Abstract
Cell-cell communication (CCC) is essential to how life forms and functions. However, accurate, high-throughput mapping of how expression of all genes in one cell affects expression of all genes in another cell is made possible only recently, through the introduction of spatially resolved transcriptomics technologies (SRTs), especially those that achieve single cell resolution. However, significant challenges remain to analyze such highly complex data properly. Here, we introduce a Bayesian multi-instance learning framework, spacia, to detect CCCs from data generated by SRTs, by uniquely exploiting their spatial modality. We highlight spacia’s power to overcome fundamental limitations of popular analytical tools for inference of CCCs, including losing single-cell resolution, limited to ligand-receptor relationships and prior interaction databases, high false positive rates, and most importantly the lack of consideration of the multiple-sender-to-one-receiver paradigm. We evaluated the fitness of spacia for all three commercialized single cell resolution ST technologies: MERSCOPE/Vizgen, CosMx/Nanostring, and Xenium/10X. Spacia unveiled how endothelial cells, fibroblasts and B cells in the tumor microenvironment contribute to Epithelial-Mesenchymal Transition and lineage plasticity in prostate cancer cells. We deployed spacia in a set of pan-cancer datasets and showed that B cells also participate in PDL1/PD1 signaling in tumors. We demonstrated that a CD8+ T cell/PDL1 effectiveness signature derived from spacia analyses is associated with patient survival and response to immune checkpoint inhibitor treatments in 3,354 patients. We revealed differential spatial interaction patterns between γδ T cells and liver hepatocytes in healthy and cancerous contexts. Overall, spacia represents a notable step in advancing quantitative theories of cellular communications.
Keywords: spatially resolved transcriptomics, cellular interaction, spacia, MERSCOPE, GeoMX
INTRODUCTION
Various types of cells form complex structures and communication networks in the tissue micro-environment, and the signaling between these cells is central to normal organ development and diseased physiological processes. Elucidating cell-cell communication (CCC) in the tissue microenvironment in different biological systems is of vital importance. Many experimental and informatics approaches have attempted to address this question 1–4.
Since the introduction of single-cell RNA-sequencing (scRNA-seq) data, many informatics approaches to infer CCCs based on scRNA-seq data have been developed, such as CellChat 5, NicheNet 6, CellphoneDB 7, NATMI 8, SingleCellSignalR 9, etc (some reviewed in Sup. Table 1). Despite their popularity and their significant contributions to the field, these methods suffer from a number of major caveats, due to the limited information provided by scRNA-seq. To begin with, many of these tools only infer interactions between cell types rather than interactions at the single-cell level, thus losing single cell resolution. Secondly, CCC is usually context-specific, and the common approach of mapping the data to pre-defined interaction pathway databases, regardless of the cellular context, inevitably results in low resolution and low specificity in the detection of true CCCs in the tissue sample of interest. Lastly, most of these tools rely on the co-expression of ligand-receptor gene pairs in signal-sending and receiving cells to claim detection of CCC. However, the expression of the receptor gene itself is not necessarily impacted by the expression of the ligand. But rather, the ligand’s impact can only be revealed by the transcriptomic regulation of (some of) the receptor’s downstream genes.
Fortunately, emerging spatially resolved transcriptomics (SRT) technologies potentially provide promising data to address these problems, by providing the extra spatial modality to aid the dissection of CCCs. Some bioinformatics tools have been developed for inferring CCCs from such data (reviewed in Sup. Table 1). However, earlier SRT methods, such as Visium/10X, Slide-Seq 10,11, and XYZeq 12, are all low resolution SRT methods, meaning that they cannot achieve single cell resolution in the SRT expression data and their usefulness for CCC inference is still very limited. For example, one Visium sequencing spot can contain 10 or even more cells. The cells that are interacting with each other could be contained in one sequencing unit, thus making it difficult to infer such types of CCCs. Therefore, bioinformatics tools developed based on such low resolution SRT data still have not addressed the problem of accurately inferring CCCs. And many of the same caveats that we mentioned above for scRNA-seq-based methods are still outstanding.
Only very recently are single cell resolution SRT technologies developed and commercialized, such as Seq-scope 13, HDST 14, MERSCOPE/Vizgen, CosMx/Nanostring and Xenium/10X, etc. First, these newest SRTs allow researchers to pinpoint each single cell with its spatial location and identify the other cells within its neighborhood. It is thus feasible to detect interactions between each pair of single cells without having to aggregate to cell types. In addition, since SRTs enable examining the expression profiles of all possible pairs of single cells, yielding very rich data that reveal ongoing CCCs, we can apply a much more data-driven approach to infer CCCs, overcoming the constraint of relying on previously known pathways. Furthermore, the high-dimensional and multi-modal information contained in these cell pair data also enables the modeling of downstream target genes in the receiving cells in detail, removing the limitation to ligand-receptor pairs documented by prior databases. Therefore, single cell SRTs overcome all major caveats encountered in mapping of CCCs from scRNA-seq data and low resolution SRT data.
However, significant data analytical challenges came with the maturation of these state-of-the-art SRT technologies. In particular, during CCCs, multiple senders in a neighborhood could confer signals to and impact the same receiver. SRTs allow us to match these potential senders and receivers. But these pairing relationships result in complex multi-to-one data structures consisting of both expression and spatial modalities that are unsuitable for classical statistical learning approaches, and have rarely been considered sufficiently by existing CCC detection approaches so far (Sup. Table 1). To address this inadequacy, we innovate spacia, a Bayesian multi-instance learning (MIL) framework, to explicitly model these multi-to-one relationships and to detect the interaction between individual signal-sending and receiving cells from SRT data.
Spacia achieved single-cell resolution detection of CCCs, models the downstream targets of CCCs, rather than being limited to ligand-receptor relationships documented in prior databases, and significantly reduced artefacts of CCC inferences while increasing specificity. We tested spacia on data from all three commercialized single cell resolution SRTs so far: MERSCOPE, CosMx, and Xenium, proving the suitability of spacia and these SRT platforms for inferring CCCs. Sup. Table 1 summarized these advances of spacia over prior works in terms of conceptual advantages and comprehensiveness of testing datasets. In real data applications, spacia out-performed benchmark tools by a large margin and revealed novel biological insights regarding CCCs from diverse biological contexts.
RESULTS
Multi-Instance Learning for Mapping CCCs from SRT Data
We present a Bayesian multiple-instance learning (MIL) model, spacia, to infer CCCs from SRT data. Under a MIL framework, the receiver cells are modeled as “bags” with labels (expression of receiving genes/pathways), and each bag is a collection of multiple instances (senders) characterized by the instance-level features (expression of genes/pathways and spatial closeness to receivers). In this way, the multiple-sender-to-one-receiver relationships during CCCs are explicitly modeled. The technical details of spacia are described in Sup. File 1 and the statistical innovation of spacia is provided in the discussion section. At a high level, spacia has two tiers. The first tier identifies signal-sending cells (senders) that are truly impacting signal-receiving cells (receivers) based on spatial closeness, indicated by a δ variable (Fig. 1ab), out of all candidate sending cells included into each bag. As different CCCs could have varying effective spatial ranges, depending on the cell types and types of interactions (contact-based or secretion-based), the model estimates a variable, indicated by b, to allow flexibility in determining δ in each specific dataset. When b is negative, the senders and receivers are determined to have stronger interactions when they are physically closer. In the second tier, spacia discovers gene co-expression patterns between the senders and the receivers (Fig. 1ab), but only for senders that are determined to be impacting each receiver cell (δ=1). We infer a variable β that indicates how the expression of the genes/pathways in the senders impacts the genes/pathways in the receiver cells. This model is solved by Markov Chain Monte Carlo (MCMC), which is an iterative process that generates a distribution for the value of each variable of interest so that we can provide both point estimates and inferences of statistical significance.
Fig. 1.
The spacia model. (a) Cartoons explaining the concept of “primary instances”, namely the sending cells that are truly interacting with the receiver cells. The purple senders refer to senders that interact with receivers through cell-to-cell contact. The green senders refer to senders that interact with receivers through secreted ligands. (b) Diagram showing the key elements of the model structure of spacia. (c) Inference results of spacia on a simulation dataset. Blue color refers to sender cells, red color refers to receiver cells and green arrows refer to CCC. (d) ROC curves measuring the accuracy of spacia finding the correct primary instances in the simulation data, with increasing numbers of total MCMC chains. (e) The distribution of the b variables across MCMC iterations after stabilization, in two MCMC chains. (f) The distribution of the β variables across MCMC iterations after stabilization, for genes in senders that are (top) or are not (bottom) truly interacting with receiving genes. (g) Point estimates of the β variables. Left: 5 truly interacting genes in the simulation data; right: 10 truly interacting genes.
Spacia infers, for each receiver cell, a set of sender cell(s) that truly interact with this receiver. This unique model design enables us to accurately infer single cell-to-single cell interactions for each cell captured by SRT. On the other hand, the sending and receiving genes that can be considered by our model do not have to be ligands and receptors, but rather, all genes captured by SRT can be considered for both the sending and receiving portions. This allows us to avoid the questionable rationale of examining the co-expression of ligands/receptors from pre-defined interaction databases for mapping CCC, while allowing us to model the upstream and downstream regulatory signals that occur during CCC.
Importantly, cell-cell communications happen in a variety of manners. As reviewed by Armingol et al 2, there are four major types of cell-cell communications: autocrine, paracrine, juxtacrine, and endocrine. The first three types of communication naturally require cells to be in close proximity. In contrast, endocrine interactions occur over long distances through systemic circulation, and it is not feasible to track such CCCs by SRT. Similarly, some CCCs, though happening within one organ, also still do not depend on spatial proximity of the interacting cells. It is important to note that spacia only considers the types of cell-cell communications that require the interacting cells to be closely localized, in addition to demonstrating co-expression patterns.
To validate its efficacy, we tested spacia on simulated data. As we demonstrated in Fig. 1c, we simulated two types of cells that are interacting. The blue cells are senders, while the red cells are receivers, but the red cells’ expression was simulated to be regulated by only nearby blue cells. As expected, spacia only infers red and blue cells that are close to each other to be interacting (Fig. 1c). We showed, in Fig. 1d, the rate of correct identification of the truly interacting cell pairs, measured by Area Under the ROC curve (AUROC). With more than 10,000 MCMC iterations, the AUROC achieves >0.95 (spacia’s default is 50,000 iterations), meaning an almost perfect detection of interacting cell pairs. We then showed in Fig. 1e that the b variables were estimated to be negative, consistent with the simulation assumption that only nearby cells are interacting. We also evaluated spacia’s estimations of the β variables, in Fig. 1f (distributions of βs across all MCMC iterations) and Fig. 1g (point estimates of βs). As is shown, the sender genes that were simulated to be truly interacting with the receiver genes had βs that were significantly different from 0, while the converse was true for non-interacting genes. Spacia allows us to perform statistical inference of the inferred b and β variables. For example, their confidence intervals are visualized in Fig. 1ef, according to the sampling from the MCMC iterations, from which we can also calculate P values. The P values are <1E-4 for both chains for b (Fig. 1e), range from 0.008 to 3.4E-5 for the truly interacting genes’ βs (top panel of Fig. 1f), and range from 0.09–0.77 for the non-interacting genes’ βs (bottom panel of Fig. 1f). Finally, the posterior samples of the b and β variables demonstrate stable convergence and minimum auto-correlations (Sup. Fig. 1). Additional analyses were provided in Sup. File 2. Taken as a whole, these results indicate that spacia exhibits excellent statistical properties.
