Abstract
Performing a motor response to a sensory stimulus creates a memory trace whose behavioral correlates are classically investigated in terms of repetition priming effects. Such stimulus–response learning entails two types of associations that are partly independent: (1) an association between the stimulus and the motor response and (2) an association between the stimulus and the classification task in which it is encountered. Here, we tested whether sleep supports long-lasting stimulus–response learning on a task requiring participants (1) for establishing stimulus–classification associations to classify presented objects along two different dimensions (“size” and “mechanical”) and (2) as motor response (action) to respond with either the left or right index finger. Moreover, we examined whether strengthening of stimulus–classification associations is preferentially linked to nonrapid eye movement (non-REM) sleep and strengthening of stimulus–action associations to REM sleep. We tested 48 healthy volunteers in a between-subjects design comparing postlearning retention periods of nighttime sleep versus daytime wakefulness. At postretention testing, we found that sleep supports consolidation of both stimulus–action and stimulus–classification associations, as indicated by increased reaction times in “switch conditions”; that is, when, at test, the acutely instructed classification task and/or correct motor response for a given stimulus differed from that during original learning. Polysomnographic recordings revealed that both kinds of associations were correlated with non-REM spindle activity. Our results do not support the view of differential roles for non-REM and REM sleep in the consolidation of stimulus–classification and stimulus–action associations, respectively.
Building associations between sensory stimuli and subsequent motor responses is a fundamental form of learning that supports efficient interactions with the environment. Such stimulus–response learning is often investigated using repetition priming paradigms (Henson et al. 2014). Repetition priming describes the phenomenon in which the same stimulus encountered under the same task conditions yields faster and more accurate responses (Logan 1990). Such improvements are thought to be due to the bypassing of one or more processing stages required during initial stimulus encounters. Conversely, if the same stimulus is re-encountered in a different context, responses tend to be slower and less accurate (i.e., negative priming) (Rothermund et al. 2005).
Classically, stimulus–response learning was assumed to reflect a unitary association between a task context and a stimulus prompting a specific action (Logan 1990; Dobbins et al. 2004; Schnyer et al. 2007). However, there is now substantial evidence indicating that the motor action performed in response to a stimulus—that is, the stimulus–action association (e.g., a left finger press to a small object)—is encoded in a memory trace separate from that underlying the stimulus–classification association (Horner and Henson 2009; Dennis and Perfect 2013). For example, if during learning, the task requires the participant to classify stimuli according to varying features (e.g., size vs. color), then during a later test, changing both the task-relevant stimulus feature and the motor response slows reaction times (RTs) and increases error rates. Intriguingly, the two effects have been shown not to interact, such that changing both the relevant task feature and the motor response at the same time yields additive slowing of RTs (Moutsopoulou and Waszak 2012, 2013; Moutsopoulou et al. 2018). This indicates that the two memory traces are at least partly independent (Dennis and Perfect 2013; Moutsopoulou et al. 2015).
Stimulus–classification and stimulus–action associations can persist over extended intervals of up to 1 wk (Moutsopoulou et al. 2018), which is substantially longer than the timescales typically examined in studies of priming. This raises the question of when and how stimulus–response learning is consolidated into long-term memory. Sleep is known to be an important factor in memory consolidation and benefits a variety of memory types (Rasch and Born 2013). Initially, a dual-process model was proposed claiming that rapid eye movement (REM) sleep preferentially consolidates procedural memory, whereas non-REM sleep (i.e., stage 2 and slow-wave sleep) is particularly implicated in consolidating declarative memories (Plihal and Born 1997; Fischer et al. 2002; for review, see Rasch and Born 2013). However, later studies revealed a benefit from non-REM sleep also for procedural types of memory (e.g., see Aeschbach et al. 2008; Rasch et al. 2009). Current concepts assume an active systems consolidation process that is established during sleep in the hippocampus-dependent episodic memory system and benefits declarative and procedural types of memory in parallel (Diekelmann and Born 2010; Sawangjit et al. 2018; Klinzing et al. 2019; Schapiro et al. 2019). According to this concept, the consolidation of memory into long-term storage is particularly reliant on slow-wave activity (SWA) during non-REM sleep via a precisely timed co-occurrence of cortically initiated slow oscillations (SOs; <1 Hz), thalamo–cortical spindles (7–15 Hz) (Steriade and Llinás 1988), and hippocampal sharp wave ripples (100–250 Hz) (Buzsáki 2015). Such SWA co-occurrence provides privileged temporal windows during which neuronal activation patterns observed during wakefulness are replayed (Wilson and McNaughton 1994), with the replay promoting the reorganization of the neuronal representations for a more effective long-term storage. REM sleep, in this view, may help stabilize the reorganized representations at the synaptic level (Diekelmann and Born 2010; Li et al. 2017).
The present study aimed to investigate whether sleep supports the consolidation of stimulus–response learning. To address this question, we used a priming paradigm for which long-term learning effects have been previously demonstrated (Hsu and Waszak 2012; Moutsopoulou et al. 2015, 2018). During a learning phase, pictures of everyday objects were primed such that each object was associated with a specific classification task (stimulus–classification association) and a specific motor response (stimulus–action association). Test sessions followed half an hour (immediate test) and (on separate stimuli) 10 h (remote test) after the learning phase. The strength of stimulus–classification and stimulus–action associations was assessed in terms of RTs to the originally learned associations as well as in “switch conditions”; that is, by changing either the classification task, the correct response action, or both task and action originally associated with the object. To study the contributions of sleep, a sleep group and a wake group of participants were examined.
We predicted, first, that sleep would benefit the consolidation of stimulus–response learning in general; that is, both stimulus–classification and stimulus–action associations would be reinforced after sleep. This would be expressed in reduced RTs to stimuli re-encountered in the learned context, as well as in enhanced switch costs; that is, slower responses to probe stimuli presented with switched task and/or response associations.
Second, we examined the hypothesis that stimulus–action and stimulus–classification associations would be enhanced by different sleep-related processes. Specifically, we expected stimulus–classification associations to primarily depend on non-REM sleep and its electroencephalogram (EEG) hallmarks (i.e., sleep spindles and slow oscillations); whereas stimulus–action associations would primarily benefit from REM sleep and associated EEG theta activity (Rasch and Born 2013). To test this second hypothesis, we recorded polysomnography (PSG) in the sleep group and correlated sleep parameters with changes in behavioral performance.
Results
The trial time line and experimental time course are shown in Figure 1. Participants saw pictures of everyday objects that they were to classify according to either (real-life) size or whether the object contained a technical mechanism. Each stimulus was preceded by a cue consisting of two letters whose identity and locations indicated the classification task to be performed as well as the response mapping for the upcoming stimulus. During an initial learning session, each object was shown twice—in the context of the same classification task and with the same response mapping. Subsequently, each object was probed once—either before or after a 10-h retention interval, and in only one of four possible switch conditions, where either the classification task, the response mapping, both, or neither differed from the learning session. Participants were instructed to respond as fast and as accurately as possible during the entire experiment.
