Abstract
Geographical differences are conspicuous in lung cancer, and the distinct molecular features of lung tumor between Western patients and Asian patients have been demonstrated. However, the etiology of non-small-cell lung cancer (NSCLC) and the distribution of associated molecular aberrations in China have not been fully elucidated. The mutational profiles of 12 lung cancer-related genes were investigated in 85 patients from eastern China and 88 patients from southern China who had been histologically confirmed NSCLC. Overall, 93.6% (162/173) of tumor samples harbored at least one somatic alteration. The most frequently mutated genes were TP53 (56.1%), EGFR (50.3%), and KRAS (14.5%). We found that EGFR mutated much more frequently (60.0% vs 40.9%, P = 0.012) and TP53 mutations had significantly lower incidence (47.1% vs 64.8%, P = 0.019) in eastern cohort than that in southern cohort. Mutational signature analysis revealed a region-related mutagenesis mechanism characterized by a high prevalence of C to T transitions mainly occurring at CpG dinucleotides in southern patients. This study reveals the difference in the mutational features between NSCLC patients in eastern and southern China. The distinct patterns of gene mutation could provide clues for the mechanism of carcinogenesis of lung cancer, offering opportunities to stratify patients into optimal treatment plans based on genomic profiles.
Keywords: Lung cancer, gene mutations, C > T transitions, Region-related
Highlights
-
•
NSCLC patients in eastern and southern China exhibited different mutational features.
-
•
In eastern and southern cohorts, the most frequently mutated genes were EGFR and TP53, respectively.
-
•
In southern patients, a high prevalence of C to T transitions occurred at CpG dinucleotides.
-
•
The distinct gene mutation patterns offer opportunities to stratify patients into optimal treatment plans based on genomic profiles.
1. Introduction
Lung cancer is a molecularly heterogeneous disease, and the molecular features of this tumor vary greatly by region. For instance, EGFR gene mutations can be observed in approximately 10%–20% in Western non-small-cell lung cancer (NSCLC) patients, but as high as 40%–50% in Asian population [1,2]. Moreover, KRAS mutations occur in about 20–30% in Western and 10%–15% in east Asian patients with NSCLC [3,4]. In China, the landscape of driver mutations in NSCLC patients from different regions displayed a region-specific mutational profile. Especially, the prevalence of EGFR, ALK, ROS1 and KRAS mutation was significantly different in NSCLC patients from Qujing City than that from other regions located in Yunnan Province of China [5]. Therefore, comprehensive understanding of the oncogenic mutations of NSCLC patients and their relationship with the regions is of great importance in unrevealing the genetic etiology.
The region-related difference of lung cancer incidence and mortality across China is significantly ranges widely [6,7]. High rates were prone to be clustered around urban areas like Beijing and Shanghai. Although the genomic profiles of lung cancer in Chinese patients were previously reported [[8], [9], [10]], the regional distribution related to etiology and carcinogenesis have yet to be elucidated clearly.
In this study, we conducted next-generation sequencing (NGS) to detect multiple lung cancer-related genes status in NSCLC patients from an eastern and a southern region from China, compared the identified molecular mutation spectrum with the clinical features, and aimed to elucidate the most geographical variation of lung cancer between the two cohorts.
2. Methods
2.1. Patient recruitment
A total of 85 patients from eastern China and 88 patients from southern China were included according to standard procedures. Samples were collected during December 2016 and December 2020 in Shanghai Changzheng Hospital for eastern cohort and Central Hospital of Guangdong Nongken for southern cohort, respectively. To clarify demographic differences, patients were also screened according to an additional procedure, i.e., only participants with household registration or permanent residence in the local area were finally included in the analysis. All the patients enrolled had been histologically confirmed NSCLC according to World Health Organization criteria based on hematoxylin and eosin staining and reviewing by experienced pathologists, and without any additional therapy when they were enrolled in our study. All tumor tissues included in the study were ensured to be derived from the patient's primary tumor site.
2.2. DNA extraction from FFPE samples
All tissue sections were reviewed by pathologists to ensure tumor cell content exceeded 10%. DNA was extracted from FFPE tissues using the QIAamp DNA FFPE Tissue Kit (Qiagen, Germany) according to the manufacturer's protocol. Concentrations were detected by the Qubit Fluorometer (Thermo scientific, USA). Only DNA samples with most fragments over 500 bp and a minimum of 50 ng were included in subsequent processes.
