Abstract
Ecological studies suggested a link between air pollution and severe COVID-19 outcomes, while studies accounting for individual-level characteristics are limited. In the present study, we aimed to investigate the impact of short-term ambient air pollution exposure on disease severity among a cohort of 569 laboratory confirmed COVID-19 patients admitted to designated hospitals in Zhejiang province, China, from January 17 to March 3, 2020, and elucidate the possible biological processes involved using transcriptomics. Compared with mild cases, severe cases had higher proportion of medical conditions as well as unfavorable results in most of the laboratory tests, and manifested higher air pollution exposure levels. Higher exposure to air pollutants was associated with increased risk of severe COVID-19 with odds ratio (OR) of 1.89 (95% confidence interval (CI): 1.01, 3.53), 2.35 (95% CI: 1.20, 4.61), 2.87 (95% CI: 1.68, 4.91), and 2.01 (95% CI: 1.10, 3.69) for PM2.5, PM10, NO2 and CO, respectively. OR for NO2 remained significant in two-pollutant models after adjusting for other pollutants. Transcriptional analysis showed 884 differentially expressed genes which mainly were enriched in virus clearance related biological processes between patients with high and low NO2 exposure levels, indicating that compromised immune response might be a potential underlying mechanistic pathway. These findings highlight the impact of short-term air pollution exposure, particularly for NO2, on COVID-19 severity, and emphasize the significance in mitigating the COVID-19 burden of commitments to improve air quality.
Keywords: Air pollution, COVID-19, Severity, Transcriptomics, Immune response
Graphical abstract
Introduction
The coronavirus disease 2019 (COVID-19) caused by infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a global pandemic with huge burden to world health and economies (World Health Organization, 2022). SARS-CoV-2 can spread effectively through several transmission routes including the fomite, airborne (or aerosol), and fecal-oral routes (Feng et al., 2021b). Infection with SARS-CoV-2 may result in a wide range of clinical manifestations from asymptomatic infection to critical illness. Several factors including demographic, economic, climate and environmental are supposed to facilitate the transmission or aggravation of COVID-19 (Bontempi and Coccia, 2021; Chelani and Gautam, 2022; Coccia, 2020, 2021a; Rahimi et al., 2021; Srivastava, 2021). More recently, ambient air pollution has been implicated as a potential driver of COVID-19 severity. It is well-established that air pollution exposure is associated with a diverse range of diseases such as hypertension, chronic obstructive pulmonary disease, and diabetes now recognized as susceptibility conditions for severe COVID-19 outcomes (Zhou et al., 2020). Additionally, air pollution exposure is also associated with suppression of immune response to respiratory virus, thus facilitating viral invasion and a worse pneumonia (Tao et al., 2020). Indeed, several studies suggested that the spatial distribution of air pollution levels coincided with COVID-19 mortality and the number of severe cases (Ran et al., 2020; Yao et al., 2020; Zheng et al., 2021). Similarly, Ogen et al. (2020) reported that 78% of the fatality cases in 66 administrative regions in four European countries including Italy, Spain, France and Germany occurred in five regions showing the highest nitrogen dioxide (NO2) concentrations.
However, previous studies assessing the impact of air pollution on COVID-19 often adopted an ecological design, while studies accounting for individual-level characteristics are limited (Villeneuve and Goldberg, 2020). The previous studies using ecological design may have several important limitations, such as aggregated exposure and outcome data from heterogeneous populations; potential misclassification of disease severity; and the lack of adjustment for important factors that are key to the infection and transmission (i.e., social characteristics, health conditions, and personal protection status). Also, most ecological studies have focused on the associations between long-term air pollution exposure and COVID-19 severity, while less is known regarding the impact of short-term air pollution exposure.
In the early stage of COVID-19 outbreak, the Health Commission of Zhejiang Province initiated an ongoing investigation of epidemiological and clinical characteristics of laboratory confirmed COVID-19 patients in Zhejiang province, China, which was designed to elucidate case transmission chains, and provide insights into the therapies for COVID-19 (Hao et al., 2020; Jin et al., 2020). In the present analyses, we aimed to evaluate the impact of short-term air pollution exposure on COVID-19 severity accounting for individual-level characteristics. To that end, we estimated exposure levels for each patient using inverse distance weighted interpolation. We then examined the associations between air pollution exposure and risk of severe COVID-19 using generalized additive models, and elucidated the possible biological processes involved using transcriptomics.
1. Material and methods
1.1. Sample and data
This was a retrospective cohort study of laboratory confirmed COVID-19 patients admitted consecutively to designated hospitals by the Health Commission of Zhejiang Province from January 17 to March 3, 2020. Patients infected with SARS-CoV-2 outside Zhejiang province, or not living in Zhejiang province were excluded. This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China (IIT20210227A).
Demographics, medical history, and laboratory findings of patients were uniformly collected in designated hospitals by the Health Commission of Zhejiang Province from electronic medical records, and reviewed by a trained team of physicians. Whole blood samples were collected from 43 COVID-19 patients admitted to the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China. The descriptive statistics of 43 participants selected were summarized in Appendix A Table S1.
