ABSTRACT
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), emerged in Wuhan, China, in December 2019. As of September 2023, there have been over 770 million confirmed cases of COVID-19, including over 6.9 million deaths. Epidemiologic studies have indicated that comorbidities of obesity and diabetes mellitus are associated with increased morbidity and mortality following SARS-CoV-2 infection. We determined how the comorbidities of obesity and diabetes affect morbidity and mortality following SARS-CoV-2 infection in unvaccinated and adjuvanted spike nanoparticle (NVX-CoV2373) vaccinated mice. We find that obese/diabetic mice infected with SARS-CoV-2 have increased morbidity and mortality compared to age-matched normal mice. Mice that were fed a high-fat diet (HFD) and then vaccinated with NVX-CoV2373 produce equivalent neutralizing antibody titers compared to those fed a normal diet (ND). However, the HFD mice have reduced viral clearance early in infection. Analysis of the inflammatory immune response in HFD mice demonstrates a recruitment of neutrophils that was correlated with increased mortality and reduced clearance of the virus. Depletion of neutrophils in diabetic/obese vaccinated mice reduced disease severity and protected mice from lethality. This model recapitulates the increased disease severity associated with obesity and diabetes in humans with COVID-19 and is an important comorbidity to study with increasing obesity and diabetes across the world.
IMPORTANCE
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused a wide spectrum of diseases in the human population, from asymptomatic infections to death. It is important to study the host differences that may alter the pathogenesis of this virus. One clinical finding in coronavirus disease 2019 (COVID-19) patients is that people with obesity or diabetes are at increased risk of severe illness from SARS-CoV-2 infection. We used a high-fat diet model in mice to study the effects of obesity and type 2 diabetes on SARS-CoV-2 infection as well as how these comorbidities alter the response to vaccination. We find that diabetic/obese mice have increased disease after SARS-CoV-2 infection and they have slower clearance of the virus. We find that the lungs of these mice have increased neutrophils and that removing these neutrophils protects diabetic/obese mice from disease. This demonstrates why these diseases have increased risk of severe disease and suggests specific interventions upon infection.
KEYWORDS: coronavirus, diabetes, immunization
INTRODUCTION
The United States of America has an obesity rate of 42%, a diabetes rate of 11%, and an additional 35% of the population has been identified as prediabetic (1). Diabetes and obesity have been linked both clinically and through physiological studies to increased severity of many types of diseases that can be traced toward various aspects of these metabolic syndromes (2 – 4). Epidemiologic studies of COVID-19 have identified obesity and diabetes as significant risk factors for increased disease severity, length of hospitalization, and death (5 – 10). The immune and molecular mechanisms behind this increased risk of COVID-19 have yet to be fully determined. SARS-CoV-2 is not the only coronavirus that displayed increased disease severity in those with diabetes or obesity (6, 11 – 17). The Middle East respiratory syndrome coronavirus (MERS-CoV), first identified in 2012, was clinically and experimentally linked to increased disease severity in those with diabetes (18, 19). Across multiple clinical and epidemiologic studies, diabetics have a threefold to sevenfold higher odds ratio of severe disease and death after MERS-CoV infection (11, 14 – 16). Mouse models of disease and viral infections have been used for studying coronaviruses and other pathogens (20). MERS-CoV also demonstrated increased morbidity and mortality in an obese/diabetic mouse model, with high-fat diet (HFD) mice presenting with increased lung pathology, viral titer, and a dysregulated immune response to MERS-CoV infection (11, 19, 21).
The etiology of type 2 diabetes (T2D) is closely linked with chronic inflammation induced by excess adipose tissue. Stressed adipocytes and adipose tissue macrophages (ATMs) secrete numerous proinflammatory cytokines that result in chronic low-grade inflammation which alters homeostatic glucose regulation by decreasing cellular responsiveness to insulin (4). This results in hyperglycemia, hyperinsulinemia, and glucose intolerance characteristic of T2D. The systemic inflammatory responses of obesity and T2D are seen in the microenvironment of the lungs. Polarization of the macrophage response is observed where a Th1 response predominates in OB/OB and DIO mice (22, 23). It is shown that fewer macrophages are recruited to the lungs which allows for the inflammatory response to recruit other cell types, including neutrophils which we observe in this manuscript (24, 25). This systemic inflammatory response leads to altered vaccine responses for a variety of vaccines in humans and can be observed in mice. Through several studies, the role of T cells is dominant where vaccinated obese or diabetic mice have reduced T-cell activation, thus altering the vaccine efficacy (26). The mechanism underlying these deficiencies is currently unknown.
To study the role of obesity and diabetes in SARS-CoV-2, we utilized a mouse model where mice are fed a high-fat diet (60% fat from diet) starting at 3 weeks of age compared to a normal diet (ND) (22% fat from diet). By 14 weeks of age, HFD mice are obese, glucose intolerant, and have high resting glucose levels; all metrics of having T2D. Infection of ND and HFD mice with SARS-CoV-2 (MA10 mouse-adapted strain) demonstrates lethal disease in only HFD mice by 7 dpi. Equivalent viral lung titers were found in both ND and HFD mice, but increased lung inflammation was observed in HFD (obese/diabetic) mice. Vaccination of ND and HFD mice with the saponin-based Matrix-M adjuvanted spike nanoparticle (NVX-CoV2373) (27, 28) resulted in the generation of similar levels of neutralizing antibodies. However, when challenged with SARS-CoV-2 (MA10), HFD mice had a delay in clearance with little reduction in lung titer early in infection. Evaluation of the inflammatory response in HFD vaccinated/infected mice found an increase in neutrophils in lung infiltrate that correlated with an increased innate inflammatory response. When neutrophils are depleted in HFD mice, we observed reduced weight loss, reduced clinical signs of disease, and increased survival demonstrating a role for neutrophils in the severity of disease in diabetic/obese mice. We conclude that this demonstrates a dysregulated immune response in obese/diabetic mice leading to increased morbidity and mortality as well as potentially skewing the quality of protection from vaccination. This supports what is seen in diabetic and obese humans throughout the COVID-19 pandemic and provides a model to identify the molecular and immunological mechanisms underlying the response to SARS-CoV-2 in this population.
RESULTS
High-fat diet-fed mice have obesity and type 2 diabetes
To study the effects of obesity and diabetes on SARS-CoV-2 infection, we established a model of SARS-CoV-2 infection based on the previously established HFD-fed model. We previously developed an HFD mouse model to study MERS-CoV, where B6/hDPP4 mice were fed an HFD for 15 weeks before evaluation for diabetic and obesity endpoints including weight, fasting glucose levels, glucose intolerance, and insulin levels (18). This demonstrated that the model of HFD chow would establish an obese and type 2 diabetes phenotype in C56B6 mice (18). We also showed that male mice developed obesity and type 2 diabetes while female mice did not. As such, the model used in this study with C57B6/K18-hACE2 mice used 15 weeks on HFD, and only male mice in this model (18). At the time of weaning (~3 weeks), C57BL/6 mice are placed on an ND (18% kcal from fat, 24% kcal from protein, and 58% kcal from carbohydrates) or an HFD (60% kcal from fat, 18% kcal from protein, and 21% from carbohydrates) (Fig. 1A). At 18 weeks of age, 15 weeks on each diet, we analyzed fasting blood glucose (Fig. 1B), weight (Fig. 1C), and ACE2 expression (Fig. 1D). The HFD mice had increased fasting blood glucose and higher weight, which are all parameters of obesity, insulin resistance, and T2D. We examined the expression of ACE2 to ensure there were no intrinsic differences that could confound results during infection. We did not observe any difference in ACE2 levels in normal vs obese/diabetic mouse cohorts. Once established, 18-week-old ND and HFD mice were then used for pathogenesis and vaccination experiments throughout the study.
Fig 1.
