Abstract
Dorsal switch protein 1 (DSP1) acts as a damage-associated molecular pattern (DAMP) molecule to activate immune responses in Tenebrio molitor. From a previous study in Spodoptera exigua, we found that DSP1 activates Toll immune signalling pathway to induce immune responses by melanisation, PLA2 activity and AMP synthesis. However, the target site of DSP1 in this pathway remains unknown. The objective of this study was to determine the role of spätzle processing enzyme in the DSP1 induced toll immune signalling pathway. To address this, we analyzed spätzle processing enzyme (Tm-SPE) of the three-step serine protease cascade of T. molitor Toll pathway. Tm-SPE expressed in all developmental stages and larval tissues. Upon immune challenge, its expression levels were upregulated but significantly reduced after RNA interference (RNAi). In addition, the induction of immune responses upon immune challenge or recombinant DSP1 injection was significantly increased. Loss of function using RNA interference revealed that the Tm-SPE is involved in connecting DSP1 induced immune responses like hemocyte nodule formation, phenoloxidase (PO) activity, phospholipase A2 (PLA2) activity and antimicrobial peptide (AMP) synthesis. These suggest that Tm-SPE controls the DSP1 induced activation of Toll immune signalling pathway required for both cellular and humoral immune responses. However, to confirm the target molecule of DSP1 in three-step proteolytic cascade, we have to check other upstream serine proteases like Spatzle activating enzyme (SAE) or modular serine protease (MSP).
Introduction
High mobility group box 1 (HMGB1) is a ubiquitously expressed, highly conserved ‘chromatin-associated protein’ in vertebrates [1]. At normal condition, it remains in nucleus to contribute gene expression, DNA repair, chromatin rearrangement etc. [2–4] while released from nucleus under stress to acts as a damage-associated molecular pattern (DAMP) that activate innate immune responses by interacting with pattern recognition receptors like Toll-like receptors (TLRs), receptor for advanced glycation end products (RAGE) etc. [5]. Dorsal Switch Protein 1 (DSP1) is the insect homolog of vertebrate HMGB1 first introduced in Drosophila melanogaster [6] as a transcriptional co-repressor and chromatin remodelling factor [7, 8]. DSP1 exhibits nuclear function like DNA binding [9], binding with zen and Rel to activate transcription in Anophiles gambie [10], regulation of Sex combs reduced (Scr), Ultrabithorax (Ubx) and Abdominal-B gene expression [8]. DSP1 also mediate immune responses upon pathogen infection in insects like Spodoptera exigua [11] and Tenebrio molitor [12] by activating eicosanoid that mediate cellular and humoral immune responses in insects [13]. Following Toll signalling pathway, DSP1 activate phospholipase A2 (PLA2), an enzyme that catalyse phospholipids to biosynthetic precursor(s) eicosanoids [14].
Toll immune signalling pathway in Drosophila is induced by Gram-positive bacteria or fungi and begins with the recognition of lysine-type peptidoglycan (PG) that triggers activation of the serine protease (SP) cascade leading to form active Spätzle [15, 16]. In Tenebrio molitor, SP cascade consists of three SPs [17, 18]. The initiating SP is a modular serine protease (modSP) which binds to the lysine-type PG recognition complex and activates the second SP, Spätzle activating enzyme (SAE), which in turn activates the last SP, Spätzle processing enzyme (SPE) [17, 19]. Inactive pro-Spätzle is cleaved by SPE to form active Spätzle, which binds to Toll receptor and triggers its specific immune signalling pathway [20, 21]. SPE also cleaves inactive prophenoloxidase (PPO) to active phenoloxidase (PO) [22] that leads to melanisation on the surface of bacteria to kill them. Thus, SPE mediates Toll immune signalling pathway to produce antimicrobial peptides (AMPs) and the melanisation immune responses [19].
DSP1 was predicted and known to mediate immune responses in T. molitor [12] and showed that DSP1 up-regulated gene expression of some AMPs and activated PLA2, PO and nodulation in response to Gram-positive bacterium containing Lys-type PG. A previous study in S. exigua reported that DSP1 activate Toll immune signaling pathways for immune mediation [14]. Another study in T. molitor reported that the Toll pathway includes a three-step proteolytic cascade such as modSP, SAE and SPE for mediating immune responses induced by peptidoglycans [17]. But we do not know the role of SPE or other upstream serine protease in DSP1 induced Toll pathway. Thus, present study aimed to know the involvement of SPE among the three-serine protease of T. molitor Toll pathway in mediating DSP1 induced immune responses. Present study showed that SPE control the immune responses induced by DSP1. This suggests that DSP1 depends on the SPE to activate Toll immune signalling pathway in T. molitor.
Materials and methods
Insect rearing and bacteria culture
Larvae of Tenebrio molitor (T. molitor) were collected from Bio Utility, Inc. (Andong, Korea) and grown on a diet of wheat bran [23] at 25 ± 2°C temperature; 60 ± 5% relative humidity and 16:8 h (L:D) photoperiod and underwent 12 larval instars (L1-L12) to become adult. Adults were provided wheat bran supplemented with cabbage [24]. Gram-positive bacterium, Enterococcus mundtii (E. mundtii) and Gram-negative Xenorhabdus hominickii (X. hominickii) were cultured overnight in tryptic soy broth (TSB) (Difco, Sparks, MD, USA) medium at 28°C with shaking at 180 rpm. For immune challenge, bacteria culture was centrifuged at 10,000 × g for 5 min, then the pellet was resuspended in sterile distilled water or PBS as needed. Bacterial cells were counted under a phase contrast microscope (BX41, Olympus, Tokyo, Japan) using a hemocytometer (Neubauer improved bright line, Superior Marienfeld, Lauda-Konigshofen, Germany). Bacteria was injected into L6 larvae (1.8 × 105 cells/larva) with a microsyringe (Hamilton, Reno, NV, USA).
