Abstract
Metastasis of colorectal cancer (CRC) is a leading cause of mortality among CRC patients. Elevated COX-2 and PD-L1 expression in colon cancer tissue has been linked to distant metastasis of tumor cells. Although COX-2 inhibitors and immune checkpoint inhibitors demonstrate improved anti-tumor efficacy, their toxicity and variable therapeutic effects in individual patients raise concerns. To address this challenge, it is vital to identify traditional Chinese medicine components that modulate COX-2 and PD-1/PD-L1: rosmarinic acid (RA) exerts striking inhibitory effect on COX-2, while ginsenoside Rg1 (GR) possesses the potential to suppress the binding of PD-1/PD-L1. In this study we investigated whether the combination of RA and GR could exert anti-metastatic effects against CRC. MC38 tumor xenograft mouse model with lung metastasis was established. The mice were administered RA (100 mg·kg−1·d−1, i.g.) alone or in combination with GR (100 mg·kg−1·d−1, i.p.). We showed that RA (50, 100, 150 μM) or a COX-2 inhibitor Celecoxib (1, 3, 9 μM) concentration-dependently inhibited the migration and invasion of MC38 cells in vitro. We further demonstrated that RA and Celecoxib inhibited the metastasis of MC38 tumors in vitro and in vivo via interfering with the COX-2-MYO10 signaling axis and inhibiting the generation of filopodia. In the MC38 tumor xenograft mice, RA administration significantly decreased the number of metastatic foci in the lungs detected by Micro CT scanning; RA in combination with GR that had inhibitory effect on the binding of PD-1 and PD-L1 further suppressed the lung metastasis of colon cancer. Compared to COX-2 inhibitors and immune checkpoint inhibitors, RA and GR displayed better safety profiles without disrupting the tissue structures of the liver, stomach and colon, offering insights into the lower toxic effects of clinical traditional Chinese medicine against tumors while retaining its efficacy.
Keywords: colon cancer, metastasis, COX-2, MYO10, filopodia, rosmarinic acid, ginsenoside Rg1, celecoxib, PD-1/PD-L1
Introduction
The progression of colorectal cancer is intricately linked to inflammation [1]. Cyclooxygenase 2 (COX-2), an inducible enzyme encoded by the Ptgs2 gene, is closely associated with inflammation, and plays a critical role in the conversion of arachidonic acid to prostaglandin E2 (PGE2), a mediator in numerous inflammatory responses. The expression of COX-2 is markedly elevated in colon cancer compared to normal colon tissue, indicating a potential role in the development of the disease [2]. Notably, distant metastasis is a significant cause of mortality in patients with colon cancer, and numerous studies have highlighted the relationship between high COX-2 expression and the occurrence of distant metastasis [3–5]. While the underlying mechanisms of COX-2 promoting tumor metastasis predominantly center on downstream inflammatory responses mediated by PGE2, the COX-2/PGE2 axis has been extensively studied in the metastasis of different cancers such as colon, liver, and ovarian cancer [6–8]. Moreover, Zheng et al. reported that COX-2 expressed by tumor cells can similarly promote metastasis of prostate cancer through non-inflammatory pathways [9], indicating that COX-2 may possess other non-inflammatory effectors that facilitate tumor metastasis.
Although COX-2 has been shown to promote tumor cell motility and metastasis, most studies have focused on the COX-2/PGE2 pathway. Actin filaments, or microfilaments, are essential cellular components of the cytoskeleton [10]. In tumor cells, thin, filopodia-like protrusions are extended from the cytoskeleton to enable cell movement. These filopodia contain proteins such as cell division cycle factor 42 (Cdc42), neural Wiskott Aldrich syndrome protein (NWASP), and actin-related 2/3 complex (Arp2/3), which are all involved in filopodia stabilization [11, 12]. Notably, myosin X (MYO10), a cytoskeletal component that stabilizes filopodia, plays a critical role in filopodia elongation and stability [13]. MYO10 is widely recognized as a mediator of tumor cell invasion and migration [14, 15], and can serve as a marker for filopodia in tumors. Dormond et al. demonstrated that COX-2 is closely associated with the Cdc42 GTP signal transduction pathway; inhibiting COX-2 activity using NSAIDs also inhibited Cdc42 GTP activity, which resulted in decreased vascular endothelial cell spreading and migration [16]. Abraham et al. also reported that Rac/Cdc42 exchange factors are responsible for filopodia formation in vascular endothelial cells, which in turn alters vessel lumen morphology [12]. However, the direct association between COX-2 and the filopodia marker MYO10 in tumor cells has not been established.
Aspirin, a widely recognized inhibitor of COX-2, has been utilized as an anti-neoplastic agent. However, its significant adverse effects limit its use as an anti-tumor agent, despite its effectiveness [17, 18]. Celecoxib (Cel), which targets COX-2, has shown promise as an alternative to aspirin, decreasing the risk of adverse side effects, but its use is still limited due to its toxicity to other organs, such as the liver, stomach, colon, and heart [19, 20]. Therefore, it is necessary to explore other aspirin-like drugs. Our research identified aromatic acids, similar to aspirin, in Salvia miltiorrhiza, an invigorating blood circulation and stasis medicine [21, 22]. Among these acids, rosmarinic acid (RA), a quality control ingredient in the Radix Salviae miltiorrhizae decoction, piqued our interest [23]. The study showed that RA has potential to inhibit COX-2. Therefore, we aimed to investigate whether RA could inhibit COX-2, suppress colon cancer metastasis, and demonstrate improved safety as compared to other COX-2 inhibitors.
Immune escape is a significant factor in the development and metastasis of colon cancer. Recent studies have focused on the role of immune checkpoints, particularly PD-1/PD-L1, in facilitating tumor immune escape. Colon cancer cells express high levels of PD-L1, which inhibits T cell activity, leading to immune escape [24–26]. Current anti-tumor therapies targeting PD-1/PD-L1 mainly involve monoclonal antibodies (mAbs) or their combination with chemotherapy. While these therapies have revolutionized cancer treatment, they come with significant adverse effects and high costs [27–30]. Panax ginseng, a traditional Chinese medicine, has been shown to enhance the immune system, and several of its components have demonstrated immune-modulating effects [31–33]. Ginsenoside Rg1 (GR), a component of Panax ginseng, has been found to have a variety of effects, including improved cognition, anti-fatigue [34–36], and anti-tumor properties [37–39]. However, the anti-tumor metastatic effects of GR on the PD-1/PD-L1 pathway have not been reported. In our study, we screened several small compounds from traditional Chinese medicine against PD-1/PD-L1 protein binding, and found that GR could inhibit this binding. We investigated whether GR could activate T cells to exert anti-tumor effects. PD-1/PD-L1 checkpoint therapies have shown a strong response in microsatellite unstable (MSI) types of colon cancer [40, 41]. The mouse colon cancer cell line MC38 belongs to microsatellite unstable tumor cells [42]. Therefore, we constructed a microsatellite unstable colon cancer lung metastasis model to investigate the efficacy of GR for colon cancer metastasis, targeting PD-1/PD-L1 pathways.
It has been known that elevated expression levels of COX-2 and PD-L1 in colorectal cancer tissues are crucial and serve as critical factors on promoting distant metastasis of colorectal cancer cells. RA was reported to demonstrate striking inhibitory effect on COX-2 [43–45], and our data indicated that GR possessed the potential to suppress the binding of PD-1/PD-L1. Therefore, in the present study, we aimed to investigate whether the combination of RA and GR could exert anti-metastatic effects against colorectal cancer. RA is a quality control component of Danshen aqueous extract, while GR acts as a major ingredient of ginseng. Danshen and ginseng are commonly co-administered traditional Chinese medicine (TCM) in clinical anti-tumor therapy. Additionally, our previous studies elucidated that the combination of Danshen and ginseng could suppress metastasis of breast cancer [46]. Thus, determining the effect and underlying mechanisms of combined therapy of RA and GR in combating colorectal cancer metastasis holds crucial clinical significance and value.
Materials and methods
Reagents and antibodies
RA (JBZ-0789) and GR (JBZ-1028) were obtained from Nanjing Jin Yibai Biological Technology Co., Ltd (Nanjing, China). Cel (HY-14398) was from MedChemExpress (New Jersey, USA). InvivoMAb anti-mouse PD-L1 (B7-H1, aPD-L1) came from BioXCell (New Hampshire, USA).
Cells and culture
The laboratory maintained a stock of the mouse colon cancer cell line MC38, which was cultured in DMEM (KeyGEN BioTECH, KGM12800-500) supplemented with 10% FBS (Cellmon, SA31102). To construct the Ptgs2-knockout MC38 cell line (Ptgs2KO MC38), we used the pCas-Puro-U6-Ptgs2-KO plasmid (Corues Biotechnology, Nanjing, China) and Lipofectamine 2000 reagents (Invitrogen, 11668-019, Massachusetts, USA), along with Opti-MEM (R) Reduced Serum Medium (Gibco, 21516800). We also generated the overexpress-Ptgs2 MC38 cell line (Ptgs2OE MC38) by transfecting lentivirus (Lentivirus-Puro-CMV-Ptgs2, Corues Biotechnology, Nanjing, China). Stable cell lines were selected by adding 3 μg/mL puromycin (InvivoGen, QLL-42-07) to the culture medium. All cell lines were maintained in a humidified incubator at 37 °C with 5% CO2 (Thermo Fisher). The sequences of KO and OE Ptgs2 plasmids are shown in Supplementary Tables S2 and S3.
Animals
We obtained male C57BL/6 wild type mice (4–5 weeks of age, weighing between 18–22 g) from GemPharmatech (Nanjing, China). The mice were housed and maintained in a specific-pathogen-free (SPF) animal facility, with 5 mice per cage. All animal experiments were approved and conducted in accordance with the guidelines outlined by the Institutional Animal Care and Use Committees of Nanjing University of Chinese Medicine (Nanjing, China).
