Abstract
Background
The Belgian Precision initiative aims to maximize the implementation of tumor-agnostic next-generation sequencing in patients with advanced cancer and enhance access to molecularly guided treatment options. Academic tumor-agnostic basket phase II studies are part of this initiative. The current investigator-driven trial aimed to investigate the efficacy of olaparib in advanced cancers with a (likely) pathogenic mutation (germline or somatic) in a gene that plays a role in homologous recombination (HR).
Patients and methods
This open-label, multi-cohort, phase II study examines the efficacy of olaparib in patients with an HR gene mutation in their tumor and disease progression on standard of care. Patients with a somatic or germline mutation in the same gene define a cohort. For each cohort, a Simon minimax two-stage design was used. If a response was observed in the first 13 patients, 14 additional patients were included. Here, we report the results on four completed cohorts: patients with a BRCA1, BRCA2, CHEK2 or ATM mutation.
Results
The overall objective response rate across different tumor types was 11% in the BRCA1-mutated (n = 27) and 21% in the BRCA2-mutated (n = 27) cohorts. Partial responses were seen in pancreatic cancer, gallbladder cancer, endocrine carcinoma of the pancreas and parathyroid cancer. One patient with a BRCA2 germline-mutated colon cancer has an ongoing complete response with 19+ months on treatment. Median progression-free survival in responding patients was 14+ months (5-34+ months). The clinical benefit rate was 63% in the BRCA1-mutated and 46% in the BRCA2-mutated cohorts. No clinical activity was observed in the ATM (n = 13) and CHEK2 (n = 14) cohorts.
Conclusion
Olaparib showed efficacy in different cancer types harboring somatic or germline mutations in the BRCA1/2 genes but not in ATM and CHEK2. Patients with any cancer type harboring BRCA1/2 mutations should have access to olaparib.
Key words: agnostic NGS, olaparib, colorectal cancer, parathyroid cancer, biliary tract cancer, BRCA1, BRCA2, CHEK2, ATM
Highlights
-
•
Advanced cancer patients harboring somatic or germline BRCA1/2 mutations respond to treatment with olaparib.
-
•
Response to olaparib in the presence of a BRCA1/2 mutation is seen in any cancer type.
-
•
Olaparib does not have clinically meaningful activity in cancers with an ATM or CHEK2 mutation.
Introduction
The mutational landscape of cancer is rapidly evolving, and next-generation sequencing (NGS) of solid tumors in various projects increasingly reveals mutations in cancer genes. It is currently estimated that >50% of cancers could harbor actionable mutations.1 The Belgian Society of Medical Oncology (BSMO), in collaboration with the different university oncological centers, major regional hospitals and other stakeholders, has launched a national Precision initiative to, firstly, promote the implementation of NGS in Belgian cancer patients and, secondly, establish a fluent path for patients’ access to drugs that match the genotype of their cancer.2 A part of the initiative was to organize tumor-agnostic basket phase II studies in specific cancer gene mutations. One of these studies recruited patients with a homologous recombination (HR) deficiency (HRD) gene mutation for treatment with olaparib. Completed cohorts of patients with a BRCA1/2, ATM or CHEK2 mutation are reported here.
During each cell division, the DNA replication machinery has a risk of error in response to which cells have developed complex systems to detect and repair these errors. When these mechanisms fail, the DNA damage response is disrupted, allowing damaged cells to survive and in some cases progress to uncontrolled cell proliferation. Dysregulation of DNA damage repair (DDR) also causes genomic instability, a hallmark of cancer.3,4 On the contrary, failure of DDR is a weakness that can be targeted therapeutically, as in the case of the poly (ADP-ribose) polymerase inhibitor (PARPi), olaparib.
Olaparib was the first PARPi to be discovered and subsequently developed. The SOLO-1 trial,5 recently updated with overall survival results, brought on the first registered indication for olaparib in ovarian cancer patients who harbor a BRCA1/2 mutation in their tumor. Later, it was also approved for the treatment of advanced and early breast cancer,6,7 advanced prostate cancer8 and pancreatic cancer9 in the presence of a BRCA1 or BRCA2 mutation. The Food and Drug Administration approval for olaparib with regard to prostate cancer also includes additional homologous recombination repair (HRR) genes.
PARPi responses have also been documented in cancers where no BRCA1/2 mutations were found, indicating molecular features shared with BRCA-mutant tumors. Other HRDs likely cause such sensitivities.10,11 When inactivated by mutation, genes that cause HRD include ATM, CHEK1, CHEK2, NBN, BRIP1, MRE11A, RAD50, RAD51B, RAD51C, RAD51D, RAD54L, PALB2, BARD1, FAM175A, CDK12, FANCL, TP53 (only germline) and PPP2R2A. This list is not limitative as other genes can, directly or indirectly, cause HRD. HR gene mutations can be found in all cancer types.12
Patients and methods
Study design and patient population
The cohorts reported here are part of an academic multicenter basket phase II study designed to investigate olaparib’s efficacy in any type of HR-deficient cancer. The study was approved by the ethical committee (EC) of every participating site (EC number 2018/442). Before entering the study, patients signed an informed consent form. Recruitment started on 1 February 2019 and ended on 1 February 2023.