Chemicals
L-3,4-dihydroxyphenylalanine (DOPA) was purchased from Sigma-Aldrich Korea (Seoul, Korea) and dissolved in 100 mM PBS before assay. Anticoagulant buffer (ACB) needed to prevent hemocyte coagulation was prepared with 186 mM NaCl, 17 mM Na2EDTA and 41 mM citric acid adjusting the pH at 4.5. Metafectene Pro, a transfection reagent, needed to mix with dsRNA was purchased from Biontex (Plannegg, Germany). Phosphoric acid (100 mM) was used to prepare phosphate-buffered saline (PBS, pH 7.4). Purified recombinant DSP1 (rDSP1) of Spodoptera exigua was prepared in a previous study [11] and used in this study.
Bioinformatics and sequence analysis
Tm-SPE was predicted from the whole genome shotgun contig (GenBank accession number: JABDTM010027791.1) using the Drosophila SPE as bait. The resulting sequence was subjected to further analysis with FGENESH Soft berry program (http://www.softberry.com/berry.phtml) to obtain an open read frame (ORF). ORF sequences were deposited to GenBank (accession number: MZ 190162.1). Phylogenetic analyses were performed using MEGA6 and Clustal W programs from EMBL-EBI (www.ebi.ac.uk) using Neighbor-joining method and the used genes accession numbers are presented in S2 Table. Bootstrapping values were obtained with 1,000 repetitions to support branching and clustering. Protein domains were predicted using SMART search program (http://smart.embl-heidelberg.de/).
RNA extraction and RT-qPCR
Total RNAs from different developmental stages were extracted using 50 eggs, 20 L1 larvae, 10 L2 larvae, 2 L6 larvae, 1 L12 larva, one pupa, and one adult per replication. To extract total RNAs from different tissues, L6 larvae were used. Hemolymph from larvae was collected in ACB by cutting the abdominal tip. The collected hemolymph was centrifuged at 500 × g for 5 min. The resulting hemocyte pellet was used to extract total RNA. The remaining body after hemolymph collection was used to isolate fat body, midgut, and epidermis. Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used for total RNAs extraction. After DNase treatment, 1 μg of total RNA was used to synthesize first-strand cDNAs using RT-Premix oligo-dT following [12]. Resulting cDNAs were used for PCR amplification with gene-specific primers (S1 Table). RT-qPCR was conducted using a Step One Real-Time PCR System (Applied Biosystem, Marsiling, Singapore) following the conditions: 95°C for 10 min for initial heat followed by 40 cycles of 95°C for 30 sec, different annealing temperature for 30 sec and 72°C for 30 sec. T. molitor specific gene, L27a, was used as reference gene [23]. Quantitative analysis was done with a comparative CT method [25] using the reference gene for normalization to estimate mRNA expression levels. Each treatment was replicated three times. Each time with three independent samples.
RNA interference (RNAi) of Tm-SPE expression
Gene-specific primers (S1 Table) containing T7 promoter sequence (5´-TAATACGACTCACTATAGGGAGA-3´) at the 5´ ends were used to amplify template DNA. The resulting PCR product was used to synthesize double-stranded RNA (dsRNA) encoding Tm-SPE (dsSPE) using a MEGAscript RNAi kit (Ambion, Austin, TX, USA) at 37°C for 4 h. Synthesized single-stranded RNAs in both directions were allowed to anneal at 25°C after heat treatment at 75°C for 5 min. Control dsRNA (dsCON) was synthesized from a viral gene, CpBV302 [26]. dsRNAs were mixed with a transfection reagent Metafectene PRO at a 1:1 (v/v) ratio and then incubated at 25°C for 30 minutes to allow liposome formation that increases RNAi efficiency [27]. One μg of dsRNA for each was injected into L6 using 10 μL Hamilton microsyringe. The RNAi efficiency was determined by RT-qPCR against Tm-SPE expression at 24, 48, 72 and 96 h post-injection (PI).
Nodulation assay
Nodule formation assay was analysed to investigate the cellular immune response against E. mundtii or rDSP1 following [28]. Heat-killed E. mundtii (1.8 × 105 cells/larva) was injected to L6 larvae through the pleuron using 10 μL Hamilton microsyringe at 24 h of dsRNA injection to induce hemocyte nodules. Number of nodules formed by T. molitor in the hemocoel as cellular immune response against bacterial challenge were counted. To rescue RNAi effect, recombinant DSP1 (rDSP1) was injected (600 ng/larva) along with bacterial challenge. After 8h incubation at 25°C, treated larvae were dissected under a stereomicroscope (Stemi SV 11, ZEISS, Jena, Germany). Nodules on the fat body were counted after removing the gut. Each treatment was replicated three times. Each time with five larvae.
Phospholipase A2 (PLA2) assay
Both sPLA2 and cPLA2 enzyme activities in treated larvae samples were analyzed as described previously [12] using PLA2 Assay Kit (Cayman Chemical, Ann Arbor, MI, USA). For induction of PLA2 enzyme activity, L6 larvae were injected with 2.2 × 105 cells of heat-killed E. mundtii. Tm-SPE was silenced using dsRNA specific to Tm-SPE by injecting 24 h before bacteria and rDSP1 injection. Hemolymph and fat body samples from treated larvae were collected at 8 h after the bacteria and rDSP1 injection. Plasma was separated from hemocyte by spinning at 500 × g for 5 min. Plasma and fat body samples were used for measuring sPLA2 and cPLA2 activity, respectively. Each treatment was replicated three times. Each time with three independent samples.
Phenoloxidase (PO) enzyme assays
PO activity in plasma of treated larvae was determined using DOPA substrate as described by [12]. To induce PO activity, L6 larvae were challenged with E. mundtii dissolved in PBS before 8h of sample (plasma) collection. Naïve larvae were injected with sterile PBS. The total reaction volume (200 μL) consisted of 180 μL of 10 mM DOPA in PBS and 20 μL of plasma collected from treated larvae. Absorbance was measured at 490 nm using a VICTOR multi label Plate reader (PerkinElmer, Waltham, MA, USA). PO activity was expressed as ΔABS/min/μL of plasma. Each treatment was replicated three times. Each time with three independent samples.