Experimental lung metastasis model and therapy
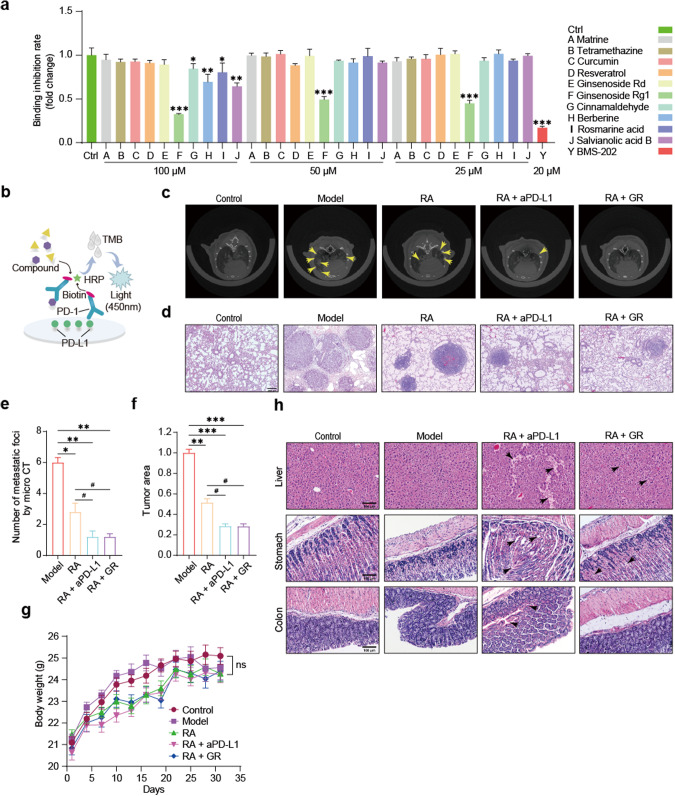
Using a blinded method, the animals were randomly divided into 6 groups (n = 10): the control group, model group, Cel 25 mg·kg−1·d−1 group (dissolved in 0.5% CMC-Na with 10% PEG300 sterile saline, Shanghai Yugong Bio-technology Co., Ltd, Shanghai, China, administered orally), RA 100 mg·kg−1·d−1 group (dissolved in 0.5% CMC-Na with 10% PEG300 sterile saline, administered orally), RA 100 mg·kg−1·d−1 + GR 100 mg·kg−1·d−1 group (dissolved in 0.5% CMC-Na with 10% PEG300 sterile saline, administered intraperitoneally), and RA 100 mg·kg−1·d−1 + aPD-L1 5 mg·kg−1·(3 d)−1 group (dissolved in sterile saline, administered intraperitoneally). For experimental lung metastasis, MC38 cells were harvested and resuspended in PBS. Except for the control group, mice in all other groups were intravenously inoculated with 2.4 × 105 MC38 cells in 200 μL of PBS via the lateral tail vein. Four weeks later, we collected peripheral blood, lungs, liver, stomach, and colorectal tissues.
Micro-computed tomography (Micro-CT)
After anesthetizing the mice with isoflurane (R510-22-16, Reward Life Technologies Co., Ltd., Shenzhen, China), they were placed inside the Micro-CT system (Quantum GX, PerkinElmer, USA). The mice were fixed to a scanning frame and subjected to 360-degree annular scanning using X-rays. The scanning parameters were set as follows: high resolution at 4.5 μm, tube voltage at 70 kV, tube current at 88 μA, FOV at 36 mm × 36 mm, and a scan time of 4 min. The CT data processing software (Analyze 12.0, PerkinElmer, USA) was utilized to export the collected images.
MTT assay
To assess the viability of MC38 cells, we employed the MTT assay. In brief, 8 × 103 cells/well were seeded into 96-well plates (Nest, Shanghai, China). Following a treatment of RA for 24 or 48 h, MTT was added to the plates and incubated at 37 °C for 4 h. DMSO was then added to the plates and shaken for 10 min at room temperature. Optical density values at 450 nm were subsequently measured using a microplate reader (Synergy2, BioTek, Burlington, USA).
Transwell assay
We employed the transwell migration assay to assess the migratory ability of MC38 cells. In detail, 1 × 105 cells/200 μL of basic culture medium containing varying concentrations of Cel or RA were seeded onto transwell inserts (3422, Corning, New York, USA). Thereafter, the insert was transferred onto a 24-well plate containing 800 μL DMEM supplemented with 30% FBS. After an incubation at 37 °C for 30 h, the migrated cells on the lower surface of the polycarbonate membrane were fixed with 4% paraformaldehyde (PFA) and stained with 1% crystal violet (C0121, Beyotime, Wuhan, China). The migrated cells were then counted under a bright-field microscope (Axio Vert A1, ZEISS, Oberkochen, Germany) and three random fields of each chamber were selected for further quantification. Following crystal violet dissolution with 3% acetic acid, OD values were tested by a microplate reader at 450 nm.
Three-dimensional (3D) tumor spheroid invasion assay
Invasion of cells was assessed using a 3D culture system. Concisely, MC38 cells were mixed with a DMEM medium and matrigel (354234, Corning, New York, USA) mixture (2:1) and seeded in a 15 mm glass-bottom confocal dish (801002, NEST, Shanghai, China) using 1 μL of cell suspension. Upon solidification of the cell suspension, 250 μL DMEM and matrigel mixture (2:1) was added to encapsulate the central matrix. After polymerization, the culture was overlaid with DMEM medium containing varying concentrations of Cel or RA. The morphology of the 3D culture system was captured by a bright-field microscope as the original image. After the cells invaded the outer matrix for 30 h, a second shot of the system was taken. Images captured at 0 and 30 h were overlaid using ImageJ to analyze the invaded cells.
Anti-PD-1/PD-L1 protein binding capacity assay by ELISA
To assess the PD-1/PD-L1 protein binding capacity of the samples, PD-L1 protein (2 µg/mL, PD1-H5258, ACRO Biosystems, USA) was coated on 96-well plates at 100 µL per well overnight at 4 °C. The plates were washed three times with wash buffer (500 µL Tween-20 in 1000 mL 1× PBS) and incubated with block buffer (P0260, Beyotime, Wuhan, China) at 37 °C for 1.5 h. After washing, 50 µL of prepared PD-1 solution (0.5 µg/mL, PD1-H82F4, ACRO Biosystems, USA) was added to each well, with dilution buffer added to the blank without PD-1 protein. After 10–15 min, 12 µL of sample was added to the drug addition group to set up three replicate wells. For the positive control, 12 µL of BMS-202 (S7921, Selleck, USA) was added to set up two replicate wells. For the DMSO and blank groups, 12 µL of DMSO was added to set up two replicate wells. Finally, 38 µL of dilution buffer (blocking solution:triple distilled water = 1:3) was added to each well to make a final volume of 100 µL per well, which was then incubated at 37 °C for 1 h. After washing, block buffer was added and incubated at 37 °C for 10 min, followed by washing. Then, 100 µL of diluted horseradish peroxidase HRP solution (A0303, Beyotime, Wuhan, China) was added to each well and incubated for 1 h in the dark at 37 °C. Washing was repeated again before 100 µL of TMB color development solution (P0209, Beyotime, Wuhan, China) was added to each well in the dark and incubated at 37 °C for 10–15 min. Next, 50 µL of TMB color development stop solution (P0215, Beyotime, Wuhan, China) was added to each well, and the solution was shaken for 3 min. Finally, the absorbance was detected within 15 min by a microplate reader (Thermo) at a wavelength of 450 nm, and the relative absorbance values were calculated using GraphPad.
Western blot analysis
Cell lysates were prepared using lysis buffer (P0013B, Beyotime, Wuhan, China) containing protease inhibitor cocktail (ST506, Beyotime, Wuhan, China) and phosphatase inhibitor cocktail (GB-0032, KeyGEN, Nanjing, China). The lysates were incubated on ice for 30 min and then subjected to centrifugation at 12,000 r/min (5417 R, Eppendorf, Germany) for 10 min at 4 °C, after which the supernatants were collected. For tissue samples, the protein was extracted by grinding the tissue using a Freezing grinder (Nanjing Qiaobeilin Biotechnology Co., Ltd) at 4 °C and then freezing it at −80 °C for 30 min. The lysates were then incubated on ice for 60 min and centrifuged at 12,000 r/min for 10 min at 4 °C, with the supernatants being collected afterwards. Total proteins were separated using 10% polyacrylamide gel electrophoresis (SDS-PAGE) gels, and transferred onto PVDF membranes (Millipore, Darmstadt, Germany). The membranes were probed with primary antibodies overnight at 4 °C, followed by incubation with corresponding secondary antibodies. Protein bands were developed using a BIORAD imaging system (chemiDOCTMXRS, Bio-Rad, Hercules, USA). The primary antibodies used were as follows: anti-COX-2 (A3560, ABclonal, Wuhan, China), anti-NWASP (A2270, ABcloanl, Wuhan, China), anti-Arp2 (A5734, ABcloanl, Wuhan, China), anti-Arp3 (A1064, ABcloanl, Wuhan China), anti-MYO10 (A12471, ABcloanl, Wuhan, China), anti-Granzyme B (A2557, ABclonal, Wuhan, China), anti-IFNG (A12450, ABcloanl, Wuhan, China), anti-GAPDH (10494-1-AP, Proteintech, Wuhan, China), and HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H + L) (SA00001-2, Proteintech, Wuhan, China).
Pulmonary lymphocyte isolation
Mouse lungs were collected and immediately placed on ice. Collagenase I (9001-12-1, BIOFROXX, Germany) was weighed and dissolved in 1× PBS to prepare a 2 mg/mL collagenase I solution. The lungs were cut into pieces and transferred to a 10 mL centrifuge tube, followed by addition of 7 mL of collagenase I solution. The mixture was thoroughly mixed and incubated for 1 h at 37 °C in a water bath. Then, the tubes were placed on ice and 70 μm filters were added onto a 6-well plate. The cut lungs were ground using a flat part of a 3 mL syringe and washed with 2–4 mL 1× PBS, repeating the process several times. The suspension was transferred to a 15 mL centrifuge tube and centrifuged at 2000 r/min (L420, Cence, Changsha, China) for 5 min at 4 °C. Fresh 40 and 70% Percoll solutions were prepared. A 100% Percoll (17089109, Cytiva, USA) solution was diluted with 10× PBS (1:9), and then 40% and 70% Percoll solutions were prepared using 1× PBS to create a 10 mL Percoll gradient system (7 mL 40% Percoll and 3 mL 70% Percoll). Three milliliter of 70% Percoll was added to a siliconized tube, followed by suspension of cells with 7 mL 40% Percoll, which was slowly added into the siliconized centrifuge tube. Since Gradient separation was sensitive to stirring, we avoided shaking the tube. The centrifuge was set at 3000 r/min (Allegra X-30R, BECKMAN COULTER, USA) at 4 °C for 30 min (increasing speed by 4 and then decreasing speed by 0). The dividing ring contained lymphocyte cells, which were then transferred to a 10 mL centrifuge tube and centrifuged at 1600 r/min for 6 min. If there was red blood cell precipitation, red blood cell lysate was used, and cell counting was performed after the red blood cells were lysed.
