Abstract
Rationale
Sleep-disordered breathing (SDB) is associated with increased complications and length of stay (LOS) after surgery. SDB-related adverse consequences for nonsurgical admissions are not well defined.
Objectives
Evaluate associations between SDB and subtypes and LOS, cost, and mortality in nonsurgical patients.
Methods
This retrospective cohort analysis used adult nonsurgical admissions from the 2017 National Inpatient Sample of the Healthcare Costs and Utilization Project. SDB associations with LOS (primary outcome), costs, and mortality were evaluated via logistic regression. Covariates included age, sex, Elixhauser Comorbidity Index, socioeconomic status, hospital type, and insurance type.
Results
The cohort included 6,046,544 hospitalizations. Compared with those without SDB, patients with SDB were older (63.6 ± 13.5 vs. 57.4 ± 20.7 yr), higher proportion male (55.8% vs. 40.9%), and more likely to be White (75.7% vs. 66.5%). SDB was associated with increased odds of increased LOS and hospitalization costs (odds ratio [OR], 1.17; 95% confidence interval [CI], 1.16–1.17 and OR, 1.67; 95% CI, 1.66–1.67 in adjusted analyses, respectively) but lower mortality (OR, 0.79; 95% CI, 0.77–0.81). The results for obstructive sleep apnea (OSA) echoed those for SDB. Obesity hypoventilation syndrome had substantially increased LOS (OR, 3.05; 95% CI, 2.98–3.13), mortality (1.76; 95% CI, 1.66–1.86), and costs (OR, 2.67; 95% CI, 2.60–2.73) even after adjustment.
Conclusions
Obesity hypoventilation syndrome is associated with higher LOS, mortality, and costs during hospitalization, whereas OSA, despite higher LOS and costs, is associated with decreased mortality. Investigation is warranted on whether paradoxically higher costs but lower mortality in OSA may be indicative of less vigilance in hospitalized patients with undiagnosed SDB.
Keywords: sleep-disordered breathing, sleep apnea, obesity hypoventilation syndrome, length of stay, healthcare utilization
Sleep-disordered breathing (SDB) is common, with obstructive sleep apnea (OSA) prevalence of 26% in the United States (1, 2). Guidelines recommend sleep apnea screening before surgical procedures because of strong evidence that untreated SDB increases risk of perioperative complications (3–6). Complications are respiratory and cardiac: an increased rate for ICU admission and either noninvasive ventilation or intubation (7). Past work in patients undergoing bariatric surgery showed lower mortality, total charges, and length of stay (LOS) but increased odds of intubation, noninvasive ventilation, and atrial fibrillation in patients with SDB (8). Patients with a high OSA risk had higher rapid response system activation than those at lower risk for OSA; furthermore, those with a high risk of OSA who were adherent to positive airway pressure (PAP) therapy had had a reduction in rapid response system activation compared with patients sans therapy (9). Moreover, in those with chronic obstructive pulmonary disease (COPD), those at high risk of OSA had higher 6-month mortality and readmission rates (10).
SDB is associated with repeated episodes of hypoxemia (from OSA) and, in obesity hypoventilation syndrome (OHS), hypercapnia (11). OHS causes additional cardiac stress and remodeling (11, 12). Respiratory system stresses may result in a lower threshold for acute respiratory failure and the need for care intensification. An unstable upper airway coupled with the use of opioids and other respiratory depressants may increase the risk of reintubation or noninvasive ventilation. Repeated intubation increases the risk for complications (e.g., ventilator-associated pneumonia). Overall, SDB may aggravate respiratory failure and lead to increased LOS and complications.
The gap this study examines is preexisting and potential risk management for SDB during nonsurgical hospitalization and, particularly, respiratory failure. Findings may justify changes in reimbursement or the need for additional interventions, monitoring, and management. We hypothesize that SDB, and particularly OHS, is associated with increased LOS, mortality, and cost during hospitalization for respiratory failure.
