Abstract
The extracellular matrix (ECM) provides structural support for cells and mediates cell-stromal communications. In addition to ECM proteins, mechanical force exerted from the ECM serves as a critical regulator of many biological processes. Epithelial- mesenchymal transition (EMT) is a cellular process by which epithelial cells loosen their cellular junctions and migrate and invade in a more mesenchymal fashion. Recent studies show that increasing ECM stiffness can impinge on cellular signaling pathways through mechanotransduction to promote carcinoma cells to undergo EMT, suggesting that mechanical force exerted by the ECM plays a critical role in tumor invasion and metastasis. Here, we highlight recent work utilizing innovative approaches to study mechanotransduction and summarize newly discovered mechanisms by which mechano-sensors and responders regulate EMT during tumor progression and metastasis.
Introduction
Epithelial – Mesenchymal Transition (EMT) is the cellular process by which epithelial cells partially lose their characteristics, such as apical-basal polarity and epithelial junctions, to migrate and invade in a mesenchymal fashion. EMT is an essential process for tissue patterning and organization during embryonic development and is also implicated in cancer pathogenesis[1]. While numerous biochemical signals have been extensively studied for their roles in regulating EMT, recent advances revealed critical roles of mechanical properties and composition of the extracellular matrix (ECM) in regulating EMT[2]. The mechanical properties of the tumor microenvironment (TME) include stiffness, topology, shear force and in the case of cancer, pressure from the surrounding tissue architecture due to increased cell proliferation. These physical properties can regulate cell morphology, proliferation, differentiation, and migration[3]. Recently, several studies show that high tumor ECM stiffness can induce tumor cells to undergo EMT and invade through mechanotransduction, the process of sensing and transmitting mechanical stimulus from the microenvironment to generate biochemical responses[4], [5]. This review highlights important advances within the last couple of years in our understanding of mechanotransduction signaling pathways that induce EMT in response to mechanical properties of the tumor microenvironment.
New experimental models to investigate mechanotransduction and EMT
To study how physical properties of the tumor microenvironment drive tumor progression, new in vitro culture models have recently been developed to recapitulate the physical properties observed in human solid tumors. In the tumor microenvironment, cancer associated-fibroblasts (CAFs) can secret and modify collagens to stiffen the ECM, which directly impacts the metastatic potential of cancer cells. A mechanically tunable 3D interpenetrating network hydrogel culture platform was fabricated with a pre-formed type I collagen matrix interposed with a second alginate network to control stiffness, pore size and architecture in individual networks. Tuning the storage modulus of this hydrogel is shown to regulate the switch between inflammatory and myofibroblastic CAF states, which altered the EMT-inducing capacity of CAFs on colorectal adenocarcinoma cells[6]. To better mimic the stroma architecture and composition, Cao, H. et al. developed a self-assembly method by combining inflammatory CAFs (iCAFs) induced from stroma-derived normal fibroblasts and an urothelium overlay based on a bladder tissue model. Their study found that the iCAF- derived stiff ECM led to increased expression of mesenchymal markers in urothelial cells, similar to a tumor response.[7] Using an epithelial monolayer system, Lin et al. utilized fibronectin microcontact printing to generate circular epithelial monolayers of cancer cells with defined shapes and sizes to investigate tumor heterogeneity and the mechanotransduction pathways that regulate EMT in response to diverse biophysical properties. They found that at peripheral regions presented greater traction forces these cells underwent EMT, whereas cells in the central regions where there was less traction force presented a more epithelial phenotype. This spatial distribution of cells with different EMT properties in the micropatterned cell monolayer only became evident with high substrate stiffness and was not observed under conditions of low substrate rigidities[8]. Overall, creating in vitro models that recapitulate the tumor mechanical microenvironment is important to contextualize various EMT phenotypes observed in human tumors.
Computational models have also been developed to investigate EMT heterogeneity regulated by matrix stiffness. Sullivan, E. et al. created a Boolean computational model to examine the effects of mechano-signals on EMT and its crosstalk with mitogen signals. Cells at the edge of a monolayer maintain adherens junctions with neighboring cells but cannot establish apical-basal polarity, thus these cells respond to stiff ECM exposure by undergoing partial EMT, as predicted by this model[9]. This partial EMT state, in which a cell acquires a mesenchymal phenotype while maintaining some epithelial markers, is functionally linked to cancer migration and invasion. In support of this model, Wei, S. et al. showed experimentally that cells cultured on a high matrix stiffness presented a partial EMT phenotype[10]. Additionally, to study how the viscoelastic properties of the matrix regulate cell spatial and temporal organization, Mooney, D. et al. utilized alginate hydrogels crosslinked to RGD-containing peptides to alter the viscoelastic properties of the matrix independent of its stiffness. Experimental observations together with computational modeling of cell behavior in this system supported the model that matrix viscoelasticity determines symmetry breaking of the tissue spheroid, leading to EMT[11]. In summary, recent research progress demonstrates that combining experimental analyses and computational modeling are fruitful to recapitulate and dissect mechanotransduction regulation of EMT observed in in vivo tumors.
