pre-coated silica gel G60 F254 plates (20 × 20 cm) (Pharmacia Biotech AB, Uppsala, Sweden). Analytical grade solvents were used as mobile phase in chromatography. TLC Plates were visualized using potable UV lamp followed by spraying with p-anisaldehyde reagent, and heating with a heat gun.
2.2. Plant material
G. livingstonei was collected from El-Nabatate Island (Aswan, Egypt) in June 2018 and identified by Dr. Hafeez R. Habeeb (Flora and Phytotaxonomy Research Department, Horticultural Research Institute, Agricultural Research Centre, Egypt). A voucher specimen (BUPD-35) was deposited in Pharmacognosy Department, Faculty of Pharmacy, Beni-Suef University. The dried G. livingstonei leaves (1.15 kg) were milled at room temperature and macerated with 70% ethanol (EtOH, 3x5L). Solvent evaporation was performed under reduced pressure at 40 °C using rotary evaporator to yield 171.3 g dried crude extract that was kept at refrigerator for further phytochemical and biological studies. The extract (161.3 g) was fractionated by partition chromatography using a separating funnel. It was suspended in water and successively fractionated using solvents of increasing polarities to give the following extractives: n-hexane (14.6 g), dichloromethane (DCM, 7.6 g), ethyl acetate (EtOAc,16 g), and n-butanol saturated with water (13.5 g) (Suppl. Fig. 1).
2.3. Phytochemical study
An aliquot of the DCM fraction (6 g) was chromatographed on silica gel (300 g, 35 × 5 cm) and eluted with n-hexane-EtOAc in 2.5% increment. Similar fractions were combined to give three sub-fractions (D1-D2). Sub-fraction D1 (449 mg, eluted with 12.5–22.5% EtOAc in n-hexane) was re-chromatographed twice on silica gel with gradient elution using n-hexane-EtOAc in 1% increments to yield compound 1 (4.4 mg). The sub-fraction D2 (490 mg, eluted with 25–30% EtOAc in n-hexane) was re-chromatographed on silica gel CC with gradient elution using n-hexane-DCM mixtures (100 % n-hexane then 50:50 n-hexane-DCM, and 100% DCM) to yield compound 2 (3.9 mg). The EtOAc fraction (12 g) was chromatographed on a silica gel column (90 × 5 cm, 363 g) and a gradient elution with DCM-MeOH mixtures with increasing polarity (by 2.5 %) was applied. Fractions were TLC-monitored and combined. The fraction eluted with 10% MeOH in DCM yielded a pure compound 3 (52.1 mg). The sub-fraction eluted with 15% MeOH in DCM (0.7619 g) was filtered on Sephadex LH-20 and eluted with 100% MeOH to yield compound 4 (18.7 mg). An isolation scheme is presented in Suppl. Fig. 1. Chemical structures of isolated compounds are presented in Fig. 1.
Fig. 1.
Chemical structures of compounds 1-4 isolated from G. livingstonei leaves.
2.4. FAS inhibition assay
The inhibitory activity against FAS from chicken liver was determined as described by Jiang et al (2019). Chicken FAS shares 63% identity with the sequence of human FAS (Tian et al., 1985). The pure compound or extract (10–200 µg/mL) was mixed with potassium phosphate buffer (100 mM), dithiothreitol (1 mM), EDTA (1 mM), malonyl–CoA (10 µM), acetyl–CoA (3 µM), NADPH (35 µM) and FAS (10 µg). The activity was determined at 37 °C by monitoring the change in NADPH absorption at 340 nm. A negative control was run without the enzyme.
2.5. α-glucosidase inhibition assay
Different concentrations (0–200 µg/mL) of G. livingstonei extract, isolated compounds and the standard inhibitor acarbose were mixed with 100 mM phosphate buffer (pH 6.8) and α-glucosidase (maltase; Sisco, India) followed by 20 min incubation at 37 °C. PNP-Glu (Sisco, India) was added and the obtained mixture was incubated for 10 min at 37 °C. 0.1 N sodium carbonate was used to terminate the reaction and the absorbance was determined at 410 nm (Pistia-Brueggeman and Hollingsworth 2001).
2.6. XO inhibition assay
XO inhibitory activity was determined as previously described (Özyürek et al., 2009). Briefly, the tested material was mixed with sodium phosphate buffer (50 mM), xanthine (0.5 mM) and XO. After incubation for 30 min at 37 °C, perchloric acid (3.2%) was used to stop the reaction. Equal volumes of the mixture, Cl2CuH4O2 (10 mM) and neocuproine (7.5 mM) were mixed with double volume of NH4CH3CO2 (1 M) and left for 30 min. The absorbance was determined at 450 nm.