2.3. Next-generation sequencing and data analysis
Probe capture-based targeted NGS was performed on 173 tumor samples by the OncoAim® kit (Singlera) as previously described [11]. In brief, the OncoAim panel consists of all exons of 12 lung cancer-related genes, including ALK, BRAF, EGFR, ERBB2, FGFR1, KRAS, MET, NRAS, PIK3CA, RET, ROS1, and TP53, as well as ALK, ROS1, and RET gene rearrangement/fusion. After DNA concentration detection, 50 ng of FFPE DNA was sheared to approximately 250 bp for library construction. End repair, A-tailing, and adapter ligation were performed before target capture with probes provided in the kit. The library product sequencing was performed on the NextSeq 500 using 150 bp paired-end runs. The median depth coverage was over 1000X with uniformity exceeding 90%. The sequencing data was then processed in accordance with the instructions provided by the OncoAim® kit. Single nucleotide variations (SNVs), short insertions and deletions (InDels), copy number variations (CNVs), and gene rearrangements were detected simultaneously. The sequencing reads in the FASTQ format were aligned to human genome reference sequence (hg19) by Burrows‐Wheeler Aligner. The threshold of variant allele frequency for somatic mutations was 1%. For CNVs, amplifications were characterized as genes with thresholds equal to or greater than 4 copies for amplification and 0 copies for homozygous deletions. Gene rearrangement was called with at least one splitting read and two discordant read‐pairs and confirmed on Integrative Genomics Viewer (IGV).
2.4. Statistical analysis
R project (version 4.0.4) was used for statistical analysis. The association of detected mutations with demographic and clinical factors was analyzed by the chi-square test or Fisher's exact test. Statistically significant defined as P value < 0.05.
3. Results
3.1. Baseline characters of included patients
The demographic characteristics of the enrolled patients, including age, gender, smoking history, histopathology, are summarized in Table 1. The median age, gender ratio, smoking history, and histological type of the two cohorts were roughly equal. Lung adenocarcinoma (LUAD) accounted for 91.9% of patients in this study, while lung squamous cell carcinoma (LUSC), large cell neuroendocrine carcinoma, and sarcomatoid carcinoma accounted for 5.8%, 1.7%, and 0.6%, respectively.
Table 1.
Characteristics of patients with NSCLC from Eastern and Southern regions.
| Characteristic |
All patients (n = 173) |
Region |
|
|---|---|---|---|
| Eastern China (n = 85) |
Southern China (n = 88) |
||
| Age | |||
| Median (range) | 63 (38–86) | 62 (38–83) | 65 (42–86) |
| Gender | |||
| Male | 99 (57.2%) | 49 (57.6%) | 50 (56.8%) |
| Female | 74 (42.8%) | 36 (42.4%) | 38 (43.2%) |
| Smoking history | |||
| Yes | 75 (43.4%) | 40 (47.1%) | 35 (39.8%) |
| No | 98 (56.6%) | 45 (52.9%) | 53 (60.2%) |
| Histopathology | |||
| Adenocarcinoma | 159 (91.9%) | 78 (91.8%) | 81 (92.0%) |
| Squamous cell carcinoma | 10 (5.8%) | 4 (4.7%) | 6 (6.8%) |
| Large cell neuroendocrine carcinoma | 3 (1.7%) | 2 (2.4%) | 1 (1.1%) |
| Sarcomatoid carcinoma | 1 (0.6%) | 1 (1.2%) | 0 (0%) |
3.2. Genetic profiling in overall population
Overall, a total of 93.6% (162/173) tumor samples harbored at least one somatic alteration (Fig. 1). SNVs and InDels were identified in 157 samples, gene rearrangements were identified in 9 samples (8 for ALK -EML4, and 1 for CD74 -ROS1), and CNVs (EGFR, KRAS and MET amplification) were identified in 9 samples. Over 50% of patients in the cohort had mutations in TP53 or EGFR gene, while KRAS mutations were present in 14.5% of patients. Mutations in other genes occurred less frequently. We performed chi-square analyses to further identify the correlations between gene mutations and gender, smoking history, or tumor stage (Supplementary Table 1). In brief, EGFR mutations were more frequent in female compared to male (63.5% vs 40.4%, P = 0.003) (Fig. 2A), in non‐smokers compared to smokers (62.2% vs 34.7%, P < 0.001) (Fig. 2B), and in patients with early-stage tumors (83.3% for stage I) compared to middle- or late-stage tumors (52%, 33.3%, and 43.2% for stage II/III/IV, respectively) (Fig. 2C). KRAS mutations tended to arise in male rather than female (21.2% vs 4.1%, P = 0.001) (Fig. 2D), and in smokers rather than non‐smokers (25.3% vs 5.1%, P < 0.001) (Fig. 2E). TP53 mutations were associated with tumor stage by a lower mutation frequency in stage I (20.8%) compared to stage II/III/IV (56%, 61.1%, and 65.4%, respectively) (Fig. 2F).