We obtained hourly concentrations of various air pollutants, including particulate matters with an aerodynamic diameter less than 2.5 µm (PM2.5), particulate matters with an aerodynamic diameter less than 10 µm (PM10), NO2, carbon monoxide (CO), and sulfur dioxide (SO2) of 41 national and 100 provincial air quality monitoring stations in Zhejiang province over 2019-2020 from the National Urban Air Quality Publishing Platform (http://106.37.208.233:20035/) and Zhejiang Province Air Quality Publishing Platform (http://aqi.zjemc.org.cn/aqi/flex/index.html), respectively.
1.2. Measures of variables
Transcriptional sequencing of the RNA isolated from whole blood samples was carried out as described elsewhere (Feng et al., 2021a). The 43 patients were divided into two subgroups, low- and high-exposure groups, based on the exposure level of NO2. Briefly, after generating complementary DNA (cDNA), sequencing libraries were constructed using the NEB Next Ultra II Library Prep Kit (New England Biolabs, MA, USA). Sequencing reads were generated using Illumina HiSeq 2500, and further mapped against human reference genome GRCh38. Per gene read counts were calculated using TopHat2 (Version 2.1.1) and RSEM (Version 1.2.31), and normalized using trimmed mean normalization. Differentially expressed genes (DEG) were estimated using the likelihood ratio test. Functional enrichment analysis was performed based on the list of DEG using Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources (https://david.ncifcrf.gov).
1.3. Models and data analysis procedure
Summary statistics were conducted for demographical characteristics of study participants and air pollutant levels. Statistical comparisons between mild cases and severe cases were evaluated by the Fisher's exact test or Mann-Whitney U-test when appropriate. Spearman's correlations between air pollutants and selected laboratory findings were assessed.
Daily concentrations of air pollutants were calculated for all state- and province-controlled stations with missing values of hourly concentrations less than 25%. Longitude and latitude coordinates for each monitoring station and participant's residential address were determined using “REmap” package (available at https://github.com/Lchiffon/REmap), and the distances from each patient's residential addresses to monitoring stations were calculated using R package “geosphere”. Daily exposure levels of PM2.5, PM10, NO2, CO, and SO2 of each patient were estimated by calculating inverse distance (1/d) weighted concentrations of the nearest three monitoring stations. One-week averaged concentrations of air pollutants before the date of symptom onset was derived as short-term exposure indicators for each patient.
Generalized additive models were conducted to estimate the odds ratio (OR) of air pollutants’ impacts on COVID-19 severity using R package “mgcv”. Log link function and binomial distribution assumption were chosen. The models were adjusted for factors related to COVID-19 severity, including age (continuous), sex (male or female), body mass index (BMI) (continuous), smoking status (yes or no), time from illness onset to hospital visiting (continuous), and hypertension (yes or no). The continuous variables were adjusted using thin plate regression spline due to the nonlinear relationship with COVID-19 severity. Two-pollutant models were further fitted to estimate independent effect of each pollutant on COVID-19 severity.
We also conducted several sensitivity analyses. Firstly, to examine the robustness of individual exposure assessment using inverse distance (1/d) weighted average of concentrations of the nearest three monitoring stations, we re-estimated the exposure levels of each patient using 1) inverse distance squared (1/d2) weighted average of concentrations of the nearest three monitoring stations; 2) inverse distance (1/d) weighted average of concentrations of the nearest five monitoring stations; 3) average concentrations of the nearest three monitoring stations. Secondly, we ran additional generalized additive models by 1) restricting patients living within 10 km from the nearest monitoring station; 2) excluding patients with time from illness onset to hospital visiting more than five days; 3) further controlling health condition (whether the patient had any underlying diseases: yes or no).
All estimates were presented as mean changes with 95% confidence intervals (CI) associated with interquartile-range (IQR) increases in pollutants. A P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R, version 3.4.3 (R Project for Statistical Computing).
2. Results
A total of 569 patients with confirmed SARS-CoV-2 infection from January17 to March 3, 2020 in Zhejiang province were enrolled in the present study, of which 48 (8.4%) were classified as severe cases and the remaining 521 mild cases (Table 1 ). There were significant differences in age, sex, BMI, smoking status, time from illness onset to hospital visiting, and underlying diseases between mild and severe cases. Compared with severe cases, mild cases had a lower proportion of ICU admission and ARDS developing, and manifested favorable results in most of the laboratory testing (Table 2 ).
Table 1.
