Summary
Background and objective
The genetic basis of dentoalveolar characteristics has been investigated by several studies, however, the findings are equivocal. The objective of this systematic review and meta-analysis was to evaluate the heritability of dental arches and occlusal parameters in different stages of human dentition.
Search methods
Electronic databases PubMed, Embase, Scopus, Web of Science, and Dentistry and Oral Science Source were searched up to August 2023 without the restriction of language or publication date.
Selection criteria
Empirical studies investigating the heritability of dentoalveolar parameters among twins and siblings were included in the review.
Data collection and analysis
Study selection, data extraction, and risk of bias assessment were performed independently and in duplicate by two authors and a third author resolved conflicts if needed. Joanna Briggs Institute’s critical appraisal tool was used to evaluate the risk of bias among studies and the certainty of evidence was assessed using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) criteria.
Results
Twenty-eight studies were included in the systematic review, of which 15 studies reporting heritability coefficients in the permanent dentition stages were deemed suitable for the meta-analysis. Random-effects meta-analyses showed high heritability estimates for maxillary intermolar width (0.52), maxillary intercanine width (0.54), mandibular intermolar width (0.55), mandibular intercanine width (0.55), maxillary arch length (0.76), mandibular arch length (0.57), and palatal depth (0.56). The heritability estimates for the occlusal parameters varied considerably, with relatively moderate values for crossbite (0.46) and overbite (0.44) and low values for buccal segment relationship (0.32), overjet (0.22), and rotation and displacement of teeth (0.16). However, the certainty of evidence for most of the outcomes was low according to the GRADE criteria.
Conclusions
Based on the available evidence, it can be concluded that the dental arch dimensions have a high heritability while the occlusal parameters demonstrate a moderate to low heritability.
Registration
PROSPERO (CRD42022358442).
Keywords: dental arch, dental occlusion, genetics, heritability, malocclusion, siblings, twins
Background
There is considerable interest in unravelling the genetic underpinnings of dentoalveolar development [1, 2]. This is especially true for orthodontists and craniofacial biologists who are striving to understand the aetiology of malocclusion. The variation in and the cause of its phenotypic display is a much-debated topic, with some investigators proposing a strong genetic influence while others emphasize environmental factors during development [3–5]. Though the nature versus nurture debate is outdated, current research acknowledges that both genetic and environmental factors have a role in the development of dental arches and occlusion, although the relative contributions of genetic and environmental factors remain unclear [6].
Heritability (H2) is the proportion of the total variation in a phenotypic trait (VP) in a population that can be attributed to genetic factors (VG), expressed as H2 = (VG/VP) and by calculating the heritability of a trait in a population, the significance of genetic and environmental factors in shaping the variation of the trait can be compared [7]. Heritability is a population-derived statistic and can, therefore, be subject to influences from the time and population from which the data is collected [8]. It can be broadly differentiated into two types—narrow-sense and broad-sense. Narrow-sense heritability specifically measures the impact of additive genetic effects on phenotypic variation, while broad-sense heritability considers both additive and non-additive genetic effects when explaining the observed variation in phenotypes [9].
Studies in twins and siblings have been frequently utilized to estimate heritability [10–13]. Due to their underlying genetic and environmental similarities in related individuals, these studies play a crucial role in understanding the aetiology of malocclusion by enabling researchers to disentangle the genetic and environmental influences on dental arches and occlusion. Even in the post-genomic era, estimation of heritability from twin and sibling studies is foundational for investigating the genes involved in complex traits [14]. However, to date, these studies report conflicting results regarding the heritability of dental arches and occlusal characteristics, with some reporting high heritability estimates [2, 15–17] and others showing low or negligible heritability [18–21].
A preliminary search of the literature did not find any systematic reviews or meta-analyses exploring the heritability of dental arches and occlusal characteristics among twins and siblings. Although one review assessed the heritability of occlusal traits, it was confined to the permanent dentition of twins [22]. Due to this limitation, the study did not provide a comprehensive understanding of the heritability of dental arches and occlusion among twins and siblings in the various stages of dental development. The purpose of this study is, therefore, to systematically review the scientific literature involving twins and siblings to evaluate the heritability of dental arches and occlusal characteristics in different stages of dentition and to conduct meta-analyses where possible.
Materials and methods
Registration and protocol
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [23]. The protocol of this review was registered (CRD42022358442) at the International Prospective Register of Systematic Reviews (PROSPERO) on 19/09/2022.
Eligibility criteria
Inclusion criteria
The inclusion criteria were based on the PECOS framework [24]:
Population (P): Twins (monozygotic or dizygotic) and siblings in different stages of dental development (primary, mixed, and permanent dentition).
Exposure (E): Normal craniofacial growth in the absence of any major syndromes or orthodontic intervention.
Comparison (C): Covariances and heritabilities between twins and siblings.
Outcomes (O): The primary dental arch and occlusal heritabilities of interest were for arch width, arch length, palatal depth, overjet, overbite, crossbite, buccal segment relationship, and rotation and displacement of teeth.
Study design (S): All empirical studies assessing the heritability of dental arches and occlusal characteristics among healthy twins and siblings.
Exclusion criteria
Studies were excluded if:
Twins or siblings were diagnosed with craniofacial anomalies or syndromes.
Twins or siblings had missing teeth except third molars in the permanent dentition.
The samples had prior orthodontic treatment.
Full-text articles were not available.
Information sources
A comprehensive literature search was conducted in five electronic databases—PubMed, Embase, Scopus, Web of Science, and Dentistry and Oral Science Source (DOSS) from inception up to 30 September 2022 and updated on 31 August 2023. No restrictions were imposed on language or date of publication. Additionally, reference lists of identified articles and review papers were hand-searched to identify pertinent articles.
Search strategy
The initial search strategy was developed for PubMed using the MeSH terms and free texts according to the eligibility criteria, and the search strategy was later adapted for other databases as per their syntax. The search strategies for all five databases are presented in a supplementary file (Supplementary Document 1). All identified articles were imported into a reference management software (EndNote® Version X9, Clarivate Analytics, Philadelphia, PA) [25] and duplicates were automatically removed. The unique articles after removing duplicates were then imported into Covidence systematic review software [26] for screening and selection.
Selection process
Two reviewers (J.G. and T.H.F.) independently and in duplicate screened the articles based on the titles and abstracts in the first stage of the selection process; articles that did not address the research questions were excluded. Full texts of the articles that passed the initial screening were reviewed by the same reviewers using defined eligibility criteria independently and in duplicate. Any disagreements were resolved by discussion and, when needed, by consultation with a third reviewer (T.H.). The inter-reviewer reliability was assessed using Cohen’s Kappa. The process is illustrated in the PRISMA study flow diagram (Fig. 1).
Figure 1.
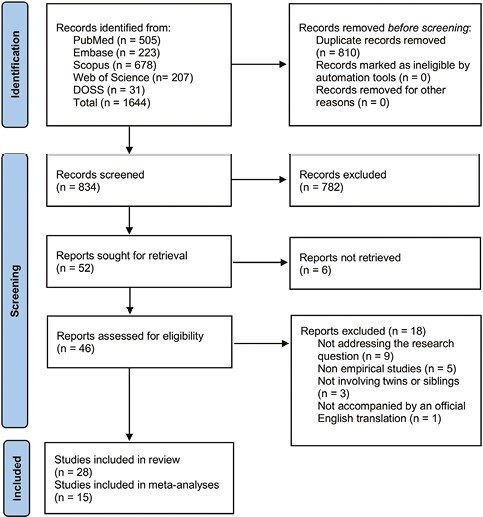
PRISMA study flow diagram.