Flow cytometry analysis
Lymphocyte cells isolated from lung tissues and peripheral blood (Mouse Peripheral Blood Lymphocyte Isolate Kit, Solarbio, China) were stimulated for 4 h at 37 °C with 50 ng/mL of PMA (Sigma-Aldrich, USA), 1 μg/mL ionomycin (Sigma-Aldrich, USA), and GolgiStopTM (BD Biosciences, USA). Then cells were suspended in PBS, and then incubated with APC anti-mouse CD8a (100711, BioLegend, USA) and PE anti-mouse Granzyme B (12-8898-80, Invitrogen, USA) or PE anti-mouse IFN-γ (12-7311-82, Invitrogen, USA) for 30 min at 4 °C in the dark. Cells were then centrifuged and suspended in 1% PFA at 4 °C overnight. The following day, all samples were passed through a flow cytometer (A00-1-1102, BECKMAN COULTER, USA) after filtration.
RNA extraction and quantitative real-time PCR
To isolate total RNA, tissue samples were lysed in TRIzol reagent (Thermo Fisher Scientific, Waltham, USA), and then extracted using the chloroform-isopropanol method. Synthesis of cDNA was carried out using Hiscript® II QRT SuperMix (Vazyme, Nanjing, China) with 500 ng of total RNA. Real-time PCR was executed using ChamQ SYBR qPCR Master Mix (Low ROX Premixed) (Vazyme, Nanjing, China) and detected by an ABI 7500 system (Applied Biosystems, CA, USA). The primer sequences employed in this study are presented in Supplementary Table S1.
H&E staining
Paraffin-embedded sections, which had been fixed with 4% PFA, were deparaffinized and dehydrated using xylene and ethanol series. Hematoxylin and eosin (DH0006, LEAGENE, Beijing, China) were used to counterstain the slides. Subsequently, the sections were visualized by the Automatic Quantitative Pathological Imaging System (Vectra 3.0, PerkinElmer, USA).
Immunofluorescent staining analysis
Paraffin-embedded sections, which were fixed with 4% PFA, were initially deparaffinized and dehydrated using xylene and ethanol series. Sodium citrate buffer was used for antigen retrieval. After blocking with 5% BSA for 30 min, the sections were incubated with indicated primary antibodies overnight at 4 °C. The following primary antibodies were utilized: anti-COX-2 (ab179800, Abcam, Cambridge, UK), anti-MYO10 (ab224120, Abcam, Cambridge, UK), anti-Granzyme B (A2557, ABclonal, Wuhan, China), and anti-IFNG (A12450, ABclonal, Wuhan, China). Then, corresponding secondary antibodies (TRITC, ab6718, Abcam) were added to the tissues and incubated for 2 h at room temperature, followed by counterstaining with DAPI (C1006, Beyotime, Wuhan, China) for the cell nucleus. The fluorescence inversion microscope (Leica Thunder, Germany) was employed to visualize the sections. For cell immunofluorescence, fixed with 4% PFA for 15 min, the cells were blocked with 5% BSA for 30 min. Thereafter, primary antibodies, including anti-COX-2 (12282, CST, Boston, USA), anti-MYO10 (ab224120, Abcam, Cambridge, UK), anti-NWASP (A2270, ABclonal, Wuhan, China), anti-Arp2 (A5734, ABclonal, Wuhan, China), and anti-Arp3 (A1064, ABclonal, Wuhan China), were added and incubated overnight at 4 °C. Corresponding secondary antibodies (TRITC, ab6718, Abcam) were then applied to the cells, incubated for 1.5 h at room temperature, followed by counterstaining with Actin-Tracker Green-488 (C2201S, Beyotime, Wuhan, China) and DAPI. Finally, the laser confocal microscope (TCS SP8, Leica, Germany) was utilized to observe the cells.
Immunohistochemistry staining analysis
The paraffin-embedded sections, which were fixed with 4% PFA, were first deparaffinized and dehydrated using a series of xylene and ethanol. Antigen retrieval was performed using sodium citrate buffer, followed by quenching of endogenous peroxidase activity with 3% H2O2. To prevent nonspecific binding, the sections were blocked with 5% BSA for 30 min. Primary antibodies, including anti-COX-2 (ab179800, Abcam, Cambridge, UK), anti-MYO10 (ab224120, Abcam, Cambridge, UK), anti-Granzyme B (A2557, ABclonal, Wuhan, China), and anti-IFNG (A12450, ABclonal, Wuhan, China), were added and incubated overnight at 4 °C. Subsequently, the sections were stained with a secondary antibody at room temperature for 1 h. The presence of HRP/peroxidase was visualized with a chromogen reaction using DAB (PV9000, ZSGB-BIO, Beijing, China), and the slides were counterstained with hematoxylin (DH0006, LEAGENE, Beijing, China). The Automatic Quantitative Pathological Imaging System (Vectra 3.0, PerkinElmer, USA) was utilized to capture the images.
Statistical analysis
The data were reported as either mean ± standard error of the mean (SEM) or mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA). Student’s t tests were used to analyze the differences between two groups, whereas one-way analysis of variance (ANOVA) was used to analyze the differences between more than two groups, followed by Tukey’s multiple comparisons test. Statistical significance was defined as *P < 0.05, **P < 0.01, ***P < 0.001.
Results
RA inhibits colon cancer metastasis in vitro and in vivo
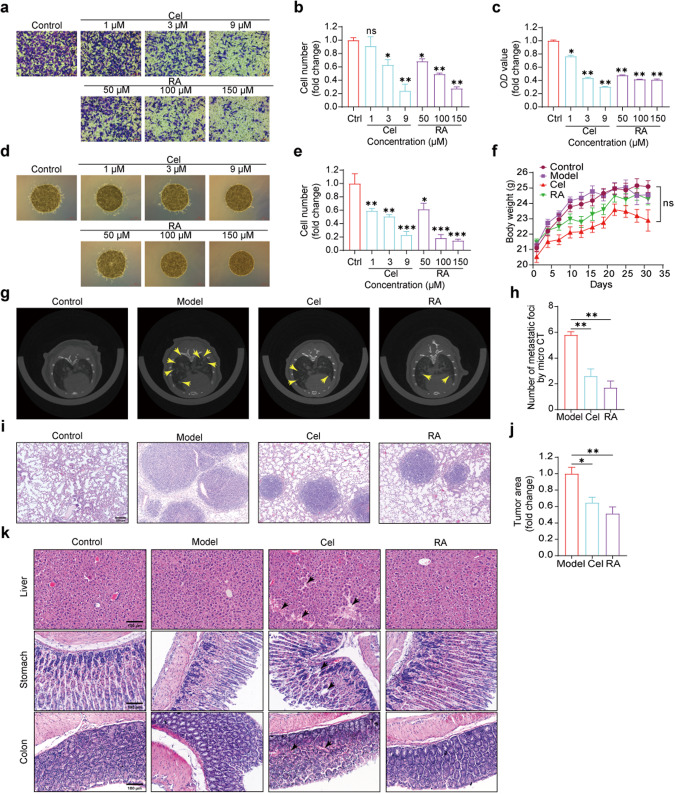
MTT assay was conducted to determine that RA had no cytotoxic effects on cell proliferation at concentrations below 150 μM, whereas Cel had no cytotoxic effects at concentrations under 9 μM (Supplementary Fig. S1). Therefore, subsequent in vitro migration and invasion experiments were carried out using concentrations below these levels. The results of the Transwell assay revealed a dose-dependent inhibition of MC38 cell migration upon RA treatment for 30 h, indicating its potential as an inhibitor of colon cancer cell migration. Cel demonstrated a similar effect (Fig. 1a–c). We further conducted a 3D invasion assay to investigate whether RA could also inhibit MC38 cell invasiveness. After 30 h of treatment, we observed a dose-dependent inhibition of MC38 cell invasion by RA. Cel also demonstrated a similar effect (Fig. 1d, e). These experiments demonstrated that RA could effectively inhibit the migration and invasion of colon cancer cells in vitro, and thereby inhibit tumor metastasis. These findings were further corroborated by the demonstrated efficacy of Cel in inhibiting colon cancer cell metastasis in vitro.
Fig. 1. RA inhibits colon cancer metastasis in vitro and in vivo.
a–c The images and quantitative analysis of the transwell migration assay depict the migration of MC38 cells treated with various concentrations of Cel or RA, and OD value is presented as well (scale bar: 100 μm, 20× magnification, compared with control group, *P < 0.05, **P < 0.01, ns, not significant, one-way ANOVA, n = 3). d, e Images and quantitative analysis of the 3D-invasion system reveal the invasion of MC38 cells treated with different concentrations of Cel or RA (scale bar: 500 μm, 4× magnification, compared with control group, *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA, n = 3). f The body weight of mice did not show significant changes during the treatment period (not significant, two-way ANOVA, n = 10). g, h The Micro-CT images and quantitative analysis demonstrate the metastatic foci in the lungs of mice (compared with model group, **P < 0.01, one-way ANOVA, n = 5). i, j The lung images with H&E staining reveal the tumor area (scale bar: 200 μm, 20× magnification, compared with model group, *P < 0.05, **P < 0.01, one-way ANOVA, n = 5). k Representative images of H&E staining of the liver, stomach, and colon tissue (scale bar: 100 μm, 20× magnification).
The mouse lung metastasis model was established by injecting 2.4 × 105 MC38 cells via tail vein into C57BL/6 mice. Drug administration treatment was initiated the day after the model was established. Micro-CT scanning of mouse lungs on day 21 revealed extensive diffuse foci in the lungs of mice in the model group, whereas fewer foci and diffuse areas were observed in the lungs of mice treated with 25 mg·kg−1·d−1 Cel and 100 mg·kg−1·d−1 RA (Fig. 1g, h). Mice were weighed every three days during the four weeks of treatment, and the body weight of mice in all groups did not show significant changes (Fig. 1f). Lung, colon, liver, and stomach tissues were extracted and subjected to H&E staining, revealing lower numbers of metastatic foci and tumor areas in the lungs of mice treated with 25 mg·kg−1·d−1 Cel and 100 mg·kg−1·d−1 RA compared to the model group, consistent with Micro-CT results (Fig. 1i, j). These findings demonstrated that 25 mg·kg−1·d−1 Cel and 100 mg·kg−1·d−1 RA could inhibit lung metastasis of colon cancer in vivo. As Cel is known to have adverse effects, whereas RA, as an extracted component of traditional Chinese medicine, is less toxic, we performed H&E staining of the liver, stomach, and colon, revealing higher safety to these organs in the RA group compared to the Cel group (Fig. 1k). These results demonstrated that while RA exerted an antitumor effect, it also has lower damaging effect on other organs than Cel.