The BSMO organized the study that recruited patients from Belgian oncological centers. The participating trial sites, 12 in total, were all university and large regional hospitals with clinical trial experience (see Appendix 1 for the list of the sites and their primary investigator).
Eligible patients were initially identified as a proband or a relative in families where a germline mutation was found in the context of diagnostic or predictive genetic testing or when a mutation was detected on historical tumor sequencing. That pathway led initially to a slow accrual. The accrual was drastically accelerated after the Geneo study was initiated. The Geneo study (NCT04641676)2 was a prospective tumor-agnostic effort of comprehensive NGS in patients with advanced cancers. In the study report of every patient, it was mentioned if a mutation was present that could be targeted with olaparib. Patients with a possible germline mutation on somatic NGS were referred for germline genetic testing. All mutations were monoallelic and only one class 4-5 variant in one of the HRR genes was present in every patient. Patients with a germline mutation carry this mutation in every tissue, the tumor cells included. A somatic mutation describes an alteration that is only present at the cellular level in somatic tissue occurring after fertilization.
Advanced cancer patients (stage IV, metastatic disease) with a germline mutation or a somatic tumor mutation (class 1, benign and class 2, possibly benign variants were excluded) in the list of the HR genes mentioned previously were eligible for the study. There were no restrictions regarding the number of lines of treatment for study participation. Patients with ovarian cancer harboring an HRD gene mutation and breast cancer patients who harbor a BRCA1 or BRCA2 mutation were excluded because they had approved or clinical trial access to olaparib.
Study treatment and assessment
All patients with an HRD mutation were treated with olaparib in an open-label study. Study medication was available as a film-coated tablet containing olaparib 150 mg or 100 mg. Every patient started with a dose of 300 mg b.i.d. continually. Two dose reductions were allowed for toxicity reasons, a first reduction to 250 mg twice daily and a second to 200 mg twice daily.
During the study, visits were carried out after 2 and 4 weeks, then every month and at the end of treatment. A computed tomography scan to evaluate response based on RECIST 1.1 was scheduled every 2 months and every 3 months after 1 year of treatment.
Statistical analysis
A cohort was defined by patients who harbor a somatic or germline mutation in the same gene. For each cohort, a Simon minimax two-stage design was used. In the first stage, 13 patients were accrued in each cohort. Only if a response was observed, 14 additional patients were accrued to a total of 27 in the second stage. The null hypothesis that the true response rate is 5% was tested against a one-sided alternative that the true response is 20%. The null hypothesis is rejected if three or more responses are observed in 27 patients. This design yields a type I error rate of 5% and a power of 80% when the true response rate is 20%. Further investigation is warranted if four or more responses are observed among the 27 subjects.
In many rare gene cohorts, only signals of activity might be detected. These data will be reported later as case histories or small case series with descriptive statistics.
Results
A data lock was carried out on 28 October 2022. The median follow-up at that time was 24.5 months. An analysis has been executed for the completed cohorts: ATM, BRCA1, BRCA2 and CHEK2.
Safety
Concerning safety, all patients included at the time of the data lock, 148 in total, were taken into account, not just those in the four complete cohorts. All safety observations were as known in the safety profile of olaparib, and the most prevalent are included in Table 1. Ninety-three (62%) of the patients experienced side-effects. In general, side-effects were mild (grades 1 and 2). Only 12 patients (8.11%) experienced a grade 3 event, and 1 (1.69%) experienced a grade 4 event. A notable high-grade event was an allergic reaction. About 30 min after taking the first dose of olaparib, the patient developed extreme facial flushing, shortness of breath and diffuse pruritus. Acute symptoms normalized after administering levocetirizine 5 mg and methylprednisolone 40 mg; cutaneous symptoms resolved after 24 h. The drug was permanently discontinued, although desensitization has been reported.13 The most common side-effects observed were nausea (40.68%), fatigue (33.89%), anorexia (25.41%), anemia (23.27%), vomiting (13.55%) and thrombocytopenia (11.68%). Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102041, displays the adverse events.
Table 1.
