Abstract
Diabetes, a chronic metabolic disorder affecting millions worldwide, presents a significant health challenge characterized by impaired glucose regulation and potential complications. This study examines the antidiabetic effects of a polyherbal formulation (PENN-DIABEX) prepared from five different medicinal plant extracts. The objective is to ascertain its efficacy in managing streptozotocin (STZ) induced diabetes in rats. To accomplish this, six distinct groups of rats were involved five with induced diabetes and one serving as a normal control. Among the diabetic groups, one received no treatment, functioning as the diabetic control group. The remaining three groups were administered PHF in three different doses while the 6th group was given metformin. On the last day of the experiment, all rats were sacrificed, and blood samples were taken in collecting tubes to analyze blood biochemical parameters. Additionally, tissue samples from the liver, kidney, and pancreas were preserved in formalin solution for subsequent histopathological activity. The results of the study revealed that treatment with PHF in diabetic rats led to a significant (P < 0.01) improvement in fasting blood glucose levels (FBG), glycated hemoglobin (HbA1c), and various biochemical markers including LFTs, RFTs, and lipid profiling. Furthermore, the histology of the liver, kidney, and pancreas indicated that the formulation did not induce any metabolic toxicity. Comparative analysis of the antidiabetic effects of PHF with those of metformin, revealed that the PHF showed better results than the standard drug. This suggests its potential utilization as a safer and alternative approach in the treatment of diabetes.
Keywords: Diabetes, STZ, Histopathology, In vivo, PENN-DIABEX, PHF
1. Introduction
In 2021, diabetes led to the loss of 6.7 million lives, while 541 million adults face Impaired Glucose Tolerance (IGT), predisposing them to an elevated risk of developing type 2 diabetes. (Sun et al., 2022). Pakistan is the 3rd most effective country with diabetes after China and India. About 33 million people in Pakistan are living with diabetes (Adnan and Aasim, 2020). This condition poses not only a daily health hurdle but also a substantial financial burden. The annual estimated worldwide economic impact of diabetes reaches $825 billion international dollars, constituting approximately 12% of the global healthcare expenditure. Moreover, the surge in diabetes cases projected for the forthcoming years is anticipated to be particularly pronounced in developing nations and low- to middle-income countries. This concerning trend is further compounded by limited access to diagnosis and treatment within these regions (Sun et al., 2022).
Diabetes mellitus type II is a complicated type of diabetes that results in other complications such as neuropathy, heart diseases, strokes, and cataracts (Ellahham, 2020). It is a complex disease influenced by more than a single gene and environmental factors such as lack of physical activity, obesity, and diet play an important role in the development of type II diabetes mellitus (Udler, 2019). Current available medicines for diabetes only focus on decreasing blood glucose and most of them consist of synthetic compounds. So, their long-term use causes serious side effects like Abdominal pain, blurred vision, hair loss, Blisters, Rashes, and Nausea (Cusi and DeFronzo, 1998, Dowarah and Singh, 2020). To deal with these issues there is a strong need to develop herbal medicines for the treatment of diabetes and associated complications. The use of herbal medicine and components derived from plants as remedies for a wide range of diseases and health problems around the world has a long history. In recent years, there has been an increasing trend towards adopting herbal remedies that are based on conventional medical procedures. These alternatives are being researched as a supplement to or a replacement for conventional treatments for issues like cancer, hypertension, hyperglycemia, and hyperlipidemia (Ngo et al., 2013).
Current polyherbal formulation possess a number of benefits over available drugs in the market for diabetes. This polyherbal not only controls hyperglycemia as well but it also controls hyperlipidemia which accounts for 95% prevalence in diabetic patients and is a major risk factor in the progression of diabetes mellitus type II.
2. Material and methods
2.1. Preparation of polyherbal formulation
The polyherbal formulation (PHF) was prepared using the five most active medicinal plants, Opuntia ficus-indica, Syzygium cumini, Rhus coriaria, Coffea arabica, and Eclipta alba, obtained from preliminary screening 51 extracts library of different parts of 35 plants against alpha-amylase, alpha-glucosidase, and lipase enzymes. The plants and fruits of the mentioned herbs were collected and washed with water and air dried for one week in a clean and controlled environment. This dried material was coarse ground before being subjected to green extraction technology via microwave-assisted extraction, in which 200 mg of each plant material was taken in a flask separately and mixed with 500 mL ethanol. The extracts were then microwaved for 6 min at 400 W and repeated in 3 cycles. Each extract was then passed through a rotary evaporator and lipolyzed to remove 99.9% water. These powder extracts were kept at −20 °C until their further use. The polyherbal formulation mentioned in Table 1 was developed by using ayurvedic antidiabetic formulation procedures which have already been reported (Bera et al., 2010).
Table 1.
Composition of ingredients present in polyherbal formulation.
| Scientific Name | Common Name | Family | Used Part | Concentration (mg) |
|---|---|---|---|---|
| Opuntia ficus-indica | prickly pear | Cactaceae | Fruit | 37.5 |
| Syzygium cumini | Java plum | Myrtaceae | Leaves | 25 |
| Rhus coriaria | Sicilian sumac | Anacardiaceae | Fruit | 25 |
| Coffea arabica | Coffee | Rubiaceae | Fruit | 25 |
| Eclipta alba | Bhangra | Asteraceae | Whole Plant | 12.5 |
2.2. Animal selection and care
This study was performed on mature Wister albino rats. Three-month-old rats, with a weight of 150 ± 10 g were acclimated in a laboratory setting for fifteen days. All rates were placed in crocus cages (five rats per cage) at a room temperature of 25 ± 2 °C and a light: dark cycle of 12 h. The rats had unlimited access to normal water and food. The Animal Ethical Committee (AEC) of Government College University Faisalabad granted permission for this experiment (Ref. No. GCUF/ERC/22/01). The procedure and rules set by the Laboratory Animal Care were followed during this study period. Animals with normal fasting blood glucose levels of 75 ± 5 mg/dL were used in this experiment (Abdelghffar et al., 2022).
2.3. In vivo assessment of the antidiabetic potential of polyherbal
2.3.1. Establishment of a high-fat diet model
To establish a high-fat rat model, normal rats were given a high-fat diet with a high level of cholesterol. The diet included 13 numbers of normal chow, 2% DL methionine, 5% cholesterol, and 5% casein. All ingredients were thoroughly mixed and fed to rats for nearly four weeks. Water and feed consumption were measured on a daily basis and weighed on a weekly basis throughout the experiment, and rats weighing 230–250 g were chosen for diabetes induction (Roy et al., 2022).