The findings of both in vitro and in vivo experiments consistently demonstrated the dose-dependent inhibition of MC38 cell migration and invasion by RA, as well as its anti-colon cancer lung metastasis effect in vivo. Compared to Cel, RA has a higher safety profile. These results collectively indicate the potent anti-metastatic effects of RA in vitro and in vivo against colon cancer metastasis.
RA inhibits colon cancer metastasis via COX-2/MYO10 axis
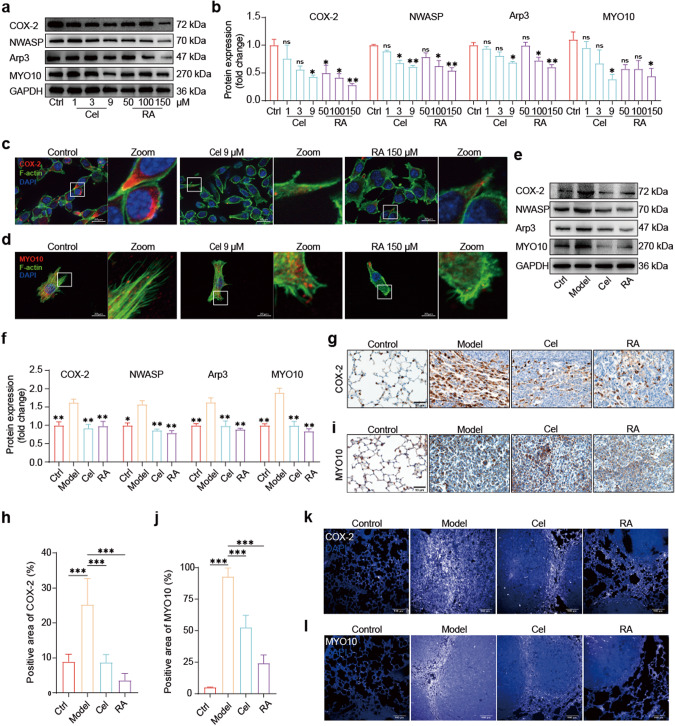
We found that RA can effectively inhibit colon cancer metastasis in vitro and in vivo. Being an aspirin-like component, we were interested in whether RA can inhibit COX-2, given that the COX-2/PGE2 axis is the most common pathway promoting colon cancer metastasis through inflammation [3, 47, 48]. Notably, we found that COX-2 can also promote cancer metastasis through non-inflammatory effects, which is a phenomenon that has rarely been reported. For example, COX-2 can promote prostate cancer lung metastasis through paracrine effects [9]. This led us to further investigate the non-inflammatory effect of COX-2 in promoting colon cancer metastasis. As it is well-established that COX-2 promotes tumor invasion and metastasis [49–51], and since tumor cell motility plays a crucial role in cancer invasion and metastasis, we focused our attention on COX-2 and cancer cell motility. Specifically, we explored the link between COX-2 and filopodia, a protrusive cytoskeletal structure that provides support for tumor cell motility. MYO10 is an important marker of filopodia, and its role in stabilizing the cytoskeleton is well-established [13, 52]. As no tight link between COX-2 and MYO10 has been reported, we investigated this association in-depth. Our in vitro experiments revealed that RA can dose-dependently inhibit the expression of COX-2 and MYO10 proteins in MC38 cells, with the greatest effect observed at 150 μM. Similarly, Cel had a significant impact on the expression of COX-2 and MYO10 proteins at 9 μM (Fig. 2a, b). Immunofluorescence assays showed that RA treatment effectively inhibited the expression of COX-2 in MC38 cells (Fig. 2c). Furthermore, double staining with actin tracker green and MYO10 revealed that RA intervention led to the remodeling of MC38 cell cytoskeleton, with changes in morphology and a significant reduction in filamentous-like synapses and MYO10 expression (Fig. 2d). These results suggest that RA can inhibit the expression of COX-2 and MYO10 in MC38 cells. In vivo experiments were also conducted to isolate lung tissue and obtain lung whole protein. Western blot analyses showed that the expression of COX-2 and MYO10 was suppressed in the RA group (100 mg·kg−1·d−1) and Cel group (25 mg·kg−1·d−1) compared to the model group, with the RA group exhibiting superior effects over the Cel group (Fig. 2e, f). Immunohistochemistry and immunofluorescence assays of the tissue samples yielded consistent results (Fig. 2g–l).
Fig. 2. RA inhibits colon cancer metastasis via COX-2 – MYO10 axis.
a, b Western blot analysis: representative images and quantitative data showing the protein levels of cells, with GAPDH used as a loading control (compared with the control group, *P < 0.05, **P < 0.01, ns not significant, one-way ANOVA, n = 3). c, d Fluorescence staining: representative images of cells showing the actin cytoskeleton and COX-2 or MYO10 (scale bar: 20 μm, 63× magnification). e, f Western blot: representative images and quantitative data showing the protein levels of lung tissues, with GAPDH used as a loading control (compared with the model group, *P < 0.05, **P < 0.01, one-way ANOVA, n = 3). g–j Immunohistochemistry staining: representative images and quantitative analysis for COX-2 or MYO10 in the lung tumor tissues (scale bar: 50 μm, 40× magnification, compared with the model group, ***P < 0.001, one-way ANOVA, n = 3). k, l Fluorescence staining: representative images of lung tumor tissues for COX-2 or MYO10 (scale bar: 100 μm, 20× magnification).
Since filopodia generation requires the involvement of precursor materials, such as the neural Wiskott Aldrich syndrome protein (NWASP) and the actin-related protein 2/3 complex (Arp2/3), we investigated their protein expression levels. Our experiments demonstrated that RA intervention led to a dose-dependent decrease in the expression of NWASP and Arp3 in MC38 cells, with the most significant effect observed at 150 μM. Similarly, the impact of Cel 9 μM on both NWASP and Arp3 was substantial (Fig. 2a, b). Consistent with the in vitro results, the expression levels of NWASP and Arp3 were significantly suppressed in the RA group (100 mg·kg−1·d−1) compared to the model mice, and the effect was better than that observed in mice treated with 25 mg·kg−1·d−1 of Cel (Fig. 2e, f). Our results suggest that RA can effectively inhibit the expression of COX-2 and MYO10 in colon cancer both in vitro and in vivo. We also demonstrate that RA could suppress the expression of NWASP and Arp3, which are key regulators of filopodia generation. Filopodia play a critical role in tumor cell motility, which is an essential step in cancer invasion and metastasis. Therefore, the ability of RA to inhibit the generation of filopodia through the suppression of NWASP and Arp3 may be a mechanism contributing to its anti-metastatic effects.
We hypothesize that RA may downregulate MYO10 expression by attenuating COX-2 expression, which serves as an internal cytoskeletal molecule that stabilizes filopodial structures. Filopodia are crucial for tumor invasion and migration, and our results suggest that COX-2 plays a significant role in filopodial formation. Nevertheless, the mechanistic link between COX-2 and MYO10 remains elusive. Overall, our findings propose that RA hinders colon cancer metastasis by inhibiting the COX-2/MYO10 axis.
COX-2 and MYO10 are highly expressed in human colon cancer
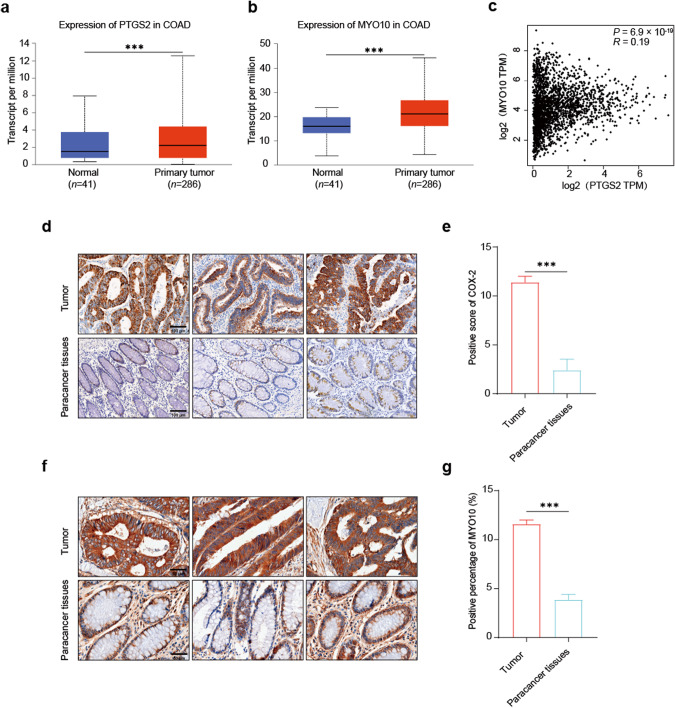
We conducted a comparative analysis of Ptgs2 and MYO10 gene expression in normal and colon cancer tissues using data obtained from TCGA database. Our analysis revealed a statistically significant upregulation of both Ptgs2 and MYO10 genes in colon cancer tissues when compared with normal colon tissues (Fig. 3a, b). Correlation analysis of PTGS2 and MYO10 in various human tumors, including colon cancer, showed a positive correlation (Fig. 3c).
Fig. 3. COX-2 and MYO10 are highly expressed in human colon cancer.
a, b Analysis of TCGA database revealed a significant upregulation of Ptgs2 or MYO10 expression in primary tumors of colon cancer compared to normal tissue samples (compared with the tumor group ***P < 0.001, t-test). c Correlation analysis between PTGS2 and MYO10 was displayed by GEPIA database (P < 0.05, Spearman’s correlation coefficient). d, e Representative images of COX-2 immunohistochemical staining in human colon cancer tissues are presented. Quantitative analysis showed a significant increase in COX-2 expression in tumor tissues compared to normal tissues (scale bar: 100 μm, 20× magnification, compared with the tumor group, ***P < 0.001, t-test, n = 7). f, g Representative images of MYO10 immunohistochemical staining in human colon cancer tissues are displayed (scale bar: 50 μm, 40× magnification, compared with the tumor group, ***P < 0.001, t-test, n = 7).
