Abstract
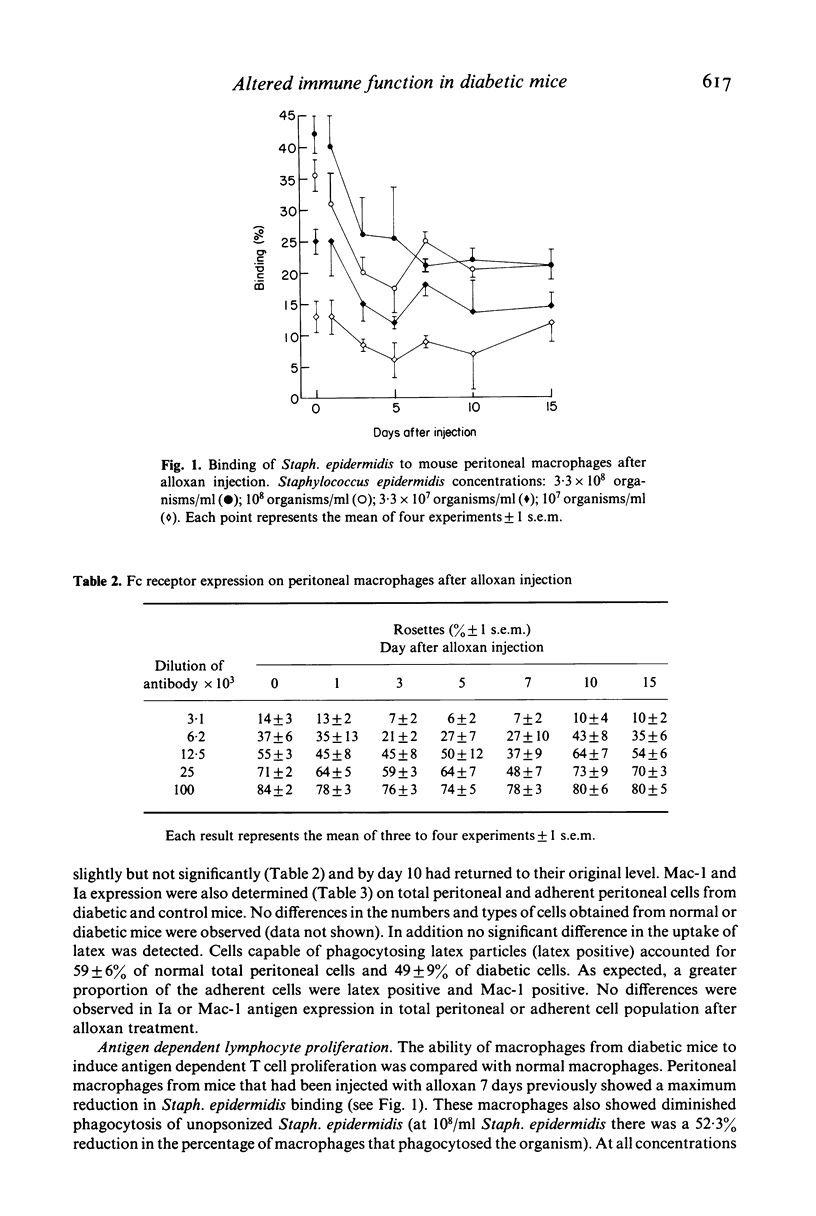
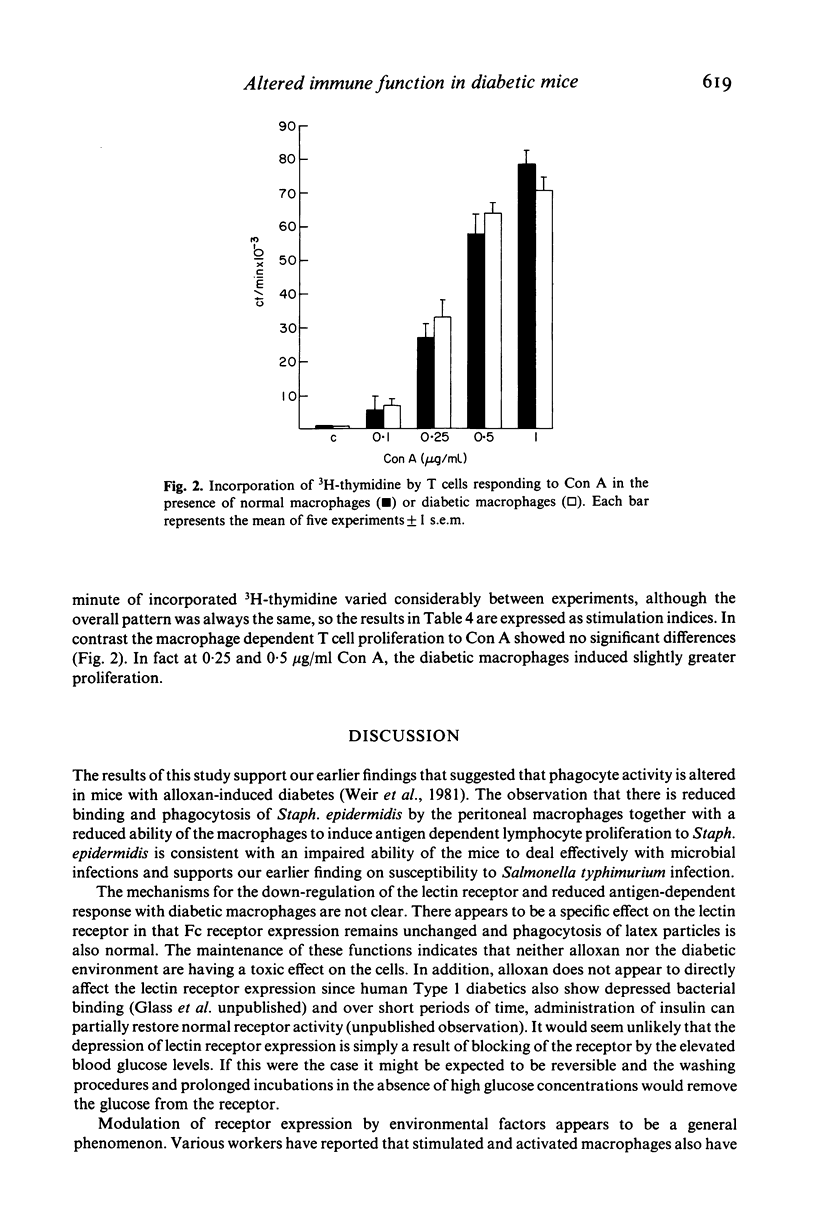
Macrophage lectin receptors that recognize bacterial cell wall sugars are reduced in alloxan diabetic mice. This is in contrast to the expression of macrophage Fc (IgG2b) receptors which remains unaltered. Peritoneal macrophages, from diabetic and normal mice, were used as a source of accessory cells in an antigen dependent T cell proliferation assay with unopsonized Staph. epidermidis as the antigen. Uptake of this antigen in the absence of serum is via the macrophage lectin receptors. We have shown that diabetic macrophages induce a level of antigen dependent T cell proliferation to Staph. epidermidis. However the T cell response to Con A was similar with both normal and diabetic macrophages. We suggest that the observed defect in antigen presentation by diabetic macrophages is at the level of uptake of antigen. High glucose levels, such as those found in diabetes, down-regulate the lectin receptor, reduce phagocytosis of Staph. epidermidis and affect antigen presentation. This has important consequences in terms of the ability of diabetics to mount an effective immune response.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann G. B., Sachs D. H., Hodes R. J. Requirement for an Ia-bearing accessory cell in Con A-induced T cell proliferation. J Immunol. 1978 Nov;121(5):1981–1989. [PubMed] [Google Scholar]
- Edwards M. S., Fuselier P. A. Enhanced susceptibility of mice with streptozotocin-induced diabetes to type II group B streptococcal infection. Infect Immun. 