Abstract
Salicylic acid (SA) has been recognized as a promising molecule for improving abiotic stress tolerance in plants due to its ability to enhance antioxidant defense system, and promote root architecture system. Recent research has focused on uncovering the mechanisms by which SA confers abiotic stress tolerance in horticultural crops. SA has been shown to act as a signaling molecule that triggers various physiological and morphological responses in plants. SA regulates the production of reactive oxygen species (ROS). Moreover, it can also act as signaling molecule that regulate the expression of stress-responsive genes. SA can directly interact with various hormones, proteins and enzymes involved in abiotic stress tolerance. SA regulates the antioxidant enzymes activities that scavenge toxic ROS, thereby reducing oxidative damage in plants. SA can also activate protein kinases that phosphorylate and activate transcription factors involved in stress responses. Understanding these mechanisms is essential for developing effective strategies to improve crop resilience in the face of changing environmental conditions. Current information provides valuable insights for farmers and plant researchers, offering new strategies to enhance crop resilience and productivity in the face of environmental challenges. By harnessing the power of SA and its signaling pathways, farmers can develop more effective stress management techniques and optimize crop performance. Plant researchers can also explore innovative approaches to breed or engineer crops with enhanced stress tolerance, thereby contributing to sustainable agriculture and food security.
Keywords: poor yield, photosynthetic impairments, stomatal conductance, signaling molecules, stunted growth
Introduction
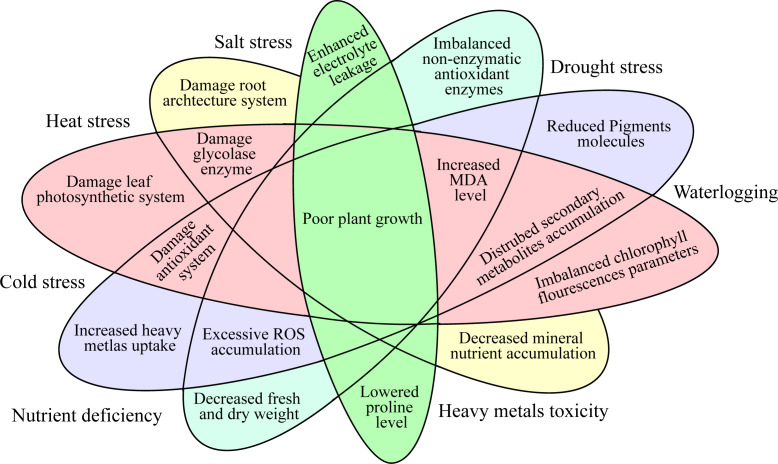
Abiotic stresses are major threats to the production of horticulture crops globally (Kreps et al., 2002). Nevertheless, significant abiotic stresses like heavy metals (HMs), drought, ozone, salinity, ultra-violet radiation, nutrients, temperature fluctuations (low and high), and anthropogenic activities in the developmental period have exacerbated the agriculture system ( Figure 1 ) (Khan et al., 2015). From the earliest stage of seed germination through maturity, the imposed abiotic stresses can affect practically all the molecular, physiological, biochemical, and metabolic functions in plants (Jia et al., 2013). This could ultimately result in serious losses in the economic output of horticulture crops (Chaudhry and Sidhu, 2022; Liu et al., 2022). The approach that plants interact with numerous environmental prompts is a furthermost vital question for plant researchers. Drought, heat, and salt stresses are the most prevalent and vital environmental stresses which have adverse effects on plant growth, yield, and quality (Altaf et al., 2022a). Phytohormones are crucial in coordinating several signal transduction pathways under stress environments (Khan et al., 2020). These hormones perform a vigorous function in the plants’ development and play an important part in the plants’ external and internal factors (Ryu and Cho, 2015). Phytohormones are recognized as environmentally acceptable for improving horticulture crops’ tolerance against abiotic stress (Mantri et al., 2012). Plants’ growth is regulated by the phytohormones as they are released in small quantities as a chemical regulator (Altaf et al., 2022b).
Figure 1.
Physiological and molecular responses of the horticultural crops under abiotic stress.
SA is the hormone that has been shown to activate several stress-reactive genes. Every aspect of the plant’s life is controlled by the biological substances known as plant hormones containing seed germination and distribution as well as the development of fruit (Klessig and Malamy, 1994). Under abiotic stress tolerance and regulation of the hormones involves in the cascade of the composite signaling from the stimulus sensitivity to the expression of the gene (Souza et al., 2017). According to previous research, the ROS-dependent signaling system and hormone responses interact to allow plants to acclimatize to biotic and abiotic challenges by improving performance (Tognetti et al., 2012).
It is challenging to exaggerate the importance of SA spray in preventing abiotic stresses on horticultural crops. SA is essential for increasing crop resistance to numerous abiotic stresses. SA causes a series of physiological processes i.e. better water retention, higher antioxidant activity, and reinforced cell walls. These reactions aid the plants in preventing damage brought on by stress, preserving metabolic balance, and boosting the growth ( Figure 2 ) (Xu et al., 2015a). SA has a crucial role in protecting horticulture crops, increasing productivity, and maintaining food security in the face of unfavorable environmental conditions by lessening the harmful effects of abiotic stress. The current review aimed to provide a comprehensive understanding of the mechanisms by which SA confers abiotic stress tolerance in horticultural crops and its potential as a tool for improving crop productivity and resilience in the face of changing environmental conditions.
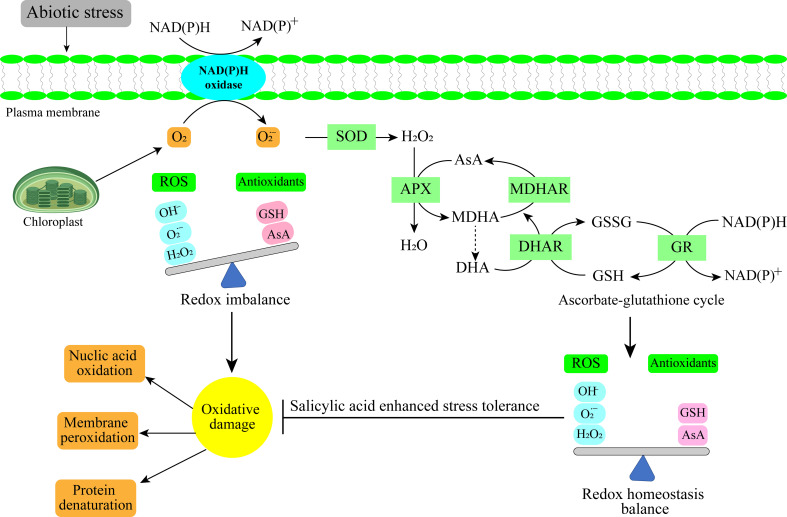
Figure 2.
Antioxidant mechanism under abiotic stress in horticultural crops.
Overview of SA and its potential as a tool for improving plant growth and development
SA sometimes referred to technically as 2-hydroxybenzoic acid, is an aromatic phenolic molecule produced by plants that contains a hydroxyl group or its functional derivative. Phenolic compounds, particularly systemic acquired resistance (SAR), have a role in trendy activating plant defenses (Vlot et al., 2009). SA improves flowering and fruit set even under normal and stress conditions (Pacheco et al., 2013), promoting root growth, stomatal closure, and reducing transpiration (Gorni and Pacheco, 2016), reversing ABA effects (Davies, 2004), regulating gravitropism, and inhibiting fruit ripening (Srivastava and Dwivedi, 2000). Additionally, it has also been observed that plants of pepper and tomato treated with 05 - 10 µM of SA had increased weight of the dry plant shoots along with the area of the leaf (Sardar et al., 2021).
Nevertheless, on plants growth and gas exchange parameters, the SA stimulatory effects depend on the application technique, exposure time, and ontogenetic plants stages and effective concentrations of the SA vary reliant on species (Horváth et al., 2007; Sardar et al., 2021). SA can indirectly affect flowering because it changes production and plant hormone signaling directions such as auxin, ethylene, and jasmonic acid (Vlot et al., 2009). The exogenous use of SA might cause an uprise in the quantity of endogenous bioactive GA in response, altering the plant’s hormonal condition (Mukherjee and Kumar, 2007). Exogenous application improved the level of phytohormones involved in enhanced plant growth, flowering, fruit set, and ultimately yield (Kim et al., 2009).
SA is the hormone that controls defense of the plants against pathogens and is connected to phenolic chemicals (Houston et al., 2016). To modify the tolerance to different abiotic stimuli, SA is also crucial. SA encourages defensive photosynthetic processes, which leads to regulation in stomatal conductance, carbon dioxide (CO2) assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase activity, and chlorophyll and carotenoid content (Feng et al., 2020). By lowering cellular lipid peroxidation and hydrogen peroxide (H2O2) buildup, SA application to plants with insufficient water lower cell membrane damage in leaves. Additionally, SA-treated plants have less H2O2 buildup, which boosts the activity of antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), and guaiacol peroxidase (GPOD) (Ahmad et al., 2023).
SA performs an important part in the plants’ developmental processes ( Table 1 ). Research has shown its functions in stomatal conductance, seeds development, glycolysis, the flowering of the plants, production of the fruit, ion transpiration and accumulation (Harper and Balke, 1981; Sardar et al., 2022), and the rate of photosynthesis (Khan, et al., 2003). SA can regulate the antioxidant defenses and reduce oxidative damage in plants ( Figure 3 ) (Banu et al., 2009). SA controlled the plant nitrogen, glycine betaine (GB) formation, photosynthesis, proline metabolism, and plant water interactions under abiotic stress (Nazar et al., 2011; Miura and Tada, 2014). Additionally, abiotic stressors have been shown to induce defense-related genes and stress tolerance (Rivas-San and Plasencia, 2011; Kumar, 2014).
Table 1.
Role of salicylic acid in abiotic stresses in horticultural crops focusing on sustainable productivity.
| Stress Name | Plant Name | References |
|---|---|---|
| Heavy Metals Stress | Malting Barley (Hordeum uhulgare L.) | Song et al. (2014) |
| Cold Stress | Bean (Phaseolus vulgaris) | Orabi et al. (2015) |
| Drought Stress | Portulaca oleracea | Saheri et al. (2020) |
| Ozone Stress | Strawberry (Fragaria × ananassa) | Drogoudi and Ashmore (2000) |
| UV-B radiation | Pepper (Capsicum annuum L.) | Mahdavian et at. (2008) |
| Cold Stress | Cucumber (Cucumus sativus L.) | Hawrylak-Nowak et al. (2010) |
| Cold stress | Tomato (Solanum lycopersicum) | Orabi et al. (2015) |
| Salinity stress | Guava (Psidium guajava L.) | Sharma et al. (2020) |
| Cold stress | Pepper (Capsicum annuum L.) | Korkmaz et al. (2010) |
| Salinity Stress | Mango (Mangifera indica) | Elsheery et al. (2020) |
| Drought stress | Tomato (Solanum lycopersicum) | Hayat et al. (2008) |
| Drought Stress | Bean (Vicia faba L.) | Abdelaal, (2015) |
| UV-B radiation | Mango (Mangifera indica) | Mavrič Čermelj et al. (2021) |
| Heat stress | Peas (Pisum sativum) | Pan et al. (2006) |
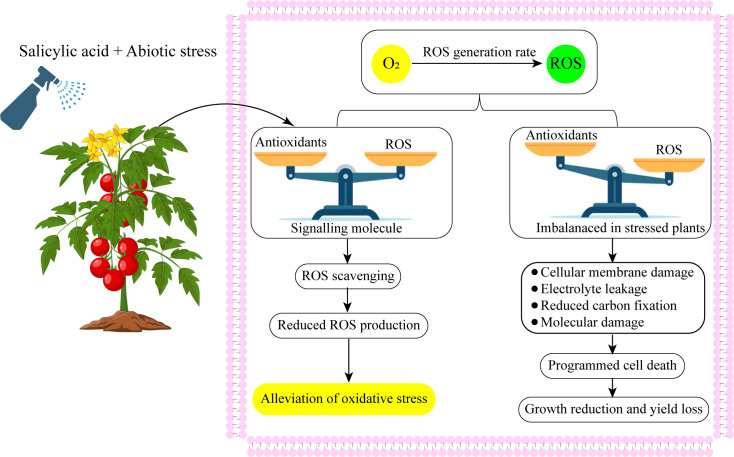
Figure 3.