Spacia Validates in SRT Data and Overcomes Limitations of Existing Approaches
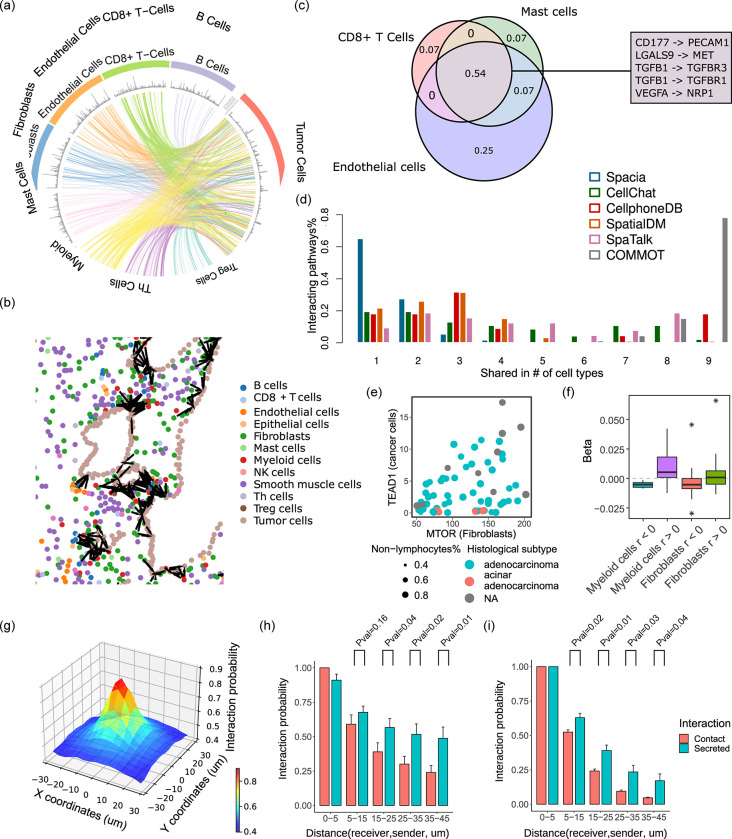
Next, we investigated a prostate cancer MERSCOPE dataset and used spacia to infer how the non-tumor cells from the tumor microenvironment impact prostate cancer cells (Fig. 2a). We compared spacia to several SRT-based CCC inference tools, including SpaTalk 15, COMMOT 16, and SpatialDM 17, that achieved high to top advantage points in Sup. Table 1, and also two popular non-SRT-based CCC inference software, CellPhoneDB 7 and CellChat 5. We visualized the interacting cell pairs in their spatial locations in Fig. 2b and Sup. Fig. 2ab. Consistent with our expectation, the inferred CCCs are all local for spacia, COMMOT, and SpaTalk. In contrast, the CCCs inferred from CellPhoneDB and CellChat indicate numerous CCCs across the entire span of the tissue, which is highly unlikely. In addition, some software such as SpatialDM and COMMOT cannot determine individual interaction strengths between single cells (or sequencing units). SpatialDM only outputs a score measuring the probability that each single cell participates in CCCs (as denoted in Sup. Fig. 2a), while not inferring the interaction partners. COMMOT only outputs a direction of interaction for each cell, without the direction pointing to any specific interacting cells.
Fig. 2.
Validating spacia in real data. (a) Spacia inferred CCCs from select immune and stromal cells to tumor cells in the prostate cancer MERSCOPE dataset. The spacia results were filtered to only visualize those that satisfy b < 0, b Pval<1×10E-30, β Pval<0.001, and top 1% of z-scored β across all results. (b) Spatial representation of the spacia results. Cells are color labeled by cell types, and significantly interacting cell pairs inferred by spacia (bs) are indicated in black. (c) Overlap between the predicted CCCs by CellphoneDB on the same MERSCOPE dataset for three example sending cell types. The numbers indicate the proportions of total CCCs found in each overlap. Some example CCCs that are inferred to exist in all three cell types are listed. (d) Comparison of the degree of overlap in inferred CCCs between different sending cell types, by spacia, CellChat, CellphoneDB, SpatialDM, SpaTalk, and COMMOT. The Y axis refers to the proportions of interactions shared in n cell types vs. all predicted interactions. (e) An example scatterplot showing the correlations between the expression of sending and receiving genes in their respective cell types in the PDX RNA-seq data. (f) Spacia β values are more likely to be positive for sending genes and receiving genes that are positively correlated in the PDX data, and vice versa. (g-i) Probability of interaction as a function of distance from the sending cells to the receiving cell. (g) Gene pairs of both secretion-based and contact-based CCCs combined in the prostate cancer MERSCOPE dataset. The sending cells are shown in their actual X-Y coordinates relative to the receiving cells and the interaction probabilities are averaged at each location. The Z axis shows the primary instance probabilities of the sending cells, explained in Sup. File 1. (h,i) The probabilities for each type of CCCs shown separately, and the sending cells are ordered and binned along the distances between sending and receiving cells. (h) the prostate cancer and (i) the lung cancer MERSCOPE datasets. For sake of consistency, for each gene pair shown in panels (g-i), all interaction probabilities are normalized by the maximum interaction probabilities and shown in the plots. T test is used to test if the sending-receiving cell pairs have higher interaction probabilities in secretion-based CCCs than in contact-based CCCs.
More importantly, we noticed the benchmark tools inferred highly similar sets of interacting genes regardless of the types of cells. For example, in Fig. 2c, we showed CellPhoneDB’s inference results for three very dissimilar cell types (as sending cells), CD8+ T cells, mast cells, and endothelial cells. Among all unique sending-receiving gene pairs that were inferred to be active in at least one of these sending cell types, 54% exists in all three sending cell types, which suggests an alarming lack of specificity. We investigated this systematically by examining how many predicted interacting genes were shared between all sender cell types (Fig. 2d). Of all the CCCs inferred by spacia, 92.7% were unique to one or two sender cell types, with no interactions found to be shared by more than four different sender cell types. In striking contrast, 63.6% of the CCCs are shared among three or more cell types according to CellphoneDB, 60.9% according to CellChat, 52.2% for SpatialDM, 71.9% for SpaTalk, and 100% for COMMOT. In particular for COMMOT, 78.3% of interactions were shared among all cell types examined, which is impossible.
Furthermore, we note that, for all of COMMOT, SpatialDM and SpaTalk, if we ran these tools on the full SRT dataset, the memory usage exceeds 1–2TB, which is far more than the maximum memory limit of regular high performance computing servers and the programs all failed. We had to downsample the number of cells in the SRT data dramatically (maximum of 5,000 for COMMOT, 1,000 for SpatialDM, and 3,000 for SpaTalk, for each cell type) so that the execution would be successful.
We utilized other forms of data to validate spacia. The NCI Patient-Derived Models Repository (PDMR, pdmr.cancer.gov) provides RNA sequencing data of 70 prostate cancer patient-derived xenografts (PDXs). In PDX models, human immune/stromal cells die out quickly and mouse T/B/NK cells are generally non-existent due to the choice of NSG mouse models 18. We separated the bulk PDX gene expression into the human tumor cell vs. mouse stromal/immune cell components with Disambiguate 19. Next, we used CIBERSORTx 20 to further demultiplex the mouse stroma/immune cell component into cell type-specific expression. We then evaluated the correlation between human tumor cells’ expression and the expression of mouse fibroblasts and myeloid cells (one example gene pair shown in Fig. 2e), which are the two most abundant cell types in the murine components, according to CIBERSORTx. Then we cross-referenced these against the pairs of signal-sending genes in senders (fibroblasts and myeloid cells) and downstream target genes in receiver tumor cells that were detected by spacia. Overall, positively correlated gene pairs from the PDX data are indeed more likely to have positive βs in the corresponding interactions inferred by spacia and vice versa (Fig. 2f).
Finally, we categorized the interacting gene pairs into “contact-based” and “secretion-based” interactions according to whether the sending gene is known to participate in contact-based or secretion-based interactions 5, and investigated cellular interaction probabilities as a function of the distances between sending and receiving cells (Fig. 2g). As is shown in Fig. 2h, the probabilities of interactions drop off along with increasing distances in both types of interactions, but the interaction probabilities drop off much faster for contact-based interactions than for secretion-based interactions, which is consistent with the fact that secretion-based interactions can happen over longer distances by moving of secreted molecules from one cell to another. We also investigated another MERSCOPE dataset from a lung cancer patient and observed the same phenomenon (Fig. 2i).
Induction of Prostate Cancer EMT and Lineage Plasticity by the Tumor Microenvironment
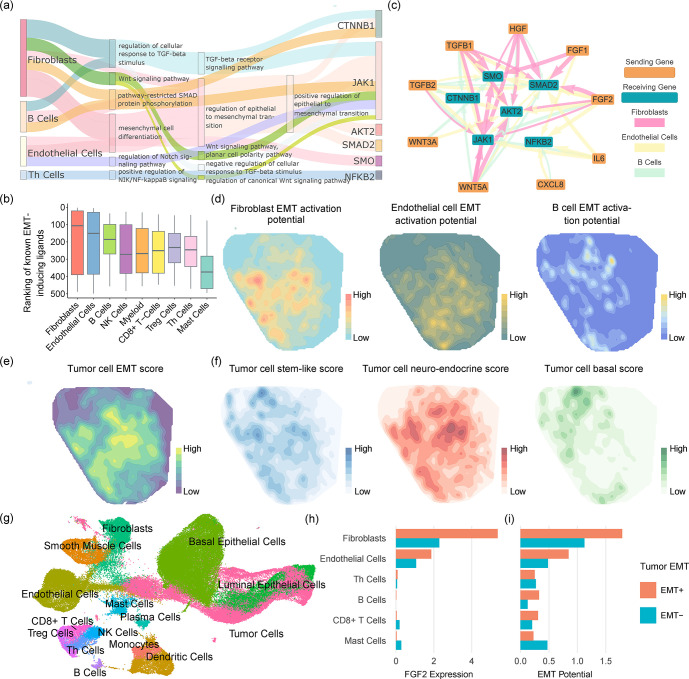
TGFBs secreted by fibroblasts induce EMT in tumor cells 21,22. We investigated whether the sender genes are enriched in signaling pathways that are associated with the induction and regulation of EMT in tumor cells. We performed Gene Ontology analysis using GOrilla 23,24 on the sending genes, for each sender cell type and for each of several receiving genes in the tumor cells that are classical markers or drivers of EMT 25–33 (Fig. 3a). We found that fibroblasts, B cells, endothelial cells and Th cells possess the highest numbers of enriched pathways (FDR<0.05) directly or closely related to the regulation of EMT (the full GO results for JAK1 as an example receiver gene are shown in Sup. Table 2). In other words, spacia correctly inferred the upstream signaling pathways in several cell types of the tumor microenvironment that regulate the downstream induction of EMT in the tumor cells.
Fig. 3.