Demographics and clinical characteristics of COVID-19 patients admitted from January 17 to March 3, 2020 in Zhejiang province.
| Characteristics | Total (N=569) | Disease severity |
P-value | |
|---|---|---|---|---|
| Mild (N=521) | Severe (N=48) | |||
| Age (year) | 49.0 (37.0-59.0) | 49.0 (36.0-57.0) | 59.5 (46.5-70.5) | <0.001 |
| Female | 305 (53.6%) | 286 (54.9%) | 19 (39.6%) | 0.05 |
| Body mass index (kg/m2) | 23.4 (21.1-25.5) | 23.3 (20.9-25.4) | 24.9 (23.4-28.4) | <0.001 |
| Smoking | 37 (6.6%) | 29 (5.6%) | 8 (17.4%) | <0.01 |
| Time from illness onset to hospital visiting (day) | 2.0 (1.0-5.0) | 2.0 (1.0-5.0) | 4.0 (1.0-5.0) | 0.05 |
| Symptoms | ||||
| Fever | 443 (78.4%) | 398 (76.8%) | 45 (95.7%) | <0.01 |
| Cough | 353 (62.5%) | 317 (61.2%) | 36 (76.6%) | 0.04 |
| Sputum | 188 (33.3%) | 161 (31.1%) | 27 (57.4%) | <0.001 |
| Headache | 46 (8.1%) | 41 (7.9%) | 5 (10.6%) | 0.57 |
| Diarrhoea | 50 (8.9%) | 44 (8.5%) | 6 (13.6%) | 0.27 |
| Myalgia | 58 (10.3%) | 50 (9.7%) | 8 (17.0%) | 0.13 |
| Fatigue | 95 (16.8%) | 79 (15.3%) | 16 (34.0%) | <0.01 |
| Underlying diseases | ||||
| Hypertension | 98 (17.3%) | 82 (15.8%) | 16 (34.0%) | <0.01 |
| Diabetes mellitus | 47 (8.3%) | 41 (7.9%) | 6 (12.8%) | 0.27 |
| Heart disease | 15 (2.7%) | 14 (2.7%) | 1 (2.1%) | 1 |
| Other | 82 (14.4%) | 69 (13.2%) | 13 (27.1%) | 0.02 |
| ICU admission | 7 (1.2%) | 7 (14.6%) | 7 (20.6%) | <0.001 |
| ARDS developing | 12 (2.1%) | 12 (25.0%) | 12 (29.3%) | <0.001 |
Data expressed as median (IQR) or number (percentage) of patients.
Table 2.
Laboratory findings of COVID-19 patients admitted from January 17 to March 3, 2020 in Zhejiang province.
| Biomarkers | Total (N=569) | Disease severity |
P-value | |
|---|---|---|---|---|
| Mild (N=521) | Severe (N=48) | |||
| Blood cell count (× 109 cells/L) | ||||
| White blood cells | 4.8 (3.8-6.1) | 4.8 (3.8-6.0) | 4.9 (3.9-6.5) | 0.57 |
| Neutrophils | 3.0 (2.3-4.0) | 3.0 (2.3-3.9) | 3.5 (2.7-4.6) | 0.04 |
| Lymphocytes | 1.2 (0.9-1.6) | 1.2 (0.9-1.6) | 0.9 (0.5-1.2) | <0.001 |
| Coagulation | ||||
| Blood platelet count (× 109 cells/L) | 185.0 (148.0-222.0) | 185.0 (148.0-225.0) | 178.5 (140.5-208.0) | 0.34 |
| International normalized ratio | 1.02 (0.97-1.09) | 1.02 (0.97-1.09) | 1.05 (1.01-1.14) | 0.01 |
| Infection related | ||||
| Hypersensitive C-reactive protein (mg/L) | 9.0 (2.8-23.9) | 8.2 (2.6-21.6) | 35.1 (8.9-57.5) | <0.001 |
| Procalcitonin (ng/mL) | 0.05 (0.04-0.09) | 0.05 (0.04-0.09) | 0.06 (0.04-0.20) | 0.06 |
| Blood biochemistry | ||||
| Alanine transaminase (U/L) | 22.0 (15.0-35.5) | 21.2 (15.0-36.0) | 25.5 (16.3-33.8) | 0.45 |
| Aspartate transferase (U/L) | 26.0 (20.0-34.0) | 26.0 (20.0-34.0) | 30.0 (22.0-41.0) | 0.04 |
| Creatinine (µmol/L) | 64.9 (55.0-76.0) | 64.0 (55.0-76.0) | 69.0 (62.5-80.5) | 0.02 |
| Creatine kinase (U/L) | 65.0 (44.0-102.0) | 65.0 (44.0-97.0) | 72.0 (50.0-125.0) | 0.09 |
| Lactate dehydrogenase (U/L) | 218.5 (173.0-281.2) | 214.0 (170.0-271.0) | 277.0 (207.0-359.0) | <0.001 |
Data expressed as median (IQR).