2.3.2. Diabetes induction
Diabetes was induced a using protocol given by Hung et al., 2012 with some modifications. To produce diabetes, 24-hour fasting rats were given 4 g/kg glucose before injecting 30 mg streptozotocin (STZ) in citrate buffer (pH 4.5) per kilogram body weight per rat, as a single intraperitoneal injection and monitored for 7 days. Their fasting blood glucose level was determined after 7 days. For this experiment, twenty-five rats with stable diabetes and fasting blood glucose levels of 250 mg/dL (11 mM/dl) were chosen as diabetic rats.
2.3.3. Experimental design
All rats were divided into equally sized six groups with five rats in each group for 28 days of study. The twenty-eight-day model was chosen because this was the threshold duration of treatment in our pilot experiment. The following grouping was done:
Group 1 (Normal Control): Normal healthy rats who were given normal diet for 28 days and free excess to water.
Group 2 (Diabetic Control): Diabetic rats were given high fat for 4 weeks after which STZ injection of 30 mg/kg was given intraperitoneally to induce diabetes.
Group 3 (Dose experiment 1): Diabetic rats orally administrated polyherbal formulation 200 mg/kg for four weeks.
Group 4 (Dose experiment 2): Diabetic rats orally administrated polyherbal formulation 400 mg/kg for four weeks.
Group 5 (Dose experiment 3): Diabetic rats orally administrated polyherbal formulation 600 mg/kg for four weeks.
Group 6 (Metformin Standard): Diabetic rats orally administrated metformin 70 mg/kg for four weeks.
After diluted ether anesthesia, animals were slaughtered at the end of the experiment with their necks slashed at the jugulars with a sharp razor blade. Blood was collected in CBC tubes and serum tubes separately for hematology and serum analysis respectively. The blood was spun at 3000 rpm for 30 min at 4 °C in the centrifuge for separation of serum. After this serum was separated into different aliquots and stored at −80 °C until further use. The kidney, Liver, and Pancreas were removed from each rat and preserved in 10% formalin solution for histopathological study (Assadi et al., 2021).
2.3.4. Fasting blood glucose measurement
Fasting blood glucose level was determined after streptozotocin injection at the grouping time and after every seventh day of treatment. All animals were fasted for eight hours before blood glucose level measurement. Fasting Blood glucose level was calculated from the punctured tail tip by using a single-touch glucometer ON CALL (Sadat et al., 2020).
2.3.5. Estimation of C-peptide level
A complimentary peptide (C-peptide) test was performed by using the Cal biotech kit (CP441S) method. In this method, 50 μl of control, C-peptide standard, and blood serum were added in separate wells. Then 100 μl of C-peptide conjugate reagent was put into selected wells and the plate was incubated for 60 min at room temperature. After removing liquid wells were washed three times with 300 μl of 1X buffer. TMB substrate 100 μl was introduced in each well and again incubated for 15 min. At the end, 50 μl of stop solution was added into each well and absorbance was taken at 450 nm. The C-peptide level was determined by comparing the absorbance of the sample with the standard curve (Bonser et al., 1984).
2.3.6. Estimation of serum profile
Serum profiles were determined using blood serum collected from serum tubes with centrifugation at 15,000 rpm for 30 min. The collected serum was used for various analyses. Lipid profiling, which included measurements of high-density lipoproteins (HDL), low-density lipoproteins (LDL), total cholesterol (TC), very low-density lipoproteins (VLDL), and triglycerides, was performed. Additionally, renal function tests (RFTs) were conducted to measure urea and creatinine levels, while liver function tests (LFTs) were performed to determine levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP). These measurements were obtained using standard protocols provided by EBRA biochemicals kits and analyzed using the Micro Lab 300 semi-automated biochemistry analyzer (Hudaya et al., 2020). The level of hemoglobin (Hb) and glycosylated hemoglobin (HbA1C) was estimated by using HbA1c EZ 2.0 m.
2.3.7. Tissue preparation and histopathology
On the 29th day of the experiment, histopathological examinations were conducted on the pancreas, liver, and kidney of the sacrificed animals. In order to perform the histology, the liver, kidney, and pancreas samples from every treated group were immersed in a 10% formaldehyde solution for 24 h. Afterward, the samples were bisected longitudinally and serial dried in 60%, 70%, 80%, and 100% ethanol and finally in xylene. These fixed tissues are then embedded in paraffin. Thin sections, approximately 3–5 µm thick, were then cut from the embedded tissues. These sections were subsequently stained with aqueous hematoxylin and alcoholic eosin, enabling visualization of cellular structures. The stained sections were observed using bright field microscopy, with images captured at a magnification of 40× (Zhou et al., 2013).
2.4. Statistical analysis
The obtained results were statistically explained by using different statistical tools such as One way ANOVA and results were represented in mean ± standard deviation. Graphs were made by using GraphPad Prism 8.0.2. Dunnett’s multiple comparison test was used to compare data and the value was considered as highly significant with P < 0.0001.
3. Results
3.1. Effect of polyherbal formulation on water consumption, food consumption, and body weight
The feed and water consumption of each group was determined on a daily basis. All groups showed a significant increase in feed and water consumption in diabetic rats as compared to the normal control group [Table 2 and 3]. Meanwhile In comparison to the normal control group, body weights in the STZ-induced diabetes control group were found to be considerably lower (P < 0.001). The use of Polyherbal formulation led to a considerable recovery of body weight loss to the level of the control group (P < 0.01). There was virtually no difference in body weights between the groups treated with PHF and “Metformin.” (Table 4).
Table 2.
Effect of polyherbal on feed consumption in high-fat diet diabetic rats.
| Groups | Feed g/d |
||||
|---|---|---|---|---|---|
| 0 Week | 1st week | 2nd week | 3rd week | 4th week | |
| NC | 110.33 ± 30.4 | 92.66 ± 10.6 | 74.71 ± 12.2 | 85.28 ± 21.5 | 84.57 ± 28.8 |
| DC | 139.5 ± 29.2 | 146.5 ± 23.8 | 84.28 ± 23.3 | 131.71 ± 43.1 | 122.57 ± 44.8 |
| DE1 | 138.16 ± 21.7 | 155.33 ± 29.4 | 158.4 ± 9 | 147.28 ± 13 | 147.28 ± 61.9 |
| DE2 | 125.33 ± 39.6 | 139.5 ± 28.7 | 169.14 ± 24.9 | 167.14 ± 14.6 | 158.71 ± 45.5 |
| DE3 | 131.39 ± 23.1 | 143.66 ± 15.5 | 162 ± 7.7 | 159 ± 15.6 | 143.57 ± 38.4 |
| MS | 129.66 ± 22.1 | 154.83 ± 31.0 | 162.42 ± 9.4 | 163.14 ± 13.4 | 153.85 ± 39.2 |
Table 3.