We collected colon cancer tissue samples from clinical patients and conducted immunohistochemical staining. The stained samples were scanned and quantified using a fully automated quantitative pathological imaging system. Our results revealed a significant overexpression of COX-2 and MYO10 in tumor tissues as compared to the paracancerous tissues. These findings were consistent with our earlier analysis of TCGA database (Fig. 3d–g). The expression of COX-2 and MYO10 in tumor tissues was quantified using the criteria suggested by Soong et al. [53]. The staining of cells was classified on the basis of the degree of cell staining, with scores ranging from 0 to 3. Further, the ratio scores of positive cells ranged from 0 to 4 depending upon the percentage of positive cells. The final score was calculated by multiplying the scores assigned for cell staining and the ratio of positive cells. Based on our results, both COX-2 and MYO10 were found to be highly expressed in colon cancer. This strengthens our expectation of uncovering the potential link between COX-2 and MYO10, which has significant clinical implications.
Knockout of COX-2 reduces MYO10 expression and inhibits MC38 cell migration and invasion
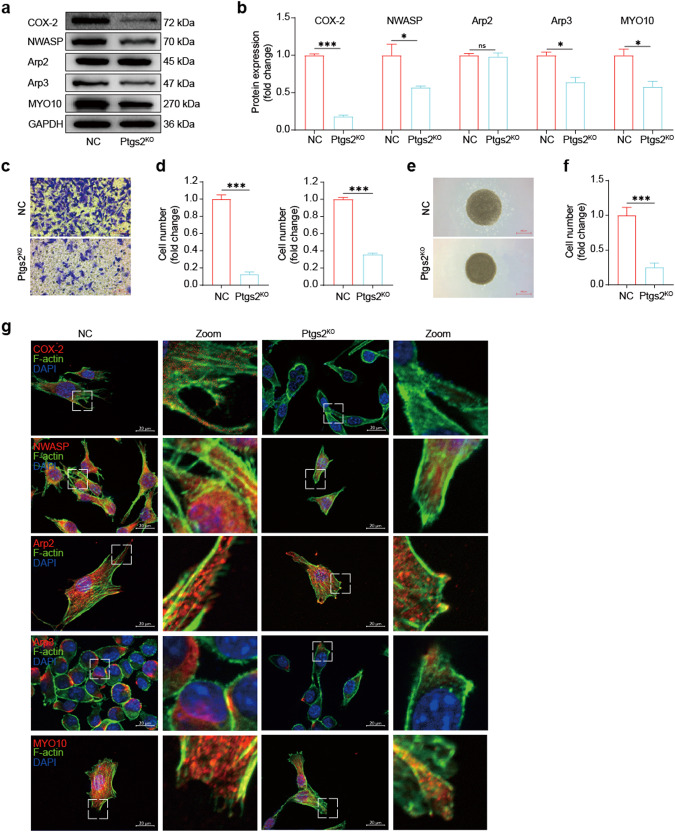
Above results demonstrated that the expression levels of COX-2 and MYO10 were markedly upregulated in human colon cancer tissues through TCGA database analysis and clinical sample IHC experiments. However, to our knowledge, the relationship between COX-2 and MYO10 in tumors has not been elucidated. Therefore, we utilized plasmids to knockout Ptgs2 in the mouse colon cancer cell line MC38, generating an MC38 Ptgs2KO cell line. We observed that the expression of MYO10, neural Wiskott Aldrich syndrome protein (NWASP), and Arp3, a subunit of the actin-related protein 2/3 complex, was significantly decreased in the absence of COX-2 through Western blotting (Fig. 4a, b). Through immunofluorescence experiments, we observed that the depletion of COX-2 in MC38 cells resulted in reduced expression levels of NWASP, Arp3, and MYO10. In contrast to the NC group, the cytoskeleton morphology of MC38 cells in the Ptgs2KO group was remodeled, where the microfilaments exhibited indistinctness and decreased in number and length. Moreover, the number and length of cellular extensions were also reduced and the occurrence of filopodia was prominently reduced, which further validated the above results (Fig. 4g). Notably, Arp2, another subunit of the Arp2/3 complex, remained unaffected, which is consistent with previous findings [54]. NWASP and Arp2/3 complex are known to regulate tumor cell filopodia generation.
Fig. 4. Knockout of COX-2 reduces MYO10 expression and inhibits MC38 cell migration and invasion.
a, b Western blot: representative images and quantitative data of MC38 or Ptgs2KO MC38 cell protein levels. GAPDH was used as a loading control (compared with NC group, *P < 0.05, ***P < 0.001, ns, not significant, t-test, n = 3). c, d Transwell migration assay: representative images and quantitative analysis of MC38 or Ptgs2KO MC38 cell migration and OD value (20× magnification, scale bar: 100 μm, compared with NC group, ***P < 0.001, t-test, n = 3). e, f 3D-invasion system: representative images and quantitative analysis of MC38 or Ptgs2KO MC38 cell invasion (4× magnification, scale bar: 500 μm, compared with NC group, ***P < 0.001, t-test, n = 3). g Fluorescence staining: representative images of MC38 or Ptgs2KO MC38 cells showing the actin cytoskeleton and expression levels of COX-2, NWASP, Arp2, Arp3, or MYO10 (63× magnification, scale bar: 20 μm).
As a molecular motor, MYO10 plays a crucial role in promoting filopodia generation, accelerating tumor cell motility, and facilitating tumor migration and invasion both in vitro and in vivo. To investigate the effect of COX-2 knockout on tumor cell migration and invasion abilities, we performed Transwell and tumor 3D invasion sphere experiments in vitro. We found that migratory and invasive abilities were greatly reduced in the Ptgs2KO group compared to the NC group (Fig. 4c–f). Overall, our findings suggest that COX-2 promotes tumor metastasis by regulating the expression of MYO10. After COX-2 knockout, MC38 cells exhibited decreased MYO10 expression, resulting in significantly reduced tumor cell migration and invasion abilities in vitro.
RA can inhibit the migration and invasion abilities of Ptgs2OE MC38 cells
To investigate the effect of COX-2 overexpression (Ptgs2OE) on tumor cell migration and invasion abilities, we constructed a stable Ptgs2OE MC38 cell line using lentivirus. The results showed that COX-2 overexpression significantly increased the migration and invasion abilities of MC38 cells (Fig. 5a–d). Western blot analysis further revealed a significant upregulation in the protein levels of MYO10, Arp3, and NWASP in the Ptgs2OE cells (Fig. 5e, f). Immunofluorescence experiments yielded similar results (Fig. 5g, h). To assess the role of COX-2 inhibitors in the Ptgs2OE cells, we treated the cells with Cel 9 μM or RA 150 μM, respectively. Interestingly, we observed a partial reduction in migration and invasion abilities in the Ptgs2OE cells following the intervention (Fig. 5a–d). These results were also supported by Western blot results, which showed a downregulation in the protein levels of MYO10, NWASP, and Arp3 in both the Cel and RA groups (Fig. 5e, f). Immunofluorescence experiments corroborated these findings (Fig. 5g, h).
Fig. 5. RA can inhibit the migration and invasion abilities of Ptgs2OE MC38 cells.
a, b Representative images and quantitative analysis for the migration of MC38 cells or Ptgs2OE MC38 cells treated with Cel or RA were obtained using Transwell migration assays (scale bar: 100 μm, 20× magnification, compared with the Ptgs2OE group, **P < 0.01, ***P < 0.001, one-way ANOVA, n = 3). c, d Representative images and quantitative analysis for the invasion of MC38 cells or Ptgs2OE MC38 cells treated with Cel or RA were obtained using 3D-invasion system assays (scale bar: 500 μm, 4× magnification, compared with the Ptgs2OE group, *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA, n = 3). e, f Western blot analysis: representative images and quantitative data for the protein levels of cells were obtained. GAPDH was used as a loading control (compared with the Ptgs2OE group, *P < 0.05, **P < 0.01, ***P < 0.001, ns no significant, one-way ANOVA, n = 3). g, h Fluorescence staining was used to visualize the actin cytoskeleton and COX-2 or MYO10 in MC38 cells or Ptgs2OE MC38 cells treated with Cel or RA. Representative images were obtained (scale bar: 20 μm, 63× magnification).
Our results suggest that overexpression of COX-2 in MC38 cells leads to an increase in their migration and invasion abilities, accompanied by upregulation of filopodia marker MYO10 and its associated regulators. Interestingly, treatment with RA partially reversed this phenomenon. Our results indicate that the inhibition of colon cancer metastasis by RA may be mediated via the COX-2-MYO10 axis.
RA combined with GR can further inhibit colon cancer metastasis in vivo
Through ELISA screening of monomeric compounds from traditional Chinese medicine (TCM) on biotin-labeled PD-1 and PD-L1 proteins, we identified GR as having a certain inhibitory effect on the binding of PD-1 and PD-L1. Repeating the experiment multiple times, we observed that GR could inhibit the PD-1/PD-L1 interaction by up to 50% at 100 μM. Subsequently, we lowered the concentration of GR to 50 μM and 25 μM, and the inhibitory ratios of PD-1/PD-L1 were both around 30% (Fig. 6a, b). Our initial hypothesis was that GR would significantly inhibit PD-1/PD-L1 binding at the protein level. Therefore, we further investigated whether GR could also function in mice.
Fig. 6. RA combined with GR can further inhibit colon cancer metastasis in vivo.
a, b Elisa experiment principle and results for PD-1/PD-L1 binding (compared with the control group: **P < 0.05, **P < 0.01, ***P < 0.001). c, e Micro-CT scans of mouse lungs: representative images and quantitative analysis for metastatic foci (compared with the model group, *P < 0.05, **P < 0.01; compared with the RA group, #P < 0.05, one-way ANOVA, n = 5). d, f H&E staining: representative images and quantitative analysis for the tumor area (20× magnification, scale bar, 200 μm; compared with the model group: **P < 0.01, ***P < 0.001; compared with the RA group: #P < 0.05, one-way ANOVA, n = 5). g Monitoring of mouse body weight (ns, not significant, two-way ANOVA, n = 10). h H&E staining: representative images of liver, stomach, and colon tissue (20× magnification, scale bar, 100 μm).
Multiple studies have demonstrated that combining COX-2 inhibitors with PD-1/PD-L1 monoclonal antibodies (mAbs) can inhibit tumor metastasis. Unfortunately, both COX-2 inhibitors and mAbs have numerous adverse effects, causing significant distress to cancer patients. To address these issues, we combined GR, which inhibits PD-1/PD-L1 binding at the protein level, with RA in an attempt to further inhibit tumor metastasis in mice. A mouse colon cancer lung metastasis model was generated by injecting 2.4 × 105 cells resuspended in 200 μL sterile PBS into C57BL/6J mice via tail vein injection. All mice were weighed once every three days, and there were no significant changes in body weight during the treatment cycle, which started the day after modeling (Fig. 6g). Mice in the combination group were given 25 mg·kg−1·d−1 Cel orally, 100 mg·kg−1·d−1 RA orally, 5 mg·kg−1·(3 d)−1 of aPD-L1 mAb i.p., and 100 mg·kg−1·d−1 of GR i.p. On day 21 after modeling, Micro-CT scanning of mouse lungs revealed a significant decrease in the number of metastatic foci in the lungs of mice in the combination treatment group (Fig. 6c, e). Consistently, H&E staining confirmed that the combination therapy significantly reduced the area of lung tumors (Fig. 6d, f). Because the use of mAbs can lead to various adverse effects, we also performed H&E staining on the liver, stomach, and colon of mice. Compared with the mAb combination group, the GR combination group showed lesser degree of damage to the hepatic, gastric, and colonic tissue structure, which indicates a certain level of drug safety (Fig. 6h).