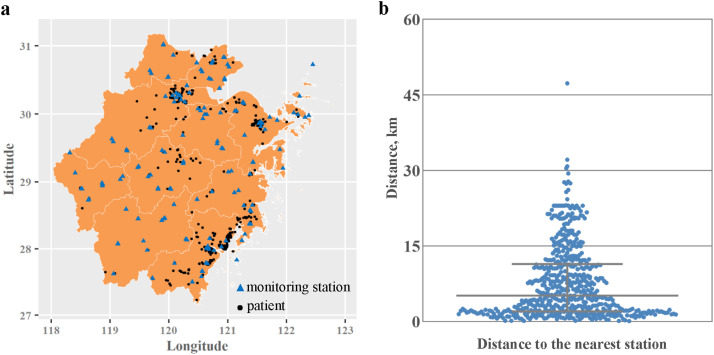
Spatial distribution of the 141 air quality monitoring stations in Zhejiang province is shown in Fig. 1 . The distance from each patient’ residential address to the nearest station ranged from 0.1 km to 47.3 km (mean: 7.7 km; median: 5.1 km), with 69.4% of patients living within 10 km from the nearest monitoring station. The median exposure levels of PM2.5, PM10, NO2, CO and SO2 for all COVID-19 patients were 31.2 µg/m3, 45.9 µg/m3, 14.8 µg/m3, 0.75 mg/m3 and 5.7 µg/m3, respectively. Significant difference of NO2 exposure level was observed between mild and severe cases (Table 3 ). Among the pollutants, PM2.5, PM10, NO2, and CO were highly correlated with each other, whereas SO2 was only moderately correlated with other pollutants (Appendix A Table S2).
Fig. 1.
Spatial distribution of monitoring stations and COVID-19 patients admitted from January 17 to March 3, 2020 in Zhejiang province. (a) Spatial distribution of 141 air quality monitoring stations and 569 COVID-19 patients’ home addresses in Zhejiang province, China; (b) The distance from each patient’ residential address to the nearest station.
Table 3.
Summary statistics of air pollutant exposure levels of COVID-19 patients admitted from January 17 to March 3, 2020 in Zhejiang province.
| Pollutants | Total (N = 569) | Disease severity |
P-value | |
|---|---|---|---|---|
| Mild (N = 521) | Severe (N = 48) | |||
| PM2.5, µg/m3 | 31.2 (24.3-39.4) | 31.2 (24.1-39.4) | 31.6 (26.4-37.5) | 0.37 |
| PM10, µg/m3 | 45.9 (32.5-54.1) | 45.4 (32.2-53.9) | 49.2 (36.9-56.6) | 0.07 |
| NO2, µg/m3 | 14.8 (9.3-23.8) | 14.5 (9.1-23.5) | 18.2 (12.3-33.2) | 0.01 |
| CO, mg/m3 | 0.75 (0.64-0.86) | 0.75 (0.63-0.86) | 0.76 (0.71-0.95) | 0.09 |
| SO2, µg/m3 | 5.7 (4.6-6.7) | 5.7 (4.6-6.7) | 5.7 (4.3-6.8) | 0.67 |
Data expressed as median (IQR).
We observed negative correlations between PM2.5 and lymphocyte, CO and platelet, and positive correlations between PM10 and international normalized ratio (INR), NO2 and lactate dehydrogenase (LDH) (Fig. 2 a). Estimated relationships between air pollutants and laboratory findings based on simple linear regression model are summarized in Appendix A Table S3. We observed significantly increased risks of severe COVID-19 with OR of 1.89 (1.01, 3.53), 2.35 (1.20, 4.61), 2.87 (1.68, 4.91), and 2.01 (1.10, 3.69) associated with IQR increases in PM2.5, PM10, NO2 and CO, respectively (Fig. 2b). In two-pollutant models, most of the significant associations observed in single-pollutant models changed to non-significant after adjusting for other pollutants. Whereas, the OR for NO2 remained significant, and were somewhat higher after adjustment for PM2.5, PM10, CO or SO2 (Table 4 ). As shown in Appendix A Fig. S1-3, the overall pollutant-severity associations remained robust when exposure levels of each patient were re-estimated using inverse distance squared (1/d2) weighted average of concentrations of the nearest three monitoring stations, inverse distance (1/d) weighted average of concentrations of the nearest five monitoring stations, or average concentrations of the nearest three monitoring stations. Excluding patients living beyond 10 km from the nearest monitoring station or with time from illness onset to hospital visiting more than five days had little impacts on the observed associations as well (Appendix A Fig. S4-5). Additionally, most of the severity-pollutant associations remained robust except PM2.5 when models were further adjusted for underlying diseases (Appendix A Fig. S6).
Fig. 2.
Air pollution exposure associated with COVID-19 severity. (a) Spearman's correlations between selected air pollutants and laboratory findings; (b) Odds ratios and respective 95% CI for the associations between exposure to air pollutants and COVID-19 severity.
Table 4.
Odds ratios of severe COVID-19 associated with IQR increase in air pollutants in two-pollutant models.
| Adjusted for pollutants below | Pollutant |
||||
|---|---|---|---|---|---|
| PM2.5 | PM10 | NO2 | CO | SO2 | |
| PM2.5 | n/a | 6.32 (0.94, 42.28) | 3.26 (1.68, 6.35) | 1.78 (0.83, 3.78) | 0.85 (0.43, 1.67) |
| PM10 | 0.35 (0.06, 2.12) | n/a | 3.13 (1.52, 6.45) | 1.46 (0.67, 3.18) | 0.81 (0.41, 1.62) |
| NO2 | 0.78 (0.36, 1.70) | 0.85 (0.34, 2.10) | n/a | 0.91 (0.39, 2.11) | 0.71 (0.35, 1.42) |
| CO | 1.24 (0.57, 2.74) | 1.80 (0.77, 4.20) | 3.03 (1.47, 6.24) | n/a | 1.06 (0.57, 1.96) |
| SO2 | 1.96 (0.99, 3.87) | 2.53 (1.22, 5.25) | 3.11 (1.77, 5.48) | 2.01 (1.10, 3.69) | n/a |
n/a: not applicable.