Methods
Study Design, Data Sources, and Study Population
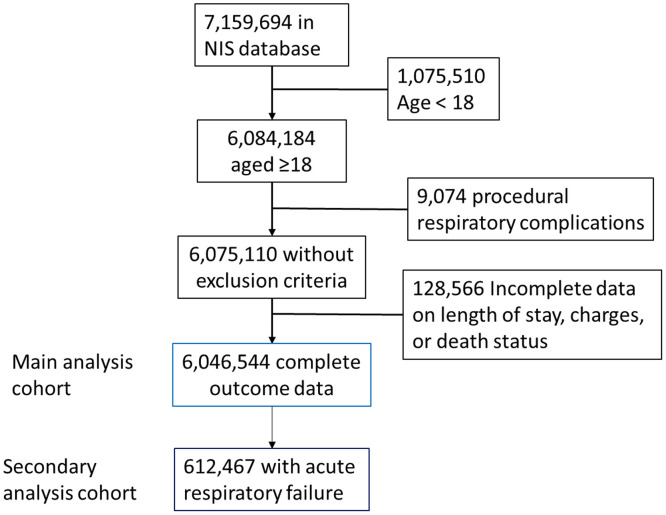
This retrospective cohort study evaluated the role of SDB in nonsurgical hospitalization using data from the 2017 U.S. Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality (13, 14). Additional information about the NIS is in the Methods section in the data supplement. To focus on medical rather than postoperative admissions, the cohort consisted of people aged ⩾18 years without respiratory failure codes related to surgical procedures (see Table E1 in the data supplement) (n = 6,075,110). Only cases with complete data on outcomes—LOS, mortality, and costs—were included. The final analytic cohort included 6,046,544 cases (Figure 1).
Figure 1.
Study flow.
Variables of interest
The primary outcome is LOS, measured in whole-day increments. Secondary outcomes include in-hospital mortality and hospitalization costs. The primary independent variable is SDB, defined as sleep apnea and obesity hypoventilation syndrome using International Classification of Diseases, 10th Revision, Clinical Modification billing codes (Table E1). We identified billing codes indicating acute respiratory failure (ARF) based on established methodology (15–17). Billing codes were used to identify comorbid asthma, COPD, and heart failure. Patient information included age at hospitalization, sex, race, insurance type, and patient zip code median income as a proxy for socioeconomic status. Race, where available, was classified as white, black, Hispanic, and other. Insurance type was classified as Medicare, Medicaid, private, self-pay, no charge, or other. The zip code median incomes were divided into quartiles: 1) $1–$24,999; 2) $25,000–$34,999; 3) $35,000–$45,000; and 4) $45,000 or more. Hospital-level variables include size (small, medium, large), location (rural or urban), and teaching status (teaching hospital vs. not). The HCUP Elixhauser comorbidity software was used to calculate the Elixhauser disease severity score for each hospital encounter (18).
Analytic approach
Participant characteristics were summarized as mean ± SD, median (interquartile range [IQR]), or n (%). We calculated the standardized mean difference (SMD) between SDB and control populations; SMD < 0.10 is considered negligible.
Multivariable logistic regression was used to evaluate associations between SDB and each outcome in turn. We dichotomized LOS and costs based on median value because of distribution skewedness. To minimize confounding from severity of disease, we used the Elixhauser Comorbidity Index, a validated score associated with in-hospital mortality, for adjustment (19). Adjusted models include age, sex, presence of comorbid disease using Elixhauser Comorbidity Index using van Walraven weights, median income quartiles (as a surrogate for socioeconomic status), insurance type, hospital size (small, medium, or large), hospital type (rural vs. urban, teaching vs. nonteaching, and government vs. private nonprofit vs. private for profit).
Participants with codes for both OHS and OSA were included in OHS but not OSA subset analyses. Subset analyses evaluated 1) OSA and OHS individually; 2) admissions with billing codes for respiratory failure; 3) stratified by age; and 4) stratified by obesity based on International Classification of Diseases, 10th Revision coding. Additional sensitivity analyses are described in the data supplement and include mortality analyses stratified by obesity status to address possible confounding by obesity, survival mortality analyses, and linear analyses of cost and LOS. All analyses were two sided, with α = 0.05, and were conducted using SAS software (SAS Institute Inc.) and R software (R Core Development Team) (20).
Results
Study Population
Table 1 reports the baseline characteristics of the cohort. There were 6,046,544 admissions in the cohort, with an average age of 57.9 ± 20.3 years, 57.8% female, and primarily White (67.3%). Most hospitalizations were in urban teaching hospitals (67.6%), with a median LOS of 3 days (IQR, 2–5). These hospitalizations were primarily paid by Medicare (47.7%). Asthma (15.8%), COPD (16.0%), and heart failure (16.9%) were common in this cohort.
Table 1.