Our results demonstrate that combining RA 100 mg·kg−1·d−1 with GR 100 mg·kg−1·d−1 interventions can more effectively suppress colon cancer lung metastasis compared to treatment with RA 100 mg·kg−1·d−1 alone. Importantly, the combination of GR and RA showed a high safety profile.
GR could activate CD8 T cells
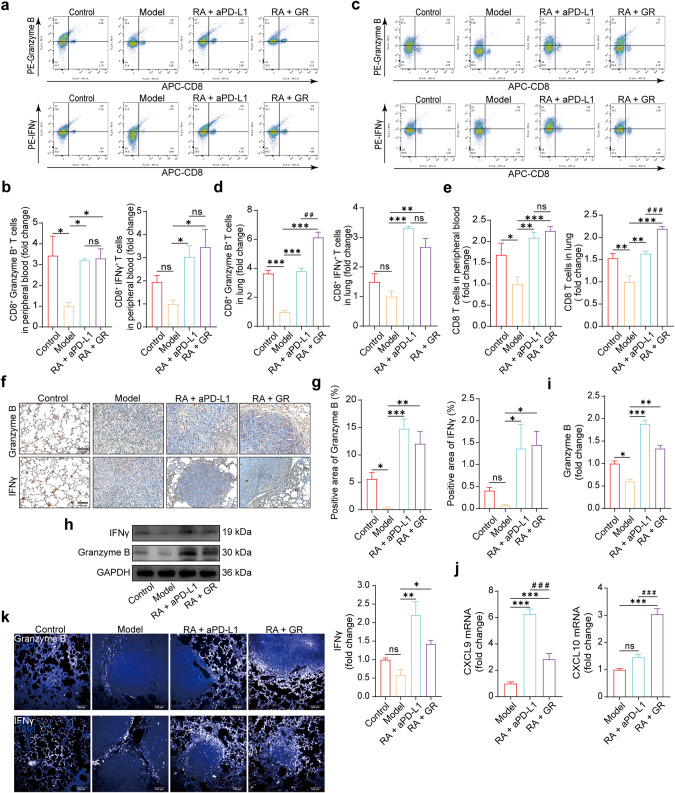
As our study focused on the inhibitory effect of GR on PD-1/PD-L1 binding, we examined whether this led to an increase in activated T cells in the blood and lungs of mice. We performed co-staining of CD8a with Granzyme B or IFN-γ, respectively. Flow analysis of peripheral blood and lung lymphocytes showed a significant increase in the proportion of Granzyme B+ CD8+ T cells and IFN-γ+ CD8+ T cells in the 5 mg·kg−1·(3 d)−1 mAb combination group and the 100 mg·kg−1·d−1 GR combination group (Fig. 7a–d), which demonstrated that GR has the capacity to activate CD8 T cells in both the bloodstream and the lung. To our surprise, the number of CD8+ T cells was also significantly higher in the GR combination group than in the model and mAb groups (Fig. 7e). Immunohistochemical staining, Western blotting, and immunofluorescence experiments showed that Granzyme B and IFN-γ were expressed highly in the GR combination group, which yielded consistent results (Fig. 7f–i, k). qPCR analysis showed that CXCL9 and CXCL10 mRNAs, which are associated with CD8 T cell activation, were significantly elevated in the GR combination groups (Fig. 7j). In order to eliminate potential interference from RA, we included separate treatment groups for RA and GR. Flow cytometry analysis revealed that RA did not induce activation of CD8 T cells (Supplementary Fig. S2). Taken together, these findings suggest that the mechanism of GR in combating colon cancer lung metastasis involves disrupting PD-1/PD-L1 binding, thereby activating CD8 T lymphocytes in the blood and lungs and enabling them to effectively eliminate tumor cells.
Fig. 7. GR could activate CD8 T cells.
a, b Peripheral blood lymphocytes were isolated and analyzed using flow cytometry. Statistical analysis revealed a significant difference compared to the model group (compared with the model group, *P < 0.05, ns not significant, one-way ANOVA; compared with the RA + aPD-L1 group, ns not significant, t-test, n = 3). c, d Lung tissue lymphocytes were isolated and analyzed using flow cytometry (compared with the model group, **P < 0.01, ***P < 0.001, ns not significant, one-way ANOVA; compared with the RA + aPD-L1 group, ##P < 0.01, ns not significant, t-test, n = 3). e Total CD8+ T cell numbers were analyzed (compared with the model group, *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant; compared with the RA + aPD-L1 group, ###P < 0.001, ns, not significant, t-test, n = 6). f, g Immunohistochemistry staining was performed for Granzyme B or IFNγ in lung tumor tissues. Representative images and quantitative analysis were obtained (20× magnification, scale bar: 100 μm, compared with the model group, *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant, one-way ANOVA, n = 3). h, i Western blot was performed to determine protein levels in lung tissues. Representative images and quantitative data were obtained. GAPDH was used as a loading control (compared with the model group, *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant, one-way ANOVA, n = 3). j qPCR analysis was performed to determine the expression of CXCL9 or CXCL10 mRNA in lung tissues (compared with the model group, ***P < 0.001, ns not significant, one-way ANOVA; compared with the RA + aPD-L1 group, ###P < 0.001, t-test, n = 5). k Fluorescence staining was performed for Granzyme B or IFNγ in lung tissues. Representative images were obtained (20× magnification, scale bar: 100 μm).
We demonstrated the mechanism by which GR counters colon cancer metastasis through the use of various techniques such as flow cytometry, immunohistochemistry, WB, qPCR, and immunofluorescence. Our results indicated that inhibition of PD-1/PD-L1 binding increased the number of activated CD8 T cells, ultimately enhancing their ability to eliminate tumor cells. Most importantly, our findings revealed that RA and GR displayed significant antitumor effects with considerably lower toxicity towards other organs of mice, when compared to COX-2 inhibitors and aPD-L1 mAbs.
Discussion
Our study demonstrates that RA can effectively inhibit colon cancer metastasis both in vitro and in vivo. Firstly, we determined the non-proliferation inhibitory concentration of RA on MC38 cells using MTT assay. Subsequently, we performed transwell and 3D tumor invasion sphere experiments, which found RA 150 μM significantly inhibited the migration and invasion of MC38 cells, indicating its potential as a tumor metastasis inhibitor in vitro. In the in vivo experiment using a mouse tail vein lung metastasis model of MC38 cells, daily administration of RA 100 mg·kg−1·d−1 starting on the second day post modeling resulted in less diffuse loss and more intact lungs in mice in the RA group, as detected by Micro-CT scanning at day 21. Furthermore, four weeks after drug administration, we performed H&E staining on multiple organs of mice and observed that RA 100 mg·kg−1·d−1 caused less damage to the liver, stomach, and intestines compared to Cel 25 mg·kg−1·d−1, a frequently used anti-tumor drug. By H&E staining and Micro-CT, we found that RA significantly reduced the size of metastatic foci in the lungs of mice compared to the model group. We also investigated the mechanism underlying RA’s inhibitory effect on colon cancer metastasis by exploring whether it acts on COX-2, a protein known to be involved in tumor metastasis by inflammatory effect. Our Western blot and immunofluorescence analysis showed that RA inhibited COX-2 expression in vitro and tumor-bearing lungs. Considering the limited studies on the possibility of COX-2 inducing tumor metastasis via non-inflammatory mechanisms, our study aims to investigate whether COX-2 mediates non-inflammatory effects to facilitate metastasis of colon cancer. We have directed our attention towards the examination of non-inflammatory effects of COX-2 on colon cancer metastasis. This led us to further explore the non-inflammatory effects of COX-2 in mediating tumor metastasis, with particular focus on its effect on tumor cell motility. Our results showed that RA 150 μM could inhibit the formation of filopodia-associated proteins by WB and immunofluorescence analysis, including NWASP, Arp3, and especially MYO10, a filopodia marker, which contributed to reduce the invasion and migration ability of colon cancer. Overall, our findings suggest that RA inhibited colon cancer metastasis by reducing COX-2 expression and inhibiting the formation of filopodia-associated marker, MYO10, which indicated COX-2 could suppress colon cancer cell metastasis by inhibiting tumor cell motility.
To further investigate the relationship between COX-2 and tumor cell motility, we constructed Ptgs2 knockout MC38 cells (Ptgs2KO MC38) by using plasmid technology. Through transwell and 3D tumor invasion sphere experiments, our results showed that knockout of COX-2 significantly reduced the migration and invasion abilities of MC38 cells, as well as downregulated the expression of MYO10 and related filopodia generation regulators. Conversely, overexpression of COX-2 in MC38 cells (Ptgs2OE MC38) significantly enhanced their migration and invasion abilities, and upregulated the expression of MYO10 and filopodia-associated proteins. However, following RA 150 μM intervention, the migration and invasion abilities of Ptgs2OE MC38 cells were significantly inhibited, and the upregulation of MYO10 and filopodia-associated proteins were downregulated. By manipulating COX-2 expression, we confirmed that COX-2 regulates the migration and invasion of tumor cells, and promotes metastasis by regulating MYO10 expression. Notably, our findings suggest that this function of COX-2 is distinct from its classical pro-inflammatory role in mediating tumor metastasis. Analysis of the TCGA database showed that COX-2 and MYO10 were highly expressed in colon cancer tissues compared to normal tissues, and immunohistochemical analysis on clinical samples of colon cancer confirmed these findings. Our experiments indicate that RA could inhibit the expression of MYO10 by inhibiting COX-2, thereby showing potential as a clinical treatment for colon cancer metastasis. Multiple studies referred to RA as a potential inhibitor of COX-2, and our findings further supported this perspective. However, we currently still lack sufficient evidence to directly confirm RA as a COX-2 inhibitor, as the exact binding sites are still unclear and require further in-depth research. Similarly, the specific mechanisms of interactions between COX-2 and MYO10 remain unclear and necessitate further investigation.