To further provide mechanistic clues for air pollution associated COVID-19 severity, we compared transcriptional profiles of the high- and low-exposure groups based on NO2 exposure level. Compared with low-exposure group, patients in high-exposure group showed 420 upregulated and 464 downregulated genes (Appendix A Table S4). The up-regulated genes were related to gene expression with the most enriched biological processes being “chromatin silencing”, “telomere organization”, and “nucleosome assembly” (Fig. 3 ). The down-regulated genes were mainly enriched in virus clearance related biological processes including “type Ⅰ interferon signaling pathway”, “defense response to virus”, and “positive regulation of immune clearance by monocytes and macrophages”.
Fig. 3.
GO-term and KEGG pathway enrichment of differentially expressed genes between low- and high-exposure groups, based on the NO2 exposure level.
3. Discussion
In the present analysis, we observed that short-term exposure to ambient air pollutants including PM2.5, PM10, NO2 and CO was associated with increased risk of severe COVID-19 among a cohort of 569 laboratory confirmed COVID-19 patients. Of the five pollutants, NO2 was identified to be the main contributor to the enhanced disease severity. Transcriptomics analyses suggested that higher air pollution levels can promote marked alterations in transcriptional profile of which mainly involved in suppression of virus clearance. These findings provide important evidence that air pollution exposure might aggravate COVID-19, and perturbation of immune response might be a potential underlying mechanistic pathway.
Several ecologic studies previously linked air pollution to the transmission and progress of COVID-19 (Copat et al., 2020), but a high wind speed was suggested to decrease the transmission dynamics of COVID-19 through improving the circulation of air and increasing the exposure of SARS-CoV-2 to the solar radiation effects (Coccia, 2021b). However, potential determinants of COVID-19 such as the position on the pandemic curve, demographic structure, and public health interventions distributed disproportionately across regions, studies relying on ecologic measures of exposures and outcomes may be biased due to residual confounding (Villeneuve and Goldberg, 2020). To date, very limited studies have been performed accounting for individual-level characteristics (Table 5 ). In a national cohort of 169102 COVID-19 positive United States veterans, Bowe et al. (2021) reported a non-linear relationship between the risk of hospitalization and satellite-based annual average 2018 PM2.5 exposure level, suggesting increased risk at PM2.5 level below the regulatory standard. Using information retrieved from UK Biobank data, Travaglio et al. (2021) assessed the association between air pollution and the risk of COVID-19 infection at the individual scale, and reported a 12% increase in COVID-19 cases associated with a single unit increase in PM2.5 level. A study conducted in Italian found a positive association between PM10 exposure and risk of developing pneumonia after infection of SARS-CoV-2 with an OR of 1.93 comparing the highest with the lowest tertile of PM10 exposure level (Pegoraro et al., 2021). Similarly, another study conducted in Mexico City reported a positive association between air pollution and the probability for an individual to die from COVID-19 (Lopez-Feldman et al., 2021). In the present study, we consistently showed a link between air pollution and COVID-19 severity after adjusting for several individual-level characteristics strongly associated with disease progression. Together with previous studies, our findings suggest that air pollution exposure might enhance the risk of severe COVID-19 outcomes.
Table 5.
Studies accounted for individual-level characteristics when assessing the impact of air pollution on COVID-19.