Baseline characteristics
| Characteristic | Overall (N = 6,046,544) |
SDB (n = 525,524) |
No SDB (n = 5,521,020) |
SMD* |
|---|---|---|---|---|
| Age, yr | 57.9 ± 20.3 | 63.6 ± 13.5 | 57.4 ± 20.7 | 0.36 |
| Female sex | 3,493,030 (57.8) | 232,078 (44.2) | 3,260,952 (59.1) | 0.30 |
| Race | 0.25 | |||
| White | 3,929,279 (67.3) | 382,691 (75.7) | 3,546,588 (66.5) | |
| Black | 881,522 (15.1) | 73,025 (14.4) | 808,497 (15.2) | |
| Hispanic | 650,072 (11.1) | 32,058 (6.3) | 618,014 (11.6) | |
| Other | 375,059 (6.4) | 17,591 (3.5) | 357,468 (6.7) | |
| Zip code median income† | 0.06 | |||
| <$25,000 | 1,806,702 (30.4) | 146,910 (28.4) | 1,659,792 (30.6) | |
| $25,000–$34,999 | 1,580,608 (26.6) | 142,777 (27.6) | 1,437,831 (26.5) | |
| $35,000–$44,999 | 1,393,730 (23.5) | 128,379 (24.8) | 1,265,351 (23.3) | |
| ⩾$45,000 | 1,160,803 (19.5) | 99,894 (19.3) | 1,060,909 (19.6) | |
| Primary insurance type | 0.35 | |||
| Medicare | 2,881,031 (47.7) | 318,636 (60.7) | 2,562,395 (46.5) | |
| Medicaid | 1,118,939 (18.5) | 53,699 (10.2) | 1,065,240 (19.3) | |
| Private | 1,612,857 (26.7) | 129,285 (24.6) | 1,483,572 (26.9) | |
| Self-pay | 235,080 (3.9) | 8,905 (1.7) | 226,175 (4.1) | |
| Other | 20,085 (0.3) | 690 (0.1) | 19,395 (0.4) | |
| Comorbidities | ||||
| Elixhauser Comorbidity Index | 5.60 ± 9.69 | 5.72 ± 9.94 | 5.58 ± 9.66 | 0.01 |
| Acute respiratory failure | 612,467 (10.1) | 102,211 (19.4) | 510,256 (9.2) | 0.29 |
| Asthma | 956,380 (15.8) | 154,654 (29.4) | 801,726 (14.5) | 0.37 |
| COPD | 966,609 (16.0) | 166,963 (31.8) | 799,646 (14.5) | 0.42 |
| Heart failure | 1,023,656 (16.9) | 182,752 (34.8) | 840,904 (15.2) | 0.46 |
| Hospital characteristics | ||||
| Hospital type | 0.14 | |||
| Government | 696,993 (11.5) | 50,064 (9.5) | 646,929 (11.7) | |
| Private nonprofit | 4,461,986 (73.8) | 415,257 (79.0) | 4,046,729 (73.3) | |
| Private for-profit | 887,565 (14.7) | 60,203 (11.5) | 827,362 (15.0) | |
| Hospital teaching/location | 0.05 | |||
| Rural | 561,971 (9.3) | 44,360 (8.4) | 517,611 (9.4) | |
| Urban nonteaching | 1,396,110 (23.1) | 116,102 (22.1) | 1,280,008 (23.2) | |
| Urban teaching | 4,088,463 (67.6) | 365,062 (69.5) | 3,723,401 (67.4) | |
| Hospital size, number of beds | 0.02 | |||
| Small | 1,213,184 (20.1) | 103,982 (19.8) | 1,109,202 (20.1) | |
| Medium | 1,781,936 (29.5) | 151,038 (28.7) | 1,630,898 (29.5) | |
| Large | 3,051,424 (50.5) | 270,504 (51.5) | 2,780,920 (50.4) | |
| Hospitalization characteristics | ||||
| Mortality | 134,938 (2.2) | 9,782 (1.9) | 125,156 (2.3) | 0.03 |
| Length of stay, d | 3 (2–5) | 3 (2–6) | 3 (2–5) | 0.06 |
| Total costs, $ | 7,960 (4,575–14,589) | 10,715 (6,272–18,103) | 7,720 (4,460–14,216) | 0.16 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; SDB = sleep-disordered breathing; SMD = standardized mean difference.
Statistics are presented as mean ± SD, median (25th percentile–75th percentile), or n (%).
SMDs of >10% are in bold.
Median income based on zip code.
In the main analytic cohort, only 8.7% of hospitalizations were coded for SDB. Most with SDB had OSA (94.7%), but 6.7% had OHS. Those with SDB were substantially older (63.6 ± 13.52 yr vs. 57.4 ± 20.7 yr without SDB; SMD, 0.36) and more likely to be male (55.8% vs. 40.9%; SMD, 0.30) and White (75.7% vs. 66.5%; SMD, 0.25). Groups had similar Elixhauser Comorbidity Index values (5.72 ± 9.94 vs. 5.58 ± 9.66, respectively; SMD, 0.01), and in-hospital mortality (1.9% vs. 2.3%; SMD, 0.03). SDB-affected individuals were more prone to respiratory failure (19.4% vs. 9.2%; SMD, 0.29) and common associated chronic underlying conditions: asthma (29.4% vs. 14.5%; SMD, 0.37), COPD (31.8% vs. 14.5%; SMD, 0.42), and heart failure (34.8% vs. 15.2%; SMD, 0.46). Twenty-four percent of people with OHS also had OSA.