Effect of polyherbal on water consumption in high fat diet diabetic rats.
| Groups | Water ml/d |
||||
|---|---|---|---|---|---|
| 0 Week | 1st week | 2nd week | 3rd week | 4th week | |
| NC | 70.71 ± 32.2 | 79.28 ± 36.3 | 68.57 ± 24.7 | 45.71 ± 19.2 | 71.42 ± 27.34 |
| DC | 124.28 ± 27.6 | 149.28 ± 49.2 | 201.42 ± 70.1 | 230.71 ± 28.9 | 209.71 ± 57.5 |
| DE1 | 117.14 ± 31.3 | 159.28 ± 46.3 | 197.85 ± 50.4 | 234.28 ± 27.1 | 232.85 ± 20.5 |
| DE2 | 102.85 ± 35.9 | 125.71 ± 59.9 | 142.85 ± 30.4 | 192.85 ± 49.9 | 113.57 ± 44.7 |
| DE3 | 110 ± 29.4 | 188.57 ± 66.1 | 227.14 ± 52.2 | 232.85 ± 31.4 | 216.42 ± 37.9 |
| MS | 102.85 ± 23.42 | 142.14 ± 57.07 | 242.85 ± 11.1 | 248.57 ± 3.77 | 226.71 ± 42.4 |
Table 4.
Effect of polyherbal on body weight in high-fat diet diabetic rats.
| Groups | Body Weight (g) |
||||
|---|---|---|---|---|---|
| 0 Week | 1st week | 2nd week | 3rd week | 4th week | |
| NC | 227 ± 7.8 | 230 ± 7.8 | 229 ± 10.5 | 231 ± 13.8 | 223 ± 12.82 |
| DC | 220 ± 14.7 | 223 ± 18.8 | 222 ± 17 | 217 ± 10.2 | 212 ± 14*** |
| DE1 | 241 ± 6.5 | 245 ± 14.5 | 244 ± 15.6 | 239 ± 12.9 | 241 ± 15.5** |
| DE2 | 211.6 ± 20.2 | 216 ± 35.9 | 206 ± 26.3 | 181 ± 17.9 | 176 ± 23.2 |
| DE3 | 216.6 ± 11.6 | 222 ± 10.4 | 218 ± 13.9 | 217 ± 11.8 | 219 ± 16.6 |
| MS | 239 ± 18.1 | 242 ± 16.3 | 242 ± 12.2 | 228 ± 24.4 | 227 ± 22.4 |
NC (normal control), DC (diabetic control), DE1 (Dose extract 200 mg/kg) DE2 (Dose extract 400 mg/kg), DE3 (dose extract 600 mg/kg), MS (metformin standard).
3.2. Effect of polyherbal formulation on fasting blood glucose (FBG) level
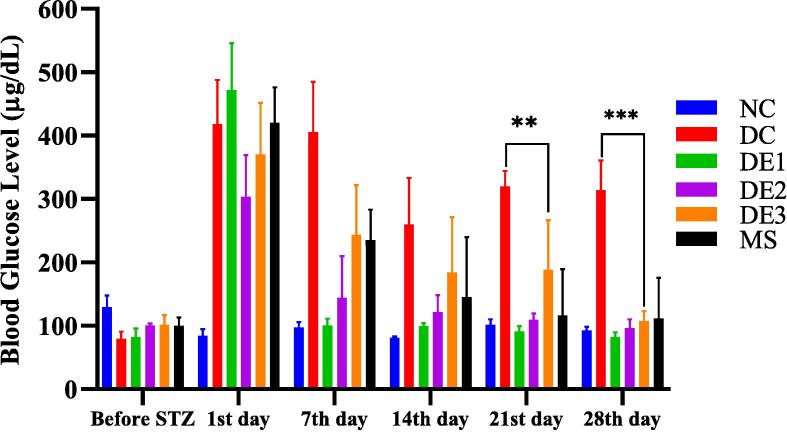
High-fat- diet streptozotocin-induced diabetic rats exhibited a significant increase (***p < 0.001) in fasting blood glucose level (FBG) in comparison to the normal control group. However, treatment with different doses (200, 400, 600 mg/kg body weight) of polyherbal formulation lower the fasting blood glucose level near to control group. Decreasing blood glucose level after treatment of 28 days exhibited significance (**p < 0.01) by the different dose treatments of polyherbal formulation as compared to both control and metformin standard group Fig. 1.
Fig. 1.
Weekly fasting blood glucose (FBG) level of treated and control groups.
3.3. Effect of polyherbal on hematological parameters
Table 5 illustrates the effect of polyherbal on blood parameters. This data indicates an increase in WBC in diabetic groups as compared to the normal control (NC) group. Similarly, the platelet level of the diabetic control (DC) group significantly decreased (P = 0.001) on the other hand different doses of polyherbal formulation maintained the platelets number in the normal control group. The average amount of hemoglobin in single cell (MCHC) and whole individual (MHC) was found higher in the diabetic control group than in other groups. The other parameters did not show many variations also indicating the nontoxicity of polyherbal formulation in diabetic rats (Table 5).
Table 5.
Effect of polyherbal formulation on the hematological parameter in high fat diet diabetic rats.