Clinical characteristics of patients in the BRCA1 cohort
| Patient number | Sex | Race | Germline mutation | Mutation | Organ or system of origin | Histology |
|---|---|---|---|---|---|---|
| 106 | M | C | Yes | NM_007249.3 (BRCA1): c.2197_2201del | Pancreas | Adenocarcinoma |
| 107 | M | C | Yes | NM_007294.3 (BRCA1): c.5309G>T | Pancreas | Adenocarcinoma |
| 110 | F | C | No | BRCA1 inversion exon 3 (Foundation One) | Gallbladder | Adenocarcinoma |
| 113 | M | C | No | NM_007294.3 (BRCA1): c.4484+1G>A | Skin | Squamous carcinoma |
| 117 | F | C | No | NM_007294.3 (BRCA1): c.1326T>A | Uterus | Adenocarcinoma |
| 216 | M | C | No | NM_007294.3 (BRCA1): c.5278-1G>A | Bladder | Adenocarcinoma |
| 225 | F | C | No | NM_007294.3 (BRCA1): c.2507_2508del | Pancreas | Adenocarcinoma |
| 304 | M | C | Yes | NM_007294.3 (BRCA1): c.694G>A | Pancreas | Adenocarcinoma |
| 311 | M | C | No | NM_007294.3 (BRCA1): c.181T>G | Skin | Carcinoma |
| 315 | F | C | No | NM_007294.3 (BRCA1): c.1314_1315delGGinsAA | Pancreas | Adenocarcinoma |
| 316 | M | C | Yes | NM_007294.3 (BRCA1): c.3841C>T | Colon | Adenocarcinoma |
| 317 | F | C | Yes | NM_007294.3 (BRCA1): c.3756_3759del | Rectum | Adenocarcinoma |
| 318 | M | C | No | NM 007294.3 (BRCA1): c.4099G>T | Biliary tract | Adenocarcinoma |
| 404 | M | C | No | NM_007294.3 (BRCA1): c.5266dup | Gallbladder | Adenocarcinoma |
| 512 | M | C | No | NM_007294.3 (BRCA1): c.68_69de1AG | Stomach | Adenocarcinoma |
| 513 | M | C | No | NM_007294.3 (BRCA1): c.3931_3934delAACA | Bladder | Carcinoma |
| 608 | M | C | No | NM_007294.3 (BRCA1): c.2722G>T | Liver | Carcinoma |
| 704 | M | C | No | NM_007294.3 (BRCA1): c.212+1G>T | Gallbladder | Adenocarcinoma |
| 706 | M | C | Yes | NM_007294.3 (BRCA1): c.1016delA | Pancreas | Adenocarcinoma |
| 711 | F | A | Yes | NM_007294.3 (BRCA1): c.981_982del | Pancreas | Adenocarcinoma |
| 714 | M | C | No | NM_007294.3 (BRCA1): c.2359dup | Gallbladder | Adenocarcinoma |
| 717 | M | C | No | NM_007294.3 (BRCA1): c.116G>A | Pancreas | Adenocarcinoma |
| 726 | F | C | Yes | NM_007294.3 (BRCA1): c.116G>A | Pancreas | Adenocarcinoma |
| 731 | M | C | Yes | NM_007294.3 (BRCA1): c.4987-1G>T | Gallbladder | Adenocarcinoma |
| 734 | M | C | No | NM_007294.3 (BRCA1): c.4987-1G>T | Unknown primary | Neuroendocrine carcinoma |
| 742 | M | C | yes | NM_00724.3 (BRCA1): c.2359dup | Gallbladder | Adenocarcinoma |
| 1107 | M | C | Yes | NM_007294.3 (BRCA1): c.1390del | Soft tissue | Sarcoma |
A, African; C, Caucasian; M, male; F, female.
Efficacy
ATM cohort
Germline and somatic pathogenic and likely pathogenic mutations were found in various cancer types (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102041).
One partial response (PR) with a short duration was seen in a patient with squamous cell carcinoma of the esophagus (patient 720). The mutation in ATM is a frameshift mutation (p.Ser571Glufs∗11) that has never been reported. This 49-year-old patient was highly pretreated.
In all other patients, immediate progressive disease was noted on the first evaluation after 2 months of treatment, illustrating the generally late-stage and aggressive nature of the disease in the patients included.
The response to treatment with olaparib in the ATM cohort is shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.102041.
BRCA1 cohort
Germline and somatic pathogenic and likely pathogenic mutations were found in various cancer types (Table 1).
The response to therapy with olaparib in the BRCA1 cohort is illustrated in Figure 1.
Figure 1.
Swimmer plot shows the response to treatment with olaparib in the BRCA1 cohort.x-axis: time in months, y-axis: patient identification number. The events in time are displayed on the bar with each patient: complete response (filled black square), partial response (filled black triangle), stable disease (filled black circle) and progressive disease (empty circle). If an arrow is shown at the end of a bar, the response is still ongoing at the time of analysis.
This cohort went to the second stage as a response was observed in the first stage. In our study no difference was seen in the response between germline and somatic mutation carriers. Three patients showed a PR. Two were diagnosed with pancreatic cancer (315, somatic protein-truncating mutation14 and 706, protein-truncating germline mutation14), and one patient had gallbladder cancer (704, somatic frameshift mutation). The pancreatic cancer patients will not be discussed in detail because there are more extensive studies available in this population9. The patient with gallbladder cancer was highly pretreated when he entered the study. He had been through four lines of chemotherapy: cisplatin/gemcitabine, intraperitoneal cisplatin, FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) and FOLFOX. Physical examination was normal, Eastern Cooperative Oncology Group (ECOG) 1 and the only abnormal value in the blood draw was a hemoglobin level of 10 g/dl. His medical history showed arterial hypertension, hypercholesterolemia and a coronary bypass. At the first evaluation at 2 months of treatment with olaparib, he showed a PR, and at the time of data lock, 34 months after the start of the study, the response was still ongoing. A first dose reduction was carried out after 2 weeks of treatment due to a grade 2 thrombocytopenia, and a second dose reduction after 10 months due to a grade 3 neutropenia.
Fourteen patients had stable disease (SD). The overall response rate (ORR) was 11%, and the clinical benefit rate (CBR) was 63%.