Applying spacia to reveal EMT and lineage plasticity induction signals from the prostate cancer tumor microenvironment. (a) Sankey diagram describing GO term enrichment in the sending genes, of each sender cell type, that are inferred by spacia to be impacting well known EMT marker genes in the tumor receiver cells. The width of the flow is scaled with the P values, and the parent term is connected with the child term if both terms were identified between the same sender cell-receiving gene pair. (b) The rankings of βs for known cytokine ligands that could induce EMT, among all the sending genes input into spacia, for each cell type. A smaller rank refers to a stronger interaction strength (larger β). (c) The top sending-receiving gene pairs inferred by spacia, for fibroblasts, endothelial cells and B cells as sending cell types. (d) The kernel density estimation of the EMT activation potentials in fibroblasts, endothelial cells and B cells. (e) The kernel density estimation of the EMT levels in prostate cancer cells. (f) The kernel density estimations of the stem-like, neuro-endocrine, and basal lineage plasticity scores in the prostate cancer cells. (g) UMAP plot showing the cell types of single cells from our prostate cancer patients. (h) The FGF expression of different sending cell types in the EMT+ and EMT− patients. (i) The EMT activation potentials of different sending cell types in the EMT+ and EMT− patients.
We focused on a number of secretory ligands that are known to induce EMT activation in tumor cells within the sending cell types above; these ligands include WNT5A, WNT3A, TGFB1, TGFB2, FGF2, IL6, CXCL8, HGF, and FGF1 21,22,25,26,28–30,32. Fig. 3b shows the ranked βs of these ligands in each sender cell type, among all 500 genes captured by MERSCOPE, where a smaller rank refers to stronger up-regulation of the EMT marker/driver genes. Fibroblasts (Pval=0.03, one-sided T-test against the mean rank of 250), endothelial cells (Pval=0.04), and B cells (Pval=0.005) demonstrate the strongest activation of tumor EMT via these ligands. While the roles of fibroblasts and endothelial cells in inducing EMT are more well established, it is surprising to see that B cells are also inferred by spacia to induce EMT in prostate cancers. We also showed more details of the inferred interactions between these ligands and the EMT genes in a network plot (Fig. 3c). It is apparent that fibroblasts, endothelial cells and B cells each employ more than one secretory factor to induce tumor cell EMT. We also uncovered EMT-inducing interactions that have not been described before in prostate cancer. For example, while it has been reported that endothelial cells secrete IL-6 and induce EMT in head and neck tumors 34 and esophageal carcinoma 35, we showed that such a mechanism also exists in prostate cancers (Fig. 3c). We next visualized the spatial co-expression patterns of these ligands in sender cells (Fig. 3d) and the EMT levels of the tumor cells (Fig. 3e, definition of EMT level in method section). In particular, we aggregated over these secretary ligands to form an “EMT activation potential” in each sender, weighted by the βs inferred by spacia, and further aggregated all senders of each receiver cell through a weighted average with weights being the probability of the senders being “primary” (δ=1). Fig. 3d confirms that the EMT activation patterns have higher correlation with the EMT levels of the tumor for fibroblasts and endothelial cells (Spearman correlation: ρ=0.822, 0.838), but much lower for B cells (ρ=0.415) (Fig. 3e, Sup. Table 3). It is interesting, however, that fibroblasts and endothelial cells have largely overlapping spatial patterns of EMT activation, while B cells show a distinct pattern with its EMT activation. It appears that the sum of the EMT activation patterns of fibroblasts/endothelial cells and B cells better corresponds to the EMT level of the tumor cells (Fig. 3e).
Our prior work showed that EMT is usually part of a lineage plasticity dys-regulation program in prostate cancer cells 36. To determine if the observed increase in EMT activity was also associated with gene expression programs involved in lineage plasticity, we studied the spatial distribution of the stem-like, neuro-endocrine, and basal lineage plasticity levels in the tumor cells. Fig. 3f shows that the stem-like and neuro-endocrine lineages are largely correlated with the prostate cancer cell EMT levels (ρ=0.666, 0.854) and the EMT activation potential of fibroblasts and endothelial cells (Sup. Table 3). In particular, the correlation between fibroblast EMT activation potential and the tumor cell neuro-endocrine lineage is the highest, achieving a Spearman correlation of 0.916.
To validate the interactions inferred by spacia at the patient level, we generated 21 scRNA-seq datasets from a cohort of prostate cancer patients (Fig. 3g). We divided the patients into two subsets of EMT high (N=10) and EMT low (N=11), according to the EMT expression in their tumor cells. We first examined the expression of FGF2 in fibroblasts, endothelial cells, B cells, and several other immune cell types as controls (Fig. 3h). Fig. 3c indicates that fibroblasts and endothelial cells are the major cell types that secrete FGF2 and induce prostate cancer cell EMT. Consistent with this observation from MERSCOPE, Fig. 3h shows that fibroblasts and endothelial cells are the only two cell types that abundantly express FGF2, and more importantly, the expression of FGF2 is higher in fibroblasts (Pval<0.001) and endothelial cells (Pval<0.001) from patients with EMT-high tumors, compared with EMT-low tumors. We again examined all the secretory factors together by calculating the EMT activation potential as in Fig. 3d. As shown in Fig. 3i, fibroblasts cells overall possess the strongest EMT activation potential. As expected, fibroblasts and endothelial cells show stronger EMT activation potential in the EMT-high patients, compared with the EMT-low patients (Pval<0.001 for both). B cells also demonstrate the same trend, though not achieving statistical significance. Next, we assessed the association between lineage plasticities of tumor cells and the EMT activation potentials of the stromal/immune cells (Sup. Fig. 3), as is done for the MERSCOPE dataset. Consistent with Sup. Table 3, the most pronounced observation is that fibroblast EMT activation potential was significantly higher in the group associated with enhanced neuro-endocrine lineage (Pval<0.001). The endothelial EMT activation potential was also higher in the stem-like positive group than in the negative group (Pval<0.001), similarly for the basal lineage (Pval<0.001). Overall, the main discoveries regarding the intra-tumor heterogeneity of EMT and lineage plasticity induction in tumor cells identified by spacia can be extrapolated to inter-tumor differences.
Finally, as a comparison, we also performed the analyses of Fig. 3b for the five benchmark software applications. However, we note that none of these software applications outputs a direction of regulation. This is in sharp contrast to spacia, which outputs a β for each sending-receiving gene pair, whose direction clearly indicates an up-regulation or down-regulation effect. This is a significant advance of spacia over these five benchmark software applications and potentially many other existing CCC inference tools listed in Sup Table 1. Nevertheless, we ignored the direction of regulation for this particular analysis, and still found that none of these benchmark software applications can identify fibroblasts, endothelial cells, and B cells as the main drivers of tumor cell EMT (Sup. File 2).
Spacia Infers the Impact of PDL1 Signaling on B cells in the Tumor Microenvironment
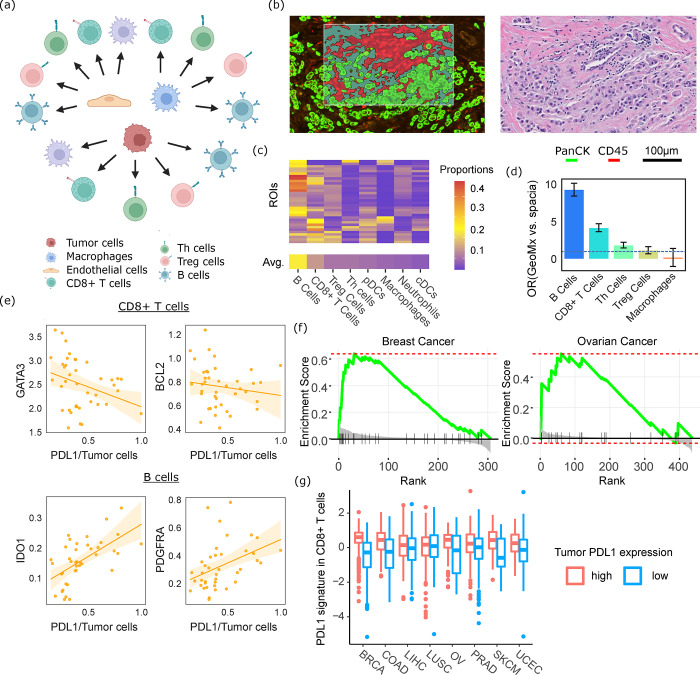
In another application, we deployed spacia to characterize the impact of PDL1 signaling on the tumor microenvironment. While it is well known that tumor cells expressing PDL1 inhibit T cells expressing PD1, whether other PDL1- and PD1-expressing cells interact with each other is not clear yet. We studied a breast cancer MERSCOPE dataset and applied spacia to investigate how the PDL1 expression of tumor cells, endothelial cells, and macrophages leads to downstream transcriptional regulation in CD8+ T cells, Th cells, Treg cells, B cells, and macrophages (Fig. 4a). We visualized the pathways enriched in the downstream target genes that spacia inferred for different cell type pairs in Sup. Fig. 4. We show that the PDL1-PD1 axis is active in almost all possible combinations of sender cell type-receiver cell type pairs, although the strength of interactions is still the strongest for PDL1 signaling from tumor cells. It is also worth noting that, in addition to the enrichment of genes in pathways typically related to immune responses, there is also an enrichment related to apoptosis.
Fig. 4.
Spacia reveals PDL1 downstream target genes. (a) Sending and receiving cell type pairs that were analyzed by spacia for the breast cancer MERSCOPE dataset. (b) Left: One example ROI of the GeoMX data; right: the region of the H&E slide corresponding to the same ROI. (c) Abundances of each type of immune cells in the GeoMx data, as predicted by BayesPrism. Cell types are ordered by their average abundance across the 40 ROIs. (d) Odds ratios showing the overlap between spacia-inferred PDL1 downstream target genes and genes that are differentially expressed in each type of immune cells in the GeoMx data, comparing ROIs that are PDL1+ and PDL1− in the tumor cells. (e) Expression of several representative receiving genes that are in the overlap of (d) for B cells and CD8+ T cells, as a function of tumor cell PDL1 expression, in the GeoMx data. The fitted curves between the X axis and the Y axis are shown as solid lines, with the shading denoting 95% CI. (f) Gene set enrichment analysis (GSEA) in the TCGA breast and ovarian cancer datasets to evaluate if the corresponding CD8-PDL1 signature genes are indeed among the top genes that are differentially expressed between PDL1+ and PDL1− patients. (g) Expression levels of the CD8-PDL1 signatures in CD8+ T cells in the TCGA samples, dichotomized by tumor cell PDL1 expression.
