Abstract
Rhizosphere soil of aromatic rice inhabits different fungal species that produce many bioactive metabolites including 2-acetyl-1-pyrroline (2AP). The mechanism for the biosynthesis of 2AP in the fungal system is still elusive. Hence, the present study investigates the role of possible nitrogen (N) precursors such as some amino acids and polyamines as well as the enzymes involved in 2AP synthesis in the fungal species isolated from the rhizosphere of aromatic rice varieties. Three fungal isolates were found to synthesize 2AP (0.32–1.07 ppm) and maximum 2AP was synthesized by Aspergillus niger (1.07 ppm) isolated from rhizosphere of Dehradun Basmati (DB). To determine the N source for 2AP synthesis, various N sources such as proline, glutamate, ornithine putrescine, spermine, and spermidine were used in place of putrescine in the synthetic medium (Syn18). The results showed that maximum 2AP synthesis was found with putrescine (1.07 ppm) followed by spermidine (0.89 ppm) and spermine (0.84 ppm). Further, LC-QTOF-MS analysis revealed the mobilization of spermine and spermidine into the putrescine, indicating that putrescine is the key N source for 2AP synthesis. Moreover, higher enzyme activity of DAO, PAO, and ODC as well as higher content of methylglyoxal metabolite in the A. niger NFCCI 5060 as compared to A. niger NFCCI 4064 (control) suggests the prominent role of these enzymes in the synthesis of 2AP. In conclusion, this study showed evidence of the polyamines mediated 2AP biosynthesis in A. niger NFCCI 5060.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01124-w.
Keywords: Aromatic rice, 2-Acetyl-1-pyrroline, Rhizosphere fungi, Aspergillus niger, Polyamines
Introduction
2-Acetyl-1-pyrroline (2AP) has been identified as a principle aroma compound imparting pleasant aroma to basmati and non-basmati aromatic rice varieties [1]. In addition to the plant species, various microbes such as bacteria and fungi have also been reported to synthesize 2AP [2–4]. The synthesis of 2AP by rhizospheric fungi of aromatic rice varieties has also been reported from our laboratory [5].
The role of various amino acids as nitrogen (N) precursors is well known in the synthesis of 2AP [6, 7]. Different amino acids such as ornithine, proline, and glutamic acid have been demonstrated to aid in the synthesis of 2AP in bacterial and plant systems [7–9]. Other than these amino acids, polyamines (putrescine, spermidine, and spermine) also act as N source for 2AP synthesis [10, 11]. Polyamines (an organic compound contains more than two amino groups) are found in a wide range of organisms, particularly spermine, spermidine, and putrescine. They are the intermediates from decarboxylation of amino acids such as ornithine, lysine, and arginine [12].
In addition to the possible N precursors, different intermediate enzymes viz, proline dehydrogenase (PDH), Δ1-pyrroline-5-carboxylate synthetase (P5CS), and ornithine amino transferase (OAT) have been identified in the biosynthetic pathway of 2AP in different plants [13]. However, polyamine degradation pathway may also contribute towards aroma synthesis [11]. Different enzymes such as ornithine decarboxylase (ODC), diamine oxidase (DAO), and polyamine oxidase (PAO) play important roles in the polyamine degradation pathway. ODC catalyzes the decarboxylation of ornithine to putrescine and PAO is responsible for the oxidation of acetylated polyamines (acetylspermine and acetylspermidine). The DAO is the only enzyme responsible for converting putrescine to either ∆1-pyrroline or Ƴ-aminobutyraldehyde (GABald) [12]. The accumulation of Ƴ-aminobutyraldehyde (GABald)/1-pyrroline, the direct precursor of 2AP, is often attributed to the loss of function of the enzyme betaine aldehyde dehydrogeanse 2 (BADH2) in several plant species [1, 6, 14].
Earlier, some fungal species such as Aspergillus awamori, A. oryzae, and Acremonium have been found to synthesize 2AP [3]. Previous study from our laboratory also reported the synthesis of 2AP by some species of rhizospheric fungi of different aromatic rice varieties [5]. Furthermore, Wongsadee et al. [15] observed that adding polyamines to Aspergillus awamori TISTR 3193 during cultivation increased the synthesis of 2AP. Although the presence and enhancement of 2AP has been reported in some fungal species, the exact N source and intermediate enzymes for 2AP synthesis have not yet been reported. The plants and bacterial systems use proline and ornithine as the most probable N precursors for the synthesis of 2AP. However, the fungal system is very much different from them so it will be interesting to investigate the possible N precursors for 2AP synthesis in fungal system along with the intermediate enzymes. Therefore, the present study deals with the unveiling of role of putative N source and intermediate enzymes involved in the biosynthetic pathway of 2AP in rhizosphere fungi of basmati and non-basmati aromatic rice varieties.
Material and methods
Collection of rhizosphere soil samples from Dehradun Basmati and Ambemohar-157 aromatic rice varieties
The rhizosphere soil of Dehradun Basmati (DB) was collected from Dudhali village (30°11′36.2396″ N 78°4′51.4336″ E), District Dehradun, Uttarakhand, India, and Ambemohar-157 (AM) from Shilimb village (18°36′34.6363″ N 73°28′2.6040″ E), Mawal Taluka, District Pune, Maharashtra, India. The soil was collected in sterile zip lock polythene bags and brought to the laboratory under low-temperature conditions.
Isolation of rhizosphere mycoflora by serial dilution plate technique
A serial dilution approach was used to isolate rhizosphere fungi, and a series of dilutions up to 103 for each sample was prepared. One milliliter of each solution was pipetted onto a potato dextrose agar (PDA) plate and incubated at 28 °C for 1 week. Every day, the culture plates were examined, and each colony was considered one colony-forming unit (CFU). Individual colonies were isolated from the same plates and re-isolated onto a fresh PDA plate after CFUs were counted. For identification, distinct morphological traits were noticed, and the plates were preserved at 4 °C. Every day, the mode of mycelia growth, color, odor, and variations in medium color were studied for each isolate. A total of 59 isolates were isolated from the rhizospheric soil of the aromatic rice varieties under study. Out of them, 35 isolates were obtained from the DB, whereas 24 fungal strains were obtained from the AM.
Primary screening of isolated pure fungal cultures for the synthesis of 2AP
The pure cultures of fungal strains isolated from the rhizosphere of DB and AM were screened for 2AP synthesis using a synthetic medium (Syn18) [3]. The chemical composition for syn18 medium (per liter) are as follows: glucose, (15.5 g), putrescine (2.0 g), MgSO4·7H2O (0.5 g), NaCl (0.1 g), CaCl2·2H2O (0.1 g), phosphate buffer, pH 7.2 (20 mM), vitamin solution (1.0 ml; biotin (50 mg), Ca. pantothenate (400 mg), inositol (2000 mg), pyridoxine·HCl (400 mg), thiamine·HCl (500 mg), mineral solution (1.0 ml; MnSO4·H2O (100 mg), ZnSO4·7H2O (300 mg), CuSO4·5H2O (100 mg), FeCl3·6H2O (250 mg), ammonium molybdate (50 mg), H3BO3 (300 mg). For screening, 3–4 inoculums (5 cm) of each fungal culture were transferred to 100 ml synthetic medium and kept under agitation in an incubator shaker at 28 °C, 100 rpm for 72 h. After every 24 h, 10 ml liquid medium containing growing mycelia was taken out and filtered. The pH of the filtrate was adjusted at pH 8.0 using sodium hydroxide and the filtrates were sniffed to detect the presence of 2AP against Pandanus amaryllifolius leaf extract as a positive control.