Mechanical forces in cancer EMT
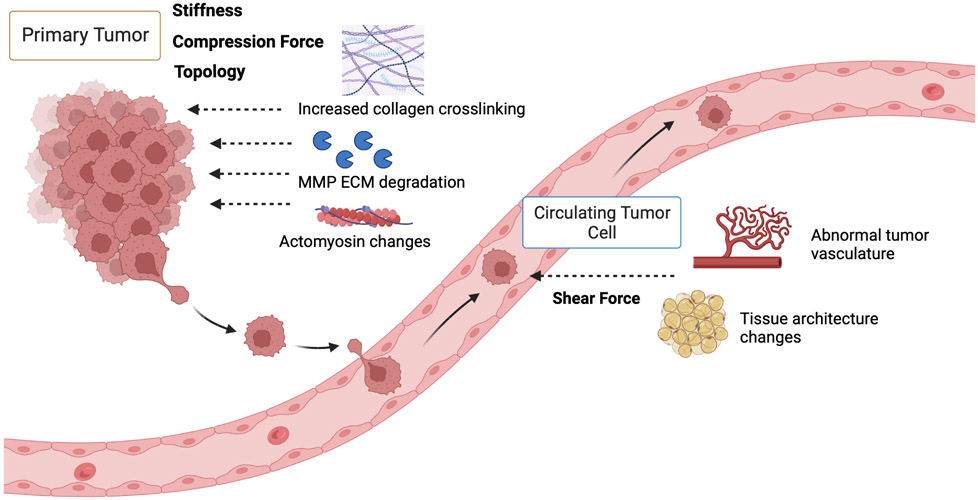
In the ever-changing tumor microenvironment, various physical forces have been shown to regulate EMT plasticity throughout the metastatic cascade. At the primary site, proliferation of tumor cells causes increased compression from the surrounding tissue architecture, and dysregulation of ECM components results in increased matrix stiffness. As tumor cells intravasate into the blood stream, they experience shear force generated by the dynamic mechanical environment, which will be discussed below (Figure 1).
Figure 1.
A schematic of various physical forces tumor cells experience during the metastatic cascade. The mechanical forces are labeled, and the upstream factors contributing to the mechanical force are depicted at both the site of primary tumor growth and in the blood circulation.
Matrix Stiffness
One of the key contributors to matrix stiffening and dysregulation during tumor development is increased deposition and cross-linking of ECM components, such as collagen, and ECM degradation by MMPs, respectively. Collagen fibers can be crosslinked to different degrees by lysyl oxidase (LOX), with more cross-linking increasing ECM stiffness. High LOXL2 expression is correlated with a higher EMT score and enrichment of ECM signaling in cervical carcinoma[12]. In liver cancer, substrate stiffness in the liver tissue plays a major role in changing tumor cell phenotypes and promoting angiogenesis, EMT, and metastasis. High ECM rigidities were reported to activate several signaling pathways, including the TGFβ1, NOTCH, and WNT/B-catenin pathways. Among them, TGFβ1 is the main driver of EMT in response to ECM stiffness during metastasis in liver cancer.[13] Increased ECM stiffness in colon tumors can also drive cancer progression through the interplays between cancer-associated fibroblasts (CAFs) and TGFβ. Mechanistically, high ECM stiffness activates CAFs to release activin A, a glycoprotein in the TGFβ family, which activates the downstream PI3K/Akt signaling to promote metastasis.[14] In breast cancer cells treated with TGFβ1 and cultured on polyacrylamide gels with increasing stiffness, high stiffness is shown to increase EMT through the PI3K/Akt signaling.[15] Additionally, under excessive mechanical force, stem-like breast cancer cells were found to be in a quiescent state, while removal of this force increased proliferation in quiescent cancer stem cells. [16] Recently, a novel method called Spatially Transformed Inferential Force Map (STIFMap) that automates atomic force microscopy (AFM) indentation was used to measure the stiffness heterogeneity in human breast tumors and found that high-elasticity regions within human breast tumors colocalize with markers of mechanical activation and EMT[17]. Taken together, increasing matrix stiffness plays a vital role in driving EMT at the primary site, thus contributing to tumor invasion and metastasis.