Primary and Secondary Analyses
SDB was associated with increased total LOS both in unadjusted (odds ratio [OR], 1.23; 95% confidence interval [CI], 1.23–1.24) and adjusted analyses (OR, 1.17; 95% CI, 1.16–1.17; Table 2). Mortality was lower in those with SDB versus those without (9,782/525,524 [1.9%] in those with vs. 125,156/5,521,020 [2.3%] in those without SDB). This was observed in regression models in unadjusted analyses (OR, 0.82; 95% CI, 0.80–0.83) and of similar magnitude after adjustment (OR, 0.79; 95% CI, 0.77–0.81). SDB was associated with higher costs in unadjusted analyses (OR, 1.92; 95% CI, 1.90–1.93), with minor attenuation in adjusted analyses (OR, 1.67; 95% CI, 1.66–1.68).
Table 2.
Results of univariate and multivariable logistic regression models of length of stay, mortality, and costs (U.S. dollars) in relation to sleep-disordered breathing and its subtypes
| Sleep-disordered Breathing |
Obstructive Sleep Apnea |
Obesity Hypoventilation |
||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Length of stay | 1.23 | 1.17 | 1.18 | 1.11 | 3.26 | 3.05 |
| (1.23–1.24) | (1.16–1.17) | (1.17–1.18) | (1.11–1.12) | (3.18–3.34) | (2.98–3.13) | |
| Mortality | 0.82 | 0.79 | 0.76 | 0.74 | 1.74 | 1.76 |
| (0.80–0.83) | (0.77–0.81) | (0.75–0.78) | (0.72–0.75) | (1.65–1.84) | (1.66–1.86) | |
| Costs | 1.92 | 1.67 | 1.88 | 1.63 | 2.72 | 2.67 |
| (1.90–1.93) | (1.66–1.68) | (1.87–1.89) | (1.62–1.64) | (2.66–2.79) | (2.60–2.73) | |
| Acute respiratory failure | ||||||
| Length of stay | 1.20 | 1.37 | 1.12 | 1.27 | 1.77 | 2.12 |
| (1.18–1.22) | (1.35–1.39) | (1.10–1.14) | (1.25–1.30) | (1.71–1.84) | (2.04–2.21) | |
| Mortality | 0.41 | 0.49 | 0.42 | 0.49 | 0.33 | 0.44 |
| (0.40–0.42) | (0.47–0.50) | (0.41–0.43) | (0.47–0.50) | (0.31–0.35) | (0.41–0.46) | |
| Costs | 1.09 | 1.26 | 1.03 | 1.17 | 1.52 | 1.83 |
| (1.08–1.11) | (1.24–1.28) | (1.01–1.05) | (1.15–1.19) | (1.47–1.58) | (1.77–1.90) | |
All results are presented as odds ratios (95% confidence intervals).
Subset and sensitivity analyses
Because the majority of SDB was OSA, effect estimates for OSA mirror those of SDB with increased LOS and costs but decreased mortality (Table 2). OSA was associated with lower in-hospital mortality (8,382/488,922 [1.9%]), whereas OHS had a substantially increased mortality (1,359/35,075 [3.9%]). However, those with OHS had substantially higher odds of longer LOS in unadjusted (OR, 3.26; 95% CI, 3.18–3.34) and adjusted analyses (OR, 3.05; 95% CI, 2.98–3.13). OHS was associated with increased mortality, with no attenuation after adjustment (OR, 1.74; 95% CI, 1.65–1.84 in unadjusted and OR, 1.76; 95% CI, 1.66–1.86 in adjusted analyses). OHS diagnosis was associated with higher odds of higher costs (OR, 2.67; 95% CI, 2.60–2.73 in adjusted analyses).
Subset analyses of respiratory failure hospitalizations largely mirror those of the overall cohort (Table 2). In contrast with other analyses, mortality in OHS with ARF is substantially lower in both unadjusted and adjusted analyses (OR, 0.33; 95% CI, 0.31–0.35 and OR, 0.44; 95% CI, 0.41–0.46, respectively), with effect estimates similar to the overall cohort and OSA subgroup with respiratory failure. Mortality odds were increased only in univariate stratified analyses only in the obese subgroup (OR, 1.12; 95% CI, 1.08–1.16) but declined after adjustment (OR, 0.87; 95% CI, 0.83–0.90); all other subgroups showed a decreased odds of mortality in those with SDB or OSA (Table E4). Additional subset and sensitivity analyses are in the Results in the data supplement and Tables E2–E5.