Exogenous salicylic acid application up-regulated antioxidant defense system and balanced redox homeostasis.
SA and drought stress
The most important abiotic stressor limiting crop output is thought to be drought ( Table 2 ). Only 10 - 30% of the available irrigation water is used each year (Szilagyi, 2003). The rise of the drought was further aided by the amalgamation of high temperatures and low precipitation (Yu et al., 2013). Global food security is facing a challenge from climate change which pollutes water resources and declining crop production (Hedhly et al., 2009; Gomes et al., 2019). Water shortages would impact agricultural land by up to 50% by 2050 (Hasanuzzaman et al., 2019). Moreover, lack of water is the main environmental barrier to the production of horticulture crops (Ullah et al., 2018; Sirisuntornlak et al., 2019). In arid and semiarid environments, the two most significant environmental stresses drought and salinity occur simultaneously (Slama et al., 2008). Extreme drought and salt stress levels affect a large portion of the earth and harm crop plant output (FAO, 2017). Drought negatively affects plant development growth, and other metabolic functions, and is a limiting factor in the effective production of crop plants worldwide (Osuagwu et al., 2010). The weight of the dry roots can be increased due to the drought stress and also had an impact on nutrient uptake, resulting in a decline in the potassium content of the plant’s roots and shoots.
Table 2.
Function of salicylic acid application in horticultural plants.
| Functions | References |
|---|---|
| Improved CO2 absorption | Saheri et al. (2020) |
| Increased the plant’s PAL (phenylalanine lyase) | Chavoushi et al. (2020) |
| Mitigates water deficits | Chakma et al. (2021) |
| Combat drought problem | Ashraf et al. (2013) |
| Opening of the stomata | Rai, 2002 |
| Resistance to cold stress | Sayyari et al. (2013) |
| Osmolytes Contents and Anti oxidative defense system modification | Kohli et al. (2018) |
| Lipid peroxidation and ROS level decline | Shi and Zhu (2008) |
| Transpiration and the rate of photosynthesis increases | Khan, et al., (2003) |
| Controls the plant nitrogen | Nazar et al. (2011) |
| metabolic and defense pathways | Bücker-Neto et al. (2017) |
| Antioxidant enzyme activity increase | Kazemi et al. (2010) |
Phytohormone supplementation is regarded as an efficient adaptation technique (El-Mageed et al., 2017). SA affects the absorption of ions, the development of the plant, and the transport of substances. It is the crucial plant signaling molecule regarding the plants’ biotic and abiotic stress tolerance (Kazemi, 2014; Gorni et al., 2017). SA treatment had a progressive effect on Portulaca oleracea growth and biomass accumulation during water deficit by improving photosynthetic pigments and CO2 absorption (Saheri et al., 2020). According to Ahmad et al. (2023), the application of SA on Carthamus tinctorius L. during a water shortage increased the plants’ phenylalanine lyase (PAL) enzyme activity and anthocyanin contents but had no impact on the dry matter (Ahmad et al., 2023). Recent findings indicate that foliar SA spray on tomato mitigates water deficits (Chakma et al., 2021). The necessity of verifying SA dosages in scenarios that farmers can use is raised by the fact that grape tomatoes are not the most widely farmed tomato in the world and that the investigational circumstances of this research cannot be reconstructed in the field.
Previous research suggested using SA, which is intricate in the stress signaling compared to abiotic and biotic stresses to combat drought problems (Rabnawaz et al., 2020). SA applied topically to plants stated to cut their susceptibility to stress such as drought and the maximum temperature (Wang and Li, 2006). Opening of the stomata, ion transport regulation, and cleaning of the HMs adverse effects are all functions of SA (Rai, 2002).
SA and cold stress
Environmental stresses including heat, salinity, cold, and drought harm plant growth and production and cause several physiological, molecular, morphological, and biochemical changes in plants that ultimately cause the plants to become unsustainable (Ahmad and Prasad, 2012). Cold stress inhibits the growth of a plant its development and plant metabolism (Lichtenthaler, 1998). The sensitivity and intolerance of many crops to low temperatures (cold stress) have an impact on crop yield. A sudden temperature change harms the plants (Schwartz et al., 2006). According to Ruelland and Zachowski, (2010), plants typically succumb to chilling and freezing stress as a result of membrane damage, change in enzyme activity, necrosis, changes in cytoplasm viscosity, and chlorosis.
Low-temperature stress is most dangerous for vegetable crops regarding their productivity and quality (Yadav, 2010). The low temperature of about 0 - 15 °C is vulnerable for summer vegetables, for the whole plant life cycle including germination of seeds, vegetative growth, and reproduction. Cold stress plants reproductive stages, cause them to blossom later and produce sterile pollen, which has a significant negative impact on agricultural productivity (Suzuki et al., 2008; Yadav, 2010). Among abiotic stresses, the low temperature has a significant impact on the existence and geographic spread of the plants, as well as on the agricultural production of plants in mountainous terrain. Disruptions to plant growth and productivity lead to significant crop failure (Hatfield and Prueger, 2015). Low-temperature initial effect on the plant is the membrane fluidity decrease may be the harm perception of the potential site (Orvar et al., 2000). ROS damaged the membrane due to freezing temperature with cell wall and membrane adhesions and causes the rupture of the cell (Olien and Smith, 1997).
SA application enhanced the number of fruits in cucumber and pepper (Elvwan and Hamahyomy, 2009; Hayat et al., 2010). SA applied on the cucumber leaves enhanced their resistance to cold (Sayyari et al., 2013). Lower acetyl SA and SA concentrations (0.1 mM and 0.5 mM) have been revealed to be highly effective against low-temperature stress in bean and tomato (Orabi et al., 2015).
SA and heat stress
When plants are subjected to abiotic stress, they cannot relocate to more hospitable conditions, which has a substantial adverse effect on the growth of the plant, development, and yield (Lippmann et al., 2019). High temperatures (HT) are a significant stress, and in recent decades, global warming has increased the rise in air temperatures (Lippmann et al., 2019). The processes by which plants adapt to extreme temperatures are therefore quite interesting. One of the most harmful pressures among the continually shifting environmental factors is the steadily rising ambient temperature. By 2100, temperatures are expected to be 1.8 – 4.0°C greater than they are now due to the global air temperature increase of 0.2°C every decade (IPCC, 2007). Plants that are subjected to heat stress (HS) experience significant, and occasionally fatal, negative impacts. Plants have developed sophisticated HS response mechanisms to deal with such circumstances. HS influences many essential physiological functions of plants, including photosynthesis, water metabolism, and respiration. Characterizing HS reactive genes, non-coding RNAs, DNA methylation, and histone amendments have made significant strides (Ruelland and Zachowski, 2010).
For plant scientists, developing new crop cultivars resistant to HT is a big task (Zhang et al., 2006; Moreno and Orellana, 2011). HT affects plants in numerous means, depending on their intensity, duration, the kinds of plants present, and other environmental conditions (Rodríguez et al., 2005; Wahid et al., 2007). However, the features that confer resistance to HT have yet to be identified and confirmed. Researchers studying plants under HT stress are attempting to understand the plant retorts that result in heat tolerance as well as how plants might be controlled in HT situations. Omics techniques and the development of transgenic plants by modifying target genes are two recently highly explored molecular approaches (Schoffl et al., 1999; Kosová et al., 2011; Duque et al., 2013). Investigating these underlying molecular mechanisms may help to develop stress-resistant crop variants and enable the HT cultivation of vital crop plants.
SA has a role in altering resistance in plants against biotic stress and abiotic stress due to the part that plays in immune system activation (Kawano and Bouteau, 2013). Researchers are very interested in the use of SA to enhance plant thermo tolerance as well as the physiological and biochemical mechanisms involved. SA foliar spraying promoted heat tolerance in essential horticultural plants like cucumbers and grapes (Wang et al., 2010; Yang et al., 2022). By controlling enzymatic activity, SA application boosts the rate of photosynthesis and preserves cell membrane stability (Waqas et al., 2021). The formation of cardenolides and antioxidants is also stimulated by SA treatments, which may be crucial in boosting resistance to HT stress (Zhang et al., 2020).
SA and salinity stress
Salinity had numerous detrimental effects on the germination of seeds, plant development, and crop yield (Munns and Tester, 2008). Various irrigated regions get salinized, primarily as a result of the usage of saline water. Approximately, 45 million hectares of land are damaged due to salinity (Munns and Tester, 2008). Plants are impacted by high salinity in a variety of aspects, including oxidative stress, water stress, nutritional issues, metabolic process changes, ion toxicity, decreased expansion and cell division, membrane disruption, and genotoxicity (Hasegawa et al., 2000; Munns, 2002; Zhu, 2007). These effects collectively by inhibiting the survival of the plant, its growth, and its development (Sardar et al., 2023).
A major factor restricting agricultural production is salinity (Slama et al., 2008). It will be difficult to feed the 9.3 billion people on the planet in 2050. According to estimates, agricultural production must increase by more than 60% from 2005 to 2007 levels by 2050. (Fita et al., 2015). However, stress factors like salinity are a constant danger to effective agricultural productivity. Climate change, groundwater penetration, seawater backflow, and human activities like fertilizer application and irrigation have all contributed to an increase in soil salinity in recent years (Arani et al., 2023). The salinity of the soil reduces plant product quality, productivity, and yield (Yang and Guo, 2018a). Salinity stress affects the land by about 950 million hectares, although the percentage of soil salinity is rising in the future (Yang and Guo, 2018b).
When salt stress initially appears, excessive salt and chloride ions restrict root water because it decreases the water potential surrounding the roots of the plants (Negrão et al., 2016). Second, too much salt and chloride in plants cause toxicity of the ion. It not only interferes with the homeostasis of ions like Na+ and K+ but also averts the efficient uptake of nutrients like Ca2+, leaving plants deficient in vital nutrients (Zhu, 2002; Wu, 2018). Plants experience oxidative stress due to the overabundance of ROS brought on by osmotic and ionic stressors (Zhu, 2016). In chloroplasts and mitochondria, excessive H2O2 accumulation impairs respiration and photosynthesis under salt stress (Balal et al., 2011).
