Abstract
Background
Social anxiety (SA) is a negative emotional response that can lead to mental health issues, which some have experienced during the coronavirus disease 2019 (COVID-19) pandemic. Little attention has been given to the neurobiological mechanisms underlying inter-individual differences in SA alterations related to COVID-19. This study aims to identify neurofunctional markers of COVID-specific SA development.
Methods
110 healthy participants underwent resting-state magnetic resonance imaging and behavioral tests before the pandemic (T1, October 2019 to January 2020) and completed follow-up behavioral measurements during the pandemic (T2, February to May 2020). We constructed individual functional networks and used graph theoretical analysis to estimate their global and nodal topological properties, then used Pearson correlation and partial least squares correlations examine their associations with COVID-specific SA alterations.
Results
In terms of global network parameters, SA alterations (T2-T1) were negatively related to pre-pandemic brain small-worldness and normalized clustering coefficient. In terms of nodal network parameters, SA alterations were positively linked to a pronounced degree centrality pattern, encompassing both the high-level cognitive networks (dorsal attention network, cingulo-opercular task control network, default mode network, memory retrieval network, fronto-parietal task control network, and subcortical network) and low-level perceptual networks (sensory/somatomotor network, auditory network, and visual network). These findings were robust after controlling for pre-pandemic general anxiety, other stressful life events, and family socioeconomic status, as well as by treating SA alterations as categorical variables.
Conclusions
The individual functional network associated with SA alterations showed a disrupted topological organization with a more random state, which may shed light on the neurobiological basis of COVID-related SA changes at the network level.
Keywords: Graph theory, Psychoradiology, Functional brain network, Social anxiety, COVID-19 pandemic, Resting-state fMRI
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has posed a serious threat to the global economy, social fabric, and public mental health (Coelho et al., 2020; Li et al., 2020). In some individuals the perceived threat of COVID-19 infection, coupled with the consequences of social isolation and movement restrictions, has led to negative psychological reactions such as fear, anxiety, depression, and posttraumatic stress (Coelho et al., 2020; Schweda et al., 2021). Among these psychological effects, social anxiety (SA) has been notable (Kindred and Bates, 2023). Characterized by fear of or anxiety about potential scrutiny by others in social situations, SA covers a spectrum from subclinical symptoms to clinical social anxiety disorder (SAD) (Miskovic and Schmidt, 2012), which can lead to significant adverse effects on social, emotional, and behavioral functioning (Stein et al., 2017). Although the impact of the COVID-19 pandemic on SA is well established (Hawes et al., 2022; Zheng et al., 2020; Zhu et al., 2021), less attention has been paid to individual variability in susceptibility to SA. Study of the underlying neuropsychological mechanisms, as well as illuminating the pathophysiology, may help in identifying practical markers of vulnerability.
Studies using magnetic resonance imaging (MRI), especially functional MRI (fMRI), have yielded insights into the neural mechanisms underlying SA/SAD (Brühl et al., 2014; Mizzi et al., 2022; Zhang et al., 2023b). Most fMRI studies have focused on SAD patients: the most consistent reported abnormalities are in activity in the frontolimbic circuitry, including the superior frontal gyrus (SFG), middle frontal gyrus (MFG), anterior cingulate cortex (ACC), hippocampus (HG)/parahippocampal gyrus (PHG), and amygdala (Brühl et al., 2014; Etkin and Wager, 2007; Gentili et al., 2016; Mizzi et al., 2022), and in resting-state functional connectivity (RSFC) in various networks including the default mode network (DMN), fronto-parietal task control network (FPN), cingulo-opercular task control network (CON), dorsal attention network (DAN), subcortical network (SCN), auditory network (AN), visual network (VN), and sensory/somatomotor network (SMN) (Kim et al., 2023; Liao et al., 2010; Liu et al., 2015; Zhang et al., 2022b). fMRI studies of SA in nonclinical populations have focused on similar brain regions: abnormalities are reported in activity in the ACC, SFG, MFG, amygdala, HG/PHG, superior temporal gyrus (STG), and insula (Bas-Hoogendam et al., 2016, 2019, 2020; Boehme et al., 2015; Kim et al., 2022; Shany et al., 2022; Tei et al., 2020; Terasawa et al., 2013), and of RSFC particularly in the DMN, FPN, DAN, SCN, AN, VN, and salience network (Avery and Blackford, 2016; Bas-Hoogendam et al., 2021; Evans et al., 2020; He et al., 2021; Kajimura et al., 2015; Luo et al., 2018; Mao et al., 2020; Schultz et al., 2019; Zhao et al., 2022). These findings suggest that SA and SAD may rely on overlapping stable dysfunction in mechanisms involved in cognitive, emotional, and sensory processing (Bas-Hoogendam and Westenberg, 2020).
Previous studies have primarily focused on exploring local neural activity or brain organization by quantifying the strength of the connectivity; this however overlooks the higher level of complex organization which is accessible via graph analysis, a powerful framework to characterize the topological patterns of functional connectivity (FC), which has been successfully applied in several psychiatric disorders (Kambeitz et al., 2016; Li et al., 2017; Sporns, 2018). A network approach to the topological organization of functional networks promises rich insights into the neurobiological basis of SA/SAD. However, few such studies have been done. Earlier functional network studies in SAD have highlighted disrupted brain networks, reflected in changes to both global and nodal network property parameters: decreased global normalized shortest path length (λ) (Yang et al., 2019), increased global shortest path length (Lp) (Zhu et al., 2017), decreased global clustering coefficient (Cp) (Zhu et al., 2017), and nodal degree centrality alterations in the PHG, posterior cingulate cortex (PCC), MFG, and insula (Yang et al., 2019). The only study of SA in a nonclinical population reported a relationship between nodal centrality in the parietal regions and fear of negative evaluation, which is a core symptom in SAD (Kajimura et al., 2015). This approach has not previously been applied to SA in the context of the COVID-19 pandemic. Our aim was to explore prospective graph theory-based global and nodal neurofunctional markers for susceptibility to COVID-related SA changes.
To this end, we explored pre-pandemic brain topological characteristics to retrospectively predict SA alterations between the pre-pandemic and pandemic periods in a group of normal adults. We used a dimensional approach, which focused on SA symptom levels, to yield more reliable neurobiological biomarkers of specific clinical presentations (Li et al., 2023, Liu et al., 2023). First, to characterize resting-state topological properties of functional network at global and nodal scales, we conducted graph theory analysis, a well-validated tool to quantify properties of structural and functional networks (Bullmore and Bassett, 2011). Next, we performed partial correlation and multivariate analysis [partial least squares correlation (PLSC) (Krishnan et al., 2011, Lai et al., 2023)] to evaluate the underlying patterns of the functional brain network linked to SA alterations. Given the previous evidence of disrupted functional brain network in SAD (Yang et al., 2019), we hypothesized that highly socially anxious individuals would manifest some common and distinct alterations. Finally, we examined the specificity and robustness of our findings by additionally controlling for the possible confounding effects of general anxiety symptoms, other stressful life events and family socioeconomic status, and also by performing categorical comparisons.
2. Methods
2.1. Participants
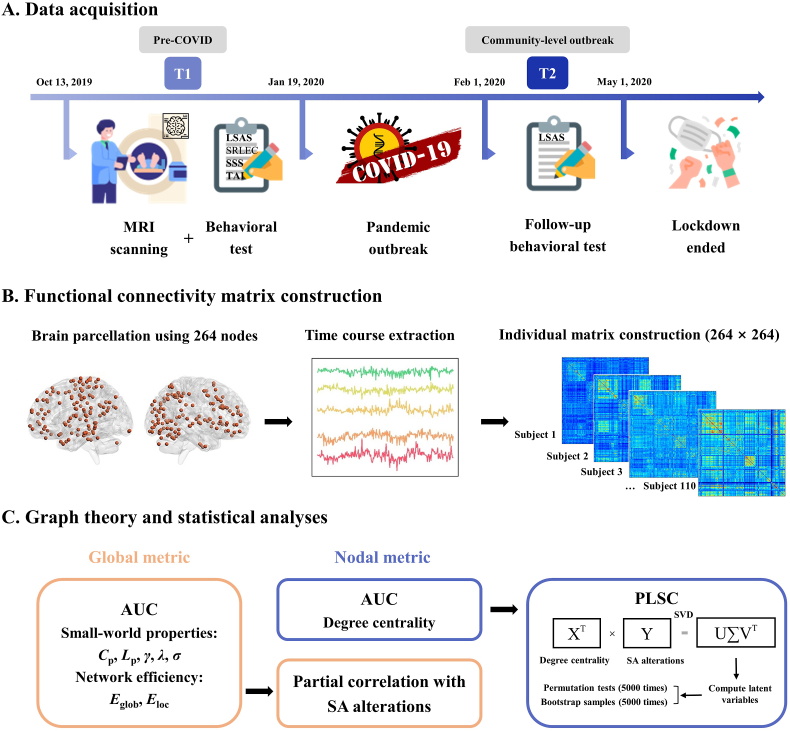
Fig. 1A gives an overview of the data collection and experimental procedures. A total of 151 general university students (77 females, mean age: 21.2 ± 2.1 years) with no history of mental or neurological diseases were recruited for brain MRI scans and self-reported behavioral measures from October 2019 to January 2020 (T1, prior to the declaration of emergency and nationwide lockdown in China). During the first COVID-19 outbreak from February to April 2020 (T2, when China was most severely impacted by the pandemic), all 151 individuals were re-contacted and invited to participate for online behavioral assessments to evaluate pandemic-specific SA changes (Zhang et al., 2023a). Of these participants, a subsample of 115 (66 females, mean age: 22.4 ± 2.1 years) responded and completed behavioral measures at T2. After excluding 1 participant with excessive head motion and 4 participants exhibiting non-typical features of small-world organization (see sections on ‘MRI data acquisition and preprocessing’ and ‘Functional brain network construction’), 110 participants (61 females, mean age: 22.4 ± 2.1 years) were included for the final analyses. The standard polymerase chain reaction test was used to ensure no participants or their household members had been diagnosed with COVID-19.