1983 Feb;39(2):580–585. doi: 10.1128/iai.39.2.580-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Austyn J., Stahl P. D., Gordon S. Surface properties of bacillus Calmette-Guérin-activated mouse macrophages. Reduced expression of mannose-specific endocytosis, Fc receptors, and antigen F4/80 accompanies induction of Ia. J Exp Med. 1981 Jul 1;154(1):60–76. doi: 10.1084/jem.154.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher-McGruder B. L., Gerritsen G. C. Deficient humoral antibody response of the spontaneously diabetic Chinese hamster. Proc Soc Exp Biol Med. 1984 Jan;175(1):74–78. doi: 10.3181/00379727-175-41769. [DOI] [PubMed] [Google Scholar]
- Ganda O. P. Morbidity and mortality from diabetes mellitus: a look at preventable aspects. Am J Public Health. 1983 Oct;73(10):1156–1158. doi: 10.2105/ajph.73.10.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass E. J., Stewart J., Weir D. M. Membrane changes in murine macrophages after in-vivo stimulation and activation. Immunology. 1983 Sep;50(1):165–173. [PMC free article] [PubMed] [Google Scholar]
- Glass E. J., Stewart J., Weir D. M. The influence of chemotactic factors on the expression of phagocyte receptors. J Clin Lab Immunol. 1982 Jan;7(1):39–41. [PubMed] [Google Scholar]
- Glass E., Stewart J., Weir D. M. Presence of bacterial binding 'lectin-like' receptors on phagocytes. Immunology. 1981 Nov;44(3):529–534. [PMC free article] [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V., Johnson W. J., Adams D. O. Selective diminution of the binding of mannose by murine macrophages in the late stages of activation. J Biol Chem. 1982 May 10;257(9):5129–5135. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Pasko K. L., Salvin S. B., Winkelstein A. Mechanisms in the in vivo release of lymphokines. V. Responses in alloxan-treated and genetically diabetic mice. Cell Immunol. 