| Study area | Pollutant types | Key observations | References |
|---|---|---|---|
| England | PM2.5, PM10, NO2 | Increased risk of COVID-19 positive test results with OR of 1.05 (1.02-1.08) and 1.05 (1.01-1.08) was associated with an interquartile range increase in PM2.5 and NO2, respectively. No significant associations for PM10 were found. | (Sheridan et al., 2022) |
| Rome, Italy | PM2.5, NO2 | Increased risk of death with HR of 1.01 (1.00, 1.02) and 1.02 (1.00, 1.04) was associated with an interquartile range increase in PM2.5 and NO2, respectively. | (Nobile et al., 2022) |
| Poland | PM2.5 and B(a)P | Exposure to PM2.5 and B(a)P exceeding the limits was associated with higher odds of early respiratory symptoms, hyperinflammatory state, oxygen therapy and death of COVID-19. | (Rzymski et al., 2022) |
| Southern California, USA | PM2.5, NO2 | OR associated with one standard deviation increase in PM2.5 was 1.24 (1.16-1.32) for COVID-19-related hospitalization, 1.33 (1.20-1.47) for intensive respiratory support, 1.32 (1.16-1.51) for ICU; the corresponding OR associated with NO2 was 1.12 (1.06-1.17) for hospitalization, 1.18 (1.10-1.27) for intensive respiratory support, and 1.21 (1.11-1.33) for ICU. | (Chen et al., 2022b) |
| Ontario, Canada | PM2.5, NO2 | OR associated with an interquartile range increase in PM2.5 was 1.06 (1.01-1.12) for hospital admission, 1.09 (0.98-1.21) for ICU, 1.00 (0.90-1.11) for death. Estimates were smaller for NO2. | (Chen et al., 2022a) |
| Jiangsu province, China | PM2.5, PM10 | Increased risk of severe COVID-19 with OR of 3.99 (1.93-8.25) and 1.82 (1.35-2.44) was associated with a unit increase in PM2.5 and PM10. | (Li et al., 2022) |
| Varese, Italy | PM2.5, PM10, NO2, NO | A unit increase in PM2.5 was associated with a 5.1% (2.7%-7.5%) increase in COVID-19 cases. Similar findings were observed for PM10, NO2 and NO. | (Veronesi et al., 2022) |
| Alberta and Ontario, Canada | PM2.5, NO2 | Increased risk of emergency department for COVID-19 with OR of 1.01 (1.004-1.015) and 1.021 (1.015-1.028) was associated with an interquartile range increase in PM2.5 and NO2, respectively. | (Lavigne et al., 2022) |
| New York City, USA | PM2.5, NO2, BC | RR associated with a unit increase in PM2.5 was 1.11 (1.02-1.21) for death and 1.13 (1.00-1.28) for ICU admission. No significant association was observed for NO2 or BC. | (Bozack et al., 2022) |
| USA | PM2.5 | RR associated with an interquartile range increase in PM2.5 was 1.10 (1.08-1.12) for COVID-19-related hospitalization. | (Bowe et al., 2021) |
| Italy | PM10 | Compared with the first tertile of PM10 exposure level, the OR for the risk of having pneumonia was 1.34 (1.09-1.65) for those of the second tertile, and 1.93 (1.55-2.39) for the third tertile. | (Pegoraro et al., 2021) |
| England | PM2.5 | A unit increase in PM2.5 was associated with a 12.7% (8.3%-17.3%) increase in COVID-19 cases. | (Travaglio et al., 2021) |
NO2 has been identified the major pollutant associated with enhanced severity of COVID-19 in this study, which is consistent with emerging evidence on association between NO2 exposure and COVID-19 mortality (Filippini et al., 2021; Liang et al., 2020; Travaglio et al., 2021). NO2 mainly generates from fossil fuel combustion, and plays an important role in the formation of secondary particulate matters and ozone. Previous researches have provided evidence that short-term exposure to NO2 might increase the susceptibility of adults to respiratory virus infections (Jakab, 1988; Kulle and Clements, 1988; Rose et al., 1989). Further, exacerbation of virus-induced asthma in children aged 8 to 11 years has also been observed in association with exposure to high level of NO2 before the start of a respiratory viral infection (Chauhan et al., 2003). More recently, it was estimated that approximately 50-60% of particulate air pollution associated COVID-19 mortality is attributable to fossil fuel use worldwide, up to 70-80% in Europe, West Asia, and North America (Pozzer et al., 2020). These findings stress the needs on supervision and management of NO2 emission to public health.
Air pollution has been recently hypothesized to enhance COVID-19 severity through upregulating expression of viral receptors, compromising immune responses to viral infection, and exacerbating underlying chronic diseases (Annesi-Maesano et al., 2021). To further provide mechanistic clues, we performed transcriptomics in the present study, and found the down-regulated genes in high-exposure group were mainly enriched in virus clearance related biological processes, suggesting the pathophysiology role of compromised immune responses in the progression of COVID-19. These findings were consistent with previous studies demonstrating that exposure to air pollution impairs the barrier function of epithelial cells, reduces antimicrobial proteins in respiratory tract lining fluid, and decreases the phagocytic capacity of macrophages (Glencross et al., 2020). Moreover, increased attachment of influenza virus with respiratory epithelial cells as well as elevated viral load in lung was observed after treatment with diesel exhaust (Gowdy et al., 2010; Jaspers et al., 2005). However, further research is still needed to determine the specific mechanisms linking the compromised immune responses and air pollution associated COVID-19 severity.
Our study has several strengths. This is a province-level cohort of hospitalized patients who tested positive for COVID-19. Detailed information of demographics and clinical characteristics was retrieved from electronic medical records, making it possible to adjust for individual-level characteristics. Several limitations should also be acknowledged. First, exposure levels of air pollution were estimated relying on the measurements at fixed-station rather than personal measurements at individual levels. It remains unclear how long the patients stayed indoors before they got infected, estimation without individual mobility might introduce exposure misclassification leading effect estimates toward null. Second, as the treatment of COVID-19 still remains diverse without a standard protocol, marked variation exists in care practices across hospitals. In the present analysis, we included all the designated hospitals in Zhejiang province, which may introduce center effects. Third, based on the study design, we were unable to distinguish which pollutant or pollutant mixtures were responsible for the different transcriptional profiles between low- and high-exposure groups. It is also inconclusive whether suppression of virus clearance related biological processes essentially mediated air pollution associated COVID-19 severity. Further animal studies are needed to verify our findings.