To address the salt issue, a variety of phenolic compounds and plant growth regulators (PGRs) are used to decrease their effects. SA is an endogenous signal molecule that functions in plants’ ability to withstand environmental challenges. Plants must be able to respond to abiotic and biotic stresses (Moustafa-Farag et al., 2020). SA also promotes several physiological processes important for plant growth (Basit et al., 2018; Guo et al., 2022). Antioxidant SA is regarded as having hormone-like properties. Abiotic stressors can raise the SA concentration in the plant, strengthening its ability to withstand these challenging circumstances (Yuan and Lin, 2008). SA is essential for stomatal conductance, photosynthesis, and transpiration. Additionally, it decreases Na+ and Cl- buildup in plant tissues, enhancing antioxidant defense (Sardar et al., 2022). SA can thereby increase salt tolerance, reducing the adverse effects that occur on plants (Manaa et al., 2014; Semida et al., 2014). The upregulation of anti-stress mechanisms and the restoration of the development process are two examples of SA’s beneficial effects (Mahmood, 2006).
SA and heavy metals
HMs have surpassed pesticides and eminent pollutants like sulfur dioxide (SO2) and CO2 (Chen et al., 2007). It is expected that HMs could overtake solid waste and nuclear waste as the most harmful contaminant (Lajayer et al., 2017). Eating is the primary way that 70% of all HMs and their derivatives enter human bodies (Jaishankar et al., 2014). HMs are emitted into the air, land, and water from numerous sources and as a result of human activity (Tchounwou et al., 2012). HMs toxicity has grown to be a significant issue in many terrestrial ecosystems all over the globe. Recently, excessive industrialization damages soil quality and agricultural yield by collecting HMs (Shahid et al., 2015).
Changes in the soil pH which damage the texture of the soil, the presence of various elements, and the HMs accumulation, may delay a variety of molecular as well as plant physiological processes, which may either directly or indirectly limit plant development (Panuccio et al., 2009). Several developmental and biological processes need HMs such as Cu, Mo, Ni, Mn, Co, and Zn at very low amounts (Salla et al., 2011; Shahid et al., 2015). However, when their concentration exceeds suboptimal levels, these metals along with four other extremely poisonous heavy metals, Be (beryllium), As (arsenic), Al (aluminum), Cd (cadmium), Pb (lead), and Hg (mercury) can significantly decrease the crop yield (Pierart et al., 2015). These harmful substances result in aberrant plant morphology and metabolic issues that lower plant production (Amari et al., 2017). ROS such as OH-, O2 -, and H2O2 are produced as a result of these irregularities, which disturb the redox balance of the cells (Ibrahim et al., 2015; Shahid et al., 2015). This redox state imbalance is known to have a significant role in the HMs toxicity in plants. Earlier studies showed that HMs accumulation in horticulture products harms human health (Uzu et al., 2011; Shahid et al., 2015).
Metal absorption and sequestration capabilities vary across plant types and plant regions. There are considerable changes in the absorption and transport of metals across species of plants and numerous varieties of the same species plant species (Shahid et al., 2017). The majority of cereal crop plants’ roots are located 25 cm below the surface of the soil, where the plant absorbs HMs. Metals like Cd and Pb are primarily collected in roots, which are the major target for metal accumulation (Kumar et al., 2012). The cellular transport system is one method that plants have progressed to lessen the toxicity of the metal ion.
Phytohormones are small signaling molecules that have a significant impact on virtually every aspect of plant development and growth. Distinct hormones can have quite different mechanisms of action for various functions. So, although several hormones may be involved in the regulation of a single function, this is shown that a single hormone may govern the range of developmental and cellular processes (Gray, 2004). Phytohormones such as IAA (indole-3-acetic acid), also known as auxin, abscisic acid (ABA), cytokinin (CK), gibberellin (GA), ethylene (ET), jasmonic acid (JA), brassinosteroids (BRs), cytokinin (CK), salicylic acid (SA) and the most recent discovered strigolactones (SLs) are essential for plant growth and development, signaling, and crosstalk. Phytohormones are much important for plants to protect them from biotic stress as well as abiotic stress (Nishiyama et al., 2011; Colebrook et al., 2014; Xu et al., 2016; Bücker-Neto et al., 2017). In agronomical crop management strategies, phytohormones have been used as regulators of HM absorption to reduce metal toxicity (Piotrowska-Niczyporuk et al., 2012). Exogenous phytohormone application demonstrated to be safe to employ and to produce favorable outcomes for plants exposed to HM toxicity (Zhu et al., 2012; Zhu et al., 2016; Masood et al., 2016). Phytohormones like IAAs, BRs, SAs, CKs, GAs, and much more play important signaling roles, metabolic and defense pathways, but the way they alleviate HM stress is at this time a subject of great attention worldwide (Bücker-Neto et al., 2017). Phytohormone priming is carried out to promote imminent research for crop stress treatment.
Under HM stress, naturally accruing SA phenolic molecule is associated with the defense response in plants ( Table 3 ). SA contributes to coordinating plant development, ripening, and abiotic stress responses (Rivas-San and Plasencia, 2011). Both the ABA and SA are engaged in managing the HMs response (Miura and Tada, 2014). The presence of SA can also control a plant’s tolerance to salt, heat, and cell death under heavy metals toxicity (Naz et al., 2022). SA shown to activate the antioxidant defense system and regulate Cd detoxification pathways, hence reducing Cd toxicity. Cd exposure increases barley SA content (Hordeum vulgare) roots. SA and other phytohormones, such as JA and ET, commonly interact with one another (Jia et al., 2013). Plant stress response as a part of hormone production, transport, and storage result in a cascade of signaling pathways (Matilla-Vazquez and Matilla, 2014). ET biosynthesis enzyme activity increases in the presence of HM, and MAPKs phosphorylate ACS2 and ACS6 (Skottke et al., 2011; Bücker-Neto et al., 2017; Ahmad and Anjum, 2023).
Table 3.
SA enhanced HMs stress in different Horticultural plants.
| Plant Name | Heavy Metal | Plants Adaptive Reaction | Reference |
|---|---|---|---|
| Cucumber | Mn | Lipid peroxidation and ROS level decline. Antioxidants (Glutathione and Ascorbate) increase |
Shi and Zhu (2008) |
| Peanut | Cd | Metal Toxicity alleviation. Photosynthesis, Mineral nutrients, Growth, and Chlorophyll enhancement |
Xu et al (2015a; 2015b) |
| Onion | Pb | Minimize the DNA damage, Increased the CAT (Catalases), APX (Ascorbate), SOD (Guaiacol Peroxidases), and GPX (Superoxide Dismutases) | Kaur et al. (2021) |
| Spinach | B | Decreased the Lipid peroxidation, H2O2 activity, and MDA contents, While increasing the Chlorophyll contents, CAT, and SOD activity. | Eraslan et al. (2008) |
| Tomato | Cd | Enhanced the plant leaves, stem and root biomass, and Photosynthetic rate | Wei et al. (2018) |
| Cucumber | Mn | Reduced the Mn transport from roots to the plant above parts, Lipid peroxidation, ROS contents, APX, and CAT activities. Increased the Ascorbate, SOD, GR (Glutathione reductase), POD, and DHAR (Dehydroascorbate reductase). | Shi and Zhu (2008) |
| Pea | B | Enhanced ROS production and degradation, Plant fresh and dry biomass, Carotenoids, and Chlorophyll contents. Reduction of the H2O2 and MDA contents. | Oliveira et al. (2020) |
| Potato | Cd | Increased the antioxidant defense system, RWC (relative water contents), and chlorophyll and proline. While decreasing the O2 - (Superoxide Anion redicle), MDA (Malondialdehyde), and H2O2 (Hydrogen Peroxide). |
Li et al. (2019) |
| Lemon balm | Ni | Improved the plant fresh and dry shoot and root biomass, Carotenoid contents, and decline in the electrolyte leakage. | Soltani Maivan et al. (2017) |
| Safflower | Zn | Reduced oxidative stress activity. Increased in the ASC (Ascorbate) level, PC (Phytochelatin), GSH (Glutathione) | Eisalou et al. (2021) |
| Cucumber | Cu | Minimized the GSH, Proline, and MDA contents. Increased plant growth. | Çanakci-Gülengül et al. (2017) |
| Lettuce | Cd | Enhancement of proline contents, Peroxidase enzyme activity, and ascorbic acid, | Talabany and Albarzinji (2023) |
| Pepper | As | Increased glutamine synthetase, Nitrogen metabolism, Nitrate reductases, and Glutamate synthase activity. | Kaya et al. (2022) |
| Periwinkle | Ni | Improvement of Alkaloid contents, Vincristine, and anticancer alkaloids vincristine | Idrees et al. (2013) |
SA and ozone stress
Tropospheric ozone (O3) is a naturally occurring element of the atmosphere; however, since industrialization, anthropogenic emissions of NO (nitrogen oxides), NMVOCs (Non-methane volatile organic compounds), CO (Carbon monoxide), and CH4 have caused the O3 to be produced as a result of photochemical reactions. O3 levels have increased as a result, rising from pre-industrial levels of less than 20 ppb to contemporary levels, which have boosted the average of Northern Mid-Latitude O3 concentrations (30-50 ppb) (Cooper et al., 2014). Since the 1950s, mean O3 levels have been increasing, with the Northern Hemisphere (NH) seeing an increase of 1 to 5 ppb/decade and the Southern Hemisphere (SH) seeing an increase of 2 ppb/decade (Cooper et al., 2014). Over the past 50 years, evidence of O3 harm and damage to vegetation as a result of O3 phytotoxicity has increased significantly along with these concentrations (Grulke and Heath, 2020). O3 is a secondary pollutant, so its concentrations vary a lot in space and time. This is because of things like the season, interactions between land and coast, monsoon systems, and the exchange of air between the stratosphere and troposphere. Accordingly, in semi-rural and rural areas O3 concentrations are typically maximum that are downwind of the industrial and the urbanization sources of precursor emissions, i.e., in areas vital to agriculture, forestry, and grasslands and the ecosystem services they provide (Mills et al., 2013). When O3 degradation (via chemical transformation and deposition) is slower than O3 creation, background O3 concentrations will be increased. O3 and its precursors are also carried around the planet in air masses. The duration, frequency, and magnitude of O3 levels are all crucial factors in determining how plants will react, and these global processes have an impact on local to regional O3 concentrations.
Limited research has been done on the ozone effects on plant reproductive characteristics. Leisner and Ainsworth (2012) examined 128 peer-reviewed studies to assess the detrimental effects of present ozone levels on the reproductive development and production of several crops. However, current levels of ambient ozone considerably reduced the number of seeds (16%), fruits (9%), and fruit weight (22%). The development and makeup of fruits vary with the growing environment and may be impacted by other environmental factors. Ozone can affect reproductive development at many distinct stages (Black et al., 2000). It was demonstrated that strawberry fruiting plants bare to ozone (184 g/m3 O3 for 69 days) experienced an initial acceleration of inflorescence formation, monitored by a decrease in inflorescence production rate and fruit setting rate (Drogoudi and Ashmore, 2000). Moreover, ozone treatment (156 g m3 ozone for 2 months of exposure) reduced ascorbic acid content, increased peroxidation of lipids, and decreased strawberry fruit taste because it altered the fruit’s carbohydrate pool (Keutgen and Pawelzik, 2008; Yi et al., 2021). This research showed that ozone modifies the development of reproductive organs to influence fruit yield and quality. Consumers’ demands for higher-quality fruit have grown.