In summary, the BRCA1 cohort includes 9 patients with pancreatic cancer and 18 patients with other tumor types. As far as the size of the cohorts allows, the CBR appears to be similar between the two groups: 55.5% (2 PR and 3 SD) for the pancreatic cancer patients and 66.6% (1 PR and 11 SD) for the other cancer patients. The partial remission was in a patient with gallbladder cancer, out of six patients with this cancer included in the study.
BRCA2 cohort
Germline and somatic pathogenic and likely pathogenic mutations were found in various cancer types (Table 2).
Table 2.
Clinical characteristics of patients in the BRCA2 cohort
| Patient number | Sex | Race | Germline mutation | Mutation | Organ or system of origin | Histology |
|---|---|---|---|---|---|---|
| 104 | M | C | Yes | Deletion exon 19 (Foundation One) | Gallbladder | Adenocarcinoma |
| 111 | F | C | Yes | NM_000059.4 (BRCA2): c.4935del | Colon | Adenocarcinoma |
| 112 | M | C | No | NM_000059.3 (BRCA2): c.3751dup | Parathyroid | Carcinoma |
| 118 | M | C | No | NM_000059.4 (BRCA2): c.8243G>A | Gallbladder | Adenocarcinoma |
| 122 | M | C | Yes | NM_000059.4 (BRCA2): c.8414_8416delinsC | Thyroid | Carcinoma |
| 201 | M | C | Yes | NM_000059.3 (BRCA2): c.4936_4939del | Bladder | Adenocarcinoma |
| 202 | M | C | Yes | NM_000059.3 (BRCA2): c.516+1G>A | Colon | Adenocarcinoma |
| 205 | F | C | Yes | NM_000059.3 (BRCA2): c.6503-6504delTT | Cervical | Squamous carcinoma |
| 302 | M | C | Yes | NM_000059.3 (BRCA2): c.4472_4475del | Pancreas | Adenocarcinoma |
| 401 | F | C | No | NM_000059.3 (BRCA2): c.9709delA | Genito-uretral | Adenocarcinoma |
| 402 | F | C | No | NM_000059.3 (BRCA2): c.2175dupA | Pancreas | Adenocarcinoma |
| 403 | M | C | No | NM_000059.3 (BRCA2): c.2588delIA | Lung | Adenocarcinoma |
| 511 | F | A | No | Rearrangement exon 10 (Foundation One) | Paraganglion | Paraganglioma |
| 601 | F | C | Yes | NM_000059.3 (BRCA2): c.516+1G>A | Pancreas | Adenocarcinoma |
| 602 | M | C | Yes | NM_000059.3 (BRCA2): c.4602delT | Pancreas | Adenocarcinoma |
| 702 | M | C | Yes | NM_000059.3 (BRCA2): c.5936del | Pancreas | Adenocarcinoma |
| 705 | M | C | Yes | NM_000059.3 (BRCA2): c.5682C>G | Pancreas | Adenocarcinoma |
| 708 | M | C | Yes | NM_000059.3 (BRCA2): c.5213_5216del | Pancreas | Adenocarcinoma |
| 713 | M | C | No | NM_000059.3 (BRCA2): 6503-6504delTT | Pancreas | Neuroendocrine carcinoma |
| 716 | M | C | No | NM_000059.3 (BRCA2): c.6600_6601del | Pancreas | Adenocarcinoma |
| 724 | M | C | Yes | NM_000059.3 (BRCA2): c.7544del | Pancreas | Adenocarcinoma |
| 727 | M | C | Yes | NM_000059.3 (BRCA2): c.6270_6271del | Colon | Adenocarcinoma |
| 729 | M | C | Yes | NM_000059.3 (BRCA2): c.5946del | Stomach | Adenocarcinoma |
| 733 | F | C | Yes | NM_000059.3 (BRCA2): c.5213_5216del | Pancreas | Adenocarcinoma |
| 735 | M | C | Yes | NM_000059.3 (BRCA2): c.3847_3848del | Gallbladder | Adenocarcinoma |
| 736 | M | C | Yes | NM_000059.3 (BRCA2): c.9117G>A | Pancreas | Adenocarcinoma |
| 1108 | M | C | No | NM_000059.3 (BRCA2): c.3860del | Soft tissue | Sarcoma |
| 1201 | M | C | No | NM_000059.3 (BRCA2): c.6275_6276del | Head and neck | Squamous carcinoma |
A, African; C, Caucasian; M, male; F, female.
