Summary
Recently published studies suggest that the paracrine substances released by mesenchymal stem cells (MSCs) are the primary motive behind the therapeutic action reported in these cells. Pre-clinical and clinical research on MSCs has produced promising outcomes. Furthermore, these cells are generally safe for therapeutic use and may be extracted from a variety of anatomical regions. Recent research has indicated, however, that transplanted cells do not live long and that the advantages of MSC treatment may be attributable to the large diversity of bioactive substances they create, which play a crucial role in the control of essential physiological processes. Secretome derivatives, such as conditioned media or exosomes, may provide significant benefits over cells in terms of manufacture, preservation, handling, longevity of the product, and potential as a ready-to-use biologic product. Despite their immunophenotypic similarities, the secretome of MSCs appears to vary greatly depending on the host’s age and the niches in which the cells live. The secretome’s effect on multiple biological processes such as angiogenesis, neurogenesis, tissue repair, immunomodulation, wound healing, anti-fibrotic, and anti-tumor for tissue maintenance and regeneration has been discovered. Defining the secretome of cultured cultivated MSC populations by conditioned media analysis will allow us to assess its potential as a novel treatment approach. This review will concentrate on accumulating data from pre-clinical and clinical trials pointing to the therapeutic value of the conditioned medium. At last, the necessity of characterizing the conditioned medium for determining its potential for cell-free treatment therapy will be emphasized in this study.
Key words: Stem cells, Conditioned media, Cell-free therapy, Regenerative medicine
Introduction
Stem cell-based therapies present potential therapeutic options for the treatment of more complex medical conditions because, unlike traditional pharmaceutical treatments, stem cells function through several pathways (multi-target therapy). Because of their ability to self-renew and differentiate into numerous cell lineages, stem cells are at the forefront of new therapeutics [1]. Embryonic stem cells and somatic stem cells are the two types of stem cells. Hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) are examples of somatic stem cells [2]. The International Society for Cellular Therapy’s Mesenchymal and Tissue Stem Cell Committee has established the minimum criteria for defining human MSCs [3]. One of the criteria is that when grown under typical growth conditions, MSCs adhere to plastic. MSCs express CD105, CD73, and CD90 but not CD45, CD34, CD14, or CD11b, CD79a, or CD19, or HLA-DR surface molecules. Last but not least, in vitro, MSCs exhibit osteogenic, adipogenic, and chondrogenic plasticity [4–6].
MSC activity appears to be mediated by a ‘hit and run’ process rather than engraftment in wounded tissues, indicating that MSCs primarily operate through bioactive substances [7–8]. MSCs are known to produce a variety of protective bioactive substances (secretome), which include cytokines, chemokines, cell adhesion molecules, lipid mediators, interleukin (IL), growth factors (GFs), hormones, exosomes, microvesicles, and others. These factors have been identified as key players in tissue healing and regeneration due to their paracrine effects that facilitate cell-to-cell communication. These secreted molecules, collectively known as the secretome or conditioned media (CM), serve an important role in modulating cross-talk contacts between cells and adjacent tissues to mediate a biological function [9].
Following MSC in vitro development in the laboratory, the cells tend to release a collection of bioactive substances into the culture medium, which is now known as the MSC conditioned media (MSC-CM) or the MSC secretome. A CM containing bioactive substances can have tissue-protective (anti-apoptotic, anti-inflammatory, anti-scarring), immunomodulatory, angiogenic, or anti-tumorigenic effects on the recipient [10,11].
The MSC-CM refers to the collection of chemicals secreted by stem cells such as proteins, microRNA, growth factors, antioxidants, proteasomes, and exomes, and it is thought to be a rich source of paracrine elements [12–14]. VEGF, SCF, HGF, IGF 1, IGF2, and SDF1 are frequent paracrine factors produced during in vitro stem cell development [15].
MSC-CM also contains extracellular vesicles (EVs) as a payload of diverse proteins, coding and non-coding RNA, short RNAs, autophagosomes, and mitophagosomes. Apoptotic bodies, microparticles, and exosomes are three types of EVs [16].