Fig. 1.
Workflow of the study. (A) Timeline of data acquisition. Before the COVID-19 pandemic (T1: October 2019 to January 2020), participants underwent brain MRI scanning and completed baseline behavioral measures. During the most severe pandemic period (T2: February 2020 to April 2020), participants were re-contacted for follow-up behavioral testing. 110 subjects were identified as eligible for the study. (B) Construction of 264 × 264 functional connectivity matrix for each subject. (C) Topological graph theory and statistical analyses. We computed both global and nodal metrics for each individual to describe the characteristics of each weighted network, and for each network metric we used the AUC over a range of network sparsity thresholds (0.02: 0.01: 0.33) in subsequent statistical analyses. We used partial correlation to investigate the association between each global metric and SA alterations (T2-T1), and PLSC to determine the degree centrality pattern of nodes linked to SA alterations. Abbreviations: AUC, area under the curve; COVID-19, coronavirus disease 2019; LSAS, Liebowitz Social Anxiety Scale; MRI, magnetic resonance imaging; PLSC, partial least squares correlation; SA, social anxiety; SRLEC, Self-Rating Life Events Checklist; SSS, Socioeconomic Status Scale; SVD, singular value decomposition; TAI, Trait Anxiety Inventory; Cp, clustering coefficient; Eglob, global efficiency; Eloc, local efficiency; Lp, shortest path length; γ, normalized clustering coefficient; λ, normalized shortest path length; σ, small-worldness.
2.2. Behavioral assessments
To investigate the changes in SA during the pandemic, we used the self-administered Liebowitz Social Anxiety Scale (LSAS) (Heimberg et al., 1999; Liebowitz, 1987; Mennin et al., 2002) in all individuals at both T1 and T2. The LSAS is commonly used in Chinese populations for its good psychometric properties (Zhang et al., 2020, 2022a), including test-retest reliability, internal consistency, convergent validity, and discriminant validity (Baker et al., 2002; Czorniej et al., 2022; Pan et al., 2006). The LSAS consists of 24 items that represent common daily scenarios pertaining to social interaction or performance conditions. Individuals are required to rate the level of fear evoked by each specific situation and the subjective probability of avoiding it on a four-point (0–3) Likert scale, corresponding to the fear factor (LSASF) and social avoidance factor (LSASA), respectively; their sum is the total score of the LSAS (LSAST), with higher scores indicating greater severity of SA symptoms (Baroni et al., 2022); Cronbach's α were 0.94 at T1 and 0.96 at T2 in our dataset, indicating satisfactory internal reliability.
To assess potential confounders of the relationship between brain network topologies and SA changes (such as general anxiety symptoms, other stressful life events, and socioeconomic status), we used several other tests at T1: the Trait Anxiety Inventory (TAI), which determines the participants' general tendency to feel anxious (Spielberger, 1983); the Self-Rating Life Events Checklist (SRLEC), which evaluates the frequency and intensity of stressful life events during the past 12 months (Liu et al., 1997); and the Socioeconomic Status Scale (SSS), which assesses participants' family sociological and economic status (Adler et al., 2000). In our sample internal reliability for TAI, SRLEC, and SSS at T1 was adequate (Cronbach's α being 0.89, 0.90, and 0.83 respectively).
2.3. MRI data acquisition and preprocessing
MRI data were acquired using a 3.0 T whole-body scanner (Siemens Trio, Erlangen, Germany), equipped with a 12-channel array coil. For details of Resting-state fMRI (RS-fMRI) data acquisition and preprocessing (see the Supplementary Methods).
2.4. Functional brain network construction
As shown in Fig. 1B, the construction of individual interregional functional brain networks was performed with GRETNA (http://www.nitrc.org/projects/gretna) (Wang et al., 2015). We defined the network nodes using the Power 264-node functional parcellation, which comprises 264 putative functional regions-of-interest (ROI) associated to 14 large-scale functional networks (Power et al., 2011). For each functional ROI, we also reported the corresponding anatomical descriptions in the Automated Anatomical Labeling (AAL) Atlas 3 (Rolls et al., 2020). The Pearson's correlation coefficients (r) between mean time courses of all pairs of nodes were considered as the network edges, and transformed using Fisher's r-to-z transformation to improve normality. Finally, a symmetric 264 × 264 functional network matrix with 34,716 (264 × 263/2) unique connectivity edges was created per participant.
Each individual network matrix was converted into a binarized matrix according to predefined sparsity thresholds, providing each network matrix with the same number of edges. Specifically, we thresholded each network matrix over a wide range from 0.02 to 0.33 with a step size of 0.01. The minimum limit, 0.02, was calculated using the widely-used GRETNA function “Gretna_get_rmax”, which estimates the minimum sparsity threshold to ensure small-world estimation (Sun et al., 2022; Wang et al., 2020); the maximum limit, 0.33, was chosen to ensure small-world characteristics for all included participants (Yun et al., 2020). Data from 4 participants were excluded due to poor small-world characteristics (the small-worldness σ of the thresholded networks was < 1) and 110 participants were included for final analyses.
2.5. Graph-theoretical analysis
As shown in Fig. 1C, to describe both global and nodal characteristics of the functional network at each sparsity threshold, we computed 7 commonly used global metrics and 1 nodal metric. There are 5 global small-world parameters: Cp (average clustering coefficient over all nodes), a measure of functional segregation; Lp (averaged shortest path length between all node pairs), which indicates how well its elements are integrated; normalized shortest path length (λ) and normalized clustering coefficient (γ), which are the normalized measures calculated by comparing each original network with its corresponding random networks; and small-worldness (σ), which reflects the network balance between functional integration and segregation. There are 2 global network efficiency parameters: global efficiency (Eglob, average inverse shortest path length), a measure of integration, and local efficiency (Eloc, average efficiency of the local subgraphs), a measure of segregation (Latora and Marchiori, 2001; Rubinov and Sporns, 2010). The nodal-level topological property was degree centrality, which indicates the functional importance of nodes within the whole brain (Zuo and Xing, 2014). For each network metric we calculated the area under the curve (AUC) for the sparsity range from 0.02 to 0.33 with an interval of 0.01, which provides a robust measure of integrated topological characteristics across the sparsity range (Reijmer et al., 2010; Zhang et al., 2011). The individual AUC for each network metric (global and nodal) was used for subsequent statistical analyses.
2.6. Statistical analysis
2.6.1. Behavioral data analyses
For each participant, SA alterations were computed by subtracting LSAST scores at T1 from LSAST scores at T2. Bivariate associations between study measures were assessed by Pearson's correlation coefficient. The paired-sample t-test was used to assess changes of LSAST at T2 relative to T1 among participants. All tests used IBM SPSS Statistics 22.0.
2.6.2. Partial correlation between global-level brain metrics and SA alterations
We examined the relationship between each global metric and SA alterations with partial correlation analyses in IBM SPSS Statistics 22.0. Correlations were computed controlling for sex, age, and mean framewise displacement (FD) to eliminate potential confounding effects. Bonferroni correction for multiple comparisons (7 tests) p < 0.007 was deemed significant.
2.6.3. PLSC for nodal-level topological property link to SA alterations
To evaluate multivariate patterns of correlation between the nodal-level topological property (degree centrality) and SA alterations across subjects, we used PLSC via the publicly available PLS toolbox (https://www.rotman-baycrest.on.ca/index.php?section=84) in MATLAB R2018b (MathWorks, Natick, USA). PLSC is a powerful method often used to jointly analyze neuroimaging and behavioral variables (Krishnan et al., 2011; McIntosh et al., 1996; Siffredi et al., 2022). Its significant advantage lies in taking into consideration the multivariate nature of brain data; importantly, no further multiple testing correction is needed in PLSC. Besides, it obviates the need for a feature selection step to reduce feature dimension, which is particularly advantageous when dealing with highly collinear and dimensional variables (Sui et al., 2020) (detailed introduction of PLSC and procedures in the Supplementary Methods). Brain networks were visualized with the BrainNet Viewer (Xia et al., 2013).
3. Results
3.1. Behavioral and sample characteristics
The descriptive statistics and bivariate correlations of study measures are shown in Table 1. No significant correlations were found between SA alterations (T2-T1 LSAS scores) and age (r = −0.120, p = 0.212), sex (r = −0.014, p = 0.887), or head motion (r = 0.080, p = 0.406) at T1. The paired-sample t-test revealed that the LSAS scores at T2 were significantly higher than those at T1 [t (109) = 2.810, p = 0.006].
Table 1.