1981 Jul 15;62(1):205–219. doi: 10.1016/0008-8749(81)90314-2. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Lehrman M. A., Imber M. J., Guthrow C. E. The clearance of glycoproteins in diabetic mice. Biochem Biophys Res Commun. 1981 Jul 30;101(2):704–708. doi: 10.1016/0006-291x(81)91315-2. [DOI] [PubMed] [Google Scholar]
- Prud'homme G. J., Fuks A., Colle E., Seemayer T. A., Guttmann R. D. Immune dysfunction in diabetes-prone BB rats. Interleukin 2 production and other mitogen-induced responses are suppressed by activated macrophages. J Exp Med. 1984 Feb 1;159(2):463–478. doi: 10.1084/jem.159.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J., Glass E. J., Weir D. M. Macrophage binding of Staphylococcus albus is blocked by anti I-region alloantibody. Nature. 1982 Aug 26;298(5877):852–854. doi: 10.1038/298852a0. [DOI] [PubMed] [Google Scholar]
- Summerfield J. A., Vergalla J., Jones E. A. Modulation of a glycoprotein recognition system on rat hepatic endothelial cells by glucose and diabetes mellitus. J Clin Invest. 1982 Jun;69(6):1337–1347. doi: 10.1172/JCI110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiel J. E., Pizzo S. V. Down regulation of macrophage mannose/N-acetylglucosamine receptors by elevated glucose concentrations. Biochim Biophys Acta. 1983 Sep 13;759(3):170–175. doi: 10.1016/0304-4165(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Weir D. M., Blackwell C. C., McLean C. A. Impaired bacterial binding to peritoneal exudate cells from mice with alloxan induced diabetes. J Clin Lab Immunol. 1981 Jan;5(1):37–40. [PubMed] [Google Scholar]