A high-fat diet (HFD) induces obesity and diabetes in C57BL/6J mice. (A) Schematic overview illustrating diet schedule and infection for control and obese/diabetic mice. Briefly, at weaning (3 weeks), mice were placed on a normal diet (ND) or HFD. At 18 weeks of age, these mice were infected with either 1 × 103 or 1 × 105 pfu of SARS-CoV-2 Mouse Adaptive 10, monitored daily for weight changes, and a subset was euthanized at days 2, 4, and 7 to analyze lung viral titer and host immune response. The (B) fasted blood glucose concentration n = 13 mice/group, (C) the starting weight of ND and HFD mice n = 45 mice/group, and (D) ACE2 expression of ND and HFD mice n = 5 mice/group prior to infection. *P < 0.5 and ****P < 0.0001 as determined by an unpaired two-sided t-test. The data are presented as mean ± SD.
SARS-CoV-2-infected obese/diabetic mice have increased morbidity and mortality
ND and HFD mice were infected with the SARS-CoV-2 (MA10) strain (29) at 103 and 105 PFU per mouse. Mice were weighed daily for 7 days, with lungs evaluated on 2, 4, and 7 days post-infection (dpi). Approximately 20% of the ND mice infected with 103 or 105 PFU MA10 died by 7 dpi compared to the HFD mice infected with 103 PFU where ~40% died by day 7 (Fig. 2A). Most notably, 100% death was observed in the 105 PFU-infected HFD group. The kinetics of lethality varied between groups as well, with 20% of the HFD mice dying by day 3, whereas the ND mice did not die until 5 dpi.
Fig 2.
Enhanced mortality in obesity/diabetes mice after SARS-CoV-2 infection. The survival (A) percent weight loss for low-dose (B) high-dose (C) gram lost for low-dose (D) and high-dose (E) challenge. Viral lung titers were assessed for control and obese/diabetic mice throughout a SARS-CoV-2 MA10 infection (F) For (A). *P < 0.05 as determined by a Log-rank (Mantel-Cox) test for survival analysis between ND and HFD for low and high infection dose with the data presented as mean ± SD with n = 10 mice/group. In (G), lung cytokine, chemokine, and growth factors were assessed at 2, 4, and 7 dpi for the 1 × 103 infectious dose using a mouse cytokine/chemokine array. The fold change was calculated compared to ND-infected mice (n = 3). For (B–D), the data are presented as mean ± SD with n = 3–5 mice/group.
Weight loss was followed through 7 dpi in all groups (Fig. 2B through E). For the low infection dose, we observed the ND mice starting to lose weight at 2 dpi with a total weight loss of 20% by 7 dpi. The HFD mice lost weight starting at 2 dpi, which continued until 4 dpi, for a total loss of around 10% and was maintained for the remainder of the experiment. For the high infection dose, the ND mice lost weight starting on 2 dpi and continued through the day 7 endpoint of this experiment with a total weight loss of ~30% (Fig. 2C). The HFD mice lost weight starting on 2 dpi and continued until succumbing to the infection on 5 dpi (Fig. 2C). As ND mice weigh less than HFD mice, analysis of by grams of weight lost intragroup, as compared to total %, can provide additional insight. Here, when we compare the ND and NFD mice, no differences were observed (Fig. 2D and E).
We next examined viral replication in the lungs of infected mice at 2, 4, and 7 dpi. For all groups, the peak viral replication occurred at 2 dpi with a viral titer ~108 pfu/g of lung and decreased through day 7 (Fig. 2F). At 4 dpi, we observed less viral titer in the 105 PFU group compared to the 103 PFU group (Fig. 2F). This was surprising; however, we suspect that the reduced viral titer in the high-dose lungs is potentially due to either reduced numbers of infectable cells at this timepoint due to virus-induced cell death or a large influx of inflammatory cells, potentially limiting replication but not to substantial levels or quality to protect from death.
Obese/diabetic mice have altered host immune responses after infection with SARS-CoV-2
As obesity/diabetes is known to augment the host immune response (30 – 34), we examined the cytokine and chemokine profile of uninfected, SARS-CoV-2-infected ND and HFD mice to determine whether differences in immune response correlated with disease. To perform a longitudinal analysis, we used the samples from mice infected with 1 × 103 pfu/mouse across 7 dpi. The 1 × 103 pfu dose was used rather than the 1 × 105 pfu dose so that we could model a moderate infection through 7 days rather than the lethal infection of the 1 × 105 pfu dose. qPCR was performed on lung RNA, and we investigated the changes in expression for a variety of chemokine and cytokine genes in both ND and HFD mice. On days 2 and 4 post-infection, we observed neutrophil recruitment-associated chemokines in HFD compared with ND mice (Fig. 2G). Cxcl1, Cxcl10, Csf3, and IL10 are all key neutrophil recruitment factors and are highly upregulated in HFD mice at day 2 of infection. Those levels are reduced at day 4 and then lower at day 7. This correlates with the neutrophil infiltration kinetics seen in the lungs as shown in Fig. 5. Interestingly, we observe that HFD mice had a ~5-fold downregulation of IL1-β, which is a mediator of lung inflammation, fever, and fibrosis and is associated with protection in human COVID-19 patients (Fig. 2G). We hypothesize that neutrophils play an early and critical role in protection from lethal disease in this model and that reduction in neutrophils may protect mice from lethal disease.
Obese/diabetic mice have no difference in lung pathology after infection with SARS-CoV-2
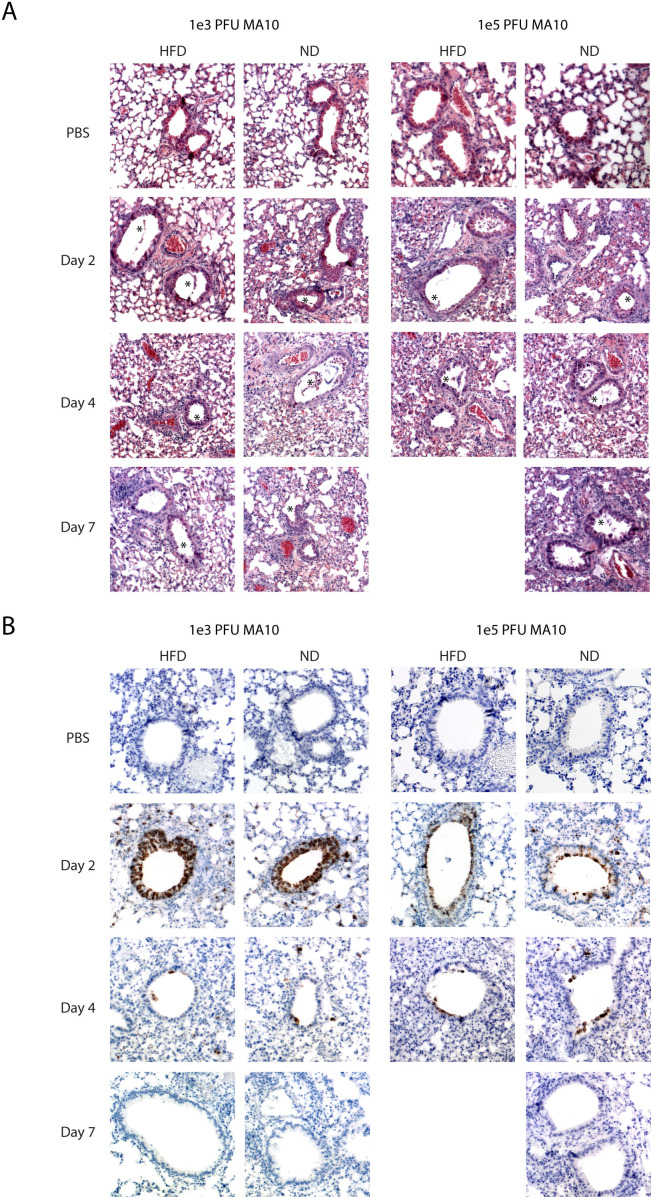
At the low infectious dose, both HFD and ND mice show similar lung pathology characterized by a mixed inflammatory infiltrate that peaks at 4 dpi and begins to resolve by 7 dpi. Epithelial cell sloughing in the bronchi is observed starting at 2 dpi with epithelial cells replaced by 7 dpi in most large airways. An observable level of edema is also visible in both HFD and ND lungs across time points (Fig. 3A).
Fig 3.