AMP genes expression pattern
Whole body tissues were evaluated for AMP genes expression that represent humoral immune responses. AMP genes expression in L6 larvae were determined after injecting 2.2 × 105 cells of heat killed E. mundtii per larvae at 24 h post injection of dsRNAs. To rescue the RNAi effect, rDSP1 (600 ng/larva) was injected to the larvae along with the bacterial cells. At 8 h of bacteria injection, samples were collected to be used for RNA extraction. Three larvae were used for sample collection and subsequent RNA extraction, for each replication. AMP genes expression levels were assessed by RT-qPCR using gene specific primers (S1 Table) using L27a as reference gene. Each treatment was replicated three times. Each time with three independent samples.
Bioassay of RNAi-treated T. molitor against E. mundtii (Em) and X. hominickii (Xh)
To assess the virulence of Gram-positive E. mundtii and Gram-negative X. hominickii on RNAi-treated T. molitor, L6 larvae were injected with 1 μg of dsRNA specific to Tm-SPE along with transfection agent, metafectene at 1:1 ratio. For control, larvae were injected with control dsRNA along with metafectene. After 24 h of dsRNA injection when the Tm-SPE is mostly silenced, same larvae were injected with either E. mundtii (2.2 x 105 cells/larvae) or X. hominickii (1.8 x 105 cells/larvae) to observe their virulence against T. molitor larvae. For injection, overnight grown bacteria were spined for 5 minute and the pallets were dissolved in PBS. The vial containing the bacterial suspension was vortexed very well to confirm almost equal number of bacterial insertion using Hamilton syringe. The treated larvae were incubated at room temperature (25 ± 2°C) providing enough diet for another 7 days under the rearing conditions and counted the mortality at each 24 h. To check the immune association of DSP1, rDSP1 was injected along with gram positive E. mundtii. Each treatment was replicated three times. Each replication consists of ten larvae.
Statistical analysis
All data for continuous variables were subjected to one-way analysis of variance (ANOVA) using PROG GLM in SAS program [29]. Mortality data were subjected to arcsine transformation and used for ANOVA. Means were compared with the least significant difference (LSD) test at Type I error = 0.05. Median lethal dose (LD50) was obtained from Probit analysis using EPA Probit Analysis Program, ver. 1.5 (U.S. Environmental Protection Agency, USA). Each treatment was replicated three times.
Results
Prediction of spätzle processing enzyme (Tm‐SPE) from T. molitor transcriptome
Tm‐SPE was predicted from a transcriptome (GenBank accession no. JABDTM010027791.1) by interrogation with D. melanogaster SPE sequence (GenBank accession no. NM_142911.4) as a query. It has 99.65 percent sequence similarity with previously predicted Tm-SPE [17] and was deposited to GenBank (Accession no. MZ 190162.1). Its ORF consists of 2217 bp encoding 738 amino acids. The predicted amino acid sequence of Tm‐SPE was compared to those of other insect SPE genes through a phylogenetic analysis where SPE genes of the insects of same order clustered together (Fig 1A). Tm‐SPE shared 52.77%, 49.40%, 32.68%, 36.91% and 36.68% amino acid sequence similarities with SPE genes of Tribolium castaneum, Leptinotarsa decemlineata, Drosophila melanogaster, Plutella xylostella and Apis cerana cerana, respectively. Phylogenetic analysis showed that Tm‐SPE was closely related to other coleopterans. Tm‐SPE comprised of Low Complexity (LC), Internal Repeat 1 (IR1), CLIP and Tryp-Spc domains (Fig 1B) whereas Drosophila SPE contains only Low Complexity (LC) and Tryp-Spc domains.
Fig 1. Molecular characterization of T. molitor Spätzle-Processing Enzyme (Tm-SPE).
(A) Phylogenetic analysis of Tm-SPE with other insect SPE genes using MEGA6 program with a Neighbor-joining method. Bootstrapping values were obtained with 1,000 repetitions to support branching and clustering. Amino acid sequences of SPE were retrieved from GenBank with accession numbers shown in S2 Table. (B) Domain analysis of Tm-SPE. Domains were predicted using Prosite (https://prosite.expasy.org/) and SMART protein (http://smart.embl-heidelberg.de/).
Expression profile of Tm-SPE in Tenebrio molitor
Tm-SPE was expressed in all developmental stages ranging from egg to adult and showed high expression levels at larval stages, especially at L6. However, its expression levels were low at the egg and pupal stage than adult stage (Fig 2A). At L6, it was expressed in all tested tissues including hemocytes, fat body, gut, and epidermis. However, its expression levels were different among tissues, showing a high expression level in the fat body and low expression level in epidermis (Fig 2B). Basal expression levels were significantly upregulated following immune challenge with E. mundtii in whole body tissue where the maximum expression (more than 7-fold) was at 8 h of injection (Fig 2C). However, at very early stage (2 h of injection), expression level was around 3-fold. The upregulation of Tm‐SPE expression after bacterial challenge suggested its immunological function.
Fig 2. Expression profiles of Tm-SPE.

(A) Expression patterns of Tm-SPE at different developmental stages: egg, some larval instar (‘L1, L2, L6, L12’), pupa and adult. (B) Expression patterns in different tissues of L6 larvae: hemocyte (‘HC’), fat body (‘FB’), midgut (‘GT’) and epidermis (‘EPD’). (C) Inducible expression of Tm-SPE in whole body sample of L6 larvae at different time intervals after bacterial infection. For bacterial challenge, heat-killed E. mundtii (‘Em’, 1.8 × 105 cells/larva) were injected into each larva. For control, larvae were injected with PBS. Expression was analyzed with real-time qPCR and up-regulated expression levels were calculated as fold change of the lowest expressed. A ribosomal gene, L27a, was used as internal control [24]. Each treatment was replicated three times with independent samples preparation. Different letters above the standard deviation bar indicate significant differences among means at Type I error = 0.05 (LSD test).