Figure 1.
Task and experimental procedure. (A) Learning trial: Each trial started with a task cue followed by presentation of an object image and ended after a response was given or the time limit of 1800 msec elapsed. (B) Task conditions during immediate and remote tests: Each object could be associated with the same classification task and response side as during learning (that is, classification repeat/action repeat [CrAr]) or presented with switched task or response associations (CrAs, CsAr, and CsAs). (C) Experimental procedure for wake and sleep groups.
Sleep increases switch costs
Mean reaction times (RTs) and variances in the different experimental conditions (i.e., sleep and wake groups, immediate and remote tests, and repeat/switch conditions) are summarized in Table 1 and were comparable with previous studies (e.g., Moutsopoulou et al. 2015, 2018). A separate analysis of the learning period confirmed participants of both groups indeed learned during this period, with responses at the second presentation of the prime being significantly faster than at the first presentation (b = −67.35, t = −13.09, P < 0.001). This learning effect was comparable between groups (b = −15.31, t = −1.49, P = 0.144, for prime × group interaction).
Table 1.
Mean RTs and SEMs in milliseconds for learning and test trials in wake and sleep groups
RTs at the tests were first analyzed for the full repeat (CrAr) condition, which can be considered as a baseline condition, because testing was performed under the same classification and action contingencies as during original learning. There was no evidence that RTs in the full repeat conditions differed as a function of sleep: We found no significant changes from immediate test to remote test in either the sleep or wake group (wake: b = 3.64, t = 0.36, P = 0.720; sleep: b = −8.87, t = −0.86, P = 0.390) and no significant group differences at the remote test (b = −16.79, t = −0.59, P = 0.557). Moreover, RTs for the immediate test were comparable, indicating that performances were matched between groups (b = −4.27, t = −0.14, P = 0.887).
Second, we analyzed RTs in the different switch conditions. For this purpose, we calculated action switch costs, classification switch costs, and full switch costs as the differences between the respective switch condition and the full repeat baseline condition (CrAr). Thus, positive switch costs refer to slowed RTs in trials where action/classification conditions differed from those during the learning period, providing evidence that memory of the originally encoded stimulus–action and stimulus–classification associations persisted and interfered with the changed stimulus conditions. On the other hand, switch costs equal to zero would indicate that the stimulus associations encoded during learning did not interfere with performance when the same stimulus is presented with different action/classification associations.
An initial contrast comparing all three switch conditions between groups indicated higher switch costs in the sleep group (b = 55.48, t = 1.92, one-tailed P = 0.028). This was followed up with more specific contrasts investigating the individual switch conditions.
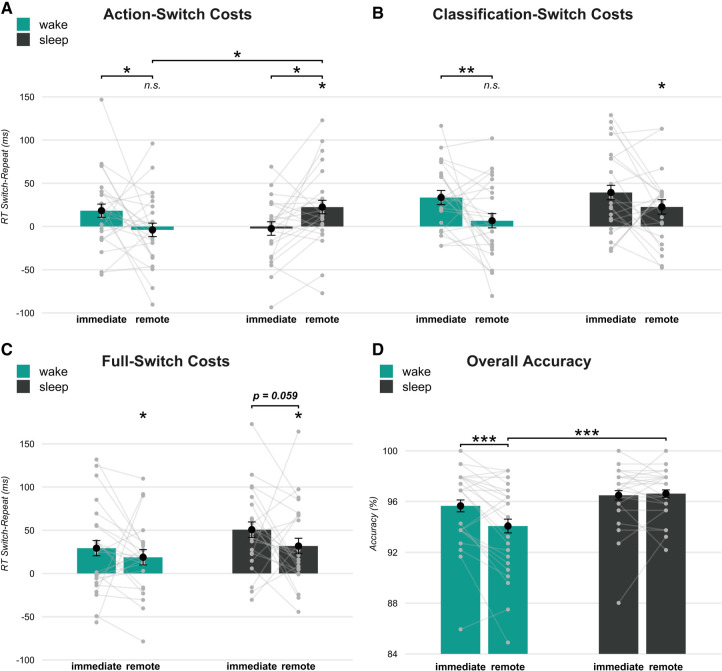
For action switch costs, we observed a decrease from immediate to remote test in the wake group (b = −22.24, t = −2.26, P = 0.024) (Fig. 2A), such that they were no longer different from zero at the remote test (b = −3.94, t = −0.51, P = 0.613). In contrast, in the sleep group, action switch costs increased from immediate to remote test (b = 24.84, t = 2.49, P = 0.013) to a level above zero (b = 22.49, t = 2.87, P = 0.004). The comparison between sleep and wake groups confirmed that action switch costs evolved differently from immediate to remote test in the two groups (b = 47.08, t = 3.36, P < 0.001), and action switch costs were significantly higher in the sleep than in the wake group at the remote test (b = 26.42, t = 2.39, P = 0.017). However, a trend in the opposite direction was present at the immediate test (b = −20.66, t = −1.89, P = 0.059) (Fig. 2A), possibly reflecting confounding influences of circadian factors. To address this possibility, we compared subjective sleepiness (obtained with the Stanford Sleepiness Scale [SSS] before learning and remote test) and vigilance (obtained before learning) between the sleep and wake groups. The results provided no evidence of differences between the groups (all P > 0.422 for respective main and interaction effects). Moreover, action switch costs at the remote test remained significantly higher in the sleep than in the wake group when performance at the immediate test was introduced as a covariate (b = 30.98, t = 2.33, P = 0.024). These control analyses suggest that sleep effects on action switch costs are not substantially confounded by baseline differences between groups at the immediate test. Taken together, S-A associations appear to preferentially persist across retention intervals containing sleep.
Figure 2.
RT switch costs and accuracy at the immediate and remote tests for the wake (green) and sleep (black) groups. (A) Action switch costs decreased across the wake retention interval but increased across the sleep interval. (B) Classification switch costs decreased across both wake and sleep retention intervals. (C) If classification task and action jointly switched, full switch costs slightly decreased across retention intervals and remained above zero in both wake and sleep groups. (D) Accuracy only decayed across the wake retention interval, leading to a higher accuracy for the sleep than the wake group at remote test. Means ± SEM are indicated (dot plots overlaid). Asterisks above individual conditions indicate significant differences from zero. (***) P < 0.001, (**) P < 0.01, (*) P < 0.05.
Classification switch costs were also reduced across the wake retention interval (b = −26.89, t = −2.71, P = 0.007) (Fig. 2B), such that they were not significantly different from zero at the remote test (b = 6.66, t = 0.81, P = 0.421). There was a similar trend toward diminished switch costs in the sleep group (b = −16.70, t = −1.66, P = 0.097), but here classification switch costs remained significantly above zero (b = 22.65, t = 2.72, P = 0.007). Direct comparisons between sleep and wake groups revealed no evidence that classification costs evolved differently over time (b = 10.19, t = 0.72, P = 0.471), and there were no group differences at the immediate (b = 5.80, t = 0.50, P = 0.619) or remote (b = 15.99, t = 1.36, P = 0.175) test.