To validate these observations, we generated a set of GeoMx data from eight treatment-naive breast cancer patients. Each patient was sampled for an average of five Regions of Interest (ROIs), resulting in a total of 40 ROIs with a mixture of tumor and immune cells. We defined the tumor and immune masks in each ROI (Fig. 4b, full images in Sup. File 2), and extracted mask-specific gene expression for the tumor cell and immune cell components. We deployed BayesianPrism 37 to dissect the immune cell gene expression into the gene expression of each immune cell type for each ROI. As Fig. 4c shows, B cells (19%) and CD8+ T cells (10%) are most abundant in these ROIs, followed by Treg cells (6%) and Th cells (6%), with macrophages accounting for the least proportions (4.5%). Next, we investigated genes in these immune cell types whose expression is positively or negatively correlated with the expression of PDL1 in tumor cells. We then calculated the overlap between these genes and the PDL1 downstream target genes that spacia inferred from MERSCOPE data, in terms of direction of expression regulation. Importantly, as BayesPrism’s authors suggested, BayesPrism is more accurate for cell types that are abundant in the tissue mixture (only B cells and CD8+ T cells achieve optimal accuracy according to their guidelines). Therefore, we focused this validation on the PDL1 target genes in B cells and CD8+ T cells. As Fig. 4d shows, there are statistically significant overlaps between GeoMx and spacia/MERSCOPE, for both B cells (Odds Ratio (OR)=9.3, Pval=0.01) and CD8+ T cells (OR=4.2, Pval=0.011). Note here that overlap is defined by a gene having the same inferred direction of regulation by PDL1 (both up-regulation or both down-regulation), in both the MERSCOPE and GeoMx data. As expected, the overlap is less pronounced for Th cells (OR=1.87, Pval=0.14) and Treg cells (OR=1.18, Pval=0.92), and there is no enrichment for macrophages (OR=0.21, Pval=0.37). Even though these two sets of PDL1-downstream genes were derived from two different technologies in two different cohorts of patients, the existence of a significant overlap speaks to the validity of spacia’s findings. Unfortunately, as the direction of regulation is critical for this analysis, we cannot perform comparison with the other five benchmark software applications, as none of them outputs the direction of regulation.
We showcase the top genes that are in these overlaps. In CD8+ T cells, both the spacia and the GeoMx analyses indicate that BCL2 is inhibited by PDL1 from tumor cells (Fig. 4e). BCL2 is a key anti-apoptosis molecule38 and therefore our results suggest that PDL1 promotes apoptosis in CD8+ T cells. We also found that PDL1 down-regulates GATA3 in CD8+ T cells (Fig. 4e), which supports the maintenance and proliferation of T cells downstream of TCR and cytokine signaling 39. Both of these observations are in alignment with the well-known immuno-suppressive functions of PDL1 for CD8+ T cells. For B cells, we found that PDL1 of tumor cells up-regulates IDO1 in B cells, in both the spacia and GeoMx analyses (Fig. 4e). The role of IDO1 for B cells is less clear so far, but recent reports have linked IDO1 with immuno-suppressive roles in B cells 40. We also found that PDL1 up-regulates PDGFRA in B cells (Fig. 4e). To our knowledge, there has been no literature on how the expression of PDGFRA in B cells is correlated with B cell functions. But we examined the breast cancer scRNA-seq data from Bassez et al 41, where data on various immune cell types pre- and post-anti-PD1 treatment were generated. These data show that PDGFRA expression in B cells decreased after anti-PD1 treatment (Sup. Fig. 5a, Pval=0.025), which suggests an activating role of PDL1 for PDGFRA.
We also analyzed a breast cancer Xenium dataset (Sup. Fig. 5b) by spacia, which has PDGFRA in its gene panel. Sup. Fig. 5c showcases the spatial distributions of the cellular interactions that spacia inferred, which are local as expected. Reassuringly, spacia also inferred tumor cell PDL1 to up-regulate PDGFRA in B cells (Sup. Fig. 5d), confirming our observations above. We systematically compared the genes that are shared between the MERSCOPE panel and Xenium panel (Sup. Fig. 5e), and observed that there is an overall concordance between the directions of βs from the spacia analyses on both the MERSCOPE and Xenium breast cancer datasets, for both B cells (OR=3.42) and CD8+ T cells (OR=1.9). Interestingly, we again observed that the concordance is higher for B cells, than for CD8+ T cells, reflecting our results in Fig. 4d.
The Spacia-derived PDL1 Signature in CD8+ T Cells Is Prognostic and Predictive
Next, we applied spacia to a set of pan-cancer MERSCOPE datasets, which include breast cancer, colon cancer, melanoma, lung cancer, liver cancer, ovarian cancer, prostate cancer, and uterine cancer (Sup. File 2). Here, we still focused on tumor cell-to-CD8+ T cell interactions. Spacia yields a set of downstream target genes for tumor-to-CD8+ T cell interactions in each cancer type, which we term the CD8-PDL1 signatures (Sup. Table 4). We first validated these gene signatures, by leveraging the RNA-seq data from The Cancer Genome Atlas Program (TCGA), for the same eight cancer types. We used BayesPrism 37 to dissect the TCGA RNA-seq data into cell type-specific gene expression in each patient sample. We investigated the differential gene expression in the CD8+ T cell components in the TCGA patients, between patients with high and low PDL1 expression in their tumor cell components. Gene Set Enrichment Analysis (GSEA) 42 confirmed that the CD8-PDL1 gene signatures identified by spacia are indeed enriched in the top differentially expressed genes. Fig. 4f showcases the GSEA results for breast cancer (Pval=0.0007) and ovarian cancer (Pval=0.025). We also calculated a composite score as the weighted average of the genes in each CD8-PDL1 signature, with weights being the inferred βs by spacia, to reflect the overall impact of PDL1 signaling on CD8+ T cells. For almost all cancer types, patients with higher PDL1 expression in the tumor cells tend to have higher expression of the CD8-PDL1 signatures (Fig. 4g, Pval(liver cancer)=0.012, Pval(lung cancer)=0.69, and P values for all other cancer types <0.002). Overall, these results validate the CD8-PDL1 signatures inferred by spacia.
We evaluated the prognostic and predictive powers of the CD8-PDL1 signatures to determine whether they possess any translational value. These signatures can potentially reflect the actual impact of tumor cells’ PDL1 signaling on T cells, which is a more direct measurement of PDL1 signaling effectiveness and could reveal stronger biological signals compared with testing tumor PDL1 expression alone. First, we tested the association between bulk tumor PDL1 expression and patient overall survival for the eight cancer types of interest in the TCGA patients (Sup. Fig. 6). Somewhat counterintuitively, we observed that higher PDL1 expression tends to predict better survival in these patients (Pval=0.016, all patients combined). One might expect higher PDL1 expression leads to worse survival as it is known to suppress anti-tumor immune functions. However, inflammatory T-cell responses could incite tumor metastasis 43, thus PDL1 can alleviate the risk for metastasis through the inhibition of inflammation. While the underlying biological mechanisms of this effect are not within the scope of this work, we assessed whether the CD8-PDL1 signatures in CD8+ T cells better capture this phenomenon.
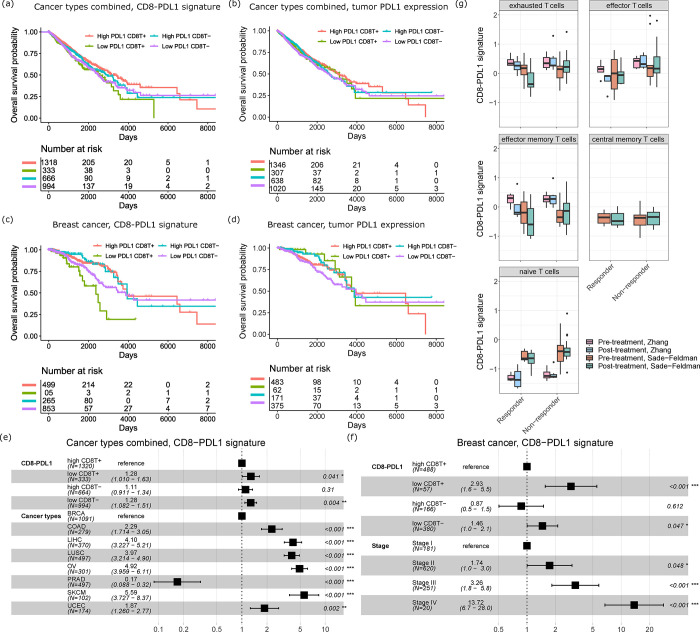
We performed Kaplan-Meier analyses for the CD8-PDL1 signatures in CD8+ T cells in all cancer types combined (Fig. 5a), and also for PDL1 expression in the tumor samples as control (Fig. 5b). Here, we also subset the patients into patients with high and low CD8+ T cell infiltrations, based on the CD8+ T cell proportions from BayesPrism. Inspired by our prior observations 44,45, we posit that the correlation between CD8-PDL1/PDL1 and survival should be stronger when the tumors have more infiltrating CD8+ T cells (otherwise, PDL1 signaling is irrelevant as there are few T cells to inhibit). For all cancer types combined, we found that high CD8-PDL1 signature expression indeed predicts better survival in the CD8+ T cell-high patients (Pval=0.004, OR=0.714, 95% CI=0.564–0.902), whereas there is no significant difference for the CD8+ T cell-low patients (Pval=0.34, OR=0.911, 95% CI=0.750–1.11) (Fig. 5a). On the other hand, the expression level of PDL1 in tumor cells is less prognostic in both patient subsets (CD8+ T cell-high: Pval=0.26, OR=0.862, 95% CI=0.667–1.11; CD8+ T cell-low: Pval=0.16, OR=0.866, 95% CI=0.710–1.05) (Fig. 5b). We next limited our analyses to breast cancer only, and again confirmed that the CD8-PDL1 signature is a more robust biomarker than tumor PDL1 expression for depicting the effect of PDL1 signaling on patient survival (Fig. 5c: CD8-PDL1 signature, CD8+ T cell-high: Pval=0.0002, OR=0.337, 95% CI=0.190–0.597, CD8+ T cell-low: Pval=0.08, OR=0.627, 95% CI=0.371–1.06; Fig. 5d: tumor PDL1 expression, CD8+ T cell-high: Pval=0.46, OR=1.35, 95% CI=0.616–2.94, CD8+ T cell-low: Pval=0.06, OR=0.593, 95% CI=0.347–1.01). We performed multivariate CoxPH analyses to adjust for the effect of confounding clinical covariates. In the pan-cancer cohort, the CD8-PDL1 signature is still significantly predictive of survival in the high CD8+ T cell patients after adjusting for the covariate of different cancer types (Fig. 5e, Pval = 0.041, Hazard Ratio=1.28, 95% CI=1.01–1.63). In breast cancer, the CD8-PDL1 signature is also still significantly prognostic in the high CD8+ T cell patients after adjusting for cancer stage (Fig. 5f, Pval < 0.0001, Hazard Ratio=2.93, 95% CI=1.6–5.5).
Fig. 5.
The PDL1-CD8 signature is prognostic and predictive. (a) Prognostic value of the PDL1-CD8 signature for overall survival of TCGA patients of all eight cancer types. (b) Prognostic value of tumor PDL1 expression for overall survival of TCGA patients of all eight cancer types. (c) Prognostic value of the PDL1-CD8 signature for overall survival of TCGA breast cancer patients. (d) Prognostic value of tumor PDL1 expression for overall survival of TCGA breast cancer patients. (e) Forest plot of hazard ratios from a Cox proportional hazard (CoxPH) model considering cancer types as confounding variables for patients of all eight cancer types. 95% CI is indicated by the whiskers. (f) Forest plot of hazard ratios from a CoxPH model considering cancer stages as confounding variables for breast cancer patients. 95% CI is indicated by the whiskers. (g) The CD8-PDL1 signature expression levels of CD8+ T cells in responders/non-responders and in pre-/post-treatment samples from the Zhang et al study and Sade–Feldman et al study.