RNAi of Tm-SPE and interruption of nodulation and PO activity
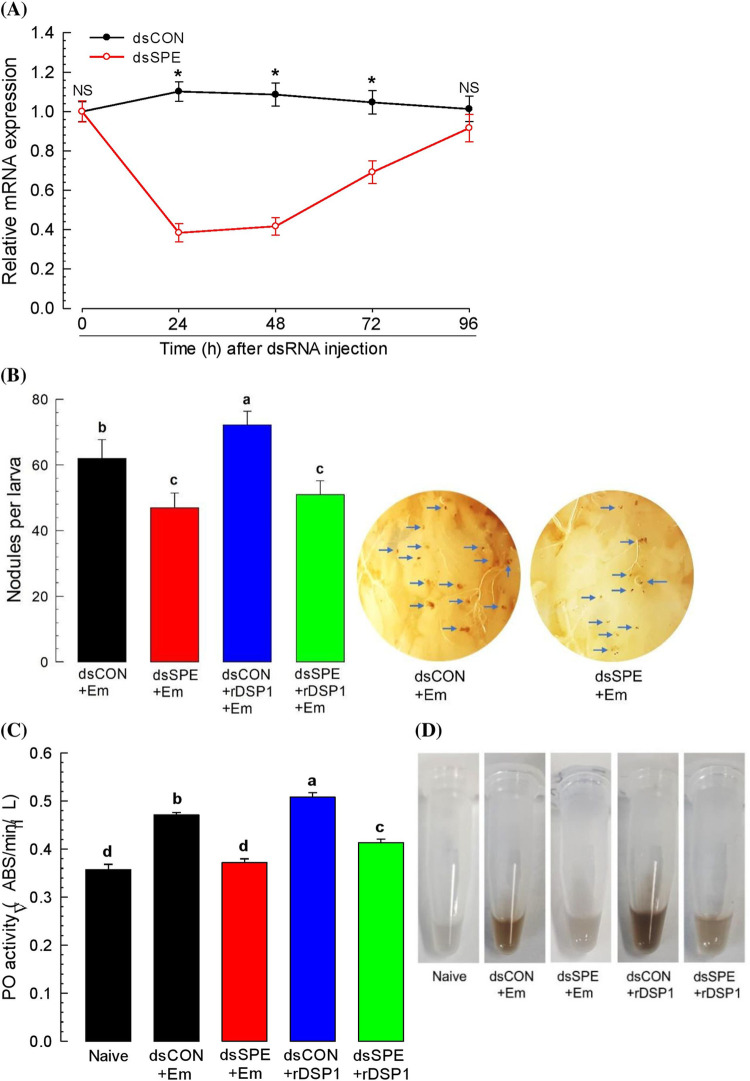
Expression of Tm-SPE was suppressed by RNAi via injection of gene‐specific dsRNA. Such RNAi treatment significantly (P < 0.05) reduced the expression levels around 60 percent at 24 h of dsRNA post injection which continued up to 48 h and then starts to increase. Such reduced levels were recovered at 96 h PI (Fig 3A). These down regulation of Tm-SPE expression denotes the efficiency of RNAi of Tm-SPE gene.
Fig 3. Role of SPE for the induction of immune responses by E. mundtii and rDSP1.
(A) RNAi efficiencies of Tm-SPE gene by injecting gene-specific dsRNA (‘dsSPE’, 1 μg/larva) to L6 larvae. A viral gene, CpBV302, was used as a control dsRNA (‘dsCON’). (B) E. mundtii and rDSP1 induced mediation of hemocyte nodulation, (C) PO activity and (D) Pictorial representation of change in plasma due of PO activity. L6 larvae of T. molitor were injected with E. mundtii (2.2 x 105 cells/larvae) dissolved in PBS or E. mundtii (2.2 x 105 cells/larvae) along with recombinant DSP1 (‘rDSP1, 0.6 μg/larva). At 8 h of E. mundtii or rDSP1 injection, nodules were counted in each larva by dissecting and plasma was collected for PO activity analysis. Naïve was injected with PBS. Knock-down of Tm-SPE expression was done by injecting dsRNA specific to Tm-SPE (1 μg/larva) into L6 larvae before 24 h of E. mundtii or rDSP1 injection. A viral gene, CpBV302, was used as a control dsRNA (‘dsCON’). Each treatment was replicated three times. Asterisks indicate significant differences between dsSPE and dsCON treated group at Type I error = 0.05 (LSD test). Different letters above standard deviation bars denotes significant difference among means at Type I error = 0.05 (LSD test).
To know the immune association of Tm-SPE, nodulation and PO activity was analyzed at both non-silenced and silenced condition of Tm-SPE. At non-suppressed condition, increased number of nodules (62 ± 5.70) formed in the insect hemocoel upon E. mundtii challenge which decreased (47 ± 4.47) significantly (P < 0.05) at the Tm-SPE silenced condition obtained from RNAi of Tm-SPE (Fig 3B). PO activity in larval plasma was also induced by E. mundtii which significantly (P < 0.05) reduced after RNAi of Tm-SPE (Fig 3C). To know the role of Tm-SPE in DSP1 induced immune mediation, nodulation and PO activity was assessed in recombinant DSP1 challenged larvae at both Tm-SPE silenced and non-silenced condition. For both the cases, induction was very high and significantly (P < 0.05) more compared to naïve and even E. mundtii which decreased significantly (P < 0.05) at silenced condition of Tm-SPE gene (Fig 3B and 3C). This result thus denotes that both E. mundtii and rDSP1 depends on SPE for the induction of hemocyte nodulation and PO activity.
Induction of PLA2 activity in T. molitor by E. mundtii or rDSP1
Upregulation of Tm-SPE after immune change indicate its immune association. To validate this, both sPLA2 and cPLA2 activity was analysed upon immune challenge. Induction of sPLA2 activity was significant (P < 0.05) upon E. mundtii challenge which was reduced after RNAi of Tm-SPE (Fig 4A). Similarly, cPLA2 is also induced significantly by E. mundtii but RNAi of Tm-SPE suppressed it (Fig 4B). To see the immune mediation by DSP1, recombinant DSP1 was injected into the larvae of T. molitor. rDSP1 injected larval plasma showed significantly higher sPLA2 (Fig 4A) and cPLA2 activity (Fig 4B) which was reduced significantly after RNAi of Tm-SPE gene. This result suggests that rDSP1 can activate both the sPLA2 and cPLA2 activity similar to E. mundtii depending on SPE for mediation of PLA2 activity.