Given that we found consistently enhancing effects of sleep on stimulus–action but not on stimulus–classification associations, we directly compared the effects of sleep versus wakefulness on the two types of switch costs at the remote test. The corresponding contrast, however, did not support the view that sleep specifically increases action switch costs (b = 10.43, t = 0.82, P = 0.412). Instead, a contrast testing the main effect of sleep across action and classification contingencies indicated consistently higher switch costs in the sleep group (b = 42.41, t = 2.24, P = 0.026). In sum, these results provide limited evidence for an enhancing effect of sleep on S-C associations similar to that on S-A associations.
When classification task and action jointly switched, the associated full switch costs remained constant across immediate and remote tests in the wake group (b = −10.58, t = −1.06, P = 0.288), whereas a marginally significant decrease was seen after sleep (b = −18.93, t = −1.89, P = 0.059) (Fig. 2C). Full switch costs remained above zero at the remote test in both groups (wake: b = 18.73, t = 2.11, P = 0.038; sleep: b = 31.80, t = 3.54, P < 0.001). There were no significant differences between sleep and wake groups at the immediate (b = 21.42, t = 1.71, P = 0.091) or remote (b = 13.07, t = 1.03, P = 0.304) test or across immediate and remote tests (b = −8.36, t = −0.59, P = 0.554). To test whether effects of sleep on switch costs differed in the full switch compared with the two single switch conditions, we implemented a contrast comparing the full switch with the single switch conditions between groups at the postretention test. This did not yield any evidence for an interactive effect with sleep (b = 16.27, t = 0.85, P = 0.394).
Sleep enhances accuracy
Error rates were low overall and comparable with previous studies. They were generally higher at the remote test [mean ± SEM; immediate test: 3.93 ± 0.419; remote test: 4.68 ± 0.471; χ2(1) = 8.15, P = 0.004 for immediate/remote main effect]. Moreover, participants performed better in classification repeat than classification switch trials [χ2(1) = 36.83, P < 0.001].
Importantly, error rates at the remote test were distinctly lower in the sleep than in the wake group [b = 0.58, z = 2.94, P = 0.003; χ2(1) = 5.17, P = 0.023 for the sleep/wake × immediate/remote interaction] (for pairwise comparison, see Fig. 2D). In the wake group, error rates increased from immediate to remote test (b = −0.39, z = −4.03, P < 0.001), while they remained unchanged in the sleep group (b = −0.04, z = −0.38, P = 0.705). Wake and sleep groups did not differ at the immediate test (b = 0.23, z = 1.15, P = 0.251).
Control analyses excluded that the reduced error rate at the remote test in the sleep group reflected a speed–accuracy trade-off in performance. First, error rates at the remote test were not correlated with RTs in the sleep group (r = −0.02, P = 0.913). Second, RTs at the remote test were closely comparable between the groups (P = 0.918). Finally, separate analyses of RTs for error responses and of omission rates at the remote test did not reveal any group differences (error RTs, P = 0.553; omissions, P = 0.481), excluding that participants in the sleep group sacrificed response speed for accuracy.
Sleep spindles predict reaction time effects
Using polysomnographic data, we analyzed whether sleep architecture and oscillatory signatures of memory processing during sleep are related to behavioral performance. These analyses focused on the conditions where sleep and wake groups differed in behavior at the remote test. Sleep parameters of interest were correlated with performance changes across the retention interval (i.e., remote test–immediate test). A summary of sleep architecture and sleep oscillations is shown in Table 2.
Table 2.
Sleep architecture and characteristics of sleep oscillations (means, with SEMs in parentheses)
We did not observe any significant correlation between sleep parameters and performance for the full-repeat baseline condition (CrAr; all P > 0.090), which matches with the absence of sleep/wake group differences in behavioral performance in this condition. There were no significant correlations between action switch costs (CrAs–CrAr) and sleep parameters when considering non-REM sleep as a whole (Table 3). Given previous data indicating that motor memory consolidation might be specifically associated with spindle activity during stage 2 sleep (Genzel et al. 2009), we conducted additional exploratory analyses separately for stage 2. Indeed, higher numbers of slow spindles and fast spindles during stage 2 sleep predicted increased action switch costs at the remote test (slow spindles, maximum correlation at F3, r = 0.45, t(20) = 2.24, P = 0.037; fast spindles, C4, r = 0.54, t(19) = 2.78, P = 0.012) (cf. Fig. 3). These coefficients also differed from those revealed for SWS (Fisher's Z = 2.42 and Z = 2.54, P = 0.016 and P = 0.011, respectively).
Table 3.
Correlations between sleep oscillations and behavioral performance
Figure 3.
(A) Slow (green triangles) and fast (black circles) spindle activity in stage 2 sleep positively correlated with action switch costs (remote test–immediate test). (B) Slow and fast spindle activity during non-REM sleep (stage 2 sleep + SWS) positively correlated with classification switch costs. Results are shown for the sites of maximum correlations.
Classification switch costs (CsAr–CrAr) (Table 3B) were positively correlated with total sleep time (r = 0.50, t(21) = 2.63, P = 0.016) and consequently negatively correlated with time awake after sleep onset (r = −0.44, t(21) = −2.25, P = 0.035). Higher numbers of slow and fast spindles during non-REM sleep predicted increased classification switch costs at the remote test (slow spindles, F3, r = 0.51, t(20) = 2.66, P = 0.015; fast spindles, P3, r = 0.58, t(21) = 3.25, P = 0.004) (Fig. 3). There were no correlations of sleep parameters with full switch costs. As to performance accuracy, larger amplitudes of slow spindles predicted higher global accuracy at the remote test (F4, r = 0.45, t(21) = 2.33, P = 0.030). In contrast, there was no evidence for links between behavioral performance and SO activity, SO–spindle coupling, REM duration, or REM theta in either action switch or classification switch conditions. Given our interest in a potential role of REM sleep in consolidating S-R learning, we conducted additional Bayesian analyses to investigate whether the data support the absence of REM effects. This was not the case for either action switch costs (maximal BF01 = 1.5) or accuracy (maximal BF01 = 2.47), and only modest evidence for the null hypothesis was seen for classification switch cost (maximal BF01 = 3.52; BF01 > 3 at three out of six electrodes).
Taken together, these results indicate a positive association of classification switch costs with non-REM spindles. For action switch costs, a similar association was seen in exploratory analyses restricted to sleep stage 2.