Data collection process and data items
Relevant data were extracted from the selected full-text articles independently by two reviewers (J.G. and T.H.F.) using a data extraction table that was pilot tested on three studies before the formal extraction. The following information was collected from the selected studies: author’s name, publication year, country of origin, the purpose of study, study design, study setting, eligibility criteria, sample size, sample characteristics, method of zygosity determination, dental and occlusal parameters assessed, method of heritability estimation, heritability coefficient, main outcomes, funding information, ethical approval, and conflicts of interest. Additional information was not sought from the authors.
Risk of bias assessment
The risk of bias in the selected studies was assessed by two reviewers (J.G. and T.H.F.) independently, using the Joanna Briggs Institute (JBI) critical appraisal tool for cross-sectional analytical studies [27]. Later, the results were compared and any disagreements were resolved by mutual consensus.
Effect measures and synthesis methods
A random-effects meta-analysis was conducted to calculate the pooled heritability estimate in R with the ‘metafor’ package [28]. Meta-analysis was conducted separately for each outcome based on available heritability estimates. When the standard errors (SEs) or confidence intervals (CIs) of the heritability estimates were not reported, the SEs or CIs from studies that reported these statistics were used to calculate them using a combined variance method [29]. The variation among studies due to heterogeneity instead of chance was assessed using I2 statistics [30]. The value of I2 lies between 0% and 100% with larger values suggesting greater heterogeneity [31].
Certainty assessment
The Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) criteria were used to assess the certainty of the evidence using the GRADEpro GDT tool [32].
Results
Study selection
The search of electronic databases yielded 1644 articles: PubMed [505], Embase [223], Scopus [678], Web of Science [207], and DOSS [31]. De-duplication in EndNote resulted in 834 unique articles that were subjected to title and abstract screening. During the screening, 782 articles were excluded (Supplementary Document 2), and 52 articles were deemed suitable for full-text review. A further 24 articles were excluded during the full-text review and reasons for exclusion were provided (Supplementary Document 3). Eventually, 28 articles were included in the qualitative synthesis, out of which 15 were subjected to meta-analysis. The Kappa values for inter-reviewer agreement at the title and abstract screening stage and full-text review were 0.93 and 1, respectively, suggesting an almost perfect agreement overall between the reviewers.
Study characteristics
Table 1 shows the characteristics of the studies included in the review. Of the 28 studies selected for the review, 23 were twin studies [2, 16–21, 33–37, 39, 40, 42–48, 51, 52] and 5 were studies conducted among siblings [15, 38, 41, 49, 50]. The reported age of the twins and the siblings ranged between 3 [16] and 88 [44] years. Three studies [33, 39, 42] did not report the age of the participants. The number of participants in the studies ranged from 32 to 412, with a total sample of 4968. In terms of study design, only 2 were longitudinal studies [21, 38] and the remaining 26 were cross-sectional studies [2, 15–20, 33–37, 39–52]. Most studies evaluated the occlusal characteristics and dental arches in the permanent dentition stage, but two studies evaluated the primary dentition alone [16, 46]. The studies originated from 12 countries, with the highest number of studies from the USA [15, 18, 19, 21, 34, 38, 41, 50], followed by Australia [2, 16, 17, 35, 36, 39], India [20, 33, 47, 51] and Lithuania [43, 45]. Bosnia [48], China [40], Croatia [37], Egypt [46], Japan [44], Poland [42], South Korea [49], and Turkey [52] accounted for one study each. The studies were published between 1980 and 2022.
Table 1.
Summary of included studies in the systematic review.
| Authors/ year/ country | Participants | Age (years) | Sample size | Sex | Method of zygosity determination | Record evaluated | Method of estimating genetic influence | Heritability coefficients for different parameters (where reported)/main outcomes |
|---|---|---|---|---|---|---|---|---|
| Corruccini et al. [18]/ 1980/USA | MZ and DZ twins | 12 to 17 | 32MZ +28 DZ (60 pairs) (total = 120) | NR | Serologic and dermatoglyphic criteria | Dental casts | Heritability coefficient* | Maxillary intermolar width: 0.16 Mandibular intermolar width: 0.22 Maxillary arch length: 0.42 Mandibular arch length: 0.28 Overjet: 0.4 Overbite: 0 Crossbite: 0.89 Buccal segment relationship: 0 Rotation and displacement: 0.27 |
| Potter et al. [19]/1981/USA | MZ and DZ twins | 12–17 | 87 MZ + 77 DZ (164 pairs; total = 328) |
162 F, 166 M | Serological | Dental casts | Heritability coefficient* | Overjet: −0.11 Overbite: 0.27 Crossbite: 0.44 Buccal segment relationship: 0.05 Rotation and displacement: −0.19 |
| Varma [33]/ 1986/ India | MZ and DZ twins | NR | 8 MZ + 8 DZ (16 pairs; total = 32) | NR | NR | Dental casts | t-test | Strong genetic influence on the palatal height (no significant difference between the means of palatal height among MZ twins but significant difference among DZ twins) |
| Sharma et al [20]/ 1986/ India | MZ and DZ twins | 17.5 (11–27) |
23MZ + 35 DZ (58 pairs; total = 116) | 61 F, 55 M | Serological | Dental casts | Heritability coefficient* | Maxillary intermolar width: 0.63 Mandibular intermolar width: 0.67 Maxillary arch length: 0.72 Mandibular arch length: 0.66 Palatal depth: 0.47 Overjet: 0.24 Overbite: 0.77 Crossbite: 0.27 Buccal segment relationship: 0.45 Rotation and displacement: 0.09 |
| Boraas et al. [34]/1988/USA | MZ and DZ twins and triplets (reared apart) | At reunion: MZ: 39.9, DZ: 42.1 |
44 twins + 3 triplets (total =97) | 64 F, 33 M | Serological | Dental casts | Heritability coefficient* | Maxillary intermolar width: −0.14 Maxillary intercanine width: −0.22 Overjet: −0.5 Overbite: 0.36 |
| Townsend et al. [35] 1988/Australia | MZ and DZ twins | 16.2 (13–26) |
48 MZ + 34 DZ (82 pairs; total = 164) | 80 F, 84 M | Serological | Dental casts | Heritability coefficient* | Overjet: 0.36 Overbite: 0.34 Buccal segment relationship: 0.24 Rotation and displacement: 0.26 |
| Richards et al. [36]/ 1990/Australia | MZ and DZ twins | 15.8 ± 3.3 | 29 MZ + 19 DZ (48 pairs) + control 45 (total = 141) | 70 F, 71 M | NR | Dental casts | Polynomial analysis | Genetic factors contributed to variation in maxillary arch shape and to a lesser extent to variation in mandibular arch shape but not to arch asymmetry |
| Lapter et al. [37]/1991/Croatia | MZ and DZ twins | 5.8–18.8 | 36 MZ + 60 DZ (96 pairs; total = 192) | NR | Test of similarity and blood tests if necessary | Dental casts | Heritability coefficient* | Maxillary intermolar width: 0.58 maxillary intercanine width: 0.69 Mandibular intermolar width: 0.11 Mandibular intercanine width: 0.31 Maxillary arch length: 0.73 Palatal depth: 0.11 Overjet: 0.76 Overbite: 0.46 |