Next, we also investigated patients who were treated with anti-PD1/PDL1 therapies. We studied two anti-PD1/PDL1-treated cohorts, Sade–Feldman et al 46, which consists of 32 scRNA-seq datasets generated from peripheral blood of melanoma patients on anti-PD1 or anti-CTLA4+PD1 treatment, and Zhang et al 47, which consists of 11 scRNA-seq datasets generated from various tissue biopsies of breast cancer patients on anti-PDL1 treatment. Both cohorts contained responders and non-responders (as defined in the original works), and the biopsies were collected before and after treatment for scRNA-seq. We performed cell typing for the scRNA-seq data. For the CD8+ T cells in particular, we further classified them into exhausted, effector, effector memory, central memory, and naive T cells, according to marker genes from Sun et al 48 in order to conduct more fine-grained analyses. We observed that exhausted T cells, effector memory T cells, and effector T cells have higher overall expression levels of the CD8-PDL1 signature, with exhausted T cells having the highest expression (Fig. 5g). This is expected as the CD8-PDL1 signature measures the immuno-supppressive effect of PDL1 signaling in T cells. In responders, we observed that the CD8-PDL1 signature significantly decreased after treatment in exhausted (Pval=0.005, testing both cohort together) and effector memory T cells (Pval=0.048), while these changes were not observed in non-responders (Pval=0.23 and 0.61 respectively). This contrast indicates that the CD8-PDL1 signature is a candidate biomarker for measuring the effectiveness of immunotherapies that block the PD1/PDL1 axis.
Taken together, the CD8-PDL1 signatures that we defined from SRT data using spacia possess both prognostic and predictive values, which demonstrates the power of these new technologies for yielding novel insights of translational value.
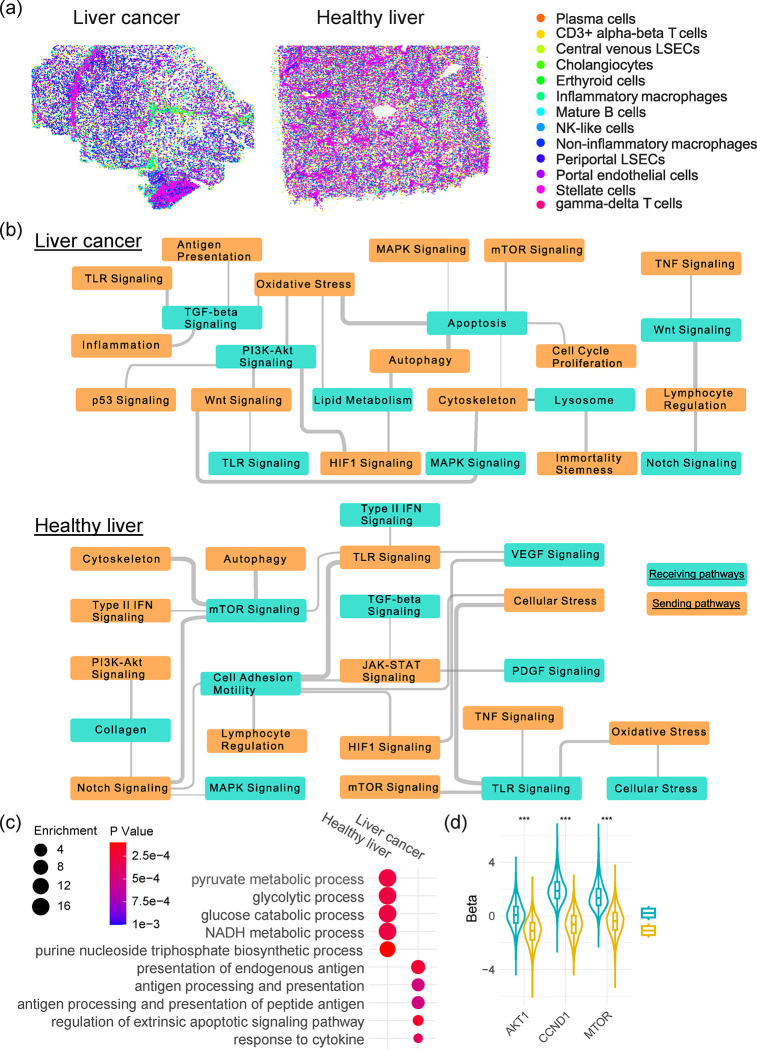
Differential Roles of γδ T Cells in Healthy Livers and Liver Cancers
We next applied spacia on two CosMX datasets, one generated from a healthy liver biospecimen and another one from a liver cancer biospecimen (Fig. 6a and Sup. Fig. 7a). We focused on a special class of T cells, γδ T cells, and characterized how these T cells impacted the transcriptomics programs of normal hepatocytes and malignant liver cancer cells. While γδ T cells are a minor class of T cells compared with αβ T cells, their abundance is much higher in the liver49,50. However, the exact roles of γδ T cells in the liver are not clear yet. In Sup. Fig. 7b, we first showed that γδ T cells surrounding hepatocytes have a generally much lower probabilities of truly interacting with the hepatocytes, while γδ T cells surrounding tumor cells have a much higher probabilities of interactions (T test Pval<1E-10). We also visualized the spatial distances between γδ T cells and their nearest healthy/cancerous hepatocytes (Sup. Fig. 7c, T test Pval=1.32E-5) and again observed that the distances are much smaller in the liver cancer specimen. These results suggested that γδ T cells likely have a different mode of action in the tumor context, as opposed to healthy livers.
Fig. 6.
Spacia infers differential roles of γδ T cells in healthy livers and liver cancers. (a) The spatial distribution of stromal/immune cells in the liver cancer and healthy liver CosMx datasets. (b) The significant interactions between sending pathways in γδ T cells and receiving pathways in healthy/cancerous hepatocytes. Wider gray lines denote the more significant interactions. (c) Significant pathways from GO analyses of top receiving genes in healthy and cancerous hepatocytes, with SQSTM1 in γδ T cells as the sending gene. (d) Heatmap showing the βs between the SQSTM1 sending gene in γδ T and the MTOR/AKT1/CCND1 receiving genes in healthy and cancerous hepatocytes. A positive β denotes a positive regulation relationship.
In Fig. 6b, we showcased the interactions that are active in each of the two samples at the pathway level, based on the recommended pathways by CosMX for these datasets (nanostring.com/resources/cosmx-smi-universal-cell-characterization-panel-gene-list), and found that the molecular pathways that are involved in the interactions between γδ T cells and healthy/cancerous hepatocytes are overall very different in the two contexts. For example, on the receiving side, apoptosis pathway shows up in the liver cancer cells, which reflects the cytotoxic role of γδ T cells. We also observed that the regulation of lipid metabolism pathway being impacted in the liver cancer cells. This might reflect an active modulation of the liver cancer cells, in response to the cytotoxic effect of γδ T cells, which recognize and are activated by lipid antigens 51,52. For the healthy liver, we observed the regulation of mTOR pathway in the hepatocytes, which has been linked with hepatocyte homeostasis and metabolism 53. This observation also resonates with the regulation of TGFβ/VEGF/PDGF pathways as shown in Fig. 6b, which are all important for hepatocyte homeostasis 54. On the sending side, we observed that the autophagy of γδ T cells impacts both the apoptosis of liver cancer cells and the mTOR pathway of healthy hepatocytes. As autophagy (especially selective autophagy) has emerged as a crucial regulator of T cell homeostasis, activation and differentiation55, our observations here seem to indicate that the homeostasis of γδ T cells is in a dynamic balance with the homeostasis of liver hepatocytes and impacts liver hepatocytes differently in different biological contexts.
We then examined individual genes and focused on SQSTM1, which is captured by the CosMx platform and is an important marker for selective autophagy 56. We examined the hepatocyte genes regulated by SQSTM1 of γδ T cells and rank-ordered by the magnitude of regulation (βs). Through Gene Ontology (GO) analyses, we confirmed again that the top pathways enriched in healthy livers are almost all related to metabolism (Fig. 6c), while the top pathways enriched in liver cancer cells are enriched with apoptosis and antigen presentation, echoing our spacia analysis at the pathway level. We further examined MTOR and AKT1, in the mTOR pathway, as well as CCND1, which is a key gene that regulates hepatocyte homeostasis downstream of the mTOR pathway 53. (PI3K is not captured in the CosMx gene panel.) While SQSTM1 in γδ T cells positively up-regulates MTOR, ATK1, and especially CCND1 in healthy liver, we observed an opposite trend in liver cancer cells, as shown in Fig. 6d. The βs are statistically significantly different between the healthy liver and liver cancer conditions, for each of the three genes (K.S. Test, Pval<1E-10). Taken together, spacia analysis revealed that γδ T cell homeostasis participates in very different biological processes through their interactions with hepatocytes in the healthy and cancerous contexts.
DISCUSSION
We developed spacia to fulfill the unmet gap in detection of cell-to-cell and gene-to-gene interactions from SRT data. Our work has five major conceptual advantages: (1) spacia achieves single cell resolution detection of CCCs, (2) spacia is not limited to prior interaction datasets and (3) not limited to ligand-receptor relationships, (4) spacia addressed the high false positive rate and low specificity issues commonly seen in prior works, and (5) spacia explicitly infers the multiple-sender-to-one-receiver relationships in SRT data through a novel Bayesian multi-instance learning framework. We proved that the quality of modern commercialized single cell resolution SRT technologies already enables the detection of CCCs with appropriate statistical models like spacia, ensuring the broad applicability of our work. We comprehensively tested spacia on simulation data and real data from diverse biological conditions, and very importantly on data from all three single cell resolution SRT technologies: MERSCOPE, CosMX, and Xenium. Practically, spacia can be executable on such SRT data that yield >50,000 cells, while we have to aggressively down-sample the number of cells to execute the other SRT-based CCC inference tools that we benchmarked to avoid memory overflow.
Whereas most existing CCC inference tools work by examining data against a known database, spacia focuses on the more general concept of CCC through accurate in situ modeling. Spacia achieves this through organically integrating the rich spatial and transcriptional information, which allows it to minimize the number of assumptions and arbitrary parameters. While this approach does not rely on existing knowledge, it offers an unbiased, independent interrogation based on first principles. Furthermore, it enables discoveries that complete known CCC pathways and uncover new pathways in a cellular context specific manner. It is important to note that the target genes identified by spacia, through modeling of transcriptional regulation as a function of sending gene expression, could be either direct targets that are within the core of the interacting pathways or represent more downstream and secondary effects. This could be an advantage or disadvantage depending on one’s vantage point. At this time, cross-referencing to existing databases could be helpful for the interpretation of the identified CCCs and distinguishing between “direct target” vs. “indirect target”, and allows for further interrogation of the regulatory relationships between genes in existing pathways. Therefore, we propose a paradigm of unbiased search of CCCs followed by filtering through prior knowledge in spacia, in contrast to those of prior works, such as CellPhoneDB, whose performance is limited to prior knowledge from the very beginning.