Shear Stress
The liquid flows surrounding endothelial and cancer cells can cause shear stress that influences cell morphology and behaviors. To investigate cellular plasticity in response to rigidity changes, prostate cancer cells were cultured on both soft and stiff matrices as well as in suspension cultures to recapitulate the forces experienced by circulating tumor cells in the blood. Gene expression analysis demonstrated increased expression of epithelial markers KRT18, MUC-1, and DSP in prostate cancer cells under soft and suspension conditions. However, increased expression of mesenchymal markers such as VIM-1, FN1, FSP1 were also observed under these conditions, suggesting the involvement of several mechanotransduction pathways. In this case, the YAP signaling did not appear to be involved; instead, p38MAPK, ERK, and Wnt were found to function in this phenotypic switching of prostate cancer cells[18]. During ovarian epithelial cancer progression, shear forces from ascitic fluid contributed to the mesenchymal and stem cell-like phenotypes through upregulating FOXS1, ERVV-2, KRT17 and RBFOX3 expression, which increased the invasive capacity of cancer cells to disseminate throughout the peritoneal cavity[19]. Similar observations are reported in hepatocellular carcinoma, where lymphoid and interstitial fluid interact directly on the surface of solid tumor cells and produce laminar flow. This constant force serves as a direct stimulus responsible for tumor cell motility[20]. In invasive breast cancer cells, exposure to fluid shear force resulted in significant changes in the ROS level, and markers for EMT and hippo signaling, which could be reversed when fluid shear force was no longer applied[21]. In summary, various fluid forces tumor cells experience in the primary tumor.
Upstream mechano-sensors and mechano-transducers in EMT regulation
Recent studies have identified various cell surface and intracellular proteins as mechano-sensors or mechano-transducers in EMT regulation (Table 1). One way by which ECM stiffness impacts EMT is via modulating the response to TGFβ1, a key biochemical inducer of EMT. At high stiffness, TGFβ1 is found to promote EMT while at low stiffness it leads to apoptosis. In examining how mammary epithelial cells respond to TGFβ1 treatment in a stiffness-dependent and time-dependent manner, Nelson et al. uncovered the involvement of biochemical pathways mediated mainly by integrin signaling. Integrin-linked kinase (ILK) can regulate the balance between cell-cell and cell-ECM adhesion and control the switch between EMT and apoptosis downstream of TGFβ1[22]. Another mechanosensitive system driven by ECM stiffening is via MENA, a member of actin regulatory proteins, and ESRP1, an RNA-binding protein that regulates alternative splicing of MENA, during the process of tumor cell intravasation. High matrix stiffness is shown to increase MENA expression, which promotes contractility and intravasation through activation of focal adhesion kinase; in the meantime, high matrix stiffness also decreases ESRP1 expression to shift alternative splicing of MENA and downregulate the expression of an anti-migratory isoform MENA11a. In basal-like breast cancer cells, there is a negative correlation between expression of MENA11a and metastatic potential of tumors[23]. Together, these studies highlight the important role of integrin-based focal adhesion in sensing and transmitting mechanical cues from the ECM to impact EMT and tumor invasion.
Table 1:
Upstream mechano-sensors and transducers in EMT regulation.
| Upstream Mechano-Sensor and Transducers |
Mechanism |
|---|---|
| TGFβ1 | Promotes EMT at high stiffness and leads to apoptosis at low stiffness through Integrin-linked Kinase |
| MENA | Downregulation of MENA11a leads to an increase in the migratory potential of breast cancer cells. |
| MGAT5 | Induces N-glycans branching on integrins, which provides a fibrillar 3D environment that generates higher matrix stiffness to enhance GSC migration. |
| LINC complex | The increased nuclear transport of β-catenin with SUN1 increased expression can induce EMT through cascades of effectors including the TCF/LEF transcription factor, the transcriptional repressor complex Grocho/Histone Deacetylase (HDAC), and EMT transcription factors such as SNAI1/2. |
| PIEZO2 | Mechanically activated through Ca2+ channel and cell depolarization, inducing Akt activation, which in turn leads to SNAI1 protein stabilization and nuclear translocation to increase motility and migration of breast cancer cells. |
| TRPV4 | Matrix stiffness and TGFβ1 cause differential expression of LncRNAs and mRNAs in a TRPV4-dependent fashion. |
Another notable mechanosensory system linking matrix stiffness and EMT is recently reported in glioblastoma multiforme (GBM), the most aggressive form of brain cancer. Increased matrix stiffness is shown to promote low-grade glioma to progress into GBM, along with changes of glioma cell morphology and nuclear volume and EMT induction. Among several signaling pathways known to be involved in cell migration, GSC invasion is found to be associated with integrin-ECM focal adhesions. Integrins have multiple sites of N-glycosylation, which can undergo N-glycans branching by the Golgi N-acetylglucosaminyltransferase (MGAT). MGAT5-induced N-glycosylation provides a fibrillar 3D environment that generates higher matrix stiffness and ultimately enhances GSC migration[24]. In radiation therapy, exposure of low-dose X-ray to the periphery of the target tumor can also increase metastatic risk because of multi-directional irradiation. Koizumi et al. explored the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex, a multifunctional assembly of nuclear proteins involved in mechanotransduction, nuclear migration, and DNA repair. They found that the LINC complex component Sad1 and UCN84 domain containing 1 (SUN1) are required for EMT. When cells are exposed to low-dose X-irradiation for 24 hours, expression of SUN1 increases along with β-catenin expression. In the downstream signaling of Wnt/ β-catenin and PI3K/Akt pathway, nuclear transport of β-catenin can induce EMT through cascades of effectors including the TCF/LEF transcription factor, the transcriptional repressor complex Grocho/Histone Deacetylase (HDAC), and EMT transcription factors such as SNAI1/2[25]. Thus, some upstream molecules in the nucleoskeleton and cytoskeleton can also contribute to the overall induction of EMT. In response to mechanical cues, these mechanotransduction programs can orchestrate various downstream signaling cascades to impact EMT and invasion.