| Harris et al. [38]/ 1991/USA # | Siblings | Assessed at ages 4, 14 and 20 | 30 sibling pairs (total = 60) | 28 F, 32 M | NA | Dental casts | Heritability coefficient | Reported higher heritability estimates at age 4 which decreased with age. Heritability estimates at age 20 Maxillary intercanine width: 0.05 Mandibular intercanine width: −0.2 Maxillary arch length: −0.01 Mandibular arch length: −0.25 Overjet: 0.43 Overbite: 0.19 Crossbite: −0.05 Buccal segment relationship: 0.24 Rotation and displacement: 0 |
| King et al. [15]/ 1993/USA | Siblings | 13.5 (9–22) |
104 sibling pairs (total =208) |
127 F, 81 M | NA | Dental casts | Heritability coefficient | Maxillary intermolar width: 0.32 maxillary intercanine width: 0.53 Mandibular intermolar width: 0.77 Mandibular intercanine width: 0.79 Maxillary arch length: 0.73 Mandibular arch length: 0.6 Overjet: 0.58 Overbite: 0.62 Crossbite: 0.45 Buccal segment relationship: 0.48 Rotation and displacement: 0.49 |
| Kasai et al. [39]/1995/Australia | MZ and DZ twins | NR | 37 MZ + 19 DZ (56 pairs; total = 112) |
112 M | NR | Dental casts | Fourier analysis | Genetic factors contribute mainly to the variation in arch size and to arch depth/arch breadth ratio than to other aspects of the dental arch. |
| Liu et al. [40]/ 1998/China | MZ and DZ twins | 6–12 | 56 MZ + 26 DZ (82 pairs; total =164) | 164 F | DNA fingerprinting | Dental casts | Heritability coefficient | Arch size demonstrated a strong heritability (0.72), but weaker heritability was associated with overjet, overbite, and buccal segment relationship |
| Cassidy et al.[41]/ 1998/USA | Siblings and triplets | 13.5 ± 1.69 | 145 sibling pairs and 10 triplets (total = 320) | NR | NA | Dental casts | Heritability coefficient | Maxillary intermolar width: 0.67 Maxillary intercanine width: 0.56 Mandibular intermolar width: 0.61 Mandibular intercanine width:0.48 Overjet: 0.23 Buccal segment relationship: 0.56 |
| Hughes et al.[16]/ 2001/Australia | MZ twins, same sexed and opposite sexed DZ twins and singletons | 3–7 | 70 MZ + 68 DZ + 11 OSDZ (149 pairs) + 114 singletons (total = 412) |
203 F, 209 M | DNA from buccal cells | Dental casts | Heritability coefficient and structural equation modelling | Interdental spacing: 0.62 to 0.81 Maxillary intermolar width: 0.87 Maxillary intercanine width: 0.84 Maxillary arch length: 0.79 Mandibular intermolar width: 0.89 Mandibular intercanine width: 0.69 Mandibular arch length: 0.87 Overbite: 0.53 Overjet: 0.28 |
| Eguchi et al.[2]/ 2004/Australia | MZ and DZ twins | MZ: 15.8 ± 3.5 DZ: 17 ± 4.7 |
44 MZ + 34 DZ (78 pairs; total = 156) | 73 F, 83 M | DNA from buccal cells | Digital dental casts | Heritability coefficient and Structural equation modelling | Maxillary intermolar width: 0.82 Maxillary intercanine width: 0.86 Mandibular intermolar width: 0.79 Mandibular intercanine width: 0.83 Maxillary arch length: 0.92 Mandibular arch length: 0.86 Palatal depth: 0.8 |
| Kawala et al.[42]/2007/Poland | MZ and DZ twins | NR | 90 MZ + 74 DZ (164 pairs; total = 328) | 136 F, 192 M | Serologic, morphologic criteria, and dermatoglyphics | NR | Heritability coefficient | The heritability coefficients had low or negative values. Only in males the values exceeded 0.1; (class II malocclusion: 0.11, mandibular crowding: 0.12) |
| Svalkauskiene et al.[43]/ 2015/Lithuania | MZ and DZ twins | MZ: 20.2 ± 6.02 DZ: 17.8 ± 2.75 |
40 MZ + 32 DZ (72 pairs; total = 144) | 70 F, 74 M | Serological | Digital dental casts | Heritability coefficient* | Maxillary intermolar width: 0.51 maxillary intercanine width: 1.04 Mandibular intermolar width: 0.65 Mandibular intercanine width: 0.78 Maxillary arch length: 1 Mandibular arch length: 0.57 |
| Kurushima et al.[44]/2015/Japan | MZ and DZ twins | 65.6 ± 10.3 | 116 MZ +16 DZ (132 pairs; total = 264) | 162 F, 102 M | Serological | Dental casts | Heritability coefficient and structural equation modelling | Maxillary interpremolar width: 0.28 Mandibular interpremolar width: 0.29 |
| Sidlauskas et al.[45]/2016/Lithuania | MZ and DZ twins | 21.73 ± 5.24 | 90 MZ + 51 DZ (141 pairs; total = 282) | 184 F, 98 M | DNA test (15 specific DNA markers) | Lateral cephalogram | Structural equation modelling | Overjet: 0 Overbite: 0.76 |
| Beltagy [46]/2017/Egypt | MZ twins | 3–5 (4.33 ± 0.7) | 20 MZ pairs (total = 40) | 24 F, 16 M | NR | Dental casts | Pearson correlation | Strong correlation for Arch width and length: 0.85 to 0.97, Molar relationship: 0.89, Canine relationship: 0.78. Moderate correlation for overbite (0.67) and overjet (0.57). |
| Anu et al. [47]/ 2018/India | MZ and DZ twins | 15–30 | 17 MZ + 13 DZ (30 pairs; total = 60) | NR | Medical records (Chorionicity and number of placental cords) |
Intra oral examination | Heritability coefficient* | Maxillary intercanine width: 0.23 Mandibular intercanine width: 0.16 Crowding: 0.29 Spacing: 1.43 Buccal segment relationship: 0.64 |
| Tiro et al.[48]/2019/Bosnia | MZ and DZ twins | 8.3–14.8 | 20 MZ + 32 DZ (52 pairs) (total = 104) | NR | Physical characteristics and by number of placentas on birth as reported by mother | Dental casts | t-test | Role of genetics on overjet and overbite could not confirmed (no significant difference between MZ and DZ twins) |
| Negishi et al.[17]/ 2020/Australia | MZ and DZ twins | MZ: 13.7 ± 1, DZ: 13.7 ± 0.8, OSDZ: 13.9 ± 0.9, range: 12–15 years | 45 MZ + 46 DZ (same sex) + 32 DZ (opposite sex) (123 pairs) (total = 246) | 124 F, 122 M | DNA from buccal cells | Digital dental casts | Heritability coefficient and structural equation modelling | Maxillary intermolar width: 0.86 Palatal depth: 0.86 |
| Kim et al[49]/ 2020/South Korea | MZ and DZ twins and siblings | 39.7 ± 9.26 | 36 MZ + 13DZ + 26 sibling pairs (75 pairs) (total = 150) | 82 F, 68 M | Questionnaire of zygosity diagnosis | Lateral cephalogram | Heritability coefficient | Functional occlusal plane to the SN plane: 0.52 Functional occlusal plane to the FH plane: 0.76 |
| Al-Qawasmi et al.[50]/ 2021/USA | Siblings | 12.7 | 148 siblings | 79 F, 69 M | NA | CBCT | Heritability coefficient | Curve of Wilson: 0.61 Curve of Spee: 1 |
| Babu et al.[51]/ 2022/India | MZ and DZ twins | 12–18 | 27 MZ + 24 DZ (51 pairs) (total = 102) | 52 F, 50 M | Facial appearance and history | Dental casts | t-test | No significant differences in arch length and arch width in both arches between MZ and DZ |
| Birant et al.[52]/ 2022/Turkey | MZ and DZ twins | MZ: 9.63, DZ: 9.47 | 59 MZ + 143 DZ (202 pairs) (total = 404) | 200 F, 204 M | NR | Dental casts | t-test, Mann–Whitney U test | Statistically significant differences in the dental arch parameters (length, width, and perimeter) among the MZ and DZ twins |
| Chaaban et al.[21]/2022/USA # | MZ and DZ twins | Mixed dentition (8–12), permanent dentition (13–16) | Mixed dentition 18 MZ +14 DZ (32 pairs) (total = 64) permanent dentition 18MZ + 19DZ (37 pairs) (total =74) |
Mixed dentition (32 F, 32 M) permanent dentition (33 F, 41 M) | NR | Digital dental casts | Heritability coefficient* | Mixed dentition Maxillary intermolar width: 0.06 maxillary intercanine width: −0.14 Permanent dentition Maxillary intermolar width: 0.43 maxillary intercanine width: 0.41 |
NA: not applicable, NR: not reported, MZ: monozygotic, DZ: dizygotic, OSDZ: opposite-sex dizygotic, F: females, M: males, CBCT: cone beam computed tomography, #: longitudinal studies, *: combined variance method used to calculate confidence interval of heritability coefficient.