Fig 4. Functional assay of spätzle processing enzyme (Tm-SPE) for PLA2 activity mediation.

(A) Mediation of sPLA2 activity induced by bacteria and rDSP1 via SPE. (B) Mediation of cPLA2 activity by bacteria and rDSP1 via SPE. L6 larvae were injected with E. mundtii (2.2 x 105 cells/larvae) or recombinant DSP1 (‘rDSP1’, 0.6 μg/larva). Naïve were injected with PBS. dsRNA specific to SPE or dsCON was injected 24 h before bacteria or rDSP1 injection. At 8 h after E. mundtii or rDSP1 injection, Fatbody and plasma was collected to determine cPLA2 and sPLA2 activity, respectively. dsRNA was made from a viral gene (CpBV302). Each treatment was replicated three times. Different letters above standard deviation bars denotes significant difference among means at Type I error = 0.05 (LSD test).
Induction of AMP genes expression in T. molitor by bacteria or rDSP1
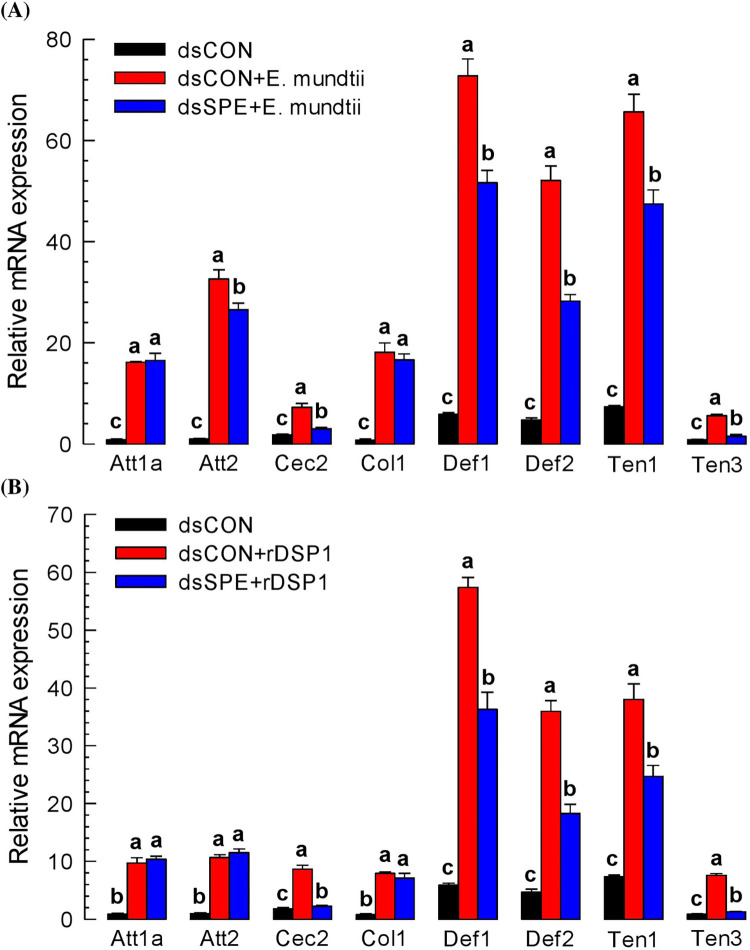
Under induced Tm-SPE expression, immunity‐associated AMP gene’s synthesis was assessed. AMP synthesis in the larval tissues was significantly (P < 0.05) increased after bacterial challenge. Eight AMP genes specific to T. molitor, such as Attachin1a (Att1a), Attachin2 (Att2), Cecropin2 (Cec2), Coleoptericin1 (Col1), Defensin1(Def1), Defensin2(Def2), Tenesin1 (Ten1) and Tenesin3 (Ten3) were analysed for their expression up on bacterial and rDSP1 challenge. All the AMP genes expressed in naïve or control tissue which strongly upregulated up on E. mundtii injection and the numerical value of AMP expression especially for Atta1a, Att2, Col1, Def1, Def2 and Ten1 convey the message (Fig 5A). After RNAi of Tm-SPE, expression of AMP genes decreased for Att2, Cec2, Def1, Def2, Ten1 and Ten3 which indicate that E. mundtii induces the expression of these AMP genes. For rDSP1 induced AMP genes expression, similar trend was observed. After RNAi of Tm-SPE, expression of Cec2, Def1, Def2, Ten1 and Ten3 reduced significantly (P < 0.05) (Fig 5B). This result concludes that Cec2, Def1, Def2, Ten1 and Ten3 AMP genes are activated or controlled by rDSP1.
Fig 5. Expression pattern of AMP genes in T. molitor.
(A) Mediation of AMP genes expression via SPE upon E. mundtii challenge (B) Mediation of AMP genes expression via SPE upon rDSP1 challenge. For AMP genes expression upon bacterial challenge, E. mundtii were dissolved in PBS (‘Em’, 1.8 × 105 cells/larva) and for rDSP1 challenge, purified rDSP1 (0.6 μg/larva) was injected into L6 larva. For analysing expression of Attachin1a (‘Att1a’), Attachin2 (‘Att2’), Cecropin2 (‘Cec2’), Coleoptericin1 (‘Col1’), Defensin1 (‘Def1’), Defensin2 (‘Def2’), Tenesin1 (‘Ten1’) and Tenesin3 (‘Ten3’) AMP genes, whole body samples were collected after 8 h of bacterial or rDSP1 challenge for RNA extraction. For knockdown of Tm-SPE gene, dsRNA specific to Tm-SPE was injected 24 h before E. mundtii or rDSP1 injection. For control, dsRNA made from a viral gene (CpBV302) was injected. Each treatment was replicated three times with independent tissue preparations. Different letters above the bar indicate significant differences among means at Type I error = 0.05 (LSD test).