HS–SPME–GC–MS-based quantification of 2AP in the fungal isolates
Optimization of 2AP extraction conditions for head space-solid-phase microextraction coupled with GC–MS
The primary screening showed that three isolates synthesized 2AP. Out of these three isolates, two isolates belonged to DB whereas one isolate was from AM. The HS-SPME parameters for extraction of 2AP were optimized with respect to the sample form (mycelial mat vs. liquid medium), sample quantity (200, 500, 800, and 1000 mg), sample incubation time (48, 72, 96, and 120 h), extraction temperature (40, 60, 80, and 100 °C), pre-incubation, and adsorption time (15, 20, 25, and 30 min) for maximum adsorption of 2AP. For optimization of 2AP extraction conditions, 2AP area count was considered.
Fungal samples were transferred into 4-ml screw cap vials (15 × 45 mm) with PTFE silicon septa (Chromatography Research Supplies, Louiseville, KY, USA) and subjected to headspace solid-phase microextraction (HS-SPME) analysis with a gas chromatography-mass spectrometer (GC–MS; Agilent Technologies, USA) with HP-5 capillary column (30 m × 0.25 m) for 2AP quantification. For the analysis of 2AP, a 1-cm-long carboxen/DVB/PDMS SPME fiber attached to the manual holder (Supelco, Bellefonte, PA, USA) was employed [16]. The fiber was conditioned according to the manufacturer’s instructions before being exposed to the sample headspace in a 4-ml vial. The SPME fiber was desorbed for 5 min on the GC injector. The injector and detector temperature were kept at 250 and 260 °C, respectively. The carrier gas nitrogen was maintained at a flow rate of 1.1 ml/min. The oven temperature was operated at 40 °C for 1 min and then programmed from 40 to 120 °C at a rate of 9 °C/min, and from 120 to 230 °C at a rate of 5 °C/min. The presence of certain ions, their relative ratio, and comparison of MS spectra with reference spectra from the National Institute of Standards and Technology (NIST, ver. 2.0f, 2008) mass spectral database were used to identify 2AP. With 2,4,6-trimethylpyridine (TMP) as a reference standard, the concentration of 2AP was determined using an external standard method. Quantification of 2AP was carried out in all three 2AP-producing fungal isolates using the optimized HS-SPME settings and GC software.
Molecular identification of 2AP synthesizing fungal isolates
Genomic DNA of three 2 AP synthesizing fungal strains was extracted from 1- to 2-week-old colonies grown on PDA using a FastPrep 24 tissue homogenizer (MP Biomedicals, Germany), followed by the salt extraction method of Aljanabi and Martinez [17]. The universal primer pairs ITS4/ITS5 were used to amplify ITS-rDNA using PCR [18]. Sequencing was performed on an ABI 3100 Avant Prism automated DNA sequencer using the Big Dye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions (Applied Biosystems). Chromas Lite v. 2.01 (http://www.technelysium.com.au) was used to modify and merge the raw DNA sequencing files. The annotated ITS sequence was performed for BLASTn analysis using the standard database nucleotide collection (nr/nt). Finally, BLAST of ITS 4 and ITS 5 region sequences of all the three isolates produced 100% similarity with A. niger, A. flavus, and A. creber sequences, respectively, deposited in NCBI GenBank. The accession numbers provided by NCBI Genbank for A. niger, A. flavus, and A. creber are OL616072, OL616035, and OL616067, respectively. Being the highest 2AP synthesizing isolate, the identified strain of 2AP-producing A. niger was deposited in the National Fungal Culture Collection of India (NFCCI), Agharkar Research Institute (ARI), Pune. The assigned strain number for this particular isolate of A. niger is A. niger NFCCI 5060.
Quantification of 2AP in A. niger NFCCI 5060 under different N sources
In place of putrescine in Syn18, other amino acids (proline, glutamate, ornithine) and polyamines (spermine and spermidine) were used (2.0 g/l) and 2AP was quantified. Being the highest 2AP synthesizing isolate, A. niger NFCCI 5060 was chosen for this analysis. The details are as follows. The plates containing each N source were inoculated with A. niger NFCCI 5060 and incubated for 3 days at 27 °C. Syn18 medium without any N source was kept as control. The quantification of 2AP was performed by the method mentioned above.
Quantification of 2AP by spermidine and spermine supplementation in combination with putrescine
The spermine and spermidine were supplemented at different concentrations in combination with putrescine (10 to 50 ppm at the interval of 10 ppm) in the syn18 to find out their influence on 2AP synthesis by A. niger NFCCI 5060. The Petri plates supplemented with these concentrations of spermidine and spermine were inoculated with A. niger NFCCI 5060 and incubated for 3 days at 28 °C and quantification of 2AP was done.
Quantification of polyamines using liquid chromatography quadrupole time of flight mass spectrometry
To understand the role of polyamines (putrescine, spermine, and spermidine) in the biosynthetic pathway of 2AP, non 2AP-synthesizing A. niger NFCCI 4064 and 2AP synthesizing A. niger NFCCI 5060 were grown on the synthetic medium containing spermine and spermidine as a sole N source (2.0 g/l). Non 2AP synthesizing strain of A. niger NFCCI 4064, procured from Agharkar Research Institute (ARI), Pune, was used as a control. The mycelial mat of both the fungal samples was collected at 48 and 72 h after inoculation and benzoylated according to Redmond and Tseng [19] with minor modifications and analyzed using LC-Q-TOF–MS. The standards of polyamines (putrescine, spermidine, and spermine) were purchased from Sisco research laboratories (SRL) Pvt. Ltd., India, and processed similarly.
For the analysis of polyamines, Agilent’s HPLC Prime Infinity II 1260 system (800 bar) equipped with Infinity Lab Poroshell 120 EC-C18 (2.1 × 150 mm, 1.9 μm particle size, Agilent, USA) column was used. The conditions and program for LC–MS were followed according to the method of Vasav et al. [20]. We used Agilent 6530 Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) mass spectrometer. For the absolute quantification of polyamines, peak area of polyamines and standards were compared using Agilent mass-hunter qualitative workflow B.08.00. software.