4. Conclusion
This statistical analysis here suggest that exposure to higher levels of air pollution, particularly for NO2, was associated with severe COVID-19. Compromised immune responses to viral infection might be partly responsible for the effects of air pollutants on COVID-19 severity. These findings provide us with a clue into toxicological mechanism of air pollution on COVID-19, and emphasize the continuation of current efforts to improve air quality. Polluted areas may implement comprehensive policies to reduce the main sources of air pollution, and improve urban ventilation meanwhile to facilitate the spread of pollutants. As NO2 mainly comes from fossil fuel combustion, society is also expected to shift toward cleaner energy sources through promotion of electric vehicles and application of clean renewable energy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Nos. 82072377, 81971919).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jes.2022.09.040.
Appendix. Supplementary materials
References
- Annesi-Maesano I., Maesano C.N., D'Amato M., D'Amato G. Pros and cons for the role of air pollution on COVID-19 development. Allergy. 2021;76:2647–2649. doi: 10.1111/all.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M. International trade as critical parameter of COVID-19 spread that outclasses demographic, economic, environmental, and pollution factors. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Gibson A.K., Cai M., van Donkelaar A., Martin R.V., et al. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: Cohort study. Environ. Int. 2021;154 doi: 10.1016/j.envint.2021.106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozack A., Pierre S., DeFelice N., Colicino E., Jack D., Chillrud S.N., et al. Long-term air pollution exposure and COVID-19 mortality a patient-level analysis from New York City. Am. J. Respir. Crit. Care Med. 2022;205(6):651–662. doi: 10.1164/rccm.202104-0845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A.J., Inskip H.M., Linaker C.H., Smith S., Schreiber J., Johnston S.L., et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361(9373):1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelani A.B., Gautam S. The influence of meteorological variables and lockdowns on COVID-19 cases in urban agglomerations of Indian cities. Stoch. Environ. Res. Risk Assess. 2022;36(9):1–12. doi: 10.1007/s00477-021-02160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Wang J., Kwong J., Kim J., van Donkelaar A., Martin R.V., et al. Association between long-term exposure to ambient air pollution and COVID-19 severity: a prospective cohort study. CMAJ. 2022;194(20):693–700. doi: 10.1503/cmaj.220068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Sidell M.A., Huang B.Z., Chow T., Eckel S.P., Martinez M.P., et al. Ambient air pollutant exposures and COVID-19 severity and mortality in a cohort of COVID-19 patients in southern California. Am. J. Respir. Crit. Care Med. 2022;206(4):440–448. doi: 10.1164/rccm.202108-1909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. High health expenditures and low exposure of population to air pollution as critical factors that can reduce fatality rate in COVID-19 pandemic crisis: a global analysis. Environ. Res. 2021;199 doi: 10.1016/j.envres.2021.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. How do low wind speeds and high levels of air pollution support the spread of COVID-19? Atmos. Pollut. Res. 2021;12(1):437–445. doi: 10.1016/j.apr.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copat C., Cristaldi A., Fiore M., Grasso A., Zuccarello P., Santo Signorelli S., et al. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: A systematic review. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., Zhang D., Wang Q., Yu F., Zou Q., Xie G., et al. Effects of angiotensin II receptor blocker usage on viral load, antibody dynamics, and transcriptional characteristics among COVID-19 patients with hypertension. J. Zhejiang Univ. Sci. B. 2021;22(4):330–340. doi: 10.1631/jzus.B2000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., Xu K., Gu S., Zheng S., Zou Q., Xu Y., et al. Multi-route transmission potential of SARS-CoV-2 in healthcare facilities. J. Hazard. Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T., Rothman K.J., Cocchio S., Narne E., Mantoan D., Saia M., et al. Associations between mortality from COVID-19 in two Italian regions and outdoor air pollution as assessed through tropospheric nitrogen dioxide. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glencross D.A., Ho T.-R., Camilla N., Hawrylowicz C.M., Pfeffer P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020;151:56–68. doi: 10.1016/j.freeradbiomed.2020.01.179. [DOI] [PubMed] [Google Scholar]
- Gowdy K.M., Krantz Q.T., King C., Boykin E., Jaspers I., Linak W.P., et al. Role of oxidative stress on diesel-enhanced influenza infection in mice. Part. Fibre. Toxicol. 2010;7:34. doi: 10.1186/1743-8977-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Zhang S., Lian J., Jin X., Ye C., Cai H., et al. Liver enzyme elevation in Coronavirus Disease 2019: a multicenter, retrospective, cross-sectional study. Am. J. Gastroenterol. 2020;115(7):1075–1083. doi: 10.14309/ajg.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G.J. Modulation of pulmonary defense mechanisms against viral and bacterial infections by acute exposures to nitrogen dioxide. Res. Rep. Health Eff. Inst. 1988;20:1–38. [PubMed] [Google Scholar]
