Abstract
This article comments on:
Mutinda S, Mobegi FM, Hale B, Dayou O, Ateka E, Wijeratne A, Wicke S, Bellis ES, Runo S. 2023. Resolving intergenotypic Striga resistance in sorghum. Journal of Experimental Botany 74, 5294–5306.
Keywords: Cell type-specific defence, cell wall-based resistance, hypersensitive response, inducible defence, lignin, parasitic plants, post-attachment resistance, Striga
Parasitic plants of the Striga species significantly damage cereals in sub-Saharan Africa. Current agricultural practices are insufficient to manage Striga infestation, necessitating sustainable approaches that harness natural resistance mechanisms. Mutinda et al. (2023) examined how different genotypes of sorghum plants resist Striga after it attacks their roots. By comparing transcriptomes, they found that sorghum activates its immune system, and molecular signatures probably associate with distinct resistance mechanisms. This study will inform the development of Striga-resistant sorghum varieties to ward off root parasitic plants.
Striga species are notorious witchweeds
Striga species, commonly named witchweeds, parasitize monocot crops, such as sorghum, maize, rice, and millet (David et al., 2022), threatening food security for nearly 300 million people in sub-Saharan Africa (Ejeta, 2007). With a specialized organ called a haustorium, Striga forms xylem–xylem connections to ‘steal’ water and nutrients from its host, which drastically hampers crop growth, sometimes leading to complete eradication of crop fields and up to 100% yield losses (Stanley et al., 2021; David et al., 2022).
The Striga life cycle depends on host-derived signals: strigolactones trigger Striga seed germination (Bouwmeester et al., 2007; Ejeta, 2007; Kountche et al., 2019; Bunsick et al., 2020, 2022, Preprint), while haustorium-inducing factors enable formation of the penetrative structure from the Striga radicle (Bandaranayake et al., 2010). Each Striga plant produces up to 0.5 million seeds, which can remain viable in the soil for 20 years (Yoneyama et al., 2010; David et al., 2022). The extremely small size of Striga seeds allows effective seed dispersal. Their outcrossing nature further maintains high genetic diversity, enabling rapid Striga adaptation to the host resistance mechanisms. These genetic characteristics make Striga challenging to eradicate.
Unveiling modes and mechanisms of post-attachment resistance
Resistance to Striga can occur before or after Striga attaches to the root. The mechanisms of pre-attachment resistance are relatively well understood (Fishman and Shirasu, 2021; Jhu and Sinha, 2022) and we introduce them in Box 1. Distinct modes of post-attachment resistance and defence mechanisms are described in Box 2. In their recent study, Mutinda and collaborators focused on likely molecular mechanisms associated with two post-attachment resistance modes: mechanical barriers and a hypersensitive response (HR).
Box 1. Host plant pre-attachment resistance to root parasitic plants.
Pre-attachment resistance refers to the strategies employed by host plants to deter or limit the number of germinated seeds to reduce the number of attachments and consequently invasion of parasitic plants (Fishman and Shirasu, 2021; Jhu and Sinha, 2022). Several mechanisms have been identified in host plants to resist root parasitic plants:
Reducing seed germination rate
The seed germination process of Striga species is triggered by the presence of strigolactones (SLs), plant hormones commonly found in the root exudates of host plants. However, when mutations occur in genes responsible for SL biosynthesis or alterations in SL composition, germination rates of Striga seeds can be significantly reduced (Fig. 1A). One example of this is observed in mutations affecting the carotenoid cleavage dioxygenase 8 (ccd8) gene, which is involved in SL biosynthesis (Gomez-Roldan et al., 2008; Umehara et al., 2008). Such mutations result in a deficiency of SLs, leading to lower germination rates in parasitic plants. Additionally, mutations in the LOW GERMINATION STIMULANT 1 (LGS1) gene in resistant sorghum genotypes cause alterations in the composition of SLs present in root exudates, consequently reducing the stimulatory effect of germination on Striga (Fig. 1A) (Gobena et al., 2017).
Toxic compound secretion
Some host plants produce toxic compounds in their root exudates that inhibit the germination or development of parasitic plant seedlings (Serghini et al., 2001; Echevarría-Zomeño et al., 2006). For instance, certain resistant sunflower varieties secrete 7-hydroxylated simple coumarins, which create a toxic and hostile environment for the root-parasitic plant Orobanche (Serghini et al., 2001). Germinated Orobanche cernua seeds near resistant sunflowers exhibit browning symptoms, stunted growth, or even die (Fig. 1B).