Enzyme assays
The activity of different enzymes (ODC, PAO, DAO, BADH2) involved in 2AP biosynthesis was measured in A. niger NFCCI 4064 (control) and A. niger NFCCI 5060 (2AP synthesizing strain) at 48 and 72 h of growth in synthetic media (syn 18). To assess the activity of ODC, ornithine (30 ppm) was supplemented in the syn 18 media. Conversely, for the evaluation of PAO and DAO activity, spermidine (30 ppm) was added to the media.
Ornithine decarboxylase activity assay
The enzyme activity of ODC was performed following the method of Legaz et al. [21]. Fungal samples (0.5 g) were homogenized in ice-cold buffer (50 mM potassium phosphate, pH 7.5, 5 mM ethylenediaminetetraacetic acid (EDTA), 0.3 mM 5′-pyridoxal phosphate (PLP), 5 mM dithiothreitol (DTT)). After centrifugation at 10,000 g for 10 min (4 °C), the supernatant was recovered and used as enzyme extract. The reaction mixture contained 2.5 mM β-mercaptoethanol, 1.5 mM EDTA, 3.0 mM l-ornithine HCl in 150 mM potassium phosphate buffer, and enzyme extract and incubated at 37 °C for 30 min. Further, the reaction was stopped by adding 200 µl 10% (w/v) TCA. After that, 1.0 mL 4N NaOH and 2.0 ml of 1-pentanol was added to the reaction mixture and incubated at 37 °C for 10 min. Samples were then centrifuged for 10 min at 4200 g. The upper phase (1.0 mL) was transferred to the test tubes containing 1.0 mL sodium borate (pH 8.0) and briefly mixed. Then, 1.0 ml of 2.5 mM 2,4,6-trinitrobenzenesulfonic acid (TNBS) and dimethyl sulfoxide (DMSO) was added to each tubes and incubated for 10 min at 37 °C. Samples were further centrifuged for 10 min at 4200 g and the lower phase’s absorbance was measured at 426 nm against a reagent blank.
Polyamine oxidase activity assay
PAO activity was assessed using a method of Cona et al. [22] based on the production of H2O2 to measure the amount of quinoneimine dye. The fungal samples (0.5 g) were extracted using a 50 mM potassium phosphate buffer at pH 7, centrifuged at 10,000 g for 10 min (4 °C) and the supernatant, thus, obtained was used as enzyme extract. The standard reaction mixture was preincubated for 3 min at 35 °C with 5 µmol of spermine or spermidine, 0.74 µmol of 4-aminoantipyrine, 0.034 µmol of phenol, and 15 units of peroxidase in 0.2 M potassium phosphate buffer (pH 7.2). The enzyme extract (500 µl) was added to initiate the reaction. The absorbance change at 505 nm was used to monitor the velocity of quinoneimine dye formation.
Diamine oxidase activity assay
For the estimation of activity of DAO enzyme, standard method of Holmstedt et al. [23] was used with slight modifications. 0.5 g of fungal samples was homogenized in 50 mM potassium phosphate buffer (pH 7). Homogenized samples were centrifuged at 10,000 g for 10 min (4 °C) and resulting supernatant was used as enzyme extract. The total reaction mixture contained 0.1 M phosphate buffer, 10 mM putrescine, and enzyme extract. The reaction was terminated by adding 10% TCA followed by 10 mg/ml of o-aminobenzaldehyde. The absorbance was measured at 430 nm.
Betaine aldehyde dehydrogenase 2 enzyme activity assay
BADH2 enzyme activity was measured following the method of Weretilnyk and Hanson [24] with some modifications. Fungal samples (0.5 g) were ground in a mortar at 0–4 °C in extraction buffer comprising of 50 mM Tris HCL (pH 8.0), 10% glycerol, 1 mM EDTA, and 0.5 mM DTT). The homogenate was cleared by centrifugation at 10,000 g for 10 min (4 °C). The total reaction mixture of 1 ml contained 50 mM Hepes–KOH, pH 8.0, l0 mM DTT, and 37 mM 4-aminobutyraldehyde (AB-ald substrate). The reactions were started by adding 1.5 mM NAD+. The reaction was initiated by adding 1.5 mM NAD+. The freshly prepared enzyme extracts were examined against the AB-aldehyde substrate. The BADH2 activity was measured by recording the increase in NADH consumption at 340 nm for 10 min.
Quantification of methylglyoxal
Methylglyoxal was measured in A. niger NFCCI 4064 (control) and 2AP synthesizing A. niger NFCCI 5060 at 48 and 72 h of growth in synthetic media (syn 18). For the estimation of methylglyoxal, initial isolation was carried out following the method of Yadav et al. [25], and quantification was performed according to Wild et al. [26]. Samples were homogenized in 0.5 M perchloric acid and incubated for 15 min at 4 °C. Further, samples were centrifuged at 4 °C for 10 min and supernatant was decolorized by adding charcoal. Decolorized supernatant was neutralized with saturated solution of potassium carbonate at room temperature and centrifuge for 10 min. For the estimation of methylglyoxal, 20 µl of N-acetyl-1-cysteine was added to the neutralized supernatant. Further, 100 µl of the reaction mixture was mixed with phosphate buffer. The absorbance was recorded at 228 nm.
Statistical analyses
The SPSS version 16 was used for all statistical analyses. The results represent the average of each experiment’s three replicates. The data were analyzed statistically using one-way analysis of variance (ANOVA), and mean separations were compared using Duncan’s multiple range tests with a significance level of P 0.05.
Results
Optimization of HS-SPME condition for quantification of 2AP
Among the two different fungal sample forms, mycelial mat was found to be the most effective form for maximum release of 2AP in the headspace over liquid medium. In terms of sample quantity and incubation duration, the 500-mg sample quantity was shown to be the most efficient in terms of 2AP release 72 h after inoculation. The most effective temperature and time combination for maximal release and adsorption of 2AP in the SPME fiber from A. niger was found to be 80 °C temperature with 20 min pre-incubation and 20 min adsorption (Figure S1). Higher temperatures may shorten adsorption time, but they also have the potential to cause premature analyte desorption from the fiber or the synthesis of new compounds. Following the optimized HS-SPME conditions, the three isolates, Aspergillus niger, A. flavus, and A. creber, recorded 1.07 ppm, 0.73 ppm, and 0.32 ppm, 2AP, respectively. The maximum synthesis of 2AP was recorded by A. niger NFCCI 5060.
2AP synthesis by A. niger NFCCI 5060 with different N sources
Among the different N sources tested, the polyamines, specifically putrescine, followed by spermidine, yielded significantly high 2AP (Fig. 1). Other than polyamines, A. niger NFCCI 5060 synthesized very less amount of 2AP with proline and glutamate.
Fig. 1.