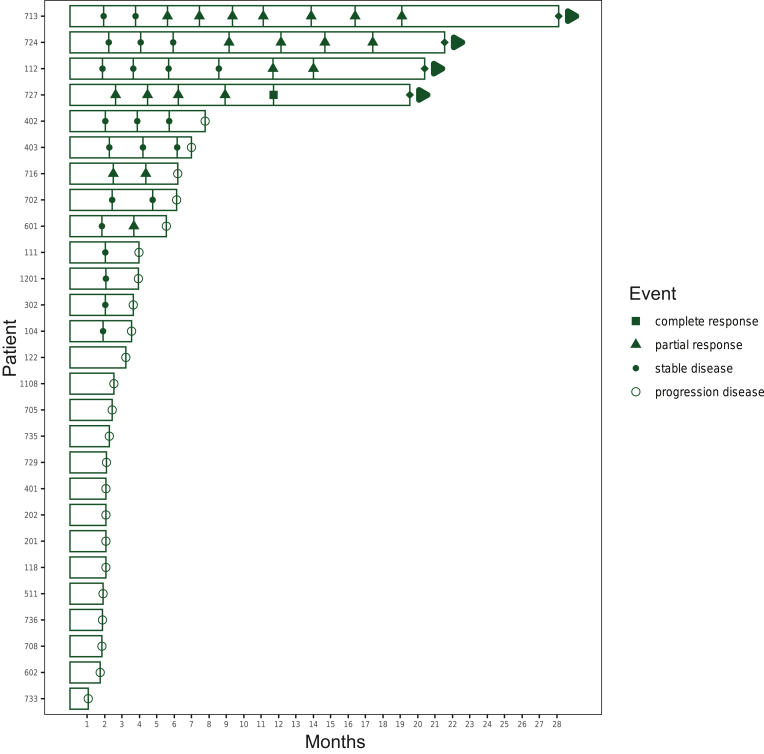
The response to treatment with olaparib in the BRCA2 cohort is shown in Figure 2.
Figure 2.
Swimmer plot shows the response to treatment with olaparib in the BRCA2 cohort.x-axis: time in months, y-axis: patient identification number. The events in time are displayed on the bar with each patient: complete response (filled black square), partial response (filled black triangle), stable disease (filled black circle) and progressive disease (empty circle). If an arrow is shown at the end of a bar, the response is still ongoing at the time of analysis.
Responses were seen in both somatic and germline mutation carriers. Five patients achieved a PR, and one patient a complete response (CR), or a response rate of 22%. Three of the partial responders had pancreatic adenocarcinomas (601, a mutation causing a splicing error; 724, germline mutation; 716, a protein-truncating somatic mutation), one patient with pancreatic neuroendocrine carcinoma (713, somatic frameshift mutation) and one patient with parathyroid carcinoma (112, somatic frameshift mutation). Since olaparib is registered and reimbursed in patients with pancreatic adenocarcinoma carrying a germline BRCA2 mutation, these cases will not be discussed in detail. It is important to emphasize that patient 716 carries a somatic BRCA2 mutation (not eligible for reimbursed drug access) and experienced a PR. This patient had metastases in the right lung and the subcarinal lymph nodes. After 2 months, a PR was seen at all sites but at 6 months, re-evaluation demonstrated progressive disease. In patient 724, the disease was localized in the lungs and at the esophagogastric junction. The first scan, at 2 months, revealed a PR and progressive disease at 4 months. The initial stage of the neuroendocrine carcinoma of the pancreas was pT4N1, for which the patient underwent a left hemi-pancreatectomy with a wedge resection of the stomach. Ten years later, the patient developed a recurrence in the abdominal lymph nodes for which carboplatin-etoposide was given as first-line chemotherapy followed by progression and FOLFOX as second-line chemotherapy. The patient’s medical history notes a pT1cN0M0 invasive ductal carcinoma of the left breast and type II diabetes mellitus. At study entry, the metastatic sites were the inguinal lymph nodes and the iliac bone. Six months after initiating therapy with olaparib, a PR was seen at all disease sites and was still ongoing at 28 months (= data cut-off). No dose reductions were required. The patient with parathyroid carcinoma was a 32-year-old, healthy Caucasian male who had undergone multiple surgeries (total thyroidectomy and central neck exploration, parathyroidectomy, partial sternotomy, mediastinal metastasectomy, bilateral cervical gland exploration) and radiotherapy (total dose of 70 Gy in 35 fractions). The physical examination was normal at inclusion and ECOG 0. The disease was localized in the lung and sternum. After 1 month, a dose reduction to 500 mg was carried out due to grade 2 nausea that did not improve with dietary changes, anti-emetic drugs and proton pump inhibitors. At 10 months, a PR was seen in all metastatic spots and was still ongoing after 20 months, the moment of data cut-off.
One patient with colon cancer achieved a CR (727, germline frameshift mutation). The patient had an impressive oncological history: diagnosed at the age of 59 years with an adenocarcinoma of the stomach, invasive ductal carcinoma of the breast when he was 63 years old and diagnosed at the age of 67 years with a pT3N0M0 adenocarcinoma of the colon. Nine years later, he had a relapse of the adenocarcinoma of the colon (histologically confirmed diagnosis) for which FOLFOX chemotherapy was started. At study entry, the disease was restricted to the ileocolic anastomosis. After 2 months on treatment with olaparib, a PR was seen, and a CR after 12 months. After 19 months (data cut-off), the CR was ongoing, and no dose adjustments were required.
Seven patients showed an SD. The ORR in this cohort is 22% and the CBR 51%.
In summary, the BRCA2 cohort consists of 11 patients with adenocarcinoma of the pancreas and 16 patients with other tumor histology. The CBR is also similar between the two groups: 54.5% (three PR, three SD) and 43.8% (one CR, two PR and four SD). The responders were diagnosed with colon cancer, parathyroid carcinoma and pancreatic neuroendocrine carcinoma. In this cohort, three patients with colon cancer were included and only one patient with parathyroid or pancreatic neuroendocrine carcinoma.
CHEK2 cohort
Germline and somatic pathogenic and likely pathogenic mutations were found in various cancer types (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102041).