| Parameters | Complete Blood Count (CBC) |
|||||
|---|---|---|---|---|---|---|
| NC | DC | DE1 | DE2 | DE3 | MS | |
| WBC ×103 | 12.64 ± 5.02 | 15.24 ± 3.04* | 18.44* ± 4.73 | 12.62 ± 5.47 | 16.42 ± 2.48 | 18.3 ± 3.5 |
| RBCs ×106 | 6.92 ± 0.5 | 6.73 ± 0.81 | 7.98 ± 0.36 | 7.5 ± 0.71 | 7.616 ± 0.82 | 7.41 ± 0.3 |
| HGB g/dl | 12.98 ± 2.1 | 14.06 ± 0.39 | 14.54 ± 0.32 | 13.88 ± 0.79 | 14.12 ± 0.57 | 13.92 ± 0.7 |
| HCT % | 36.72 ± 2.47 | 35.22 ± 4.67 | 41.42 ± 1.25 | 39.5 ± 2.35 | 40.62 ± 1.54 | 39.64 ± 1.9 |
| MCV fL | 53.04 ± 0.82 | 52.2 ± 0.9 | 51.9 ± 1.52 | 52.82 ± 3.36 | 51.08 ± 0.81 | 52.54 ± 0.5 |
| MCH pg | 18.68 ± 2.21 | 21.18 ± 2.9* | 18.24 ± 0.71 | 18.6 ± 1.52 | 18.1 ± 0.41 | 19.14 ± 0.5 |
| MCHC g/dl | 35.24 ± 4.55 | 40.76 ± 6.3* | 35.12 ± 0.62 | 35.16 ± 0.98 | 35.32 ± 0.99 | 36.1 ± 0.6 |
| Platelets ×103/μl | 1041.4 ± 464.3 | 733.4** ± 297.5 | 921 ± 230 | 1077 ± 57.4 | 1083 ± 57.38 | 1069 ± 15.8 |
| Lymphocytes % | 69.72 ± 5.29 | 71.98 ± 3.17 | 67.24 ± 7.06 | 65.54 ± 7.20 | 65.86 ± 3.49 | 73.08 ± 7.0 |
| Neutrophiles% | 29.06 ± 5.15 | 28.02 ± 3,17 | 32.76 ± 7.06 | 34.46 ± 7.20 | 34.14 ± 3.49 | 30.28 ± 6.0 |
| PDW fL | 7.72 ± 0.55 | 8.46 ± 0.93 | 8.2 ± 0.46 | 12.04 ± 9.15 | 8.06 ± 0.11 | 8.9 ± 0.61 |
| MPV% | 6.9 ± 0.33 | 7.26 ± 0.39 | 6.98 ± 0.22 | 12.1 ± 7.53 | 7.1 ± 0.08 | 7.3 ± 0.1 |
| P-LCR% | 5.84 ± 1.45 | 6.74 ± 2.35 | 5.44 ± 1.14 | 34 ± 40.33 | 5.86 ± 0.55 | 7.54 ± 0.9 |
| PCT (µg/mL) | 0.728 ± 0.33 | 0.51 ± 0.21 | 0.63 ± 0.15 | 5.65 ± 9.51 | 0.77 ± 0.04 | 0.73 ± 0.04 |
Here, WBC stands for white blood cells, RBC for red blood cells, HGB for hemoglobin, HCT for hematocrit, MCV for mean corpuscular volume, MCH for mean corpuscular hemoglobin, MCHC for mean corpuscular hemoglobin concentration, PDW for platelet distribution width, MPV for mean platelet volume, P-LCR for platelet-large cell ratio, and PCT for procalcitonin test. All parameters are written in mean ± Standard deviation.
3.4. Effect of polyherbal on C-peptide, glycated hemoglobin (HbA1c), and lipid profiling
The C-peptide test clearly indicated the induction of Type II diabetes in all groups, as the levels were found to be similar to those of the normal control group, indicating normal insulin secretion across all these groups. However, the HbA1c level increased by 9.74% in the diabetic rat groups as compared to the normal rat control group at 4.88%. Treatment with different doses of the polyherbal formulation resulted in a decrease in HbA1c levels, bringing them near to the levels determined in the control group as shown in Table 6. The results of the serum lipid profiling showed elevated levels of total cholesterol (TC) by 49% in the diabetic control group as compared to the normal control, triglycerides (TG) level increased by 20% in the diabetic group then normal control, low-density lipoproteins (LDL-C) increased 212%, very low-density lipoproteins (VLDL-C) 14%, and high-density lipoproteins (HDL-C) 17% increase in diabetic group as compared to control group. However, in the polyherbal treated groups, there was a significant reduction (P < 0.001) was noted in the levels of TC, TG, LDL, and HDL compared to the DC group. In contrast, the metformin-treated groups showed a non-significant reduction (P ≥ 0.05) in these values with the comparison to the DC.
Table 6.
Effect of polyherbal formulation on C-peptide, glycated hemoglobin (HbA1c) and lipid profiling in high fat diet diabetic rats.
| Groups | C-peptides (ng/ml) | HbA1c (%) | TC (mg/dl) | TG (mg/dl) | LDL-C (mg/dl) | VLDL-C (mg/dl) | HDL-C (mg/dl) |
|---|---|---|---|---|---|---|---|
| NC | 0.48 ± 0.1 | 4.88 ± 0.7 | 69 ± 2.54 | 206 ± 2.54 | 9.62 ± 1.59 | 41.42 ± 0.26 | 17.3 ± 0.71 |
| DC | 0.53 ± 0.02 | 9.74 ± 1.18 | 103.2 ± 29.8 | 248.2 ± 29.8 | 30.20 ± 18.8 | 47.58 ± 5.47 | 30.06 ± 7.2 |
| DE1 | 0.55 ± 0.03 | 5.75 ± 1.2 | 82.8 ± 9.57 | 183.6 ± 28.5 | 26.25 ± 10.8 | 32 ± 5.74 | 20.71 ± 1.8 |
| DE2 | 0.51 ± 0.009 | 5.84 ± 0.91 | 71.8 ± 16.7 | 122.6 ± 11.2 | 26.16 ± 13.5 | 25.54 ± 2.3 | 19.8 ± 3.43 |
| DE3 | 1.0 ± 0.9 | 5.72 ± 1.3 | 65.6 ± 8.08 | 148.6 ± 22.3 | 19.27 ± 2.78 | 30.12 ± 4.56 | 17.75 ± 1.4 |
| MS | 0.56 ± 0.09 | 5.24 ± 0.8 | 88 ± 20.89 | 211.8 ± 18.4 | 32.4 ± 9.34 | 41.9 ± 4.76 | 25.06 ± 4.6 |
3.5. Effect of polyherbal formulation on liver and kidneys
3.5.1. Effect of polyherbal formulation on alanine phosphatase (ALP)
The mean alanine phosphatase level in normal rats was found 234.6 ± 6.2 which increases in the diabetic control group by 542.4 ± 147. This increased level significantly decreased in the polyherbal treated group (DE1; 200, DE2;400 and DE3; 600 mg/kg). On the other hand, ALP levels in metformin-treated rats were increased by 964.8 ± 263.5 indicating ALP causing toxicity in the liver (Table 7).
Table 7.