Summary and correlations of demographics and behavioral measures.
| Variable | Mean ± SD | Range | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sexa (T1) | – | – | – | |||||||||
| 2. Age (years) (T1) | 22.4 ± 2.1 | 19.4–27.7 | −0.086 | – | ||||||||
| 3. FD (T1) | 0.18 ± 0.07 | 0.06–0.43 | −0.081 | −0.115 | – | |||||||
| 4. SA (T1) | 41.4 ± 19.3 | 4–99 | −0.084 | 0.117 | −0.042 | – | ||||||
| 5. TAI (T1) | 42.0 ± 8.0 | 24–61 | 0.251** | −0.024 | −0.094 | 0.362*** | – | |||||
| 6. SRLEC-Number (T1) | 12.1 ± 5.6 | 1–27 | −0.063 | −0.077 | −0.140 | 0.119 | 0.229* | – | ||||
| 7. SRLEC-Impact (T1) | 27.7 ± 16.2 | 2–77 | 0.016 | −0.020 | −0.117 | 0.143 | 0.262** | 0.922*** | – | |||
| 8. SSS (T1) | 4.9 ± 1.5 | 1.5–9 | −0.013 | 0.056 | −0.013 | −0.066 | −0.312*** | −0.246** | −0.234* | – | ||
| 9. SA (T2) | 46.4 ± 24.5 | 3–106 | −0.077 | 0.001 | 0.027 | 0.663*** | 0.328*** | 0.070 | 0.083 | −0.103 | – | |
| 10. SA-alterations (T2 - T1) | 5.0 ± 18.6 | −31–72 | −0.014 | −0.120 | 0.080 | −0.166 | 0.056 | −0.032 | −0.040 | −0.067 | 0.628*** | – |
Note: There were 110 subjects (61 females). Abbreviations: FD, framewise displacement during scanning; SA, social anxiety; SD, standard deviation; SRLEC, Self-Rating Life Events Checklist; SSS, Socioeconomic Status Scale; TAI, Trait Anxiety Inventory; T1, sampling point October 2019 to January 2020; T2, sampling point February to April 2020. ***p < 0.001; **p < 0.01; *p < 0.05. a Analysed as Male = 0; Female = 1.
3.2. Associations between global metrics and SA alterations
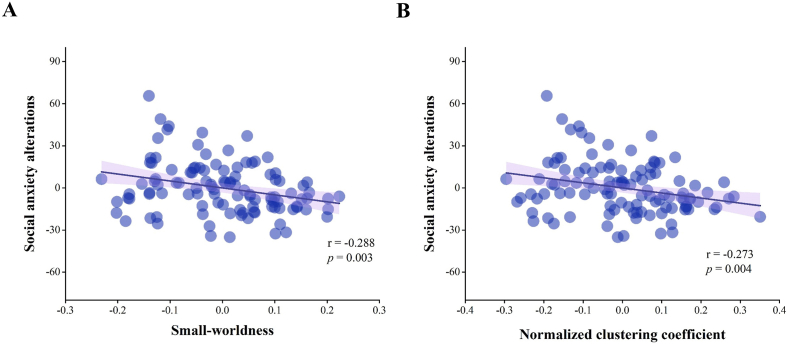
We investigated the relationships between global functional network metrics and SA alterations. In the 0.02–0.33 range of sparsity threshold, all subjects had small-world properties, with λ ≈ 1, γ > 1, and σ > 1 (Bullmore and Bassett, 2011). In partial correlations adjusted for age, sex, and mean FD, SA alterations were negatively associated with AUC of the parameters σ (r = −0.288, p = 0.003) and γ (r = −0.273, p = 0.004) (Fig. 2). In contrast, there were no significant relations between SA alterations and AUC of the parameters Lp (r = −0.097, p = 0.320), λ (r = 0.107, p = 0.272), Cp (r = 0.174, p = 0.073), Eglob (r = 0.133, p = 0.172), and Eloc (r = 0.174, p = 0.074).
Fig. 2.
Relationships between social anxiety alterations and two global metrics of the brain functional network. The y-axis represents the alterations in social anxiety from T1 to T2; the x-axis represents the residuals of the global metrics' AUCs after controlling for age, sex, and mean framewise displacement during scanning. (A) Higher AUC of the small-worldness parameter (σ) is associated with smaller SA alterations. (B) Higher AUC of the normalized clustering coefficient (γ) is associated with smaller SA alterations. Abbreviations: AUC, area under the curve; SA, social anxiety.
3.3. Degree centrality pattern linked to SA alterations
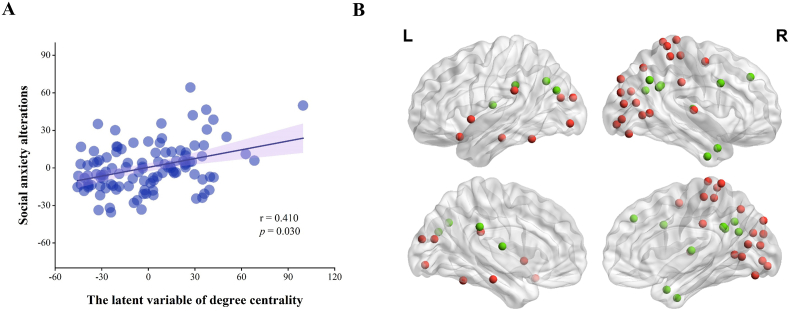
After controlling for age, sex, and mean FD, PLSC analyses revealed a generalizable degree centrality pattern that contributed to stable individual differences in SA alterations. As shown in Fig. 3A, the degree centrality pattern was positively correlated with SA alterations [r = 0.410, p(permutated) = 0.030]; i.e. if this degree centrality pattern was more evident in an individual's functional network, the individual had a larger increase in SA at T2 relative to T1. The bootstrap approach indicated substantial contributions of 40 nodes to this identified mixed pattern, where a large positive/negative weighting indicates a larger contribution of the specific brain feature in shaping this pattern (Fig. 3B and Supplementary Table S1). In pursuit of a deeper understanding of the complex interactions, we grouped these nodes into networks based on the Power's brain atlas (Power et al., 2011): 27 nodes with positive weightings came from DAN [right superior occipital gyrus (SOG), right SFG, and right middle temporal gyrus (MTG)], CON [left STG and right supramarginal gyrus (SMG)], DMN [left PHG and left inferior temporal gyrus (ITG)], FPN (left posterior orbital gyrus), SMN [right postcentral gyrus (PoCG), right precuneus (PCUN), and right precentral gyrus (PreCG)], AN (right STG and left SMG), and VN [right cuneus, right fusiform gyrus (FG), right lingual gyrus (LING), bilateral middle occipital gyrus (MOG), and right SOG]; 13 nodes with negative weightings came from DMN [bilateral angular gyrus (AG), right ITG, right middle cingulate and paracingulate gyri (MCC), left MOG, right PCUN, right SFG, and right MTG], memory retrieval network (MRN, left PCC), FPN [right inferior frontal gyrus (IFG)], and SCN (bilateral thalamus). For the details of the brain regions in the functional networks, see Supplementary Table S1.
Fig. 3.
Partial Least Squares Correlation analysis of the relationship between social anxiety alterations and degree centrality of nodes of the brain functional network. (A) SA alterations are positively linked to a latent variable that comprised a degree centrality pattern of the functional network. (B) 40 regions had absolute values of standardized weighting >2, so made a substantial contribution to the identified degree centrality pattern: for 27 of these regions the contribution was positive (red), and for 13 it was negative (green). Abbreviations: SA, social anxiety; L, left; R, right. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To test whether there were sex differences in the association between SA alterations and brain features, condition-by-covariate interaction analyses (Pan et al., 2023, Wang et al., 2021a) were performed with sex as a condition, SA alterations as covariates of interest, and age and mean FD as covariates of no interest. No sex differences were observed in the association between SA alterations and both global and nodal metrics after Bonferroni correction.
3.4. Specificity and robustness of the findings
Given the impact of general anxiety symptoms (Kuo et al., 2011), other stressful life events (Chou, 2009), and family socioeconomic status (Itani et al., 2021) on SA, we performed all the above analyses additionally controlling for scores of TAI, SRLEC, and SSS as confounding variables. Second, having investigated SA alterations as continuous variables (as described above), we categorized subjects into two groups of higher SA (HSA) and lower SA (LSA). Two main results were consistent with the main analysis, and can therefore be deemed specific and robust (see the Supplementary Results).
4. Discussion
In this prospective study of healthy young adults, we applied graph theory analysis to RS-fMRI data in order to define a widespread brain functional network topology underlying COVID-related alterations in SA. There were two key findings: (1) lower pre-pandemic small-worldness and lower normalized clustering coefficient of the functional brain network were related to SA exacerbation; (2) a latent variable consisting of a generalizable degree centrality pattern positively contributed to inter-individual variability in SA alterations. Nodes showing substantial contributions to this pattern came from a highly integrated set of brain connections involving multiple internal networks, including both high-level cognitive networks (DAN, CON, DMN, MRN, FPN, and SCN) and low-level perceptual networks (SMN, AN, and VN). Our main findings survived additional adjustment for pre-pandemic general anxiety, other stressful life events, and family socioeconomic status, and they were consistent with the results of the categorical analyses, suggesting substantial specificity and robustness. Our study provides insight into the neurobiological basis underlying COVID-related changes in SA and suggests potential functional network neural markers for the prediction of individual susceptibility to SA.
4.1. Global topology associated with SA alterations
Although the brain functional network of all subjects had, as expected, stable small-world characteristics, lower pre-pandemic small-worldness and lower normalized clustering coefficient were related to SA exacerbation during the outbreak. Consistent with this, lower global clustering coefficient of functional network has been reported in individuals with SAD (Kim et al., 2023; Zhu et al., 2017), along with lower clustering coefficient, normalized clustering coefficient, and small-worldness in gray matter networks (Chen et al., 2022; Zhao et al., 2020), suggesting a possible common network-topology pathophysiological profile in SAD. Segregation (reflected by high clustering and local efficiency) and integration (reflected by short path length and high global efficiency) are two major brain network organizational principles (Bullmore and Sporns, 2012; Fan et al., 2022). Our finding of lower normalized clustering coefficient in high-SA individuals implies impaired local processing capacity (a less segregated network organization) associated with SA changes. ‘Small-worldness’ describes networks that are simultaneously highly segregated and integrated (Rubinov and Sporns, 2010), and our finding of lower small-worldness in high-SA individuals reflects an imbalance between local specialization and global integration which may underpin less efficient information transfer, contributing to cognitive, emotional, and behavioral impairments in SA/SAD. We did not find a significantly higher normalized shortest path length, so the lower small-worldness is predominantly due to the lower normalized clustering coefficient. Collectively, our findings of reduced local segregation and maintained global integration demonstrate a trend toward more random topology in high-SA individuals (Fan et al., 2022). In comparison to small-world architecture, a more random network exhibits lower fault tolerance (Latora and Marchiori, 2001), signal-propagation speed, computational power, and synchronizability (Watts and Strogatz, 1998). This shift toward randomization may be a key way in which suboptimal brain network topology contributed to excessive SA syndromes during the pandemic.