Discussion
In this large, representative nationwide hospitalization sample, we found associations with higher LOS and costs in SDB and its subtypes. In particular, OHS is associated with threefold odds of higher LOS. Odds of in-hospital mortality were lower in those with SDB and OSA but substantially higher in OHS. Lower mortality odds in SDB were not explained by obesity based on stratified analyses. Mortality odds were even lower when considering only those with respiratory failure diagnoses (odds less than half). Race-stratified analyses did not find a negative effect on LOS, mortality, or costs in Black people but did find increased costs and LOS in Hispanic people with SDB without an effect on mortality.
Past work in OHS and surgical outcomes is aligned with this study in showing increased mortality, LOS, and complications (21, 22). However, outcomes in OSA are more heterogeneous. Numerous studies have linked OSA, which makes up the vast majority of cases of SDB, with cardiovascular mortality, heart failure progression, arrhythmia, and other cardiovascular sequelae, which would be expected to increase mortality (11, 23–25). In addition, there is a sizeable literature base for OSA leading to respiratory failure, the need for increase in the level of care or rapid response activation, and mortality among surgical and obstetric patients (9, 10, 26–29). However, past work in those with pneumonia, nonsurgical patients on the wards, and bariatric patients also observed lower mortality with sleep apnea (8, 30–33). Perhaps the difference in these outcomes is secondary to the root admission reason. For instance, bariatric patients are generally required to be adherent to PAP therapy to qualify for the surgery and receive therapy during hospitalization; therefore, rates of complications may be expected to be lower because their sleep apnea is treated. In contrast, other postsurgical patients may be less likely to use PAP after extubation. In addition, postsurgical patients are on average more likely to receive opioids, which are respiratory depressants and the most common pain-relieving modality after surgery, compared with medical patients. Although opioids have often been seen as standard of care in the postsurgical population, physicians may have a higher threshold to prescribe such medications to medical patients with a sleep apnea diagnosis, particularly if untreated. The clinical context may be key in understanding different hospitalization outcomes in hospitalized patients with OSA.
Past work shows OHS increases mortality to 23% compared with 9% in people with similar obesity but without OHS (12). However, OHS coding may be misclassifying individuals, because the diagnosis requires careful evaluation for obstructive, restrictive, and neuromuscular disorders causing hypercapnia and the frequency of COPD in hospitalized populations. People with OHS are likely to have a multimorbidity complex rather than sole OHS. OHS recognition is often delayed, and adverse events outside of the ICU care are common, because many physicians lack familiarity with how to diagnose and treat this disorder. The combination of these factors may predispose to substantially increased LOS, mortality, and costs.
This analysis is novel in another way. Racial disparities in care have been well documented over decades in numerous health-related areas. In particular, it is common to find worse care given to Black people than to White (15, 34). Our analysis does not find a difference in SDB LOS, mortality, or costs between White and Black people. Notwithstanding, our analysis did find increased costs and LOS in Hispanic folks, with scant effect on mortality. Differences in these results may be caused by an imbalance of Hispanic individuals (higher percentage in those without SDB). The Hispanic people diagnosed with SDB may be sicker or have more interaction with the healthcare system. Furthermore, it may be that there is a higher concentration of OHS in this population than in other races, but that would need to be explored further, because it has not to this point been demonstrated in other studies.
SDB, and particularly OHS, is part of a multimorbidity network rather than a lone disease: OSA and OHS represent pathobiology bridging respiratory, cardiac, renal, and CNS morbidity. This multimorbidity network has a complicated interplay that is still being untangled. Therefore, adjustment for one variable may open a back door for confounding by another. Looking at groups of associated conditions rather than individual conditions may more comprehensively address risk and illuminate causality for it.
Limitations
This study has weaknesses, generally representative of such analyses. Billing data are not collected for research; therefore, there is always a question of data quality. When studying a disorder that is primarily treated in the outpatient setting, there is a high likelihood of undercoding for SDB and its variants during a hospitalization (35). In particular, even though sleep apnea cooccurs with OHS in 90% of patients (12), only 24% of our cohort were coded for both diagnoses; this likely reflects undercoding of sleep apnea in the presence of OHS, either as a misunderstanding of OHS and sleep apnea comorbidity or a lowered priority of coding OSA in the setting of OHS compared with other diagnoses that played a larger role during hospital management. Furthermore, although SDB affects a quarter of adults (2), epidemiologic studies suggest that 80–90% of cases are undiagnosed. Therefore, there is a substantial concern for misclassification. The misclassification would be differential, because it would be uncommon to classify someone not having SDB as having it. This information bias could skew results. In addition, SDB is diagnosed almost exclusively in outpatients. Therefore, access to care may affect SDB diagnosis or preexisting care. Although we tried to account for socioeconomic status via median zip code income, and we did not see a difference in this in the baseline characteristics, looking at median income by zip code is a meager measure compared with the panoply of characteristics that determine this domain. The increased proportion of people with SDB on Medicaid suggest a lower socioeconomic status may predispose to SDB or SDB identification. Such population-level summaries are insufficient for a granular understanding and individualized care.