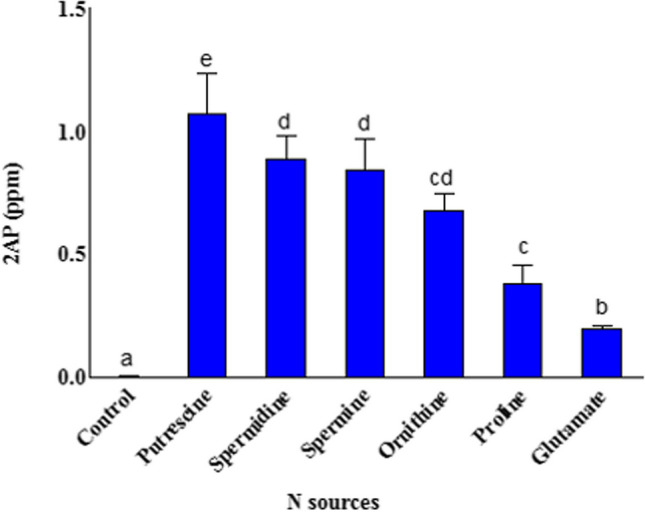
Effect of different N sources on the synthesis of 2AP by A. niger NFCCI 5060. The vertical bars indicate the standard deviation of the mean. The different letters indicate significant differences at P ≤ 0.05
Effect of spermidine and spermine supplementation on 2AP synthesis by A. niger NFCCI 5060
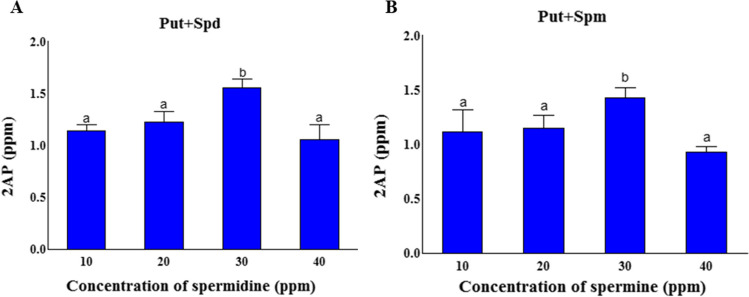
When the polyamines (spermidine and spermine) were supplemented in combination with putrescine, A. niger NFCCI 5060 synthesized maximum 2AP (1.55 ppm) supplemented with 30 ppm spermidine followed by spermine (1.42 ppm) (Fig. 2A, B). The maximum synthesis of 2AP was recorded in spermidine supplemented medium by A. niger NFCCI 5060 viz. 1.44-fold higher over non-supplemented medium.
Fig. 2.
2AP synthesis by A. niger NFCCI 5060 under polyamine supplementation. A Medium with spermidine (Put + Spd). B Medium with spermine (Put + Spm). The vertical bars indicate the standard deviation of the mean. The different letters indicate significant differences at P ≤ 0.05
Quantification of putrescine produced by 2AP synthesizing A. niger NFCCI 5060
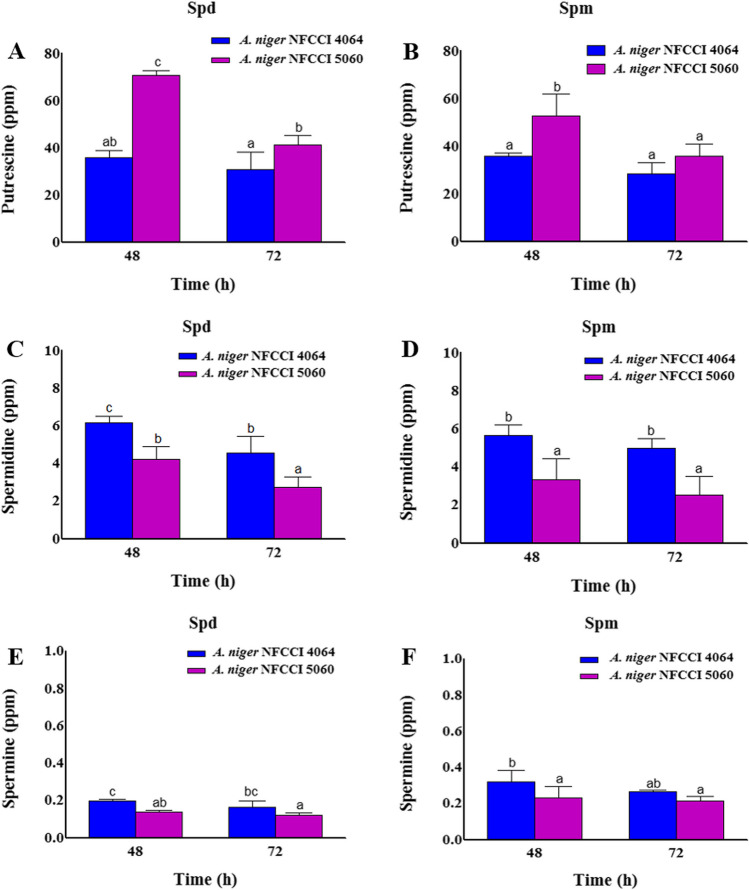
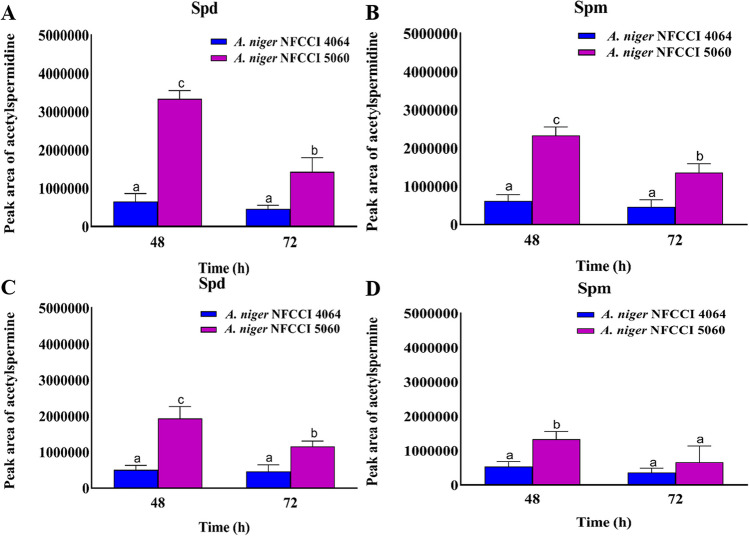
A. niger NFCCI 5060 (2AP-producing strain) grown on medium with spermidine as a sole source of N synthesized 1.97-fold higher putrescine at 48 h compared to A. niger NFCCI 4064 (non 2AP synthesizing strain; control), whereas with spermine, the amount of putrescine was 1.47-fold higher than A. niger NFCCI 4064. (Fig. 3A, B). The other polyamines, spermine, and spermidine were also detected in less concentrations (Fig. 3C, D, E, F). However, the intermediates viz. acetylspermine and acetylspermidine were present in higher amount in A. niger NFCCI 5060 as compared to A. niger NFCCI 4064 (Fig. 4). The acetytspermidine and acetylspermine were 5.11- and 3.77-fold higher in A. niger, respectively, over control (Fig. 4A, B). A. niger NFCCI 5060 grown in spermine-containing medium showed 2.49 and 3.81 higher acetylspermine and acetylspermidine, respectively, over A. niger NFCCI 4064 (Fig. 4C, D).