The core of spacia is a fully integrated Bayesian MIL model. Due to the complexity of learning the multiple-to-one functions, MIL problems are much more difficult than typical machine learning problems. Spacia’s MIL model allows solving this difficult mathematical problem in a graceful manner. Whereas existing MIL methods mainly focus on predictive performance 57–59, our two-tier MIL approach enables concurrent identification of primary instances (the sender cells responsible for the reaction in the receiver cell) and elucidation of the relationships between bags and instances (βs). This technical innovation is important for both the fields of MIL in general and the specific application of finding CCC in SRT data. Critically, in the era of data explosion in biomedical research, MIL can offer elegant solutions to disentangle complex interactions and large, diverse data from various fields. For example, in biochemistry, one might be interested in how the many conformations of a chemical compound are related to its bioactivity 60. We envision that our work will propel the wide adoption of MIL in biomedical research, by providing a success case and also by providing a model that can be further improved upon.
Admittedly, one major limitation associated with Bayesian MIL model is that spacia’s runtime quickly increases with increasing sample sizes, namely numbers of bags, instances and gene features, to attain convergence. Our current spacia already leverages C++ programming to enhance the computational efficiency, implemented by the Rcpp package in the R language. In our future iterations, we will deploy mean-field variational inference to approximate the joint posterior and to further enhance computational efficiency, where our Bayesian formulation will allow a close-form solution in each step of the mean-field algorithm.
Despite the exciting results presented in our study, mapping CCC is still challenging. Perhaps one of the biggest challenges is associated with the low signal-to-noise ratios (SNRs) of the SRT data. The inaccuracy in cell segmentation is a major contributor to this low SNR. Most, if not all, high-resolution SRT technologies generate the raw expression counts at subcellular or even pixel levels. To aggregate such data to single cell levels, cell boundaries are usually created from the matching H&E staining or fluorescence images via segmentation techniques. However, improper cell segmentation can lead to serious errors. For example, two cells that are close by (potentially two cells that are interacting) can be segmented as the same cell. In this scenario, two genes that are interacting with each other from two different cells can be mistaken as a pair of genes that are transcriptionally linked within the same cell. However, the field has seen continuous improvement in the cell segmentation procedure and other pre-processing procedures of SRT data, either in academically generated tools 61–64 or commercially available software. We expect such caveats to become less pronounced with future iterations of SRT technologies and the corresponding bioinformatics analysis software.
As discussed above, we recommend SRT users to visually assess the image segmentation quality to determine if the cell types of interest have been accurately segmented out. In addition, we suggest users to deploy de-noising software, such as Sprod 62, to increase the SNRs of the expression data from SRTs, and use dimension-reduction/visualization tools 65–67 to assess the overall clustering quality of the cells.
In summary, we built a general and principled framework for the analysis of CCCs from SRT data that can be applied to a vast number of biological systems. More broadly, when coupled with remarkable experimental and analytical advances in single cell and spatial approaches 68–77, spacia will enable us to understand how complex cell states arise from communications in the local cellular community and to move towards holistic models and theories of entire organisms.
METHODS
Simulation data creation
Simulated datasets were generated with the following steps. First, 8,000 cells were generated in a two-dimensional space of 2,000 × 2,000 units. These cells were classified into three types, with the receivers forming the core of several blobs, senders lining the perimeters, and non-interacting cells filling the space between blobs. Senders were divided into two categories: primary senders and non-primary senders based on their distances to a given receiver (distance cutoff = 50). Next, we simulated expression data (50 genes) associated with sender and receiver cells. Expression of each gene of the sender cells was generated with normal distribution (mean = 0, s.d. = 1). The first 5 or 10 genes (in two simulation settings) were designated as truly interacting genes, in two different settings, while the remaining genes were designated as non-interacting genes. Expression for receivers was generated as a weighted sum of gene expression from their primary senders, with weights (βs) generated from uniform distributions. The uniform distribution ranges from 10 to 20 for the truly interacting genes and 0 to 1 for the other genes. The signs of these weights were randomly assigned to be positive or negative to model upregulation and downregulation of receiving genes by sending genes. Lastly, we added noise to the receiver genes, with noises sampled from a normal distribution (mean = 0, s.d. = 0.5).
MERSCOPE data pre-processing and annotation
The pan-cancer MERSCOPE datasets consisting of 8 different cancer types were downloaded from the publically available MERFISH FFPE Human Immuno-oncology Data Set. The R Seurat package was used to load the “cell_by_gene.csv” file for each MERSCOPE experiment to create a Seurat object for downstream processing and analysis. To ensure we only retain high-quality cells, each experiment was subset so that cells with more than 100 total counts were kept. After the initial clustering and annotation, we subset the T cell population and the epithelial population and performed a second round of the standard workflow for each population to identify the fine-grained clusters for the T cell subpopulations and to segregate epithelial cells into tumor epithelial cells and normal epithelial cells. The final version of the annotated clusters in the UMAP space and the average gene expression of cell type markers in each cluster can be found in Sup. File 2.
Benchmarking with existing CCC software
CellPhoneDB: Normalized counts and cell type labels were converted into CellPhoneDB compatible TXT files. A microenvironments file was generated to limit the predictions to stromal-tumor interactions only. CellphoneDB 3.1.0 was run using method statistical_analysis, options --counts-data hgnc_symbol, --pvalue 1, and --threads 24 options. Since CellPhoneDB does not designate sending and receiving cells, they were defined by which of the interacting genes was labeled as receptor. For each interaction, the cell type with the gene labeled as receptor was designated as the receiving cell. Interactions where both or neither genes were labeled as receptor were discarded due to ambiguity of the direction of interaction. For consistency with spacia results, results with smooth muscle and normal epithelial cells as sending cells were removed, and only interactions with tumor cells as the receiving cell were kept. Interactions with Pval > 0.05 were removed.
CellChat: CellChat 1.5.0 was used with the default CellChatDB.human ligand-receptor interaction database. Functions identifyOverExpressedGenes and identifyOverExpressedInteractions were run with default options; computeCommunProb was run with type = “truncatedMean” and trim = 0.05; filterCommunication used min.cells = 10, and computeCommunProbPathway, aggregateNet, and subsetCommunication were all run with default options. For the outputs, “source” was considered equivalent to spacia’s sending cell, “target” equal to receiving cell, “ligand” as sending gene, and “receptor” as receiving gene. For consistency, results with smooth muscle and normal epithelial cells as sending cells were removed, and only interactions with tumor cells as the receiving cell were kept. Interactions with Pval > 0.05 were removed.
COMMOT: COMMOT version 0.0.3 was used in the analysis. The function commot.pp.ligand_receptor_database was run with options database=CellPhoneDB_v4.0, and species=human to generate the ligand-receptor dataframe. Function commot.tl.spatial_communication was run with option database_name=cpdb, dis_thr=500, heteromeric=True, and pathway_sum=True to infer spatial communication. Results were filtered by sending and receiving cell types to match that of spacia, and cell type level results were generated using the funtion commot.tl.cluster_communication with option database_name=cpdb. For visualization, commot.pl.plot_cell_communication was run with options: database_name=cpdb, plot_method=cell, scale=0.03, ndsize=10, background=cluster, background_legend=True, cmap=Alphabet, and clustering=cell type labels.
SpatialDM: SpatialDM version 0.1.0 was used. Weight matrix was calculated using sdm.weight_matrix with options l=50, cutoff=0.2, and single_cell=True. The value for l was found according to the method described in the authors’ tutorial. Following the tutorial, sdm.extract_lr was run with species=human, and min_cell=3, then sdm.spatialdm_global was run using method=both, and sdm.sig_pairs with method=permutation. Local results were calculated by running functions sdm.spatialdm_local with option method=both, and sdm.sig_spots with options method=permutation and fdr=False.
SpaTalk: SpaTalk version 1.0 was used. The function createSpaTalk was run with options species=Human, if_st_is_sc=T, and spot_max_cell=1 to generate the SpaTalk object. For the analysis, first the function find_lr_path was run using the default ligand-receptor pairs and pathways, then function dec_cci was run using the same sending and receiving cell types as those in the spacia analysis.
Validation with PDMR data
Bulk prostate cancer PDX RNA-sequencing data were downloaded from The NCI Patient-Derived Models Repository (PDMR), NCI-Frederick, Frederick National Laboratory for Cancer Research, Frederick, MD (pdmr.cancer.gov). Disambiguate 19 was used to segregate the PDX RNA-seq data into the human and mouse components. CIBERSORTx was used to further deconvolute the mouse stromal/immune component into cell type specific expression values. The CIBERSORTx web portal (cibersortx.stanford.edu) was used and the signature matrix for CIBERSORTx was created on the web portal using the expression matrix and cell typing results from the prostate cancer MERSCOPE data. To comply with the computational limits of the web server, for cell types with large numbers of cells, 5,000 cells were randomly sampled to create the reference sample file. CIBERSORTx was then run in high resolution mode for the PDMR data using default options.
EMT process calculation in the prostate MERSCOPE dataset
We define the “EMT activation potential” scores as the sum of the strengths of EMT-induction signals from nearby sender cells for each tumor cell. This is calculated with the following steps. The β values from the sender-to-tumor cells spacia results are filtered to keep those with sending genes included in a list of secreted factors that have been reported to impact EMT (HGF, WNT3A, WNT5A, FGF2, IL6, CXCL8, FGF1, TGFB1, and TGFB2), and receiving genes being EMT upstream regulators that have some prior evidence of being impacted by these factors (JAK1, AKT2, SMO, CTNNB1, SMAD2, and NFKB2) 25–33. For each sender cell and each sending gene, a set of scores are calculated by multiplying the β of each sending gene-receiving gene pair with the corresponding expression of the sending gene. These scores, one for each receiving gene, are averaged. The averaged scores for all sending genes are further averaged to arrive at a composite score to be assigned as the EMT activation potential of this sender cell. Finally, an EMT activation potential score for each tumor cell is computed by the weighted sum of the EMT activation potentials of the sender cells in each tumor cell bag with the weights being the primary instance probabilities of the senders.
The “EMT score” of a tumor cell is defined to be the mean expression of the EMT marker genes from Gorgola et al 78: FN1, TWIST1, SNAI1, SNAI2, ZEB1, TGFB1, TGFB2, and CTNNB1, which represents the activity level of EMT in that tumor cell. We performed additional analyses to validate that this EMT score is valid in our scRNA-seq datasets (Sup. File 2).
Lineage score calculation in the prostate cancer MERSCOPE dataset
We collected gene markers from Deng et al 36 to calculate the lineage plasticity scores for the basal, neuro-endocrine and stem-like lineages. We were not able to investigate the luminal lineage, as the most important canonical markers such as AR and KLK3 were missing. For the basal lineage, TP63, CAV1, and LAMB3 were used. For the neuro-endocrine lineage, EZH2 and NCAM1 were used. For the stem-like lineage, KIT, LGR5, and LGR6 were used.