The response to therapy with olaparib in the CHEK2 cohort is illustrated in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2023.102041.
No clinical benefit was seen in most patients, although two patients had SD for several months. Patient 121, a female with an extensively pretreated stage IV melanoma, had SD for 2 months. Patient 306, a female with stage IV breast cancer with only hormonal pretreatment, experienced SD for 11 months.
Discussion
We report the clinical activity of olaparib in advanced cancers that carry a BRCA1/2, CHEK2 or ATM mutation. These are completed cohorts of a tumor-agnostic Belgian basket phase II trial that investigates the clinical activity of olaparib in cancers with a somatic or germline mutation in genes that play a role in HR. PARP inhibition might be active in cancers with an HRD caused by other gene mutations or cancer types than currently documented or approved.
At the time of study design, only BRCA mutation testing was an approved biomarker for PARPi use. In recent years, new methods to measure HRD have been developed.15 HRD testing may include the detection of tumor mutations in genes beyond BRCA1/BRCA2 that are implicated in HRR (the approach used in this study) and genomic instability to evaluate the molecular phenotype of HRD beyond BRCA mutations. Currently, different methodologies and genomic markers are used in HRD testing.16 In future studies, the different methods must be integrated to predict response sensitivity to PARPi better.
At the time of writing, the European Medicines Agency (EMA) approval for olaparib as monotherapy includes BRCA1/2-mutated ovarian, breast, prostate and pancreatic cancer.17 EMA also approved olaparib in combination with bevacizumab for the treatment of HRD-positive ovarian cancer patients and in combination with abiraterone for the treatment of HRR-mutated prostate cancer.
No novel safety signals were observed besides those included in the product information.18,19 We had one patient with a rare allergy to olaparib leading to discontinuation of the treatment, although desensitization and subsequent continuation of olaparib would be possible.13
This study observed no clinically relevant activity in patients with an ATM mutation. The literature shows conflicting data concerning ATM-mutated cancer and response to PARPi. The Profound study20 showed a benefit of olaparib in prostate cancers harboring an ATM, BRCA1 or BRCA2 mutation. In a study with talazoparib and avelumab in patients with BRCA1/2- or ATM-mutated advanced tumor types (JAVELIN trial), recruitment in the ATM cohort was terminated early because the ORR was 10.5% (two responses in unspecified tumor types).21 One report describes a patient with a small-cell ATM-mutated esophageal cancer who had a response for 5.9 months with the addition of olaparib to etoposide.22. The preclinical evidence of PARPi in ATM-mutated cancers is conflicting. ATM-mutated prostate cancer was not sensitive to PARPi but sensitive to ATR inhibition.23,24 PARPi can be active in the presence of homozygous kinase-dead mutations in the ATM gene,23 consistent with sensitization to PARPi when ATM is experimentally depleted.25
No response was also seen in the CHEK2 mutation cohort. A phase II study in patients with CHEK2-mutated advanced breast cancer also failed to find olaparib activity.26
In contrast, in the BRCA1/2-mutated cancers, treatment with olaparib is effective. Before the start of this study, olaparib had already been approved and reimbursed as a treatment for patients with BRCA-mutated ovarian cancer (SOLO-1 trial)5,27 and breast cancer (OlympiAD trial28). Studies in ovarian cancer patients with an HRD were ongoing (e.g. PAOLA-1 trial, olaparib in combination with bevacizumab29). As a result, these patient populations were not eligible for the current study.
The response rate in BRCA1-mutated cancers was modest (11%) and observed in two pancreatic and one gall bladder cancer cases. The responsiveness of BRCA1/2-mutated pancreatic cancer to PARPi has, since the initiation of our study, been solidly documented, leading to regulatory approval of olaparib in this indication.30, 31, 32
The extended response (34+ months) to olaparib in a gallbladder adenocarcinoma with a BRCA1 mutation is an index case, consistent with the fact that BRCA1/2 mutations predispose to biliary tract cancer, supporting a strong driver role.33 BRCA2 mutations are more prevalent (3%) in biliary tract cancers than BRCA1 (0.6%), and several cases of response to PARPi have been reported in BRCA2-mutated biliary tract cancer.33
We also observed a prolonged, slowly achieved response to olaparib in a somatic BRCA2-mutated parathyroid carcinoma with an 18-month ongoing PR. Parathyroid cancer is a very rare cancer (0.005% of all malignancies). This index case is an extreme example of the clinical utility of agnostic sequencing in any cancer type, as neither BRCA1/2 mutations nor responses to olaparib have ever been reported before in this cancer type. And this is even more valid in rare cancers lacking effective therapies. The important response supports a driver function for BRCA2 in parathyroid cancer.
A patient with a BRCA2-mutated adenocarcinoma of the colon, an index case, also slowly developed a long-term complete and ongoing remission. BRCA1/2 mutation carriers have a possible modest increase in colorectal cancer risk.34 However, no prior reports of PARPi response in BRCA2-mutant colorectal cancer exist. Preclinical studies have found that a small proportion of colorectal cancer cell lines are sensitive to olaparib when TP53 is mutated but without an HRD.35 One patient with CHEK2-mutated metastatic colorectal cancer was reported to respond to olaparib.36 A more recent publication in colon cancer organoids revealed preclinical activity of olaparib in a BRCA2-mutated colorectal cancer, but the patient could not be treated to confirm this activity.37 A prospective phase II study specifically in BRCA1/2-mutated colorectal cancer seems warranted.