Characterization of the MSC-CM is critical since its therapeutic potential has been linked to cytokine combinations and their associated paracrine actions. Molecular investigations of MSC-CM can reveal crucial therapeutically active components that can be isolated and exploited further. Furthermore, recognizing the methods by which such important components exercise their therapeutic benefits would be of particular importance [17].
When a cell defect occurs, it secretes paracrine factors, resulting in a paracrine factor gradient between the diseased region and stem cell niches. This gradient would attract stem cells to the afflicted organ, which would then develop into organ-specific cells, facilitating the process of tissue regeneration. The scientific community believes that adding conditioned medium obtained from mesenchymal stem cell cultures to an afflicted organ will aid in raising the paracrine gradient, resulting in a faster tissue regeneration process. MSC-CM might be employed as an alternative to the cell-based therapeutic strategy based on the notion of paracrine signaling [15].
Because MSC-CM is cell-free, one important advantage of employing CM is its immunological compatibility for the recipients; as a result, a donor-recipient match, which is a requirement in cell-based treatment, is not necessary. In contrast to using stem cells, which need sterile circumstances during the application process to the patient, the application technique of MSC-CM does not require such regulated settings, which increases the simplicity of use. Preparation of CM would decrease the time required for stem cell multiplication, resulting in cost efficiency compared to mass manufacturing [14]. Frozen or lyophilized and well-packaged forms of CM would ease transportation needs by eliminating the need for cryopreservation, which is presently the technique of preservation employed in cell-based treatment. In comparison to traditional cell-based therapeutic approaches, the preserved CM would provide convenient and easy access to regenerative medicine [14,18].
Although distinct MSC populations exhibit similar phenotypic traits and therapeutic potential, they inhabit diverse anatomic regions, and their secretome is expected to differ. There are differences in therapeutic potential based on the origin of the MSCs [19–21]. MSCs have been shown to differ significantly in their gene expression patterns and, not surprisingly, in their secretome profile, with the differences attributed to the MSCs’ source, the host’s age, and the culture medium in which they are propagated [20–22].
The goal of this review is to cover some of the pre-clinical, and clinical research that looked at the MSC secretome as a therapeutic option with the larger goal of developing a viable cell-free based therapy. The application of MSC-CM in regenerative medicine is still in its infancy, hence it is necessary to develop suitable animal models to study how MSC-CM affects the process of regeneration. Most notably, when implementing MSC-CM-based treatment, the fundamental question that must be addressed is the confirmation of its safety and therapeutic efficacy in people.
Secretome identification techniques
Although many issues about the intricate components of MSC-CM remain unanswered, efforts are being made to identify and describe the secretome from various MSC sources. Shot-gun and immunological tests are the two most often used modern proteomics techniques for defining the MSC-CM [23]. Immunological tests have good specificity, sensitivity, and repeatability when it comes to a wide variety of known proteins. Antibody-based methods such as enzyme-linked immunosorbent assay (ELISA), Luminex antibody bead-based array, microarray, western blotting, and cytokine antibody array may detect and quantify these proteins. The shotgun technique to proteomics is more exploratory in nature, yet it aids in the identification of any unknown and unique secreted proteins. Accessing publicly available datasets, employing bioinformatics tools, and doing pathway analysis can help establish the role of such unusual proteins. Gel-based methods such as 2-D gel electrophoresis (2-DE), liquid chromatography with tandem mass spectrometry (LC-MS/MS), stable isotope labeling by amino acids in cell culture (SILAC), matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF), MS/MS, quadrupole time-of-flight mass spectrometry (QTOF-MS), and others are used in shotgun approaches [24–26].