Prostate patients for scRNA-seq
The study was performed following protocols approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. There are two studies from which we obtained patient biopsies. STU 072010–098: Tissue Procurement and Outcome Collection for Radiotherapy Treated Patients & Healthy Participants, and STU062014–027: Phase I Clinical Trial of Stereotactic Ablative Radiotherapy (SABR) of Pelvis and Prostate Targets for Patients with High Risk Prostate Cancer. We obtained a total of 21 androgen deprivation (ADT)-treated patients, 4 untreated prostate cancer patients, and 4 healthy donors. The scRNA-seq experiments were done in Dr. Douglas Strand’s lab following the protocol in Henry et al 79. Briefly, we performed a 1 hour digestion with 5mg/ml collagenase type I, 10mM ROCK inhibitor, and 1mg DNase. 3’ GEX barcoding was performed on a 10X controller and sequencing was performed on an Illumina NextSeq 500 sequencer. These 29 scRNA-seq datasets were processed through QC, integration, and cluster annotation prior to the analysis. We utilized the R DoubletFinder package and followed the recommended workflow for filtering the doublets or multiplets in the data. For the joint analysis of multiple patient scRNA-seq datasets, we followed the Seurat integration vignette. Each dataset was processed through the ScaleData and RunPCA functions. The anchors for integration were found with the “rpca” mode of the FindIntegrationAnchors function. Utilizing these anchors, we proceeded with the IntegrateData function for the integration of the 29 scRNA datasets. Initially, the clusters were automatically annotated with the R SingleR package and the annotated dataset from Song et al 80.
(1) The patients were dichotomized into EMT+ and EMT− groups with the following steps. First, the EMT level of each tumor cell is defined as above in the prostate cancer MERSCOPE dataset. The global median EMT level is calculated from all the tumor cells. Then, for each patient, the percentage of tumor cells with higher EMT levels than the global median cutoff is calculated. The patients were divided into a EMT+ group and a EMT− group based on the median of these percentages. (2) The calculation of the EMT activation potential in the scRNA-seq data follows the same method as in the MERSCOPE dataset, except for that we calculated the activation potential for each sender cell, and did not aggregate to receiver cells through spatial averaging (as these are not SRT data). (3) Finally, the lineage scores for basal, neuro-endocrine, and stem-like were calculated as in the MERSCOPE dataset, utilizing the complete marker set from Deng et al 36.
Breast cancer patients for GeoMx
The study was performed following protocols approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. The IRB Protocol Number is STU2018–0015: Pre-surgical trial of letrozole in post-menopausal patients with operable hormone-sensitive breast cancer. Formalin-fixed, paraffin-embedded (FFPE) tumor tissues were obtained from diagnostic core needle biopsies from postmenopausal patients with stage I to stage III operable ER+/HER2− breast cancer enrolled in a clinical trial (UT Southwestern SCCC-11118).
To perform the GeoMx® assays, 5 μm FFPE sections were mounted on charged slides, baked, and prepared on the Leica Biosystem, following the manufacturer’s automated slide preparation user manual. After hybridization with RNA probes that are conjugated to barcoded oligonucleotide tags with an ultraviolet (UV) photocleavable linker and staining with fluorescent morphology markers consisting of pan-cytokeratin (epithelial and tumoral regions), CD45 (immune cells), SYTO13 (nuclear) and Ki67 (proliferation marker), the slides were loaded onto a GeoMx® digital spatial profiler (DSP, NanoString Technologies) and scanned. After labeling, the tissues were imaged, and we selected specific regions of interest (ROIs) sized 222 × 354.6 μm after consultation with a pathologist. Each ROI was further segmented into Areas of Illumination (AOI) based on morphological features. Subsequently, the selected areas were exposed to UV light, and the barcoded oligos were released, aspirated, and dispensed into a collection plate for library construction for next-generation sequencing (NGS).
GeoMx NGS libraries were prepared according to the manufacturer’s instructions. Briefly, after the collection of the probes was completed, aspirates in the collection plate were dried at 65°C for 1 hour in a thermal cycler with an open lid and resuspended in 10 μL of nuclease-free water. 4 μL of rehydrated aspirates were mixed with 2 μL of 5×PCR Master Mix and 4 μL of SeqCode primers. PCR amplification was then performed with 18 cycles. The indexed libraries were pooled equally and purified twice with 1.2×AMPure XP beads (Beckman Coulter). The final libraries were evaluated and quantified using Agilent’s High Sensitivity DNA Kit and Invitrogen’s Qubit dsDNA HS assay, respectively. Total sequencing reads per DSP collection plate were calculated using the NanoString DSP Worksheet. The libraries were sequenced using 38 bp paired-end sequencing (PE 38) on an Illumina NovaSeq 6000 system with a 100-cycle S1 kit (v1.5). FASTQ files were processed into digital count conversion digital files using Nanostring’s GeoMx NGS Pipeline software. Quality control, data filtering and normalization (Q3) were performed using the GeoMx DSP Data Analysis suite.
BayesPrism bulk expression data deconvolution
The BayesPrism R package was installed according to its authors’ instructions at github.com/Danko-Lab/BayesPrism. The histology-matching MERSCOPE dataset was used as the single cell reference in order to maximize consistency with spacia’s results. BayesPrism was run using the run.prism function with default options. The cell type assignments we produced for the MERSCOPE datasets were used as the “cell.type.labels”. Cell type fractions were extracted using the get.fraction function. Predicted cell type-specific expression was extracted by running the get.exp function on each cell type.
The Cancer Genome Atlas Program (TCGA) RNA-seq data analysis
TCGA data of eight cancer types that are of the same histologies as the MERSCOPE data were downloaded from Broad GDAC Firehose. These include: BRCA, COAD, LIHC, LUSC, OV, PRAD, SKCM, and UCEC. We only considered primary tumor samples of the TCGA cohort since all MERSCOPE datasets are from primary tumor samples. As we expected, inclusion of the TCGA metastatic samples (data not shown) in all downstream analyses yields similar results but with less statistical strength. BayesPrism was then run with default options, using the corresponding MERSCOPE data as the reference.
To define the CD8-PDL1 signature for each cancer type, we extracted, from spacia’s results, all receiving genes with b < 0, b Pval < 0.01, and β Pval <0.1. The genes of each cancer type were further filtered to keep those that have appeared in the gene list from breast cancer and at least two other cancer types. This was done due to our promising analysis results in breast cancer in Fig. 4a–e. The genes that passed all filters were designated as the CD8-PDL1 signature for each cancer type. The R Fast Gene Set Enrichment Analysis (fgsea) package was used to perform GSEA using the CD8-PDL1 signatures for the TCGA BayesPrism-dissected expression data. GSEA was run using the fgsea function with options eps=0, minsize=10, and scoreType=“pos”. The results were plotted using the plotEnrichmentData function from the fgsea package and the R ggplot2 package.
TCGA patient survival analysis
The same TCGA datasets and CD8-PDL1 signature genes as above were used. For each patient sample in the TCGA datasets, the CD8-PDL1 signature expression level was calculated as the dot product of the normalized and log-transformed gene expression values of the BayesPrism-dissected CD8+ T cells and the β values of the genes in the corresponding CD8-PDL1 signature gene set. Survival analyses were performed using the survfit function from the R survival package with the CD8-PDL1 signature or tumor PDL1 as the covariate. The Kaplan-Meier curves were plotted using the ggsurvplot function from the R survminer package. For the forest plots, the coxph function from the R survival package was used to fit Cox proportional hazards regression models with CD8-PDL1 signature and cancer type or stage as covariates. The forest plots were then generated using the ggforest function from the survminer package.
Anti-PD1/PDL1 scRNA-seq data analyses
The Zhang et al 47 study originally identified naive (CD8Tn), effector (CD8Teff), and effector memory (CD8Tem) CD8+ T cell subsets in the patient peripheral blood, while the Sade–Feldman et al 46 study originally classified CD8Tem, central memory (CD8Tcm), exhausted (CD8Tex) subtypes. To homogenize classification and nomenclature, we loaded each dataset as a Seurat object to recluster and tried to identify all CD8+ T subtypes in both studies. Apart from the 20 dimensions used in FindNeighbors and FindClusters, all default parameters were used. Utilizing the gene marker sets from Sun et al 48, we annotated 28 clusters in the melanoma dataset and 13 clusters in the breast cancer dataset, compared to 4 and 6 clusters originally. All CD8+ T subtypes were identified, except for CD8Tcm in the Zhang et al dataset. The CD8-PDL1 signature score was calculated as in the TCGA data analysis, except that we have the actual CD8+ T cell gene expression in these scRNA-seq data, as opposed to BayesPrism-dissected expression for the TCGA samples.
Statistics & reproducibility
Computations were performed in the R (3.6.3 and 4.1.3) and Python (3.7 and 3.8) programming languages. All statistical tests were two-sided unless otherwise described. Spacia was run in the PCA mode (see our documentation) for all our analyses in this work. Unless otherwise stated, the spacia results were filtered to keep those interactions satisfying b < 0, b Pval<1×10E-30, and β Pval<0.01. For pre-processing of all SRT and scRNA-seq data, we loaded the original data into Seurat objects and analyzed them through a standard workflow, using NormalizeData, FindVariableFeatures, ScaleData, RunPCA, FindNeighbors, FindClusters, and RunUMAP functions, before other custom operations. We executed the pipeline with default parameters except for dims = 1:20 for FindNeighbors and RunUMAP in the prostate cancer scRNA-seq datasets to capture sufficient variability in the principal components. GOrilla 23,24 pathway enrichment analyses were performed by inputting the genes on the MERSCOPE gene panel into GOrilla, where the genes were filtered to keep only those that satisfy b < 0 and sorted by β values for each sending cell type (tumor cells are the receiving cells). The Kernel Density Estimation for the visualization of spatial EMT patterns was calculated from the gaussian_kde function from the SciPy package with bandwidth less than the silverman factor, to demonstrate robustness from the spatial noise.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Dr. Shijia Zhu for creating Fig. 1c.
FUNDING
This study was supported by the National Institutes of Health (NIH) [R01CA258584/TW, RC2DK129994/DS, R01DK115477/DS, R01DK135535/DS, R01CA222405/SZ, R01CA255064/SZ], Cancer Prevention Research Institute of Texas [RP230363/TW, RP190208/TW, RR170061/CA,RR220024/SZ], Dedman Family Scholars in Clinical Care [ND].
Footnotes
COMPETING INTEREST STATEMENT
Tao Wang is one of the scientific co-founders of NightStar Biotechnologies, Inc. Ariella Hanker receives or has received research grants from Takeda and Lilly and nonfinancial support from Puma Biotechnology and Tempus. Carlos Arteaga receives or has received research grants from Pfizer, Lilly, and Takeda; holds minor stock options in Provista; serves or has served in an advisory role to Novartis, Merck, Lilly, Daiichi Sankyo, Taiho Oncology, OrigiMed, Puma Biotechnology, Immunomedics, AstraZeneca, Arvinas, and Sanofi; and reports scientific advisory board remuneration from the Susan G. Komen Foundation.
Data and code availability statement
The MERSCOPE datasets were downloaded from vizgen.com/data-release-program. The breast cancer Xenium dataset was downloaded from www.10xgenomics.com/resources/datasets. The PDX RNA-seq datasets were downloaded from pdmdb.cancer.gov/web/apex/f?p=101:41:0:. The TCGA data were downloaded from gdac.broadinstitute.org. The scRNA-Seq datasets by Zhang et al 47 and Sade–Feldman et al 46 were accessed via Gene Expression Omnibus with accession numbers GSE169246 and GSE120575, respectively. The scRNA-Seq datasets by Bassez et al41 were accessed from biokey.lambrechtslab.org. The CosMx data are available from nanostring.com/products/cosmx-spatial-molecular-imager/ffpe-dataset/human-liver-rna-ffpe-dataset. The prostate cancer scRNA-seq data that we generated were archived at doi.org/10.5281/zenodo.8270765. The breast cancer GeoMx data that we generated were archived at github.com/yunguan-wang/Spacia/data. The spacia software is available at the Database for Actionable Immunology 76,81,82 (dbai.biohpc.swmed.edu, Tools page).