Dysregulation of mechano-sensitive ion channels also plays a role in regulating invasive cellular behaviors in triple-negative breast cancer. One of such mechanically gated ion channel is PIEZO that has been linked to increase in motility and migration of breast cancer cells. PIEZO2 is linked to carcinogenesis and cancer progression via the mechanical activation of Ca2+ channel and cell depolarization[26]. The transient receptor potential (TRP) channel of the vanilloid subfamily, TRPV4, is one example of a matrix stiffness-sensing ion channel that plays a role in EMT. TRPV4 is required to sense and transmit ECM mechanical signals to regulate the expression of long non-coding RNAs (LncRNAs). Dysregulation of LncRNAs can affect EMT in fibrotic diseases and cancers. In primary mouse normal epidermal keratinocytes, matrix stiffness and TGFβ1 cause differential expression of LncRNAs and mRNAs in a TRPV4-dependent fashion[27]. Together, these recent studies revealed a novel function of mechano-sensitive ion channels in mechano-regulation of EMT and tumor invasion.
Downstream mechano- responding transcription factors in EMT induction
The EMT program is regulated by a network of transcription factors, including TWIST1/2, SNAI1/2, and ZEB1/2[28]. Our group reported that increasing matrix stiffness can induce a partial EMT and promotes tumor invasion and metastasis in a TWIST1-dependent manner in mammary carcinoma cells. Mechanistically, TWIST1 is sequestered in the cytoplasm by binding to G3BP2 at low ECM stiffness; upon increasing matrix stiffness, the Lyn kinase is activated and phosphorylates TWIST1 to prevent its binding to G3BP2, thus allowing TWIST1 to enter into the nucleus to induce EMT[29], [30]. As mentioned above, MGAT increases EMT, cell migration speed, and focal adhesion turnover through regulation of integrin clustering in glioblastoma. At high stiffness, MGAT5 activity augments focal adhesion turnover and maturation and galectin 3 upregulation, which increases the expression of ZEB1 and subsequently increases N-Cadherin. In this case, ZEB1 integrates ECM stiffness signals via MGAT5, as well as other receptor tyrosine kinase signaling pathways that regulate focal adhesion[24]. By examining the phenotypic switch in response to TGFβ1 at soft and stiff matrixes, Nihan, A. et al. discovered a critical role for integrin-linked kinase (ILK) in regulating EMT and invasion. In stiff matrices, cells decrease the expression of E-cadherin and ZO-1 and upregulated ILK, SNAI1, αSMA and vimentin. Knockdown of ILK abolishes the induction of EMT by TGFβ1, suggesting SNAI1 can serve as the mechano-responder downstream of ILK activation[22]. In triple-negative breast cancers (TNBC), high expression of Piezo2 is correlated with increased expression of vimentin and reduced expression of E-Cadherin. Mechanistically, the authors show that Piezo2 induces Akt activation, which in turn leads to SNAI1 protein stabilization and nuclear translocation[26]. In osteosarcoma, high matrix stiffness regulates EMT morphological changes through cytoskeleton remodeling and polymerization of actin, resulting in nuclear translocation of Myocardin-Related Transcription Factor A (MRTF-A). Inhibiting MRTF-A reduces EMT and migration on rigid gels[31]. Overall, the force exerted by a stiff matrix can be sensed and transduced downstream to a number of transcription factors that induce phenotypic and functional changes of EMT (Table 2).
Table 2:
Mechano-responsive transcription factors involved in EMT
| Mechano-responsive Transcription Factors in EMT |
Mechanism |
|---|---|
| TWIST1 | Upon increasing matrix stiffness, the Lyn kinase is activated and phosphorylates TWIST1 to prevent its binding to G3BP2, allowing TWIST1 to enter into the nucleus to induce EMT. |
| ZEB1 | Responds to ECM stiffness signals via MGAT5, as well as other receptor tyrosine kinase signaling pathways that regulate focal adhesions to increase N-Cadherin expression and induce EMT. |
| SNAI1 | In stiff matrices, cells decrease expression of E-cadherin and ZO-1 and upregulate ILK, SNAI1, αSMA and vimentin, suggesting ILK results in downstream SNAI1 EMT induction. |
| MRTF-A | High matrix stiffness results in cytoskeleton remodeling and polymerization of actin, which induces nuclear translocation of MRTF-A to induce EMT. |
YAP/TAZ as mechano-responders
In cancers, YAP/TAZ transcription activators have also been reported to mediate mechano-responses to the tumor microenvironment. YAP/TAZ in the unphosphorylated state enters the nucleus and binds to the transcription factor TEAD, thus promoting transcription of target genes regulating various cellular processes (Figure 2). YAP/TAZ responds to various mechanical signals including changes in actin cytoskeleton, disruption of cell polarity or junctions, as well as increases in ECM stiffness. YAP/TAZ, in close association with ZEB1/2, SNAI1/2, and TWIST1, can regulate EMT. Overexpression of TWIST1 and SNAI1 leads to delocalization of Scribble, relieving TAZ from the cell polarity complex to promote cancer stem cell traits in breast cancer cells. Both ZEB1 and SNAI1/2 bind to YAP or the YAP/TAZ complex respectively to regulate target gene transcription.[28] The role of YAP/TAZ in EMT is also revealed by luteolin treatment to induce YAP/TAZ degradation in TNBC cells. Inhibiting YAP/TAZ led to a decrease in mesenchymal markers and an increase in epithelial markers[32].