Fig. 3.
Quantification of putrescine, spermidine and spermine by LC–MS in A. niger NFCCI 4064 (non 2AP-producing strain; control) and A. niger NFCCI 5060 (2AP-producing strain). A, C, E Spermidine (Spd) only as a nitrogen source. B, D, F Spermine (Spm) only as a nitrogen source. The vertical bars indicate the standard deviation of the mean. The different letters indicate significant differences at P ≤ 0.05
Fig. 4.
LC–MS-based relative quantification of acetylspermidine and acetylspermine in A. niger NFCCI 4064 (non 2AP-producing strain; control) and A. niger NFCCI 5060 (2AP-producing strain). A, C With spermidine only. B, D With spermine only. The vertical bars indicate the standard deviation of the mean. The different letters indicate significant differences at P ≤ 0.05
Enzyme assays
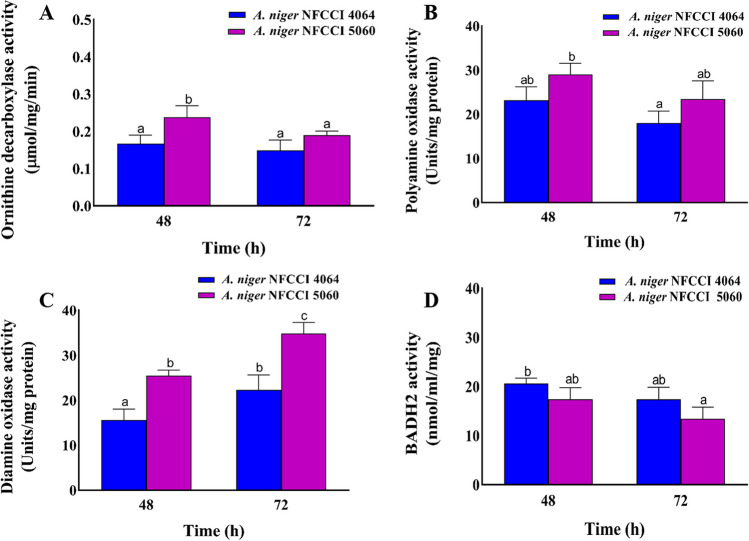
The activities of PAO, DAO, and ODC were found higher in the A. niger NFCCI 5060 as compared to the A. niger NFCCI 4064 (Fig. 5). The activities of PAO and ODC enzymes were found higher at 48 h and decrease thereafter in both the samples. The maximum activity of DAO was however observed at 72 h in both the samples. The activities of ODC and PAO in A. niger NFCCI 5060 were found higher by 1.43- and 1.25-fold compared to A. niger NFCCI 4064 (Fig. 5A, B). In the case of DAO, A. niger NFCCI 5060 recorded 1.5-fold higher activity when compared with A. niger NFCCI 4064 control (Fig. 5C). There was no significant difference found in the BADH2 enzyme activity of both the A. niger NFCCI 4064 control and A. niger NFCCI 5060. The A. niger NFCCI 4064 and A. niger NFCCI 5060 showed 20.58 and 18.7 nmol/ml/mg BADH2 enzyme activities, at 48 h, respectively (Fig. 5D).
Fig. 5.
Enzyme assays of different enzymes involved in the 2AP biosynthetic pathway in A. niger NFCCI 4064 (non 2AP-producing strain; control) and A. niger NFCCI 5060 (2AP-producing strain), A ODC, B PAO, C DAO, and D BADH2. The vertical bars indicate the standard deviation of the mean. The different letters indicate significant differences at P ≤ 0.05
Quantification of methylglyoxal
The results showed that methylglyoxal (MG) content was found to be higher in A. niger NFCCI 5060 (20.74 µM/g) as compared to A. niger NFCCI 4064 (13.73 µM/g) (Fig. 6). MG content was 1.51-fold greater in A. niger NFCCI 5060 than in A. niger NFCCI 4064.
Fig. 6.
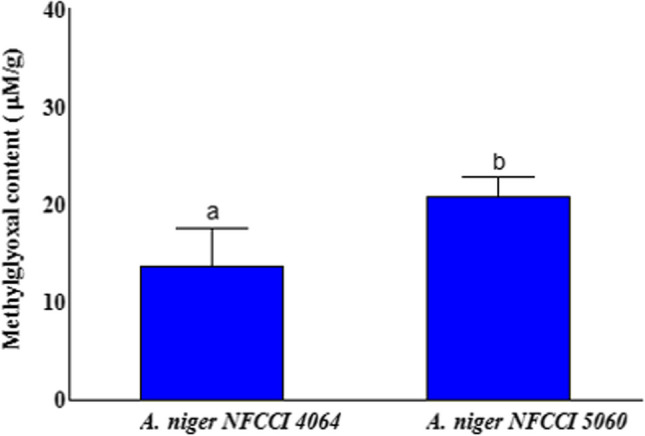
Quantification of methylglyoxal inA. niger NFCCI 4064 (non 2AP-producing strain; control) and A. niger NFCCI 5060 (2AP-producing strain). The vertical bars indicate the standard deviation of the mean. The different letters indicate significant differences at P ≤ 0.05
Discussion
2AP which is a major component of aromatic rice flavors has also been synthesized by rhizosphere fungi. Several fungal strains were recovered from the rhizosphere soil of the fragrant rice cultivars DB and AM in this study. Out of these fungal strains, three fungal isolates were found to synthesize 2AP which was confirmed by HS–SPME–GC–MS analysis. The optimization of HS-SPME conditions is very important for the maximum quantification of 2AP. In our study, 80 °C temperature for 20 min pre-incubation and adsorption showed maximum release of 2AP. The HS-SPME extraction parameters for maximum 2AP release were observed to differ between microorganisms and plant species. After 48 h of inoculation, the temperature of 80 °C for 25 min was shown to be the most effective for maximum release of 2AP by 500 mg sample quantity of Bacillus cereus bacteria [27]. Grimm et al. [18] found that a temperature of 80 °C and a time of 40 min were the best conditions for maximum 2AP adsorption from rice samples. The greatest release of 2AP was seen in fragrant mung bean plants at 80 °C with 30 min of pre-incubation and 20 min of adsorption [14]. According to Wakte et al. [6], the most efficient combination for maximum release of 2AP in HS of Bassia latifolia corolla and effective adsorption by SPME fiber is 60 °C temperature for 25 min. The optimal conditions for maximum release of 2AP in different fragrant rice cultivars were extraction at 80 °C for 30 min pre-incubation followed by 20 min adsorption from 1 g rice [28]. Our study showed that the maximum 2AP was found to synthesize by A. niger NFCCI 5060. Similar observations were also recorded by some previous studies. 2AP were identified in the different strains of A. oryzae viz. A.oryzae TISTR 3088 and A. oryzae TISTR 3232 [29]. Further, Rungsardthong and Noohoom [3] also reported the maximum synthesis of 2AP by the different species of Aspergillus such as A. nigricans, A. awamori, and A. oryzae. Similarly, among the diverse 2AP-producing fungal species inhabiting the rhizosphere, Aspergillus species including A. terreus, A. fumigatus, A. flavus, and A. niger emerged as the prominent 2AP producers [5].