Among the 23 twin studies, 22 were classic twin studies comparing monozygotic and dizygotic twins [2, 16–21, 33–37, 39, 40, 42–45, 47, 48, 51, 52] and 1 study was conducted among monozygotic twins only [46]. One of the classic twin studies compared twins reared apart [34]. Only one twin study was longitudinal, evaluating dental arches from the mixed to the permanent dentition [21]. The most common method of zygosity determination in twin studies was serological testing, followed by the buccal swab method. Six studies, however, did not report the method of zygosity determination [21, 33, 36, 39, 46, 52]. Among the five sibling studies, only one was longitudinal in design, comparing the dental occlusion of siblings from age 4 to 20 years [38].
Synthesis of results
The studies employed various approaches for determining the heritability of dental arches and occlusal characteristics. Estimation of the heritability coefficient using the intraclass correlation coefficient was the most common approach, but studies also used structural equation modelling, Pearson correlation coefficient, and even conventional tests like t-tests to compare monozygotic (MZ) and dizygotic (DZ) twins. Although all studies meeting the selection criteria were included in the systematic review, only studies reporting the heritability coefficient were included in the meta-analysis.
Heritability coefficient
Heritability typically varies between 0 and 1, and the reported coefficients for various outcomes spanned the entire range. Some studies, however, reported heritability coefficients of less than 0 or greater than 1. Most studies failed to specify the type of heritability, with only six studies explicitly indicating it: five [2, 16, 17, 38, 45] reported narrow-sense heritability, and one [21] reported broad-sense heritability. The meta-analyses of the heritability estimates were conducted for individual outcomes from the studies where the outcomes were measured uniformly using the same landmarks and were limited to 15 studies reporting dental arch dimensions and occlusal characteristics in the permanent dentition.
Dental arch parameters
For dental arch width, meta-analyses of heritability estimates for intermolar and inter-canine widths were conducted separately in maxillary and mandibular arches. Heritability for the maxillary intermolar width from 10 studies was estimated at 0.52 (95% CI, 0.34–0.7) and for the maxillary intercanine width from 9 studies was estimated at 0.54 (95% CI, 0.31–0.78; Fig. 2). Likewise, heritability for the mandibular intermolar and intercanine widths from seven studies were estimated at 0.55 (95% CI, 0.35–0.76) and 0.55 (95% CI, 0.33–0.77) respectively (Fig. 3).
Figure 2.
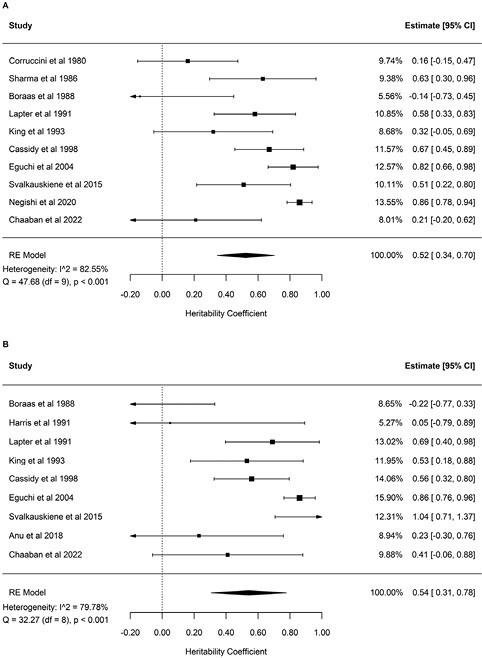
Forest plots of the heritability estimates for maxillary intermolar width (A) and maxillary intercanine width (B).
Figure 3.

Forest plots of the heritability estimates for mandibular intermolar width (A) and mandibular intercanine width (B).
For the maxillary and mandibular arch lengths, heritability estimates were 0.76 (95% CI, 0.59–0.92) and 0.57 (95% CI, 0.34–0.80) respectively (Figure 4). Heritability of palatal depth was estimated at 0.56 (95% CI, 0.22–0.90; Fig. 5). High heterogeneity (I2 between 56.24% and 98.58%) was observed in the meta-analyses of the dental arch parameters.
Figure 4.
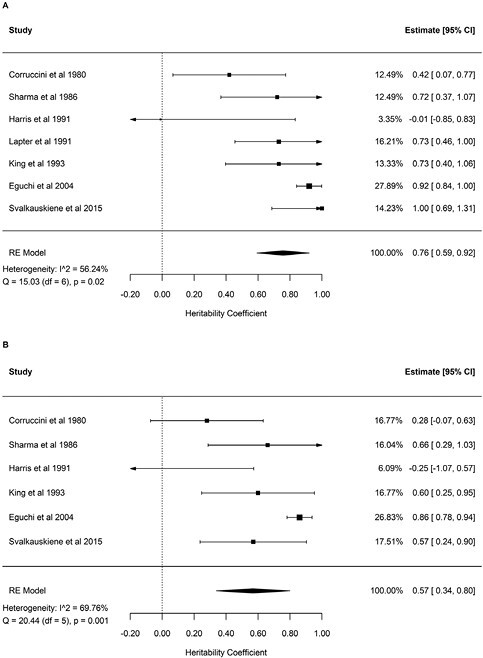
Forest plots of the heritability estimates for maxillary arch length (A) and mandibular arch length (B).
Figure 5.

Forest plot of the heritability estimate of palatal depth.
Occlusal parameters
The heritability coefficients for the crossbite and overbite were relatively higher compared to other occlusal parameters with coefficients estimated at 0.46 (95% CI, 0.27–0.65) and 0.44 (95% CI, 0.31–0.56), respectively. The heritability coefficient for the buccal segment relationship was 0.32 (95% CI, 0.15–0.49) and for overjet, it was 0.25 (95% CI, 0.04–0.46). Among the occlusal parameters, the rotation and displacement of teeth demonstrated the lowest heritability with the coefficient estimated at 0.16 (95% CI, −0.08 to 0.4; Supplementary Document 4). High heterogeneity (I2 between 41.37% and 65.23%) was observed in most of the meta-analyses of the occlusal parameters except crossbite (I2 = 0%).
Other findings in the permanent dentition
For some of the outcomes, meta-analysis was not deemed suitable due to a limited number of studies and methodological heterogeneity. The review identified three studies reporting the heritability of spacing in dental arches in permanent dentition. The heritability coefficients reported in these studies ranged from 0.02 to 1.43, indicating considerable variability and possible issues with the estimation of heritability [19, 20, 47]. One study each evaluated the heritability of the occlusal plane and Curve of Spee and Wilson. Among the occlusal planes, the functional occlusal plane demonstrated the highest heritability followed by the bisected occlusal plane [49]. The heritability estimates for the functional occlusal plane to the Sella–Nasion line was 0.52 and to the Frankfort horizontal line was 0.76. The curve of spee was highly heritable between siblings, with a heritability estimate of 1, while the curve of Wilson was moderately heritable, with a heritability estimate of 0.6 [50].