4.2. Networks positively contributing to the multivariate pattern associated with SA alterations
In addition to the global topologies, high-SA individuals displayed a generalizable degree centrality pattern that was characterized by a positive correlation with alterations in SA.
We found one such correlation for higher nodal degree centrality in the DAN (right SOG, right SFG, and right MTG). This is broadly in accordance with RS-fMRI studies providing evidence for abnormal intrinsic FC within DAN in families genetically enriched for SAD (Bas-Hoogendam et al., 2021), patients with confirmed SAD (Liao et al., 2010), and participants with high levels of social inhibition (a basic human behavior and core feature of SA) (Blackford et al., 2014). Furthermore, task-based fMRI studies in SA/SAD have linked brain activation within DAN to negative attention bias (Kreifelts et al., 2014), social threats (harsh faces) (Goldin et al., 2009), and cognitive control during the perception of negative emotional stimuli (Brühl et al., 2013). There is evidence that the DAN plays a role in top-down spatial and feature-based attention (i.e. orienting to specific regions across the visual field), regulating attention in favor of task-relevant goals, as well as emotion regulation (Liao et al., 2010; Sripada et al., 2014). Our finding of increased nodal centrality in DAN may reflect maladaptive emotional regulation, a high level of self-awareness, and hypervigilance toward social stressors (e.g. the COVID-19 pandemic), all typical of those who become excessively social anxious.
We found another such correlation for higher nodal degree centrality in the CON (left STG and right SMG). This is consistent with previous reports of altered connectivity of the CON in SAD (Liao et al., 2010) and in participants experiencing perceived social isolation (a potential risk factor for SAD) (Layden et al., 2017). The CON is broadly associated with executive functions, including maintenance of tonic alertness, stable maintenance of task contexts (i.e. instructed rules) (Dosenbach et al., 2007), salience encoding, and detecting errors to signal the need for increased cognitive control (Botvinick et al., 2001; Sylvester et al., 2012). Therefore, it is possible that higher centrality levels of CON reflect these neural mechanisms, including a heightened tonic alertness (e.g. increased gaze fixations on negative social scenes), greater personal salience and maintenance of mental task sets involved in negative social information processing (Layden et al., 2017), and a hyperactive ‘false-positive’-prone error monitoring mechanism (Becker et al., 2023), which may predispose to developing pandemic-related SA.
We found such correlations for higher nodal degree centrality in the FPN (left posterior orbital gyrus) and in the DMN (left PHG and left ITG). These regions have consistently been implicated in SA symptoms in meta-analyses of neuroimaging studies in SAD (Brühl et al., 2014; Mizzi et al., 2022). The orbital gyrus has a crucial role in recognition and perception of emotional response and fear modulation via the amygdala, including evaluating the negative emotion state and mediating the response toward negative stimuli (Geiger et al., 2016; Klumpp et al., 2014). Higher nodal centrality in the orbital gyrus may align with high-SA individuals’ enhanced response to stressors or fear stimuli (Hahn et al., 2011), resulting in severe impairments in social behavior. In addition, the PHG is typically involved in fear conditioning and assigning accurate saliency to a stimulus (Hattingh et al., 2013). Thus these findings may reflect a neural substrate of emotional and cognitive dysfunction that contributed to pandemic-specific SA (Yang et al., 2013).
Further, we found higher degree centrality in several low-level perceptual networks, including the SMN (right PoCG, right PCUN, and right PreCG), AN (right STG and left SMG), and VN (right cuneus, right FG, right LING, bilateral MOG, and right SOG). In line with this, previous neuroimaging studies in SAD have reported abnormal activity and connectivity of SMN, AN, and VN during socially related tasks (i.e. social threat stimuli and negative self-beliefs) (Dixon et al., 2020; Goldin et al., 2009) and at resting state (Geiger et al., 2016; Liao et al., 2010; Liu et al., 2015; Zhang et al., 2022b). A pilot structural MRI study revealed that SAD patients had significant bilateral cortical thinning in the visual and sensory regions relative to healthy controls (Syal et al., 2012). The SMN is involved in multi-sensory processing of body perception and motion preparation, e.g. increased bodily awareness and various somatic symptoms (Northoff, 2020); this aligns well with the fact that in SA/SAD, enhanced awareness of bodily sensation (such as blushing, heart rate, and muscle tension) and preparation for coping with a physical threat are core symptoms (Wood et al., 2022). Additionally, beyond the traditional view that sensory cortices have merely perceptual functions, AN is responsible for emotion processing of socially important auditory information (Koelsch et al., 2018). Our results may reflect aberrant auditory information perception and affective processes in SA/SAD, resulting in difficulties in identifying social signals from voice expressions (Kreifelts et al., 2010). Moreover, VN is involved in emotional facial processing, which is crucial for social and communicative functioning (Nickl-Jockschat et al., 2015). A meta-analysis in SAD identified significant clusters in visual cortex in which faces evoked a higher neural response (Gentili et al., 2016). As the constant alertness to social-evaluative threats in the environment serves as a maintenance factor of SAD (Wong and Rapee, 2016), increased degree centrality in VN may be related to constant hypervigilance to social threats (e.g. angry faces) (Mizzi et al., 2022). In summary, the increased nodal centrality of the low-level perceptual networks in our study may reflect perceptual and emotional impairments responding to social intercourse during the pandemic.
4.3. Networks negatively contributing to the multivariate pattern associated with SA alterations
We found lower nodal degree centrality in DMN (bilateral AG, right ITG, right MCC, left MOG, right PCUN, right SFG and right MTG) and in the MRN (left PCC) high-SA individuals. This align with fMRI studies in SAD reporting lower deactivation in the PCC/PCUN during task conditions (Gentili et al., 2009) and decreased perfusion of the right PCUN in the resting state (Warwick et al., 2008); these networks have also been previously identified as displaying aberrant intrinsic FC patterns in SAD (Arnold Anteraper et al., 2014; Liao et al., 2010; Liu et al., 2015). The DMN comprises several interconnected brain regions and is activated during rest or internally-oriented mental processes (Schmaal et al., 2013). The DMN is hypothesized to perform multiple cognitive-social processes, including autobiographical/episodic memory retrieval, self-referential processing, scene construction, emotion regulation as well as social cognition (Sambuco et al., 2022; Sreenivas et al., 2012; Xu et al., 2016; Yeshurun et al., 2021). Thus, our results support the notion of aberrant processing in the DMN leading to disturbed self-evaluative and self-referential processing, heightened public self-consciousness, post-event rumination, and disruptions in emotion regulation (Cremers and Roelofs, 2016; Pan et al., 2022; Yoon et al., 2019), all of which underlie the psychopathology of SA/SAD. In addition, in the parcellation by Power et al. (2011), the MRN consists of PCC, posterior medial and lateral parietal cortex, regions frequently included in the DMN in other parcellations (Allen et al., 2011; Yeo et al., 2011). Considering the key role of MRN in autobiographical, episodic, and semantic memory retrieval, the altered nodal degree centrality of the MRN may contribute to the bias of retrieving negative life events (e.g. the COVID-19 pandemic) in SA/SAD (Fan et al., 2019). Our results therefore offer further evidence for general SA psychopathology associated with cognitive, emotional and self-social deficits.
We also revealed lower nodal degree centrality in the FPN (right IFG) and SCN (bilateral thalamus). Emerging literature links SA/SAD to structural/functional abnormalities and network connectivity involving the IFG (Dixon et al., 2020; Liao et al., 2010; Qiu et al., 2015) and thalamus (Duval et al., 2013; Heitmann et al., 2016; Mizzi et al., 2022; Terasawa et al., 2013; Wang et al., 2018; Zhang et al., 2022a). The IFG is implicated in top-down modulation of the fear response (Wang et al., 2021b), emotional stimuli processing (e.g. emotional content of speech and emotional face identification), and emotional empathy (Camacho et al., 2019; Dixon et al., 2020). Furthermore, the thalamus is an integral part of the emotional salience, emotion modulation, and cognitive/executive networks (Yamamura et al., 2016). Moreover, significant habituation effects were found in the thalamus of SAD patients (Sladky et al., 2012). Together with the literature, our results suggest that the IFG and thalamus are important network hubs for dysfunction in emotional processing and impaired cognitive control over emotion (e.g. worse inhibitory control over the emotional responses) in the socially-anxious brain during the pandemic (Wang et al., 2021b).