2.7. In silico molecular docking study
The binding affinity of the isolated compounds and pharamacological inhibitors with XO, FAS and α-glucosidase was carried out as previously reported. The DFT calculations performed in this study were executed using Gaussian 09 software package. The geometrical structures of isolated phytochemicals were optimized at the B3LYP level of theory without constrains using the 6-311G (d, p) basis set. UCSF Chimera software was employed for generating the.pdb 3D structures of ligands (Pettersen et al., 2004). Autodock Tools (ADT) v1.5.6 and AutoDock Vina software packages were used for performing the molecular docking assessment (Trott and Vina 2010). The isolated compounds were optimized for docking by means of ADT software. PyMOL v2.3.2 program was used for molecular visualization, binding modes inspection and drug-enzyme interactions analysis. The three-dimensional X-ray crystal structures of XO and FAS were obtained from the protein data bank (PDB), where PDB ID: 3NVY was employed for XO, two FAS domains were used, namely KS (PDB ID: 3HHD) and TE (PDB ID: 1XKT) and α-glucosidase (PDB ID: 3A4A). The PDB structures of enzymes under investigation were optimized for docking by solvent and nonstandard residues removal, addition of polar hydrogens and adjusting the grid box to the most proper configuration of the active site (Cheng et al., 2008, Kamel and Lamsabhi, 2020).
3. Results
3.1. Phytochemical study
Fractionation of the ethanolic extract of G. livingstonei leaves afforded compounds 1–4 (Fig. 1, Suppl. Table I and Suppl. Figs. 2-10). The structures of the compounds were established by comparison of their observed data (supplementary data) with previously reported literature and they were identified as lupeol (1) (Abdullahi et al., 2013), betulin (2) (Tijjani et al., 2012), podocarpusflavone (3) (Suárez et al., 2003), and amentoflavone (4) (Elghondakly et al., 2020).
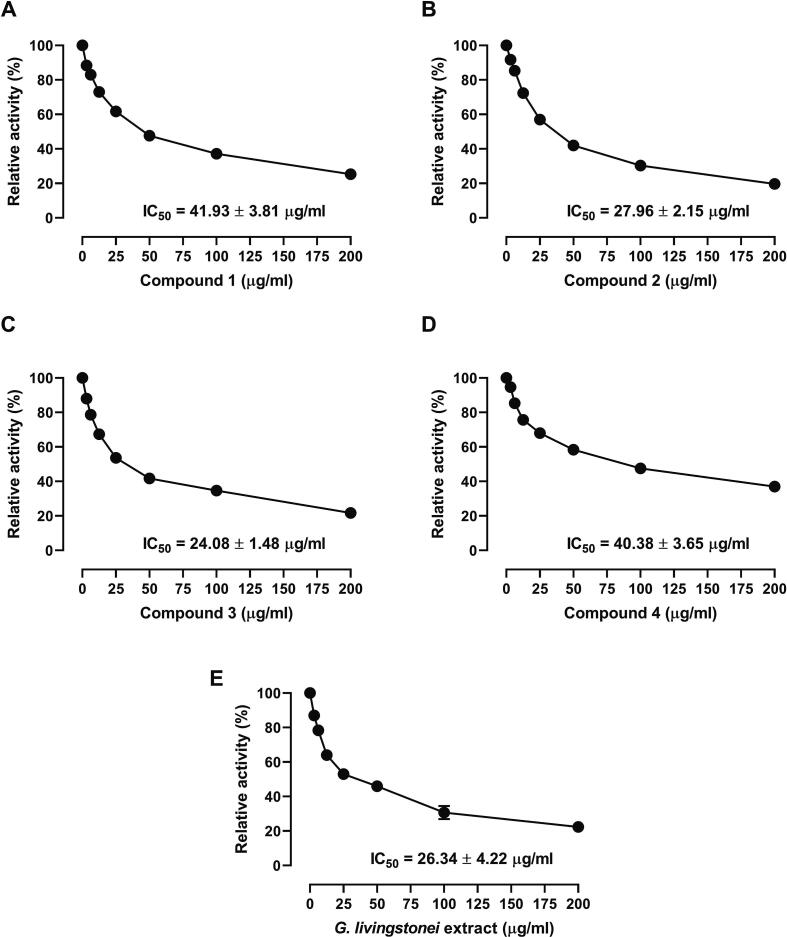
3.2. FAS inhibitory activity of G. livingstonei extract and the isolated compounds
The data represented in Fig. 2 show FAS inhibition by the extract and the isolated compounds and the IC50 values. G. livingstonei extract and isolated compounds (1-4) exhibited inhibitory effects against FAS with IC50 values of 26.34 ± 4.22, 41.93 ± 3.81, 27.96 ± 2.15, 24.04 ± 1.48, and 40.38 ± 3.65 µg/mL, respectively (Fig. 2A-E).