Similar lung histology and viral clearance in control and obese/diabetic mice. Control and obese/diabetic mice were infected intranasally with either 1 × 103 or 1 × 105 pfu/ mouse. Lungs were collected on 2, 4, and 7 dpi and fixed in 10% neutral buffered formalin for greater than 24 hours. Tissue was embedded in paraffin. (A) 5 µm sections were cut and stained with hematoxylin and eosin and (B) immunostained with anti-SARS nucleocapsid protein antibody. Images are shown at original magnification ×10 and are representative of n= 3–5 mice/group.
At the high infectious dose, the course of inflammation in the lung is similar but more severe than at the low dose. Mixed inflammatory infiltrates in the alveolar space are observed beginning at 2 dpi and increasing through 7 dpi for the surviving ND mice. The infiltrates are predominantly comprised of neutrophils and monocytes, as initially observed by H&E and quantified by flow cytometry analysis in Fig. 5; Fig. S1. Bronchiolar sloughing is also more pronounced at the high infectious dose, with visible damage still present at 7 dpi. Perivascular cuffing and edema were also observed in the high-infectious dose mice (Fig. 3A).
Lung sections were stained for the presence of nucleocapsid (N) protein using immunohistochemistry (IHC) to visualize viral antigen localization within the lung sections and to determine whether HFD caused a broader spread of infection than ND-fed mice. Lungs from the low 1 × 103 PFU dose and the higher 1 × 105 PFU dose were assessed by IHC. For the lower dose infection, similar to what was observed by viral titer, the amount of N staining is highest at 2 dpi, with the majority of N localizing in the ciliated epithelial cells of the large airways for both the control and HFD mice (Fig. 3B). Few positive cells are seen at day 4 and no positive lung cells are seen by day 7 post-infection. We do detect a small amount of N-positive cells in the alveolar epithelial cells, most noticeably at 4 dpi (Fig. 3B). Sections from 7 dpi show little to no N staining positivity, correlating with the viral clearance pattern observed by the viral titer (Fig. 3B). At the higher dose, we find few positive cells at day 2 compared to the low-dose infection. We have observed this in previous experiments as well and hypothesize that after infection the infected epithelial cells are sloughing off quickly after the large dose of virus is administered, leading to cell death and less N-positive cells at day 2 compared to the low-dose infection. Through days 4 and 7, there is little difference in the two models. No differences are observed between ND and HFD mice demonstrating that HFD mice have no change in virus tropism. The data demonstrate that obesity/diabetes increases the mortality of a SARS-CoV-2 infection.
Vaccinated HFD mice suffer from weight loss and have delayed clearance after SARS-CoV-2 infection
Obesity/diabetes decreases vaccine efficacy toward viral respiratory pathogens (30 – 36). In humans receiving SARS-CoV-2 mRNA vaccination, those with obese/diabetic comorbidity demonstrate the highest rates of severe disease and hospitalization (37 – 40). Thus, to mimic the timing of vaccination in the general population, we assessed the efficacy of a clinically licensed adjuvanted protein subunit vaccine (NVX-CoV2373) vaccine in obese/diabetic mice. NVX-CoV2373 is an adjuvanted full-length Spike protein vaccine based on the SARS-CoV-2/Wuhan1 sequence that is highly effective at reducing SARS-CoV-2 infection in young ND mice (28). At 18 weeks of age, ND and HFD mice were vaccinated with 1 µg of NVX-CoV2373 and then received a second dose 14 days later. On day 28 post-vaccination, blood was collected to evaluate neutralizing antibodies toward SARS-CoV-2 (MA10). On day 32 (~2 weeks after the second vaccination), mice were challenged with 1 × 105 pfu/mouse of SARS-CoV-2 (MA10) (Fig. 4A). The 1 × 105 pfu is lethal in obese/diabetic mice by 7 dpi, as seen in Fig. 2A, so for this experiment, the mice were only analyzed through 4 dpi. We monitored weight daily and analyzed lungs on 2 and 4 dpi to determine viral lung titer and immune responses (Fig. 4A). As expected, the unvaccinated mice had no detectable neutralizing antibodies against MA10 (Fig. 4B). Vaccinated ND mice had ~1×104 neut99, as did the vaccinated HFD mice (Fig. 4B), demonstrating no difference between groups. Next, we observed that non-vaccinated mice in both ND or HFD mouse groups had ~50% survival rate by day 3, with no further mortality through 4 dpi (Fig. 4C). Unvaccinated ND mice lost 10% of their starting weight through 4dpi (Fig. 4D), which was prevented with vaccination. However, vaccination in HFD mice did not protect from a ~10% starting weight loss by 4 dpi.
Fig 4.
Viral clearance is delayed in obese/diabetic vaccinated mice. (A) Schematic overview illustrating vaccination and challenge for ND and HFD mice. Briefly, at 18 weeks of age, mice were vaccinated as well as 14 days later. On day 28 post-vaccination, mice were bled to determine (B) serum-neutralizing antibodies prior to infection, n = 12–15 mice/group. On day 32, these mice were challenged with 1 × 105 pfu of SARS-CoV-2 MA 10, monitored daily for weight changes, and a subset was euthanized on days 2 and 4 to analyze lung viral titer and host immune response. The (C) mortality, weight loss of (D) ND, (E) HFD, and (F) viral titer of sham and vaccinated mice after the SARS-2 MA10 challenge. n = 5–10 mice/group. For (D), *P < 0.5 and ***P < 0.001 as determined by a mixed-effect analysis followed by a Sidak test for multiple comparisons. For (F), P *** <0.001 and P **** <0.0001, data were log-transformed and analyzed by mixed-effect analysis followed by Sidik for multiple comparisons. The data are pooled from two independent experiments and presented as mean ± SD.
Lungs of infected mice were next analyzed for viral titer at 2 and 4 dpi. The unvaccinated ND mice had a viral titer of ~1010 pfu/g (Fig. 4F) and the other two mice with moderate levels in their lungs cleared the virus by 2 dpi. By 4 dpi, the unvaccinated ND mice had 2 logs lower virus compared to 2 dpi, and the vaccinated ND mice had almost no detectable virus in their lungs (Fig. 4F). For the HFD mice, at 2 dpi, there was little difference in lung titer between vaccinated and unvaccinated mice although these mice had equivalent levels of neutralizing antibody (Fig. 4F). At 4 dpi, the unvaccinated HFD mice had equivalent lung titers to the ND mice, and the vaccinated HFD mice had no detectable virus in their lungs (Fig. 4F). In separate experiments, the mice were tested at day 7 for lung titer in the same scheme of vaccination in HFD mice and there was no detectable virus in either ND or HFD groups. This demonstrates that despite comparable neutralizing titers in vaccinated HFD and ND mice, there is significantly delayed clearance of SARS-CoV-2 in obese/diabetic mice.
HFD mice have increased neutrophil infiltration and higher inflammatory chemokine response after SARS-CoV-2 infection
Our data demonstrating that HFD mice suffer from increased weight loss and have delayed clearance suggest they have a distinct immune response that alters infection. To assess the innate immune response of HFD mice after challenge with SARS-CoV-2, we performed both flow cytometry-based immunophenotyping and quantification of cytokine and chemokine protein levels in the lungs. Our previous analysis of RNA levels in the lungs of obese/diabetic mice suggested that neutrophils may be present at much higher concentrations in obese/diabetic lungs.
As obesity is known to augment the immune system, we established the baseline immune cell population in the lungs of uninfected ND and HFD mice to determine what the initial skew was of inflammatory infiltrates. When we examined the cells of the innate immune system, we observed that HFD mice had about fivefold fewer neutrophils and threefold fewer monocyte-derived dendritic cells (moDCs) compared to ND mice (Fig. 5A and C; Fig. S1). For adaptive immune system cells, we observed that HFD mice had around twofold higher total T cells, CD4+, and CD8+ T cells (Fig. 6C through E; Fig. S2). With established baselines, we could examine how infection and infection plus vaccination change the landscape of the immune cells.
Fig 5.