In response to relating the ozone effects to concentrations averaged at one-hour intervals, which might serve as a substitute for dosage, it is crucial to summarize concentrations in a physiologically significant way (Lefohn, 1992). The exposure index should, in theory, be based on the idea of effective dose (Runeckles, 1974), that is, it should capture the exposure features that are most closely related to the quantity of ozone that is absorbed by the plants. In this case, the absorbed dosage would be the integral of the rate of absorption (flux) over time, which could be determined for ozone by dividing the concentration at the leaf surface by the leaf conductance (Fowler and Cape, 1982).
The conduction of the atmosphere could be incorporated into this conception (Grunhage and Jager, 1994), when there is adequate air mixing (high air conductivity), ozone concentration, and conductance of the leaf control the diurnal pattern of ozone flux. In open-top contact chambers, this is the case. In crops, the use of radiation as a substitute for leaf conductance has been proposed due to the lack of data on leaf conductance (Rawlings et al., 1988). It is necessary to characterize exposure by using ozone concentrations during the daylight hours measured (for example, >50 W/m2 global radiation). However, no such distinction should be established for species that exhibit significant leaf conductivity at night. Although it is also known that other parameters, such as air humidity, soil water availability, and temperature affect leaf conductance, these factors have not yet been employed in long-term research to quantify ozone uptake or dose.
SA and UV-B radiation
Sunlight, the supreme energy source on Earth, is required for all vital biological activities. On the earth’s surface, UV light is one of the principal solar spectrum components (Dotto et al., 2018). The geomagnetic field and stratospheric ozone layer are only two examples of the many natural barriers that exist to lessen the effects of hazardous natural radiations on biotic components (Herndon et al., 2018). Approximately, 90% of (290-320 nm) of UV-B and 100% of (100-290 nm) light cannot reach the Earth’s surface due to the stratospheric ozone layer. Although over the past two decades, the thinning of the stratospheric ozone layer has gained attention due to the massive buildup of environmental pollutants that eventually cause UV-B rays to reach the earth’s surface (Barnes et al., 2019; Neale et al., 2021). As a result, concern over UV-B radiation’s effects on plants has increased due to its increased penetration.
UV-B-induced morphological alterations in plants may have an impact on how well they compete for light. Deformed morphological characteristics are the result of UV-B radiation’s harmful impacts. UV-B exposure decreased plant leaf area, height, and dry weight while increasing auxiliary branching and leaf curling (Greenberg et al., 1997; Furness et al., 1999). After a few weeks of UV-B exposure, the leaf area, and plant dry weight of rice plants significantly reduced. Weih et al. (1998) found that improved UV-A decreased the amount of leaf area per unit plant biomass (leaf area ratio), but increased the amount of biomass produced per unit leaf area (leaf area productivity) and per unit leaf nitrogen (leaf nitrogen productivity). It was clear that these variables affected the relative growth rate and nitrogen productivity since high UV-B levels decreased the leaf area ratio, leaf area productivity, and leaf nitrogen productivity (Manzoor et al., 2023). The principal causes of UV-B-induced the loss of photosynthetic pigments, thylakoid membrane integrity, reduced rubisco enzymatic activity, and the down-regulation of photosynthesis-related gene transcript levels (Jansen et al., 1996; Hollósy, 2002). Adverisites occur from abiotic stress tolerance can be mitigated by SA application ( Figure 4 ; Table 4 ).
Figure 4.
Salicylic acid supplementation enhanced abiotic stress tolerance in horticultural crops.
Table 4.
Effects of Salicylic acid use in different abiotic stresses in the plants.
| Plant name | Stress Type | Antioxidant Enzyme | Non-enzymatic antioxidant | Stress biomarkers | References |
|---|---|---|---|---|---|
| Tomato | Salinity | APX decreased and GPX increased | Damage DNA, inactivate enzymes, and cause lipid peroxidation | H2O2 increased | Monteiro et al. (2011) |
| Tobacoo | Ozone | antioxidant enzyme decreased; enzyme BAZ-hydroxylase increased | Photosynthesis decreased | Antioxidant enzymes (CAT and SOD) decline | Yalpani et al. (1994) |
| Bluegrass | UV-B Radiation | Antioxidant enzymes (CAT and SOD) decreased | Inhibition of photosynthesis, premature senescence, and altered biomass partitioning |
Anthocyanin and a-tocopherol contents decreased |
Ervin et al. (2004) |
| Pea | Heat stress | MDA contents decreased | Promote plant thermotolerance, PAL, and BA2H. | Induce the synthesis of Hsp (Heat shock protein) | Pan et al. (2006) |
| Cucumber | Cold Stress | Benzoic acid 2-hydroxylase activity increased | Significantly decreased the shoot biomass | Reduced the H2O2 accumulation | Dong et al. (2014) |
| Spinach | Salinity | Reduction of electrolyte linkage | Enhanced root and shoot length and dry weight of the plant | Increased Chlorophyll a and b and total chlorophyll contents | Gilani et al. (2020) |
| Potato | HMs | Declined the MDA contents | Increased the RWC (relative water contents), Chlorophyll, and Proline | Enhanced the ROS metabolism, SOD, CAT, EC, GR, and APX contents | Li et al. (2019) |
| Carrot | Salinity | Enhanced the total antioxidant activity | Increased the root’s fresh and dry weight | Regulation of B and Cl ion and Proline | Eraslan et al. (2007) |
| Cucumber | Heat Stress | Increased the CAT and SOD activity | Maximize the Photosystem II photochemical reactions | Decreased the H2O2 and Thiobarbituric acid reactive substances. | Shi et al. (2006) |
| Pepper | Heat Stress | Decreased the ROS and Increased the protective enzymes activity | Maintain root vigor and cell structure integrity. Also, inhibit the water boss | Reduced the oxidative damage | Zhang et al. (2020) |
| Common Bean | HMs | Increased the Catalase, Peroxidase, Superoxide dismutase and antioxidant enzymes, and proline | Improvements in photosynthetic pigments, Nitrogen contents, and growth traits | Decreased the electrolyte leakage and mineral ions uptake | Khalil et al. (2021) |
| Mung Bean | UV B Radiation | Reduce the Superoxide dismutase, peroxidase, and Catalase | Increased the chlorophyll contents an | Enhanced the Protein contents and carotenoids | Bano and Singh (2017) |
| Onion | Drought stress | Reduce the osmoprotectants | Increased the RWC, Photosynthetic activity, and membrane stability index | Increased Chlorophyll a contents | Semida et al. (2017) |
| Garlic | Salinity | Increased the bulb diameter and nick bulb diameter | Increase the average clove weight, bulb diameter, number of cloves per bulb, and bulb fresh weight | Enhanced the potassium concentration | Shama et al. (2016) |
Conclusion and future horizon
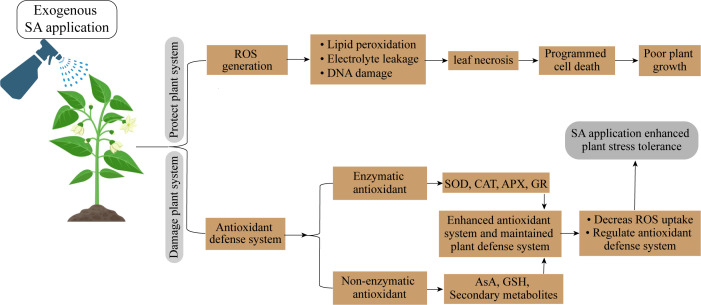
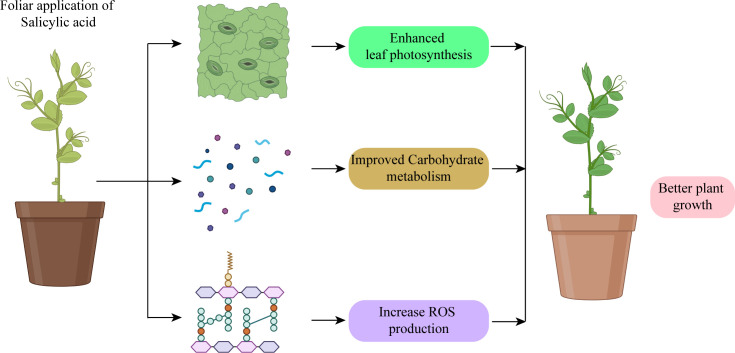
SA works as a signaling molecule and regulates physiological activities in plants such as photosynthesis, metabolic, and growth processes ( Figure 5 ). Numerous studies indicate that SA plays a significant part in regulating how plants react to different abiotic stressors. Depending on the plant type, the SA was proven to be efficacious in various foliar sprays/incorporation with growing media treatment forms. SA helps crops withstand the effects of salinity, cold, heat, heavy metals, and drought stressors. Foliar SA treatment proved effective in reducing the negative effects of water deficiency on plants. By boosting the activities of antioxidant enzymes, SA administered to drought-stressed seedlings improved physiological traits and decreased ROS generation, which lessened drought stress and increased plant growth. One method to reduce the low-temperature stress on crops is the application of PGRs and the unitization of genetic tools and plant breeding. PGRs are more important in increasing resistance to cold stress. There has been a lot of research on the use of exogenous SA to reduce heat exhaustion.
Figure 5.
Salicylic acid application protected photosynthetic system and promoted plant growth.
Future research should focus on how SA controls cross-talk with PGR operating at high, medium, and short ranges (auxins, gibberellins, cytokinins, ethylene, etc.). It will be possible to identify plant systems devoted to maintaining an optimum balance between growth and defense with further study into the double roles in the development and stress response of salicylic acid. Moreover, this innovative growth regulator has prodigious potential as a running tool for allowing crops to attain greater yields.
Author contributions
HY and RF: Conceptualization, Literature survey, Writing major original draft, Review structure. LL and WY: Literature survey, Writing- review and editing, Figure designing. QH: Literature survey, Writing- review and editing. CY: Reviewing and editing, References collection. HY: Supervision, WH and WG, JW: Preparing figures and tables: All authors contributed to the article and approved the submitted version.