Low heritability estimates for different malocclusions were reported by Kawala et al. [42], with the highest estimates being 0.12 and 0.11, respectively, for mandibular crowding and class II malocclusion among male samples. On the other hand, Liu et al. [40] reported a high heritability estimate (0.72) for arch size but did not specify whether the heritability estimate was related to arch length or arch width in the maxillary or mandibular arch. The study also reported a ‘weaker’ heritability for occlusal traits, namely overjet, overbite, and molar relationship, without specifying any heritability estimate. The study was therefore excluded from the meta-analysis.
Some studies did not report heritability estimates per se. A study using polynomial coefficients found that genetic factors influenced the variations in the shape of maxillary and mandibular arches but not the asymmetry [36]. Similarly, another study employing a Fourier analysis concluded that the arch size and shape were under genetic influence [39].
Other studies used conventional tests to assess the genetic influence on the traits with an assumption that a trait is under a strong genetic influence if there is a statistically significant difference between the MZ and DZ twins. One such study reported that palatal height was under strong hereditary influence, but the arch length and widths were influenced by environmental factors [33]. Likewise, another study could not attribute the influence of heredity to overjet and overbite as there was no statistically significant difference between the MZ and DZ twins [48] Furthermore, Birant et al. [52] reported statistically significant differences in the dental arch parameters (length, width, and perimeter) among MZ and DZ twins and suggested a genetic influence on dental arches. However, Babu et al. [51] found no significant differences in the dental arch parameters based on zygosity.
Primary and mixed dentition
Three studies assessing the heritability of dental arches and occlusal traits in primary dentition were identified. Hughes et al. [16] reported high heritability (0.62–0.81) for the interdental spacing in the primary dentition and the arch width and length in the primary dentition were also highly heritable, with estimates in the range of 0.69–0.89. This study also found a moderate to low heritability for overbite (0.53) and overjet (0.28), respectively [16]. Harris and Johnson [38] similarly reported high heritability for arch width and length in the primary dentition among siblings, with estimates in the range of 0.79–1.0, except for overjet (0), rotation (0.09), and posterior crossbite (0.14); the heritability estimates for other occlusal parameters were in the range of 0.53–0.66. Similarly, Beltagy [46] reported a strong correlation among monozygotic twins in relation to the arch width and length, with the Pearson correlation coefficient in the range of 0.85–0.97, as well as for molar (0.89) and canine (0.78) relationship, but the correlation was moderate for overbite (0.67) and overjet (0.57). In the only study conducted in the mixed dentition, Chaaban et al. [21] reported a very low heritability estimate for intermolar and intercanine widths, with estimates in the range of 0–0.06. There were a limited number of studies assessing the heritability of dental arches and occlusal traits in the primary and mixed dentition, which also presented with methodological heterogeneity, so meta-analysis was confined to studies in the permanent dentition.
Risk of bias
Among 28 studies, 15 studies demonstrated a low risk of bias with a score of 7 or above out of 8 as per the JBI critical appraisal tool [27]. The most common reason for bias was the inability to measure outcomes in a valid and reliable way. However, studies were not excluded based on their methodological qualities (Supplementary Document 5). Although the primary intention was to investigate reporting bias through the assessment of funnel plot asymmetry, it was not performed due to the unavailability of meta-analyses comprising more than ten studies [53].
Certainty of evidence
The overall certainty of the evidence for most of the outcomes was ‘low’ as per the GRADE assessment criteria [32]. However, for two outcomes related to the occlusal characteristics (rotation and displacement of teeth and overjet), the certainty of evidence was ‘very low’. As all the outcomes were derived from observational studies, they started at a ‘low’ certainty of evidence and for two of the occlusal outcomes, they were further downgraded for inconsistency resulting in an overall ‘very low’ certainty (Table 2).
Table 2:
GRADE certainty of evidence for the heritability of dental arches and occlusal characteristics
| Quality assessment | Heritability coefficient (95% CI) | Overall certainty of evidence | |||||
|---|---|---|---|---|---|---|---|
| Participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ||
| Maxillary intermolar width | |||||||
| 1673 (10 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.52 (0.34–0.70) |
⨁⨁◯◯ Low |
| Maxillary intercanine width | |||||||
| 1294 (9 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.54 (0.31–0.78) |
⨁⨁◯◯ Low |
| Mandibular intermolar width | |||||||
| 1256 (7 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.55 (0.35–0.76) |
⨁⨁◯◯ Low |
| Mandibular intercanine width | |||||||
| 1123 (7 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.55 (0.33–0.77) |
⨁⨁◯◯ Low |
| Maxillary arch length | |||||||
| 979 (7 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.76 (0.59–0.92) |
⨁⨁◯◯ Low |
| Mandibular arch length | |||||||
| 787 (6 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.57 (0.34–0.80) |
⨁⨁◯◯ Low |
| Palatal depth | |||||||
| 710 (4 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.56 (0.22– 0.90) |
⨁⨁◯◯ Low |
| Overjet | |||||||
| 1870 (10 OS) |
Not serious | Seriousa | Not serious | Not serious | None | 0.25 (0.04–0.46) |
⨁◯◯◯ Very low |
| Overbite | |||||||
| 1548 (9 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.44 (0.31–0.56) |
⨁⨁◯◯ Low |
| Crossbite | |||||||
| 815 (5 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.46 (0.27–0.65) |
⨁⨁◯◯ Low |
| Buccal segment relationship | |||||||
| 1362 (8 OS) |
Not serious | Not serious | Not serious | Not serious | None | 0.32 (0.15–0.49) |
⨁⨁◯◯ Low |
| Rotation and displacement | |||||||
| 979 (6 OS) |
Not serious | Seriousa | Not serious | Not serious | None | 0.16 (−0.08–0.40) |
⨁◯◯◯ Very low |
OS = Observational studies, 95% CI = 95% confidence interval, a = inconsistency among results of the studies (downgrade by 1 level).
Discussion
This systematic review analyzed 28 studies that aimed to determine the heritability of dental arch dimensions and occlusal characteristics among a total of 4968 twins and siblings. The meta-analyses included 15 studies which demonstrated high heritability estimates for several of the evaluated parameters. To the best of our knowledge, this is the first meta-analysis of heritability estimates of dental arch dimensions and occlusal parameters. All the dental arch dimensions, namely the maxillary intermolar width (0.52), maxillary intercanine width (0.54), mandibular intermolar width (0.55), mandibular intercanine width (0.55), maxillary arch length (0.76), mandibular arch length (0.57), and palatal depth (0.56) demonstrated high heritability estimates. For the occlusal parameters, the heritability coefficient varied considerably, with relatively moderate values for crossbite (0.46) and overbite (0.44), but the heritability coefficients for buccal segment relationship (0.32), overjet (0.22), and rotation and displacement of teeth (0.16) were low. The overall certainty of the evidence, however, was graded as ‘low’ according to the GRADE criteria. Given that the evidence was derived exclusively from the observational studies that was expected.