Increased neutrophil recruitment in the lungs of HFD mice after the SARS-CoV-2 challenge. After the SARS-2 MA10 challenge, lungs were harvested, cells were isolated, and stained for surface markers for (A) neutrophils, (B) CD11b+ DCs, (C) monocyte-derived DCs, (D) CD103+ cDCs, and (E) plasmacytoid DCs at days 2 and 4 post-challenge. *P < 0.05 and **P < 0.01 as data were analyzed by mixed-effect analysis followed by Tukey for multiple comparisons n = 3–5 mice/group.
Fig 6.
T-cell dysregulation in the lungs of HFD mice after the SARS-CoV-2 challenge. After the SARS-2 MA10 challenge, lungs were harvested, cells were isolated, and stained for surface markers for (A) natural Killer cells, (B) B cells, (C) total T cells, (D) T-helper cells, and (E) cytotoxic T cells at days 2 and 4 post-challenge n = 3–5 mice/group. *P < 0.05 as data were analyzed by mixed-effect analysis followed by Tukey for multiple comparisons n = 3–5 mice/group (F) CD4+ SARS-CoV-2-specific T cells and (G) CD8+ SARS-CoV-2-specific T cells from naïve and vaccinated ND and HFD mice after ex vivo stimulation n = 6 mice/group. Cells were collected at day 28 after prime and boost vaccination in vaccine experiments. *P < 0.5 as determined by an unpaired two-sided t-test. The data are presented as mean ± SD.
As neutrophils are the first cells recruited to the site of infection, we quantified the percentage of neutrophils in the lungs. On day 2 post-infection, unvaccinated HFD mice recruited ~5-fold the neutrophils to the lungs as compared to uninfected HFD mice (Fig. 5A). Interestingly, this recruitment was to the level of unvaccinated ND mice, which did not increase neutrophil recruitment compared to uninfected (Fig. 5A). On day 4 post-infection, around a fivefold decrease in neutrophils was observed between the sham HFD and ND mice, which indicated the HFD mice had returned to baseline (Fig. 6A). To determine whether other innate immune system cells were augmented during infection, we next examined the different subsets of DCs. Regardless of vaccine status, ND and HFD had ~20-fold increase in CD11b+ DCs and ~2-fold decrease in CD103+ DCs compared to uninfected. We did not observe changes in the moDCs of ND vaccinated or unvaccinated compared to uninfected ND mice (Fig. 6C). However, on day 2 post-infection, sham-vaccinated HFD mice had around a threefold increase in moDCs compared to uninfected, which was comparable to ND-uninfected mice (Fig. 5C). On day 4 post-infection, the unvaccinated ND mice had ~3-fold higher moDCs than vaccinated ND mice. Collectively, the data demonstrate that HFD mice preferentially recruit neutrophils to the site of infection. Their immune system is below the baseline of ND mice and must compensate for this. After examining myeloid lineage cells, we wanted to determine how cells of lymphoid origin responded to infection.
NK cells are lymphoid origin but are cells of the innate immune system and could play a role in the differential protection seen. Unvaccinated ND mice had about a significant sixfold increase in NK cells at day 2 post-infection, with a trend at day 4 post-infection compared to uninfected (Fig. 6A). Vaccinated ND mice did not increase their percentage of NK cells compared to unvaccinated, which is not surprising as vaccination should elicit a robust adaptive immune response. Regardless of vaccination status, HFD mice had a twofold increase in NK cells on days 2 and 4 post-infection (Fig. 6A), suggesting that vaccination was not as efficient in these mice. Next, we wanted to examine the cells of the adaptive immune system.
For B cells that produce antibodies, we observed that HFD-vaccinated mice had ~2-fold higher B cells in their lungs at day 4 post-infection than HFD-unvaccinated mice (Fig. 6B). Finally, we wanted to examine the T-cell response between these mouse groups. When we compared uninfected ND and HFD mice, we observed ~2-fold higher total T cells, CD4+, and CD+8 T cells (Fig. 6C through E). We hypothesized that HFD mice had a lower percentage of innate immune cells which allows for the percentage of T cells to contribute more to the overall CD45+ cell population. On day 2 post-infection, sham-vaccinated ND had ~2-fold higher T cells, CD4+ and CD8+ T cells compared to sham HFD mice (Fig. 6C through E), and around twofold lower CD8+ T cells compared to ND-vaccinated mice (Fig. 6E). Vaccinated ND mice had ~2-fold CD4+ T cells compared to HFD-vaccinated mice (Fig. 6D). On day 2 post-infection, unvaccinated HFD mice had ~2-fold less total T cells and CD4+ T cells when compared to HFD- and ND-vaccinated mice, respectively (Fig. 6C and D). The data suggest that HFD mice have a lower percentage of T cells compared to ND mice and have a poorer CD4+ T-cell response to a challenge after vaccination.
HFD mice have altered antigen-specific T-cell activation
We performed ex vivo stimulation of T cells with SARS-CoV-2 spike peptide pool from the lungs of vaccinated and unvaccinated ND and HFD mice. At 7 days post-vaccination, mice were euthanized and analyzed for the amount of CD4 and CD8 T-cell activation in ND and HFD mice. For unvaccinated ND and HFD mice, we observed negligible amounts of cytokine production from CD4+ or CD8+ T cells after stimulation (Fig. 6F and G). It was determined that vaccinated mice produced IFN-γ and TNF-α after peptide stimulation, indicating a T-cell response skewed toward a Th1 response (Fig. 6F and G). For HFD-vaccinated mice, IFN-γ and TNF-α were secreted from CD4+ and CD8+ T cells, but only CD8+ T cells significantly increased compared to unvaccinated (Fig. 6F and G). However, ND-vaccinated mice produced IFN-γ and TNF-α from CD4+ and CD8+ T cells and produced more from CD4+ T cells compared to HFD mice (Fig. 7F and G). Taken together, vaccination induces a Th1 response in ND and HFD mice, but vaccination does not induce a robust response in HFD mice compared to ND mice which collaborates with could be due to a lower percentage of CD4+ T cells in HFD mice (Fig. 6D).
Fig 7.
Increased neutrophil recruitment and cytokine production in HFD-vaccinated mice after challenge. On days 2,4, and 7 post-infection, lungs were harvested and used in a Bio-plex pro-inflammatory mouse chemokine panel. The pg/mL was generated from a standard curve. *P < 0.05 and **P < 0.01 as analyzed by mixed-effect analysis followed by Sidak for multiple comparisons (n = 3–5 mice/group).
HFD mice have higher levels of cytokine and chemokines in the lungs after challenge
To further characterize the immune responses in ND and HFD mice, we next performed a multiplexing cytokine assay to examine the concentration of soluble immune mediators in the lungs of vaccinated ND and HFD SARS-CoV-2-infected mice. When we compared the ND and HFD mice at 2 dpi, we observed higher levels of CCL2, CCL5, CCL7, CXCL10, and IL-6, and at 4 dpi, CCL2, CCL5, CXCL12, CXCL1, GM-CSF, and TNF-α in HFD mice compared to ND mice (Fig. 7). These chemokines are all produced from or attract neutrophils and are concordant with the increased neutrophil infiltration we observe in flow cytometry analysis of infected mice. As we observed this infiltration of neutrophils in HFD mice compared to ND mice, we wanted to determine whether neutrophils played a role in the increased mortality of HFD mice.
To address the role neutrophils play in the increased mortality of HFD mice, we depleted neutrophils with an anti-Ly6G antibody (clone, 1A8) in the context of a lethal (1 × 105 pfu/mouse) SARS-CoV-2 mouse model. A day before infection, mice were intra-peritoneally injected with either anti-Ly6G or control (IgG2a isotype) antibody, and administration continued every other day throughout the infection. We examined mortality as our first parameter to determine the effect neutrophils have on increased mortality in HFD mice. ND mice with neutrophils depleted compared to isotype control showed no differences in survivability (Fig. 8A). However, HFD mice lacking neutrophils demonstrated increased survivability compared to HFD isotype and anti-Ly6G ND mice (Fig. 8A). After determining the effect on mortality, we wanted to examine weight loss. HFD mice given the isotype antibody lost around 10% body weight, which was similarly observed in the previous study (Fig. 8B and 2C). As previously observed, ND mice lost around 30% of their body weight (Fig. 8B and 2C). Neutrophil-depleted ND mice lost around 20% of body weight before death (Fig. 8B and 2C). Surprisingly, neutrophil-depleted HFD mice lost around 10% body weight, but they survived the experiment’s conclusion, which was not previously observed (Fig. 8B and 2C). The data suggest that neutrophil recruitment has a direct role in the increased mortality of SARS-CoV-2 infection.