Funding Statement
This work was supported financially by the National Key Research and Development Program of China (Program No. 2020YFD1000700) and the Sichuan Characteristic Economic Crops Innovation Team Project of National Modern Agricultural Technology Systems (sccxtd-2020-12).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdelaal K. A. (2015). Effect of salicylic acid and abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. 6 (11), 1771–1788. doi: 10.21608/jpp.2015.52096 [DOI] [Google Scholar]
- Ahmad R., Anjum M. A. (2023). “Mineral nutrition management in fruit trees under salt stress: a review,” Erwerbs-Obstbau, 1–9. doi: 10.1007/s10341-023-00830-x [DOI] [Google Scholar]
- Ahmad R., Manzoor M., Muhammad H. M. D., Altaf M. A., Shakoor A. (2023). Exogenous melatonin spray enhances salinity tolerance in Zizyphus germplasm: a brief theory. Life 13, 493. doi: 10.3390/life13020493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P. M., Prasad N. V. (2012). Abiotic stress responses in plants: metabolism, productivity and sustainability (New York, NY, USA: Springer; ). doi: 10.1007/978-1-4614-0634-1 [DOI] [Google Scholar]
- Altaf M. A., Shahid R., Altaf M. M., Kumar R., Naz S., Kumar A., et al. (2022. a). Melatonin: first-line soldier in tomato under abiotic stress current and future perspective. Plant Physiol. Biochem. 185, 188–197. doi: 10.1016/j.plaphy.2022.06.004 [DOI] [PubMed] [Google Scholar]
- Altaf M. A., Shahid R., Ren M. X., Naz S., Altaf M. M., Khan L. U., et al. (2022. b). Melatonin mitigates cadmium toxicity by promoting root architecture and mineral homeostasis of tomato genotypes. J. Soil Sci. Plant Nutr. 22 (1), 1112–1128. doi: 10.1007/s42729-021-00720-9 [DOI] [Google Scholar]
- Amari T., Ghnaya T., Abdelly C. (2017). Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S. Afr. J. Bot. 111, 99–110. doi: 10.1016/j.sajb.2017.03.011 [DOI] [Google Scholar]
- Arani E. A., Hosseini S. A., Javadi S., Ghazavi R. (2023). Field studies, ion analysis, and modeling of Kashan plain aquifer to predict the source of salinization. Environ. Monit. Assess. 195 (1), 23. doi: 10.1007/s10661-022-10644-1 [DOI] [PubMed] [Google Scholar]
- Ashraf Q. H., Weil D. N., Wilde J. (2013). The effect of fertility reduction on economic growth. Popul Dev. Rev. 39 (1), 97–130. doi: 10.1111/j.1728-4457.2013.00575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balal R. M., Ashraf M. Y., Khan M. M., Jaskani M. J., Ashfaq M. (2011). Influence of salt stress on growth and biochemical parameters of citrus rootstocks. Pak. J. Bot. 43, 2135–2141. Available at: https://www.pakbs.org/pjbot/PDFs/43(4)/PJB43(4)2135.pdf [Google Scholar]
- Bano C., Singh N. B. (2017). Interactive effects of UV-B radiation and salicylic acid on Vigna radiata L. Russian Agri Sci. 43, 128–133. doi: 10.3103/S1068367416060057 [DOI] [Google Scholar]
- Banu M. N. A., Hoque M. A., Watanabe-Sugimoto M., Matsuoka K., Nakamura Y., Shimoishi Y., et al. (2009). Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 166 (2), 146–156. doi: 10.1016/j.jplph.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Barnes P. W., Williamson C. E., Lucas R. M. (2019). Ozone depletion, ultraviolet radiation, climate change and prospects for a sustainable future. Nat. Sustain. 2 (7), 569–579. doi: 10.1038/s41893-019-0314-2 [DOI] [Google Scholar]
- Basit A., Shah K., Rahman M. U., Xing L., Zuo X., Han M., et al. (2018). Salicylic acid an emerging growth and flower inducing hormone in marigold (Tagetes sp. L.). Pure Appl. Biol. 7 (4), 1301–1308. doi: 10.19045/bspab.2018.700151 [DOI] [Google Scholar]
- Black V. I., Black C. R., Roberts I. A., Stewart C. A. (2000). Impact of ozone on the reproductive development of plants. New Phytol. 147, 421–447. doi: 10.1046/j.1469-8137.2000.00721.x [DOI] [PubMed] [Google Scholar]
- Bücker-Neto L., Paiva A. L. S., MaChado R. D., Arenhart R. A., Margis-Pinheiro M. (2017). Interactions between plant hormones and HMs responses. Genet. Mol. Biol. 40, 373–386. doi: 10.1590/1678-4685-gmb-2016-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çanakci-Gülengül S., Yildiz T. U. Ğ. B. A., Deveci D. (2017). Some physiological and biochemical responses to copper of detached cucumber (Cucumis sativus L.) cotyledons pre-floated in salicylic acid. Pak. J. Bot. 49 (2), 487–492. Available at: https://www.pakbs.org/pjbot/PDFs/49(2)/13.pdf [Google Scholar]
- Chakma R., Biswas A., Saekong P., Ullah H., Datta A. (2021). Foliar application and seed priming of salicylic acid affect growth, fruit yield, and quality of grape tomato under drought stress. Sci. Hortic. 280, 109904. doi: 10.1016/j.scienta.2021.109904 [DOI] [Google Scholar]
- Chaudhry S., Sidhu G. P. S. (2022). Climate change regulated abiotic stress mechanisms in plants: a comprehensive review. Plant Cell Rep. 41 (1), 1–31. doi: 10.1007/s00299-021-02759-5 [DOI] [PubMed] [Google Scholar]
- Chavoushi M., Najafi F., Salimi A., Angaji S. A. (2020). Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci. Hortic. 259, 108823. doi: 10.1016/j.scienta.2019.108823 [DOI] [Google Scholar]
- Chen T. M., Gokhale J., Shofer S., Kuschner W. G. (2007). Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 333, 249–256. doi: 10.1097/MAJ.0b013e31803b900f [DOI] [PubMed] [Google Scholar]
- Colebrook E. H., Thomas S. G., Phillips A. L., Hedden P. (2014). The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 217, 67–75. doi: 10.1242/jeb.089938 [DOI] [PubMed] [Google Scholar]
- Cooper O. R., Parrish D. D., Ziemke J., Balashov N. V., Cupeiro M., Galbally I. E., et al. (2014). Global distribution and trends of tropospheric ozone: an observationbased review. Elementa 2, 1–28. doi: 10.12952/journal.elementa.000029 [DOI] [Google Scholar]
- Davies P. J. (2004). “Plant hormones: their nature, occurrence and functions,” in Plant hormones: biosynthesis, signal transduction, action. Ed. Davies P. J. (London: Kluwer Academic Publishers; ), 1–15. [Google Scholar]
- Dong C. J., Li L., Shang Q. M., Liu X. Y., Zhang Z. G. (2014). Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 240, 687–700. doi: 10.1007/s00425-014-2115-1 [DOI] [PubMed] [Google Scholar]
- Dotto M., Go´mez M. S., Soto M. S., Casati P. (2018). UV-B radiation delays flowering time through changes in the PRC2 complex activity and miR156 levels in Arabidopsis thaliana . Plant Cell Environ. 41 (6), 1394–1406. doi: 10.1111/pce.13166 [DOI] [PubMed] [Google Scholar]
- Drogoudi P. D., Ashmore M. R. (2000). Does elevated ozone have differing effects in flowering and deblossomed strawberry? New Phytol. 147, 561–569. doi: 10.1046/j.1469-8137.2000.00718.x [DOI] [PubMed] [Google Scholar]
- Duque A. S., de Almeida A. M., da Silva A. B., da Silva J. M., Farinha A. P., Santos D., et al. (2013). “Abiotic stress responses in plants: Unraveling the complexity of genes and networks to survive,” in Abiotic Stress—Plant Responses and Applications in Agriculture. Eds. Vahdati K., Leslie C. (Rijeka, Croatia: InTech; ), 3–23. [Google Scholar]
- Eisalou A. V., Namdjoyan S., Soorki A. A. (2021). The combined effect of melatonin and salicylic acid improved the tolerance of safflower seedlings to zinc toxicity. Acta Physiologiae Plantarum 43, .1–14. doi: 10.1007/s11738-021-03311-4 [DOI] [Google Scholar]
- El-Mageed T. A., Semida W. M., Rady M. M. (2017). Moringa leaf extract as bio stimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manage. 193, 46–54. doi: 10.1016/j.agwat.2017.08.004 [DOI] [Google Scholar]
- Elsheery N. I., Helaly M. N., El-Hoseiny H. M., Alam-Eldein S. M. (2020). Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 10 (4), 558. doi: 10.3390/agronomy10040558 [DOI] [Google Scholar]
- Elvwan M. W. M., Hamahyomy M. A. M. (2009). Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci. Hortic. 122, 521–526. doi: 10.1016/j.scienta.2009.07.001 [DOI] [Google Scholar]
- Eraslan F., Inal A., Gunes A., Alpaslan M. (2007). Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hortic. 113, 120–128. doi: 10.1016/j.scienta.2007.03.012 [DOI] [Google Scholar]
- Eraslan F., Inal A., Pilbeam D. J., Gunes A. (2008). Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. cv. Matador) grown under boron toxicity and salinity. Plant Growth Regul. 55, 207–219. doi: 10.1007/s10725-008-9277-4 [DOI] [Google Scholar]
- Ervin E. H., Zhang X. Z., Fike J. H. (2004). Ultraviolet-B radiation damage on Kentucky Bluegrass II: hormone supplement effects. Hortic. Sci. 39, 1471–1474. doi: 10.21273/HORTSCI.39.6.1471 [DOI] [Google Scholar]
- FAO (2017). The future of food and agriculture – Trends and challenges (Rome: ). [Google Scholar]
- Feng B. H., Li G. Y., Islam M., Fu W. M., Zhou Y. Q., Chen T. T., et al. (2020). Strengthened antioxidant capacity improves photosynthesis by regulating stomatal aperture and ribulose-1, 5-bisphosphate carboxylase/oxygenase activity. Plant Sci. 290, 110245. doi: 10.1016/j.plantsci.2019.110245 [DOI] [PubMed] [Google Scholar]
- Fita A., Rodriguez-Burruezo A., Boscaiu M., Prohens J., Vicente O. (2015). Breeding and domesticating crops adapted to drought and salinity: a new paradigm for increasing food production. Front. Plant Sci. 6, 978. doi: 10.3389/fpls.2015.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D., Cape J. N. (1982). “Air pollutants in agriculture and horticulture,” in Effects of gaseous air pollution in agriculture and horticulture. Eds. Unsworth M. H., Ormrod D. P. (London: Butterworth Scientific; ), 3–26. [Google Scholar]
- Furness N., Upadhyaya M. K., Ormrod D. P. (1999). Seedling growth and leaf surface morphological responses of three rangeland weeds to ultraviolet-B radiation. Weed Sci. 47, 427–434. doi: 10.1017/S0043174500092031 [DOI] [Google Scholar]
- Gilani M., Danish S., Ahmed N., Rahi A. A., Akrem A., Younis U., et al. (2020). Mitigation of drought stress in spinach using individual and combined applications of salicylic acid and potassium. Pak. J. Bot. 52, 1505–1513. doi: 10.30848/PJB2020-5(18) [DOI] [Google Scholar]
- Gomes A. M. F., Nhantumbo N., Ferreira-Pinto M., Massinga R., Ramalho J. C., Ribeiro-Barros A. (2019). “Breeding elite cowpea [Vigna unguiculata (L.) walp] varieties for improved food security and income in Africa: opportunities and challenges,” in Legume Crops—Characterization and Breeding for Improved Food Security (London, UK: IntechOpen; ), 626–640. [Google Scholar]
- Gorni P. H., Brozulato M. O., Lourenção R. S., Konrad E. C. G. (2017). Increased biomass and salicylic acid elicitor activity in fennel (Foeniculum vulgare Miller). Braz. J. Food Technol. 20, e2016172. doi: 10.1590/1981-6723.17216 [DOI] [Google Scholar]
- Gorni P. H., Pacheco A. C. (2016). Growth promotion and elicitor activity of salicylic acid in Achillea millefolium L. Afr. J. Biotechnol. 15 (16), 657–665. Available at: https://www.ajol.info/index.php/ajb/article/view/134362 [Google Scholar]
- Gray W. M. (2004). Hormonal regulation of plant growth and development. PloS Biol. 2, e311. doi: 10.1371/journal.pbio.0020311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. M., Wilson M. I., Huang X. D., Duxbury C. L., Gerhaddt K. E., Gensemer R. W. (1997). “The effects of ultraviolet-B radiation on higher plants,” in Plants for environmental studies, vol. 1–35 . Eds. Wang W., Goursuch J., Hughes J. S. (Boca Raton, FL: CRC Press; ). [Google Scholar]
- Grulke N. E., Heath R. L. (2020). Ozone effects on plants in natural ecosystems. Plant Biol. 22 (S1), 12–37. doi: 10.1111/plb.12971 [DOI] [PubMed] [Google Scholar]
- Grunhage L., Jager H. J. (1994). Influence of the atmospheric conductivity on the ozone exposure of plants under ambient conditions: considerations for establishing ozone standards to protect vegetation. Environ. pollut. 85, 125–129. doi: 10.1016/0269-7491(94)90078-7 [DOI] [PubMed] [Google Scholar]