A last important remark is the fact that most patients in this study are highly pretreated (median therapeutic lines in metastatic setting is 2). The phase III SOLO-127 (maintenance olaparib after first-line platinum-based chemotherapy) and SOLO-3 trials38 (olaparib monotherapy in third line) in advanced BRCA-mutated ovarian cancer taught us that the early introduction of olaparib offers the most significant benefit. Patient 112, diagnosed with parathyroid carcinomas with a somatic BRCA2 mutation, is one of the patients who supports this in the current study. This patient was never previously exposed to systemic treatment and shows a sustained response.39
Our study results are in favor of the utility of tumor-agnostic NGS in all cancer types. Without agnostic NGS, the patients who benefited from the treatment in our study would not have had access to the treatment. These data and data from additional cohorts could, hopefully, move regulators and health insurers to allow implementation of broad agnostic NGS and corresponding drug access in clinical practice. It would be very difficult to accrue enough patients with rare cancer type–genotype associations in phase III trials.
Conclusion
In this study, olaparib does not have clinically meaningful activity in cancers with an ATM or CHEK2 mutation.
In BRCA1/2-mutated cancers, olaparib can be active and produce durable responses in various cancer types.
Funding
This work was supported by pharmaceutical companies AstraZeneca (global) and Merckx for medication supply and financial contributions. Financial support was provided by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society (project ID: 1145) and the Stichting Tegen Kanker (no grant number).
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
Supplementary Figure 1.
Supplementary Figure 2.
References
- 1.Bailey M.H., Tokheim C., Porta-Pardo E., et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;173(2):371–385.e18. doi: 10.1016/j.cell.2018.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thouvenin J., Van Marcke C., Decoster L., et al. PRECISION: the Belgian molecular profiling program of metastatic cancer for clinical decision and treatment assignment. ESMO Open. 2022;7(4) doi: 10.1016/j.esmoop.2022.100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopacinska-Joergensen J., Oliveira D., Poulsen T.S., Hoegdall C.K., Hoegdall E.V. Somatic variants in DNA damage response genes in ovarian cancer patients using whole-exome sequencing. Anticancer Res. 2023;43(5):1891–1900. doi: 10.21873/anticanres.16348. [DOI] [PubMed] [Google Scholar]
- 4.Dalmasso B., Puccini A., Catalano F., et al. Beyond BRCA: the emerging significance of DNA damage response and personalized treatment in pancreatic and prostate cancer patients. Int J Mol Sci. 2022;23(9):4709. doi: 10.3390/ijms23094709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiSilvestro P., Banerjee S., Colombo N., et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J Clin Oncol. 2023;41:609–617. doi: 10.1200/JCO.22.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griguolo G., Dieci M.V., Miglietta F., Guarneri V., Conte P. Olaparib for advanced breast cancer. Future Oncol. 2020;16(12):717–732. doi: 10.2217/fon-2019-0689. [DOI] [PubMed] [Google Scholar]
- 7.Robson M., Im S.A., Senkus E., et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 8.Inderjeeth A.J., Topp M., Sanij E., Castro E., Sandhu S. Clinical application of poly(ADP-ribose) polymerase (PARP) inhibitors in prostate cancer. Cancers (Basel) 2022;14(23):5922. doi: 10.3390/cancers14235922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golan T., Hammel P., Reni M., et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh T., Casadei S., Lee M.K., et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108(44):18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Brakeleer S., De Greve J., Desmedt C., et al. Frequent incidence of BARD1-truncating mutations in germline DNA from triple-negative breast cancer patients. Clin Genet. 2016;89(3):336–340. doi: 10.1111/cge.12620. [DOI] [PubMed] [Google Scholar]
- 12.Riaz N., Blecua P., Lim R.S., et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat Commun. 2017;8(1):857. doi: 10.1038/s41467-017-00921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabowski J.P., Sehouli J., Glajzer J., et al. Olaparib desensitization in a patient with recurrent peritoneal cancer. N Engl J Med. 2018;379(22):2176–2177. doi: 10.1056/NEJMc1810168. [DOI] [PubMed] [Google Scholar]
- 14.Nykamp K., Anderson M., Powers M., et al. Sherloc: a comprehensive refinement of the ACMG-AMP variant classification criteria. Genet Med. 2017;19(10):1105–1117. doi: 10.1038/gim.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppe M.M., Sundar R., Tan D.S.P., Jeyasekharan A.D. Biomarkers for homologous recombination deficiency in cancer. J Natl Cancer Inst. 2018;110(7):704–713. doi: 10.1093/jnci/djy085. [DOI] [PubMed] [Google Scholar]
- 16.Pujade-Lauraine E., Brown J., Barnicle A., et al. Homologous recombination repair gene mutations to predict olaparib plus bevacizumab efficacy in the first-line ovarian cancer PAOLA-1/ENGOT-ov25 trial. JCO Precis Oncol. 2023;7 doi: 10.1200/PO.22.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynparza European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/lynparza#authorisation-details-section