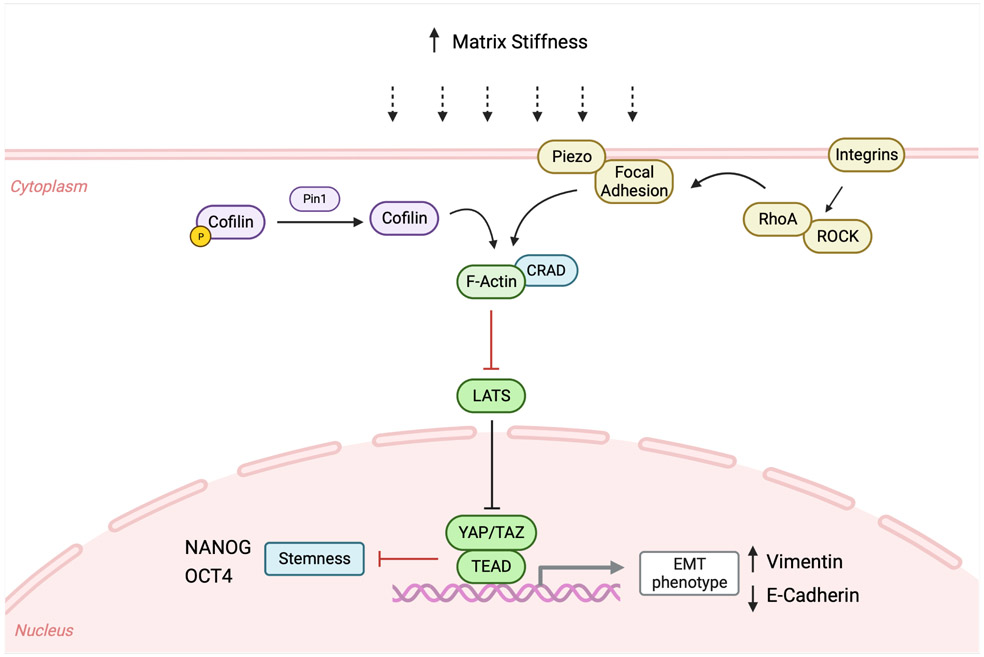
Figure 2.
A schematic of the signaling pathways by which YAP/TAZ function as mechano-responders to induce EMT at high matrix stiffness in various human cancers.
YAP/TAZ could impact EMT via various downstream signaling pathways in response to mechanical cues. At the rigid ECM stiffness, increased interactions between ECM and focal adhesions promote YAP/TAZ nuclear location and their transcriptional activity[33]. In breast cancer cells, mechanical stress activates YAP to promote the transcription of Skp2, an F-box protein of the SCF E3 ubiquitin ligase complex important in cell cycle progression[34]. In pancreatic cancer, one of the stiffest solid carcinomas due to deposition of a dense and crosslinked ECM, increased vimentin expression and decreased E-cadherin expression are correlated with nuclear localization of β-catenin, YAP and TAZ[35]. In cervical cancer, matrix stiffness induces EMT through a Pin1/YAP nuclear localization through F-actin remodeling independent of the Hippo pathway. Increased Pin1 expression affected F-actin remodeling by inducing Cofilin, thereby increasing YAP nuclear translocation in a phosphorylation-independent manner. Inhibition of Pin1 led to downregulation of YAP expression and subsequent downregulation of vimentin expression in vivo[36]. In colorectal cancer, the F-actin regulator CRAD protein level is decreased and promotes YAP cytoplasmic retention in soft matrices. Surprisingly, soft matrices promote stemness and metastasis due to the lack of transcriptional repression by YAP on stemness markers, such as NANOG and OCT4. Tumor repopulating cells isolated from soft matrices presented a hybrid EMT signature that mostly overlapped with the RNA expression profile of cells without CRAD, thus contributing to the high metastatic capability. These downstream effects could be reversed once these cells from a soft matrix were cultured on the rigid plastic surface[37].