- Guo Y., Khan R. A. A., Xiong Y., Fan Z. (2022). Enhanced suppression of soil-borne phytopathogenic bacteria Ralstonia solanacearum in soil and promotion of tomato plant growth by synergetic effect of green synthesized nanoparticles and plant extract. J. App. Microbiol. 132 (5), 3694–3704. doi: 10.1111/jam.15459 [DOI] [PubMed] [Google Scholar]
- Harper J. R., Balke N. E. (1981). Characterization of the inhibition of K+ absorption in oats roots by salicylic acid. Plant Physiol. 68, 1349–1353. doi: 10.1104/pp.68.6.1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman M., Fugita M., Oku H., Islam M. T. (2019). Plant Tolerance to Environmental Stress: Role of Phytoprotectants. 1st ed (Boca Raton, FL, USA: CRC Press; ), 488. [Google Scholar]
- Hasegawa P. M., Bressan R. A., Zhu J. K., Bohnert H. J. (2000). Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. 51, 463–499. doi: 10.1146/annurev.arplant.51.1.463 [DOI] [PubMed] [Google Scholar]
- Hatfield J. L., Prueger J. H. (2015). Temperature extremes: effect on plant growth and development. Weather Clim. Extrem. 10, 4–10. doi: 10.1016/j.wace.2015.08.001 [DOI] [Google Scholar]
- Hawrylak-Nowak B., Matraszek R., Szymańska M. (2010). Selenium modifies the effect of short-term chilling stress on cucumber plants. Biol. Trace Elem. Res. 138, 307–315. doi: 10.1007/s12011-010-8613-5 [DOI] [PubMed] [Google Scholar]
- Hayat S., Hasan S. A., Fariduddin Q., Ahmad A. (2008). Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 3, 297–304. doi: 10.1080/17429140802320797 [DOI] [Google Scholar]
- Hayat Q., Hayat S., Irfan M., Ahmad A. (2010). Effect of exogenous salicylic acid under changing environment-a review. Environ. Experi. Bot. 68, 14–25. doi: 10.1016/j.envexpbot.2009.08.005 [DOI] [Google Scholar]
- Hedhly A., Hormaza J., Herrero M. (2009). Global warming and sexual plant reproduction. Trends Plant Sci. 14, 30–36. doi: 10.1016/j.tplants.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Herndon J. M., Hoisington R. D., Whiteside M. (2018). Deadly ultraviolet UV-C and UV-B penetration to Earth’s surface: human and environmental health implications. J. Geog. Environ. Earth Sci. Intn. 14 (2), 1–11. doi: 10.9734/jgeesi/2018/40245 [DOI] [Google Scholar]
- Hollósy F. (2002). Effects of ultraviolet radiation on plant cells. Micron. 33 (2), 179–197. doi: 10.1016/S0968-4328(01)00011-7 [DOI] [PubMed] [Google Scholar]
- Horváth E., Szalai G., Janda T. (2007). Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 26, 290–300. doi: 10.1007/s00344-007-9017-4 [DOI] [Google Scholar]
- Houston K., Tucker M. R., Chowdhury J., Shirley N., Little A. (2016). The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 7, 984. doi: 10.3389/fpls.2016.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. A., Khan P. R., Hegazy S. S., Hashim E. A., Azamal H., Ansari M. K. A., et al. (2015). Improving the phytoextraction capacity of plants to scavenge heavy-metal infested sites. Environ. Rev. 23, 1–22. [Google Scholar]
- Idrees M., Naeem M., Aftab T., Khan M. M. A. (2013). Salicylic acid restrains nickel toxicity, improves antioxidant defence system and enhances the production of anticancer alkaloids in Catharanthus roseus (L.). J. Hazard. Mat. 252, 367–374. doi: 10.1016/j.jhazmat.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) (2007). “Climate change 2007–The physical science basis,” in Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge, UK: Cambridge University Press; ). [Google Scholar]
- Jaishankar M., Tseten T., Anbalagan N., Mathew B. B., Beeregowda K. N. (2014). Toxicity, mechanism and health effects of some HMs. Interdiscip. Toxicol. 7 (2), 60–72. doi: 10.2478/intox-2014-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M. A., Greenberg B. M., Edelman M., Mattoo A. K., Gaba V. (1996). Accelerated degradation of the D2 protein of photosystem II under ultraviolet radiation. Photochem. Photobiol. 63 (6), 814 817. doi: 10.1111/j.1751-1097.1996.tb09636.x [DOI] [Google Scholar]
- Jia C., Zhang L., Liu L., Wang J., Li C., Wang Q. (2013). Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternate f. sp. lycopersici. J. Exp. Bot. 64 (2), 637–650. doi: 10.1093/jxb/ers360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Sharma P., Rathee S., Singh H. P., Batish D. R., Kohli R. K. (2021). Salicylic acid pre-treatment modulates Pb2+-induced DNA damage vis-à-vis oxidative stress in Allium cepa roots. Environ. Sci. Poll Res. 28, 51989–52000. doi: 10.1007/s11356-021-14151-7 [DOI] [PubMed] [Google Scholar]
- Kawano T., Bouteau F. (2013). Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Rep. 32 (7), 1125–1138. doi: 10.1007/s00299-013-1451-0 [DOI] [PubMed] [Google Scholar]
- Kaya C., Sarıoglu A., Ashraf M., AlYemeni M. N., Ahmad P. (2022). The combined supplementation of melatonin and salicylic acid effectively detoxifies arsenic toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants. Environ. Poll 297, 118727. doi: 10.1016/j.envpol.2021.118727 [DOI] [PubMed] [Google Scholar]
- Kazemi N., Khavari-Nejad R. A., Fahimi H., Saadatmand S., Nejad-Sattari T. (2010). Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci. Hortic. 126 (3), 402–407. doi: 10.1016/j.scienta.2010.07.037 [DOI] [Google Scholar]
- Kazemi M. (2014). Effect of foliar application with salicylic acid and methyl jasmonate on growth, flowering, yield and fruit quality of tomato. Bull. Environ. Pharmacol. Life Sci. 3, 154–158. doi: 10.12816/0031770 [DOI] [Google Scholar]
- Keutgen A. J., Pawelzik E. (2008). Influence of pre-harvest ozone exposure on quality of strawberry fruit under simulated retail conditions. Postharvest Biol. Tech. 49, 10–18. doi: 10.1016/j.postharvbio.2007.12.003 [DOI] [Google Scholar]
- Khalil R., Haroun S., Bassyoini F., Nagah A., Yusuf M. (2021). Salicylic acid in combination with kinetin or calcium ameliorates heavy metal stress in Phaseolus vulgaris plant. J. Agri Food Res. 5, 100182. doi: 10.1016/j.jafr.2021.100182 [DOI] [Google Scholar]
- Khan W., Prithiviraj B., Smith D. L. (2003). Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 160 (5), 485–492. doi: 10.1078/0176-1617-00865 [DOI] [PubMed] [Google Scholar]
- Khan N., Bano A., Ali S., Babar M. A. (2020). Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 90, 189–203. doi: 10.1007/s10725-020-00571-x [DOI] [Google Scholar]
- Khan M. I. R., Fatma M., Per T. S., Anjum N. A., Khan N. A. (2015). Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 6, 462. doi: 10.3389/fpls.2015.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S. K., Handa N., Bali S., Arora S., Sharma A., Kaur R., et al. (2018). Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol. Environ. Saf. 147, 382–393. doi: 10.1016/j.ecoenv.2017.08.051 [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Hamayun M., Khan A. L., Kang S. M., Han H. H., Lee I. J. (2009). Exogenous application of plant growth regulators increased the total flavonoid content in Taraxacum officinale (Wigg). Afr. J. Biotechnol. 8, 5727–5732. doi: 10.5897/AJB09.927 [DOI] [Google Scholar]
- Klessig D. F., Malamy J. (1994). The salicylic acid signal in plants. Plant Mol. Biol. 26, 1439–1458. doi: 10.1007/BF00016484 [DOI] [PubMed] [Google Scholar]
- Korkmaz A., Korkmaz Y., Demirkıran A. R. (2010). Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 67 (3), 495–501. doi: 10.1016/j.envexpbot.2009.07.009 [DOI] [Google Scholar]
- Kosová K., Vítámvás P., Prášil I. T., Renaut J. (2011). Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J. Proteom. 274, 1301–1322. doi: 10.1016/j.jprot.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Kreps J. A., Wu Y., Chang H. S., Zhu T., Wang X., Harper J. F. (2002). Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130, 2129–2141. doi: 10.1104/pp.008532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D. (2014). Salicylic acid signaling in disease resistance. Plant Sci. 228, 127–124. doi: 10.1016/j.plantsci.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Kumar A., Prasad M. N. V., Sytar O. (2012). Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangulare grown hydroponically. Chemosphere 89, 1056–1065. doi: 10.1016/j.chemosphere.2012.05.070 [DOI] [PubMed] [Google Scholar]
- Lajayer A. B., Ghorbanpour M., Nikabadi S. (2017). HMs in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 145, 377–390. doi: 10.1016/j.ecoenv.2017.07.035 [DOI] [PubMed] [Google Scholar]
- Lefohn A. S. (1992). “The characterization of ambient ozone exposures,” in Surface level ozone exposures and their effects on vegetation. Ed. Lefohn A. S. (Chelsea, MI: Lewis Publishers; ), 31–92. [Google Scholar]
- Leisner C. P., Ainsworth E. A. (2012). Quantifying the effects of ozone on plant reproductive growth and development. Glob. Change Biol. 18, 606–616. doi: 10.1111/j.1365-2486.2011.02535.x [DOI] [Google Scholar]
- Li Q., Wang G., Wang Y., Yang D., Guan C., Ji J. (2019). Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotoxicol. Environ. Saf. 172, 317–325. doi: 10.1016/j.ecoenv.2019.01.078 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H. K. (1998). The stress concept in plants: an introduction. Ann. NY Acad. Sci. 851, 187–198. doi: 10.1111/j.1749-6632.1998.tb08993. [DOI] [PubMed] [Google Scholar]
- Lippmann R., Babben S., Menger A., Delker C., Quint M. (2019). Development of wild and cultivated plants under global warming conditions. Curr. Biol. 29, R1326–R1338. doi: 10.1016/j.cub.2019.10.016 [DOI] [PubMed] [Google Scholar]
- Liu J., Qiu G., Liu C., Li H., Chen X., Fu Q., et al. (2022). Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life 12 (6), 886. doi: 10.3390/life12060886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavian K., Ghorbanli M., Kalantari K. (2008). The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annuum L. Turk. J. Bot. 32 (1), 25–33. doi: 10.1007/s10535-008-0037-0 [DOI] [Google Scholar]
- Mahmood R. (2006). In vitro effect of salt on the vigor of potato (Solanum tuberosum L.) plantlets. Int. J. Biotechnol. 1, 73–77. [Google Scholar]
- Manaa A., Gharbi E., Mimouni H., Wasti S., AschiSmiti S., Lutts S., et al. (2014). Simultaneous application of salicylic acid and calcium improves salt tolerance in two contrasting tomato (Solanum lycopersicum) cultivars. S. Afr. J. Bot. 95, 32–39. doi: 10.1016/j.sajb.2014.07.015 [DOI] [Google Scholar]
- Mantri N., Patade V., Penna S., Ford R. (2012). “Abiotic stress responses in plants: present and future,” in Abiotic Stress Responses in Plants. Eds. Ahmad P., Prasad M. N. V. (New York, NY: Springer; ), 1–19. doi: 10.1007/978-1-4614-0634-1_1 [DOI] [Google Scholar]
- Manzoor M., Naz S., Muhammad H. M. D., Ahmad R. (2023). Smart reprogramming of jujube germplasm against salinity tolerance through molecular tools. Funct. Integ. Genom. 23, 222. doi: 10.1007/s10142-023-01140-x [DOI] [PubMed] [Google Scholar]
- Masood A., Khan M. I., Fatma M., Asgher M., Per T. S., Khan N. A. (2016). Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol. Biochem. 104, 1–10. doi: 10.1016/j.plaphy.2016.03.017 [DOI] [PubMed] [Google Scholar]
- Matilla-Vazquez M. A., Matilla A. J. (2014). “Ethylene:Role in plants under environmental stress,” in Physiological mechanisms and adaptation strategies in plants under changing environment, vol. 2 . Eds. Ahmad P., Wani M. R. (New York: Springer; ), 189–222. [Google Scholar]
- Mavrič Čermelj A., Golob A., Vogel-Mikuš K., Germ M. (2021). Silicon mitigates negative impacts of drought and UV-b radiation in plants. Plants 11 (1), 91. doi: 10.3390/plants11010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills G., Wagg S., Harmens H. (2013) Ozone pollution: Impacts on ecosystem services and biodiversity. Available at: http://nora.nerc.ac.uk/id/eprint/502675/1/N502\penalty-\@M675CR.