- 18.Roubaud G., Ozguroglu M., Penel N., et al. Olaparib tolerability and common adverse-event management in patients with metastatic castration-resistant prostate cancer: further analyses from the PROfound study. Eur J Cancer. 2022;170:73–84. doi: 10.1016/j.ejca.2022.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cottrell K., Clark C.L., Penson R.T. An update on the safety of olaparib for treating ovarian cancer. Expert Opin Drug Saf. 2022;21(4):447–451. doi: 10.1080/14740338.2022.2047176. [DOI] [PubMed] [Google Scholar]
- 20.de Bono J., Mateo J., Fizazi K., et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 21.Schram A.M., Colombo N., Arrowsmith E., et al. Avelumab plus talazoparib in patients with BRCA1/2- or ATM-altered advanced solid tumors: results from JAVELIN BRCA/ATM, an open-label, multicenter, phase 2b, tumor-agnostic trial. JAMA Oncol. 2023;9:29–39. doi: 10.1001/jamaoncol.2022.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Zhang X., Fang Y., et al. Case report: olaparib shows satisfactory clinical outcomes against small cell esophageal carcinoma with ATM mutation. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.808801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakr A., Oing C., Kocher S., et al. Involvement of ATM in homologous recombination after end resection and RAD51 nucleofilament formation. Nucleic Acids Res. 2015;43(6):3154–3166. doi: 10.1093/nar/gkv160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtin N.J. Targeting the DNA damage response for cancer therapy. Biochem Soc Trans. 2023;51(1):207–221. doi: 10.1042/BST20220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvin S., Akter J., Takenobu H., et al. ATM depletion induces proteasomal degradation of FANCD2 and sensitizes neuroblastoma cells to PARP inhibitors. BMC Cancer. 2023;23(1):313. doi: 10.1186/s12885-023-10772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung N.M., Robson M.E., Ventz S., et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee S., Moore K.N., Colombo N., et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1721–1731. doi: 10.1016/S1470-2045(21)00531-3. [DOI] [PubMed] [Google Scholar]
- 28.Hodgson D., Lai Z., Dearden S., et al. Analysis of mutation status and homologous recombination deficiency in tumors of patients with germline BRCA1 or BRCA2 mutations and metastatic breast cancer: OlympiAD. Ann Oncol. 2021;32(12):1582–1589. doi: 10.1016/j.annonc.2021.08.2154. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Martin A., Desauw C., Heitz F., et al. Maintenance olaparib plus bevacizumab in patients with newly diagnosed advanced high-grade ovarian cancer: main analysis of second progression-free survival in the phase III PAOLA-1/ENGOT-ov25 trial. Eur J Cancer. 2022;174:221–231. doi: 10.1016/j.ejca.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Devico Marciano N., Kroening G., Dayyani F., et al. BRCA-mutated pancreatic cancer: from discovery to novel treatment paradigms. Cancers (Basel) 2022;14(10):2453. doi: 10.3390/cancers14102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garajova I., Balsano R., Gelsomino F., Leonardi F. Olaparib as a single agent treatment in pre-treated metastatic pancreatic cancer patient harboring BRCA2 mutation: what could we expect? Tumori. 2022;108(6):NP30–NP33. doi: 10.1177/03008916221132589. [DOI] [PubMed] [Google Scholar]
- 32.Teke M.E., Saif A., Ryan C.E., Lux S.C., Hernandez J.M., Reiss K.A. A randomized study of olaparib or placebo in patients with surgically removed pancreatic cancer who have a BRCA1, BRCA2 or PALB2 mutation (the APOLLO trial) Ann Surg Oncol. 2022;29(9):5375–5376. doi: 10.1245/s10434-022-11917-2. [DOI] [PubMed] [Google Scholar]
- 33.Spizzo G., Puccini A., Xiu J., et al. Molecular profile of BRCA-mutated biliary tract cancers. ESMO Open. 2020;5(3) doi: 10.1136/esmoopen-2020-000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katona B.W., Stadler Z.K., Robson M.E., Domchek S.M. RE: BRCA1 and BRCA2 gene mutations and colorectal cancer risk: systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(5):522–523. doi: 10.1093/jnci/djz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smeby J., Kryeziu K., Berg K.C.G., et al. Molecular correlates of sensitivity to PARP inhibition beyond homologous recombination deficiency in pre-clinical models of colorectal cancer point to wild-type TP53 activity. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghiringhelli F., Richard C., Chevrier S., Vegran F., Boidot R. Efficiency of olaparib in colorectal cancer patients with an alteration of the homologous repair protein. World J Gastroenterol. 2016;22(48):10680–10686. doi: 10.3748/wjg.v22.i48.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martini G., Belli V., Napolitano S., et al. Establishment of patient-derived tumor organoids to functionally inform treatment decisions in metastatic colorectal cancer. ESMO Open. 2023;8(3) doi: 10.1016/j.esmoop.2023.101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penson R.T., Valencia R.V., Cibula D., et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol. 2020;38(11):1164–1174. doi: 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberti A., Smussi D., Zamparini M., et al. Treatment and outcome of metastatic parathyroid carcinoma: a systematic review and pooled analysis of published cases. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.997009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.