4.4. Limitations and future directions
This study has several limitations. First, while the Power 264 atlas is commonly used to define the network nodes (Power et al., 2011), the reasons for variations in graph-based theoretical parameters caused by different predefined template parcellations are still unclear (Wang et al., 2009) and thus need to be investigated. Second, the measure of SA relied on a classic self-report scale. Despite the appropriate reliability and validity of this standard scale, self-report measurements are inherently subject to response bias (Van de Mortel, 2008). In future work, structured interview assessment and task performance will enhance the robustness of the findings (Brach et al., 2002; Ganellen, 2007). Third, given that graph theory faces challenges regarding interpretation and sensitivity to arbitrary parameters (Stam and Reijneveld, 2007), our findings need to be compared and tested by employing other RS-fMRI methodologies (e.g. connectome-based predictive model, seed-based correlation analysis, and independent component analysis) (Geiger et al., 2016, Lin et al., 2023, Wang et al., 2023). Besides, no connections were observed between SA and other anticipated regions of interest (e.g. amygdala), therefore future studies using different analytic approaches may aid our developing understanding of the neurofunctional markers of SA alterations during stressful events. Fourth, since our study relied on a relatively small and homogeneous sample of general college students, the results should be interpreted with caution. To enhance the generalizability of our findings, future research should be conducted using larger, independent samples, encompassing populations with more diverse backgrounds (e.g. age, occupation, and mental illness). Finally, the applicability of the current findings in clinical settings is constrained due to the fundamental nature of our research as an exploratory study. Subsequent investigations are essential to validate and build upon our findings.
5. Conclusion
In conclusion, using graph theory analyses based on RS-fMRI data, this study provided evidence for pre-pandemic brain functional network topology associated with SA alterations during the COVID-19 pandemic. We found the individual functional networks of high SA individuals tended toward a more randomly organized network, and a pronounced degree centrality pattern positively associated with SA alterations, including both the high-level cognitive networks (DAN, CON, DMN, MRN, FPN, and SCN) and low-level perceptual networks (SMN, AN, and VN). Our findings throw new light on the neurobiology underlying the development of SA at the network level, and may help to identify robust neurofunctional biomarkers which can be used to prescribe and monitor interventions such as psychosocial strategies (Mayo-Wilson et al., 2014) and noninvasive brain stimulation techniques (Rosson et al., 2022), for individuals who be more susceptible to SA symptoms in response to stress- and trauma-related events such as a global pandemic, all in line with the goals of psychoradiology (Gong, 2020, Li et al., 2021, Lui et al., 2016, Sun et al., 2015).
Funding
This study was supported by the Key Research and Development Program of Sichuan Province (Grant No. 2023YFS0084), the National Key R&D Program of China (2022YFC2009900) and the National Natural Science Foundation of China (Grant Nos. 31800963, 81621003, 81761128023, 81820108018, 82027808, and 31700964).
Ethics approval statement
The study was approved by the Research Ethics Committee of West China Hospital of Sichuan University, and signed ethical consents were obtained for all participants in line with the Declaration of Helsinki.
Data and code availability statement
Data will be made available on request.
CRediT authorship contribution statement
Qingyuan Li: Formal analysis, Visualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. Xun Zhang: Formal analysis, Visualization, Writing – original draft. Xun Yang: Data curation. Nanfang Pan: Data curation. Xiao Li: Writing – review & editing. Graham J. Kemp: Writing – review & editing. Song Wang: Supervision, Writing – review & editing. Qiyong Gong: Supervision.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We are most grateful to all participants for their cooperation in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2023.100578.
Contributor Information
Song Wang, Email: wangs_psych@163.com.
Qiyong Gong, Email: qiyonggong@hmrrc.org.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Adler N.E., Epel E.S., Castellazzo G., Ickovics J.R. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Allen E., Erhardt E., Damaraju E., Gruner W., Segall J., Silva R., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A., Caprihan A., Turner J., Eichele T., Adelsheim S., Bryan A., Bustillo J., Clark V., Feldstein Ewing S., Filbey F., Ford C., Hutchison K., Jung R., Kiehl K., Kodituwakku P., Komesu Y., Mayer A., Pearlson G., Phillips J., Sadek J., Stevens M., Teuscher U., Thoma R., Calhoun V. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5(2) doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold Anteraper S., Triantafyllou C., Sawyer A.T., Hofmann S.G., Gabrieli J.D., Whitfield-Gabrieli S. Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connect. 2014;4(2):81–90. doi: 10.1089/brain.2013.0180. [DOI] [PubMed] [Google Scholar]
- Avery S.N., Blackford J.U. Slow to warm up: the role of habituation in social fear. Soc. Cognit. Affect Neurosci. 2016;11(11):1832–1840. doi: 10.1093/scan/nsw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.L., Heinrichs N., Kim H.J., Hofmann S.G. The liebowitz social anxiety scale as a self-report instrument: a preliminary psychometric analysis. Behav. Res. Ther. 2002;40(6):701–715. doi: 10.1016/s0005-7967(01)00060-2. [DOI] [PubMed] [Google Scholar]
- Baroni D., Caccico L., Ciandri S., Di Gesto C., Di Leonardo L., Fiesoli A., Grassi E., Lauretta F., Lebruto A., Marsigli N., Policardo G.R., Rosadoni M., Chiorri C. Measurement invariance of the liebowitz social anxiety scale-self-report. J. Clin. Psychol. 2022;79(2):391–414. doi: 10.1002/jclp.23413. [DOI] [PubMed] [Google Scholar]
- Bas-Hoogendam J.M., Blackford J.U., Brühl A.B., Blair K.S., van der Wee N.J.A., Westenberg P.M. Neurobiological candidate endophenotypes of social anxiety disorder. Neurosci. Biobehav. Rev. 2016;71:362–378. doi: 10.1016/j.neubiorev.2016.08.040. [DOI] [PubMed] [Google Scholar]
- Bas-Hoogendam J.M., van Steenbergen H., Blackford J.U., Tissier R.L.M., van der Wee N.J.A., Westenberg P.M. Impaired neural habituation to neutral faces in families genetically enriched for social anxiety disorder. Depress. Anxiety. 2019;36(12):1143–1153. doi: 10.1002/da.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Hoogendam J.M., van Steenbergen H., Cohen Kadosh K., Westenberg P.M., van der Wee N.J.A. Intrinsic functional connectivity in families genetically enriched for social anxiety disorder – an endophenotype study. EBioMedicine. 2021;69 doi: 10.1016/j.ebiom.2021.103445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Hoogendam J.M., van Steenbergen H., Tissier R.L.M., van der Wee N.J.A., Westenberg P.M. Altered neurobiological processing of unintentional social norm violations: a multiplex, multigenerational functional magnetic resonance imaging study on social anxiety endophenotypes. Biol. Psychiatr.: Cognitive Neuroscience and Neuroimaging. 2020;5(10):981–990. doi: 10.1016/j.bpsc.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Bas-Hoogendam J.M., Westenberg P.M. vol. 9. 2020. (Imaging the Socially-Anxious Brain: Recent Advances and Future Prospects). F1000Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C., Norman L.J., Yang H., Monk C.S., Phan K.L., Taylor S.F., Liu Y., Mannella K., Fitzgerald K.D. Disorder-specific cingulo-opercular network hyperconnectivity in pediatric OCD relative to pediatric anxiety. Psychol. Med. 2023;53(4):1468–1478. doi: 10.1017/S0033291721003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Clauss J.A., Avery S.N., Cowan R.L., Benningfield M.M., VanDerKlok R.M. Amygdala–cingulate intrinsic connectivity is associated with degree of social inhibition. Biol. Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S., Miltner W.H., Straube T. Neural correlates of self-focused attention in social anxiety. Soc. Cognit. Affect Neurosci. 2015;10(6):856–862. doi: 10.1093/scan/nsu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brach J.S., VanSwearingen J.M., Newman A.B., Kriska A.M. Identifying early decline of physical function in community-dwelling older women: performance-based and self-report measures. Phys. Ther. 2002;82(4):320–328. [PubMed] [Google Scholar]
- Brühl A.B., Delsignore A., Komossa K., Weidt S. Neuroimaging in social anxiety disorder-A meta-analytic review resulting in a new neurofunctional model. Neurosci. Biobehav. Rev. 2014;47:260–280. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Brühl A.B., Herwig U., Delsignore A., Jäncke L., Rufer M. General emotion processing in social anxiety disorder: neural issues of cognitive control. Psychiatr. Res. Neuroimaging. 2013;212(2):108–115. doi: 10.1016/j.pscychresns.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Bullmore E.T., Bassett D.S. Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Camacho M.C., Karim H.T., Perlman S.B. Neural architecture supporting active emotion processing in children: a multivariate approach. Neuroimage. 2019;188:171–180. doi: 10.1016/j.neuroimage.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang X., Zhang X., Cao H., Gong Q. Altered single-subject gray matter structural networks in social anxiety disorder. Cerebr. Cortex. 2022;33(6):3311–3317. doi: 10.1093/cercor/bhac498. [DOI] [PubMed] [Google Scholar]