Fig 8.
Increased HFD survival with neutrophil depletion after the challenge. The (A) survival and percent weight loss for (B) isotype antibody control or (C) anti-Ly6G antibody ND and HFD mice. *P < 0.05 as analyzed by Gehan-Breslow-Wilcoxon test for survival n = 5 mice/group.
DISCUSSION
SARS-CoV-2 infection is more severe in obese and diabetic individuals (5 – 7, 21). This has been observed in epidemiologic data across many countries and economic levels (8). Increased disease in diabetic and obese individuals is seen following several viral infections, including influenza virus, hepatitis B, hepatitis C, and another highly pathogenic coronavirus, MERS-CoV (19, 41, 42). We have previously demonstrated that in a mouse model of MERS-CoV, there is increased inflammation and a dysregulated immune response (18). In this work, we utilized an obese and diabetic mouse model for the study of SARS-CoV-2 pathogenesis to determine whether we could observe increased disease as well as assess the effect of obesity and diabetes on SARS-CoV-2 vaccine responses.
We find that there are significant differences in the obese and diabetic (HFD) mice compared to normal mice after SARS-CoV-2 infection. First, the HFD mice have increased mortality after SARS-CoV-2 infection compared to ND mice. There are no observed differences in lung pathology or virus titer to explain the reason for the mortality other than the increased neutrophil infiltration we observed in the lungs of mice, which may have an overreaching effect on other aspects of host response not accounted for in this work.
Upon vaccination of the ND and HFD mice, we identified severe effects of obesity and diabetes on the vaccine-mediated protection in mice. While neutralizing antibody levels are similar between ND and HFD mice vaccinated with NVX-2373 and lethality is protected in both the ND- and HFD-vaccinated mice, even with their advanced age to the initial testing, there is no protection from weight loss in the vaccinated HFD mice compared to ND mice. When virus levels in the lung were analyzed, we observed a significant delay in clearance of the virus at 2 dpi with the HFD mice showing no reduction in virus titer of vaccinated mice while the ND mice reduced virus titer by 4 logs in lungs at the same timepoint. Analysis of the inflammatory response in the mice also shows differences with the key difference being higher levels of neutrophils in the lungs of sham and vaccinated HFD mice compared to ND mice. This was corroborated by assaying for chemokines and cytokines in these lungs. We find that neutrophil-produced chemokines and cytokines are significantly upregulated in the HFD mice, potentially associated with the reduction in clearance of virus from HFD-vaccinated mice. Analysis of adipose tissue in humans also displays increased inflammation which correlates with what we observe in mice (43, 44).
We interpret these results to demonstrate that the HFD mouse model reflects what is seen in humans with obesity and diabetes throughout the COVID-19 pandemic. This comorbid population has significantly more hospitalizations, irrespective of vaccine status. This model also allows us to begin to differentiate the mechanism behind this lack of protection from SARS-CoV-2. The receptor for SARS-CoV-2 is ACE2 and in humans, there is a modest increase in ACE2 levels in some reports suggesting that increased disease could be due to increased entry and thus infection with SARS-CoV-2 (45). In mice, we do not see an increase in ACE2 levels in the lungs in the diabetic and obese model used here. While this could be a reason for increased disease in diabetic humans, we do not believe this is the reason for increased disease that we see in mice. With equivalent neutralizing antibody levels but less clearance in the lungs, we hypothesize that this is due to changes in the quality of the antibodies produced. Potentially, the antigens that the antibodies recognize, or the subtypes of the antibodies are changed in the obese/diabetic mice compared to normal mice. There are reports of altered T-cell activation in humans with obesity in response to COVID-19 vaccination suggesting that T cells may be involved in the altered inflammatory response as well (46). We also observe the strong association of a hyperactive neutrophil response in the lungs. This could either be due to increased neutrophil recruitment or once there, the neutrophils are not responding correctly. We have shown that depleting neutrophils in diabetic/obese mice protect them from lethal disease suggesting neutrophil activity in the lungs is pathologic in this model.
We hypothesize that the dysregulated immune response leads to increased morbidity and mortality as well as skewing the ability of antibodies produced by vaccination to clear viral infection. It is yet to be determined whether it is the metabolic disease associated with obesity that is driving this response or the hyperglycemia of type 2 diabetes; however, alterations to therapeutic efficacy from vaccination to anti-viral drugs can be affected by either type of metabolic change and will be studied further in the future.
MATERIALS AND METHODS
Cells and virus strain
Viruses and cells were processed as described previously (47). Briefly, Vero E6 cells (ATCC# CRL 1586) were cultured in Dulbecco’s modified Eagle medium (DMEM; Quality Biological), supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gibco), 1% (vol/vol) penicillin/streptomycin (Gemini Bio-products), and 1% (vol/vol) L-glutamine (2 mM final concentration, Gibco) (Vero media). Cells were maintained at 37°C and 5% CO2. SARS-CoV-2 (MA10) was provided by Dr. Ralph Baric (UNC). Stocks were prepared by infection of VeroE6 cells for 2 days when cytopathic effect (CPE) was starting to be visible (48). Media were collected and clarified by centrifugation before being aliquoted for storage at −80°C. The stock titer was determined by plaque assay using Vero E6 cells as described previously (49). All work with infectious viruses was performed in a Biosafety Level 3 laboratory and approved by our Institutional Biosafety Committee.
Animal ethics statement
The University of Maryland School of Medicine is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International). All animal procedures were in accordance with the NRC Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and the CDC/NIH Biosafety in Microbiological and Biomedical Laboratories. Mouse studies were approved by The University of Maryland School of Medicine IACUC. Studies were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH publication 8023, Revised 1978). All mouse infections are carried out in an animal BSL-3 facility in accordance with approved practices.
Husbandry of diet-induced obesity mice
Eighteen-week-old male C57BL/6 diet included obesity (DIO) and C57BL/6 control mice were purchased from Jackson Laboratory. After arrival, mice were kept on a diet of normal chow (22% kcal from fat; Teklad, 2019S) or a diet consisting of high-fat chow (60% kcal from fat; Teklad, TD.06414) and maintained on their respective diets throughout the course of the experiments. To evaluate the diabetic status of the mice, their fasting blood glucose and starting weight were evaluated before infection. Animals were fasted with water only for 6 hours prior to glucose testing to obtain fasting blood glucose measurements. Sub-mandibular blood was used to obtain blood glucose measurements on a Contour One blood glucose meter strip (Ascensia, Parsippany, NJ, USA).
Mouse infections
Eighteen-week-old male C57BL/6 diet included obesity (DIO) and C57BL/6 control mice were purchased from Jackson Laboratory. On day 0, mice were anesthetized by intraperitoneal injection of 50 µL of a mix of xylazine (0.38 mg/mouse) and ketamine (1.3 mg/mouse) diluted in phosphate-buffered saline (PBS). While anesthetized, mice were intranasally inoculated with 50 µL sterile PBS, 1 × 103 PFU, or 1 × 105 PFU of SARS-CoV-2 MA10. Mice are monitored daily for weight loss and signs of morbidity. On days 2, 4, and 7, animals were euthanized, and lung tissue was collected for further analysis.
Mouse challenge study
Eighteen-week-old male C57BL/6 diet included obesity (DIO) and C57BL/6 control mice were purchased from Jackson Laboratory and immunized by intramuscular (IM) injection with two doses spaced 14 days apart (days 0 and 14) of 1 µg rS-WU1, with 5 µg saponin-based Matrix-M adjuvant (Novavax, AB, Uppsala, SE) as a prime/boost. A placebo group was injected with a vaccine formulation buffer as a negative control. Serum was collected for analysis on days 0 and 28. Vaccinated and control animals were intranasally challenged with SARS-CoV-2 on study day 32. On days 2 and 4 post-challenge, animals were euthanized, and lung tissue was collected for further analysis.