There was a difference in the heritability estimates between the dental arch dimensions and occlusal traits, with a greater heritability estimate for the dental arch dimensions. The strong genetic influence on dental arch dimensions resembled the genetic influence on the craniofacial skeleton documented in earlier studies [54, 55]. This suggests a stronger genetic influence on the skeletal component compared to dental occlusion. A meta-analysis evaluating twin studies across 50 years reported the overall heritability estimate across all human traits to be 0.49 [56]. The heritability estimates for the dental arch parameters were higher than the overall heritability across all human traits, but the estimates for occlusal parameters were lower than the overall estimate. Maxillary and mandibular dental arches develop independently, and the occlusal traits depend upon the development of both arches, so it could be hypothesized that a minor variation in the development of either of the arches could result in a significant change in occlusal characteristics. This might explain the low heritability of occlusal parameters compared to the dental arch parameters.
The overall heritability estimates for maxillary and mandibular arch widths were similar, but the heritability estimates for the arch lengths differed considerably, with higher estimates for the maxillary arch compared to the mandibular arch. Earlier studies reported higher heritability estimates for dental arch dimensions in the posterior region of the arch compared to the anterior region and it was hypothesized that the anterior region of the arch could be more influenced by environmental factors [2, 17, 57]. The findings from this review do not support that hypothesis as the heritability estimates for the mandibular intermolar and intercanine widths were equal. Contrary to the hypothesis, in the maxillary arch, the heritability estimate for the intercanine width was slightly higher than that of the intermolar width (0.54 vs. 0.52). Further research, however, is needed to better understand the influence of genetic factors in different regions of the dental arch.
The heritability of a trait is not a fixed value and can change with time due to fluctuations in genetic influences, environmental factors, and gene-environment interactions [8]. Earlier research suggested that the heritability of the dentoalveolar trait diminishes with age (from primary to permanent dentition) [38]. This review could not verify that claim due to a limited number of studies in primary and mixed dentition compared to permanent dentition. Though two studies in the primary dentition reported relatively high heritability estimates for evaluated traits, a meta-analysis could not be conducted due to methodological differences [16, 38]. A single paper in the mixed dentition, however, reported a very low heritability estimate close to zero. which could be due to a small sample size underpowering the study [21]. It is disappointing that this review could not provide further evidence regarding the temporal changes in heritability, though it was initially planned. This, however, suggests that more research is necessary in primary and mixed dentition to better understand this topic.
Studies included in the review used various methods to evaluate heritability, but the most common method involved calculating the heritability coefficient using the classical correlation approach. One of the limitations of the heritability coefficient is that it does not estimate the influence of shared environment, so the calculated heritability coefficient could be inflated [10]. Few studies used a more sophisticated model-fitting approach to estimate the relative contribution of the genetic and environmental factors for a trait and reported a narrow sense heritability for the same. In general, studies failed to distinguish between narrow-sense and broad-sense heritability while reporting the heritability estimate. Thus, for the purpose of this meta-analysis, heritability, in general, was reported without any distinction between the narrow and broad sense heritability. Furthermore, some studies merely compared the mean difference between the monozygotic and dizygotic twins using conventional tests such as t-test and did not report heritability per se, rendering them unsuitable for meta-analysis. A more comprehensive meta-analysis would have been possible if the studies had adopted a uniform and consistent methodology.
Due to considerable heterogeneity among the studies, a random-effects meta-analysis was deemed suitable to estimate the average heritability coefficient across studies. The heterogeneity (I2) was high for most of the analyses, which could be attributed to methodological differences and variations in the sample population among the studies. However, it has been reported that the I2 value is generally biased and high in prevalence meta-analyses and should not be a cause for significant concern [58]. Only crossbite reported a low heterogeneity (0%), which could be attributed to similarity in heritability coefficients among the five studies reporting the heritability of crossbite and overlapping of confidence intervals of the heritability coefficients among the studies [59].
Heritability estimates are population-specific and are influenced by ethnicity [7]. This review, however, could not estimate the ethnic variation among the heritability estimates of dental arches and occlusal parameters because not all the studies specified the ethnicity of the participants. The studies reported the heritability estimates in general without sex distinction, so inference could also not be made on sex-specific heritability estimates.
Strengths and limitations
The strengths of this systematic review include its pre-registered protocol [60], comprehensive search of the literature [61], assessment of the quality of evidence using GRADE, and transparent review process. However, there are also several limitations of this review. One of the primary limitations of this review was the methodological differences among the studies, resulting in the inclusion of only 15 studies in the meta-analysis, out of the possible 28. This methodological inconsistency presented a challenge in conducting a quantitative synthesis of the outcomes across studies and calls for the need for a more rigorous and consistent approach to estimating heritability. A lack of distinction between narrow-sense and broad-sense heritability in the articles was another limitation, as it restricted a meaningful comparison of heritability estimates across studies. Furthermore, it should be noted that heritability is a population-level concept that estimates the genetic contribution to the variation of a trait within a population and cannot be directly applied to individuals [7]. For instance, a heritability estimate of 0.76 for the maxillary arch length means that 76% of the variability in the maxillary arch length in the population is due to genetic differences among people. Therefore, it is important to avoid overgeneralizing heritability estimates while describing traits at an individual level.
Considerations for future research
This review identified limited research in the primary and mixed dentition stages, with a bulk of papers targeting the permanent dentition. More research, therefore, is required in the primary and mixed dentition stages of occlusal development. If possible, longitudinal studies from the primary to the permanent dentition with larger sample sizes using a classic twin study design should be conducted to assess genetic influences in the different stages of dentition. It must be stressed that considerable heterogeneity in the selection of landmarks for measuring different arch and occlusal parameters and methods of heritability estimation used in different studies was present, which limited the number of studies to be included in the meta-analysis. Uniformity in the landmark selection and measurement along with the method of heritability estimation should be given due consideration in future research. Instead of merely estimating the heritability of various dental and occlusal parameters, there is a need to evaluate the relative contribution of genetic and environmental factors using more robust structural equation modelling approaches. Like other dental variations such as missing and supernumerary teeth, the development of dental arches and subsequently the occlusion could be influenced by a complex interaction among genetic, environmental, and epigenetic factors [62]. The epigenetic influences on the dental arch and occlusion could be investigated by evaluating the phenotypic differences among the monozygotic twins [63].
In the post-genomic era, although genome-wide association studies have received significant attention, twin/family studies remain equally important. With different assumptions and sample size requirements, these methods complement each other in understanding various aspects of human genetics [64].
Conclusions
Overall, with a low certainty in the body of evidence, it can be concluded that the dental arch dimensions, namely arch width, arch length, and palatal depth, are under a strong genetic influence with high heritability estimates in the range of 0.52–0.76. However, occlusal parameters demonstrate moderate to low heritability estimates (0.46–0.16) with crossbite and overbite at the upper end and rotation and displacement of teeth at the lower end of the spectrum.
Supplementary Material
Acknowledgements
The authors would like to thank Vikki Langton for her support in refining the search strategy. Jamal Giri and Taseef Hasan Farook are the recipients of The University of Adelaide Research Scholarship.
Contributor Information
Jamal Giri, Adelaide Dental School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Michelle Bockmann, Adelaide Dental School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Alan Brook, Adelaide Dental School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Taseef Hasan Farook, Adelaide Dental School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Maurice Meade, Adelaide Dental School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Toby Hughes, Adelaide Dental School, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Author Contributions
Jamal Giri (Conceptualization [Equal], Data curation [Equal], Formal analysis [Equal], Investigation [Equal], Methodology [Equal], Project administration [Equal], Resources [Equal], Software [Equal], Validation [Equal], Visualization [Equal], Writing—original draft [Lead], Writing—review & editing [Equal]), Michelle Bockmann (Conceptualization [Equal], Methodology [Equal], Project administration [Equal], Supervision [Equal], Writing—review & editing [Equal]), Alan Brook (Conceptualization [Equal], Methodology [Equal], Project administration [Equal], Supervision [Equal], Writing—review & editing [Equal]), Taseef Farook (Conceptualization [Equal], Data curation [Equal], Investigation [Equal], Methodology [Equal], Validation [Equal], Writing—review & editing [Equal]), maurice meade (Conceptualization [Equal], Methodology [Equal], Supervision [Equal], Writing—review & editing [Equal]), and Toby Hughes (Conceptualization [Equal], Investigation [Equal], Methodology [Equal], Project administration [Equal], Supervision [Equal], Validation [Equal], Writing—review & editing [Equal])
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
None.