Reducing haustorium initiation
Haustorium initiation is a crucial step for parasitic plants to establish a connection with the host plant. Multiple studies have highlighted the importance of host-derived haustorium-inducing factors (HIFs) in root-parasitic plants for this initiation process (Kokla and Melnyk, 2018). In response, host plants can exhibit resistance by interfering with the induction of HIFs or disrupting the signalling pathways involved (Rich et al., 2004) (Fig. 1B). Previous studies suggest that certain genotypes of sorghum have a limited ability to initiate the formation of Striga asiatica haustoria (Rich et al., 2004). This could also be attributed to the release of substances in the host root exudate that inhibit the induction of haustoria (Keyes et al., 2000).
These findings highlight diverse strategies of host plants to defend against Striga and other root parasitic plants. Understanding the mechanisms of pre-attachment resistance opens up possibilities for developing novel approaches to combat Striga infestations and improve crop productivity. Further research is necessary to explore the full potential of these mechanisms for their applicability in sustainable agriculture.
Box 2. Role of the defence mechanisms in post-attachment resistance against parasitic plants.
Natural variation in sorghum cultivar resistance to Striga can be used to elucidate target pathways to enhance resistance. One can then deploy gene editing of the target gene(s) or pathway(s) within susceptible cultivars to facilitate host resistance. Recent studies have indicated that when attacked by root parasitic plants, such as Striga or Orobanche, host plant cells can trigger the immune response, resembling gene-for-gene interactions between hosts and pathogens such as microbes or insects (Fishman and Shirasu, 2021; Jhu and Sinha, 2022). Initially, pathogen-triggered immunity (PTI) is induced upon Striga detection, which activates mechanical and biochemical defences in host plant cells. However, Striga suppresses PTI and promotes parasitism by injecting effector-like molecules into host plant cells (Li and Timko, 2009). If the host is resistant, effector-triggered immunity (ETI) is activated, leading to programmed cell death, preventing further parasitic growth.
Previously, diverse modes of post-attachment resistance—including mechanical-based resistance and a likely HR—were found in a variety of sorghum cultivars (Kavuluko et al., 2021). Collectively, these show a mechanical barrier-type resistance where increased cell wall thickness could prevent Striga ingress, a HR with localized cell necrosis at the host–parasite junction, or deposition of secondary metabolites such as polyphenols. Since these represent a typical onset of an immune response, it is likely that these mechanisms can be attributed to PTI, ETI, or both.
Mutinda et al. investigated five sorghum genotypes with documented Striga post-attachment resistance phenotypes (Kavuluko et al., 2021) and revealed genotype-specific gene expression signatures underlying two major resistance mechanisms. Biological processes enriched within these molecular signatures were used to classify responses to Striga into pattern-triggered immunity (PTI) and effector-triggered immunity (ETI) in these five cultivars (Mutinda et al., 2023).
To date, it was unclear whether a sorghum genotype could possess PTI and ETI simultaneously. Mutinda et al. discovered that downstream transcriptional responses of PTI and ETI could co-exist within a single genotype, although some genotypes probably rely predominantly on only one mode of defence. This discovery explains previously observed differences in the efficiency of inducing HR and changes in cell wall composition (Kavuluko et al., 2021). HRs were observed at 90% of the host–parasite contact sites within the root system, while the formation of a cell wall-based barrier was observed in only 50% of Striga attachment sites of a given root system, depending on the sorghum genotype. Therefore, certain resistant sorghum genotypes may have evolved the ability to deploy multiple forms of resistance, enhancing their defence mechanisms and increasing their resilience against root-parasitic plants.
Mutinda et al. further compiled candidate gene lists for PTI and ETI, which revealed the up-regulation of several sorghum genes whose homologues are associated with HRs and systemic acquired resistance (SAR) pathways in other species, including a pathogenesis-related thaumatin-like gene. They hypothesized that sorghum recognizes specific molecules released during Striga infection, such as damage-associated pathogen patterns (DAMPs), and then triggers downstream HRs and mechanical barrier resistance.