Histology and immunohistochemistry
Lung sections were fixed in 4% paraformaldehyde in PBS for a minimum of 48 hours, after which they were sent to the Histology Core at the University of Maryland, Baltimore, for paraffin embedding and sectioning. Five-micrometer sections were prepared and used for H&E staining by the Histology Core Services.
For SARS-CoV-2 nucleocapsid immunohistochemistry staining, 5-μm sections were cut from paraffin-embedded blocks and placed onto positively charged slides. The sections were immunostained using the Dako EnVision FLEX + detection system (DAKO, Carpinteria, CA, USA). The Dako PT link was used for deparaffinization and heat-induced epitope retrieval using Dako Target Retrieval Solution, high pH, for 20 min. Endogenous peroxidase activity was blocked with DAKO Peroxidase-Blocking Reagent for 5 min before incubation with rabbit polyclonal SARS nucleocapsid protein antibody (NB100-56576; Novus Biologicals, Centennial, CO, USA), 1:400 for 20 min at room temperature, followed by DAKO anti-rabbit HRP detection reagent for 20 min. Finally, the sections were incubated for 10 min with DAKO diaminobenzene (DAB) before they were counterstained with Dako FLEX hematoxylin, rinsed, and mounted in Cytoseal XYL (Thermo Scientific, Waltham, MA, USA). Sections were imaged at 10× magnification, and figures were put together using Adobe Photoshop and Illustrator software (Adobe, San Jose, CA).
Plaque assay
VeroE6/TMPRSS2 cells were cultured in DMEM (Quality Biological), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Sigma), 1% (vol/vol) penicillin/streptomycin (Gemini Bio-products), and 1% (vol/vol) L-glutamine (2 mM final concentration, Gibco) (Vero Media). Cells were maintained at 37°C (5% CO2). SARS-CoV-2 lung titers were quantified by homogenizing mouse lungs in 1 mL PBS (Quality Biological, Inc.) using 1.0 mm glass beads (Sigma Aldrich) and a Beadruptor (Omni International, Inc.). VeroE6 cells are plated in 12-well plates with 1.5 × 105 cells per well. SARS-CoV-2 virus titer in plaque-forming units was determined by plaque assay. In the plaque assay, 25 µL of the lung homogenate is added to 225 µL of DMEM and diluted 10-fold across a 6-point dilution curve with 200 uL of diluent added to each well. After 1 hour, a 2 mL agar overlay containing DMEM is added to each well. Plates are incubated for 3 days at 37°C (5% CO2) before plaques are counted.
SARS-CoV-2 neutralization titer determination
SARS-CoV-2 neutralizing antibody titers were determined as described previously (50). Briefly, serum samples were heat-inactivated to remove complement and allowed to equilibrate to room temperature before processing. Samples were diluted in duplicate for a 12-point, 1:2 dilution series with an initial dilution of 1:40 dilution (1:80 following virus addition). Dilution plates were then transported into the BSL-3 laboratory, and an equal volume of SARS-CoV-2 inoculum was added to each plate. A non-treated, virus-only control and a mock infection control were included on every plate. The sample/virus mixture was then incubated at 37°C (5.0% CO2) for 1 hour before transferring to 96-well titer plates with confluent VeroE6 cells. Titer plates were incubated at 37°C (5.0% CO2) for 72 hours, followed by CPE determination for each well in the plate. The first sample dilution to show CPE was reported as the minimum sample dilution required to neutralize >99% of the concentration of SARS-CoV-2 tested (neut99).
Gene expression
Mouse lung tissue was homogenized in 1 mL of TRIzol (Ambion) using 1.4 mm glass beads (Omni International, Inc.) and a beadruptor (Omni International, Inc.) RNA was extracted using the DIRECT-zol Mini RNA Extraction Kit (Zymo Research) per the manufacturer’s instructions. RNA was transcribed into cDNA using RT2 First Strand Kit (Qiagen). Gene expression was performed using Real-time PCR for RT2 Profile PCR Array Mouse Cytokine and Chemokines using RT2 SYBR Green Mastermixes (Qiagen). Using the RT2 qPCR Array Data Analysis spreadsheet, gene expression was calculated using the ΔΔCt method with housekeeping genes as the control, and fold change was calculated relative to control mice-infected lungs. Fold regulation, which represents fold change in a biologically meaningful way, was calculated using fold change. A fold-change value greater than one indicates an upregulation, and the fold regulation is equal to the fold change. A fold-change value less than one indicates a down-regulation, and the fold regulation is the negative inverse of the fold change.
Cytokine and chemokine quantification
For the quantification of secreted cytokine and chemokines induced by SARS-CoV-2 infection, mice lung tissue was homogenized in 1 mL of PBS (Quality Biologicals, Inc.) using 1.4 mm glass beads (Omni International, Inc.) and a beadruptor (Omni International, Inc.). Bio-Plex Pro Mouse Chemokine Panel 31-plex (BIO-RAD) was used to quantify cytokines and chemokines in lung homogenate according to the manufacturer’s protocol with the addition of a fixation step after the final wash. Samples underwent a 24-hour fixation (4% formaldehyde, Sigma-Aldrich) at 4°C. After fixation, the plate was washed three times in wash buffer, microparticles were resuspended in 100 µL wash buffer, and analyzed on a Luminex MagPix and xPONENT Software version 4.3. Based on the standard curve, concentrations (pg/mL) of each analyte were calculated for all samples.
Flow cytometry
Single cells were isolated from the lungs using the Lung Dissociation Kit, mouse and gentleMACS Dissociator (Miltenyi Biotec). Lungs were placed in C tubes (Miltenyi Biotec) containing 2.2 mL Buffer S, 15 µL of Enzyme A, and 100 µL of Enzyme D. Lungs were run on m_lung_01 and allowed to digest for 30 min at 37°C followed by running m_lung_02. Cell suspensions were filtered on a 70 µm filter (BD), and cells were pelleted by centrifugation. Red blood cells were lysed in ammonium-chloride-potassium lysis buffer (Quality Biological, Inc.) and subsequently washed with PBS containing 3% FBS. Approximately, 1 × 106 cells were plated and washed twice with PBS containing. Cells were stained for viability using the Live/Dead Fixable NIR Dead Cell Stain Kit (Molecular Probes). Cells were washed with PBS containing 3% FBS. Cells were stained with antibody cocktails made in BD Horizon Brilliant Stain Buffer (BD). The antibodies used were as follows: CD45 Alexa Fluor 700 (BioLegend, clone 30-F11), CD11b Brilliant Violet 650 (BioLegend, clone M1/70), Ly6G Brilliant Violet 785 (BioLegend, clone 1A8), Ly6C Brilliant Violet 510 (BioLegend, clone HK1.4), CD3 BUV 563 (BD, clone 145-2C11), CD4 FITC (BioLegend, clone GK1.5), CD8a APC (BioLegend clone 53-6.7), CD19 PE (BioLegend clone 6D5), and NK1.1 Pacific Blue (BioLegend, clone PK136), CD65 Brilliant Violet 605 (BioLegend, clone X54-5/7.1), CD103 BUV 661 (BD, clone 2e7), Siglec-H BUV 563 (BD, clone 440 c), and Siglec-F Brilliant Violet 711 (BD, clone E50-2440). Stained cells were washed twice and fixed for 1 hour in FluoroFix (BioLegend). For intracellular cytokine staining, lung leukocytes 2 × 106 cells/well from vaccinated and sham mice were cultured at 37°C for 5 hours in the presence of SARS-CoV-2 spike peptide pool (1 µg/mL) kindly provided by Alessandro Sette LA Jolla Institute, Brefeldin A and Monensin (BioLegend). The peptide pool consisted of overlapping 15-mers by 10 spanning the entire protein. The treated cells were incubated with LIVE/DEAD Fixable NIR Dead Cell stain (Invitrogen) for 20 min at room temperature. Cells were then labeled for cell surface markers at 4°C for 15 min in the dark followed by fixation and permeabilization for 20 min at 4°C (BD CytoFix/CytoPerm) then labeled with intracellular antibody cocktail. The antibodies used were as follows: IFN-γ BV421 (BioLegend, clone: XMG1.2), TNF-α BV650 (BioLegend, clone: MP6-XT22), IL-4 BV605 (BioLegend, clone: 11B11), and IL-17a BV711 (BioLegend, clone: TC11-18H10.1). Fixed cells were washed once in PBS containing 3% FBS and resuspended in PBS containing 3% FBS. Samples were acquired (approximately 50,000 events) using the Aurora-UV spectral flow cytometer (Cytek), and data were analyzed using FCS Express analysis software (DeNova Software, Pasadena, CA, USA).