The PD-1/PD-L1 signaling pathway plays a crucial role in tumor immune escape. Binding of the ligand PD-L1 on tumor cells to its receptor PD-1 on T cells inhibits the secretion of factors such as interferon γ (IFN γ), tumor necrosis factor (TNF), interleukin 2 (IL-2), thus impairing the killing function of T cells [55, 56]. Clinically, immunotherapy for colon cancer involves drugs such as K-drug-pabolizumab, O-drug-nivolumab, and bevacizumab [57, 58]. However, these treatments can cause serious adverse effects such as bleeding, gastrointestinal perforation, arterial thrombosis, pneumonia, enteritis, hepatitis, adrenal insufficiency, diabetes, heart disease, etc [59–62], which are additional burdens for oncology patients. In recent years, the development of small molecule inhibitors for PD-1/PD-L1 has garnered significant attention, and research on TCM-based PD-1/PD-L1 inhibition has also become popular. Panax ginseng is known for its Qi-tonic effects and can enhance the immune function of the body. Ginseng’s anti-tumor effects have also been widely recognized, and the saponin components ginsenoside Rh2 and ginsenoside Rg3 have been extensively studied [63, 64]. Based on this information, we speculated whether Ginseng contains components that can inhibit PD-1/PD-L1. Through screening the major active components of panax ginseng and other Chinese medicinal herbs, we found that GR could inhibit PD-1/PD-L1 binding at the protein level in vitro. Moreover, dual inhibition of COX-2 and PD-1/PD-L1 has been reported to effectively inhibit tumor metastasis. Thus, we constructed a mouse colon cancer lung metastasis model and found that combined administration of RA 100 mg·kg−1·d−1 and GR 100 mg·kg−1·d−1 further inhibited colon cancer lung metastasis compared to single administration of RA 100 mg·kg−1·d−1. Flow cytometry analysis showed that GR increased the number of CD8 T cells in the peripheral blood and lungs, and CO staining of IFN γ and Granzmye B indicated that GR activated CD8 T cells in the peripheral blood and lungs. We hypothesized that GR not only increased the number of CD8 T cells, but also enhanced their killing function towards tumor cells to some extent in vivo. Our findings from immunohistochemistry, immunofluorescence, Western blot, and PCR analysis showed that GR could activate CD8 T cells, possibly by inhibiting PD-1/PD-L1. Given that mAb drugs can cause serious adverse reactions, we evaluated the degree of GR toxicity to the liver, stomach, and colon by using H&E staining, and found that the GR combination group exhibited lower drug toxicity compared to the mAb combination group.
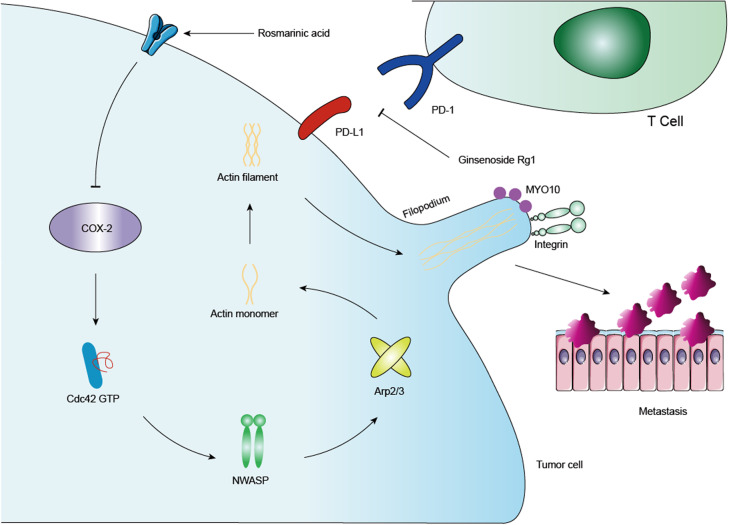
Our results suggest that RA suppresses colon cancer metastasis both in vitro and in vivo by inhibiting the COX-2-MYO10 axis. Specifically, we found that by inhibiting the non-inflammatory effects of COX-2, RA could effectively inhibit colon cancer metastasis. It is worth noting that the non-inflammatory role of COX-2 in mediating tumor metastasis should not be overlooked. Furthermore, GR was found to activate cytotoxic T cells in vivo, leading to increased killing capacity against tumor cells in the blood and lungs. When RA combined with GR, the inhibitory effect on lung metastasis for colon cancer was further augmented. Importantly, RA and GR have better safety profiles than that of COX-2 inhibitors and aPD-L1 mAbs, suggesting potential clinical strategies for treating colon cancer metastasis without serious adverse effects (Fig. 8).
Fig. 8. Mechanism diagram.
In this study, after administration of RA, the expressions of COX-2 and MYO10 were suppressed, and the related proteins regulating the expression of filamentous pseudopodia were also inhibited to varying degrees. By constructing COX-2 knockout and overexpression cell lines, we discovered that COX-2 can regulate the expression of MYO10, thus influencing the migration and invasion of colon cancer cells. Additionally, we found that GR can inhibit PD-1/PD-L1 binding at the protein level. In vivo experiments have demonstrated that GR can activate CD8 T cells and enhance their killing function against tumor cells. Combining RA and GR has shown a beneficial effect on inhibiting colon cancer metastasis, the underlying mechanism of which was previously unknown.
Conclusion
Our results demonstrate that the combination of RA and GR effectively curbed colon cancer metastasis, with lower visceral toxicity, which provided some help in explaining the anti-tumor mechanism of traditional Chinese medicine. The above findings have certain clinical significance.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81973734, 81961128020 and 82204802), the Jiangsu Specially Appointed Professorship Foundation (013038021001) and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX22_2014).
Author contributions
HL and RD performed most of the experiments and analyzed the data. CWZ, GFZ and HKH participated in part of the experiments. LR, PC and ZHW provided helps for the statistical analysis. YZ, SYY, and YL conceived supervised the study. HL and SYY wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Huan Liu, Rui Deng
Contributor Information
Yang Zhao, Email: y.zhao@njucm.edu.cn.
Su-yun Yu, Email: yusuyun@njucm.edu.cn.
Yin Lu, Email: luyingreen@njucm.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01158-8.
References
- 1.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Uddin MJ, Crews BC, Blobaum AL, Kingsley PJ, Gorden DL, McIntyre JO, et al. Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents. Cancer Res. 2010;70:3618–27. doi: 10.1158/0008-5472.CAN-09-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L, Stevens J, Hilton MB, Seaman S, Conrads TP, Veenstra TD, et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6:242ra84. doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidalgo-Estévez AM, Stamatakis K, Jiménez-Martínez M, López-Pérez R, Fresno M. Cyclooxygenase 2-regulated genes an alternative avenue to the development of new therapeutic drugs for colorectal cancer. Front Pharmacol. 2020;11:533. doi: 10.3389/fphar.2020.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E, et al. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through β-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun. 2016;58:91–8. doi: 10.1016/j.bbi.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong XF, Liu TQ, Zhi XT, Zou J, Zhong JT, Li T, et al. COX-2/PGE2 axis regulates HIF2α activity to promote hepatocellular carcinoma hypoxic response and reduce the sensitivity of sorafenib treatment. Clin Cancer Res. 2018;24:3204–16. doi: 10.1158/1078-0432.CCR-17-2725. [DOI] [PubMed] [Google Scholar]
- 7.Garrido MP, Hurtado I, Valenzuela-Valderrama M, Salvatierra R, Hernández A, Vega M, et al. NGF-enhanced vasculogenic properties of epithelial ovarian cancer cells is reduced by inhibition of the COX-2/PGE2 signaling axis. Cancers. 2019;11:1970. doi: 10.3390/cancers11121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Araújo WM, Tanaka MN, Lima PHS, de Moraes CF, Leve F, Bastos LG, et al. TGF-β acts as a dual regulator of COX-2/PGE2 tumor promotion depending of its cross-interaction with H-Ras and Wnt/β-catenin pathways in colorectal cancer cells. Cell Biol Int. 2021;45:662–73. doi: 10.1002/cbin.11519. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Comaills V, Burr R, Boulay G, Miyamoto DT, Wittner BS, et al. COX-2 mediates tumor-stromal prolactin signaling to initiate tumorigenesis. Proc Natl Acad Sci USA. 2019;116:5223–32. doi: 10.1073/pnas.1819303116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harb Perspect Biol. 2018;10:a018267. doi: 10.1101/cshperspect.a018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izdebska M, Zielińska W, Hałas-Wiśniewska M, Grzanka A. Involvement of actin and actin-binding proteins in carcinogenesis. Cells. 2020;9:2245. doi: 10.3390/cells9102245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham S, Scarcia M, Bagshaw RD, McMahon K, Grant G, Harvey T, et al. A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nat Commun. 2015;6:7286. doi: 10.1038/ncomms8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Berg JS, Li Z, Wang Y, Lång P, Sousa AD, et al. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6:523–31. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 14.Peuhu E, Jacquemet G, Scheele C, Isomursu A, Laisne MC, Koskinen LM, et al. MYO10-filopodia support basement membranes at pre-invasive tumor boundaries. Dev Cell. 2022;57:2350–64. doi: 10.1016/j.devcel.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XJ, Wang CZ, Dai PG, Xie Y, Song NN, Liu Y, et al. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol. 2007;9:184–92. doi: 10.1038/ncb1535. [DOI] [PubMed] [Google Scholar]
- 16.Dormond O, Foletti A, Paroz C, Rüegg C. NSAIDs inhibit alpha V beta 3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis. Nat Med. 2001;7:1041–7. doi: 10.1038/nm0901-1041. [DOI] [PubMed] [Google Scholar]
- 17.Han Y, Liao Z, Li Y, Zhao X, Ma S, Bao D, et al. Magnetically controlled capsule endoscopy for assessment of antiplatelet therapy-induced gastrointestinal injury. J Am Coll Cardiol. 2022;79:116–28. doi: 10.1016/j.jacc.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TNM, Sha S, Chen LJ, Holleczek B, Brenner H, Schöttker B. Strongly increased risk of gastric and duodenal ulcers among new users of low-dose aspirin: results from two large cohorts with new-user design. Aliment Pharmacol Ther. 2022;56:251–62. doi: 10.1111/apt.17050. [DOI] [PubMed] [Google Scholar]
- 19.Kang DO, An H, Park GU, Yum Y, Park EJ, Park Y, et al. Cardiovascular and bleeding risks associated with nonsteroidal anti-inflammatory drugs after myocardial infarction. J Am Coll Cardiol. 2020;76:518–29. doi: 10.1016/j.jacc.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Szeto CC, Sugano K, Wang JG, Fujimoto K, Whittle S, Modi GK, et al. Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: joint APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut. 2020;69:617–29. doi: 10.1136/gutjnl-2019-319300. [DOI] [PubMed] [Google Scholar]