Fig. 2.
FAS inhibitory activity of compounds 1–4 and G. livingstonei extract. Data are mean ± SD, (N = 3).
Molecular docking simulations revealed the binding affinity of the isolated compounds towards FAS TE (Fig. 3) and KS domains (Fig. 4). The binding affinities of orlistat and cerulenin with FAS-TE and FAS-KS domains, respectively, are represented in Suppl. Fig. 11 and Suppl. Table II. The reported lowest binding energy were −9.9, −10.4, −10.3 and −8.7 kcal/mol with FAS/TE and −9.4, −9.6, −8.7 and −7.3 kcal/mol with FAS/KS for compounds 1, 2, 3, and 4, respectively (Table 1). The residues involved in polar bonding and hydrophobic interactions are summarized in Table 1.
Fig. 3.
Binding interactions of compounds 1–4 with TE domain of FAS.
Fig. 4.
Binding interactions of compounds 1–4 with KS domain of FAS.
Table 1.
Binding affinities, polar bonds, and hydrophobic interactions of phytochemicals (1-4) with FAS domains TE and KS.
| Compound |
FAS/TE |
FAS/KS |
||||
|---|---|---|---|---|---|---|
| Affinity (kcal/mol) | Polar bonds | Hydrophobic interactions | Affinity (kcal/mol) | Polar bonds | Hydrophobic interactions | |
| 1 | −9.9 | Leu2222, Ile2250, Glu2251, Ser2308, Glu2366, Ala2367, Phe2370, Phe2371, Phe2423, Phe2427 and Arg2482 | −9.4 | Arg224 and Glu333 | Ser112, Gly113, Glu115, Glu118, Arg137, Asp158, Thr159, Ala160, Gln199, Phe200 and Phe395 | |
| 2 | −10.4 | Ser2221, Gln2374 and Arg2482 | Asn2218, Leu2222, Ile2250, Glu2251, Thr2255, Val2256, Phe2370, Phe2371, Phe2375 and Phe2423 | −9.6 | Arg224 and Glu333 | Ser112, Gly113, Glu115, Glu118, Asp158, Thr159, Ala160, Phe200, Phe395 and Gly396 |
| 3 | −10.3 | Ile12250, Ser2308, Ala2367, Phe2370, Phe2423, Leu2427 and Arg2482 | −8.7 | Val133, Met139, Asn142, Arg143, Phe146, Asp149, Phe150 and Arg151 | ||
| 4 | −8.7 | Ile12250, Ser2308, Glu2366, Ala2367, Phe2370, Phe2423, Leu2427, Glu2431 and Arg2482 | −7.3 | Thr126, Val844, Ala846 and Asp849 | Thr74, Leu120, Arg122, Leu127, Val128, Asp843 and Pro845 | |
3.3. α-glucosidase inhibitory activity of G. livingstonei extract and the isolated compounds
The inhibitory activity of the plant extract and isolated compounds on α-glucosidase has been explored and all exhibited inhibitory activity (Fig. 5A-E). Betulin (2) showed the highest activity with IC50 value of 38.96 ± 5.57 µg/mL followed by lupeol (1) and amentoflavone (4) with IC50 values of 43.15 ± 4.08 and 49.96 ± 1.83 µg/mL, respectively, as compared to that of acarbose (29.72 ± 3.11 µg/mL; Fig. 5F). In silico investigation (Fig. 6, Fig. 7 and Suppl. Fig. 12, and Table 2 and Suppl. Table II) revealed the binding affinity of the isolated compounds and acarbose towards α-glucosidase. Compounds 1, 2, 3 and 4 exhibited binding energy values of −9.3, −8.9, −10.8, and −10.3 kcal/mol, respectively and all interact through polar and hydrophobic interactions (Table 2).
Fig. 5.
α-glucosidase inhibitory activity of compounds 1–4, G. livingstonei extract and acarbose. Data are mean ± SD, (N = 3).
Fig. 6.
Binding interactions of compounds 1 and 2 with α-glucosidase.
Fig. 7.
Binding interactions of compounds 3 and 4 with α-glucosidase.
Table 2.