Neutrophil depletion
Eighteen-week-old male C57BL/6 diet included obesity (DIO) and C57BL/6 control mice were purchased from Jackson Laboratory and neutrophils were depleted with 500 µg of anti-Ly6G (clone 1A8) or isotype antibody (IgG2a isotype) (Bio-X-Cell, Lebanon, NH), via i.p. injection 1 day prior to infection with SARS-CoV-2 and every 2 days afterward. Mice were infected with either 50 µL sterile PBS or 1 × 105 PFU of SARS-CoV-2 MA10. Mice are monitored daily for weight loss and signs of morbidity. On day 7, animals were euthanized, and lung tissue was collected for further analysis.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 9.2 software (GraphPad Software, San Diego, CA, USA). Data were analyzed using unpaired t-test, Log-rank (Mantel-Cox) test, one-way or two-way ANOVA followed by Tukey, Sidak, or Dunnett multiple comparison post hoc test as indicated. All statistical analyses were two-sided and a P < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This work is partially funded by NIH R01 AI148166, NIH HHSN272201400007C, NIH HHSN272201400008C/ 0258-0689-4609 to M.B.F. RJ is funded by T32AI007524. N.P. and G.S are employees of and funded by Novavax.
Contributor Information
Matthew Frieman, Email: mfrieman@som.umaryland.edu.
Stacey Schultz-Cherry, St. Jude Children's Research Hospital, Memphis, Tennessee, USA .
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.01336-23.
FACS cell number data.
FACS cell number data.
Legends for Figures S1 and S2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. CDC . n.d. National diabetes statistics report. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. 2014. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105:141–150. doi: 10.1016/j.diabres.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 3. Ritchie SA, Connell JMC. 2007. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis 17:319–326. doi: 10.1016/j.numecd.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM. 2004. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600. doi: 10.1210/jc.2004-0372 [DOI] [PubMed] [Google Scholar]
- 5. Kompaniyets L, Goodman AB, Belay B, Freedman DS, Sucosky MS, Lange SJ, Gundlapalli AV, Boehmer TK, Blanck HM. 2021. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death - United States, March-December 2020. MMWR Morb Mortal Wkly Rep 70:355–361. doi: 10.15585/mmwr.mm7010e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. 2020. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 8:782–792. doi: 10.1016/S2213-8587(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rawshani A, Kjölhede EA, Rawshani A, Sattar N, Eeg-Olofsson K, Adiels M, Ludvigsson J, Lindh M, Gisslén M, Hagberg E, Lappas G, Eliasson B, Rosengren A. 2021. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg Health Eur 4:100105. doi: 10.1016/j.lanepe.2021.100105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozonoff A, Schaenman J, Jayavelu ND, Milliren CE, Calfee CS, Cairns CB, Kraft M, Baden LR, Shaw AC, Krammer F, et al. 2022. Phenotypes of disease severity in a cohort of hospitalized COVID-19 patients: results from the IMPACC study. EBioMedicine 83:104208. doi: 10.1016/j.ebiom.2022.104208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heubner L, Petrick PL, Güldner A, Bartels L, Ragaller M, Mirus M, Rand A, Tiebel O, Beyer-Westendorf J, Rößler M, Schmitt J, Koch T, Spieth PM. 2022. Extreme obesity is a strong predictor for in-hospital mortality and the prevalence of long-COVID in severe COVID-19 patients with acute respiratory distress syndrome. Sci Rep 12:18418. doi: 10.1038/s41598-022-22107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosero PA, Realpe JS, Farinango CD, Restrepo DS, Salazar-Cabrera R, Lopez DM. 2022. Risk factors for COVID-19: a systematic mapping study. Stud Health Technol Inform 299:63–74. doi: 10.3233/SHTI220964 [DOI] [PubMed] [Google Scholar]
- 11. Badawi A, Ryoo SG. 2016. Prevalence of diabetes in the 2009 influenza A (H1N1) and the Middle East respiratory syndrome Coronavirus: a systematic review and meta-analysis. J Public Health Res 5:733. doi: 10.4081/jphr.2016.733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rathnasinghe R, Jangra S, Cupic A, Martínez-Romero C, Mulder LCF, Kehrer T, Yildiz S, Choi A, Mena I, De Vrieze J, Aslam S, Stadlbauer D, Meekins DA, McDowell CD, Balaraman V, Richt JA, De Geest BG, Miorin L, Krammer F, Simon V, García-Sastre A, Schotsaert M. 2021. The N501Y mutation in SARS-CoV-2 spike leads to morbidity in obese and aged mice and is neutralized by convalescent and post-vaccination human sera. medRxiv:2021.01.19.21249592. doi: 10.1101/2021.01.19.21249592 [DOI] [Google Scholar]
- 13. Johnson R, Ardunay J, Hammond H, Logue J, Jackson L, Baracco L, McGrath M, Dillen C, Patel N, Smith G, Frieman M. 2023. Diet induced obesity and diabetes enhance mortality and reduces vaccine efficacy for SARS-CoV-2. bioRxiv:2022.10.15.512291. doi: 10.1101/2022.10.15.512291 [DOI] [PMC free article] [PubMed]
- 14. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ, Zumla AI, Memish ZA. 2013. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome Coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 13:752–761. doi: 10.1016/S1473-3099(13)70204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cha R-H, Joh J-S, Jeong I, Lee JY, Shin H-S, Kim G, Kim Y, Critical Care Team of National Medical Center . 2015. Renal complications and their prognosis in Korean patients with Middle East respiratory syndrome-Coronavirus from the central MERS-CoV designated hospital. J Korean Med Sci 30:1807–1814. doi: 10.3346/jkms.2015.30.12.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi WS, Kang C-I, Kim Y, Choi J-P, Joh JS, Shin H-S, Kim G, Peck KR, Chung DR, Kim HO, et al. 2016. Clinical presentation and outcomes of Middle East respiratory syndrome in the Republic of Korea. Infect Chemother 48:118–126. doi: 10.3947/ic.2016.48.2.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Y-D, Ding M, Dong X, Zhang J-J, Kursat Azkur A, Azkur D, Gan H, Sun Y-L, Fu W, Li W, Liang H-L, Cao Y-Y, Yan Q, Cao C, Gao H-Y, Brüggen M-C, van de Veen W, Sokolowska M, Akdis M, Akdis CA. 2021. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy 76:428–455. doi: 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 18. Kulcsar KA, Coleman CM, Beck SE, Frieman MB. 2019. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight 4:20. doi: 10.1172/jci.insight.131774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Y-M, Hsu C-Y, Lai C-C, Yen M-F, Wikramaratna PS, Chen H-H, Wang T-H. 2017. Impact of comorbidity on fatality rate of patients with Middle East respiratory syndrome. Sci Rep 7:11307. doi: 10.1038/s41598-017-10402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rydell-Törmänen K, Johnson JR. 2019. The applicability of mouse models to the study of human disease. Methods Mol Biol 1940:3–22. doi: 10.1007/978-1-4939-9086-3_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alanazi KH, Abedi GR, Midgley CM, Alkhamis A, Alsaqer T, Almoaddi A, Algwizani A, Ghazal SS, Assiri AM, Jokhdar H, Gerber SI, Alabdely H, Watson JT. 2020. Diabetes mellitus, hypertension, and death among 32 patients with MERS-CoV infection, Saudi Arabia. Emerg Infect Dis 26:166–168. doi: 10.3201/eid2601.190952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee FY, Li Y, Yang EK, Yang SQ, Lin HZ, Trush MA, Dannenberg AJ, Diehl AM. 1999. Phenotypic abnormalities in macrophages from leptin-deficient, obese mice. Am J Physiol 276:C386–94. doi: 10.1152/ajpcell.1999.276.2.C386 [DOI] [PubMed] [Google Scholar]