- Miura K., Tada Y. (2014). Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C. C., Carvalho R. F., Grata˜o P. L., Carvalho G., Tezotto T., Medici L. O., et al. (2011). Biochemical responses of the ethylene-insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environ. Exp. Bot. 71, 306–320. doi: 10.1016/j.envexpbot.2010.12.020 [DOI] [Google Scholar]
- Moreno A. A., Orellana A. (2011). The physiological role of the unfolded protein response in plants. Biol. Res. 44, 75–80. doi: 10.4067/S0716-97602011000100010 [DOI] [PubMed] [Google Scholar]
- Moustafa-Farag M., Mohamed H. I., Mahmoud A., Elkelish A., Misra A. N., Guy K. M., et al. (2020). Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 9, 724. doi: 10.3390/plants9060724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D., Kumar R. (2007). Kinetin regulates plant growth and biochemical changes during maturation and senescence of leaves, flowers, and pods of Cajanus cajan (L.). Biol. Plant 50, 80–85. doi: 10.1007/s10535-007-0016-x [DOI] [Google Scholar]
- Munns R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25 (2), 239–250. doi: 10.1046/j.0016-8025.2001.00808.x [DOI] [PubMed] [Google Scholar]
- Munns R., Tester M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Naz S., Mushtaq A., Ali S., Muhammad H. M. D., Saddiq B., Ahmad R., et al. (2022). Foliar application of ascorbic acid enhances growth and yield of lettuce (Lactuca sativa) under saline conditions by improving antioxidant defence mechanism. Funct. Plant Biol. doi: 10.1071/FP22139 [DOI] [PubMed] [Google Scholar]
- Nazar R., Iqbal N., Sayeed S., Khan N. A. (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mung bean cultivars. J. Plant Physiol. 168, 807–815. doi: 10.1016/j.jplph.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Neale R. E., Barnes P. W., Robson T. M., Neale P. J., Williamson C. E., Zepp R. G., et al. (2021). Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP environmental effects assessment panel, update 2020. Photochem. Photobiol. Sci. 20 (1), 1–67. doi: 10.1007/s43630-020-00001-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrão S., Schmöckel S. M., Tester M. (2016). Evaluating physiological responses of plants to salinity stress. Ann. Bot. 119, 1–11. doi: 10.1093/aob/mcw191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama R., Watanabe Y., Fujita Y., Le D. T., Kojima M., Werner T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 23, 2169–2183. doi: 10.1105/tpc.111.087395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olien C. R., Smith M. N. (1997). Ice adhesions in relation to freeze stress. Plant Physiol. 60, 499–503. doi: 10.1104/pp.60.4.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira K. R., Junior J. P. S., Bennett S. J., Checchio M. V., de Cássia Alves R., Felisberto G., et al. (2020). Exogenous silicon and salicylic acid applications improve tolerance to boron toxicity in field pea cultivars by intensifying antioxidant defence systems. Ecotoxicol. Environ. Saf. 201, 110778. doi: 10.1016/j.ecoenv.2020.110778 [DOI] [PubMed] [Google Scholar]
- Orabi S. A., Dawood M. G., Saleem S. R. (2015). Comparative study between the physiological role of hydrogen peroxide and salicylic acid in alleviating low temperature stress on tomato plants grown under sandponic culture. Sci. Agric. 9, 49–59. doi: 10.15192/pscp.sa.2015.1.9.4959 [DOI] [Google Scholar]
- Orvar B. L., Sangwan V., Omann F., Dhindsra R. S. (2000). Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J. 23, 785–794. doi: 10.1046/j.1365-313x.2000.00845.x [DOI] [PubMed] [Google Scholar]
- Osuagwu G., Edeoga H., Osuagwu A. (2010). The influence of water stress (drought) on the mineral and vitamin potential of the leaves of Ocimum gratissimum (L). Recent Res. Sci. Technol. 2, 27–33. Available at: https://core.ac.uk/download/pdf/236007834.pdf [Google Scholar]
- Pacheco A. C., Cabral C. D. S., Fermino E. S., Aleman C. C. (2013). Salicylic acid-induced changes to growth, flowering and flavonoids production in marigold plants. J. Med. Plants Res. 7 (42), 3158–3163. Available at: https://citeseerx.ist.psu.edu/document?repid=rep1type=pdf&doi=e7b8c7e567c365897a6f45c4a221b6835dce68c3 [Google Scholar]
- Pan Q., Zhan J., Liu H., Zhang J., Chen J., Wen P., et al. (2006). Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci. 171, 226–233. doi: 10.1016/j.plantsci.2006.03.012 [DOI] [Google Scholar]
- Panuccio M. R., Sorgona A., Rizzo M., Cacco G. (2009). Cadmium adsorption on vermiculite, zeolite and pumice: batch experiment studies. J. Environ. Manage. 90, 364–374. doi: 10.1016/j.jenvman.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Pierart A., Shahid M., Séjalon-Delmas N., Dumat C. (2015). Antimony bioavailability: knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 289, 219–234. doi: 10.1016/j.jhazmat.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Piotrowska-Niczyporuk A., Bajguz A., Zambrzycka E., Godlewska-Żyłkiewicz B. (2012). Phytohormones as regulators of HM biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 52, 52–65. doi: 10.1016/j.plaphy.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Rabnawaz A., Ahmad R., Anjum M. A. (2020). Effect of seed priming on growth, flowering and cut flower quality of carnation. Indian J. Hortic. 77, 527–531. doi: 10.5958/0974-0112.2020.00076.6 [DOI] [Google Scholar]
- Rai V. K. (2002). Role of amino acids in plant responses to stresses. Biol. Plant 45, 481–487. doi: 10.1023/A:1022308229759 [DOI] [Google Scholar]
- Rawlings J. O., Lesser V. M., Heagle A. S., Heck W. W. (1988). Alternative ozone dose metrics to characterize ozone impact on crop yield. J. Environ. Qual. 17, 285–291. doi: 10.2134/jeq1988.00472425001700020021x [DOI] [Google Scholar]
- Rivas-San V. M., Plasencia J. (2011). Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 62 (10), 3321–3338. doi: 10.1093/jxb/err031 [DOI] [PubMed] [Google Scholar]
- Rodríguez M., Canales E., Borrás-Hidalgo O. (2005). Molecular aspects of abiotic stress in plants. Biotechnol. Appl. 22, 1–10. Available at: https://elfosscientiae.cigb.edu.cu/PDFs/Biotecnol20Apl/2005/22/1/BA002201RV001-010.pdf [Google Scholar]
- Ruelland E., Zachowski A. (2010). How plants sense temperature. Environ. Exp. Bot. 69, 225–232. doi: 10.1016/j.envexpbot.2010.05.011 [DOI] [Google Scholar]
- Runeckles V. C. (1974). Dosage of air pollutants and damage to vegetation. Environ. Conserv. 1, 305–308. doi: 10.1017/S0376892900004963 [DOI] [Google Scholar]
- Ryu H., Cho Y. G. (2015). Plant hormones in salt stress tolerance. J. Plant Biol. 58, 147–155. doi: 10.1007/s12374-015-0103-z [DOI] [Google Scholar]
- Saheri F., Barzin G., Pishkar L., Boojar M. M. A., Babaeekhou L. (2020). Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under Drought Stress. Biologia 75, 2189–2200. doi: 10.2478/s11756-020-00571-2 [DOI] [Google Scholar]
- Salla V., Hardaway C. J., Sneddon J. (2011). Preliminary investigation of Spartina alterniflora for phytoextraction of selected heavy metals in soils from Southwest Louisiana. Microchem. J. 97, 207–212. doi: 10.1016/j.microc.2010.09.005 [DOI] [Google Scholar]
- Sardar H., Nisar A., Anjum M. A., Naz S., Ejaz S., Ali S., et al. (2021). Foliar spray of moringa leaf extract improves growth and concentration of pigment, minerals and stevioside in stevia (Stevia rebaudiana Bertoni). Ind. Crops Products 166, 113485. doi: 10.1016/j.indcrop.2021.113485 [DOI] [Google Scholar]
- Sardar H., Ramzan M. A., Naz S., Ali S., Ejaz S., Ahmad R., et al. (2023). Exogenous application of melatonin improves the growth and productivity of two broccoli (Brassica oleracea L.) cultivars under salt stress. J. Plant Growth Regul. 42, 5152–5166. doi: 10.1007/s00344-023-10946-9 [DOI] [Google Scholar]
- Sardar H., Waqas M., Naz S., Ejaz S., Ali S., Ahmad R. (2022). Evaluation of different growing media based on agro-industrial waste materials for the morphological, biochemical and physiological characteristics of stevia. Cleaner Waste Syst. 3, 100038. doi: 10.1016/j.clwas.2022.100038 [DOI] [Google Scholar]
- Sayyari M., Ghanbari F., Fatahi S., Bavandpour F. (2013). Chilling tolerance improving of watermelon seedling by salicylic acid seed and foliar application. Not. Sci. Biol. 1, 67–73. doi: 10.15835/nsb518293 [DOI] [Google Scholar]
- Schöffl F., Prandl R., Reindl A. (1999). “Molecular responses to heat stress,” in Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants. Eds. Shinozaki K., Yamaguchi Shinozaki K. (Austin, TX, USA: R.G. Landes Co; ), 81–98. [Google Scholar]
- Schwartz M., Ahas R., Aasa A. (2006). Onset of spring starting earlier across the northern hemisphere. Glob. Change Biol. 12, 343–351. doi: 10.1111/j.1365-2486.2005.01097.x [DOI] [Google Scholar]
- Semida W. M., Abd El-Mageed T. A., Mohamed S. E., El-Sawah N. A. (2017). Combined effect of deficit irrigation and foliar-applied salicylic acid on physiological responses, yield, and water-use efficiency of onion plants in saline calcareous soil. Arch. Agron. Soil Sci. 63, 1227–1239. doi: 10.1080/03650340.2016.1264579 [DOI] [Google Scholar]
- Semida W. M., Taha R. S., Abdelhamid M. T., Rady M. M. (2014). Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 95, 24–31. doi: 10.1016/j.sajb.2014.08.005 [DOI] [Google Scholar]
- Shahid M., Dumat C., Khalid S., Schreck E., Xiong T., Niazi N. K. (2017). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 325, 36–58. doi: 10.1016/j.jhazmat.2016.11.063 [DOI] [PubMed] [Google Scholar]
- Shahid M., Khalid S., Abbas G., Shahid N., Nadeem M., Sabir M., et al. (2015). “Heavy metal stress and crop productivity,” in Crop Production and Global Environmental Issues. Ed. Hakeem K. R. (Cham: Springer International Publishing; ), 1–25. [Google Scholar]