Misadventures after surgery and procedures often cause increased LOS and costs. We excluded only those records with diagnosis codes for respiratory failure associated with a procedure, which could misclassify periprocedural adverse events if not coded as such. There is also the possibility that adverse events in patients with OSA may be more easily reversible (e.g., with ventilatory support) than adverse events in other populations (e.g., sepsis development). However, we would then expect decreased LOS as well as mortality, which is not see in this study. LOS can be impacted by a wide variety of nonclinical factors and continuous PAP use (36, 37); LOS is still a patient-centered medical outcome, and increases in LOS, even when secondary to ancillary factors, are meaningful because we treat patients holistically rather than as a set of disconnected disease processes.
Administrative data also suffer from lack of specificity as to the origin of some of the data. For instance, it is possible that the patient self-identifies a particular race, but it is equally possible that race was assumed by a healthcare team member based on external characteristics, which can lead to misclassification. However, this misclassification is likely to be nondifferential. Thankfully, only a minimal amount of race (3.5%) and other patient characteristic data included in the model are missing. Another limitation is that this administrative database does not capture disease severity, including severity of both SDB and ARF. Severe SDB may differ significantly in terms of unexpected adverse events compared with mild SDB. This is particularly salient when evaluating a broad diagnosis like ARF, which can encompass anything from needing supplemental oxygen to needing a ventilator. Residual confounding can arise when important variables may not have been included because of the limitations of this administrative dataset. For instance, PAP therapy status and laboratory/imaging results are not available via this dataset. The NIS sample, by its structure, does not allow for evaluation of certain patient-centered outcomes, such as readmissions.
We caution that findings in this manuscript should not decrease provider vigilance for and treatment of SDB in hospitalized settings. It is possible that the diagnosis of sleep apnea makes medical professionals more wary, perhaps increasing monitoring or lowering the threshold to send to a higher level of care. This could also explain the increased LOS for these patients even after accounting for other common conditions. Such additional safeguards may improve the quality of SDB patients’ care but increase their LOS and cost. The lack of SDB diagnosis in 80–90% of those affected may increase their risk for adverse events by foregoing standard safeguards (and the possibility of treatment while in the hospital) for people with this condition. In marginalized groups, prevalence may be higher, but diagnosis lower, because testing is expensive and time consuming (38, 39). Perioperative adverse events are associated with untreated SDB and seem to be ameliorated by SDB treatment; the same is likely to be true in the nonsurgical realm (4, 7).
The next logical step is to further investigate the heterogeneity of effects and validate these findings in other hospitalization samples. In particular, the question of what features of the presentation or care path account for increased LOS, costs, and especially mortality in OHS is particularly relevant to health promotion and clinical care. In addition, a better understanding of why mortality seems to be lower in those with SDB and respiratory failure may improve resource allocation. The addition of clinical information and measures of disease severity and multimorbidity may help elucidate these patterns. To assess whether increased vigilance is the cause of the improvement in mortality for patients with SDB, an investigation into whether there is an increased transfer to a higher level of care may be helpful. Prospective studies would be able to address the discussed limitations of large databases and are sorely needed to clarify the impact of SDB in hospitalized populations. These studies would help to pinpoint where interventions may benefit patient-centered outcomes.
Conclusions
Patients with SDB or OSA had lower in-hospital mortality but increased LOS and costs. Those with OHS had increases in all examined outcomes: LOS, mortality, and costs. We identified racial disparities in costs and LOS associated with being Hispanic but no such differences in Black individuals.