Binding affinities, polar bonds, and hydrophobic interactions of phytochemicals (1-4) with α-glucosidase.
| Compound | Affinity (kcal/mol) | Polar bonds | Hydrophobic interactions |
|---|---|---|---|
| 1 | −9.3 | Arg315 and Asp352 | Tyr158, Ser240, Gln279, Phe303, Pro312, Leu313, Gln353, Glu411 and Arg442 |
| 2 | −8.9 | Arg315 and Asp352 | Lys156, Tyr158, Ser240, Gln279, Phe303, Pro312, Leu313, Gln353, Glu411 and Arg442 |
| 3 | −10.8 | Thr310 and Asn415 | Lys156, Tyr158, Asp242, His280, Phe303, Asp307, Ser311, Pro312, Phe314, Arg315, Tyr316, Asp352, Gln353 and Glu411 |
| 4 | −10.3 | Thr310 and Asn415 | Tyr158, Asp242, His280, Phe303, Asp307, Ser311, Pro312, Phe314, Arg315, Tyr316 and Glu411 |
3.4. XO inhibition activity of G. livingstonei extract and its isolated compounds
Investigation of XO inhibitory activity of the isolated compounds (Fig. 8A-D) and the extract (Fig. 8E) revealed IC50 values of compound 2 (30.94 ± 3.61 µg/ml) and the extract (33.05 ± 2.51 µg/ml) were the lowest and allopurinol (Fig. 8F) exhibited an IC50 value of 9.04 ± 0.64 µg/ml. The results of our molecular docking simulations revealed the activities of the compounds and allopurinol against XO (Fig. 9, Fig. 10 and Suppl. Fig. 12). Interestingly, all compounds were shown to dock into the main active site of XO with polar interactions with Tyr592, His741, Phe742 and Gln 1194 only with compounds 1, 2 and 4 (Table 3).
Fig. 8.
XO inhibitory activity of compounds 1–4, G. livingstonei extract and allopurinol. Data are mean ± SD, (N = 3).
Fig. 9.
Binding interactions of compounds 1 and 2 with XO.
Fig. 10.
Binding interactions of compounds 3 and 4 with XO.
Table 3.
Binding affinities, polar bonds, and hydrophobic interactions of phytochemicals (1-4) with XO.
| Compound | Affinity (kcal/mol) | Polar bonds | Hydrophobic interactions |
|---|---|---|---|
| 1 | −8.6 | His741, Phe742 and Gln1194 | His579, Leu744, Phe798, Gly1039, Gly1193, Val1200, Ile1229 and Ile1235 |
| 2 | −9.8 | Tyr592, His741 and Phe742 | Gln585, Leu744, Phe798 Gly795, Gly1039, Gly1193 Gln1194, Gly1197, Val1200, Gln1201 and Ile1229 |
| 3 | −8.6 | Gln585, Leu744, Gly795, Phe798, Gly1197, Val1200, Gln1201, Ile1229 and Pro1230 | |
| 4 | −8.2 | Gly1197 | Phe742, Leu744, Gly795, Phe798, Val1200, Gln1201, Ile1229 and Pro1230 |
4. Discussion
G. livingstonei is traditionally used for the treatment of respiratory problems and tuberculosis due to its rich content of phytoconstituents (Kaikabo and Eloff 2011). The ability of G. livingstonei extract as well as isolated compounds (1–4) to inhibit FAS, α-glucosidase, and XO enzymes was evaluated in this study. A visual inspection of the interactions between isolated secondary metabolites and the three enzymes has been performed through computational studies.
FAS is a critical enzyme in de novo lipogenesis (Jiang et al., 2010), and previous studies proved that FAS inhibition is involved in controlling appetite and body weight (Loftus et al., 2000). Moreover, it was observed that FAS is over-expressed in various types of cancer (Zhang et al., 2016). Consequently, it emerged as a potential target for anticancer and body weight control drugs. G. cambogia extract has been previously proved to inhibit FAS (Kim et al., 2013). In this context, the isolated secondary metabolites from G. mangostana exhibited inhibitory effects on FAS (Jiang et al., 2010, Liang et al., 2018). G. livingstonei extract and the isolated compounds (1-4) were evaluated for FAS inhibition. To figure out the modulatory influences of isolated phytochemicals on FAS, we carried out molecular docking assessment to estimate their binding modes. Our compounds were shown to invade an active binding pocket on the surface of TE domain of FAS. Only compound 2 showed polar interactions with the FAS/TE domain active site residues Ser2221, Gln2374 and Arg2482. Meanwhile, all the remaining compounds were encased in the active site surrounded by dense network of hydrophobic residues. The binding energies ranged from −8.7 to −10.4 kcal/mol, indicating a high probability for forming a stable drug-enzyme complex. In addition, many phenylalanine residues were observed in these hydrophobic interactions. Such residues can exhibit thermodynamically favorable π-π interactions and can contribute successfully to the binding of the compounds with FAS/TE. The in silico docking model of the isolated compounds with the KS domain of FAS was built by molecular docking. The isolated phytochemicals were in the main binding site on the enzyme surface with large number of polar and hydrophobic interactions. Compounds 1, 2 and 4 formed hydrogen bonds with the active site residues Arg224, Glu333, Thr126, Val844, Ala846, Asp849, Trp836 and His838. Also, many hydrophobic residues were involved in this drug-enzyme interaction, and the binding energies for the formed complexes ranged from −7.3 to −9.6 kcal/mol. These outputs lead us to the conclusion that stable complexes are formed between the isolated compounds and FAS/KS. Similarly, phenylalanine residues involved in these complexes hydrophobic interactions could exhibit the favorable π-π interactions results in these complexes energy minimization. Given its high expression levels in adipocytes and cancer cells (Bauerschlag et al., 2015), the inhibitory activity of G. livingstonei phytoconstituents towards FAS could be beneficial for the development of new anti-cancer and anti-obesity agents.