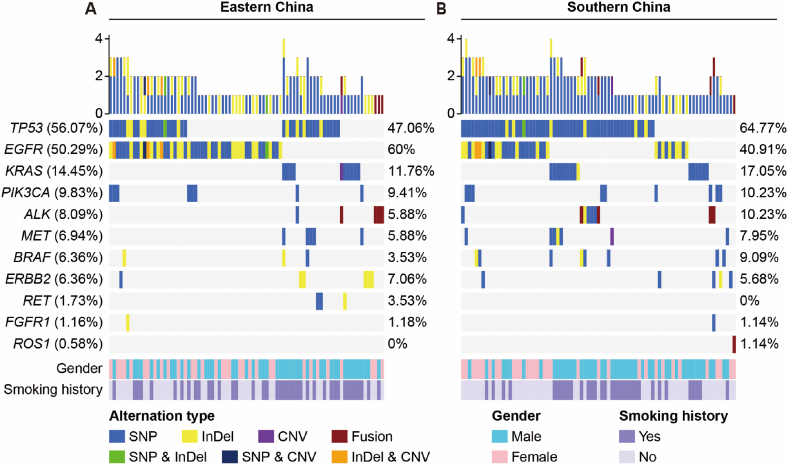
Fig. 1.
Mutational landscape and clinical characteristics of 162 NSCLC patients with at least one somatic alteration. The X-axis represents 81 patients from eastern China (A) and 81 patients from southern China (B), respectively. The Y-axis represents the mutated genes and the corresponding mutation frequency in all patients (the left column), eastern patients (the middle column) and southern patients (the right column). Clinical characteristics and alteration types are annotated in different colors.
Fig. 2.
Correlation between clinical characteristics and genetic alterations in NSCLC patients. (A-C) Correlation between gender (A), smoking history (B), and stage (C) with EGFR mutation in all patients. (D, E) Correlation between gender (D) and smoking history (E) with KRAS mutation in all patients. (F) Correlation between stage with TP53 mutation in all patients.
3.3. The difference of molecular alterations in NSCLC between eastern and southern cohorts
The landscape of driver mutations in patients with NSCLC from Eastern (Fig. 1A) and Southern (Fig. 1B) regions displayed a region-specific mutational profile. Chi-square analyses were performed to investigate the difference in gene mutation frequency between eastern and southern cohorts (Supplementary Table 1). For instance, the prevalence of EGFR mutation was significantly higher in patients from eastern than that in those from southern China (60.0% vs 40.9%, P = 0.012; Fig. 3A). On the contrary, TP53 mutated less in eastern patients compared with southern patients (47.1% vs 64.8%, P = 0.019; Fig. 3B). Furthermore, the mutation frequencies of EGFR 19Del were more common in female (P = 0.004) and nonsmokers (P < 0.001) in southern NSCLC, while the prevalence of EGFR L858R occurred more in female (P = 0.009) and nonsmokers (P < 0.001) in eastern NSCLC (Table 2). ERBB2 exon 20 insertions (20ins) were identified in 6 patients, of which 4 patients from eastern region harboring A775_G776insYVMA, one patient from eastern region harboring A775_G776insAVMA, and one patient from southern region harboring Tyr742_Ala745dup (Fig. 3C and D).
Fig. 3.
Comprehensive analysis of genetic mutations in eastern and southern NSCLC patients. (A, B) Comparison of number of patients with EGFR or TP53 mutation between the two cohorts. (C, D) The clinical characteristics of patients with common driver mutations in eastern and southern cohorts.
Table 2.