Hypersensitive responses (HRs)
HRs are a well-known defence mechanism in plants, characterized by localized cell death and necrosis at the site of pathogen infection. The observations of localized necrosis upon Striga infection in sorghum cultivars IS14963 and IS41724 support an HR. Similar HR mechanisms have been observed in the Striga resistance responses of several other host species where haustorium penetration is disrupted (Fig. 1C) (Li and Timko, 2009; Fishman and Shirasu, 2021; Jhu and Sinha, 2022). For example, the rice cultivar Nipponbare, which exhibits strong post-attachment resistance, up-regulates a HR-induced protein upon Striga infection (Swarbrick et al., 2008). Similarly, one resistant cowpea cultivar detects unidentified signals from Striga gesnerioides, potentially pathogen-associated molecular patterns (PAMPs) or DAMPs, or avirulence (Avr) proteins, triggering activation of a positive regulator of HR (Li and Timko, 2009). This activation leads to localized necrosis upon infestation by S. gesnerioides, providing post-attachment resistance in cowpea. However, a specific race of S. gesnerioides can overcome this defence response by secreting a small effector that inhibits the positive regulator of HR, resulting in rendering the resistant host cultivar susceptible (Li and Timko, 2009). Critical to any HR is a host gene which has co-evolved with a respective pathogen to detect a pathogen effector via a specific receptor. This interaction initiates signal transduction cascades, culminating in an HR (Fig. 1C). The target receptors in these sorghum cultivars remain to be determined, although there was evidence of several candidate nucleotide-binding site leucine-rich repeat (NBS-LRR) genes up-regulated in IS9830 and N13.
Fig. 1.
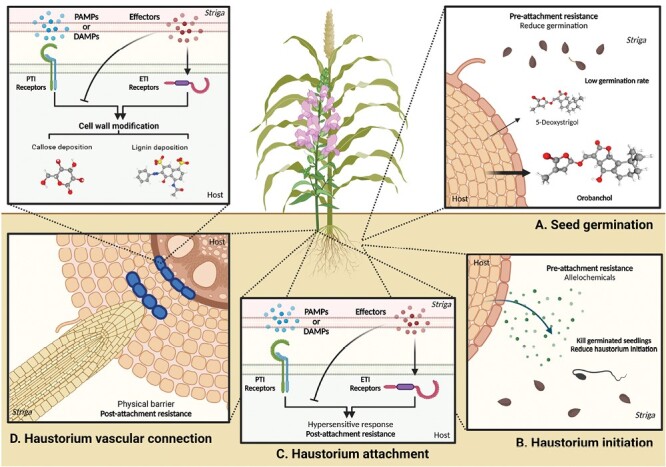
Host resistance responses during different stages of the Striga life cycle. (A) Pre-attachment resistance response during Striga seed germination. Host plants growing in nutrient-poor soil release strigolactones, promoting beneficial arbuscular mycorrhizal fungus symbiosis. Striga seeds perceive these host strigolactones as germination stimulants. However, mutations in genes responsible for strigolactone biosynthesis or alterations in their composition significantly reduce Striga seed germination rates. For example, mutations in the LOW GERMINATION STIMULANT 1 (LGS1) gene in resistant sorghum plants alter the composition of strigolactones in root exudates, reducing their stimulatory effect on Striga germination. (B) Pre-attachment resistance response during haustorium initiation. Germinated Striga seedlings grow towards host roots and perceive haustorium induction factors (HIFs) for haustorium initiation. Resistant host plants produce toxic compounds in root exudates that inhibit the development of parasitic plant seedlings (Box 1). Some resistant host plants produce lower levels of HIFs, reducing Striga haustorium formation (Box 1). (C) Post-attachment resistance response during haustorium attachment. Following the detection of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) from Striga, plants initiate pattern-triggered immunity (PTI) to obstruct haustorium attachment. However, parasitic plant effectors can suppress PTI to facilitate parasitism. Consequently, effector-triggered immunity (ETI) overcomes this suppression and triggers hypersensitive responses (HRs) to discourage parasite penetration. (D) Post-attachment resistance response during haustorium vascular connection. Plants fortify cell walls to create physical barriers that hinder the establishment of vascular connections. According to the study by Mutinda et al., cell wall enhancement-based resistance responses probably occur downstream of PTI and ETI. Examples of these barriers include accumulating substances such as lignin or callose in the cortex, impeding the progress of parasites. Moreover, the endodermis serves as a barrier by inducing lignin accumulation, effectively preventing parasitic plant penetration and vascular connection. More details are described in Fig. 2. Three-dimensional structure images of orobanchol (Compound CID: 10665247), 5-deoxystrigol (Compound CID: 15102684), lignin (Compound CID: 175586), and callose (beta-d-glucose, Compound CID: 64689) are exported from PubChem. This figure was created with https://www.biorender.com/.