Inhibition of α-glucosidase is of value for reducing postprandial hyperglycemia and the inhibitors of this enzyme modestly decrease glycated hemoglobin. Various Garcinia species extract and secondary metabolites can inhibit α-glucosidase (Ngoupayo et al., 2008, Ryu et al., 2011, Fouotsa et al., 2012, Raksat et al., 2019, Nguyen et al., 2022). The inhibitory activity of the plant extract and isolated compounds on α-glucosidase have been explored and all exhibited inhibitory activity. Previous studies on lupeol and betulin and betulinic acid mixture reported good α-amylase and α-glucosidase inhibitory activities, and another work on amentoflavone showed its potent inhibitory activity against α-glucosidase. (Rathinavel et al., 2021, Yuca et al., 2022, Li et al., 2023). Molecular docking analysis was performed to explore the binding modes of our isolated phytochemicals against α-glucosidase. The three-dimensional crystal structure of α-glucosidase is still under dispute. Therefore, the structure of the isomaltose from S. cerevisiae was used because it exhibits 84% similarity to S. cerevisiae α-glucosidase. Our docking model was validated by re-docking the native inhibitor to the binding site of the protein. The compounds showed good binding affinities (-8.9 to −10.8 kcal/mol), reflecting the compatibility of isolated compounds. Interestingly, all isolated compounds occupied the same binding pocket of the protein encased by dense network of hydrophobic interacting residues. Also, two polar bonds were detected for each tested ligand. These outputs led to the conclusion that the isolated phytochemicals displayed inhibitory activity against the target protein. Previous in silico testing for lupeol showed excellent binding affinities to α-glucosidase and α-amylase (Rathinavel et al., 2021).
The enzymatic activity of XO results in the production of uric acid through the metabolism of purine nucleotides (Wang et al., 2016). Inhibition of XO has been connected to weight gain suppression via induction of salvage pathway (Nakamura et al., 2021). G. mangostana extract has been reported to inhibit XO (Kosem et al., 2007), and various XO inhibitors were isolated from Garcinia species (Lin et al., 2011, Zhu et al., 2014). The ability of G. livingstonei extract and the isolated compounds to inhibit XO was tested and the results revealed that betulin (2) and the extract were the most active with IC50 values of 30.94 and 33.05 µg/mL, respectively. Lupeol showed inhibitory activity with IC50 value of 46.35 µg/mL compared to the positive control, allopurinol (9.04 µg/mL). The results of molecular docking simulations revealed the activities of the compounds against XO where all compounds were shown to dock into the main active site with polar interactions with Tyr592, His741, Phe742 and Gln 1194 only with compounds 1, 2 and 4. The common residues included in the polar interaction of this complex reflect the compatibility of these compounds to the main binding pocket of XO. This inference is mainly explained by the relatively low binding energies obtained for these complexes (-8.2 to −9.8 kcal/mol). These low binding affinities suggest the potency of isolated phytochemicals as XO inhibitors. All compounds occupied the same binding cavity with many common amino acid residues involved in the drug-enzyme hydrophobic interactions.
5. Conclusion
The inhibitory potential of G. livingstonei leaves extract and isolated secondary metabolites against FAS, XO and α-glucosidase was studied via in vitro testing and molecular docking simulations. Both the extract and compounds exhibited inhibitory activities against the studied enzymes. Podocarpusflavone A showed the strongest FAS inhibition activity followed by betulin. Inhibition of α-glucosidase and XO was best performed by lupeol. The in silico docking studies revealed interactions and remarkable binding affinities between the isolated metabolites and target enzymes which may illustrate their inhibition efficacy. Therefore, G. livingstonei is rich in phytochemicals with inhibitory activities against enzymes involved in metabolism and oxidative stress. However, in vivo studies are needed to explore the efficacy of this plant and its secondary metabolites in disease models of obesity and other diseases associated with oxidative stress.