- Chou K.L. Social anxiety disorder in older adults: evidence from the National Epidemiologic Survey on alcohol and related conditions. J. Affect. Disord. 2009;119(1–3):76–83. doi: 10.1016/j.jad.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Coelho C.M., Suttiwan P., Arato N., Zsido A.N. On the nature of fear and anxiety triggered by COVID-19. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.581314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers H.R., Roelofs K. Social anxiety disorder: a critical overview of neurocognitive research. Wiley Interdiscip Rev Cogn Sci. 2016;7(4):218–232. doi: 10.1002/wcs.1390. [DOI] [PubMed] [Google Scholar]
- Czorniej K.P., Krajewska-Kułak E., Kułak W. Assessment of anxiety disorders in students starting work with coronavirus patients during a pandemic in Podlaskie Province, Poland. Front. Psychiatr. 2022;13 doi: 10.3389/fpsyt.2022.980361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M.L., Moodie C.A., Goldin P.R., Farb N., Heimberg R.G., Gross J.J. Emotion regulation in social anxiety disorder: reappraisal and acceptance of negative self-beliefs. Biol. Psychiatr.: Cognitive Neuroscience and Neuroimaging. 2020;5(1):119–129. doi: 10.1016/j.bpsc.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval E.R., Hale L.R., Liberzon I., Lepping R., J N.P., Filion D.L., Savage C.R. Anterior cingulate cortex involvement in subclinical social anxiety. Psychiatr. Res. 2013;214(3):459–461. doi: 10.1016/j.pscychresns.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatr. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.C., Bar-Haim Y., Fox N.A., Pine D.S., Britton J.C. Neural mechanisms underlying heterogeneous expression of threat-related attention in social anxiety. Behav. Res. Ther. 2020;132 doi: 10.1016/j.brat.2020.103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Tso I.F., Maixner D.F., Abagis T., Hernandez-Garcia L., Taylor S.F. Segregation of salience network predicts treatment response of depression to repetitive transcranial magnetic stimulation. Neuroimage: Clinic. 2019;22 doi: 10.1016/j.nicl.2019.101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Fan Z., Qiu T., Hu L., Shi Y., Xia Y., Sun X., Liu Y., Li S., Xia M., Zhu W. Altered topological properties of the intrinsic functional brain network in patients with right-sided unilateral hearing loss caused by acoustic neuroma. Brain Imaging and Behavior. 2022;16(4):1873–1883. doi: 10.1007/s11682-022-00658-1. [DOI] [PubMed] [Google Scholar]
- Ganellen R.J. Assessing normal and abnormal personality functioning: strengths and weaknesses of self-report, observer, and performance-based methods. J. Pers. Assess. 2007;89(1):30–40. doi: 10.1080/00223890701356987. [DOI] [PubMed] [Google Scholar]
- Geiger M.J., Domschke K., Ipser J., Hattingh C., Baldwin D.S., Lochner C., Stein D.J. Altered executive control network resting-state connectivity in social anxiety disorder. World J. Biol. Psychiatr. 2016;17(1):47–57. doi: 10.3109/15622975.2015.1083613. [DOI] [PubMed] [Google Scholar]
- Gentili C., Cristea I.A., Angstadt M., Klumpp H., Tozzi L., Phan K.L., Pietrini P. Beyond emotions: a meta-analysis of neural response within face processing system in social anxiety. Exp. Biol. Med. 2016;241(3):225–237. doi: 10.1177/1535370215603514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C., Ricciardi E., Gobbini M.I., Santarelli M.F., Haxby J.V., Pietrini P., Guazzelli M. Beyond amygdala: default Mode Network activity differs between patients with social phobia and healthy controls. Brain Res. Bull. 2009;79(6):409–413. doi: 10.1016/j.brainresbull.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatr. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q. Neuroimaging Clinics of North America. Vol. 30. Elsevier Inc; New York: 2020. Psychoradiology; pp. 1–123. [Google Scholar]
- Hahn A., Stein P., Windischberger C., Weissenbacher A., Spindelegger C., Moser E., Kasper S., Lanzenberger R. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- Hattingh C., Ipser J., Tromp S., Syal S., Lochner C., Brooks S., Stein D. Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front. Hum. Neurosci. 2013;6:347. doi: 10.3389/fnhum.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes M.T., Szenczy A.K., Klein D.N., Hajcak G., Nelson B.D. Increases in depression and anxiety symptoms in adolescents and young adults during the COVID-19 pandemic. Psychol. Med. 2022;52(14):3222–3230. doi: 10.1017/S0033291720005358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Wei D.T., Yang F., Zhang J., Cheng W., Feng J.F., Yang W.J., Zhuang K.X., Chen Q.L., Ren Z.T., Li Y., Wang X.Q., Mao Y., Chen Z.Y., Liao M., Cui H.R., Li C.B., He Q.H., Lei X., Feng T.Y., Chen H., Xie P., Rolls E.T., Su L.Y., Li L.J., Qiu J. Functional connectome prediction of anxiety related to the COVID-19 pandemic. Am. J. Psychiatr. 2021;178(6):530–540. doi: 10.1176/appi.ajp.2020.20070979. [DOI] [PubMed] [Google Scholar]
- Heimberg R.G., Horner K.J., Juster H.R., Safren S.A., Brown E.J., Schneier F.R., Liebowitz M.R. Psychometric properties of the liebowitz social anxiety scale. Psychol. Med. 1999;29(1):199–212. doi: 10.1017/S0033291798007879. [DOI] [PubMed] [Google Scholar]
- Heitmann C.Y., Feldker K., Neumeister P., Zepp B.M., Peterburs J., Zwitserlood P., Straube T. Abnormal brain activation and connectivity to standardized disorder-related visual scenes in social anxiety disorder. Hum. Brain Mapp. 2016;37(4):1559–1572. doi: 10.1002/hbm.23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani M.H., Eltannir E., Tinawi H., Daher D., Eltannir A., Moukarzel A.A. Severe social anxiety among adolescents during COVID-19 lockdown. J Patient Exp. 2021;8 doi: 10.1177/23743735211038386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S., Kochiyama T., Nakai R., Abe N., Nomura M. Fear of negative evaluation is associated with altered brain function in nonclinical subjects. Psychiatr. Res. 2015;234(3):362–368. doi: 10.1016/j.pscychresns.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kambeitz J., Kambeitz-Ilankovic L., Cabral C., Dwyer D.B., Calhoun V.D., van den Heuvel M.P., Falkai P., Koutsouleris N., Malchow B. Aberrant functional whole-brain network architecture in patients with schizophrenia: a meta-analysis. Schizophr. Bull. 2016;42(Suppl. l_1):S13–S21. doi: 10.1093/schbul/sbv174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Niu X., Zhang F. Functional connectivity strength and topology differences in social phobia adolescents with and without ADHD comorbidity. Neuropsychologia. 2023;178 doi: 10.1016/j.neuropsychologia.2022.108418. [DOI] [PubMed] [Google Scholar]
- Kim B.H., Kim M.K., Jo H.J., Kim J.J. Predicting social anxiety in young adults with machine learning of resting-state brain functional radiomic features. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-17769-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred R., Bates G.W. The influence of the COVID-19 pandemic on social anxiety: a systematic review. Int. J. Environ. Res. Publ. Health. 2023;20(3):2362. doi: 10.3390/ijerph20032362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H., Fitzgerald D.A., Angstadt M., Post D., Phan K.L. Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychol. Med. 2014;44(14):3109–3121. doi: 10.1017/S0033291714000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S., Skouras S., Lohmann G. The auditory cortex hosts network nodes influential for emotion processing: an fMRI study on music-evoked fear and joy. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifelts B., Brück C., Ritter J., Ethofer T., Domin M., Lotze M., Jacob H., Schlipf S., Wildgruber D. They are laughing at me: cerebral mediation of cognitive biases in social anxiety. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreifelts B., Ethofer T., Huberle E., Grodd W., Wildgruber D. Association of trait emotional intelligence and individual fMRI-activation patterns during the perception of social signals from voice and face. Hum. Brain Mapp. 2010;31(7):979–991. doi: 10.1002/hbm.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Kuo J.R., Goldin P.R., Werner K., Heimberg R.G., Gross J.J. Childhood trauma and current psychological functioning in adults with social anxiety disorder. J. Anxiety Disord. 2011;25(4):467–473. doi: 10.1016/j.janxdis.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H., Zhao Y., Li J., Gong Q., Wang S. Neuroanatomical signatures associated with dispositional optimism predict COVID-19-related posttraumatic stress symptoms. Cereb Cortex. 2023;33(15):9387–9398. doi: 10.1093/cercor/bhad211. [DOI] [PubMed] [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87(19) doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Layden E.A., Cacioppo J.T., Cacioppo S., Cappa S.F., Dodich A., Falini A., Canessa N. Perceived social isolation is associated with altered functional connectivity in neural networks associated with tonic alertness and executive control. Neuroimage. 2017;145:58–73. doi: 10.1016/j.neuroimage.2016.09.050. [DOI] [PubMed] [Google Scholar]
- Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Sun H., Biswal B.B., Sweeney J.A., Gong Q. Artificial intelligence applications in psychoradiology. Psychoradiology. 2021;1:94–107. doi: 10.1093/psyrad/kkab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhou H., Yang Y., Wang H., Zhong N. More randomized and resilient in the topological properties of functional brain networks in patients with major depressive disorder. J. Clin. Neurosci. 2017;44:274–278. doi: 10.1016/j.jocn.2017.06.037. [DOI] [PubMed] [Google Scholar]
- Li M., Dahmani L., Hubbard C.S., Hu Y., Wang M., Wang D., Liu H. Individualized functional connectome identified generalizable biomarkers for psychiatric symptoms in transdiagnostic patients. Neuropsychopharmacology. 2023;48(4):633–641. doi: 10.1038/s41386-022-01500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Chen H., Feng Y., Mantini D., Gentili C., Pan Z., Ding J., Duan X., Qiu C., Lui S., Gong Q., Zhang W. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52(4):1549–1558. doi: 10.1016/j.neuroimage.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Liebowitz M.R. Social phobia. Mod. Probl. Pharmacopsychiatr. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Lin J., Li L., Pan N., Liu X., Zhang X., Suo X., Kemp G.J., Wang S., Gong Q. Neural correlates of neuroticism: a coordinate-based meta-analysis of resting-state functional brain imaging studies. Neurosci. Biobehav. Rev. 2023;146:105055. doi: 10.1016/j.neubiorev.2023.105055. [DOI] [PubMed] [Google Scholar]