The distribution of EGFR hotspots in eastern and southern regions.
| Characteristic | Eastern China |
Southern China |
||
|---|---|---|---|---|
| EGFR L858R | P-value | EGFR 19Del | P-value | |
| Gender | 0.009 | 0.004 | ||
| Male | 7 (14.3%) | 3 (6.0%) | ||
| Female | 14 (38.9%) | 11 (28.9%) | ||
| Smoking history | <0.001 | <0.001 | ||
| Yes | 3 (7.5%) | 0 | ||
| No | 18 (40.0%) | 14 (26.4%) | ||
3.4. Mutational signature for NSCLC according to the base substitutions
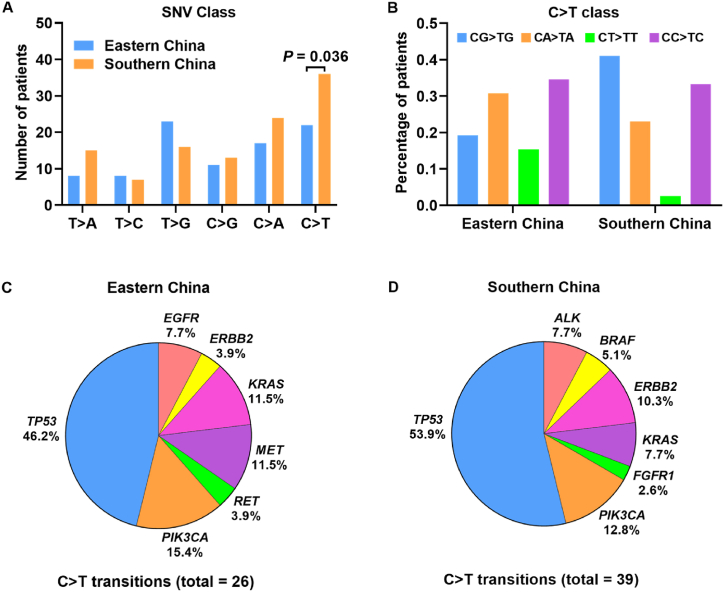
We have found that the base substitutions of single-nucleotide variants were different between eastern and southern cohorts (Fig. 4A). The thymine to guanine (T > G) transversion was the most common base substitution type in eastern cohort, while the cytosine to thymine (C > T) transition was the most frequent in southern cohort. Mutations with C > T transition were identified much more in southern samples than that in eastern samples (P = 0.036). Nearly 19.2% and 41% of C > T mutations occurred in CpG dinucleotides in patients from eastern and southern regions, respectively (Fig. 4B). Amino acid changes caused by C > T substitutions were mainly related with arginine (Arg) (Table 3). Among all the mutated genes related with C > T transition, TP53 mutated most frequently in either eastern or southern NSCLC patients (Fig. 4C and D).
Fig. 4.
Base substitutions of SNVs in eastern and southern NSCLC. (A) Different class of the base substitutions of SNVs between eastern and southern patients. (B) Percentage of patients with C > T transitions localized in CG, CT, CA, and CC dinucleotides. (C, D) The prevalence of mutated genes with C > T transitions in eastern and southern patients.
Table 3.
Distribution of gene mutations with C > T transitions occurred in the context of CpG dinucleotides.
| Southern China |
Eastern China |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Mutant | Codon | Cases | Gene | Mutant | Codon | Cases |
| ALK | p.Arg1231Gln | CGG > CAG | 1 | EGFR | p.Thr790Met | ACG > ATG | 1 |
| ALK | p.Arg1575His | CGT > CAT | 1 | PIK3CA | p.Val344Met | GTG > ATG | 1 |
| ALK | p.Arg291His | CGC > CAC | 1 | TP53 | p.Arg156Cys | CGC > TGC | 1 |
| FGFR1 | p.Gly849Arg | GGA > AGA | 1 | TP53 | p.Arg248Trp | CGG > TGG | 1 |
| PIK3CA | p.Val344Met | GTG > ATG | 1 | TP53 | p.Arg213Ter | CGA > TGA | 1 |
| TP53 | p.Arg158His | CGC > CAC | 1 | ||||
| TP53 | p.Arg175His | CGC > CAC | 1 | ||||
| TP53 | p.Arg213Ter | CGA > TGA | 1 | ||||
| TP53 | p.Arg248Gln | CGG > CAG | 1 | ||||
| TP53 | p.Arg248Trp | CGG > TGG | 2 | ||||
| TP53 | p.Arg273His | CGT > CAT | 1 | ||||
| TP53 | p.Val31Ile | GTT > ATT | 1 | ||||
4. Discussion
Genomic alterations of Chinese NSCLC patients were investigated in two independent cohorts. Similar to recent studies [[1], [2], [3], [4]], Chinese patients had a much higher frequency of EGFR mutations but a significantly lower frequency of KRAS mutation than Western populations. Additionally, higher rate of EGFR mutations was correlated with female and non-smokers, whereas the KRAS mutation was more common in male and patients with smoking history, which was in accordance with previous studies [12,13]. Moreover, EGFR mutation frequency was higher in early-stage patients, whereas TP53 mutation had a correspondingly lower incidence, which may be due to the significant malignancy of TP53 mutation leading to higher initial diagnosis stages.