Discussion
The association of perceived stimuli with subsequent motor responses is a fundamental form of learning. Based on findings that such stimulus–response learning comprises at least two partly independent memory traces (that is, stimulus–action and stimulus–classification associations) (Moutsopoulou and Waszak 2012, 2013), we examined whether its long-term persistence relies on sleep-dependent mechanisms of memory consolidation (Diekelmann and Born 2010; Moutsopoulou et al. 2018) and, in addition, whether these mechanisms differ between action- and classification-related associations. In terms of reaction times, we found that sleep does not improve stimulus–response performance when stimuli are re-encountered in the learned context; that is, in the full repeat baseline condition, where both task and response mapping were the same as during learning. However, when the response mapping changed at the test after the retention interval, the sleep group showed increased RTs, indicating improved consolidation of stimulus–action associations. A weaker sleep effect was seen when the classification task changed: Here, sleep preserved higher RTs across the retention interval, although this effect did not reach significance in a direct comparison with the wake condition. Finally, sleep effects were least pronounced in comparisons limited to the full switch condition, where both response mapping and classification task changed compared with the learning context. Nevertheless, a global beneficial effect of sleep was seen when data from all three switch conditions were compared between sleep and wake groups. Moreover, sleep improved performance accuracy compared with the wake group across all conditions. Behavioral signs of enhanced stimulus–action and stimulus–classification associations (i.e., increased switch costs) in the sleep group were predicted by fast and slow spindle activity during non-REM sleep. For action switch costs, this association was only observed for spindles during sleep stage 2. Taken together, these findings support the hypothesis that sleep consolidates stimulus–response learning, with a pronounced effect on stimulus–action associations and a weaker effect on stimulus–classification associations. Both effects appear related to non-REM sleep spindles. On the other hand, our findings do not provide evidence for the notion that differential mechanisms related to non-REM versus REM sleep are selectively implicated in strengthening stimulus–action and stimulus–classification associations, respectively.
Despite its effects on stimulus–action and stimulus–classification associations, sleep did not lead to behavioral changes in the full repeat condition, which represents the simplest task condition. This means that postlearning sleep does not alter the memory traces to an extent that would lead to immediate RT advantages. Nevertheless, the combination of equivalent reaction times in this condition with higher accuracy after sleep confirms the notion that sleep improves stimulus–response learning overall. The fact that beneficial effects of sleep were expressed in switch costs—that is, increased RTs in conditions conflicting with original learning rather than decreased RTs in the full repeat condition—might also point to effects of sleep on higher-level aspects of the associative representation (e.g., related to decision-making) rather than on lower-level priming-related aspects. Indeed, previous studies on the effects of sleep on priming tasks yielded mixed results (Gaskell et al. 2019; Shaikh and Coulthard 2019; Sánchez-Mora and Tamayo 2021).
Sleep also did not affect RTs in the most complex condition; that is, the full switch condition, where both the classification task and the response mapping differed from the original learning context. This result was unexpected, given previous studies showing that stimulus–action and stimulus–classification associations can independently vary, such that effects on action and classification switch costs add up linearly (Moutsopoulou et al. 2018). Against this backdrop, one might have expected that effects of sleep on stimulus–action and stimulus–classification switch costs likewise add up to an even more prominent increase in RTs. Instead, we did not observe any difference in full switch performance between the sleep and wake groups. This outcome does not question the view that stimulus–action and stimulus–classification associations are at least partially independent but hints at the presence of currently unknown processes involved in the consolidation (or retrieval) of these associations that interact with the brain states of sleep and wakefulness, underlining the multifaceted nature of sleep's contribution to sensorimotor learning (King et al. 2017). An intriguing speculation in this regard is that consolidation processes during sleep promote the integrative (and interdependent) processing of both kinds of association, resulting in relatively diminished switch costs specifically in the full switch condition. A similar sleep-induced promotion of integrated processing has been revealed in the spatial domain, where sleep after encoding fostered the integration of landmark-based hippocampal and local, boundary-based striatal representations (Noack et al. 2021).
Indeed, the view that sleep simultaneously strengthens separate stimulus–action and stimulus–classification association as well their integration would nicely concur with the main finding of our sleep analysis, linking the strengthening of both stimulus–action and stimulus–classification associations to the same oscillatory mechanism; that is, non-REM spindle activity. Using optogenetic induction of thalamic spindles, studies in mice have demonstrated a causal role of non-REM sleep spindles in triggering systems consolidation processes in the hippocampal–neocortical episodic memory system (Latchoumane et al. 2017). In particular, sleep spindles appear to coordinate hippocampal memory replay with processes of synaptic plasticity underlying the strengthening of associative memories at the cortical level (Bergmann et al. 2012; Niethard et al. 2018). In our study, the relationship between spindles and stimulus–action associations was more pronounced in separate analyses of stage 2 sleep (Malerba et al. 2022), and there were no consistent correlations between behavioral performance and slow oscillations. These findings suggest that slow oscillatory activity is less essential for the consolidation of S-R learning, consistent with previous reports using a range of experimental paradigms (e.g., see Payne et al. 2009; Cunningham et al. 2021; Lutz et al. 2021). This might be related to the permissive role of slow oscillations, which, although driving thalamic spindle generation, cannot overcome refractoriness in spindle generation (Ngo et al. 2015).
While our sleep analyses revealed a link to non-REM spindles for the effects of sleep on both stimulus–action and stimulus–classification associations, we obtained no cues pointing to a possible contribution of REM sleep in this consolidation process. Although additional Bayesian analyses did not provide support for the null hypothesis, the absence of evidence for an effect of REM sleep argues against the view derived from early studies that REM sleep plays a specific role in consolidating non-hippocampus-dependent procedural skills (e.g., see Plihal and Born 1997). In fact, dual-process concepts of sleep-dependent memory consolidation assuming differential functions of non-REM and REM sleep for hippocampus-dependent declarative memories and non-hippocampus-dependent procedural memories have been disproven by recent studies showing that the hippocampus is critical for the beneficial effects of sleep on memories traditionally considered not to depend on hippocampal function (Sawangjit et al. 2018, 2022; Schapiro et al. 2019). In combination with those studies, the present findings linking enhancements in both stimulus–action and stimulus–classification association to the same non-REM spindle mechanism concur with the view of an active systems consolidation process in which the strengthening of associative memories in general is mediated by non-REM sleep. Here, spindles are thought to coordinate the hippocampal replay of episodic memory features with synaptic changes mediating the transformation of memory traces into more persisting, and perhaps more abstract, representations residing in neocortical networks (Klinzing et al. 2019). However, although the association of increased switch costs with non-REM spindle activity is consistent with active consolidation based on concurrent reactivation of newly encoded representations, the effects of sleep observed here might as well reflect a passive action of sleep protecting newly formed associations from forgetting (Mednick et al. 2011).
Regardless of whether actively supported or passively protected by sleep, it is tempting to speculate that systems consolidation of stimulus–response learning in our experiment partly derives from the fact that participants encountered the overall task structure, including all switch conditions, already during learning before the retention interval, thus enabling sleep to support representations of higher-order contingencies and task–structure information that, after sleep, lead to an enhanced activation and reinstatement of stimulus–action and stimulus–classification associations. This view is to be scrutinized in further experiments.