Cell wall modifications
Many resistant host species modify their cell wall composition to enhance this physical barrier against Striga, often through lignin deposition. Mutinda et al. emphasized the significance of the lignin biosynthesis pathway in Striga resistance, observing that genotypes with cell wall-based resistance showed up-regulation of essential lignin biosynthesis genes (Fig. 1D). This finding is consistent with previous studies conducted on Striga-infected cowpea and rice (Huang et al., 2012; Mutuku et al., 2019), further highlighting the role of lignin biosynthesis in conferring resistance (Yoshida and Shirasu, 2009).
In addition to lignification, callose deposition is another defence mechanism employed by plants that strengthens the plant cell wall to resist pathogen infections (Bacete et al., 2018). Mutinda et al. identified a gene called Glucan Synthase-Like 10 (GSL10), which catalyses callose production. This discovery aligns with previous studies highlighting the importance of callose deposition in resisting attacks from other parasitic plant–host systems (Fig. 1D), although callose remains to be observed within the sorghum cultivars characterized.
Future research and perspectives
Overall, Mutinda et al. shed light on the transcriptional basis of observed sorghum resistance mechanisms against Striga and identified candidate genes for further enhancing sorghum through breeding or genetic modifications. Future research should focus on localized, inducible modes of resistance. The transcriptome changes should be assessed specifically at the attachment sites, comparing responses at successful and blocked attachment sites. Finally, it is vital to acknowledge the practical challenges of implementing host resistant varieties and promote integrated management approaches. We propose future research directions for integrated crop management to enhance crop improvement.
Inducible defence responses
Inducible defence responses activated upon pathogen detection allow a plant to allocate resources efficiently and to enhance survival and reproductive success (Shudo and Iwasa, 2001). Known post-attachment resistances types are mainly inducible mechanisms triggered by the presence of parasitic plants (Fishman and Shirasu, 2021; Jhu and Sinha, 2022; Jhu et al., 2022). This interaction between the host and parasitic plant might direct the co-evolution of resistance mechanisms and lead to different sorghum genotypes domesticated in different regions of Africa exhibiting various gene expression profiles and resistance responses (Kavuluko et al., 2021; Mutinda et al., 2023).
Constitutive defence mechanisms could hamper crop growth. For example, knocking out a negative regulator in resistant tomato cultivars leads to constitutive lignin accumulation, increasing resistance to Cuscuta but stunting vegetative growth (Jhu et al., 2022). Thus, genetic engineering and breeding towards Striga resistance should focus on inducible defence responses (Gurr and Rushton, 2005).
Cell type- or tissue-specific defences
Cell type-specific barriers and defence mechanisms are crucial for plants to resist root-parasitic plant invasion (Hu et al., 2020; Kawa and Brady, 2022, preprint). Epidermal phenolic compounds physically redirect or impede parasitic plant growth, while lignin or callose barriers in the cortex hinder parasite progression (Fig. 2) (Yoshida and Shirasu, 2009; Yoder and Scholes, 2010). Increased accumulation of lignin or other phenolic compounds and silica deposition in the endodermis prevent the parasitic plant from reaching the vasculature, while reinforcing xylem vessels with additional lignin restricts establishment of xylem–xylem connections (Fig. 2) (Yoshida and Shirasu, 2009; Mutuku et al., 2019). These cell type-specific defence mechanisms play a crucial role in preventing the invasion and establishment of parasitic plants. Localized defence mechanisms at the parasite–root interface minimize energy costs and unintended effects. New technologies such as single-cell RNA-sequencing or spatial transcriptomics could yield more comprehensive information on the spatiotemporal patterns of regulation of the post-attachment resistance. Incorporating cell-specific defences into Striga resistance breeding programmes could enable the development of crop cultivars with enhanced protection.