To the best of our knowledge, somatic mutations of EGFR are key cancerous drivers in non-small-cell lung cancer (NSCLC), contributing to the vast majority of reported NSCLC cases in Asians [14,15]. However, limited studies have been reported about the EGFR mutation distribution across different regions of lung cancer patients. It was reported that uncommon or complex EGFR mutation rates were found to be higher in specimens from Xuanwei compared with other areas in China [5,16,17], however, the underlying molecular mechanism of this feature has not been fully understood yet. In our study, the frequency of EGFR mutations in eastern cohort was higher than that in southern cohort, indicating a region-related EGFR mutation frequency. This difference in EGFR incidence between the two cohorts might be due to the discrepancies in geographic location disparity or the corresponding variety in environmental characteristics. Smoking history [18,19], indoor cooking [20,21], air pollution exposure (e.g., PM2.5) [22,23], and exposure to toxic chemicals (e.g., radon, asbestos, and arsenic) [24,25] have been reported as key factors in the incidence of NSCLC. However, except for the smoking history discussed broadly in several research [[26], [27], [28]] and this study, other factors are challenging to quantify at the level of a certain individual, which made it difficult to accurately determine the correlation and potential mechanisms between geographic or environmental factors and certain types of gene mutations. In the future, larger cohorts and better consideration of patient information collection may make systematic analysis possible.
In the present study, the C > T mutations was the most frequent mutation type in the patients from southern China. Transition of C > T is one of the most common DNA modifications across somatic mutational signatures [29]. Most of these mutations are likely related to the relatively elevated rate of spontaneous deamination of 5-methyl-cytosine, which results in C > T transitions predominantly occur at CpG dinucleotides [30]. This also helps explain why so many mutations in this study alter arginine residues, as four of the six codons encoding arginine have CpG dinucleotides in the first two positions of their respective codons. Similar results have been described previously in TP53 gene mutations [31]. In this study, the frequency of TP53 mutation was significantly higher in NSCLC patients from southern region than that from eastern region. Studies reported that TP53 was frequently mutated in nonmelanoma skin cancer, and C > T transitions had been observed as signature mutations after UV irradiation [32,33]. Southern China with more sufficient sunshine may indeed have more TP53-related mutations than eastern China. These findings therefore suggest that C > T mutagenesis likely to be a potential factor contributing to the pathogenesis and progression of lung cancer in southern cohort.
According to the National Comprehensive Cancer Network (NCCN) guidelines for NSCLC, biomarker tests for EGFR mutation, ALK rearrangement, ROS1 rearrangement, BRAF V600E mutation, NTRK1/2/3 gene fusion, METex14 skipping mutation, RET rearrangement, and PD-L1 expression are recommended for all LUAD patients and partially recommended for LUSC patients. Incorporation of the above genetic tests could help to guide targeted therapies at the individual level. Given that all patients enrolled in our study were primarily diagnosed as NSCLC without any additional therapy, the positive results of EGFR mutation (87 patients), ALK rearrangement (8 patients), ROS1 rearrangement (1 patient), BRAF V600E mutation (1 patient), and METex14 skipping mutation (1 patient) have provided great guidance to help these patients with individually targeted medications.