Materials and Methods
Participants
The study was conducted in accordance with the principles set forth in the Declaration of Helsinki (World Medical Association 2013) and was approved by the Ethics Committee of the Medical Faculty at the University of Tübingen. Sample size was calculated using G*Power (Faul et al. 2009) to achieve 80% power at 5% α level to detect an estimated effect of . The latter was based on results of a previous study, where the same task paradigm as in the present study was used (Moutsopoulou et al. 2018). Forty-nine participants reporting no previous or current neurological or psychiatric disorders, drug abuse, or sleep problems were recruited for the experiment and paid for participation. All participants had regular sleep/wake cycles and did not work night shifts during the 4 wk before the experiment. Two participants were excluded from analysis—one due to insomnia during the experimental night (sleep duration of 180.5 min compared with sample level of [mean ± SD] 453.4 min ± 39.3 min), and the other for exceptionally slow RTs and fatigue following the retention interval (mean RT of 1023 msec compared with sample level of 739 msec ± 99 msec, in line with self-report of powerlessness, lack of concentration, and bad sleep quality during the experimental night). The remaining 47 participants were between 18 and 33 yr old (mean age: 24.26; SD: 3.48; 30 women and 17 men). All were naïve to the study paradigm and signed an informed consent form before the start of the experiment. They were instructed not to consume alcohol or caffeine throughout the experiment. All participants spoke German fluently and were able to follow task instructions.
Stimuli and tasks
A total of 384 pictures of everyday objects was taken from an online library (Brady et al. 2008). All pictures were centrally presented in color against a white background with the same size of 256 × 256 pixels on a 19-in LCD monitor at a resolution of 1280 × 1024 pixels. The participants’ task during the learning phase was to classify each object based on one of two features. One feature was real-life stimulus size, specifically the question of whether the object was smaller or larger than a basketball. The other feature referred to whether the stimulus contained a technical mechanism. Thus, a pair of scissors would be an object that is smaller than a basketball and comprising a mechanism, whereas a sofa would be larger than a basketball and a nonmechanical object.
Each stimulus was preceded by a cue consisting of two letters presented to the left and right of a fixation cross. The letters indicated both the classification task to be performed on the stimulus and the response side for the upcoming stimulus. Specifically, the letters M (presented left) and N (presented right) indicated that a mechanical versus nonmechanical classification was to be performed, with mechanical objects requiring a left-hand response and nonmechanical objects requiring a right-hand response. Similarly, the letter K (for German “kleiner”—smaller, presented left) and G (for German “größer”—bigger, presented right) indicated the size classification task, with “smaller” responses mapped to the left hand and “bigger” responses mapped to the right hand. Left-hand and right-hand responses were given via a standard keyboard using the “A” and “L” keys, respectively. Participants were instructed to respond as fast and as accurately as possible during the entire experiment.
Each trial started with the presentation of the task cue, with the letters M/N (or N/M) or K/G (or G/K) 200 pixels to the left and right of the fixation cross. The task cue disappeared after 700 msec and was immediately followed by the central target stimulus. The target stimulus stayed on the screen for maximally 1800 msec or until a response was given. Responses were followed by performance feedback (“correct,” “wrong,” and “no response”) presented centrally on the screen for 700 msec. For a schematic depiction of trial and experimental time lines, see Figure 1.
Design and procedure
The experiment was based on a four-factorial mixed design. Two within-subject factors (classification contingency and action contingency) represented the different conditions used to probe the strength of the learned stimulus–classification and stimulus–action associations at the immediate and remote tests. The resulting four conditions were full repeat (classification repeat/action repeat [CrAr]; that is, classification task and response side for the presented object stimulus were the same as during the learning phase), action switch (classification repeat/action switch [CrAS]), classification switch (classification switch/action repeat [CsAr]), and full switch (classification switch/action switch [CsAs]) (Fig. 1B). Stimuli were randomly assigned to one of the four conditions while maintaining an equal number of stimuli (i.e., 48) per condition in each test session. The comparison between immediate and remote tests yielded a third within-subject factor.
The influence of sleep on stimulus–classification and stimulus–action associations was investigated via a fourth, between-subjects factor. Upon recruitment, participants were randomly allocated to the wake or sleep group. The wake group (nwake = 24; mean age: 24 yr; SD: 3.48 yr) performed the learning phase and the immediate test in the morning and the remote test in the evening of the same day. During the intervening retention interval, the participants left the laboratory to pursue their everyday activities, with instructions not to take any naps. Participants in the sleep group (nsleep = 23, 24.52 ± 3.54) completed the learning phase and immediate test in the evening and the remote test the next morning. During the night, they slept in the laboratory and PSG was recorded. Participants in the sleep group spent one night in the sleep laboratory 3–7 d before the main experiment to adapt to the new environment.
The learning task was performed in a quiet, dimly lit room with the participant seated ∼50 cm away from the computer screen. Following task instructions, participants performed 16 practice trials to become familiar with the task. Stimuli used during practice were not presented during the main experiment. The latter consisted of learning, immediate test, and remote test sessions. During learning, each of 384 stimuli was presented twice in random order. The same classification task and action mapping applied to both stimulus instances, thus creating initial stimulus–classification and stimulus–action associations. The total of 768 stimuli was equally divided into eight blocks, with breaks of 30 sec between blocks. After 30 sec, participants were free to initiate the next block themselves, thus giving them the opportunity to take longer breaks if needed. The learning session lasted ∼1 h.
Following learning, participants were told to rest quietly for 30 min. They were not allowed to fall asleep, but their activities were not otherwise restricted. Then, the immediate test was carried out, followed by a 10-h retention interval. During this time, the sleep group were equipped with PSG electrodes and slept in the laboratory. Participants were woken up at 7:00 a.m., leaving 1 h between wake-up and the remote test, thus ensuring the dissipation of any sleep inertia (Achermann et al. 1996; Hilditch and McHill 2019; Occhionero et al. 2021).
During the immediate and remote tests, each of the trained 384 stimuli was shown only once, with an equal number of stimuli randomly allocated to each of the two test sessions. Both sessions consisted of two blocks separated by a break of minimally 30 sec (see above) and lasted ∼15 min each. During learning and test sessions, in addition to trial-by-trial feedback, participants also received accuracy feedback at the end of each block to increase motivation. In addition, a vigilance test (Diekelmann et al. 2013) and the Stanford Sleepiness Scale (SSS) (Hoddes et al. 1972) were applied before learning, and the SSS was applied once more before the remote test.