Bibliography
- 1.Bechtel T. J., Reyes-Robles T., Fadeyi O. O. & Oslund R. C. Strategies for monitoring cell-cell interactions. Nat. Chem. Biol. 17, 641–652 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Armingol E., Officer A., Harismendy O. & Lewis N. E. Deciphering cell-cell interactions and communication from gene expression. Nat. Rev. Genet. 22, 71–88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitrov D. et al. Comparison of methods and resources for cell-cell communication inference from single-cell RNA-Seq data. Nat. Commun. 13, 3224 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S. et al. A systematic evaluation of the computational tools for ligand-receptor-based cell-cell interaction inference. Brief. Funct. Genomics 21, 339–356 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browaeys R., Saelens W. & Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat. Methods 17, 159–162 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Efremova M., Vento-Tormo M., Teichmann S. A. & Vento-Tormo R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 15, 1484–1506 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Hou R., Denisenko E., Ong H. T., Ramilowski J. A. & Forrest A. R. R. Predicting cell-to-cell communication networks using NATMI. Nat. Commun. 11, 5011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabello-Aguilar S. et al. SingleCellSignalR: inference of intercellular networks from single-cell transcriptomics. Nucleic Acids Res. 48, e55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stickels R. R. et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat. Biotechnol. 39, 313–319 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriques S. G. et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y. et al. XYZeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci. Adv. 7, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho C.-S. et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell 184, 3559–3572.e22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vickovic S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao X. et al. Knowledge-graph-based cell-cell communication inference for spatially resolved transcriptomic data with SpaTalk. Nat. Commun. 13, 4429 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cang Z. et al. Screening cell-cell communication in spatial transcriptomics via collective optimal transport. Nat. Methods 20, 218–228 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z., Wang T., Liu P. & Huang Y. SpatialDM for rapid identification of spatially co-expressed ligand-receptor and revealing cell-cell communication patterns. Nat. Commun. 14, 3995 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T. et al. An empirical approach leveraging tumorgrafts to dissect the tumor microenvironment in renal cell carcinoma identifies missing link to prognostic inflammatory factors. Cancer Discov. 8, 1142–1155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahdesmäki M. J., Gray S. R., Johnson J. H. & Lai Z. Disambiguate: An open-source application for disambiguating two species in next generation sequencing data from grafted samples. [version 2; peer review: 3 approved]. F1000Res. 5, 2741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman A. M. et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 37, 773–782 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirino V. et al. TGF-β1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133+ A549 cell fraction. Cell Death Dis. 4, e620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ping Q. et al. TGF-β1 dominates stromal fibroblast-mediated EMT via the FAP/VCAN axis in bladder cancer cells. J. Transl. Med. 21, 475 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eden E., Navon R., Steinfeld I., Lipson D. & Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eden E., Lipson D., Yogev S. & Yakhini Z. Discovering motifs in ranked lists of DNA sequences. PLoS Comput. Biol. 3, e39 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai J. et al. Tumor-Associated Macrophages Derived TGF-β‒Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad2,3–4/Snail Signaling Pathway. Cancer Res. Treat. 51, 252–266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D. et al. M2-polarized tumor-associated macrophages promote epithelial-mesenchymal transition via activation of the AKT3/PRAS40 signaling pathway in intrahepatic cholangiocarcinoma. J. Cell. Biochem. 121, 2828–2838 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Zhang W. et al. Interaction with neutrophils promotes gastric cancer cell migration and invasion by inducing epithelial-mesenchymal transition. Oncol. Rep. 38, 2959–2966 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Qu J. et al. Mast cells induce epithelial-to-mesenchymal transition and migration in non-small cell lung cancer through IL-8/Wnt/β-catenin pathway. J. Cancer 10, 5567 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X. et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 8, 20741–20750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L. et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 8, 76116–76128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y. et al. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 110, 724–732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labelle M., Begum S. & Hynes R. O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigurdsson V. et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PLoS ONE 6, e23833 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav A., Kumar B., Datta J., Teknos T. N. & Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol. Cancer Res. 9, 1658–1667 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebbing E. A. et al. Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc Natl Acad Sci USA 116, 2237–2242 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng S. et al. Ectopic JAK-STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance. Nat. Cancer 3, 1071–1087 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu T., Wang Z., Pe’er D. & Danko C. G. Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat. Cancer 3, 505–517 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3, 697–707 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Wang Y. et al. GATA-3 controls the maintenance and proliferation of T cells downstream of TCR and cytokine signaling. Nat. Immunol. 14, 714–722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merlo L. M. F., Peng W. & Mandik-Nayak L. Impact of IDO1 and IDO2 on the B cell immune response. Front. Immunol. 13, 886225 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassez A. et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat. Med. 27, 820–832 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hibino S. et al. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu T. et al. Netie: inferring the evolution of neoantigen-T cell interactions in tumors. Nat. Methods 19, 1480–1489 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu T. et al. Tumor neoantigenicity assessment with CSiN score incorporates clonality and immunogenicity to predict immunotherapy outcomes. Sci. Immunol. 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sade–Feldman M. et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013.e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y. et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 39, 1578–1593.e8 (2021). [DOI] [PubMed] [Google Scholar]
- 48.Sun D. et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 49, D1420–D1430 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren H., Li W., Liu X. & Zhao N. γδ T cells: The potential role in liver disease and implications for cancer immunotherapy. J. Leukoc. Biol. 112, 1663–1668 (2022). [DOI] [PubMed] [Google Scholar]
- 50.Hou W. & Wu X. Diverse functions of γδ T cells in the progression of hepatitis B virus and hepatitis C virus infection. Front. Immunol. 11, 619872 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X. et al. Host-derived lipids orchestrate pulmonary γδ T cell response to provide early protection against influenza virus infection. Nat. Commun. 12, 1914 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribot J. C., Lopes N. & Silva-Santos B. γδ T cells in tissue physiology and surveillance. Nat. Rev. Immunol. 21, 221–232 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Wei Y. et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 371, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura M., Moteki H. & Ogihara M. Role of hepatocyte growth regulators in liver regeneration. Cells 12, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Botbol Y., Guerrero-Ros I. & Macian F. Key roles of autophagy in regulating T-cell function. Eur. J. Immunol. 46, 1326–1334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar A. V., Mills J. & Lapierre L. R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 10, 793328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong D., Zhang Z., Wang T. & Wang X. A comparative study of multiple instance learning methods for cancer detection using T-cell receptor sequences. Comput. Struct. Biotechnol. J. 19, 3255–3268 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y., Wang T., Xiong D., Wang X. & Park S. Multiple instance neural networks based on sparse attention for cancer detection using T-cell receptor sequences. BMC Bioinformatics 23, 469 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S. et al. Bayesian multiple instance regression for modeling immunogenic neoantigens. Stat. Methods Med. Res. 29, 3032–3047 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu G. et al. Implementation of multiple-instance learning in drug activity prediction. BMC Bioinformatics 13 Suppl 15, S3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stringer C., Wang T., Michaelos M. & Pachitariu M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods 18, 100–106 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Wang Y. et al. Sprod for de-noising spatially resolved transcriptomics data based on position and image information. Nat. Methods 19, 950–958 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rong R. et al. A Deep Learning Approach for Histology-Based Nucleus Segmentation and Tumor Microenvironment Characterization. Mod. Pathol. 36, 100196 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S., Yang D. M., Rong R., Zhan X. & Xiao G. Pathology image analysis using segmentation deep learning algorithms. Am. J. Pathol. 189, 1686–1698 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang K. et al. Comparative analysis of dimension reduction methods for cytometry by time-of-flight data. Nat. Commun. 14, 1836 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Maaten L. & Hinton G. Visualizing Data using t-SNE. Journal of Machine Learning Research 9, 2579–2605 (2008). [Google Scholar]
- 67.McInnes L., Healy J. & Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv (2018) doi: 10.48550/arxiv.1802.03426. [DOI] [Google Scholar]
- 68.Holler K. et al. Spatio-temporal mRNA tracking in the early zebrafish embryo. Nat. Commun. 12, 3358 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan M. M. et al. Molecular recording of mammalian embryogenesis. Nature 570, 77–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKenna A. et al. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu T. et al. Overcoming Expressional Drop-outs in Lineage Reconstruction from Single-Cell RNA-Sequencing Data. Cell Rep. 34, 108589 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Rodriques S. G. et al. RNA timestamps identify the age of single molecules in RNA sequencing. Nat. Biotechnol. 39, 320–325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheth R. U. & Wang H. H. DNA-based memory devices for recording cellular events. Nat. Rev. Genet. 19, 718–732 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adamson B. et al. A Multiplexed Single-Cell CRISPR Screening Platform Enables Systematic Dissection of the Unfolded Protein Response. Cell 167, 1867–1882.e21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dixit A. et al. Perturb-Seq: Dissecting Molecular Circuits with Scalable Single-Cell RNA Profiling of Pooled Genetic Screens. Cell 167, 1853–1866.e17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z., Xiong D., Wang X., Liu H. & Wang T. Mapping the functional landscape of T cell receptor repertoires by single-T cell transcriptomics. Nat. Methods 18, 92–99 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z. et al. Interpreting the B-cell receptor repertoire with single-cell gene expression using Benisse. Nat. Mach. Intell. (2022) doi: 10.1038/s42256-022-00492-6. [DOI] [Google Scholar]
- 78.Gogola S. et al. Epithelial-to-Mesenchymal Transition-Related Markers in Prostate Cancer: From Bench to Bedside. Cancers (Basel) 15, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henry G. H. et al. A cellular anatomy of the normal adult human prostate and prostatic urethra. Cell Rep. 25, 3530–3542.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song H. et al. Single-cell analysis of human primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states. Nat. Commun. 13, 141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu T. et al. Deep learning-based prediction of the T cell receptor-antigen binding specificity. Nat. Mach. Intell. 3, 864–875 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu J. et al. BepiTBR: T-B reciprocity enhances B cell epitope prediction. iScience 25, 103764 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MERSCOPE datasets were downloaded from vizgen.com/data-release-program. The breast cancer Xenium dataset was downloaded from www.10xgenomics.com/resources/datasets. The PDX RNA-seq datasets were downloaded from pdmdb.cancer.gov/web/apex/f?p=101:41:0:. The TCGA data were downloaded from gdac.broadinstitute.org. The scRNA-Seq datasets by Zhang et al 47 and Sade–Feldman et al 46 were accessed via Gene Expression Omnibus with accession numbers GSE169246 and GSE120575, respectively. The scRNA-Seq datasets by Bassez et al41 were accessed from biokey.lambrechtslab.org. The CosMx data are available from nanostring.com/products/cosmx-spatial-molecular-imager/ffpe-dataset/human-liver-rna-ffpe-dataset. The prostate cancer scRNA-seq data that we generated were archived at doi.org/10.5281/zenodo.8270765. The breast cancer GeoMx data that we generated were archived at github.com/yunguan-wang/Spacia/data. The spacia software is available at the Database for Actionable Immunology 76,81,82 (dbai.biohpc.swmed.edu, Tools page).