- 23. Manicone AM, Gong K, Johnston LK, Giannandrea M. 2016. Diet-induced obesity alters myeloid cell populations in naïve and injured lung. Respir Res 17:24. doi: 10.1186/s12931-016-0341-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Brien KB, Vogel P, Duan S, Govorkova EA, Webby RJ, McCullers JA, Schultz-Cherry S. 2012. Impaired wound healing predisposes obese mice to severe influenza virus infection. J Infect Dis 205:252–261. doi: 10.1093/infdis/jir729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milner JJ, Sheridan PA, Karlsson EA, Schultz-Cherry S, Shi Q, Beck MA. 2013. Diet-induced obese mice exhibit altered heterologous immunity during a secondary 2009 pandemic H1N1 infection. J Immunol 191:2474–2485. doi: 10.4049/jimmunol.1202429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bandaru P, Rajkumar H, Nappanveettil G. 2011. Altered or impaired immune response to hepatitis B vaccine in WNIN/GR-Ob Rat: an obese rat model with impaired glucose tolerance. ISRN Endocrinol 2011:980105. doi: 10.5402/2011/980105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guebre-Xabier M, Patel N, Tian J-H, Zhou B, Maciejewski S, Lam K, Portnoff AD, Massare MJ, Frieman MB, Piedra PA, Ellingsworth L, Glenn G, Smith G. 2020. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine 38:7892–7896. doi: 10.1016/j.vaccine.2020.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian J-H, Patel N, Haupt R, Zhou H, Weston S, Hammond H, Logue J, Portnoff AD, Norton J, Guebre-Xabier M, et al. 2021. SARS-Cov-2 spike glycoprotein vaccine candidate NVX-CoV2373 Immunogenicity in baboons and protection in mice. Nat Commun 12:372. doi: 10.1038/s41467-020-20653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leist SR, Dinnon KH 3rd, Schäfer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, Gully KL, Scobey T, Brown AJ, Sheahan TP, Moorman NJ, Boucher RC, Gralinski LE, Montgomery SA, Baric RS. 2020. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183:1070–1085. doi: 10.1016/j.cell.2020.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paich HA, Sheridan PA, Handy J, Karlsson EA, Schultz-Cherry S, Hudgens MG, Noah TL, Weir SS, Beck MA. 2013. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity (Silver Spring) 21:2377–2386. doi: 10.1002/oby.20383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim Y-H, Kim J-K, Kim D-J, Nam J-H, Shim S-M, Choi Y-K, Lee C-H, Poo H. 2012. Diet-induced obesity dramatically reduces the efficacy of a 2009 pandemic H1N1 vaccine in a mouse model. J Infect Dis 205:244–251. doi: 10.1093/infdis/jir731 [DOI] [PubMed] [Google Scholar]
- 32. Costanzo AE, Taylor KR, Dutt S, Han PP, Fujioka K, Jameson JM. 2015. Obesity impairs gammadelta T cell homeostasis and antiviral function in humans. PLoS One 10:e0120918. doi: 10.1371/journal.pone.0120918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fisher-Hoch SP, Mathews CE, McCormick JB. 2013. Obesity, diabetes and pneumonia: the menacing interface of non-communicable and infectious diseases. Trop Med Int Health 18:1510–1519. doi: 10.1111/tmi.12206 [DOI] [PubMed] [Google Scholar]
- 34. Hagau N, Slavcovici A, Gonganau DN, Oltean S, Dirzu DS, Brezoszki ES, Maxim M, Ciuce C, Mlesnite M, Gavrus RL, Laslo C, Hagau R, Petrescu M, Studnicska DM. 2010. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care 14:R203. doi: 10.1186/cc9324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Painter SD, Ovsyannikova IG, Poland GA. 2015. The weight of obesity on the human immune response to vaccination. Vaccine 33:4422–4429. doi: 10.1016/j.vaccine.2015.06.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tagliabue C, Principi N, Giavoli C, Esposito S. 2016. Obesity: impact of infections and response to vaccines. Eur J Clin Microbiol Infect Dis 35:325–331. doi: 10.1007/s10096-015-2558-8 [DOI] [PubMed] [Google Scholar]
- 37. Caimi B, Franzetti M, Velleca R, Lai A, Gatti A, Rossi PL, D’Orso M, Pregliasco F, Balotta C, Calicchio G. 2022. Sero-survey on long-term care facility residents reveals increased risk of sub-optimal antibody response to BNT162b2: implications for breakthrough prevention. BMC Geriatr 22:191. doi: 10.1186/s12877-022-02884-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soetedjo NNM, Iryaningrum MR, Lawrensia S, Permana H. 2022. Antibody response following SARS-CoV-2 vaccination among patients with type 2 diabetes mellitus: a systematic review. Diabetes Metab Syndr 16:102406. doi: 10.1016/j.dsx.2022.102406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, Morgan C, Kerschmann R, Beyer P, Dittrich M, Block GA, Chertow GM, Parsonnet J. 2022. SARS-CoV-2 vaccine antibody response and breakthrough infection in patients receiving dialysis. Ann Intern Med 175:371–378. doi: 10.7326/M21-4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitsunaga T, Ohtaki Y, Seki Y, Yoshioka M, Mori H, Suzuka M, Mashiko S, Takeda S, Mashiko K. 2021. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: a prospective study in Japan. PeerJ 9:e12316. doi: 10.7717/peerj.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Almond MH, Edwards MR, Barclay WS, Johnston SL. 2013. Obesity and susceptibility to severe outcomes following respiratory viral infection. Thorax 68:684–686. doi: 10.1136/thoraxjnl-2012-203009 [DOI] [PubMed] [Google Scholar]
- 42. Honce R, Schultz-Cherry S. 2019. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol 10:1071. doi: 10.3389/fimmu.2019.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stefan N. 2023. SARS-CoV-2 fires up inflammation in adipose tissue. Nat Rev Endocrinol 19:8–9. doi: 10.1038/s41574-022-00778-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El-Sayed Moustafa JS, Jackson AU, Brotman SM, Guan L, Villicaña S, Roberts AL, Zito A, Bonnycastle L, Erdos MR, Narisu N, et al. 2022. ACE2 expression in adipose tissue is associated with cardio-metabolic risk factors and cell type composition-implications for COVID-19. Int J Obes (Lond) 46:1478–1486. doi: 10.1038/s41366-022-01136-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wijnant SRA, Jacobs M, Van Eeckhoutte HP, Lapauw B, Joos GF, Bracke KR, Brusselle GG. 2020. Expression of ACE2, the SARS-CoV-2 receptor, in lung tissue of patients with type 2 diabetes. Diabetes 69:2691–2699. doi: 10.2337/db20-0669 [DOI] [PubMed] [Google Scholar]
- 46. Kelly NEW, De Barra C, Shaamile F, Holland A, Shaw L, Mallon PWG, O’Connell J, Hogan AE, O’Shea D. 2023. Antigen specific T cells in people with obesity at five months following ChAdOx1 COVID-19 vaccination. Int J Obes (Lond) 47:83–86. doi: 10.1038/s41366-022-01235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weston S, Coleman CM, Haupt R, Logue J, Matthews K, Li Y, Reyes HM, Weiss SR, Frieman MB. 2020. Broad anti-Coronavirus activity of food and drug administration-approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo J Virol 94:21. doi: 10.1128/JVI.01218-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, Nagata N, Sekizuka T, Katoh H, Kato F, Sakata M, Tahara M, Kutsuna S, Ohmagari N, Kuroda M, Suzuki T, Kageyama T, Takeda M. 2020. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A 117:7001–7003. doi: 10.1073/pnas.2002589117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coleman CM, Frieman MB. 2015. Growth and quantification of MERS-CoV infection. Curr Protoc Microbiol 37:15E. doi: 10.1002/9780471729259.mc15e02s37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. 2020. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 383:2320–2332. doi: 10.1056/NEJMoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FACS cell number data.
FACS cell number data.
Legends for Figures S1 and S2.