Polysomnographic recordings and sleep scoring
Polysomnographic recordings of the sleep group were obtained and digitized at a sampling rate of 500 Hz with a BrainAmp DC system (Brain Products GmbH). All data were stored using the Brain Vision Recorder (version 1.20.0701, Brain Products GmbH). The electroencephalogram (EEG) was recorded from electrodes at F3, F4, C3, C4, P3, and P4 according to the international 10–20 system. In addition, the diagonal electrooculogram (EOG) and submental electromyogram (EMG) were recorded. All electrodes were referenced to electrodes attached to the mastoids (A1 and A2). A ground electrode was attached to the nasion. EOGs and EMGs were recalculated offline as bipolar montages. Before sleep scoring, signals were filtered between 0.16 and 35 Hz (EEG and EOG) and between 0.16 and 70 Hz (EMG). In addition, a 50-Hz notch filter was applied to all channels. We used fourth-order zero-phase-shift Butterworth filters, as implemented in BrainVision Analyzer (Brain Products GmbH). Visual sleep scoring was conducted offline supported by custom Matlab scripts and performed based on EOG, EMG, and EEG (C3 and C4) data in 30-sec epochs, according to standard criteria (Rechtschaffen and Kales 1968). Total sleep time (TST; starting with sleep onset) and time spent in different sleep stages, including the wake time after sleep onset (WASO), were determined for each participant. Slow-wave sleep (SWS) was defined by the sum of stage 3 and 4 sleep.
Data preprocessing
All 384 pictures were shown once for all 47 participants as a probe either in the immediate or remote tests, yielding a total of 18,048 trials. All trials were included in the analysis of performance accuracy (both errors and omissions included). To analyze RTs, stimuli were removed if an error was committed or if no response was given during the time limit in one of three presentations of this stimulus (two presentations during learning plus one presentation in either the immediate or the remote test). This ensured that only well-learned stimulus–classification and stimulus–action associations were analyzed and led to the exclusion of 16.67% of the trials. To further avoid potential influences from fast guesses or lack of attention, we excluded another 7.5% of the trials because they were above or below two SDs from individual mean RTs within a specific test condition (Berger and Kiefer 2021).
In the sleep EEG, slow and fast spindles were automatically identified during non-REM sleep (stages 2–4) using an algorithm implemented in the SleepTrip toolbox (http://www.sleeptrip.org; RRID:SCR_017318) based on previous publications (Mölle et al. 2011; Weber et al. 2021). Individual frequency peaks for slow spindles were identified in the averaged frontal channels (F3 and F4) from SWS epochs (10.2–12.1 Hz), and peaks for fast spindles were identified in the averaged central and parietal channels (C3, C4, P3, and P4) from stage 2 sleep epochs (12.5–14.9 Hz). At these sites and stages, slow and fast spindles are expected to display maximum power (Gennaro and Ferrara 2003; Ayoub et al. 2013; Fernandez and Lüthi 2020). Slow and fast spindle power peaks were identified for all participants by visual inspection of the normalized power spectrum. Next, the signal of all non-REM sleep epochs in all channels was filtered with a band-pass of ±1 Hz (−3-dB cutoff, Butterworth filter) around the identified individual peak frequency of slow and fast spindles, respectively. A 200-msec sliding window was subsequently used for calculating the root mean square (RMS) of the signal, and the resulting signal was smoothed with a moving average of the same window size. We then calculated the standard deviation (SD) of the filtered signal in each channel across all non-REM sleep epochs. A spindle event was detected if the smoothed signal exceeded an amplitude threshold of 1.5 SD in a given channel for 0.5–2 sec. Local minima and maxima in the filtered spindle signal were marked as peaks and troughs. Initial and final threshold crossings were taken as the beginning and end of a spindle. The largest trough was defined as the spindle peak. Spindle amplitude was defined by the potential difference between the largest trough and largest peak. The frequency of an individual spindle event was determined by summing the number of peaks and troughs divided by twice its duration. Spindles events occurring <0.25 sec apart were merged.
The SleepTrip toolbox was also used to detect SOs based on an automated algorithm validated elsewhere (Ngo et al. 2013). In brief, in all channels and for all non-REM sleep epochs, the preprocessed signal was filtered between 0.3 and 3.5 Hz (fourth-order zero-phase Butterworth filter). Potential SOs were derived between consecutive positive-to-negative zero crossings with a frequency range of 0.5–1.11 Hz (corresponding to 0.9–2 sec). The mean potential from the down zero crossings to the maximum trough (negative peak) and the mean amplitude from maximum trough to peak potential were separately calculated in each channel for all putative SOs. SOs were detected if their negative peak amplitude was <1.25 times the mean negative peak and its amplitude difference (positive peak minus negative peak) was >1.25 times the mean amplitude difference across all putative SOs.
The co-occurrence of spindles and SOs was determined based on time points of previously detected events, also as implemented in SleepTrip. The co-occurrences were considered as SO–spindle couplings if the maximal trough of a spindle fell within the time window between the beginning and the end of a SO (see above). The SO–spindle couplings were determined for each channel and for slow and fast spindles, respectively.
Statistical analysis
Statistical analyses were conducted using R (version 4.1.1; R Core Team 2021). The significance level of all statistical tests was set at 5%, and we report two-tailed results unless noted otherwise.
To analyze RT and accuracy data, we used a linear mixed model and a generalized linear mixed model, respectively (Baayen et al. 2008; R package lme4, Bates et al. 2015). The R package lmerTest (version 3.1-3) was used to obtain significance values via Satterthwaite's approximation of degrees of freedom (Kuznetsova et al. 2017). The design comprised one between-subjects factor group (sleep/wake) and three within-subject factors: test session (immediate/remote), classification contingency (classification switch/repeat), and action contingency (action switch/repeat).
For RT data, we ran a linear mixed model with all four factors and their interactions as fixed effects and a random effect structure consisting of the by-subject random intercepts, by-subject random slopes for the test sessions (immediate/remote), classification contingency (switch/repeat), action contingency (switch/repeat), task type (mechanical/nonmechanical vs. size classification), by-item random intercept, and by-item random slope for task type. This model takes into account that participants and items usually show different baseline levels and may evolve differently across factor levels. This overall model revealed a significant sleep/wake × recent/remote × action contingency × classification contingency four-way interaction (b = −65.62, t = −3.28, P = 0.001) (see Supplemental Table S1). We thus used planned contrasts to compare sleep/wake differences for the factor combinations of central interest. Specifically, we divided switch costs into three subtypes; that is, action switch costs (caused by a switch of response side between learning and testing, calculated as CrAs − CrAr), classification switch costs (caused by a switch of tasks, calculated as CsAr − CrAr), and full switch costs (caused by concurrent switches of task and response side, calculated as CsAs − CrAr). Each type of switch cost was then submitted to the same planned contrasts to answer the following questions: (1) Do switch costs diminish over the retention interval within sleep and wake groups, indicating memory decay? This was calculated for each group as CrAr RTremote test − CrAr RTimmediate test and switch costremote test − switch costimmediate test, respectively. (2) Are switch costs significantly different from zero within groups at the remote test, indicating persistence of memory traces? This was calculated for each group as switch costremote test − 0. (3) Do changes in switch costs from recent to remote test differ between sleep and wake groups, indicating an effect of sleep on memory consolidation with sleep? This was calculated as (switch costsleep, remote test − switch costsleep, immediate test) − (switch costwake, remote test − switch costwake, immediate test). If so, then (4) differences between sleep and wake groups were separately assessed at immediate and remote test, calculated as CrAr RTsleep − CrAr RTwake and switch costsleep − switch costwake, respectively.