Effect of spätzle processing enzyme (SPE) on susceptibility of T. molitor
Toll-spätzle immune signaling pathway is activated by Gram-positive bacteria. To confirm the involvement of Tm-SPE in Toll pathway activation, we checked virulence of both Gram-positive (E. mundtii) and Gram-negative (X. hominickii) bacteria against T. molitor larvae after individual RNAi treatment with dsSPE. Control larvae were treated with dsCON. For control larvae, E. mundtii found less virulent compared to X. hominickii whereas E. mundtii showed more virulence than X. hominickii against dsSPE treated larvae (Fig 6A). This indicate that SPE of T. molitor Toll pathway is essential for immune activation in Gram positive E. mundtii bacterium. To confirm the immune role of DSP1, recombinant DSP1 was injected into T. molitor larvae which significantly (P < 0.05) decreased the mortality in control larvae but no significant change in SPE silenced larvae (Fig 6B). This suggested that DSP1 is dependent on SPE for immune mediation. This finding concludes that DSP1 depend on SPE to follow the Toll-spätzle pathway to induce the immune activation.
Fig 6. Comparative virulence analysis of E. mundtii (‘Em’) and X. hominickii (‘Xh’) bacteria against T. molitor larvae.
(A) Individual RNAi treatment targeting SPE gene and (B) Immune induction by rDSP1 to increase survivability. For survivability test, Gram positive E. mundtii (3 x 104 cells/larva) and Gram-negative X. hominickii (2.5 x 104 cells/larva) were injected to L6 larvae of T. molitor already (24 h before) injected with dsSPE and dsCON (1 μg/larva). dsCON was made from a viral gene CpBV302. For immune mediation test of rDSP1, at 24 h post injection of dsRNA, E. mundtii (3 x105 cells/larva) or E. mundtii along with rDSP1 (0.6 μg/larva) was injected to larvae with a Hamilton micro syringe. Mortality was recorded up to 7 days after treatment (‘DAT’). Each treatment was replicated three times and each replication used 10 larvae. Different letters above standard deviation bars denote significant difference among means at Type I error = 0.05 (LSD test).
Discussion
Dorsal switch protein 1 (DSP1), an insect homolog of vertebrate HMGB1 protein was characterized as a co-repressor of Dorsal protein in Drosophila [7]. Dorsal is one of the transcriptional activators from the Rel/NF-kB family [7]. In Drosophila, DSP1 also play role in embryo development, differentiation, and segmentation [6, 30, 31]. In mosquito, Aedes aegypti, another HMGB1 having high homologies with mammalian HMGB1 and Drosophila DSP1 [32] has been identified. This mosquito HMGB1 facilitate the binding of transcriptional factor ‘Rel’ to the nuclear factor kappa B (NF-kB) promoter for antiviral gene expression against a dengue viral infection [33]. A lepidopteran insect (Plodia interpunctella), apart from dipterans, also possesses HMGB1-like proteins [34]. Recent study revealed the DAMP role of DSP1 in lepidopteran S. exigua [11], coleopteran T. molitor [12] and dipteran Aedes albopictus [35] that mediate different immune responses like PLA2 activity, nodulation formation, AMP synthesis, PO activity etc. [11–13]. Subsequent study in S. exigua reports that DSP1 uses Toll-spätzle pathway for immune mediation [14]. But we do not know the role of SPE in this pathway. To address this, we coined this study in T. molitor to know the involvement of SPE in DSP1 induced immune mediation through Toll-spatzle pathway. For this, recombinant DSP1 was injected into the larvae to observe gain of function or additive immune responses and lose of function after RNAi of SPE.
Toll immune signalling pathway initiated with the recognition of lysine-type peptidoglycan (PG) triggering the activation of serine protease (SP) cascade needs to form active Spätzle [15]. This active Spatzle binds with Toll receptor and Toll signaling is activated. In Tenebrio molitor, SP cascade consists of three SPs [17], starting with modular serine protease (modSP) that binds to the lysine-type PG recognition complex in upstream and activate the second SP, Spätzle activating enzyme (SAE), which in turn activates Spätzle processing enzyme (SPE), the last SP [17, 19]. SPE cleave the inactive pro-Spätzle to form active Spätzle, which binds to Toll receptor and triggers its specific immune signalling pathway [21, 36]. In other direction, SPE cleaves inactive prophenoloxidase (PPO) to active phenoloxidase (PO) [22]. Thus, SPE mediates Toll immune signalling pathway to produce antimicrobial peptides (AMPs), PLA2 activity and the melanisation immune responses [19] including PO activity, nodulation etc. In this study, we found that Gram-positive E. mundtii and rDSP1 upregulated the expression of Attachin1a (‘Att1a’), Attachin2 (‘Att2’), Cecropin2 (‘Cec2’), Coleoptericin1 (‘Col1’), Defensin1 (‘Def1’), Defensin2 (‘Def2’), Tenesin1 (‘Ten1’) and Tenesin3 (‘Ten3’) AMP genes (Fig 5A). A previous study in T. molitor reported that Att1, Att2, Cec and Def was upregulated by both Gram-positive bacteria and rDSP1 [12]. Another study in S. exigua revealed that Apolipophorin (Apol), Attachin (Att1, Att2), Cecropin (Cec), Defensin (Def), Gallerimycin (Gal), Gloverin (Glv), Lysozyme (Lyz) etc. AMP genes were induced by E. mundtii [14]. Gram-positive E. mundtii and rDSP1 also increase the PLA2 activity (Fig 4A and 4C), PO activity (Fig 3C) and hemocyte nodule formation (Fig 3A). In contrast, RNAi of SPE gene interrupt the immune responses of AMP synthesis (Fig 5B), PLA2 activity (Fig 4B and 4D), PO activity (Fig 3D) and hemocyte nodulation (Fig 3B). This indicates that SPE control these immune responses. Interruption of these immune responses by RNAi of SPE lead to increased mortality of T. molitor larvae upon E. mundtii infection (Fig 6). However, in controlled condition, X. hominickii shows more virulence to T. molitor larvae because X. hominickii is an entomopathogenic bacterium that release different chemicals [28, 37] to host for immune suppression [38, 39], Toxemia or apoptosis [40] resulting host mortality. There was no significant difference in virulence of X. hominickii at Tm-SPE silenced condition indicating non-association of SPE to X. hominickii (Fig 6A). Thus, we can conclude that Spatzle processing enzyme (SPE) is essential for E. mundtii and DSP1 to activate Toll immune signaling pathway. To confirm the exact target of DSP1 protein in Toll pathway we need to check the upstream targets of SAE or modSP also.