The role of different N sources as 2AP precursors is well understood. In our study, maximum synthesis of 2AP was found by A. niger NFCCI 5060 when grown in putrescine containing medium followed by spermidine and spermine. Therefore, this study indicates that the polyamines especially putrescine is the most probable N precursor for the synthesis of 2AP in A. niger NFCCI 5060. The various reports revealed contradictory findings in this regard. Romanczyk et al. [8] detected an increase in 2AP content in Bacillus cereus cells when high quantities of proline, ornithine, and glutamate were present. Ornithine was proposed as a nitrogen source for 2AP in Lactobacillus hilgardii DSM 20176 by Costello and Henschke [4]. The intermediate 1-pyrroline may acylate acyl-CoA derivatives at the C-2 position, resulting in the production of 2AP. When an amino acid (l-lysine or l-ornithine) reacted with acetyl-CoA in the bacteria L. hilgardii DSM 20176, acetaldehyde and, finally, 2AP were formed.
The addition of different supplements can improve fungi’s ability to synthesize 2AP. Our study showed that the maximum synthesis of 2AP by A. niger was recorded in spermidine supplemented medium than the non-supplemented medium. The current study correlates with Wongsadee et al. [15], who discovered that Aspergillus awamori has the ability to synthesize 2AP, and that this ability is enhanced in the presence of polyamine supplementation. The enhancement of 2AP by polyamine supplementation might be due to the conversion of spermine and spermidine into the putrescine and high concentration of putrescine might lead to the high synthesis of 2AP.
In different plant species, the role of proline is well deciphered in the synthesis of 2AP. In different aromatic rice varieties, the role of proline as a key precursor along with various enzymes has been shown in the biosynthetic pathway of 2AP [30, 31]. Similarly, in Pandanus amaryllifolius, proline has been identified as a main precursor in the synthesis of 2AP [32]. Proline has also been reported as a probable precursor in aromatic varieties of mung bean [14]. In Bassia latifolia, the role of proline has been described in the synthesis of 2AP [6]. In bacteria, the role of ornithine as a key precursor has been identified in the synthesis of 2AP [7]. However, in our study, there is a shift from proline to putrescine in fungal system as a probable N source that contributes in the synthesis of 2AP. The results of our study suggest that the spermine and spermidine mobilized into the putrescine with the formation of acetylspermine and acetylspermidine intermediates. The higher content of putrescine in A. niger NFCCI 5060 suggests its role as a N precursor for the 2AP synthesis through polyamine degradation pathway. In their investigation, Duhaze et al. [33] discovered the production of putrescine from spermidine in the roots of Limonium tataricum. The decreased spermidine level could imply that spermidine is being cyclized more to putrescine, resulting in increased 1-pyrroline production. Because less spermidine is accessible for conversion to spermine by the enzyme spermine synthase, this cyclization of spermidine to putrescine also explains for lower spermine level, as seen in our study. The polyamine pathway, according to Vanavichit and Yoshihashi [11], also contributes to the synthesis of 2AP. The involvement of putrescine was also suggested by Costello and Henschke [4], since Δ1-pyrroline, via putrescine oxidation, is the immediate precursor of ∆1-pyrroline ring of 2AP.
The activity of different enzymes involved in the synthesis of 2AP has been examined in our study. The DAO is the sole enzyme responsible for the conversion of putrescine to ∆1-pyrroline [12]. In our study, presence of high activity of DAO in the A. niger NFCCI 5060 as compared to control might lead to the synthesis of 2AP. The activity of DAO has been found varied among the different strains of fungal species [34]. PAO is required for the conversion of acetylated polyamines into the putrescine. High concentration of putrescine in A. niger NFCCI 5060 supports the high activity of enzyme PAO in our study. Furthermore, high activity of ODC, which catalyzes the conversion of ornithine to putrescine, is also explained by the high concentration of putrescine in A. niger NFCCI 5060. In rice, high activities of DAO, PAO, and ODC have been reported in some aromatic varieties of rice as compared to non-aromatic ones [31]. Furthermore, our results showed the presence of BADH2 enzyme activity in A. niger NFCCI 5060. A similar result was also observed by Bhatt et al. [32] in P. amaryllifolius.
Methylglyoxal (MG) is a highly reactive compound that is primarily generated endogenously during glycolytic pathways. Our study revealed that the A. niger NFCCI 5060 had a greater MG content than the A. niger NFCCI 4064 (control). Similar results were also reported by several workers. Wu et al. [35] found that scented soybeans had considerably higher MG levels than non-scented soybeans. Likewise, Huang et al. [36] found that scented rice calli had a greater MG content than non-scented rice calli. Further, Attar et al. [14] mentioned the higher MG level in Sona Mung Bean (SMB) variety than in NSMB. Higher level of MG was also observed in 2AP-producing strains of bacteria as compared to non-producing species [9]. Greater levels of MG in A. niger NFCCI 5060 may be contributing to higher levels of 2AP in this study. Overall, the present study suggests that the high concentration of putrescine, methylglyoxal, and activity of DAO might lead to the synthesis of 2AP in A. niger NFCCI 5060 as compared to A. niger NFCCI 4064.
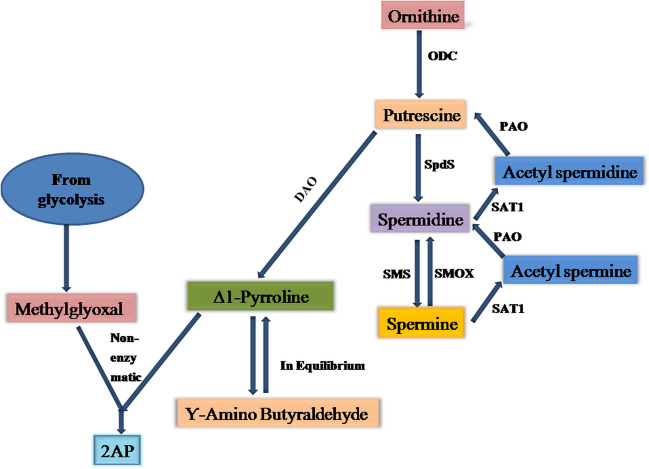
Biosynthetic pathway of 2AP in aromatic varieties of rice has been proposed by several researchers [13, 30]. In this particular study, the presence and activity of polyamines (putrescine, spermine, spermidine, acetylspermine, acetylspermidine, and three enzymes, namely ODC, PAO, and DAO) were determined in relation to the biosynthetic pathway for 2AP synthesis in A. niger NFCCI 5060 (fungal system). Based on our scientific considerations and some previous literatures [30, 37], a biosynthetic pathway has been proposed to correlate and integrate the polyamines and certain enzymes thereby defining the likely metabolic pathway of 2AP synthesis in A. niger NFCCI 5060 (Fig. 7). The polyamine degradation pathway can be considered the most prominent pathway for 2AP synthesis in A. niger NFCCI 5060. The polyamines, especially putrescine, spermine, and spermidine, which are essential for several biological activities as well as responses to biotic and abiotic stresses [37], are utilized for 2AP synthesis in A. niger NFCCI 5060. High concentration of putrescine in A. niger NFCCI 5060 growing in spermine- and spermidine-containing medium as a sole N source suggests that spermidine and spermine might have converted first to N-acetylspermidine/N-acetylspermine through spermidine/spermine N-acetyltransferases, followed by its degradation via PAO into putrescine. Further, high amount of putrescine synthesized through this route might have led to enhanced ∆1-pyrroline production because of higher DAO activity to non-enzymatically react with methylglyoxal to form 2AP. The synthesis of methylglyoxal is linked to the Embedn-Meyerhof pathway’s excess glucose metabolism [38]. Hence, it can be said that in the case of 2AP synthesizing A. niger NFCCI 5060 strain, probably this polyamine degradation pathway is highly operative, resulting in more spermidine and spermine being broken down into putrescine and higher amount of putrescine being converted into ∆1-pyrroline, which subsequently may contribute towards 2AP synthesis.