Based on previous studies (Moutsopoulou et al. 2018), our hypotheses regarding accuracy data were less specific. Thus, we ran a generalized linear mixed model with the same four factors and their interactions as fixed factors using a binomial link function to represent the binomial distribution of the dependent variable. Only by-subject random intercept, by-item random intercept, and by-item random slope for task type were included as random effects. This reduced random effect structure was adopted due to singular-fit issues, indicating that the variances in categorical accuracy data were insufficient to reliably estimate the more complex model adopted for RT analysis. The linear mixed model and generalized linear mixed model were fitted using the restricted maximum likelihood (REML) and the maximum likelihood (Laplace approximation) methods, respectively.
To connect behavioral performance to sleep architecture and signatures of memory processing, we calculated Pearson correlations for the sleep group. We focused on the relationship between behavioral performance in those conditions where it was affected by sleep and time spent in the different sleep stages (S2, SWS, and REM), as well as slow and fast spindles, SOs, SO–spindle coupling, and REM theta activity. Specifically, we analyzed slow spindle activity at frontal electrodes (F3 and F4), fast spindle activity at central and parietal electrodes (C3, C4, P3, and P4), and slow oscillations and REM theta across all six electrodes. Coupling between SOs and spindles was separately analyzed for slow spindles at frontal channels and for fast spindles at centroparietal channels. Three of a total of 138 channels across participants were excluded from analysis due to bad signal quality. Comparisons between correlations were conducted using Fisher's Z transformation procedure, implemented in R package cocor (Diedenhofen and Musch 2015). Given that these analyses were performed to explore potential neurophysiological correlates of behavioral sleep effects, we did not correct for multiple testing. An overview of all correlations (Supplemental Tables S2–S4) indicated that the pattern of significant results is highly specific and unlikely to be explained by randomly distributed false positives, as they would occur under the null hypothesis. Additional Bayesian correlation analyses were conducted in JASP v0.17.1 using a standard stretched beta prior width of 1.0 to investigate whether null effects in classical analyses reflected evidence in favor of the null hypothesis.
Supplementary Material
Acknowledgments
This work was supported by grants from the European Research Council (ERC AdG 883098 SleepBalance) and the German Research Foundation (FOR 5434) to J.B. X.M. is supported by a China Scholarship Council grant. We thank Steffen Gais for providing sleep scoring software.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053753.123.
References
- Achermann P, Werth E, Dijk D-J, Borbely A. 1996. Time course of sleep inertia after nighttime and daytime sleep episodes. Arch Ital Biol 134: 109–119. [PubMed] [Google Scholar]
- Aeschbach D, Cutler AJ, Ronda JM. 2008. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci 28: 2766–2772. 10.1523/jneurosci.5548-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub A, Aumann D, Hörschelmann A, Kouchekmanesch A, Paul P, Born J, Marshall L. 2013. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep 36: 905–911. 10.5665/sleep.2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. 2008. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 59: 390–412. 10.1016/j.jml.2007.12.005 [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Berger A, Kiefer M. 2021. Comparison of different response time outlier exclusion methods: a simulation study. Front Psychol 12: 675558. 10.3389/fpsyg.2021.675558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. 2012. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59: 2733–2742. 10.1016/j.neuroimage.2011.10.036 [DOI] [PubMed] [Google Scholar]
- Brady TF, Konkle T, Alvarez GA, Oliva A. 2008. Visual long-term memory has a massive storage capacity for object details. Proc Natl Acad Sci 105: 14325–14329. 10.1073/pnas.0803390105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. 2015. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25: 1073–1188. 10.1002/hipo.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Bottary R, Denis D, Payne JD. 2021. Sleep spectral power correlates of prospective memory maintenance. Learn Mem 28: 291–299. 10.1101/lm.053412.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis I, Perfect TJ. 2013. Do stimulusaction associations contribute to repetition priming? J Exp Psychol Learn Mem Cogn 39: 85–95. 10.1037/a0028479 [DOI] [PubMed] [Google Scholar]
- Diedenhofen B, Musch J. 2015. Cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One 10: e0121945. 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. 2010. The memory function of sleep. Nat Rev Neurosci 11: 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Wagner U, Born J. 2013. Sleep improves prospective remembering by facilitating spontaneous-associative retrieval processes. PLoS One 8: e77621. 10.1371/journal.pone.0077621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Schnyer DM, Verfaellie M, Schacter DL. 2004. Cortical activity reductions during repetition priming can result from rapid response learning. Nature 428: 316–319. 10.1038/nature02400 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang A-G. 2009. Statistical power analyses using GPower 3.1: tests for correlation and regression analyses. Behav Res Methods 41: 1149–1160. 10.3758/brm.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Fernandez LMJ, Lüthi A. 2020. Sleep spindles: mechanisms and functions. Physiol Rev 100: 805–868. 10.1152/physrev.00042.2018 [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. 2002. Sleep forms memory for finger skills. Proc Natl Acad Sci 99: 11987–11991. 10.1073/pnas.182178199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell MG, Cairney SA, Rodd JM. 2019. Contextual priming of word meanings is stabilized over sleep. Cognition 182: 109–126. 10.1016/j.cognition.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Gennaro LD, Ferrara M. 2003. Sleep spindles: an overview. Sleep Med Rev 7: 423–440. 10.1053/smrv.2002.0252 [DOI] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Wehrle R, Grözinger M, Steiger A. 2009. Slow wave sleep and REM sleep awakenings do not affect sleep dependent memory consolidation. Sleep 32: 302–310. 10.1093/sleep/32.3.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Eckstein D, Waszak F, Frings C, Horner AJ. 2014. Stimulus–response bindings in priming. Trends Cogn Sci 18: 376–384. 10.1016/j.tics.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilditch CJ, McHill AW. 2019. Sleep inertia: current insights. Nat Sci Sleep 11: 155–165. 10.2147/nss.s188911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddes E, Dement W, Zarcone V. 1972. The development and use of the Stanford Sleepiness Scale (SSS). Psychophysiology 9: 150. 10.1111/j.1469-8986.1972.tb00747.x [DOI] [Google Scholar]
- Horner AJ, Henson RN. 2009. Bindings between stimuli and multiple response codes dominate long-lag repetition priming in speeded classification tasks. J Exp Psychol Learn Mem Cogn 35: 757–779. 10.1037/a0015262 [DOI] [PubMed] [Google Scholar]