CRediT authorship contribution statement
Azza M. Abdul-Rahman: Methodology, Investigation, Data curation, Writing – original draft. Ahlam Elwekeel: Methodology, Investigation. Reem S. Alruhaimi: Methodology, Resources, Funding acquisition. Emadeldin M. Kamel: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing – original draft, Visualization, Supervision. Albandari Bin-Ammar: Methodology, Resources, Validation, Visualization. Ayman M. Mahmoud: Conceptualization, Methodology, Investigation, Resources, Data curation, Visualization, Supervision, Formal analysis, Validation, Writing – original draft, Writing – review & editing. Abeer S. Moawad: Methodology, Investigation, Resources, Writing – original draft, Supervision. Mohamed A. Zaki: Conceptualization, Methodology, Investigation, Resources, Visualization, Supervision, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R381), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.101762.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abdullahi S., Musa A., Abdullahi M., et al. Isolation of Lupeol from the Stem-bark of Lonchocarpus sericeus (Papilionaceae) Scholars Acad. J. Biosci. 2013;1:18–19. [Google Scholar]
- Abukhalil M.H., Hussein O.E., Bin-Jumah M., et al. Farnesol attenuates oxidative stress and liver injury and modulates fatty acid synthase and acetyl-CoA carboxylase in high cholesterol-fed rats. Environ. Sci. Pollut. Res. 2020;27:30118–30132. doi: 10.1007/s11356-020-09296-w. [DOI] [PubMed] [Google Scholar]
- Acuna U.M., Dastmalchi K., Basile M.J., et al. Quantitative high-performance liquid chromatography photo-diode array (HPLC-PDA) analysis of benzophenones and biflavonoids in eight Garcinia species. J. Food Compos. Anal. 2012;25:215–220. [Google Scholar]
- Antar S.A., Abdo W., Taha R.S., et al. Telmisartan attenuates diabetic nephropathy by mitigating oxidative stress and inflammation, and upregulating Nrf2/HO-1 signaling in diabetic rats. Life Sci. 2022;291 doi: 10.1016/j.lfs.2021.120260. [DOI] [PubMed] [Google Scholar]
- Baggett S., Protiva P., Mazzola E.P., et al. Bioactive benzophenones from Garcinia xanthochymus fruits. J. Nat. Prod. 2005;68:354–360. doi: 10.1021/np0497595. [DOI] [PubMed] [Google Scholar]
- Bauerschlag D.O., Maass N., Leonhardt P., et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J. Transl. Med. 2015;13:146. doi: 10.1186/s12967-015-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F., Wang Q., Chen M., et al. Molecular docking study of the interactions between the thioesterase domain of human fatty acid synthase and its ligands. Proteins: Struct., Funct. Bioinf. 2008;70:1228–1234. doi: 10.1002/prot.21615. [DOI] [PubMed] [Google Scholar]
- Delle Monache G., Delle Monache F., Waterman P.G., et al. Minor xanthones from Rheedia gardneriana. Phytochemistry. 1984;23:1757–1759. [Google Scholar]
- Elghondakly M., Moawad A., Hetta M. Cytotoxicity and chromatographic analysis of Dioon spinulosum, family Zamiaceae. J. Appl. Pharm. Sci. 2020;10:75–82. [Google Scholar]
- Elsayed R.H., Kamel E.M., Mahmoud A.M., et al. Rumex dentatus L. phenolics ameliorate hyperglycemia by modulating hepatic key enzymes of carbohydrate metabolism, oxidative stress and PPARγ in diabetic rats. Food Chem. Toxicol. 2020;138 doi: 10.1016/j.fct.2020.111202. [DOI] [PubMed] [Google Scholar]
- Fouotsa H., Lannang A.M., Mbazoa C.D., et al. Xanthones inhibitors of α-glucosidase and glycation from Garcinia nobilis. Phytochem. Lett. 2012;5:236–239. [Google Scholar]
- Golzarand M., Omidian M., Toolabi K. Effect of Garcinia cambogia supplement on obesity indices: A systematic review and dose-response meta-analysis. Complement. Ther. Med. 2020;52 doi: 10.1016/j.ctim.2020.102451. [DOI] [PubMed] [Google Scholar]
- Gustafson K.R., Blunt J.W., Munro M.H., et al. The guttiferones, HIV-inhibitory benzophenones from Symphonia globulifera, Garcinia livingstonei, Garcinia ovalifolia and Clusia rosea. Tetrahedron. 1992;48:10093–10102. [Google Scholar]
- Jiang H.Z., Quan X.F., Tian W.X., et al. Fatty acid synthase inhibitors of phenolic constituents isolated from Garcinia mangostana. Bioorg. Med. Chem. Lett. 2010;20:6045–6047. doi: 10.1016/j.bmcl.2010.08.061. [DOI] [PubMed] [Google Scholar]