Acknowledgments
Acknowledgment
The author thanks Siran M. Koroukian for being a sounding board for the project and Dr. Kingman Strohl for proofreading the manuscript. The author also thanks the HCUP Data Partners that contribute to the NIS: Alaska Department of Health and Social Services, Alaska State Hospital and Nursing Home Association, Arizona Department of Health Services, Arkansas Department of Health, California Office of Statewide Health Planning and Development, Colorado Hospital Association, Connecticut Hospital Association, Delaware Division of Public Health, District of Columbia Hospital Association, Florida Agency for Health Care Administration, Georgia Hospital Association, Hawaii Laulima Data Alliance, Hawaii University of Hawaii at Hilo, Illinois Department of Public Health, Indiana Hospital Association, Iowa Hospital Association, Kansas Hospital Association, Kentucky Cabinet for Health and Family Services, Louisiana Department of Health, Maine Health Data Organization, Maryland Health Services Cost Review Commission, Massachusetts Center for Health Information and Analysis, Michigan Health & Hospital Association, Minnesota Hospital Association (provides data for Minnesota and North Dakota), Mississippi State Department of Health, Missouri Hospital Industry Data Institute, Montana Hospital Association, Nebraska Hospital Association, Nevada Department of Health and Human Services, New Hampshire Department of Health & Human Services, New Jersey Department of Health, New Mexico Department of Health, New York State Department of Health, North Carolina Department of Health and Human Services, North Dakota (data provided by the Minnesota Hospital Association), Ohio Hospital Association, Oklahoma State Department of Health, Oregon Association of Hospitals and Health Systems, Oregon Office of Health Analytics, Pennsylvania Health Care Cost Containment Council, Rhode Island Department of Health, South Carolina Revenue and Fiscal Affairs Office, South Dakota Association of Healthcare Organizations, Tennessee Hospital Association, Texas Department of State Health Services, Utah Department of Health, Vermont Association of Hospitals and Health Systems, Virginia Health Information, Washington State Department of Health, West Virginia Department of Health and Human Resources, West Virginia Health Care Authority, Wisconsin Department of Health Services, and Wyoming Hospital Association.
Footnotes
Supported by U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service Career Development Award IK2CX001882. The contents of this work do not represent the views of the Department of Veterans Affairs or the U.S. government.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol . 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med . 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology . 2014;120:268–286. doi: 10.1097/ALN.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 4. Gaddam S, Gunukula SK, Mador MJ. Post-operative outcomes in adult obstructive sleep apnea patients undergoing non-upper airway surgery: a systematic review and meta-analysis. Sleep Breath . 2014;18:615–633. doi: 10.1007/s11325-013-0925-1. [DOI] [PubMed] [Google Scholar]
- 5. Bryson GL, Gomez CP, Jee RM, Blackburn J, Taljaard M, Forster AJ. Unplanned admission after day surgery: a historical cohort study in patients with obstructive sleep apnea. Can J Anaesth . 2012;59:842–851. doi: 10.1007/s12630-012-9746-0. [DOI] [PubMed] [Google Scholar]
- 6. Foldvary-Schaefer N, Kaw R, Collop N, Andrews ND, Bena J, Wang L, et al. Prevalence of undetected sleep apnea in patients undergoing cardiovascular surgery and impact on postoperative outcomes. J Clin Sleep Med . 2015;11:1083–1089. doi: 10.5664/jcsm.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasu TS, Grewal R, Doghramji K. Obstructive sleep apnea syndrome and perioperative complications: a systematic review of the literature. J Clin Sleep Med . 2012;8:199–207. doi: 10.5664/jcsm.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg . 2013;23:1842–1851. doi: 10.1007/s11695-013-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma S, Chowdhury A, Tang L, Willes L, Glynn B, Quan SF. Hospitalized patients at high risk for obstructive sleep apnea have more rapid response system events and intervention is associated with reduced events. PLoS One . 2016;11:e0153790. doi: 10.1371/journal.pone.0153790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naranjo M, Willes L, Prillaman BA, Quan SF, Sharma S. Undiagnosed OSA may significantly affect outcomes in adults admitted for COPD in an inner-city hospital. Chest . 2020;158:1198–1207. doi: 10.1016/j.chest.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 11. May AM, Mehra R. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Semin Respir Crit Care Med . 2014;35:531–544. doi: 10.1055/s-0034-1390023. [DOI] [PubMed] [Google Scholar]
- 12. Mokhlesi B, Masa JF, Brozek JL, Gurubhagavatula I, Murphy PB, Piper AJ, et al. Evaluation and management of obesity hypoventilation syndrome: an Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2019;200:e6–e24. doi: 10.1164/rccm.201905-1071ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. 2017. www.hcup-us.ahrq.gov/databases.jsp [DOI] [PubMed]
- 14.Houchens R, Ross D, Elizhauser A.2015. http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp
- 15. Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979–1996) Crit Care Med . 2002;30:1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 16. Cooke CR, Erickson SE, Eisner MD, Martin GS. Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med . 2012;40:1532–1538. doi: 10.1097/CCM.0b013e31824518f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones N, Schneider G, Kachroo S, Rotella P, Avetisyan R, Reynolds MW. A systematic review of validated methods for identifying acute respiratory failure using administrative and claims data. Pharmacoepidemiol Drug Saf . 2012;21:261–264. doi: 10.1002/pds.2326. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp [DOI] [PubMed]
- 19. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care . 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. http://www.R-project.org/ [Google Scholar]
- 21. Kaw R, Bhateja P, Paz Y Mar H, Hernandez AV, Ramaswamy A, Deshpande A, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest . 2016;149:84–91. doi: 10.1378/chest.14-3216. [DOI] [PubMed] [Google Scholar]
- 22. Chindamporn P, Wang L, Bena J, Zajichek A, Milinovic A, Kaw R, et al. Obesity-associated sleep hypoventilation and increased adverse postoperative bariatric surgery outcomes in a large clinical retrospective cohort. J Clin Sleep Med . 2022;18:2793–2801. doi: 10.5664/jcsm.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gami AS, Rader S, Svatikova A, Wolk R, Herold DL, Huyber C, et al. Familial premature coronary artery disease mortality and obstructive sleep apnea. Chest . 2007;131:118–121. doi: 10.1378/chest.06-1404. [DOI] [PubMed] [Google Scholar]
- 24. Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep . 2007;30:755–766. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest . 2017;151:225–241. doi: 10.1016/j.chest.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology . 2008;108:822–830. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 27. Sharma S, Mukhtar U, Kelly C, Mather P, Quan SF. Recognition and treatment of sleep-disordered breathing in obese hospitalized patients may improve survival: the HoSMed database. Am J Med . 2017;130:1184–1191. doi: 10.1016/j.amjmed.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 28. Stundner O, Zubizarreta N, Mazumdar M, Memtsoudis SG, Wilson LA, Ladenhauf HN, et al. Differential perioperative outcomes in patients with obstructive sleep apnea, obesity, or a combination of both undergoing open colectomy: a population-based observational study. Anesth Analg . 2021;133:755–764. doi: 10.1213/ANE.0000000000005638. [DOI] [PubMed] [Google Scholar]
- 29. Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep . 2014;37:843–849. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindenauer PK, Stefan MS, Johnson KG, Priya A, Pekow PS, Rothberg MB. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest . 2014;145:1032–1038. doi: 10.1378/chest.13-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyons PG, Zadravecz FJ, Edelson DP, Mokhlesi B, Churpek MM. Obstructive sleep apnea and adverse outcomes in surgical and nonsurgical patients on the wards. J Hosp Med . 2015;10:592–598. doi: 10.1002/jhm.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen NT, Masoomi H, Laugenour K, Sanaiha Y, Reavis KM, Mills SD, et al. Predictive factors of mortality in bariatric surgery: data from the Nationwide Inpatient Sample. Surgery . 2011;150:347–351. doi: 10.1016/j.surg.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 33. Opperer M, Cozowicz C, Bugada D, Mokhlesi B, Kaw R, Auckley D, et al. Does obstructive sleep apnea influence perioperative outcome? A qualitative systematic review for the society of anesthesia and sleep medicine task force on preoperative preparation of patients with sleep-disordered breathing. Anesth Analg . 2016;122:1321–1334. doi: 10.1213/ANE.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 34. Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and ethnic disparities in early-stage lung cancer survival. Chest . 2017;152:587–597. doi: 10.1016/j.chest.2017.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McIsaac DI, Gershon A, Wijeysundera D, Bryson GL, Badner N, van Walraven C. Identifying obstructive sleep apnea in administrative data: a study of diagnostic accuracy. Anesthesiology . 2015;123:253–263. doi: 10.1097/ALN.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 36. Brasel KJ, Lim HJ, Nirula R, Weigelt JA. Length of stay: an appropriate quality measure? Arch Surg . 2007;142:461–465. doi: 10.1001/archsurg.142.5.461. [DOI] [PubMed] [Google Scholar]
- 37. Spurr KF, Graven MA, Gilbert RW. Prevalence of unspecified sleep apnea and the use of continuous positive airway pressure in hospitalized patients, 2004 National Hospital Discharge Survey. Sleep Breath . 2008;12:229–234. doi: 10.1007/s11325-007-0166-2. [DOI] [PubMed] [Google Scholar]
- 38. Billings ME, Cohen RT, Baldwin CM, Johnson DA, Palen BN, Parthasarathy S, et al. Disparities in sleep health and potential intervention models: a focused review. Chest . 2021;159:1232–1240. doi: 10.1016/j.chest.2020.09.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson DA, Guo N, Rueschman M, Wang R, Wilson JG, Redline S. Prevalence and correlates of obstructive sleep apnea among African Americans: the Jackson Heart Sleep Study. Sleep . 2018;41:zsy154. doi: 10.1093/sleep/zsy154. [DOI] [PMC free article] [PubMed] [Google Scholar]