It has been believed that both EGFR 19Del and L858R are sensitive to EGFR tyrosine kinase inhibitors (TKIs) [34]. Recent studies have demonstrated that patients with L858R showed significantly less response to TKIs than those with 19Del [35,36]. The distribution of EGFR 19Del and L858R were found different in NSCLC between the two cohorts, suggesting more accurate therapeutic approaches are necessary for patients from different regions. In the present study, it is worth noting that different ERBB2 20ins subtypes was identified in patients between the two regions. TKIs targeting ERBB2 have limited activity in patients with ERBB2-positive tumors [37,38], although some case series have reported that lung cancer patients with ERBB2 20ins can benefit from TKIs, such as afatinib [39] and poziotinib [40]. However, response heterogeneity was observed in patients harboring different 20ins subtypes [39,41]. Thus, further efforts are needed to understand the underlying mechanism, and additional clinical validations in larger cohorts will be necessary to confirm our findings.
NGS technology is widely applied in clinical practice for monitoring genetic landscape of different types of tumors [42,43]. For instance, detection of several driver mutations is recommended for improving personal medication in NSCLC patients. The advantages of NGS include the ability to sequence a large panel of genes at the same time, the high sensitivity of rare mutations, and a relatively low cost [44]. However, the balance between sequencing panel size, sequencing depth, and cost must be considered in clinical applications. Moreover, NGS has potential deficiencies in the clinical sensitivity of detecting technically challenging variants (e.g., large indels, small CMVs, and variants in highly homologous regions) [[45], [46], [47]]. As investigated in a recent interlaboratory pilot study, it is temporarily impossible to capture all challenging variant types in a single NGS platform or variant calling algorithm [48]. For the benefit of our research, the engagement of larger NGS panels and improvement of clinical-related challenging variants identification may further clearly state the distinction between genetic alteration characteristics of different cohorts.
Finally, it is essential to discuss the existing limitations of this study. The sample size of our cohorts was relatively small, which may impact the generalizability of the findings. Moreover, we recruited over 90% of LUAD patients and fewer other types of NSCLC patients. However, accounting for the different genetic characteristics between LUAD and LUSC, the conclusions drawn in this article may be more applicable to LUAD patients. In the future, larger cohorts and an average enrollment of participants would help us to validate the current findings and better explore the mutational profile of NSCLC in the eastern and southern Chinese cohorts. Moreover, our research was initially limited to exploring the genetic alterations in primary NSCLC patients between different cohorts, and further analysis of healthy controls or metastatic tumors would enable us to better understand the tumor-specific or metastatic-associated gene mutation in larger cohorts.
5. Conclusions
The differences in the mutational features of NSCLC patients between eastern and southern China have been revealed. Results of eastern cohort showed higher frequency of EGFR mutations, lower prevalence of TP53 mutations and fewer C > T transitions compared with those in the southern patients. These distinct patterns of mutation could provide clues for the mechanism of carcinogenesis of lung cancer, offering opportunities to stratify patients into optimal treatment plans based on genomic profiles.
Funding sources
This work was supported by the Shanghai Municipal Health Commission Clinical Research Project (No. 202240394).
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all patients before genetic analysis of biological samples. The study was approved by the Ethics Committee of Shanghai Changzheng Hospital (2019SL009) and Central Hospital of Guangdong Nongken (No.2018001), separately.
Author contribution statement
Chengdong Liu: Conceived and designed the experiments; Wrote the paper.
Kangbao Li: Conceived and designed the experiments.
Yi Sui: Analyzed and interpreted the data; Wrote the paper.
Hongmei Liu, Tianmin Xiang: Analyzed and interpreted the data.
Yunzhi Zhang, Yuan Lu: Contributed reagents, materials, analysis tools or data.
Wei Lu, Suqian Xu, Yongfeng Chen: Performed the experiments.
Gehui Wang: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Yongguang Cai, Kenan Huang: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data included in article/supp. Material/referenced in article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
YS, HL, YZ, GW, SX and TX were employed by company Singlera Genomics (Shanghai). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all researchers for their contributions in this study, including those whose relevant contributions were not cited due to space limitations.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e20171.
Contributor Information
Yongguang Cai, Email: caiyongguang@126.com.