Effect of polyherbal formulation on LFTs and RFTs in high fat diet diabetic rats.
| Groups | ALP (U/L) | AST (U/L) | ALT (U/L) | IB (mg/dl) | DB (mg/dl) | Urea (mg/dl) | Creatinine (mg/dl) |
|---|---|---|---|---|---|---|---|
| NC | 234.6 ± 6.2 | 35.5 ± 3.6 | 49.2 ± 11.1 | 0.6 ± 0.07 | 0.3 ± 0.0 | 43.8 ± 2.7 | 0.8 ± 0.1 |
| DC | 542.4 ± 147.3 | 83.8 ± 17.2 | 110 ± 29.2 | 0.58 ± 0.08 | 0.32 ± 0.04 | 29.6 ± 4.8 | 0.8 ± 0.07 |
| DE1 | 394.8 ± 72.06 | 57.6 ± 6.1 | 81.2 ± 9.8 | 0.7 ± 0.1 | 0.34 ± 0.05 | 20.2 ± 5.3 | 0.7 ± 0.1 |
| DE2 | 379.8 ± 46.4 | 58.6 ± 10.3 | 81 ± 2.9 | 0.72 ± 0.2 | 0.32 ± 0.04 | 20.4 ± 5.5 | 0.7 ± 0.05 |
| DE3 | 359.2 ± 79.9 | 63 ± 9.6 | 124.8 ± 2.3 | 0.64 ± 0.1 | 0.36 ± 0.08 | 25.2 ± 8.5 | 0.7 ± 0.05 |
| MS | 964.8 ± 263.5 | 117.4 ± 9.6 | 141 ± 32.9 | 0.68 ± 0.2 | 0.32 ± 0.04 | 22 ± 2.9 | 0.7 ± 0.05 |
3.5.2. Effect of polyherbal formulation on aspartate aminotransferase (AST)
The mean aspartate aminotransferase level in normal rats was found 35.5 ± 3.6 which increases in the diabetic control group to 83.8 ± 17.2. This increased level was significantly lowered in the polyherbal-treated group as compared to metformin-treated rats where its level increased up to 117.4 ± 9.6 indicating AST-induced toxicity in the liver (Table 7).
3.5.3. Effect of polyherbal formulation on alanine transferase (ALT)
The mean aspartate aminotransferase level in normal rats was found 49.2 ± 11.1 which increases in the diabetic control group to 110 ± 29.2. This increased level was significantly lowered in polyherbal treated groups except DE3 as compared to metformin-treated rats where its level increased up to 141 ± 32.9 indicating ALT-induced toxicity in the liver (Table 7).
3.5.4. Effect of polyherbal formulation on urea and creatinine
The urea and creatinine levels in diabetic rats decreased as compared to the normal control group which indicates diabetic-induced damage in the liver and kidney which disturbed the normal urea cycle in the liver. Direct bilirubin (DB) and indirect bilirubin (IB) level also exhibited non-significant variations in both control and diabetic rats (Table 7).
3.6. Histopathological studies
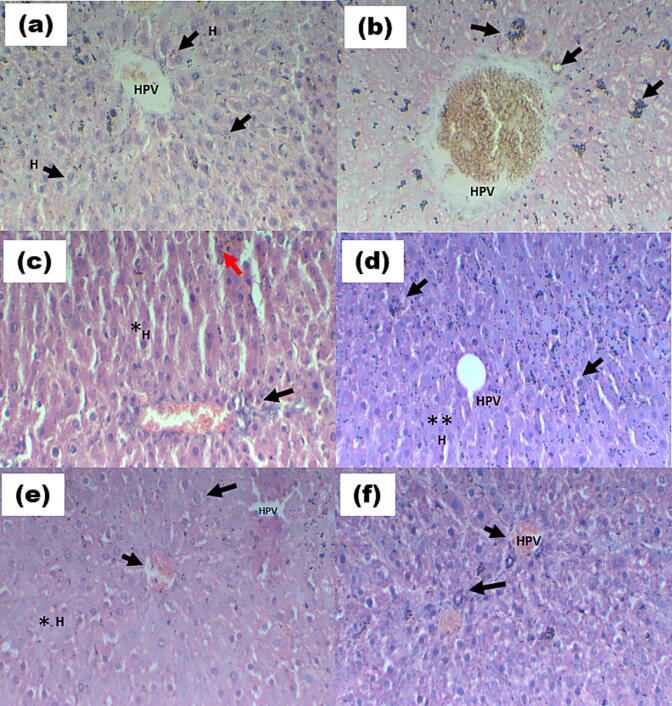
The diabetic control group showed central vein dilation in the hepatic portal vein, hepatic parenchyma, and bile duct in the portal area. It also showed high centrilobular hepatocellular necrosis which is associated with hemorrhage and mononuclear inflammatory cell infiltration. Infiltration in diffused leukocytes inflammatory cells, ballooning degeneration of hepatocytes, and Kupper cell proliferation between degraded and fatty changed hepatocytes were also observed in diabetic rats. These all changes were decreased near to normal by the treatment with polyherbal formulation as compared to the metformin standard (Fig. 2a).
Fig. 2a.
Rat liver (a) Normal group (NC) displaying the typical histological organization of the hepatic lobule. Notable are the normal hepatocytes (H) and the normal hepatic portal vein (HPV). (b) A diabetic control (DC) with extensive centrilobular hepatocellular necrosis, hemorrhage, and infiltration of mononuclear inflammatory cells (black arrow) (c) Hepatocyte ballooning degeneration with pyknosis of their nuclei (red arrow), necrosis of sporadic hepatocytes (black arrow), and infiltration of mononuclear inflammatory cells in treated diabetic rat liver (T1 200 mg/kg). (d) Hepatocyte ballooning degradation and apoptosis are visible in the treated diabetic rat liver (T2 400 mg/kg). (e) A diabetic rat liver treated with T3 600 mg/kg demonstrates a considerable (*) decline in hepatocyte ballooning degeneration and cytoplasmic vacuolization (black arrow). (f) Hepatic portal injury in the metformin standard (MS) diabetic group with necrosis of sporadic hepatocytes (long arrow). (H&E, inserted images ×400).