Fig. 2.

Cell type-specific barriers and defence mechanisms safeguarding host plants against root-parasitic plant invasion. Plant cell type-specific barriers and defence mechanisms play a vital role in protecting plants against root-parasitic plant invasion. Notable examples of these protective barriers include: (A) concentrated accumulation of phenolic compounds in the epidermis and endodermis, (B) localized deposition of lignin in the cortex and endodermis, (C) targeted accumulation of silica in the endodermis, and (D) confined build up of callose in the cortex. Three-dimensional structure images of phenolic compounds (4-hydroxycinnamic-acid, Compound CID: 637542), lignin (Compound CID: 175586), silica (silicon dioxide, Compound CID: 24261) and callose (beta-d-glucose, Compound CID: 64689) are exported from PubChem. This figure is created with https://www.biorender.com/.
Integrated management of Striga
To effectively control Striga, an integrated approach is recommended, combining cultural practices, chemical control, breeding solutions, and bioinoculants. Strategies such as intercropping, water management, crop rotation, trap crops, and fertilization have been employed to manage Striga (David et al., 2022). Chemical compounds that mimic strigolactone action, known as suicidal germination agents, offer promise in allowing Striga to sprout without a host before crop planting (Kountche et al., 2019). Fungal isolates pathogenic to Striga have been successfully used in maize fields to reduce Striga infection (Nzioki et al., 2016). Bacterial species degrading haustorium-inducing factors or inducing mechanical barriers in sorghum roots have been isolated (Kawa et al., 2022, Preprint). Additionally, using beneficial microorganisms as bioinoculants can promote plant health and inhibit Striga growth and attachment (Jamil et al., 2021; Abdullahi et al., 2022).
Combining two or more methods has shown significant results. For instance, combining host resistance with a beneficial fungus that produces myco-herbicides has considerably reduced Striga (Bàrberi, 2019). The combination of phosphate fertilizer, a resistant host variety, and rhizobium inoculation reduced Striga and increased the grain yield of cowpea (Abdullahi et al., 2022; David et al., 2022). Intercropping maize cultivars that are Striga resistant and herbicide resistant with legume crops has suppressed Striga seed germination and reduced the Striga seed bank in the soil (Kanampiu et al., 2018). Utilizing host plant varieties with pre-attachment resistance could induce suicidal germination in Striga and therefore be an effective approach for reduction of Striga seed banks (Kountche et al., 2019). Furthermore, when combined with germination stimulants, cereal–legume crop rotation can significantly reduce Striga seeds in the soil (Jamil et al., 2021). These results demonstrate that to achieve effective and sustainable Striga control, an integrated approach is recommended (Mwangangi et al., 2021; Abdullahi et al., 2022).
Acknowledgements
We thank Eli Marable for feedback on the early draft of this manuscript.
Contributor Information
Min-Yao Jhu, Crop Science Centre, Department of Plant Sciences, University of Cambridge, Cambridge, UK.
Dorota Kawa, Department of Plant Biology and Genome Center, University of California, Davis, CA, USA; Plant Stress Resilience, Department of Biology, Utrecht University, The Netherlands; Plant Environment Signaling, Department of Biology, Utrecht University, The Netherlands.
Siobhán M Brady, Department of Plant Biology and Genome Center, University of California, Davis, CA, USA.
Conflict of interest
The authors declare that no commercial or financial relationships were present during the research that could be perceived as a potential conflict of interest.