- Jiang H.-Z., Yuan J.-J., Ma Q.-Y., et al. Phenolic compounds from Mangifera indica. Chem. Nat. Compd. 2019;55:147–150. [Google Scholar]
- Kaikabo A.A., Eloff J.N. Antibacterial activity of two biflavonoids from Garcinia livingstonei leaves against Mycobacterium smegmatis. J. Ethnopharmacol. 2011;138:253–255. doi: 10.1016/j.jep.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Kamel E.M., Lamsabhi A.M. The quasi-irreversible inactivation of cytochrome P450 enzymes by paroxetine: a computational approach. Org. Biomol. Chem. 2020;18:3334–3345. doi: 10.1039/d0ob00529k. [DOI] [PubMed] [Google Scholar]
- Kamel E.M., Lamsabhi A.M. Water biocatalytic effect attenuates cytochrome P450-mediated carcinogenicity of diethylnitrosamine: A computational insight. Org. Biomol. Chem. 2021;19:9031–9042. doi: 10.1039/d1ob01439k. [DOI] [PubMed] [Google Scholar]
- Khanum S.A., Shashikanth S., Deepak A. Synthesis and anti-inflammatory activity of benzophenone analogues. Bioorg. Chem. 2004;32:211–222. doi: 10.1016/j.bioorg.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kim Y.-J., Choi M.-S., Park Y.B., et al. Garcinia Cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013;19:4689. doi: 10.3748/wjg.v19.i29.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kosem N., Han Y.-H., Moongkarndi P. Antioxidant and cytoprotective activities of methanolic extract from Garcinia mangostana hulls. Sci. Asia. 2007;33:283–292. [Google Scholar]
- Li H., Yang J., Wang M., et al. Studies on the inhibition of α-glucosidase by biflavonoids and their interaction mechanisms. Food Chem. 2023;420 doi: 10.1016/j.foodchem.2023.136113. [DOI] [PubMed] [Google Scholar]
- Liang Y., Luo D., Gao X., et al. Inhibitory effects of garcinone E on fatty acid synthase. RSC Adv. 2018;8:8112–8117. doi: 10.1039/c7ra13246h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K.-W., Huang A.-M., Tu H.-Y., et al. Xanthine oxidase inhibitory triterpenoid and phloroglucinol from guttiferaceous plants inhibit growth and induced apoptosis in human NTUB1 cells through a ROS-dependent mechanism. J. Agric. Food Chem. 2011;59:407–414. doi: 10.1021/jf1041382. [DOI] [PubMed] [Google Scholar]
- Loftus T.M., Jaworsky D.E., Frehywot G.L., et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- Lyles J.T., Negrin A., Khan S.I., et al. In vitro antiplasmodial activity of benzophenones and xanthones from edible fruits of Garcinia species. Planta Med. 2014;80:676–681. doi: 10.1055/s-0034-1368585. [DOI] [PubMed] [Google Scholar]
- Mahboubi M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019;146 doi: 10.1016/j.phrs.2019.104341. [DOI] [PubMed] [Google Scholar]
- Mbwambo Z.H., Kapingu M.C., Moshi M.J., et al. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J. Nat. Prod. 2006;69:369–372. doi: 10.1021/np050406v. [DOI] [PubMed] [Google Scholar]
- Merza J., Aumond M.-C., Rondeau D., et al. Prenylated xanthones and tocotrienols from Garcinia virgata. Phytochemistry. 2004;65:2915–2920. doi: 10.1016/j.phytochem.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Muriithi E., Bojase-Moleta G., Majinda R.R. Benzophenone derivatives from Garcinia livingstonei and their antioxidant activities. Phytochem. Lett. 2016;18:29–34. [Google Scholar]
- Nakamura T., Nampei M., Murase T., et al. Influence of xanthine oxidoreductase inhibitor, topiroxostat, on body weight of diabetic obese mice. Nutr. Diabetes. 2021;11:12. doi: 10.1038/s41387-021-00155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaee A., Sanjari M. Evaluation of effect of acarbose consumption on weight losing in non-diabetic overweight or obese patients in Kerman. J. Res. Med. Sci. 2013;18:391. [PMC free article] [PubMed] [Google Scholar]
- Ngoupayo J., Tabopda T.K., Ali M.S., et al. α-Glucosidase inhibitors from Garcinia brevipedicellata (Clusiaceae) Chem. Pharm. Bull. 2008;56:1466–1469. doi: 10.1248/cpb.56.1466. [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.H., Nguyen V.T., Van Cuong P., et al. A new flavonoid from the leaves of Garcinia mckeaniana Craib and α-glucosidase and acetylcholinesterase inhibitory activities. Nat. Prod. Res. 2022;36:5074–5080. doi: 10.1080/14786419.2021.1916019. [DOI] [PubMed] [Google Scholar]