Matrix stiffness controls YAP/TAZ activation via several signaling pathways. One study examined YAP activation by culturing PDAC cell lines with different EMT-related phenotypes in both 2D monolayers with high stiffness and 3D spheroids floating in the medium with low rigidities to determine how cell characteristics could influence mechanotransduction. In 3D spheroid cultures with PDAC cells exhibiting both an epithelial and mesenchymal phenotype, YAP/TAZ expression was predominantly cytoplasmic, indicating inactive YAP. As 3D spheroids do not exhibit focal adhesions and experience lower mechanical inputs, YAP activation was not affected by 3D conditions or by inhibition of actin cytoskeleton. With a more intense mechanical stimuli in the 2D monolayers than in 3D spheroids, YAP is found to be mostly nuclear in PDAC cells and not affected by actin cytoskeleton inhibition, suggesting that these mechanical forces act on focal adhesions rather than on cell junctions[38]. Using the ocular lens as a model to study mechanotransduction pathways of both YAP and Piezo, a study shows that Rho/ROCK-induced FAK signaling enhanced cell migration[39]. Piezo1 is found to colocalize with focal adhesion at the extrusion edge in epithelial cells in a force-dependent manner. These focal adhesion complexes are controlled by myosin-II contractility via the Rho/ROCK pathway and when this pathway was blocked, there was a decrease in Piezo1 at the tip of spreading cells[40]. Because YAP/TAZ also responds to changes in cell polarity, shape and cell-cell junctions, their function in matrix stiffness-induced EMT is likely to be coupled with their roles in responses to these other changes involving cytoskeleton remodeling.
Conclusion and perspective
A growing body of studies have recently been published reporting critical roles of mechanotransduction in cancer development and progression, particularly through its regulation of EMT. While significant progress has been made in linking matrix stiffness with EMT regulation, the underlying molecular mechanisms remain to be elucidated to fully understand the complex interplay between matrix stiffness, EMT, and cancer progression. Especially how different mechanical cues from the tumor microenvironment are sensed and translated into specific and unique transcriptional responses requires new experimental approaches to dissect. Given the important roles of EMT in tumor metastasis and therapy resistance, many new mechanotransduction pathways linking mechanical forces from the tumor microenvironment to EMT and tumor invasion could be promising novel targets for new cancer therapeutics.
Acknowledgements
We apologize to many researchers whose work we were unable to cite due to space restrictions. Our research is supported by grants from NCI (1RO1CA262794, 1R01CA174869, 1R01CA206880, and 1R01CA236386), CDMRP DOD Breast Cancer Program BC170283, METAvivor Research Award 20200586, California Tobacco-Related Disease Research Program (TRDRP) 28IP-0023, and Padres Pedal the Cause #PTC2021. C.H. and K.D. were supported in part by the UCSD Graduate Training Program in Cellular and Molecular Pharmacology through an institutional training grant from the National Institute of General Medical Sciences T32 GM007752. The figure in this review was created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
Declaration of Interest Statement
The authors declare no conflict of interest related to this work.
Reference:
- [1].Prieto-García E, Díaz-García CV, García-Ruiz I, and Agulló-Ortuño MT, “Epithelial-to-mesenchymal transition in tumor progression,” Med. Oncol. Northwood Lond. Engl, vol. 34, no. 7, p. 122, Jul. 2017, doi: 10.1007/s12032-017-0980-8. [DOI] [PubMed] [Google Scholar]
- [2].Scott LE, Weinberg SH, and Lemmon CA, “Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition,” Front. Cell Dev. Biol, vol. 7, 2019, Accessed: Mar. 20, 2023. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fcell.2019.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou H et al. , “Functions and clinical significance of mechanical tumor microenvironment: cancer cell sensing, mechanobiology and metastasis,” Cancer Commun., vol. 42, no. 5, pp. 374–400, 2022, doi: 10.1002/cac2.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tian H, Shi H, Yu J, Ge S, and Ruan J, “Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT,” Glob. Chall. Hoboken NJ, vol. 6, no. 6, p. 2100094, Jun. 2022, doi: 10.1002/gch2.202100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deng B, Zhao Z, Kong W, Han C, Shen X, and Zhou C, “Biological role of matrix stiffness in tumor growth and treatment,” J. Transl. Med, vol. 20, no. 1, p. 540, Nov. 2022, doi: 10.1186/s12967-022-03768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cao H, Cheng HS, Wang JK, Tan NS, and Tay CY, “A 3D physio-mimetic interpenetrating network-based platform to decode the pro and anti-tumorigenic properties of cancer-associated fibroblasts,” Acta Biomater, vol. 132, pp. 448–460, Sep. 2021, doi: 10.1016/j.actbio.2021.03.037. [DOI] [PubMed] [Google Scholar]
- [7].Millet M et al. , “Cancer-Associated Fibroblasts in a 3D Engineered Tissue Model Induce Tumor-like Matrix Stiffening and EMT Transition,” Cancers, vol. 14, no. 15, Art. no. 15, Jan. 2022, doi: 10.3390/cancers14153810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Lin F et al. , “Spontaneous formation and spatial self-organization of mechanically induced mesenchymal-like cells within geometrically confined cancer cell monolayers,” Biomaterials, vol. 281, p. 121337, Feb. 2022, doi: 10.1016/j.biomaterials.2021.121337. Here the regulation of mechanic heterogeneity on the EMT phenotype switch is investigated, showing cell contraction and substrate stiffness are involved in the mesenchymal-like phenotype in micropattened cell monolayers.