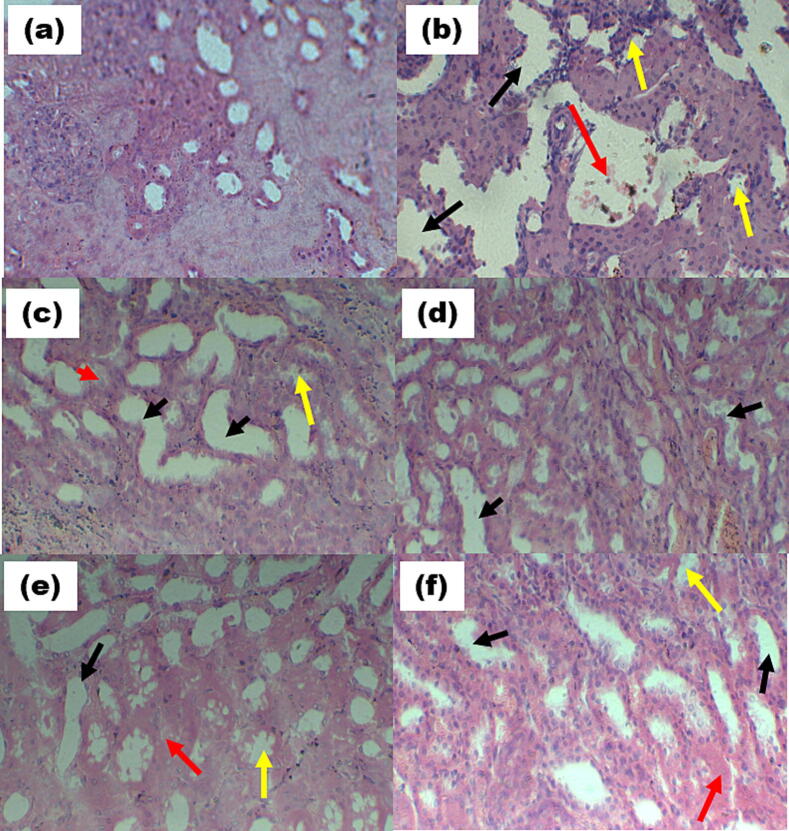
The normal kidney’s histology showed a healthy glomerulus which is surrounded with the Bowman's capsule, as well as intact proximal and distal tubules, without any sign of inflammation. In contrast, the kidneys of untreated diabetic control rats exhibited destruction in glomeruli with invading inflammatory cells, leading to vasodilation and basement membrane thickening (Fig. 2b). However, the groups treated with polyherbal formulations demonstrated signs of recovery, including restoration of the normal glomerular structure, the absence of inflammation and vasodilation, and the presence of a normal basement membrane and capillaries.
Fig. 2b.
Kidney of rat (a) Normal group (NC) showing normal tubules of the kidney. (b) Diabetic control (DC) showing massive cell necrosis and hemorrhage (red arrow) and glomerular/ tubule granular damage (yellow arrow) and tubule dilation and vacuolization (black arrow) (c) Treated diabetic rat pancreas (T1 200 mg/kg), showing significant (*) decrease cellular necrosis (red arrow) and recovery of vasodilation. (d) Treated diabetic rat pancreas (T2 400 mg/kg), showing a more significant (**) decrease in cell necrosis and recovery of tubule granular damage (black arrow) (e) Treated diabetic rat pancreas (T3 600 mg/kg), showing normal vasodilation, glomerular damage (f) Metformin standard (MS) diabetic group showing mild cellular necrosis (red arrow) and vasodilation in kidney (black arrow). (H&E, inserted images ×400).
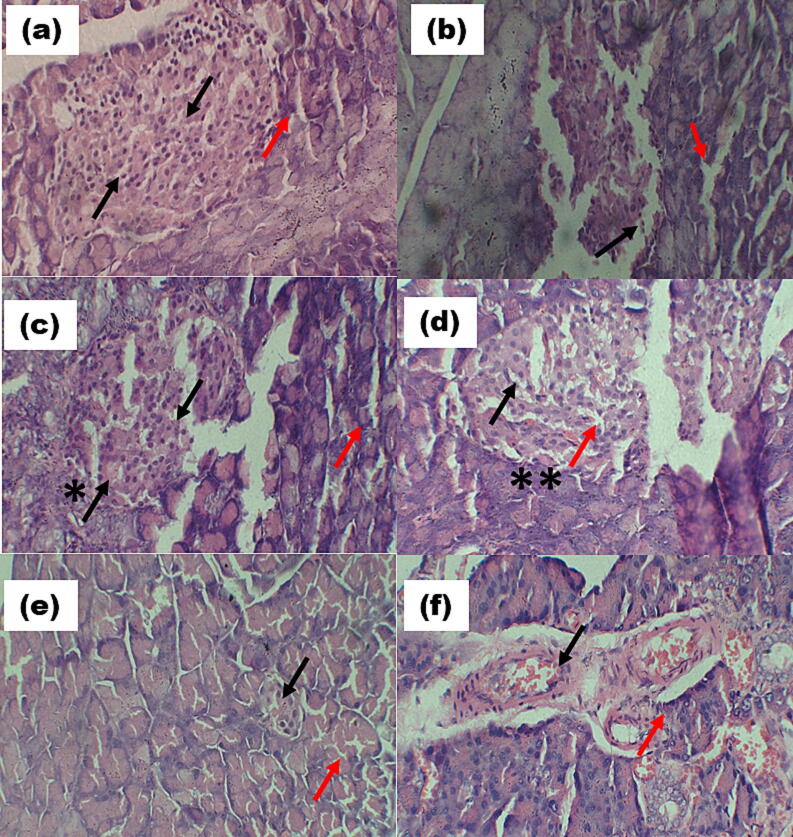
Pancreatic histopathology of the control group (Fig. 2c) showed normal parenchymatic cells and Islets of Langerhans. The pancreas portion in the diabetes control group showed moderate islet cell hyperplasia, severe pancreatic parenchymal congestion, minor inflammatory cell infiltration, and deposition of lipids around beta cells. Upon treatment with the polyherbal formulation, these pathological changes were significantly improved, bringing them closer to the conditions observed in the control group, with only mild hyperplasia. In contrast, the metformin-treated diabetic group did not show significant β-cell regeneration and exhibited a higher rate of necrosis compared to the polyherbal formulation-treated groups.
Fig. 2c.
Pancreas of rat (a) Normal group (NC) showing normal Islets (red arrow) and normal β cells (black arrow). (b) Diabetic control (DC) showed massive β cells necrosis (black arrow) and damaged islets (red arrow). (c) Treated diabetic rat pancreas (T1 200 mg/kg), showing significant (*) decrease β cells necrosis (black arrow) and recovery of islets (red arrow). (d) Treated diabetic rat pancreas (T2 400 mg/kg), showing significant (**) decrease β cells necrosis (black arrow) and recovery of islets (red arrow). (e) Treated diabetic rat pancreas (T3 600 mg/kg), showing normal β cells and normal islets (f) Metformin standard (MS) diabetic group showing mild β cells necrosis and inflammation in islets (red arrow). (H&E, inserted images ×400).