- Hsu Y-F, Waszak F. 2012. Stimulus–classification traces are dominant in response learning. Int J Psychophysiol 86: 262–268. 10.1016/j.ijpsycho.2012.10.002 [DOI] [PubMed] [Google Scholar]
- King BR, Hoedlmoser K, Hirschauer F, Dolfen N, Albouy G. 2017. Sleeping on the motor engram: the multifaceted nature of sleep-related motor memory consolidation. Neurosci Biobehav Rev 80: 1–22. 10.1016/j.neubiorev.2017.04.026 [DOI] [PubMed] [Google Scholar]
- Klinzing JG, Niethard N, Born J. 2019. Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22: 1598–1610. 10.1038/s41593-019-0467-3 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J Stat Softw 82: 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S. 2017. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95: 424–435.e6. 10.1016/j.neuron.2017.06.025 [DOI] [PubMed] [Google Scholar]
- Li W, Ma L, Yang G, Gan W-B. 2017. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci 20: 427–437. 10.1038/nn.4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. 1990. Repetition priming and automaticity: common underlying mechanisms? Cognit Psychol 22: 1–35. 10.1016/0010-0285(90)90002-l [DOI] [Google Scholar]
- Lutz ND, Admard M, Genzoni E, Born J, Rauss K. 2021. Occipital sleep spindles predict sequence learning in a visuo–motor task. Sleep 44: zsab056. 10.1093/sleep/zsab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerba P, Whitehurst L, Mednick SC. 2022. The space-time profiles of sleep spindles and their coordination with slow oscillations on the electrode manifold. Sleep 45: zsac132. 10.1093/sleep/zsac132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. 2011. An opportunistic theory of cellular and systems consolidation. Trends Neurosci 34: 504–514. 10.1016/j.tins.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Bergmann TO, Marshall L, Born J. 2011. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34: 1411–1421. 10.5665/sleep.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulou K, Waszak F. 2012. Across-task priming revisited: response and task conflicts disentangled using ex-Gaussian distribution analysis. J Exp Psychol Hum Percept Perform 38: 367–374. 10.1037/a0025858 [DOI] [PubMed] [Google Scholar]
- Moutsopoulou K, Waszak F. 2013. Durability of classification and action learning: differences revealed using ex-Gaussian distribution analysis. Exp Brain Res 226: 373–382. 10.1007/s00221-013-3445-0 [DOI] [PubMed] [Google Scholar]
- Moutsopoulou K, Yang Q, Desantis A, Waszak F. 2015. Stimulus–classification and stimulus–action associations: effects of repetition learning and durability. Q J Exp Psychol 68: 1744–1757. 10.1080/17470218.2014.984232 [DOI] [PubMed] [Google Scholar]
- Moutsopoulou K, Pfeuffer C, Kiesel A, Yang Q, Waszak F. 2018. How long is long-term priming? Classification and action priming in the scale of days. Q J Exp Psychol 72: 1183–1199. 10.1177/1747021818784261 [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Martinetz T, Born J, Mölle M. 2013. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78: 545–553. 10.1016/j.neuron.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Miedema A, Faude I, Martinetz T, Molle M, Born J. 2015. Driving sleep slow oscillations by auditory closed-loop Stimulation self-limiting process. J Neurosci 35: 6630–6638. 10.1523/jneurosci.3133-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethard N, Ngo H-VV, Ehrlich I, Born J. 2018. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc Natl Acad Sci 115: E9220–E9229. 10.1073/pnas.1805517115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack H, Doeller CF, Born J. 2021. Sleep strengthens integration of spatial memory systems. Learn Mem 28: 162–170. 10.1101/lm.053249.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhionero M, Fabbri M, Tonetti L, Martoni M, Natale V. 2021. Time course of sleep inertia dissipation in memory tasks. Appl Sci 11: 3354. 10.3390/app11083354 [DOI] [Google Scholar]
- Payne JD, Schacter DL, Propper RE, Huang L-W, Wamsley EJ, Tucker MA, Walker MP, Stickgold R. 2009. The role of sleep in false memory formation. Neurobiol Learn Mem 92: 327–334. 10.1016/j.nlm.2009.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W, Born J. 1997. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9: 534–547. 10.1162/jocn.1997.9.4.534 [DOI] [PubMed] [Google Scholar]
- Rasch B, Born J. 2013. About sleep's role in memory. Physiol Rev 93: 681–766. 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Pommer J, Diekelmann S, Born J. 2009. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci 12: 396–397. 10.1038/nn.2206 [DOI] [PubMed] [Google Scholar]
- R Core Team. 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Rechtschaffen A, Kales A. 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. U.S. Department of Health, Education, and Welfare, Washington, DC. [Google Scholar]
- Rothermund K, Wentura D, Houwer JD. 2005. Retrieval of incidental stimulus-response associations as a source of negative priming. J Exp Psychol Learn Mem Cogn 31: 482–495. 10.1037/0278-7393.31.3.482 [DOI] [PubMed] [Google Scholar]
- Sánchez-Mora J, Tamayo RM. 2021. From incidental learning to explicit memory: the role of sleep after exposure to a serial reaction time task. Acta Psychol 217: 103325. 10.1016/j.actpsy.2021.103325 [DOI] [PubMed] [Google Scholar]
- Sawangjit A, Oyanedel CN, Niethard N, Salazar C, Born J, Inostroza M. 2018. The hippocampus is crucial for forming non-hippocampal long-term memory during sleep. Nature 564: 109–113. 10.1038/s41586-018-0716-8 [DOI] [PubMed] [Google Scholar]
- Sawangjit A, Harkotte M, Oyanedel CN, Niethard N, Born J, Inostroza M. 2022. Two distinct ways to form long-term object recognition memory during sleep and wakefulness. Proc Natl Acad Sci 119: e2203165119. 10.1073/pnas.2203165119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro AC, Reid AG, Morgan A, Manoach DS, Verfaellie M, Stickgold R. 2019. The hippocampus is necessary for the consolidation of a task that does not require the hippocampus for initial learning. Hippocampus 29: 1091–1100. 10.1002/hipo.23101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Dobbins IG, Nicholls L, Davis S, Verfaellie M, Schacter DL. 2007. Item to decision mapping in rapid response learning. Mem Cognit 35: 1472–1482. 10.3758/bf03193617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N, Coulthard E. 2019. Nap-mediated benefit to implicit information processing across age using an affective priming paradigm. J Sleep Res 28: e12728. 10.1111/jsr.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Llinás RR. 1988. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742. 10.1152/physrev.1988.68.3.649 [DOI] [PubMed] [Google Scholar]
- Weber FD, Supp GG, Klinzing JG, Mölle M, Engel AK, Born J. 2021. Coupling of gamma band activity to sleep spindle oscillations a combined EEG/MEG study. Neuroimage 224: 117452. 10.1016/j.neuroimage.2020.117452 [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679. 10.1126/science.8036517 [DOI] [PubMed] [Google Scholar]
- World Medical Association. 2013. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc 310: 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.