- [9].Sullivan E, Harris M, Bhatnagar A, Guberman E, Zonfa I, and Ravasz Regan E, “Boolean modeling of mechanosensitive epithelial to mesenchymal transition and its reversal,” iScience, vol. 26, no. 4, p. 106321, Mar. 2023, doi: 10.1016/j.isci.2023.106321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wei SC et al. , “Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway,” Nat. Cell Biol, vol. 17, no. 5, pp. 678–688, May 2015, doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Elosegui-Artola A et al. , “Matrix viscoelasticity controls spatiotemporal tissue organization,” Nat. Mater, vol. 22, no. 1, Art. no. 1, Jan. 2023, doi: 10.1038/s41563-022-01400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cao C et al. , “LOXL2 Expression Status Is Correlated With Molecular Characterizations of Cervical Carcinoma and Associated With Poor Cancer Survival via Epithelial-Mesenchymal Transition (EMT) Phenotype,” Front. Oncol, vol. 10, p. 284, Mar. 2020, doi: 10.3389/fonc.2020.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu X, Zhang Y, Wang X, Li S, and Tang L, “Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer,” Int. J. Mol. Sci, vol. 22, no. 21, p. 12066, Nov. 2021, doi: 10.3390/ijms222112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bauer J et al. , “Increased stiffness of the tumor microenvironment in colon cancer stimulates cancer associated fibroblast-mediated prometastatic activin A signaling,” Sci. Rep, vol. 10, no. 1, Art. no. 1, Jan. 2020, doi: 10.1038/s41598-019-55687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bauer J et al. , “Activin and TGFβ use diverging mitogenic signaling in advanced colon cancer,” Mol. Cancer, vol. 14, p. 182, Oct. 2015, doi: 10.1186/s12943-015-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li C et al. , “Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling,” Signal Transduct. Target. Ther, vol. 8, no. 1, Art. no. 1, Jun. 2023, doi: 10.1038/s41392-023-01453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stashko C et al. , “A convolutional neural network STIFMap reveals associations between stromal stiffness and EMT in breast cancer,” Nat. Commun, vol. 14, no. 1, Art. no. 1, Jun. 2023, doi: 10.1038/s41467-023-39085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Aw Yong KM, Sun Y, Merajver SD, and Fu J, “Mechanotransduction-Induced Reversible Phenotypic Switching in Prostate Cancer Cells,” Biophys. J, vol. 112, no. 6, pp. 1236–1245, Mar. 2017, doi: 10.1016/j.bpj.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martinez A et al. , “Understanding the effect of mechanical forces on ovarian cancer progression,” Gynecol. Oncol, vol. 162, no. 1, Art. no. 1, Jul. 2021, doi: 10.1016/j.ygyno.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xin Y, Li K, Yang M, and Tan Y, “Fluid Shear Stress Induces EMT of Circulating Tumor Cells via JNK Signaling in Favor of Their Survival during Hematogenous Dissemination,” Int. J. Mol. Sci, vol. 21, no. 21, Art. no. 21, Oct. 2020, doi: 10.3390/ijms21218115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brown SR, Bates JC, Avera AD, and Kim Y, “Relationship between Stemness, Reactive Oxygen Species, and Epithelial-to-Mesenchymal Transition in Model Circulating Tumor Cells,” Cells Tissues Organs, vol. 211, no. 3, pp. 282–293, 2022, doi: 10.1159/000516574. [DOI] [PubMed] [Google Scholar]
- [22].Kilinc AN, Han S, Barrett LA, Anandasivam N, and Nelson CM, “Integrin-linked kinase tunes cell–cell and cell-matrix adhesions to regulate the switch between apoptosis and EMT downstream of TGFβ1,” Mol. Biol. Cell, vol. 32, no. 5, pp. 402–412, Mar. 2021, doi: 10.1091/mbc.E20-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Wang W et al. , “Matrix stiffness regulates tumor cell intravasation through expression and ESRP1-mediated alternative splicing of MENA,” Cell Rep., vol. 42, no. 4, Art. no. 4, Apr. 2023, doi: 10.1016/j.celrep.2023.112338. ECM stiffening resulted in increased intravasation through a novel splicing regulation of MENA by ESRP1, a known regulator of EMT. Therefore, suggesting a link between ECM stiffness and EMT.