- Liu F., Guo W., Fouche J.-P., Wang Y., Wang W., Ding J., Zeng L., Qiu C., Gong Q., Zhang W., Chen H. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Struct. Funct. 2015;220(1):101–115. doi: 10.1007/s00429-013-0641-4. [DOI] [PubMed] [Google Scholar]
- Liu X., Liu L., Yang J., Chai F., Wang A., Sun L., Zhao G., Ma D. The adolescent self-rating life events checklist and its reliability and validity. Chin. J. Clin. Psychol. 1997;5(1):34–36. [Google Scholar]
- Liu X., Zhao Y., Suo X., Zhang X., Pan N., Kemp G.J., Gong Q., Wang S. Psychological resilience mediates the protective role of default-mode network functional connectivity against COVID-19 vicarious traumatization. Transl. Psychiatry. 2023;13(1):231. doi: 10.1038/s41398-023-02525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S., Zhou X.J., Sweeney J.A., Gong Q. Psychoradiology: the frontier of neuroimaging in psychiatry. Radiology. 2016;281(2):357–372. doi: 10.1148/radiol.2016152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Becker B., Zheng X., Zhao Z., Xu X., Zhou F., Wang J., Kou J., Dai J., Kendrick K.M. A dimensional approach to determine common and specific neurofunctional markers for depression and social anxiety during emotional face processing. Hum. Brain Mapp. 2018;39(2):758–771. doi: 10.1002/hbm.23880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Zuo X.N., Ding C., Qiu J. OFC and its connectivity with amygdala as predictors for future social anxiety in adolescents. Developmental Cognitive Neuroscience. 2020;44 doi: 10.1016/J.Dcn.2020.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo-Wilson E., Dias S., Mavranezouli I., Kew K., Clark D.M., Ades A.E., Pilling S. Psychological and pharmacological interventions for social anxiety disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatr. 2014;1(5):368–376. doi: 10.1016/S2215-0366(14)70329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Bookstein F.L., Haxby J.V., Grady C.L. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3(3):143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., Fresco D.M., Heimberg R.G., Schneier F.R., Davies S.O., Liebowitz M.R. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J. Anxiety Disord. 2002;16(6):661–673. doi: 10.1016/S0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L.A. Social fearfulness in the human brain. Neurosci. Biobehav. Rev. 2012;36(1):459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Mizzi S., Pedersen M., Lorenzetti V., Heinrichs M., Labuschagne I. Resting-state neuroimaging in social anxiety disorder: a systematic review. Mol. Psychiatr. 2022;27(1):164–179. doi: 10.1038/s41380-021-01154-6. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T., Rottschy C., Thommes J., Schneider F., Laird A.R., Fox P.T., Eickhoff S.B. Neural networks related to dysfunctional face processing in autism spectrum disorder. Brain Struct. Funct. 2015;220(4):2355–2371. doi: 10.1007/s00429-014-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Anxiety disorders and the brain's resting state networks: from altered spatiotemporal synchronization to psychopathological symptoms. Adv. Exp. Med. Biol. 2020;1191:71–90. doi: 10.1007/978-981-32-9705-0_5. [DOI] [PubMed] [Google Scholar]
- Pan N., Qin K., Yu Y., Long Y., Zhang X., He M., Suo X., Zhang S., Sweeney J.A., Wang S., Gong Q. Pre-COVID brain functional connectome features prospectively predict emergence of distress symptoms after onset of the COVID-19 pandemic. Psychol. Med. 2022:1–12. doi: 10.1017/s0033291722002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N., Yang C., Suo X., Shekara A., Hu S., Gong Q., Wang S. Sex differences in the relationship between brain gray matter volume and psychological resilience in late adolescence. Eur Child Adolesc Psychiatry. Online ahead of print. doi:10.1007/s00787-023-02231-7. 2023 doi: 10.1007/s00787-023-02231-7. [DOI] [PubMed] [Google Scholar]
- Pan J., Zhang J., Ma P., Liang H., Wang H., Tao J., Wen S., Zhang J. The utility of Liebowitz social anxiety scale in the patients with social anxiety disorder in Chinese. Chin. J. Nerv. Ment. Dis. 2006;12:206–210. [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Feng Y., Meng Y., Liao W., Huang X., Lui S., Zhu C., Chen H., Gong Q., Zhang W. Analysis of altered baseline brain activity in drug-naive adult patients with social anxiety disorder using resting-state functional MRI. Psychiatry Investigation. 2015;12(3):372–380. doi: 10.4306/pi.2015.12.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer Y.D., van den Berg E., Ruis C., Jaap Kappelle L., Biessels G.J. Cognitive dysfunction in patients with type 2 diabetes. Diabetes/metabolism research and reviews. 2010;26(7):507–519. doi: 10.1002/dmrr.1112. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Huang C.-C., Lin C.-P., Feng J., Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206 doi: 10.1016/j.neuroimage.2019.116189. [DOI] [PubMed] [Google Scholar]
- Rosson S., de Filippis R., Croatto G., Collantoni E., Pallottino S., Guinart D., Brunoni A.R., Dell'Osso B., Pigato G., Hyde J., Brandt V., Cortese S., Fiedorowicz J.G., Petrides G., Correll C.U., Solmi M. Brain stimulation and other biological non-pharmacological interventions in mental disorders: an umbrella review. Neurosci. Biobehav. Rev. 2022;139 doi: 10.1016/j.neubiorev.2022.104743. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sambuco N., Bradley M.M., Lang P.J. Narrative imagery: emotional modulation in the default mode network. Neuropsychologia. 2022;164 doi: 10.1016/j.neuropsychologia.2021.108087. [DOI] [PubMed] [Google Scholar]
- Schmaal L., Goudriaan A.E., Joos L., Krüse A.M., Dom G., van den Brink W., Veltman D.J. Modafinil modulates resting-state functional network connectivity and cognitive control in alcohol-dependent patients. Biol. Psychiatr. 2013;73(8):789–795. doi: 10.1016/j.biopsych.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Schultz J., Willems T., Gadeke M., Chakkour G., Franke A., Weber B., Hurlemann R. A human subcortical network underlying social avoidance revealed by risky economic choices. Elife. 2019;8 doi: 10.7554/eLife.45249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweda A., Weismüller B., Bäuerle A., Dörrie N., Musche V., Fink M., Kohler H., Teufel M., Skoda E.-M. Phenotyping mental health: age, community size, and depression differently modulate COVID-19-related fear and generalized anxiety. Compr. Psychiatr. 2021;104 doi: 10.1016/j.comppsych.2020.152218. [DOI] [PubMed] [Google Scholar]
- Shany O., Dunsky N., Gilam G., Greental A., Gilboa-Schechtman E., Hendler T. Self-evaluation of social-rank in socially anxious individuals associates with enhanced striatal reward function. Psychol. Med. 2022:1–11. doi: 10.1017/S0033291722001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siffredi V., Liverani M.C., Freitas L.G.A., Tadros D., Farouj Y., Borradori Tolsa C., Van De Ville D., Hüppi P.S., Ha-Vinh Leuchter R. Large-scale brain network dynamics in very preterm children and relationship with socio-emotional outcomes: an exploratory study. Pediatr. Res. 2022 doi: 10.1038/s41390-022-02342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Höflich A., Atanelov J., Kraus C., Baldinger P., Moser E., Lanzenberger R., Windischberger C. Increased neural habituation in the amygdala and orbitofrontal cortex in social anxiety disorder revealed by FMRI. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. Mind Garden, Inc; 1983. State-Trait Anxiety Inventory for Adults. [Google Scholar]
- Sporns O. Graph theory methods: applications in brain networks. Dialogues Clin. Neurosci. 2018;20(2):111–121. doi: 10.31887/DCNS.2018.20.2/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas S., Boehm S.G., Linden D.E. Emotional faces and the default mode network. Neurosci. Lett. 2012;506(2):229–234. doi: 10.1016/j.neulet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Sripada C., Angstadt M., Kessler D., Phan K.L., Liberzon I., Evans G.W., Welsh R.C., Kim P., Swain J.E. Volitional regulation of emotions produces distributed alterations in connectivity between visual, attention control, and default networks. Neuroimage. 2014;89:110–121. doi: 10.1016/j.neuroimage.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., Reijneveld J.C. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed. Phys. 2007;1(1):3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D.J., Lim C.C.W., Roest A.M., de Jonge P., Aguilar-Gaxiola S., Al-Hamzawi A., Alonso J., Benjet C., Bromet E.J., Bruffaerts R., de Girolamo G., Florescu S., Gureje O., Haro J.M., Harris M.G., He Y., Hinkov H., Horiguchi I., Hu C., Karam A., Karam E.G., Lee S., Lepine J.P., Navarro-Mateu F., Pennell B.E., Piazza M., Posada-Villa J., Ten Have M., Torres Y., Viana M.C., Wojtyniak B., Xavier M., Kessler R.C., Scott K.M. The cross-national epidemiology of social anxiety disorder: data from the world mental health survey initiative. BMC Med. 2017;15(1):143. doi: 10.1186/s12916-017-0889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Jiang R., Bustillo J., Calhoun V. Neuroimaging-based individualized prediction of cognition and behavior for mental disorders and health: methods and promises. Biol. Psychiatr. 2020;88(11):818–828. doi: 10.1016/j.biopsych.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Lui S., Yao L., Deng W., Xiao Y., Zhang W., Huang X., Hu J., Bi F., Li T., Sweeney J.A., Gong Q. Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry. 2015;72(7):678–686. doi: 10.1001/jamapsychiatry.2015.0505. [DOI] [PubMed] [Google Scholar]