4. Discussion
This is the first study to evaluate the antidiabetic effect of a novel polyherbal formulation using Opuntia ficus-indica, Syzygium cumini, Rhus coriaria, Coffea arabica, and Eclipta alba extracts. Before the preparation of this polyherbal formulation, all plants were accessed individually in an in vitro enzyme-based biochemical study and their combination showed highly significant results in an in vitro study. To access the antidiabetic activity of this polyherbal formulation fat diet albino rat model was developed by continually giving a high fat diet to normal rats for one month as reported by Roy et al. (2022). After gaining a maximum weight above 250 g diabetes was induced with a low dose of STZ 30 mg/kg (Hung et al., 2012). HFD-fed rats may experience insulin resistance due to the accumulation of fat deposits that hinder the function of receptor cells. Additionally, a low dose of STZ (streptozotocin) can enhance the diabetic effects. Consequently, various studies have indicated that the combination of a high-fat diet and a low dose of STZ could serve as an effective model for non-insulin dependent diabetes mellitus. The increase in blood glucose level (Fig. 1) and body weight (Table 4) could be due to dietary change via a high-fat diet and streptozotocin administration. However, this hyperglycemic condition was significantly reduced in groups treated with polyherbal formulation and metformin as compared to the diabetic control group. This decrease in blood glucose may be due to inhibitory effect of polyherbal constituents on metabolic enzymes such as α-glucosidase, α-amylase, lipase, and Aldo reductase involved in hyperglycemic activity. The inhibition of these enzymes has the effect of delaying the absorption of glucose into the bloodstream and reducing postprandial hyperglycemia. This, in turn, leads to better glycemic control, as supported by various sources (Sarkar et al., 2022). Opuntia fucus indica is reported as a potent inhibitor of α-glucosidase and lipase enzymes (Rabi et al., 2020, Padilla-Camberos et al., 2015). The food amelioration of HFD plus STZ administration may be the reason for the type 2 diabetic rats which showed significantly higher body weight and blood glucose levels (Table 4) as compared to normal control rats. However, in groups receiving metformin and PHF treatment, the high glucose level in diabetic control rats was gradually controlled. The observed drop in blood sugar levels following treatment with the formulation may indicate the presence of antihyperglycemic agents in individual plant extracts which were used in this formulation. The antidiabetic effects of these extracts could potentially arise from their ability to inhibit both intestinal α-glucosidase and α-amylase (Gök et al., 2020, Rabi et al., 2020). These inhibitory effects were assessed separately before the formulation's preparation and glucose uptake activity by tannins present in these extracts (Kumari and Jain, 2012).
Moreover, the inhibition of these enzymes leads to a delay in glucose absorption into the bloodstream, effectively reducing postprandial hyperglycemia and thereby enhancing glycemic control (Wang et al., 2010). Additionally, one of the primary active components in Eclipta alba, wedelolactone, has shown the ability to activate proliferator-activated receptors PPARα and AMPK in human HepG2 cells. It also enhances the expression and activity of PPAR-α-dependent lipoprotein lipase in HepG2 cells (Zhao et al., 2015). Furthermore, this compound exhibits anti-glycation activity, which can contribute to slowing down the onset or progression of diabetes (Shahab et al., 2018).
The PHF's antihyperlipidemic effect is demonstrated by a reduction in lipid profile, which may be brought on by the presence of flavonoids like quercetin and hesperidin. Different studies showed that both quercetin and hesperidin are involved in lipolysis regulation, adiponectin modulation, and fat oxidation which conclusively take part in fat reduction (Jung et al., 2013, Iskender et al., 2017). The histopathological study also revealed the protective effect of PHF by reducing cell necrosis, hemorrhage, and tubule granular damage in kidneys, recovering massive centrilobular hepatocellular necrosis in liver and regeneration of β-cells in damaged pancreas of diabetic treated rats as compared to non-treated diabetic rats. Conclusively this study revealed the antidiabetic potential of new PHF which can be used as an alternate and safer drug source with better efficacy for diabetes treatment. However, careful clinical trials are recommended before administration of this polyherbal formulation.
5. Conclusion
This study aimed to assess the potential antidiabetic effects of a polyherbal formulation containing extracts from Opuntia ficus-indica, Syzygium cumini, Rhus coriaria, Coffea arabica, and Eclipta alba. To achieve this, a high-fat diet diabetic rat model was established, and these rats were administered multiple doses of the polyherbal formulation (PHF). Subsequent to the treatment period, a comprehensive analysis was conducted, involving both biochemical and histopathological examinations. The results revealed noteworthy outcomes in the context of diabetes management attributed to the administration of PHF. Notably, the treatment involving the polyherbal formulation on diabetic rats induced through a high-fat diet and streptozotocin (STZ) displayed, there was a significant reduction in glucose levels, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and recovery weight loss. These findings suggest a potential role for the polyherbal formulation in controlling the progression of diabetes, along with its associated complications. The histopathological analysis further indicated that the PHF treatment exhibited the capability to ameliorate the damage induced by STZ in diabetic rats. However, both pre-clinical and clinical studies are recommended before considering the utilization of this formulation as an alternative approach to diabetes treatment.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully thanks the Punjab Higher Education Commission (PHEC) for providing funding grant (PHEC/ARA/PIRCA/20316/13) and the Department of Zoology Government College University Faisalabad for providing lab access.
Patent
This polyherbal formulation, PENN-DIABEX, has been submitted for patent consideration to the Intellectual Property Organization of Pakistan (Patent Application No. 506/2023 Filing date 03/08/2023).
Contributor Information
Mudassir Hassan, Email: mudassirhassan22@gcuf.edu.pk.
Azhar Rasul, Email: azharrasul@gcuf.edu.pk, drazharrasul@gmail.com.
Muhammad Ajmal Shah, Email: ajmalshah@hu.edu.pk.
Farhat Jabeen, Email: farhatjabeen@gcuf.edu.pk.
Ayesha Sadiqa, Email: ayshasadiqa536@gcuf.edu.pk.