Funding
MYJ is supported by the Bill and Melinda Gates Foundation and the UK Foreign, Commonwealth and Development Office (OPP1028264) through Engineering the Nitrogen Symbiosis for Africa (ENSA) project. DK and SMB acknowledge support from the Bill and Melinda Gates Foundation, Seattle, WA via grant OPP1082853 ‘RSM Systems Biology for Sorghum’. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abdullahi WM, Dianda M, Boukar O, et al. 2022. Integrated management of Striga gesnerioides in cowpea using resistant varieties, improved crop nutrition and rhizobium inoculants. Plant and Soil 473, 197–213. [Google Scholar]
- Bacete L, Mélida H, Miedes E, Molina A.. 2018. Plant cell wall-mediated immunity: cell wall changes trigger disease resistance responses. The Plant Journal 93, 614–636. [DOI] [PubMed] [Google Scholar]
- Bandaranayake PCG, Filappova T, Tomilov A, Tomilova NB, Jamison-Mcclung D, Ngo Q, Inoue K, Yoder JI.. 2010. A single-electron reducing quinone oxidoreductase is necessary to induce haustorium development in the root parasitic plant Triphysaria. The Plant Cell 22, 1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bàrberi P. 2019. Ecological weed management in Sub-Saharan Africa: prospects and implications on other agroecosystem services. Advances in Agronomy 156, 219–264. [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G.. 2007. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends in Plant Science 12, 224–230. [DOI] [PubMed] [Google Scholar]
- Bunsick M, Toh S, Wong C, et al. 2020. SMAX1-dependent seed germination bypasses GA signalling in Arabidopsis and Striga. Nature Plants 6, 646–652. [DOI] [PubMed] [Google Scholar]
- Bunsick M, Xu Z, Pescetto G, Ly G, Hountalas J, Boyer F-D, McErlean CSP, Scholes JD, Lumba S.. 2022. HTL/KAI2 signalling substitutes for light to control plant germination. bioRxiv. 10.1101/2022.03.30.486460. [Preprint]. [DOI]
- David OG, Ayangbenro AS, Odhiambo JJO, Babalola OO.. 2022. Striga hermonthica: a highly destructive pathogen in maize production. Environmental Challenges 8, 100590. [Google Scholar]
- Echevarría-Zomeño S, Pérez-De-Luque A, Jorrín J, Maldonado AM.. 2006. Pre-haustorial resistance to broomrape (Orobanche cumana) in sunflower (Helianthus annuus): cytochemical studies. Journal of Experimental Botany 57, 4189–4200. [DOI] [PubMed] [Google Scholar]
- Ejeta G. 2007. The Striga scourge in Africa: a growing pandemic. In: Ejeta G, Gressel J, eds. Integrating new technologies for Striga control. World Scientific, 3–16. [Google Scholar]
- Fishman MR, Shirasu K.. 2021. How to resist parasitic plants: pre- and post-attachment strategies. Current Opinion in Plant Biology 62, 102004. [DOI] [PubMed] [Google Scholar]
- Gobena D, Shimels M, Rich PJ, Ruyter-Spira C, Bouwmeester H, Kanuganti S, Mengiste T, Ejeta G.. 2017. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proceedings of the National Academy of Sciences USA 114, 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. 2008. Strigolactone inhibition of shoot branching. Nature 455, 189–194. [DOI] [PubMed] [Google Scholar]
- Gurr SJ, Rushton PJ.. 2005. Engineering plants with increased disease resistance: what are we going to express? Trends in Biotechnology 23, 275–282. [DOI] [PubMed] [Google Scholar]
- Hu L, Wang J, Yang C, Islam F, Bouwmeester HJ, Muños S, Zhou W.. 2020. The effect of virulence and resistance mechanisms on the interactions between parasitic plants and their hosts. International Journal of Molecular Sciences 21, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Mellor KE, Paul SN, Lawson MJ, Mackey AJ, Timko MP.. 2012. Global changes in gene expression during compatible and incompatible interactions of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. BMC Genomics 13, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil M, Kountche BA, Al-Babili S.. 2021. Current progress in Striga management. Plant Physiology 185, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhu MY, Farhi M, Wang L, et al. 2022. Heinz-resistant tomato cultivars exhibit a lignin-based resistance to field dodder (Cuscuta campestris) parasitism. Plant Physiology 189, 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhu M-Y, Sinha NR.. 2022. Parasitic plants: an overview of mechanisms by which plants perceive and respond to parasites. Annual Review of Plant Biology 73, 433–455. [DOI] [PubMed] [Google Scholar]