- [24]. Marhuenda E et al. , “Glioma stem cells invasive phenotype at optimal stiffness is driven by MGAT5 dependent mechanosensing,” J. Exp. Clin. Cancer Res. CR, vol. 40, no. 1, p. 139, Apr. 2021, doi: 10.1186/s13046-021-01925-7. Using a 3D-nanofibre scaffold to adjust stiffness, MGAT5 induced N-glycosylation of integrins modulated mechano-sensing to induce downstream EMT and migration in gliobastoma.
- [25].Imaizumi H, Minami K, Hieda M, Narihiro N, and Koizumi M, “The linker of nucleoskeleton and cytoskeleton complex is required for X-ray-induced epithelial-mesenchymal transition,” J. Radiat. Res. (Tokyo), p. rrac104, Jan. 2023, doi: 10.1093/jrr/rrac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Katsuta E et al. , “Mechano-Sensing Channel PIEZO2 Enhances Invasive Phenotype in Triple-Negative Breast Cancer,” Int. J. Mol. Sci, vol. 23, no. 17, Art. no. 17, Aug. 2022, doi: 10.3390/ijms23179909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sharma S, Ma L, and Rahaman SO, “Role of TRPV4 in matrix stiffness-induced expression of EMT-specific LncRNA,” Mol. Cell. Biochem, vol. 474, no. 1–2, Art. no. 1–2, Nov. 2020, doi: 10.1007/s11010-020-03844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Noguchi S, Saito A, and Nagase T, “YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer,” Int. J. Mol. Sci, vol. 19, no. 11, p. 3674, Nov. 2018, doi: 10.3390/ijms19113674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wei SC et al. , “Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway,” Nat. Cell Biol, vol. 17, no. 5, Art. no. 5, May 2015, doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fattet L et al. , “Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex,” Dev. Cell, vol. 54, no. 3, pp. 302–316.e7, Aug. 2020, doi: 10.1016/j.devcel.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dai J et al. , “Matrix stiffness regulates epithelial-mesenchymal transition via cytoskeletal remodeling and MRTF-A translocation in osteosarcoma cells,” J. Mech. Behav. Biomed. Mater, vol. 90, pp. 226–238, Feb. 2019, doi: 10.1016/j.jmbbm.2018.10.012. [DOI] [PubMed] [Google Scholar]
- [32].Cao D et al. , “Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity,” Biomed. Pharmacother. Biomedecine Pharmacother, vol. 129, p. 110462, Sep. 2020, doi: 10.1016/j.biopha.2020.110462. [DOI] [PubMed] [Google Scholar]
- [33].Ortega Á et al. , “The YAP/TAZ Signaling Pathway in the Tumor Microenvironment and Carcinogenesis: Current Knowledge and Therapeutic Promises,” Int. J. Mol. Sci, vol. 23, no. 1, Art. no. 1, Jan. 2022, doi: 10.3390/ijms23010430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jang W, Kim T, Koo JS, Kim S-K, and Lim D-S, “Mechanical cue-induced YAP instructs Skp2-dependent cell cycle exit and oncogenic signaling,” EMBO J., vol. 36, no. 17, pp. 2510–2528, Sep. 2017, doi: 10.15252/embj.201696089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rice AJ et al. , “Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells,” Oncogenesis, vol. 6, no. 7, Art. no. 7, Jul. 2017, doi: 10.1038/oncsis.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Yang L et al. , “Pin1/YAP pathway mediates matrix stiffness-induced epithelial–mesenchymal transition driving cervical cancer metastasis via a non-Hippo mechanism,” Bioeng. Transl. Med, vol. 8, no. 1, p. e10375, 2023, doi: 10.1002/btm2.10375. The finding of a non-Hippo YAP-mediated mechanotransduction pathway is novel in that it suggests a link from the ECM stiffness to EMT though a novel upstream regulator of YAP transcriptional activity.
- [37].Chang Y et al. , “Substrate Rigidity Dictates Colorectal Tumorigenic Cell Stemness and Metastasis via KIAA1211 Dependent Mechanotransduction.” Rochester, NY, Jul. 09, 2021. doi: 10.2139/ssrn.3883635. [DOI] [PubMed] [Google Scholar]
- [38].Bianchi F et al. , “Mechanical Cues, E-Cadherin Expression and Cell ‘Sociality’ Are Crucial Crossroads in Determining Pancreatic Ductal Adenocarcinoma Cells Behavior,” Cells, vol. 11, no. 8, p. 1318, Apr. 2022, doi: 10.3390/cells11081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Taiyab A and West-Mays J, “Lens Fibrosis: Understanding the Dynamics of Cell Adhesion Signaling in Lens Epithelial-Mesenchymal Transition,” Front. Cell Dev. Biol, vol. 10, p. 886053, 2022, doi: 10.3389/fcell.2022.886053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jetta D, Bahrani Fard MR, Sachs F, Munechika K, and Hua SZ, “Adherent cell remodeling on micropatterns is modulated by Piezo1 channels,” Sci. Rep, vol. 11, no. 1, Art. no. 1, Mar. 2021, doi: 10.1038/s41598-021-84427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]