In summary, DSP1 depends on SPE to activate the Toll signaling pathway required for innate immune response like nodulation, PLA2 and PO activity; AMP gene synthesis in Tenebrio molitor (Fig 7). However, all the AMP genes in T. molitor are not activated by this DSP1-Toll signaling pathway.
Fig 7. A working hypothesis of Tm-DSP1 for mediating immune responses via Toll-Spatzle (‘Spz’) signalling pathway.
Upon challenge with gram positive bacteria including a non-entomopathogenic bacterium, E. mundtii, Tm-DSP1 is secreted to the plasma to activate serine protease (SP) cascade for activating phenoloxidase (‘PO’) and Spz. Activated PO can catalyze melanin formation to suppress the growth of pathogenic bacteria. Besides, activated Spz can bind to Tm-Toll receptor to activate PLA2 and the expression of antimicrobial peptide [Cecropine2 (‘Cec2’), Defencine1 (‘Def1’), Defencine2 (‘Def2’), Tenecine1 (‘Ten1’) and Tenecine3 (‘Ten3’)) genes. Activated PLA2 can catalyze eicosanoid biosynthesis to mediate cellular immune responses to defend bacterial infection along with AMPs.
Supporting information
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
The author express sincere gratefulness to Professor Yonggyun Kim for supports to conduct the study and Youngim Song for supplying experimental materials.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author received no specific funding for this work.
References
- 1.Bianchi ME. HMGB1 loves company. J Leukoc Biol. 2009; 86: 573–576. doi: 10.1189/jlb.1008585 [DOI] [PubMed] [Google Scholar]
- 2.Ivics Z, Kaufman CD, Zayed H, Miskey C, Walisco O, Izsvak Z. The Sleeping Beauty transposable element: Evolution, regulation, and genetic applications. Current Issues in Molecular Biol. 2004; 6(1): 43–55. 10.21775/cimb.006.043. [DOI] [PubMed] [Google Scholar]
- 3.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta. 2010; 1799: 101–113. doi: 10.1016/j.bbagrm.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 4.Malarkey CS, Churchill MEA. The high mobility group box: The ultimate utility player player of a cell. Trends Biochem Sci. 2012; 37: 553–562. doi: 10.1016/j.tibs.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jong SR, Dong HS. Damage-associated molecular patterns in inflammatory diseases. Immune Network. 2018; 18: 1–14. 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosrin-Huaman C, Canaple L, Locker D, Decoville M. DSP1 gene of Drosophila melanogaster encodes an HMG-domain protein that plays multiple roles in development. Dev Genet. 1998; 23: 324–334. doi: [DOI] [PubMed] [Google Scholar]
- 7.Lehming N, Thanos D, Brickman JM, Ma J, Maniatis T, et al. An HMG like protein that can switch a transcriptional activator to a repressor. Nature. 1994; 371: 175–179. doi: 10.1038/371175a0 [DOI] [PubMed] [Google Scholar]
- 8.Decoville M, Giacomello E, Leng M, Locker D. DSP1, an HMG-like protein, is involved in the regulation of homeotic genes. Genetics. 2001; 157: 237–244. doi: 10.1093/genetics/157.1.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janke C, Martin D, Giraud-Panis MJ, Decoville M, Locker D. Drosophila DSP1 and Rat HMGB1 Have Equivalent DNA Binding Properties and Share a Similar Secondary Fold. J. Biochem. 2003; 133: 533–539. doi: 10.1093/jb/mvgO63 [DOI] [PubMed] [Google Scholar]
- 10.Decoville M, Giraud-Panis MJ, Mosrin-Huaman C, Leng M, Locker D. HMG boxes of DSP1 protein interact with the Rel homology domain of Transcription factors. Nucleic Acids Res. 2000; 28(2): 454–462. doi: 10.1093/nar/28.2.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mollah MMI, Choi HW, Yeam I, Lee JM, Kim Y. Salicylic acid, a plant hormone, suppresses phytophagous insect immune response by interrupting HMG-like DSP1. Front Physiol. 2021a; 12: 744272. doi: 10.3389/fphys.2021.744272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mollah MMI, Kim Y. HMGB1‐like dorsal switch protein 1 of the mealworm, Tenebrio molitor, acts as a damage‐associated molecular pattern. Arch Insect Biochem Physiol.2021; e21795. 10.1002/arch.21795. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Ahmed S, Stanley D, An C. Eicosanoid-mediated immunity in insects. Dev Comp Immunol. 2018; 83: 130–143. doi: 10.1016/j.dci.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Mollah MMI, Ahmed S, Kim Y. Immune mediation of HMG‐like DSP1 via Toll‐Spätzle pathway and its specific inhibition by salicylic acid analogs. PLoS Pathog. 2021. b; 17(119): e1009467. 10.1371/journal.ppat.1009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemaitre B, Hoffmann J. The host defence of Drosophila melanogaster. Annu Rev Immunol. 2007; 25: 697–743. https://10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 16.Valanne S, Wang JH, Ramet M. The Drosophila Toll Signaling Pathway. J Immunol. 2011; 186 (2): 649–656. doi: 10.4049/jimmunol.1002302 [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Kim SJ, Kan H, Kwon HM, Roh KB, Jiang R, et al. A Three-step Proteolytic Cascade Mediates the Activation of the Peptidoglycan-induced Toll Pathway in an Insect. J Boil Chem. 2008; 283 (12): 7599–7607. 10.1074/jbc.M710216200. [DOI] [PubMed] [Google Scholar]
- 18.Roh KB, Kim CH, Lee H, Kwon HM, Park JW, Ryu JH, et al. Proteolytic Cascade for the Activation of the Insect Toll Pathway Induced by the Fungal Cell Wall Component. J Biol Chem. 2009; 284 (29): 19474–19481. doi: 10.1074/jbc.M109.007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kan H, Kim CH, Kwon HM, Park JW, Roh KB, Lee H, et al. Molecular control of phenol-oxidase-induced melanin synthesis in an insect. J Biol Chem. 2008; 283:25316–25323. doi: 10.1074/jbc.M804364200 [DOI] [PubMed] [Google Scholar]