- Kanampiu F, Makumbi D, Mageto E, Omanya G, Waruingi S, Musyoka P, Ransom J.. 2018. Assessment of management options on striga infestation and maize grain yield in Kenya. Weed Science 66, 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavuluko J, Kibe M, Sugut I, Kibet W, Masanga J, Mutinda S, Wamalwa M, Magomere T, Odeny D, Runo S.. 2021. GWAS provides biological insights into mechanisms of the parasitic plant (Striga) resistance in sorghum. BMC Plant Biology 21, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa D, Brady SM.. 2022. Root cell types as an interface for biotic interactions. Trends in Plant Science 27, 1173–1186. [DOI] [PubMed] [Google Scholar]
- Kawa D, Thiombiano B, Shimels M, et al. 2022. The soil microbiome reduces Striga infection of sorghum by modulation of host-derived signaling molecules and root development. bioRxiv. 10.1101/2022.11.06.515382. [Preprint]. [DOI]
- Keyes WJ, O’Malley RC, Kim D, Lynn DG.. 2000. Signaling organogenesis in parasitic angiosperms: xenognosin generation, perception, and response. Journal of Plant Growth Regulation 19, 217–231. [DOI] [PubMed] [Google Scholar]
- Kokla A, Melnyk CW.. 2018. Developing a thief: haustoria formation in parasitic plants. Developmental Biology 442, 53–59. [DOI] [PubMed] [Google Scholar]
- Kountche BA, Jamil M, Yonli D, Nikiema MP, Blanco-Ania D, Asami T, Zwanenburg B, Al-Babili S.. 2019. Suicidal germination as a control strategy for Striga hermonthica (Benth.) in smallholder farms of sub-Saharan Africa. Plants, People, Planet, 1, 107–118. [Google Scholar]
- Li J, Timko MP.. 2009. Gene-for-gene resistance in Striga–cowpea associations. Science 325, 1094. [DOI] [PubMed] [Google Scholar]
- Mutinda S, Mobegi FM, Hale B, Dayou O, Ateka E, Wijeratne A, Wicke S, Bellis ES, Runo S.. 2023. Resolving intergenotypic Striga resistance in sorghum. Journal of Experimental Botany 74, 5294–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku JM, Cui S, Hori C, et al. 2019. The structural integrity of lignin is crucial for resistance against Striga hermonthica parasitism in rice. Plant Physiology 179, 1796–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangangi IM, Büchi L, Haefele SM, Bastiaans L, Runo S, Rodenburg J.. 2021. Combining host plant defence with targeted nutrition: key to durable control of hemiparasitic Striga in cereals in sub-Saharan Africa? New Phytologist 230, 2164–2178. [DOI] [PubMed] [Google Scholar]
- Nzioki HS, Oyosi F, Morris CE, Kaya E, Pilgeram AL, Baker CS, Sands DC.. 2016. Striga biocontrol on a toothpick: a readily deployable and inexpensive method for smallholder farmers. Frontiers in Plant Science 7, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PJ, Grenier C, Ejeta G.. 2004. Striga resistance in the wild relatives of sorghum. Crop Science 44, 2221–2229. [Google Scholar]
- Serghini K, de Luque AP, Castejón-Muñoz M, García-Torres L, Jorrín JV.. 2001. Sunflower (Helianthus annuus L.) response to broomrape (Orobanche cernua Loefl.) parasitism: induced synthesis and excretion of 7-hydroxylated simple coumarins. Journal of Experimental Botany 52, 2227–2234. [DOI] [PubMed] [Google Scholar]
- Shudo E, Iwasa Y.. 2001. Inducible defense against pathogens and parasites: optimal choice among multiple options. Journal of Theoretical Biology 209, 233–247. [DOI] [PubMed] [Google Scholar]
- Stanley AE, Menkir A, Ifie B, et al. 2021. Association analysis for resistance to Striga hermonthica in diverse tropical maize inbred lines. Scientific Reports 11, 24193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbrick PJ, Huang K, Liu G, Slate J, Press MC, Scholes JD.. 2008. Global patterns of gene expression in rice cultivars undergoing a susceptible or resistant interaction with the parasitic plant Striga hermonthica. New Phytologist 179, 515–529. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. [DOI] [PubMed] [Google Scholar]
- Yoder JI, Scholes JD.. 2010. Host plant resistance to parasitic weeds; recent progress and bottlenecks. Current Opinion in Plant Biology 13, 478–484. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y.. 2010. Strigolactones as germination stimulants for root parasitic plants. Plant and Cell Physiology 51, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Shirasu K.. 2009. Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. New Phytologist 183, 180–189. [DOI] [PubMed] [Google Scholar]