- Sun L., Zhang W., Wang M., Wang S., Li Z., Zhao C., Lin M., Si Q., Li X., Liang Y., Wei J., Zhang X., Chen R., Li C. Reading-related brain function restored to normal after articulation training in patients with cleft lip and palate: an fMRI study. Neurosci. Bull. 2022;38(10):1215–1228. doi: 10.1007/s12264-022-00918-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal S., Hattingh C.J., Fouché J.P., Spottiswoode B., Carey P.D., Lochner C., Stein D.J. Grey matter abnormalities in social anxiety disorder: a pilot study. Metab. Brain Dis. 2012;27(3):299–309. doi: 10.1007/s11011-012-9299-5. [DOI] [PubMed] [Google Scholar]
- Sylvester C.M., Corbetta M., Raichle M.E., Rodebaugh T.L., Schlaggar B.L., Sheline Y.I., Zorumski C.F., Lenze E.J. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35(9):527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S., Kauppi J.P., Jankowski K.F., Fujino J., Monti R.P., Tohka J., Abe N., Murai T., Takahashi H., Hari R. Brain and behavioral alterations in subjects with social anxiety dominated by empathic embarrassment. Proc. Natl. Acad. Sci. U. S. A. 2020;117(8):4385–4391. doi: 10.1073/pnas.1918081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa Y., Shibata M., Moriguchi Y., Umeda S. Anterior insular cortex mediates bodily sensibility and social anxiety. Soc. Cognit. Affect Neurosci. 2013;8(3):259–266. doi: 10.1093/scan/nss108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Mortel T.F. Faking it: social desirability response bias in self-report research. Aust. J. Adv. Nurs. 2008;25(4):40–48. doi: 10.3316/informit.210155003844269. [DOI] [Google Scholar]
- Wang J., Wang L., Zang Y., Yang H., Tang H., Gong Q., Chen Z., Zhu C., He Y. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum. Brain Mapp. 2009;30(5):1511–1523. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., Xia M., Liao X., Evans A., He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015;9:386. doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhao Y., Li J. True grit and brain: trait grit mediates the connection of DLPFC functional connectivity density to posttraumatic growth following COVID-19. J. Affect Disord. 2023;325:313–320. doi: 10.1016/j.jad.2023.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhao Y., Wang X., Yang X., Cheng B., Pan N., Suo X., Gong Q. Emotional intelligence mediates the association between middle temporal gyrus gray matter volume and social anxiety in late adolescence. Eur. Child Adolesc. Psychiatr. 2021;30(12):1857–1869. doi: 10.1007/s00787-020-01651-z. [DOI] [PubMed] [Google Scholar]
- Wang X., Cheng B., Luo Q., Qiu L., Wang S. Gray matter structural alterations in social anxiety disorder: a voxel-based meta-analysis. Front. Psychiatr. 2018;9:449. doi: 10.3389/fpsyt.2018.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cheng B., Wang S., Lu F., Luo Y., Long X., Kong D. Distinct grey matter volume alterations in adult patients with panic disorder and social anxiety disorder: a systematic review and voxel-based morphometry meta-analysis. J. Affect. Disord. 2021;281:805–823. doi: 10.1016/j.jad.2020.11.057. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang C., Miao P., Liu J., Wei Y., Wu L., Wang K., Cheng J. An imbalance between functional segregation and integration in patients with pontine stroke: a dynamic functional network connectivity study. Neuroimage: Clinic. 2020;28 doi: 10.1016/j.nicl.2020.102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwick J.M., Carey P., Jordaan G.P., Dupont P., Stein D.J. Resting brain perfusion in social anxiety disorder: a voxel-wise whole brain comparison with healthy control subjects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32(5):1251–1256. doi: 10.1016/j.pnpbp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wong Q.J.J., Rapee R.M. The aetiology and maintenance of social anxiety disorder: a synthesis of complementary theoretical models and formulation of a new integrated model. J. Affect. Disord. 2016;203:84–100. doi: 10.1016/j.jad.2016.05.069. [DOI] [PubMed] [Google Scholar]
- Wood H., Rusbridge S., Lei J., Lomax C., Elliston J., Russell A. Exploring the cognitive model of social anxiety in autistic young people—the central role of bodily symptoms. J. Autism Dev. Disord. 2022;52(12):5500–5514. doi: 10.1007/s10803-021-05359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yuan H., Lei X. Activation and connectivity within the default mode network contribute independently to future-oriented thought. Sci. Rep. 2016;6(1) doi: 10.1038/srep21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T., Okamoto Y., Okada G., Takaishi Y., Takamura M., Mantani A., Kurata A., Otagaki Y., Yamashita H., Yamawaki S. Association of thalamic hyperactivity with treatment-resistant depression and poor response in early treatment for major depression: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Transl. Psychiatry. 2016;6(3):e754. doi: 10.1038/tp.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Kendrick K.M., Wu Q., Chen T., Lama S., Cheng B., Li S., Huang X., Gong Q. Structural and functional connectivity changes in the brain associated with shyness but not with social anxiety. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Liu J., Meng Y.J., Xia M.R., Cui Z.X., Wu X., Hu X.Y., Zhang W., Gong G., Gong Q.Y., Sweeney J.A., He Y. Network analysis reveals disrupted functional brain circuitry in drug-naive social anxiety disorder. Neuroimage. 2019;190:213–223. doi: 10.1016/j.neuroimage.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y., Nguyen M., Hasson U. The default mode network: where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 2021;22(3):181–192. doi: 10.1038/s41583-020-00420-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H.J., Seo E.H., Kim J.J., Choo I.H. Neural correlates of self-referential processing and their clinical implications in social anxiety disorder. Clinical Psychopharmacology and Neuroscience. 2019;17(1):12–24. doi: 10.9758/cpn.2019.17.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J.-Y., Boedhoe P.S.W., Vriend C., Jahanshad N., Abe Y., Ameis S.H., Anticevic A., Arnold P.D., Batistuzzo M.C., Benedetti F., Beucke J.C., Bollettini I., Bose A., Brem S., Calvo A., Cheng Y., Cho K.I.K., Ciullo V., Dallaspezia S., Denys D., Feusner J.D., Fouche J.-P., Giménez M., Gruner P., Hibar D.P., Hoexter M.Q., Hu H., Huyser C., Ikari K., Kathmann N., Kaufmann C., Koch K., Lazaro L., Lochner C., Marques P., Marsh R., Martínez-Zalacaín I., Mataix-Cols D., Menchón J.M., Minuzzi L., Morgado P., Moreira P., Nakamae T., Nakao T., Narayanaswamy J.C., Nurmi E.L., O'Neill J., Piacentini J., Piras F., Piras F., Reddy Y.C.J., Sato J.R., Simpson H.B., Soreni N., Soriano-Mas C., Spalletta G., Stevens M.C., Szeszko P.R., Tolin D.F., Venkatasubramanian G., Walitza S., Wang Z., van Wingen G.A., Xu J., Xu X., Zhao Q., group E.-O.w., Thompson P.M., Stein D.J., van den Heuvel O.A., Kwon J.S. Brain structural covariance networks in obsessive-compulsive disorder: a graph analysis from the ENIGMA Consortium. Brain. 2020;143(2):684–700. doi: 10.1093/brain/awaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y., Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatr. 2011;70(4):334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang X., Li Q., Yang X., Pan N., Suo X., He M., Wang S., Kemp G.J., Gong Q. Pre-coronavirus disease 2019 brain structure might be associated with social anxiety alterations during the pandemic. Chin. Med. J. 2023 doi: 10.1097/cm9.0000000000002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Luo Q., Wang S., Qiu L., Pan N., Kuang W., Lui S., Huang X., Yang X., Kemp G.J., Gong Q. Dissociations in cortical thickness and surface area in non-comorbid never-treated patients with social anxiety disorder. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Suo X., Yang X., Lai H., Pan N., He M., Li Q., Kuang W., Wang S., Gong Q. Structural and functional deficits and couplings in the cortico-striato-thalamo-cerebellar circuitry in social anxiety disorder. Transl. Psychiatry. 2022;12(1):26. doi: 10.1038/s41398-022-01791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yang X., Wu B., Pan N., He M., Wang S., Kemp G.J., Gong Q. Large-scale brain functional network abnormalities in social anxiety disorder. Psychol. Med. 2022:1–11. doi: 10.1017/s0033291722003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen J., Gao W., Chen W., Xiao Z., Qi Y., Turel O., He Q. From fears of evaluation to social anxiety: the longitudinal relationships and neural basis in healthy young adults. Int. J. Clin. Health Psychol. : IJCHP. 2023;23(2) doi: 10.1016/j.ijchp.2022.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Zhang X., Zhou X., Song X., Zhang Z., Xu L., Zhou F., Kendrick K.M. Depression mediates the association between insula-frontal functional connectivity and social interaction anxiety. Hum. Brain Mapp. 2022;43(14):4266–4273. doi: 10.1002/hbm.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Niu R., Lei D., Shah C., Xiao Y., Zhang W., Chen Z., Lui S., Gong Q. Aberrant gray matter networks in non-comorbid medication-naive patients with major depressive disorder and those with social anxiety disorder. Front. Hum. Neurosci. 2020;14:172. doi: 10.3389/fnhum.2020.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Miao M., Lim J., Li M., Nie S., Zhang X. Is lockdown bad for social anxiety in COVID-19 regions?: a national study in the SOR perspective. Int. J. Environ. Res. Publ. Health. 2020;17(12):4561. doi: 10.3390/ijerph17124561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Qiu C., Meng Y., Yuan M., Zhang Y., Ren Z., Li Y., Huang X., Gong Q., Lui S., Zhang W. Altered topological properties of brain networks in social anxiety disorder: a resting-state functional MRI study. Sci. Rep. 2017;7(1) doi: 10.1038/srep43089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Zhuang Y., Lee P., Wong P.W.C. The changes of suicidal ideation status among young people in Hong Kong during COVID-19: a longitudinal survey. J. Affect. Disord. 2021;294:151–158. doi: 10.1016/j.jad.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.-N., Xing X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014;45:100–118. doi: 10.1016/j.neubiorev.2014.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.
Data will be made available on request.