- Jaspers I., Ciencewicki J.M., Zhang W.L., Brighton L.E., Carson J.L., Beck M.A., et al. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol. Sci. 2005;85(2):990–1002. doi: 10.1093/toxsci/kfi141. [DOI] [PubMed] [Google Scholar]
- Jin X., Lian J., Hu J., Gao J., Zheng L., Zhang Y., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulle T.J., Clements M.L. Susceptibility to virus infection with exposure to nitrogen dioxide. Res. Rep. Health Eff. Inst. 1988;15:5–21. [PubMed] [Google Scholar]
- Lavigne E., Ryti N., Gasparrini A., Sera F., Weichenthal S., Chen H., et al. Short-term exposure to ambient air pollution and individual emergency department visits for COVID-19: a case-crossover study in Canada. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-217602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Tao B., Hu Z., Yi Y., Wang J. Effects of short-term ambient particulate matter exposure on the risk of severe COVID-19. J. Infect. 2022;84(5):684–691. doi: 10.1016/j.jinf.2022.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Shi L., Zhao J., Liu P., Schwartz J., Gao S., et al. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. Innovation. 2020;1(3) doi: 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Feldman A., Heres D., Marquez-Padilla F. Air pollution exposure and COVID-19: A look at mortality in Mexico City using individual-level data. Sci. Total. Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile F., Michelozzi P., Ancona C., Cappai G., Cesaroni G., Davoli M., et al. Air pollution, SARS-CoV-2 incidence and COVID-19 mortality in Rome–a longitudinal study. Eur. Respir. J. 2022;60(3) doi: 10.1183/13993003.00589-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro V., Heiman F., Levante A., Urbinati D., Peduto I. An Italian individual-level data study investigating on the association between air pollution exposure and Covid-19 severity in primary-care setting. BMC Public Health. 2021;21:902. doi: 10.1186/s12889-021-10949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzer A., Dominici F., Haines A., Witt C., Munzel T., Lelieveld J. Regional and global contributions of air pollution to risk of death from COVID-19. Cardiovasc. Res. 2020;116(14):2247–2253. doi: 10.1093/cvr/cvaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi N.R., Fouladi-Fard R., Aali R., Shahryari A., Rezaali M., Ghafouri Y., et al. Bidirectional association between COVID-19 and the environment: A systematic review. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran J., Zhao S., Han L., Qiu Y., Cao P., Yang Z., et al. Effects of particulate matter exposure on the transmissibility and case fatality rate of COVID-19: A Nationwide Ecological Study in China. J. Travel. Med. 2020;27(6):taaa133. doi: 10.1093/jtm/taaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R.M., Pinkston P., Skornik W.A. Altered susceptibility to viral respiratory infection during short-term exposure to nitrogen dioxide. Res. Rep. Health Eff. Inst. 1989;24:1–24. [PubMed] [Google Scholar]
- Rzymski P., Poniedzia B., Rosinsk J., Rogalska M., Zarebska-Michaluk D., Rorat M., et al. The association of airborne particulate matter and benzo[a]pyrene with the clinical course of COVID-19 in patients hospitalized in Poland. Environ. Pollut. 2022;306 doi: 10.1016/j.envpol.2022.119469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C., Klompmaker J., Cummins S., James P., Fecht D., Roscoe C. Associations of air pollution with COVID-19 positivity, hospitalisations, and mortality: observational evidence from UK Biobank. Environ. Pollut. 2022;308 doi: 10.1016/j.envpol.2022.119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. COVID-19 and air pollution and meteorology-an intricate relationship: a review. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., Cao W., Li M., Yang L., Dai R., Luo X., et al. PM2.5 compromises antiviral immunity in influenza infection by inhibiting activation of NLRP3 inflammasome and expression of interferon-beta. Mol. Immunol. 2020;125:178–186. doi: 10.1016/j.molimm.2020.07.001. [DOI] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi G., De Matteis S., Calori G., Pepe N., Ferrario M.M. Long-term exposure to air pollution and COVID-19 incidence: a prospective study of residents in the city of Varese, Northern Italy. Occup. Environ. Med. 2022;79(3):192–199. doi: 10.1136/oemed-2021-107833. [DOI] [PubMed] [Google Scholar]
- Villeneuve P.J., Goldberg M.S. Methodological considerations for epidemiological studies of air pollution and the SARS and COVID-19 coronavirus outbreaks. Environ. Health Persp. 2020;128(9):95001. doi: 10.1289/EHP7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization) World Health Organization; Geneva, Switzerland: 2022. WHO Coronavirus (COVID-19) Dashboard.https://covid19.who.int/ Available: Accessed September 27, 2022. [Google Scholar]
- Yao Y., Pan J., Wang W., Liu Z., Kan H., Qiu Y., et al. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Chen Z., Liu Y., Song H., Wu C.H., Li B., et al. Association between coronavirus disease 2019 (COVID-19) and long-term exposure to air pollution: Evidence from the first epidemic wave in China. Environ. Pollut. 2021;276 doi: 10.1016/j.envpol.2021.116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.