- Shama M., A. M., Moussa S., I Abo El Fadel. N. (2016). Salicylic acid efficacy on resistance of garlic plants (Allium sativum, L.) to water salinity stress on growth, yield and its quality. Alexandria Sci. Exchange J. 37 (April-June), 165–174. doi: 10.21608/asejaiqjsae.2016.2238 [DOI] [Google Scholar]
- Sharma A., Ruiz-Manriquez L. M., Serrano-Cano F. I., Reyes-Pérez P. R., Tovar Alfaro C. K., Barrón Andrade Y. E., et al. (2020). Identification of microRNAs and their expression in leaf tissues of guava (Psidium guajava L.) under salinity stress. Agronomy 10 (12), 1920. doi: 10.3390/agronomy10121920 [DOI] [Google Scholar]
- Shi Q., Bao Z., Zhu Z., Ying Q., Qian Q. (2006). Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 48, 127–135. doi: 10.1007/s10725-005-5482-6 [DOI] [Google Scholar]
- Shi Q., Zhu Z. (2008). Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ. Exp. Bot. 63, 317–326. doi: 10.1016/j.envexpbot.2007.11.003 [DOI] [Google Scholar]
- Sirisuntornlak N., Ghafoori S., Datta A., Arirob W. (2019). Seed priming and soil incorporation with silicon influence growth and yield of maize under water-deficit Stress. Arch. Agron. Soil Sci. 65, 197–207. doi: 10.1080/03650340.2018.1492713 [DOI] [Google Scholar]
- Skottke K. R., Yoon G. M., Kieber J. J., DeLong A. (2011). Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PloS Genet. 7 (4), e1001370. doi: 10.1371/annotation/b4fc15d6-b3ae-4fbb-8d88-b7d674a79697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama I., Ghnaya T., Savoure A., Abdelly C. (2008). Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum . C. R. Biol. 331, 442–451. doi: 10.1016/j.crvi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Soltani Maivan E., Radjabian T., Abrishamchi P., Talei D. (2017). “Physiological and biochemical responses of Melissa officinalis L. @ to nickel stress and the protective role of salicylic acid,” Arch. Agron. Soil Sci., vol. 63. , 330–343. [Google Scholar]
- Song W. Y., Yang H. C., Shao H. B., Zheng A. Z., Brestic M. (2014). The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by heavy metals. Clean - Soil Air Water. 42 (1), 88–97. doi: 10.1002/clen.201200310 [DOI] [Google Scholar]
- Souza L. A., Monteiro C. C., Carvalho R. F., Gratão P. L., Azevedo R. A. (2017). Dealing with abiotic stresses: an integrative view of how phytohormones control abiotic stress-induced oxidative stress. Theor. Exp. Plant Physiol. 29, 109–127. doi: 10.1007/s40626-017-0088-8 [DOI] [Google Scholar]
- Srivastava M. K., Dwivedi U. N. (2000). Delaying ripening of banana fruits by salicylic acid. Plant Sci. 158, 87–96. doi: 10.1016/S0168-9452(00)00304-6 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Nagasuga K., Okada M. (2008). The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol. 49, 433–442. doi: 10.1093/pcp/pcn020 [DOI] [PubMed] [Google Scholar]
- Szilagyi L. (2003). Influence of drought on seed yield components in common bean. Bulg. J. Plant, 320-330. Available at: http://www.bio21.bas.bg/ippg/bg/wp-content/uploads/2011/06/03_essa_320-330.pdf [Google Scholar]
- Talabany N. K., Albarzinji I. M. (2023). Effects of Salicylic Acid on some Growth and Physiological Characteristics of Lettuce (Lactuca sativa L.) under Cadmium Stress Conditions. Sci. J. Univ. Zakho 11 (1), 37–44. doi: 10.25271/sjuoz.2023.11.1.987 [DOI] [Google Scholar]
- Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J. (2012). HM toxicity and the environment. EXS 101, 133–164. doi: 10.1007/978-3-7643-8340-4_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti V. B., Muhlenbock P., Van Breusegem F. (2012). Stress homeostasis-the redox and auxin perspective. Plant Cell Environ. 35, 321–333. doi: 10.1111/j.1365-3040.2011.02324.x [DOI] [PubMed] [Google Scholar]
- Ullah H., Luc P. D., Gautam A., Datta A. (2018). Growth, yield and silicon uptake of rice (Oryza sativa) as influenced by dose and timing of silicon application under water-deficit stress. Arch. Agron. Soil Sci. 64, 318–330. doi: 10.1080/03650340.2017.1350782 [DOI] [Google Scholar]
- Uzu G., Sauvain J. J., Baeza-Squiban A., Riediker M., Hohl M. S. S., Val S., et al. (2011). In vitro assessment of the pulmonary toxicity and gastric availability of lead rich particles from a lead recycling plant. Environ. Sci. Technol. 45, 7888–7895. doi: 10.1021/es200374c [DOI] [PubMed] [Google Scholar]
- Vlot A. C., Dempsey M. A., Klessig D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 47, 177–206. doi: 10.1146/annurev.phyto.050908.135202 [DOI] [PubMed] [Google Scholar]
- Wahid A., Gelani S., Ashraf M., Foolad M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011 [DOI] [Google Scholar]
- Wang L., Fan L., Loescher W., Duan W., Liu G., Cheng J., et al. (2010). Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 10 (1), 34. doi: 10.1186/1471-2229-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. J., Li S. H. (2006). Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 170, 685–694. doi: 10.1016/j.plantsci.2005.09.005 [DOI] [Google Scholar]
- Waqas M. A., Wang X., Zafar S. A., Noor M. A., Hussain H. A., Azher Nawaz M., et al. (2021). Thermal stresses in maize: effects and management strategies. Plants 10, 293. doi: 10.3390/plants10020293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T., Lv X., Jia H., Hua L., Xu H., Zhou R., et al. (2018). Effects of salicylic acid, Fe (II) and plant growth-promoting bacteria on Cd accumulation and toxicity alleviation of Cd tolerant and sensitive tomato genotypes. J. Environ. Manage. 214, 164–171. doi: 10.1016/j.jenvman.2018.02.100 [DOI] [PubMed] [Google Scholar]
- Weih M., Johanson U., Gwynn-Jones D. (1998). Growth and nitrogen utilization in seedlings of mountain birch (Betula pubescens ssp. tortuosa) as affected by ultraviolet radiation (UV-A and UV-B) under laboratory and outdoor conditions trees. Struct. Funct. 12, 201–204. doi: 10.1007/PL00009711 [DOI] [Google Scholar]
- Wu H. (2018). Plant salt tolerance and Na+ sensing and transport. Crop J. 6, 215–225. doi: 10.1016/j.cj.2018.01.003 [DOI] [Google Scholar]
- Xu L. L., Fan Z. Y., Dong Y. J., Kong J., Bai X. Y. (2015. a). Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol. Plant 59, 171–182. doi: 10.1007/s10535-014-0475-9 [DOI] [Google Scholar]
- Xu Z., Jiang Y., Zhou G. (2015. b). Response and adaptation of photosynthesis, respiration, and anti-oxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 6, 701. doi: 10.3389/fpls.2015.00701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. X., Mao J., Chen W., Qian T. T., Liu S. C., Hao W. J., et al. (2016). Identification and expression profiling of the auxin response factors (ARFs) in the tea plant (Camellia sinensis (L.) under various abiotic stresses. Plant Physiol. Biochem. 98, 46–56. doi: 10.1016/j.plaphy.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Yadav S. (2010). Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 30, 515–527. doi: 10.1051/agro/2009050 [DOI] [Google Scholar]
- Yalpani N., Enyedi A. J., Leon J., Raskin I. (1994). Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193, 372–376. doi: 10.1007/BF00201815 [DOI] [Google Scholar]
- Yang Y., Guo Y. (2018. a). Elucidating the molecular mechanisms mediating plant salt stress responses. New Phytol. 217, 523–539. doi: 10.1111/nph.14920 [DOI] [PubMed] [Google Scholar]
- Yang Y., Guo Y. (2018. b). Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 60, 796–804. doi: 10.1111/jipb.12689 [DOI] [PubMed] [Google Scholar]
- Yang L., Zhang Z., Hu X., You L., Khan R. A. A., Yu Y. (2022). Phenolic contents, organic acids, and the antioxidant and bio activity of wild medicinal Berberis plants-as sustainable sources of functional food. Molecules 27 (8), 2497. doi: 10.3390/molecules27082497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X., Guo Y., Khan R. A. A., Fan Z. (2021). Understanding the pathogenicity of Pochonia chlamydosporia to root knot nematode through omics approaches and action mechanism. Biol. Control 162, 104726. doi: 10.1016/j.biocontrol.2021.104726 [DOI] [Google Scholar]
- Yu O. Y., Raichle B., Sink S. (2013). Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 4 (1), 44. doi: 10.1186/2251-6832-4-44 [DOI] [Google Scholar]
- Yuan S., Lin H. H. (2008). Role of salicylic acid in plant abiotic stress. Z. Naturforsch. C. J. Biosci. 63, 313–320. doi: 10.1515/znc-2008-5-601 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lan M., Han X., Wu J., Wang-Pruski G. (2020). Response of ornamental pepper to high-temperature stress and role of exogenous salicylic acid in mitigating high temperature. J. Plant Growth Reg. 39, 133–146. doi: 10.1007/s00344-019-09969-y [DOI] [Google Scholar]
- Zhang Y., Mian M. A. R., Bouton J. H. (2006). Recent molecular and genomic studies on stress tolerance of forage and turf grasses. Crop Sci. 46, 497–511. doi: 10.2135/cropsci2004.0572 [DOI] [Google Scholar]
- Zhu J. K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273. doi: 10.1146/annurev.arplant.53.091401.143329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. K. (2007). Plant Salt Stress (Newyork: John Wiley & Sons, Ltd; ). [Google Scholar]
- Zhu J. K. (2016). Abiotic stress signaling and responses in plants. Cell 167, 313–324. doi: 10.1016/j.cell.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X. F., Jiang T., Wang Z. W., Lei G. J., Shi Y. Z., Li G. X., et al. (2012). Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana . J. Hazard. Mater. 239–240, 302–307. doi: 10.1016/j.jhazmat.2012.08.077 [DOI] [PubMed] [Google Scholar]