- 21.Zhang DY, Peng RQ, Wang X, Zuo HL, Lyu LY, Yang FQ, et al. A network pharmacology-based study on the quality control markers of antithrombotic herbs: using salvia miltiorrhiza - ligusticum chuanxiong as an example. J Ethnopharmacol. 2022;292:115197. doi: 10.1016/j.jep.2022.115197. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y, Yang T, Huang K, Shen L, Tao Y, Liu C. Salvia miltiorrhiza ameliorates liver fibrosis by activating hepatic natural killer cells in vivo and in vitro. Front Pharmacol. 2018;9:762. doi: 10.3389/fphar.2018.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao L, Wang S, Zhao Y, Sheng X, Wang A, Zheng S, et al. Phenolcarboxylic acids from medicinal herbs exert anticancer effects through disruption of COX-2 activity. Phytomedicine. 2014;21:1473–82. doi: 10.1016/j.phymed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Cen B, Wei J, Wang D, Xiong Y, Shay JW, DuBois RN. Mutant APC promotes tumor immune evasion via PD-L1 in colorectal cancer. Oncogene. 2021;40:5984–92. doi: 10.1038/s41388-021-01972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki T, Chung S, Sakai H, Ohata H, Obata Y, Shiokawa D, et al. Stemness and immune evasion conferred by the TDO2-AHR pathway are associated with liver metastasis of colon cancer. Cancer Sci. 2022;113:170–81. doi: 10.1111/cas.15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruck T, Barman S, Schulte-Mecklenbeck A, Pfeuffer S, Steffen F, Nelke C, et al. Alemtuzumab-induced immune phenotype and repertoire changes: implications for secondary autoimmunity. Brain. 2022;145:1711–25. doi: 10.1093/brain/awac064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–92. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977–90. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 30.Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 31.Long J, Liu XK, Kang ZP, Wang MX, Zhao HM, Huang JQ, et al. Ginsenoside Rg1 ameliorated experimental colitis by regulating the balance of M1/M2 macrophage polarization and the homeostasis of intestinal flora. Eur J Pharmacol. 2022;917:174742. doi: 10.1016/j.ejphar.2022.174742. [DOI] [PubMed] [Google Scholar]
- 32.Ren Z, Chen X, Hong L, Zhao X, Cui G, Li A, et al. Nanoparticle conjugation of ginsenoside Rg3 inhibits hepatocellular carcinoma development and metastasis. Small. 2020;16:e1905233. doi: 10.1002/smll.201905233. [DOI] [PubMed] [Google Scholar]
- 33.Xia J, Ma S, Zhu X, Chen C, Zhang R, Cao Z, et al. Versatile ginsenoside Rg3 liposomes inhibit tumor metastasis by capturing circulating tumor cells and destroying metastatic niches. Sci Adv. 2022;8:eabj1262. doi: 10.1126/sciadv.abj1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Wu T, Qi Z, Zhao L, Pei J, Tang F. Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum. BMC Biotechnol. 2018;18:29. doi: 10.1186/s12896-018-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Wu JJ, Yang Y, Wan Y, Xia J, Xu JF, Zhang L, et al. New insights into the role and mechanisms of ginsenoside Rg1 in the management of Alzheimer’s disease. Biomed Pharmacother. 2022;152:113207. doi: 10.1016/j.biopha.2022.113207. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Gou X, Gao H. Doxorubicin nanomedicine based on ginsenoside Rg1 with alleviated cardiotoxicity and enhanced antitumor activity. Nanomedicine. 2021;16:2587–604. doi: 10.2217/nnm-2021-0329. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Chen J, Li J, Zhou C, Huang X, Chen B. Ginsenoside Rg1 as a promising adjuvant agent for enhancing the anti-cancer functions of granulocytes inhibited by noradrenaline. Front Immunol. 2023;14:1070679. doi: 10.3389/fimmu.2023.1070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong J, Gwon D, Jang CY. Ginsenoside Rg1 suppresses cancer cell proliferation through perturbing mitotic progression. J Ginseng Res. 2022;46:481–8. doi: 10.1016/j.jgr.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–75. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng W, Tan S, Xu Y, Wang L, Qiu D, Cheng C, et al. LC‑MS/MS metabolome analysis detects the changes in the lipid metabolic profiles of dMMR and pMMR cells. Oncol Rep. 2018;40:1026–34. doi: 10.3892/or.2018.6510. [DOI] [PubMed] [Google Scholar]
- 42.Augustine T, John P, Friedman T, Jiffry J, Guzik H, Mannan R, et al. Potentiating effect of reovirus on immune checkpoint inhibition in microsatellite stable colorectal cancer. Front Oncol. 2022;12:1018767. doi: 10.3389/fonc.2022.1018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CP, Liu JX, Gu J, Liu F, Li JH, Bin Y, et al. Combination effect of three main constituents from sarcandra glabra inhibits oxidative stress in the mice following acute lung injury: a role of MAPK-NF-κB pathway. Front Pharmacol. 2020;11:580064. doi: 10.3389/fphar.2020.580064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talukder S, Ahmed KS, Hossain H, Hasan T, Liya IJ, Amanat M, et al. Fimbristylis aestivalis Vahl: a potential source of cyclooxygenase-2 (COX-2) inhibitors. Inflammopharmacology. 2022;30:2301–15. doi: 10.1007/s10787-022-01057-0. [DOI] [PubMed] [Google Scholar]
- 45.Pintha K, Chaiwangyen W, Yodkeeree S, Suttajit M, Tantipaiboonwong P. Suppressive effects of rosmarinic acid rich fraction from perilla on oxidative stress, inflammation and metastasis ability in A549 cells exposed to PM via C-Jun, P-65-NF-κB and AKT signaling pathways. Biomolecules. 2021;11:1090. doi: 10.3390/biom11081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han H, Qian C, Zong G, Liu H, Wang F, Tao R, et al. Systemic pharmacological verification of Salvia miltiorrhiza-Ginseng Chinese herb pair in inhibiting spontaneous breast cancer metastasis. Biomed Pharmacother. 2022;156:113897. doi: 10.1016/j.biopha.2022.113897. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H, et al. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One. 2015;10:e0123478. doi: 10.1371/journal.pone.0123478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Karpisheh V, Nikkhoo A, Hojjat-Farsangi M, Namdar A, Azizi G, Ghalamfarsa G, et al. Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Other Lipid Mediat. 2019;144:106338. doi: 10.1016/j.prostaglandins.2019.106338. [DOI] [PubMed] [Google Scholar]
- 49.Gai JQ, Sheng X, Qin JM, Sun K, Zhao W, Ni L. The effect and mechanism of bufalin on regulating hepatocellular carcinoma cell invasion and metastasis via Wnt/β-catenin signaling pathway. Int J Oncol. 2016;48:338–48. doi: 10.3892/ijo.2015.3250. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Yang L, Chien S, Lv Y. Suspension state promotes metastasis of breast cancer cells by up-regulating cyclooxygenase-2. Theranostics. 2018;8:3722–36. doi: 10.7150/thno.25434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim KM, Im AR, Kim SH, Hyun JW, Chae S. Timosaponin AIII inhibits melanoma cell migration by suppressing COX-2 and in vivo tumor metastasis. Cancer Sci. 2016;107:181–8. doi: 10.1111/cas.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makowska KA, Hughes RE, White KJ, Wells CM, Peckham M. Specific myosins control actin organization, cell morphology, and migration in prostate cancer cells. Cell Rep. 2015;13:2118–25. doi: 10.1016/j.celrep.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soong R, Shah N, Peh BK, Chong PY, Ng SS, Zeps N, et al. The expression of RUNX3 in colorectal cancer is associated with disease stage and patient outcome. Br J Cancer. 2009;100:676–9. doi: 10.1038/sj.bjc.6604899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang S, Li D, Zhuang L, Sun L, Wu J. Identification of Arp2/3 complex subunits as prognostic biomarkers for hepatocellular carcinoma. Front Mol Biosci. 2021;8:690151. doi: 10.3389/fmolb.2021.690151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai WY, Huang BT, Wang JW, Lin PY, Yang PC. A novel PD-L1-targeting antagonistic DNA aptamer with antitumor effects. Mol Ther Nucleic Acids. 2016;5:e397. doi: 10.1038/mtna.2016.102. [DOI] [PubMed] [Google Scholar]
- 56.van Asten SD, de Groot R, van Loenen MM, Castenmiller SM, de Jong J, Monkhorst K, et al. T cells expanded from renal cell carcinoma display tumor-specific CD137 expression but lack significant IFN-γ, TNF-α or IL-2 production. Oncoimmunology. 2021;10:1860482. doi: 10.1080/2162402X.2020.1860482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boukouris AE, Theochari M, Stefanou D, Papalambros A, Felekouras E, Gogas H, et al. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit Rev Oncol Hematol. 2022;173:103663. doi: 10.1016/j.critrevonc.2022.103663. [DOI] [PubMed] [Google Scholar]
- 58.Jin Z, Sinicrope FA. Mismatch repair-deficient colorectal cancer: building on checkpoint blockade. J Clin Oncol. 2022;40:2735–50. doi: 10.1200/JCO.21.02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao BB, Robertson S, Philpott J. Checkpoint inhibitor-induced hemorrhagic gastritis with pembrolizumab. Am J Gastroenterol. 2019;114:196. doi: 10.1038/s41395-018-0366-3. [DOI] [PubMed] [Google Scholar]
- 60.Parikh M, Bajwa P. Immune checkpoint inhibitors in the treatment of renal cell carcinoma. Semin Nephrol. 2020;40:76–85. doi: 10.1016/j.semnephrol.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell. 2020;182:655–71. doi: 10.1016/j.cell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol. 2022;13:779691. doi: 10.3389/fimmu.2022.779691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang Z, Yang Y, Yang Y, Zhang Y, Yue Z, Pan Z, et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed Pharmacother. 2017;96:378–83. doi: 10.1016/j.biopha.2017.09.129. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y, Zhang Y, Song W, Zhang Y, Dong X, Tan M. Ginsenoside Rh2 improves the cisplatin anti-tumor efect in lung adenocarcinoma A549 cells via superoxide and PD-L1. Anticancer Agents Med Chem. 2020;20:495–503. doi: 10.2174/1871520619666191209091230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.