Kenan Huang, Email: renrenhuanghe@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Shi Y., et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J. Thorac. Oncol. 2014;9(2):154–162. doi: 10.1097/jto.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh J.H., et al. Comprehensive genomic profiling facilitates implementation of the national comprehensive cancer Network guidelines for lung cancer biomarker testing and identifies patients who may benefit from enrollment in mechanism-driven clinical trials. Oncol. 2016;21(6):684–691. doi: 10.1634/theoncologist.2016-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L., et al. Comprehensive genomic profiling of lung cancer using a validated panel to explore therapeutic targets in East Asian patients. Cancer Sci. 2017;108(12):2487–2494. doi: 10.1111/cas.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheffler M., et al. K-Ras mutation subtypes in NSCLC and associated Co-occurring mutations in other oncogenic pathways. J. Thorac. Oncol. 2019;14(4):606–616. doi: 10.1016/j.jtho.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y., et al. Unique profile of driver gene mutations in patients with non-small-cell lung cancer in qujing city, yunnan Province, southwest China. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.644895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen X., Wang L., Zhu L. Spatial analysis of regional factors and lung cancer mortality in China, 1973-2013. Cancer Epidemiol. Biomarkers Prev. 2017;26(4):569–577. doi: 10.1158/1055-9965.Epi-16-0922. [DOI] [PubMed] [Google Scholar]
- 7.Cao M., Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. doi: 10.1111/1759-7714.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo W., et al. Characteristics of genomic alterations of lung adenocarcinoma in young never-smokers. Int. J. Cancer. 2018;143(7):1696–1705. doi: 10.1002/ijc.31542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C., et al. Whole-genome sequencing reveals genomic signatures associated with the inflammatory microenvironments in Chinese NSCLC patients. Nat. Commun. 2018;9(1):2054. doi: 10.1038/s41467-018-04492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X.C., et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat. Commun. 2019;10(1):1772. doi: 10.1038/s41467-019-09762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S., et al. Investigating the genomic alteration improved the clinical outcome of aged patients with lung carcinoma. BMC Genom. 2022;23(1):55. doi: 10.1186/s12864-021-08289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shigematsu H., et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 13.Colombino M., et al. EGFR, KRAS, BRAF, ALK, and cMET genetic alterations in 1440 Sardinian patients with lung adenocarcinoma. BMC Pulm. Med. 2019;19(1):209. doi: 10.1186/s12890-019-0964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham R.P., et al. Worldwide frequency of commonly detected EGFR mutations. Arch. Pathol. Lab Med. 2018;142(2):163–167. doi: 10.5858/arpa.2016-0579-CP. [DOI] [PubMed] [Google Scholar]
- 15.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., et al. Distinct epithelial growth factor receptor mutation profile in non-small-cell lung cancer patients from the Xuanwei area of China. Mol Clin Oncol. 2016;4(5):749–755. doi: 10.3892/mco.2016.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv L., et al. Distinct EGFR mutation pattern in patients with non-small cell lung cancer in Xuanwei region of China: a systematic review and meta-analysis. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.519073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyczynski J.E., Bray F., Parkin D.M. Lung cancer in Europe in 2000: epidemiology, prevention, and early detection. Lancet Oncol. 2003;4(1):45–55. doi: 10.1016/s1470-2045(03)00960-4. [DOI] [PubMed] [Google Scholar]
- 19.Molina J.R., et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu I.T., et al. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66(9):4961–4967. doi: 10.1158/0008-5472.Can-05-2932. [DOI] [PubMed] [Google Scholar]
- 21.Kim C., et al. Home kitchen ventilation, cooking fuels, and lung cancer risk in a prospective cohort of never smoking women in Shanghai, China. Int. J. Cancer. 2015;136(3):632–638. doi: 10.1002/ijc.29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckel S.P., et al. Air pollution affects lung cancer survival. Thorax. 2016;71(10):891–898. doi: 10.1136/thoraxjnl-2015-207927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R., Zhou R., Zhang J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol. Lett. 2018;15(5):7506–7514. doi: 10.3892/ol.2018.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J.B., et al. Attributable causes of lung cancer incidence and mortality in China. Thorac Cancer. 2011;2(4):156–163. doi: 10.1111/j.1759-7714.2011.00067.x. [DOI] [PubMed] [Google Scholar]