Data Availability
All data generated or analysed in this review are included in the published article and supplementary files.
Ethics approval
Not applicable.
References
- 1. Harris, EF, Smith, RJ.. A study of occlusion and arch widths in families. Am J Orthod 1980;78:155–63. 10.1016/0002-9416(80)90057-3 [DOI] [PubMed] [Google Scholar]
- 2. Eguchi, S, Townsend, GC, Richards, LC, Hughes, T, Kasai, K.. Genetic contribution to dental arch size variation in Australian twins. Arch Oral Biol 2004;49:1015–24. 10.1016/j.archoralbio.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 3. Lundström, A. Nature versus nurture in dento-facial variation. Eur J Orthod 1984;6:77–91. 10.1093/ejo/6.2.77 [DOI] [PubMed] [Google Scholar]
- 4. Mossey, P. The heritability of malocclusion: part 2. The influence of genetics in malocclusion. Br J Orthod 1999;26:195–203. [DOI] [PubMed] [Google Scholar]
- 5. Carlson, DS. Evolving concepts of heredity and genetics in orthodontics. Am J Orthod Dentofac Orthop 2015;148:922–38. 10.1016/j.ajodo.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 6. Proffit, WR. On the Aetiology of Malocclusion: The Northcroft Lecture, 1985 Presented to the British Society for the Study of Orthodontics, Oxford, April 18, 1985. Br J Orthod 1986;13:1–11. [PubMed] [Google Scholar]
- 7. Visscher, PM, Hill, WG, Wray, NR.. Heritability in the genomics era—concepts and misconceptions. Nat Rev Genet 2008;9:255–66. 10.1038/nrg2322 [DOI] [PubMed] [Google Scholar]
- 8. Wray, N, Visscher, P.. Estimating trait heritability. Nat Educ 2008;1:29. [Google Scholar]
- 9. Townsend, G, Hughes, T, Luciano, M, Bockmann, M, Brook, A.. Genetic and environmental influences on human dental variation: a critical evaluation of studies involving twins. Arch Oral Biol 2009;54:S45–51. 10.1016/j.archoralbio.2008.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenesa, A, Haley, CS.. The heritability of human disease: estimation, uses and abuses. Nat Rev Genet 2013;14:139–49. 10.1038/nrg3377 [DOI] [PubMed] [Google Scholar]
- 11. Lauweryns, I, Carels, C, Vlietinck, R.. The use of twins in dentofacial genetic research. Am J Orthod Dentofac Orthop 1993;103:33–8. 10.1016/0889-5406(93)70101-S [DOI] [PubMed] [Google Scholar]
- 12. Harris, EF. Interpreting heritability estimates in the orthodontic literature. Semin Orthod 2008;14:125–34. 10.1053/j.sodo.2008.02.003 [DOI] [Google Scholar]
- 13. Simon, B, Aschheim, K, Vág, J.. The discriminative potential of palatal geometric analysis for sex discrimination and human identification. J Forensic Sci 2022;67:2334–42. 10.1111/1556-4029.15110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin, N, Boomsma, D, Machin, G.. A twin-pronged attack on complex traits. Nat Genet 1997;17:387–92. 10.1038/ng1297-387 [DOI] [PubMed] [Google Scholar]
- 15. King, L, Harris, EF, Tolley, EA.. Heritability of cephalometric and occlusal variables as assessed from siblings with overt malocclusions. Am J Orthod dentofac Orthop 1993;104:121–31. 10.1016/S0889-5406(05)81001-7 [DOI] [PubMed] [Google Scholar]
- 16. Hughes, T, Thomas, C, Richards, L, Townsend, G.. A study of occlusal variation in the primary dentition of Australian twins and singletons. Arch Oral Biol 2001;46:857–64. 10.1016/s0003-9969(01)00026-7 [DOI] [PubMed] [Google Scholar]
- 17. Negishi, S, Richards, LC, Hughes, T, Kondo, S, Kasai, K.. Genetic contribution to palatal morphology variation using three-dimensional analysis in Australian twins. Arch Oral Biol 2020;115:104740. 10.1016/j.archoralbio.2020.104740 [DOI] [PubMed] [Google Scholar]
- 18. Corruccini, RS, Potter, RH.. Genetic analysis of occlusal variation in twins. Am J Orthod. 1980;78:140–54. 10.1016/0002-9416(80)90056-1 [DOI] [PubMed] [Google Scholar]
- 19. Potter, RHY, Corruccini, RS, Green, LJ.. Variance of occlusion traits in twins. J Craniofac Genet Dev Biol 1981;1:217–27. [PubMed] [Google Scholar]
- 20. Sharma, K, Corruccini, R.. Genetic basis of dental occlusal variations in northwest Indian twins. Eur J Orthod 1986;8:91–7. 10.1093/ejo/8.2.91 [DOI] [PubMed] [Google Scholar]
- 21. Chaaban, M, AlSulaiman, A, Kantarci, A, Stashenko, P, Will, LA, Motro, M.. Longitudinal changes in the dental arch width and symmetry in identical and fraternal twins. Am J Orthod Dentofacial Orthop 2022;162:704–13. 10.1016/j.ajodo.2021.06.026 [DOI] [PubMed] [Google Scholar]
- 22. Santana, LG, Flores-Mir, C, Iglesias-Linares, A, Pithon, MM, Marques, LS.. Influence of heritability on occlusal traits: a systematic review of studies in twins. Prog Orthod 2020;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page, MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (London, England) 2021;88:105906. 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 24. Morgan, RL, Whaley, P, Thayer, KA, Schünemann, HJ.. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018;121:1027–31. 10.1016/j.envint.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The EndNote Team. EndNote. EndNote X9 ed. Philadelphia, PA: Clarivate Analytics, 2013. [Google Scholar]
- 26. Software CSR. Covidence systematic review software. Melbourne: Veritas Health Innovation Melbourne, 2018. [Google Scholar]
- 27. Moola, S, Munn, Z, Tufanaru, C, Aromataris, E, Sears, K, Sfetcu, R, et al. Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer’s Manual 2017;5, 217–69. [Google Scholar]
- 28. Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 29. Li, MD, Cheng, R, Ma, JZ, Swan, GE.. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 2003;98:23–31. 10.1046/j.1360-0443.2003.00295.x [DOI] [PubMed] [Google Scholar]
- 30. Higgins, JP, Thompson, SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 31. Higgins, JP, Thompson, SG, Deeks, JJ, Altman, DG.. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schünemann, H, Brożek, J, Guyatt, G, Oxman, A.. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available fromguidelinedevelopment.org/handbook
- 33. Varma, RK. Genetic environmental interaction on dental arch dimensions. J Indian Dent Assoc 1986;58:99–102. [PubMed] [Google Scholar]
- 34. Boraas, JC, Messer, LB, Till, MJ.. A genetic contribution to dental caries, occlusion, and morphology as demonstrated by twins reared apart. J Dent Res 1988;67:1150–5. 10.1177/00220345880670090201 [DOI] [PubMed] [Google Scholar]
- 35. Townsend, GC, Corruccini, RS, Richards, LC, Brown, T.. Genetic and environmental determinants of dental occlusal variation in South Australian twins. Aust Orthod J 1988;10:231–5. [PubMed] [Google Scholar]