Fig. 7.
Proposed 2AP biosynthetic pathway in A. niger NFCCI 5060. (ODC, ornithine decarboxylase; SpdS, spermidine synthase; SMS, spermine synthase; SMOX, spermine oxidase; SAT1, spermidine/spermine-N1-acetyltransferase; PAO, polyamine oxidase; DAO, diamine oxidase)
Conclusions
To the best of our knowledge, this is the first report that identified the probable precursors and intermediate enzymes in the biosynthetic pathway of 2AP in A. niger NFCCI 5060 (fungal system). The present study revealed that in the biosynthesis pathway of 2AP in the A. niger NFCCI 5060, there is a shift from proline to putrescine as a possible N precursor. Through this study, we found that putrescine serves as a probable N source in the 2AP synthesis and polyamine degradation pathway is the main 2AP synthesis pathway in A. niger NFCCI 5060. The significantly higher level of putrescine, DAO, and methylglyoxal in A. niger NFCCI 5060 might be responsible for the accumulation of 2AP. These findings expanded our understanding of 2AP synthesis in the fungal system and provided a new aspect for polyamine metabolism. The molecular genetic analysis of the genes especially BADH2 may throw further light on the mechanism of 2AP synthesis in these 2AP-producing fungal isolates.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AZ acknowledges the financial assistance from the UGC, New Delhi, India, for Dr. D. S. Kothari Post-doctoral Fellowship.
Abbreviations
- 2AP
2-Acetyl-1-pyrroline
- HS-SPME-GC-MS
Head space-solid phase micro extraction gas chromatography
- LC-Q-TOF-MS
Liquid chromatography quadrupole time of flight mass spectrometry
- ODC
Ornithine decarboxylase
- DAO
Diamine oxidase
- PAO
Polyamine oxidase
- BADH2
Betaine aldehyde dehydrogenase 2
Author contribution
ABN and VTB conceived and designed research. AZ performed the research, analyzed the data, and wrote the manuscript. HVD and SKS assisted in some experimental sections. ABN and VTB reviewed the manuscript. All authors read and approved the manuscript.
Data availability
The authors confirm that the datasets supporting the findings and conclusions of this study are available within the article and its supplementary information file.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vitthal T. Barvkar, Email: bvitthal@unipune.ac.in
Altafhusain B. Nadaf, Email: abnadaf@unipune.ac.in
References
- 1.Hinge VR, Patil HB, Nadaf AB. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice. 2016;9(1):1–22. doi: 10.1186/s12284-016-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams A, De Kimpe N. Formation of pyrazines and 2-acetyl-1-pyrroline by Bacillus cereus. Food Chem. 2007;101(3):1230–1238. doi: 10.1016/j.foodchem.2006.03.027. [DOI] [Google Scholar]
- 3.Rungsardthong V, Noomhoom A. Production of 2-acetyl-1-pyrroline by microbial cultures. Flavour Frag J. 2005;20(6):710–714. doi: 10.1002/ffj.1645. [DOI] [Google Scholar]
- 4.Costello PJ, Henschke PA. Mousy off-flavor of wine: precursors and biosynthesis of the causative N-heterocycles 2-ethyltetrahydropyridine, 2-acetyltetrahydropyridine, and 2-acetyl-1-pyrroline by Lactobacillus hilgardii DSM 20176. J Agric Food Chem. 2002;50(24):7079–7087. doi: 10.1021/jf020341r. [DOI] [PubMed] [Google Scholar]
- 5.Shaikh MN, Nadaf AB. Qualitative analysis of 2-acetyl-1-pyrroline from the rhizosphere fungal species of basmati rice varieties by GC-FID. Int J Curr Res. 2013;5:1663–1665. [Google Scholar]
- 6.Wakte KV, Kad TD, Zanan RL, Nadaf AB. Mechanism of 2-acetyl-1-pyrroline biosynthesis in Bassia latifolia Roxb. flowers. Physiol Mol Biol Plants. 2011;17(3):231–237. doi: 10.1007/s12298-011-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deshmukh Y, Khare P, Patra DD. Mechanism of 2-acetyl-1-pyrroline biosynthesis by bacterial system. Int J Sci Res Pub. 2015;5(11):575–581. [Google Scholar]
- 8.Romanczyk LJ, Jr, McClelland CA, Post LS, Aitken WM. Formation of 2-acetyl-1-pyrroline by several Bacillus cereus strains isolated from cocoa fermentation boxes. J Agric Food Chem. 1995;43(2):469–475. doi: 10.1021/jf00050a040. [DOI] [Google Scholar]
- 9.Dhondge HV, Pable AA, Barvkar VT, Dastager SG, Nadaf AB. Rhizobacterial consortium mediated aroma and yield enhancement in basmati and non-basmati rice (Oryza sativa L.) J Biotechnol. 2021;328:47–58. doi: 10.1016/j.jbiotec.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Yang Y, Shi W, Ji Q, He F, Zhang Z, et al. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;20(7):1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanavichit A, Yoshihashi T. Molecular aspects of fragrance and aroma in rice. Adv Botanical Res, Acad Press. 2010;56:49–73. doi: 10.1016/B978-0-12-381518-7.00002-9. [DOI] [Google Scholar]
- 12.Gill SS, Tuteja N. Polyamines and abiotic stress tolerance in plants. Plant Sig Behav. 2010;5(1):26–33. doi: 10.4161/psb.5.1.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang TC, Teng CS, Chang JL, Chuang HS, Ho CT, Wu ML. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with Δ1-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J Agric Food Chem. 2008;56(16):7399–7404. doi: 10.1021/jf8011739. [DOI] [PubMed] [Google Scholar]
- 14.Attar U, Hinge V, Zanan R, Adhav R, Nadaf AB. Identification of aroma volatiles and understanding 2-acetyl-1-pyrroline biosynthetic mechanism in aromatic mung bean (Vigna radiata (L.) Wilczek) Physiol Mol Biol Plants. 2017;23(2):443–451. doi: 10.1007/s12298-017-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wongsadee T, Vatanyoopaisarn S, Rungsardthong V, Thumthanaruk B, Puttanlek C, Uttapap D, et al. Effect of polyamine on growth, intermediates and 2-acetyl-1-pyrroline formation by Aspergillus awamori. Flavour Frag J. 2021;36(3):395–403. doi: 10.1002/ffj.3651. [DOI] [Google Scholar]