- 25.Hubaux R., et al. Arsenic, asbestos and radon: emerging players in lung tumorigenesis. Environ. Health. 2012;11:89. doi: 10.1186/1476-069x-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindan R., et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman A.M., et al. Lung cancer mutation profile of EGFR, ALK, and KRAS: meta-analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–134. doi: 10.1016/j.lungcan.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Ernst S.M., et al. Tobacco smoking-related mutational signatures in classifying smoking-associated and nonsmoking-associated NSCLC. J. Thorac. Oncol. 2023;18(4):487–498. doi: 10.1016/j.jtho.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Alexandrov L.B., et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeifer G.P. Mutagenesis at methylated CpG sequences. Curr. Top. Microbiol. Immunol. 2006;301:259–281. doi: 10.1007/3-540-31390-7_10. [DOI] [PubMed] [Google Scholar]
- 31.Baugh E.H., et al. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25(1):154–160. doi: 10.1038/cdd.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tornaletti S., Rozek D., Pfeifer G.P. The distribution of UV photoproducts along the human p53 gene and its relation to mutations in skin cancer. Oncogene. 1993;8(8):2051–2057. [PubMed] [Google Scholar]
- 33.Kucab J.E., Phillips D.H., Arlt V.M. Linking environmental carcinogen exposure to TP53 mutations in human tumours using the human TP53 knock-in (Hupki) mouse model. FEBS J. 2010;277(12):2567–2583. doi: 10.1111/j.1742-464X.2010.07676.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S.V., et al. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 35.He Q., et al. The impact of epidermal growth factor receptor mutations on the prognosis of resected non-small cell lung cancer: a meta-analysis of literatures. Transl. Lung Cancer Res. 2019;8(2):124–134. doi: 10.21037/tlcr.2019.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang H., et al. Concomitant mutations in EGFR 19Del/l858r mutation and their association with response to EGFR-TKIs in NSCLC patients. Cancer Manag. Res. 2020;12:8653–8662. doi: 10.2147/cmar.S255967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazières J., et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 2013;31(16):1997–2003. doi: 10.1200/jco.2012.45.6095. [DOI] [PubMed] [Google Scholar]
- 38.Costa D.B., et al. Pulse afatinib for ERBB2 exon 20 insertion-mutated lung adenocarcinomas. J. Thorac. Oncol. 2016;11(6):918–923. doi: 10.1016/j.jtho.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z., et al. Clinical characterization of ERBB2 exon 20 insertions and heterogeneity of outcomes responding to afatinib in Chinese lung cancer patients. OncoTargets Ther. 2018;11:7323–7331. doi: 10.2147/ott.S173391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y., et al. HER2 exon 20 insertion mutations in lung adenocarcinoma with leptomeningeal metastasis: a case report and response to poziotinib. Ann. Palliat. Med. 2022;11(4):1582–1588. doi: 10.21037/apm-21-213. [DOI] [PubMed] [Google Scholar]
- 41.Kosaka T., et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res. 2017;77(10):2712–2721. doi: 10.1158/0008-5472.Can-16-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yohe S., Thyagarajan B. Review of clinical next-generation sequencing. Arch. Pathol. Lab Med. 2017;141(11):1544–1557. doi: 10.5858/arpa.2016-0501-RA. [DOI] [PubMed] [Google Scholar]
- 43.Sabour L., Sabour M., Ghorbian S. Clinical applications of next-generation sequencing in cancer diagnosis. Pathol. Oncol. Res. 2017;23(2):225–234. doi: 10.1007/s12253-016-0124-z. [DOI] [PubMed] [Google Scholar]
- 44.Zhong Y., et al. Application of next generation sequencing in laboratory medicine. Ann Lab Med. 2021;41(1):25–43. doi: 10.3343/alm.2021.41.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Telenti A., et al. Deep sequencing of 10,000 human genomes. Proc Natl Acad Sci U S A. 2016;113(42):11901–11906. doi: 10.1073/pnas.1613365113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eichler E.E. Genetic variation, comparative genomics, and the diagnosis of disease. N. Engl. J. Med. 2019;381(1):64–74. doi: 10.1056/NEJMra1809315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebbert M.T.W., et al. Systematic analysis of dark and camouflaged genes reveals disease-relevant genes hiding in plain sight. Genome Biol. 2019;20(1):97. doi: 10.1186/s13059-019-1707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lincoln S.E., et al. One in seven pathogenic variants can be challenging to detect by NGS: an analysis of 450,000 patients with implications for clinical sensitivity and genetic test implementation. Genet. Med. 2021;23(9):1673–1680. doi: 10.1038/s41436-021-01187-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. Material/referenced in article.