- Ogunwa T.H. Computer-aided modeling of interaction between aldehyde dehydrogenase and Garcinia biflavonoids. Int. J. Comput. Appl. 2018;975:8887. [Google Scholar]
- Özyürek M., Bektaşoğlu B., Güçlü K., et al. Measurement of xanthine oxidase inhibition activity of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta. 2009;636:42–50. doi: 10.1016/j.aca.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pistia-Brueggeman G., Hollingsworth R.I. A preparation and screening strategy for glycosidase inhibitors. Tetrahedron. 2001;57:8773–8778. [Google Scholar]
- Raksat A., Phukhatmuen P., Yang J., et al. Phloroglucinol benzophenones and xanthones from the leaves of Garcinia cowa and their nitric oxide production and α-glucosidase inhibitory activities. J. Nat. Prod. 2019;83:164–168. doi: 10.1021/acs.jnatprod.9b00849. [DOI] [PubMed] [Google Scholar]
- Rathinavel T., Iqbal M.N., Kumarasamy S. Lupeol from Crateva adansonii DC exhibits promising enzymes inhibition: Play a crucial role in inflammation and diabetes. S. Afr. J. Bot. 2021;143:449–456. [Google Scholar]
- Ryu H.W., Cho J.K., Curtis-Long M.J., et al. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry. 2011;72:2148–2154. doi: 10.1016/j.phytochem.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Sordat-Diserens I., Rogers C., Sordat B., et al. Prenylated xanthones from Garcinia livingstonei. Phytochemistry. 1992;31:313–316. [Google Scholar]
- Suárez, A. r. I., B. Diaz, F. Delle Monache, et al., 2003. Biflavonoids from Podocalyx loranthoides. Fitoterapia. 74, 473-475. [DOI] [PubMed]
- Tabit F.T., Komolafe N.T., Tshikalange T.E., et al. Phytochemical constituents and antioxidant and antimicrobial activity of selected plants used traditionally as a source of food. J. Med. Food. 2016;19:324–329. doi: 10.1089/jmf.2015.0099. [DOI] [PubMed] [Google Scholar]
- Tian W.X., Hsu R.Y., Wang Y.S. Studies on the reactivity of the essential sulfhydryl groups as a conformational probe for the fatty acid synthetase of chicken liver. Inactivation by 5,5'-dithiobis-(2-nitrobenzoic acid) and intersubunit cross-linking of the inactivated enzyme. J. Biol. Chem. 1985;260:11375–11387. [PubMed] [Google Scholar]
- Tian W.-X., Li L.-C., Wu X.-D., et al. Weight reduction by Chinese medicinal herbs may be related to inhibition of fatty acid synthase. Life Sci. 2004;74:2389–2399. doi: 10.1016/j.lfs.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Tijjani A., Ndukwe I., Ayo R. Isolation and characterization of lup-20 (29)-ene-3, 28-diol (Betulin) from the stem-bark of Adenium obesum (Apocynaceae) Trop. J. Pharm. Res. 2012;11:259–262. [Google Scholar]
- Trott O., Vina O. improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading Oleg Public Access. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Zhang C., Xing X.H. Xanthine dehydrogenase: An old enzyme with new knowledge and prospects. Bioengineered. 2016;7:395–405. doi: 10.1080/21655979.2016.1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuca H., Özbek H., Demirezer L.Ö., et al. Assessment of the α-glucosidase and α-amylase inhibitory potential of Paliurus spina-christi Mill. and its terpenic compounds. Med. Chem. Res. 2022;31:1393–1399. [Google Scholar]
- Zhang J.-S., Lei J.-P., Wei G.-Q., et al. Natural fatty acid synthase inhibitors as potent therapeutic agents for cancers: A review. Pharm. Biol. 2016;54:1919–1925. doi: 10.3109/13880209.2015.1113995. [DOI] [PubMed] [Google Scholar]
- Zhang A.J., Rimando A.M., Fish W., et al. Serviceberry [Amelanchier alnifolia (Nutt.) Nutt. ex. M. Roem (Rosaceae)] leaf extract inhibits mammalian α-glucosidase activity and suppresses postprandial glycemic response in a mouse model of diet-induced obesity and hyperglycemia. J. Ethnopharmacol. 2012;143:481–487. doi: 10.1016/j.jep.2012.06.054. [DOI] [PubMed] [Google Scholar]
- Zhu L.-L., Fu W.-W., Watanabe S., et al. Xanthine oxidase inhibitors from Garcinia esculenta twigs. Planta Med. 2014;80:1721–1726. doi: 10.1055/s-0034-1383193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.