- 20.Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, Ochiai M, et al. A Spatzle-Processing Enzyme Required for Toll Signaling Activation in Drosophila Innate Immunity. Develop Cell. 2006; 10: 45–55. 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury M, Li CF, He Z, Lu Y, Liu XS, Wang YF, et al. Toll family members bind multiple Spätzle proteins and activate antimicrobial peptide gene expression in Drosophila. J Biol Chem. 2019; 294: 10172–10181. 10.1074/jbc.RA118.006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon TH, Kim MS, Choi HW, Joo CH, Cho MY, Lee BL. A masquerade-like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae. Eur J Biochem. 2000; 267: 6188–6196. 10.1046/j.1432-1327.2000.01695.x [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Masri J, Perez V, Maya C, Zhao J. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials‐supplemented diets. Foods. 2020; 9: 1–10. doi: 10.3390/foods9020151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keshavarz M, Jo YH, Patnaik BB, Park KB, Ko HJ, Kim CE, et al. TmRelish is required for regulating the antimicrobial responses to Escherichia coli and Staphylococcus aureus in Tenebrio molitor. Sci Rep. 2020; 10: 425. 10.1038/s41598-020-61157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐ΔΔCT method. Methods. 2001; 25: 402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Park B, Kim Y. Transient transcription of a putative RNase containing BEN domain encoded in Cotesia plutellae bracovirus induces an immunosuppression of the diamondback moth, Plutella xylostella. J Invertebr Pathol. 2010; 105: 156–163. 10.1016/j.jip.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Cooper AMW, Song H, Yu Z, Biondi M, Bai J, Shi X, et al. Comparison of strategies for enhancing RNA interference efficiency in Ostrinia nubilalis. Pest Manag Sci. 2021; 77(2): 635–645. doi: 10.1002/ps.6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollah MMI, Kim Y. Virulent secondary metabolites of entomopathogenic bacteria genera, Xenorhabdus and Photorhabdus, inhibit phospholipase A2 to suppress host insect immunity. BMC Microbiol. 2020; 20: 359. 10.1186/s12866-020-02042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute Inc. SAS/STAT 15.1 user’s guide, Release 6.03, Ed. Cary, NC (1989). [Google Scholar]
- 30.Salvaing J, Decoville M, Mouchel-Vielh E, Bussiere M, Daulny A, et al. Corto and DSP1 interact and bind to a maintenance element of the Scr Hox gene: understanding the role of Enhancers of trithorax and Polycomb. BMC Biol. 2006; 4: 9. doi: 10.1186/1741-7007-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamiable O, Rabhi M, Peronnet F, Locker D, Decoville M. Rm62, a DEAD-box RNA helicase, complexes with DSP1 in Drosophila embryos. Genesis. 2010; 48: 244–253. doi: 10.1002/dvg.20609 [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro FS, de Abreu da Silva IC, Carneiro VC, dos Santos Belgrano F Mohana-Borges R, de Andrade Rosa I, et al. The dengue vector Aedes aegypti contains a functional high mobility group box 1 (HMGB1) protein with a unique regulatory C-terminus. PLoS One. 2012; 7: e40192. doi: 10.1371/journal.pone.0040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Mendonça Amarante A, Jupatanakul N, de Abreu da Silva IC, Carneiro VC, Vicentino ARR, Dimopolous G, et al. The DNA chaperone HMGB1 potentiates the transcriptional activity of Rel1A in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2017; 80: 32–41. 10.1016/j.ibmb.2016. 11.006. [DOI] [PubMed] [Google Scholar]
- 34.Aleporou-Marinou V, Drosos Y, Ninios Y, Agelopoulou B, Patargias T. High mobility group-like proteins of the insect Plodia interpunctella. Biochem Genet. 2003; 41: 39–46. 10.1023/A:1020922512440. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S, Sajjadian SM, Mollah MMI, Kim Y. HMGB1-Like Dorsal Switch Protein 1 Triggers a Damage Signal in Mosquito Gut to Activate Dual Oxidase via Eicosanoids. J Innate Immun. 2022; 14(6): 657–672. doi: 10.1159/000524561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang R, Kim EH, Gong JH, Kwon HM, Kim CH, Ryu KH, et al. Three pairs of protease serpin complexes cooperatively regulate the insect innate immune responses. J Biol Chem. 2009; 284: 35652–35658. doi: 10.1074/jbc.M109.071001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mollah MMI, Roy MC, Choi DY, Hasan MA, Al Baki MA, Yeom HS, et al. Variations of Indole Metabolites and NRPS-PKS Loci in Two Different Virulent Strains of Xenorhabdus hominickii. Front Microbiol. 2020. a; 11: 583594. doi: 10.3389/fmicb.2020.583594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mollah MMI, Dekebo A, Kim Y. Immunosuppressive Activities of Novel PLA2 Inhibitors from Xenorhabdus hominickii, an Entomopathogenic Bacterium. Insects. 2020. b; 11: 505 doi: 10.3390/insects11080505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasan MA, Ahmed S, Mollah MMI, Lee D, Kim Y. Variation in pathogenicity of different strains of Xenorhabdus nematophila; Differential immunosuppressive activities and secondary metabolite production. J Invertebr Pathol. 2019; 166(2019) 107221. 10.1016/j.jip.2019.107221. [DOI] [PubMed] [Google Scholar]
- 40.Mollah MMI, Yeasmin F, Kim Y. Benzylideneacetone and other phenylethylamide bacterial metabolites induce apoptosis to kill insects. J Asia-Pacific Entomol. 2020. c; 23 (2): 449–457. 10.1016/j.aspen.2020.03.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.