References
- Abdelghffar E.A., Mostafa N.M., El-Nashar H.A., Eldahshan O.A., Singab A.N.B. Chilean pepper (schinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high fat diet/streptozotocin-induced type 2 diabetic rat model. Industrial Crops and Products. 2022;183:114953. [Google Scholar]
- Adnan M., Aasim M. Prevalence of type 2 diabetes mellitus in adult population of pakistan: A meta-analysis of prospective cross-sectional surveys. Annals of global health. 2020;86(1) doi: 10.5334/aogh.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assadi S., Shafiee S.M., Erfani M., Akmali M. Antioxidative and antidiabetic effects of capparis spinosa fruit extract on high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats. Biomedicine & Pharmacotherapy. 2021;138:111391. doi: 10.1016/j.biopha.2021.111391. [DOI] [PubMed] [Google Scholar]
- Bera T.K., De D., Chatterjee K., Ali K.M., Ghosh D. Effect of diashis, a polyherbal formulation, in streptozotocin-induced diabetic male albino rats. International Journal of Ayurveda Research. 2010;1(1):18. doi: 10.4103/0974-7788.59939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser A.M., Garcia-Webb P., Harrison L.C. C-peptide measurement: Methods and clinical utility. CRC Critical Reviews in Clinical Laboratory Sciences. 1984;19(4):297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- Cusi K., DeFronzo R. Metformin: A review of its metabolic effects. Diabetes Reviews. 1998;6(2):89–131. [Google Scholar]
- Dowarah J., Singh V.P. Anti-diabetic drugs recent approaches and advancements. Bioorganic & medicinal chemistry. 2020;28(5):115263. doi: 10.1016/j.bmc.2019.115263. [DOI] [PubMed] [Google Scholar]
- Ellahham S. Diabetes and its associated cardiovascular complications in the arabian gulf: Challenges and opportunities. Journal of Clinical and Experimental Cardiology. 2020;11:1–5. [Google Scholar]
- Gök H.N., Deliorman Orhan D., Gürbüz İ., Aslan M. Activity‐guided isolation of α‐amylase, α‐glucosidase, and pancreatic lipase inhibitory compounds from rhus coriaria l. Journal of Food Science. 2020;85(10):3220–3228. doi: 10.1111/1750-3841.15438. [DOI] [PubMed] [Google Scholar]
- Hudaya M.F., Sitaresmi P.I., Noviandi C.T., Widyobroto B.P., Widayati D.T. Behavior and blood profile in friesian-holstein dairy cows in the special region of yogyakarta, indonesia. Journal of Animal Behaviour and Biometeorology. 2020;8(4):244–249. [Google Scholar]
- Hung H.-Y., Qian K., Morris-Natschke S.L., Hsu C.-S., Lee K.-H. Recent discovery of plant-derived anti-diabetic natural products. Natural product reports. 2012;29(5):580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- Iskender H., Dokumacioglu E., Sen T.M., Ince I., Kanbay Y., Saral S. The effect of hesperidin and quercetin on oxidative stress, nf-κb and sirt1 levels in a stz-induced experimental diabetes model. Biomedicine & Pharmacotherapy. 2017;90:500–508. doi: 10.1016/j.biopha.2017.03.102. [DOI] [PubMed] [Google Scholar]
- Jung C.H., Cho I., Ahn J., Jeon T.I., Ha T.Y. Quercetin reduces high‐fat diet‐induced fat accumulation in the liver by regulating lipid metabolism genes. Phytotherapy Research. 2013;27(1):139–143. doi: 10.1002/ptr.4687. [DOI] [PubMed] [Google Scholar]
- Kumari M., Jain S. Tannins: An antinutrient with positive effect to manage diabetes. Research Journal of Recent Sciences ISSN. 2012;2277:2502. [Google Scholar]
- Ngo L.T., Okogun J.I., Folk W.R. 21st century natural product research and drug development and traditional medicines. Natural product reports. 2013;30(4):584–592. doi: 10.1039/c3np20120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabi L., Altamimi M., Jaradat N. Phytoconstituents, antioxidant and inhibitory activity against α-amylase and α-glucosidase of opuntia ficus-indica. An-Najah University Journal for Research-A (Natural Sciences) 2020;34(1):31–48. [Google Scholar]
- Sarkar D., Christopher A., Shetty K. Phenolic bioactives from plant-based foods for glycemic control. Frontiers in Endocrinology. 2022;12:727503. doi: 10.3389/fendo.2021.727503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Du Y.-J., Song H.-C. Α-glucosidase and α-amylase inhibitory activities of guava leaves. Food chemistry. 2010;123(1):6–13. [Google Scholar]
- Padilla-Camberos, E., Flores-Fernandez, J.M., Fernandez-Flores, O., Gutierrez-Mercado, Y., Carmona-de la Luz, J., Sandoval-Salas, F., Mendez-Carreto, C., Allen, K., 2015. Hypocholesterolemic effect and in vitro pancreatic lipase inhibitory activity of an opuntia ficus-indica extract. BioMed research international, 2015. [DOI] [PMC free article] [PubMed]
- Roy J.R., Janaki C.S., Jayaraman S., Periyasamy V., Balaji T., Vijayamalathi M., Veeraraghavan V.P. Carica papaya reduces muscle insulin resistance via ir/glut4 mediated signaling mechanisms in high fat diet and streptozotocin-induced type-2 diabetic rats. Antioxidants. 2022;11(10):2081. doi: 10.3390/antiox11102081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadat H., Alami K., Mousavi S.Y. Effect of afghan senjed (elaeagnus angustifolia l.) leaves aqueous alcoholic extract on blood glucose level of diabetic rats. Pharmacognosy Journal. 2020;12(6) [Google Scholar]
- Shahab U., Faisal M., Alatar A.A., Ahmad S. Impact of wedelolactone in the anti‐glycation and anti‐diabetic activity in experimental diabetic animals. IUBMB life. 2018;70(6):547–552. doi: 10.1002/iub.1744. [DOI] [PubMed] [Google Scholar]
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C., Basit A., Chan J.C., Mbanya J.C. Idf diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes research and clinical practice. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udler M.S. Type 2 diabetes: Multiple genes, multiple diseases. Current diabetes reports. 2019;19:1–9. doi: 10.1007/s11892-019-1169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Peng L., Yang L.-C., Xu X.-D., Li W.-J., Luo X.-M., Jin X. Wedelolactone regulates lipid metabolism and improves hepatic steatosis partly by ampk activation and up-regulation of expression of pparα/lpl and ldlr. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhou S., Zeng S. Experimental diabetes treated with trigonelline: Effect on β cell and pancreatic oxidative parameters. Fundamental & clinical pharmacology. 2013;27(3):279–287. doi: 10.1111/j.1472-8206.2011.01022.x. [DOI] [PubMed] [Google Scholar]