- 36. Richards, LC, Townsend, GC, Brown, T, Burgess, VB.. Dental arch morphology in south Australian twins. Arch Oral Biol 1990;35:983–9. 10.1016/0003-9969(90)90018-6 [DOI] [PubMed] [Google Scholar]
- 37. Lapter, V, Slaj, M, Muretić, Z, Weber, D.. Orthodontic anomalies and differences in gnathometric variables in twins. Acta stomatologica Croatica. 1991;25:25–31. [PubMed] [Google Scholar]
- 38. Harris, EF, Johnson, MG.. Heritability of craniometric and occlusal variables: a longitudinal sib analysis. Am J Orthod Dentofac Orthop 1991;99:258–68. 10.1016/0889-5406(91)70007-J [DOI] [PubMed] [Google Scholar]
- 39. Kasai, K, Richards, LC, Townsend, GC, Kanazawa, E, Iwasawa, T.. Fourier analysis of dental arch morphology in South Australian Twins. Anthropol Sci 1995;103:39–48. 10.1537/ase.103.39 [DOI] [Google Scholar]
- 40. Liu, H, Deng, H, Cao, CF, Ono, H.. Genetic analysis of dental traits in 82 pairs of female-female twins. Chinese Journal of Dental Research 1998;1:12–6. [PubMed] [Google Scholar]
- 41. Cassidy, KM, Harris, EF, Tolley, EA, Keim, RG.. Genetic influence on dental arch form in orthodontic patients. Angle Orthod 1998;68:445–54. [DOI] [PubMed] [Google Scholar]
- 42. Kawala, B, Antoszewska, J, Necka, A.. Genetics or environment? a twin-method study of malocclusions. World J Orthod 2007;8:405–10. [PubMed] [Google Scholar]
- 43. Švalkauskienė, V, Šmigelskas, K, Šalomskienė, L, Andriuškevičiūtė, I, Šalomskienė, A, Vasiliauskas, A, Šidlauskas, A.. Heritability estimates of dental arch parameters in Lithuanian twins. Stomatologija. 2015;17:3–8. [PubMed] [Google Scholar]
- 44. Kurushima, Y, Ikebe, K, Matsuda, K, Enoki, K, Ogata, S, Yamashita, M, Murakami, S, Hayakawa, K, Maeda, Y.. Influence of genetic and environmental factors on oral diseases and function in aged twins. J Oral Rehabil 2015;42:49–56. 10.1111/joor.12228 [DOI] [PubMed] [Google Scholar]
- 45. Sidlauskas, M, Salomskiene, L, Andriuskeviciute, I, Sidlauskiene, M, Labanauskas, Z, Vasiliauskas, A, Kupčinskas, L, Juzėnas, S, Šidlauskas, A.. Heritability of mandibular cephalometric variables in twins with completed craniofacial growth. Eur J Orthod 2016;38:493–502. 10.1093/ejo/cjv062 [DOI] [PubMed] [Google Scholar]
- 46. Beltagy, TM. Variance of occlusion among preschool monozygotic twins. Tanta Dental J 2017;14:148. 10.4103/tdj.tdj_25_17 [DOI] [Google Scholar]
- 47. Anu, V, Arsheya, GS, Anjana, V, Annison, GK, Aruna, MRL, Alice, AP, et al. Dental caries experience, dental anomalies, and morphometric analysis of canine among monozygotic and dizygotic twins. Contemp Clin Dent 2018;9:S314–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tiro, A, Dzemidzic, V, Salaga-Nefic, S, Redzic, I, Nakas, E.. Heritability of craniofacial characteristics in twins—cephalometric study. Medical Arch (Sarajevo, Bosnia and Herzegovina). 2019;73:205–8. 10.5455/medarh.2019.73.205-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim, JH, Kim, YH, Kim, SJ, Sung, J, Song, YM, Shin, JW, Park, JH, Chae, HS.. Twin study-genetic comparison of matrix versus intramatrix rotation in the mandible and three different occlusal planes. Prog Orthod 2020;21:44. 10.1186/s40510-020-00344-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Al-Qawasmi, R, Coe, C.. Genetic influence on the curves of occlusion in children seeking orthodontic treatment. Int Orthodont. 2021;19:82–7. 10.1016/j.ortho.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 51. Babu, KLG, Doddamani, GM, Kavyashree, GH.. Dental arch characteristics among South Indian twins—a morphometric study. Braz J Oral Sci 2022;21:e225388. [Google Scholar]
- 52. Birant, S, Koruyucu, M, Kasimoglu, Y, Veznikli, M, Seymen, F.. Assessment of dental arch parameters in Turkish twins. J Clin Pediatr Dent 2022;46:160–70. 10.17796/1053-4625-46.2.12 [DOI] [PubMed] [Google Scholar]
- 53. Dalton, JE, Bolen, SD, Mascha, EJ.. Publication bias: the elephant in the review. Anesth Analg 2016;123:812–3. 10.1213/ANE.0000000000001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakata, M, Yu, P-I, Davis, B, Nance, WE.. The use of genetic data in the prediction of craniofacial dimensions. Am J Orthodont. 1973;63:471–80. 10.1016/0002-9416(73)90160-7 [DOI] [PubMed] [Google Scholar]
- 55. Lobb, WK. Craniofacial morphology and occlusal variation in monozygous and dizygous twins. Angle Orthod 1987;57:219–33. [DOI] [PubMed] [Google Scholar]
- 56. Polderman, TJ, Benyamin, B, De Leeuw, CA, Sullivan, PF, Van Bochoven, A, Visscher, PM, Posthuma, D.. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 2015;47:702–9. 10.1038/ng.3285 [DOI] [PubMed] [Google Scholar]
- 57. Hu, J, Nakasima, A, Takahama, Y.. Familial similarity in dental arch form and tooth position. J Craniofac Genet Dev Biol 1992;12:33–40. [PubMed] [Google Scholar]
- 58. Migliavaca, CB, Stein, C, Colpani, V, Barker, TH, Ziegelmann, PK, Munn, Z, Falavigna, M; Prevalence Estimates Reviews-Systematic Review Methodology Group (PERSyst). Meta‐analysis of prevalence: I 2 statistic and how to deal with heterogeneity. Res Synth Methods 2022;13:363–7. 10.1002/jrsm.1547 [DOI] [PubMed] [Google Scholar]
- 59. Chang, Y, Phillips, MR, Guymer, RH, Thabane, L, Bhandari, M, Chaudhary, V, et al. The 5 min meta-analysis: understanding how to read and interpret a forest plot. Eye 2022;36:673–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sideri, S, Papageorgiou, SN, Eliades, T.. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J Clin Epidemiol 2018;100:103–10. 10.1016/j.jclinepi.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 61. Bramer, WM, Rethlefsen, ML, Kleijnen, J, Franco, OH.. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brook, A. Multilevel complex interactions between genetic, epigenetic and environmental factors in the aetiology of anomalies of dental development. Arch Oral Biol 2009;54:S3–S17. 10.1016/j.archoralbio.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Townsend, G, Richards, L, Hughes, T, Pinkerton, S, Schwerdt, W.. Epigenetic influences may explain dental differences in monozygotic twin pairs. Aust Dent J 2005;50:95–100. 10.1111/j.1834-7819.2005.tb00347.x [DOI] [PubMed] [Google Scholar]
- 64. Friedman, NP, Banich, MT, Keller, MC.. Twin studies to GWAS: there and back again. Trends Cogn Sci 2021;25:855–69. 10.1016/j.tics.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed in this review are included in the published article and supplementary files.