- 16.Grimm CC, Bergman C, Delgado JT, Bryant R. Screening for 2-acetyl-1-pyrroline in the headspace of rice using SPME/GC-MS. J Agric Food Chem. 2001;49(1):245–249. doi: 10.1021/jf0008902. [DOI] [PubMed] [Google Scholar]
- 17.Aljanabi SM, Martinez I. Universal and rapid salt-extraction of high-quality genomic DNA for PCR-based techniques. Nuc Acids Res. 1997;25(22):4692–4693. doi: 10.1093/nar/25.22.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White TJ, Bruns T, Lee SJWT, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a Guide Methods App. 1990;18(1):315–322. [Google Scholar]
- 19.Redmond JW, Tseng A. High-pressure liquid chromatographic determination of putrescine, cadaverine, spermidine and spermine. J Chroma A. 1979;170(2):479–481. doi: 10.1016/S0021-9673(00)95481-5. [DOI] [Google Scholar]
- 20.Vasav AP, Pable AA, Barvkar VT. Differential transcriptome and metabolome analysis of Plumbago zeylanica L. reveal putative genes involved in plumbagin biosynthesis. Fitoterapia. 2020;147:104761. doi: 10.1016/j.fitote.2020.104761. [DOI] [PubMed] [Google Scholar]
- 21.Legaz ME, Fontaniella B, De Armas R, Vicente C. Determination by high performance liquid chromatography of ornithine and lysine decaboxylases in sugar cane juices. Chromatographia. 2002;53(1):S260–S265. [Google Scholar]
- 22.Holmstedt B, Larsson L, Tham R. Further studies of a spectrophotometric method for the determination of diamine oxidase activity. Biochem Biophys Acta. 1961;48(1):182–186. doi: 10.1016/0006-3002(61)90530-3. [DOI] [PubMed] [Google Scholar]
- 23.Cona A, Rea G, Botta M, Corelli F, Federico R, Angelini R. Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J Exp Bot. 2006;57(10):2277–2289. doi: 10.1093/jxb/erj195. [DOI] [PubMed] [Google Scholar]
- 24.Weretilnyk EA, Hanson AD. Betaine aldehyde dehydrogenase from spinach leaves: purification, in vitro translation of the mRNA, and regulation by salinity. Arch Biochem Biophy. 1989;271(1):56–63. doi: 10.1016/0003-9861(89)90255-5. [DOI] [PubMed] [Google Scholar]
- 25.Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK. Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophy Res Comm. 2005;337(1):61–67. doi: 10.1016/j.bbrc.2005.08.263. [DOI] [PubMed] [Google Scholar]
- 26.Wild R, Ooi L, Srikanth V, Münch G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal Bioanal Chem. 2012;403(9):2577–2581. doi: 10.1007/s00216-012-6086-4. [DOI] [PubMed] [Google Scholar]
- 27.Deshmukh Y, Khare P, Patra DD, Nadaf AB. HS-SPME-GC-FID method for detection and quantification of Bacillus cereus ATCC 10702 mediated 2-acetyl-1-pyrroline. Biotechnol Prog. 2014;30(6):1356–1363. doi: 10.1002/btpr.1989. [DOI] [PubMed] [Google Scholar]
- 28.Mathure S, Shaikh A, Renuka N, Wakte K, Jawali N, Thengane R, et al. Characterisation of aromatic rice (Oryza sativa L.) germplasm and correlation between their agronomic and quality traits. Euphytica. 2011;179(2):237–246. doi: 10.1007/s10681-010-0294-9. [DOI] [Google Scholar]
- 29.Nagsuk A, Winichphol N, Rungsarthong V (2003) Identification of 2-acetyl-1-pyrroline, the principal aromatic rice flavor compound, in fungus cultures. In: Proceedings of the 2nd International Conference on Medicinal Mushrooms & International Conference on Biodiversity and Bioactive Compounds, Pattaya Exhibition Center, Cholburi, Thailand 395–400.
- 30.Wakte K, Zanan R, Hinge V, Khandagale K, Nadaf A, Henry R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L): a status review. J Sci Food Agric. 2017;97(2):384–395. doi: 10.1002/jsfa.7875. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh P, Roychoudhury A. Differential levels of metabolites and enzymes related to aroma formation in aromatic indica rice varieties: comparison with non-aromatic varieties. 3 Biotech. 2018;8(1):1–13. doi: 10.1007/s13205-017-1045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt V, Barvkar VT, Furtado A, Henry RJ, Nadaf AB. Fragrance in Pandanus amaryllifolius Roxb. despite the presence of a betaine aldehyde dehydrogenase 2. Int J Mol Sci. 2021;22(13):6968. doi: 10.3390/ijms22136968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duhazé C, Gouzerh G, Gagneul D, Larher F, Bouchereau A. The conversion of spermidine to putrescine and 1, 3-diaminopropane in the roots of Limonium tataricum. Plant Sci. 2002;163(3):639–646. doi: 10.1016/S0168-9452(02)00172-3. [DOI] [Google Scholar]
- 34.Yamada H, Adachi O, Ogata K. Amine oxidases of microorganisms: part I. Formation of amine oxidase by fungi. Agric Bio Chem. 1965;29(2):117–123. [Google Scholar]
- 35.Wu ML, Chou KL, Wu CR, Chen JK, Huang TC. Characterization and the possible formation mechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine max L) J Food Sci. 2009;74(5):S192–S197. doi: 10.1111/j.1750-3841.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- 36.Huang JX, Xiao D, Duan MY, Tian H, Li GX, Zhong KY, et al. Effects of different applications of ZnCl2 on the yield and aroma content of aromatic rice. Acta Agric Boreali-Sinica. 2008;23(B10):290–292. [Google Scholar]
- 37.Valdés-Santiago L, Ruiz-Herrera J. Stress and polyamine metabolism in fungi. Front Chem. 2014;1:42. doi: 10.3389/fchem.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips SA, Thornalley PJ. The formation of methylglyoxal from triose phosphates: investigation using a specific assay for methylglyoxal. Eur J Biochem. 1993;212(1):101–115. doi: 10.1111/j.1432-1033.1993.tb17638.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the datasets supporting the findings and conclusions of this study are available within the article and its supplementary information file.