Manufacturing options
The primary aspects of MSC-CM preparation are stem cell isolation and growth, followed by CM collection and storage. Tissue origin influences the types of paracrine factors produced. As previously noted, the most appropriate stem cell type may differ based on the paracrine components necessary for the treatment of certain disorders. Allogenic MSCs are preferable to autologous MSCs since CM may be prepared by these people ahead of time. Allogenic MSCs may be prescreened for pathogens, and huge quantities of secretory profiles can be acquired. This also enables control over the quality of CM derived from allogeneic MSCs. Immunogenic antigens are absent in CM since they are produced from immunogen-free MSCs. As a result, the immunogenicity of CM derived from allogenic MSCs is of minor relevance. However, the prevalence of batch-to-batch fluctuation due to donor reliance is a serious problem while manufacturing MSC-CM. Immortalized stem cell lines can be created to overcome phenotypic alterations that may occur during long-term cultivation. This will enable the bulk production of MSC-CM while still maintaining MSC properties. MSCs obtained from dental pulp were immortalized by adding the human papillomavirus genes E6 and E7, as well as the hTERT genes [27].
To obtain a therapeutically adequate number of isolated MSCs, their multiplication in tissue culture flasks is performed in the laboratory. These flasks have surface areas of 25, 75, 150, and 225 cm2. Larger bioreactors, however, are necessary for the mass production of CM. Roller bottles, multilayered cell factories, hollow fiber, packed beds, and stirred suspension bioreactors are some of the most often utilized bioreactors for the generation of clinical-grade stem cells [28].
To improve the neuroregulatory characteristics of human bone marrow stem cells (hBMSCs), MSC-CM was collected in computer-controlled stirred suspension bioreactors. The implementation of a dynamic bioreactor allowed for the manipulation of variables such as pH, temperature, and oxygen concentration, resulting in a greater level of differentiation of stem cells [29].
In this review, we address 10 recently published studies concerning the investigation of MSC-CM as a possibility for the future treatment of several different disorders (Table 1). From the total number of reviewed reports, 6 were in pre-clinical stages, utilizing animal models, and 4 of the reports were performed at the clinical stage, focused on efficacy and safety in humans and the ability of MSC-CM to be applied in regenerative medicine practices.
Table 1.
Overview of the studies employing MSC-CM for therapeutic purposes in specific scenarios.
| Authors | Trial Stage | Model Type | Disease | Year |
|---|---|---|---|---|
| Leeman et al [30] | Pre-clinical | cells, mouse | bronchopulmonary dysplasia | 2019 |
| Takeuchi et al. [31] | Pre-clinical | rat | calvaria bone lesion | 2019 |
| Gregorio et al. [32] | Pre-clinical | cells, mice | diabetic polyneuropathy | 2020 |
| Yang et al. [33] | Pre-clinical | cells, mice | non-alcoholic fatty liver disease | 2021 |
| Dorronsoro, et al. [34] | Pre-clinical | cells, mice | aging | 2021 |
| Sun et al. [35] | Pre-clinical | cells, mice | cancer | 2021 |
| Harell et al. [36] | Clinical | cells, mice, human | chronic obstructive pulmonary disease | 2020 |
| Zhang et al. [37] | Clinical | cells, mice, human | systemic lupus erythematosus | 2019 |
| Oh et al. [38] | Clinical | cells, human | androgenetic alopecia | 2020 |
| Dahbour et al. [39] | Clinical | human | multiple sclerosis | 2017 |
Pre-clinical reports utilizing MSC-derived CM
The pre-clinical research results have shown that MSC secretome has therapeutic potential for treating a wide range of morbidities. To name a few of the MSC-CM applications reviewed in this study, they include bone regeneration, anti-aging, bronchopulmonary dysplasia (BPD), diabetic polyneuropathy (DPN), and others.
Leeman et al. published a pre-clinical study, which shows that MSC-secreted factors have a direct influence on lung progenitor cell development. In this study, MSC-CM was sufficient to induce alveolar organoid development, indicating that the reaction is most likely due to soluble substances produced by MSCs [30].
Normal functioning of alveolar stem/progenitor cells is likely required for effective healing. The development of lung disease is influenced by lung epithelial cell injury and defective restoration. In a mice model of bronchopulmonary dysplasia (BPD), for example, we found that MSCs protect against hyperoxic lung damage by increasing the amount of Epcam+ Sca-1+ distal lung epithelial cells. In three-dimensional (3D) organoid preparations, these cells can differentiate into both small airway (CCSP+) and alveolar (SPC+) epithelial cells. Leeman and their team introduced MSCs to lung progenitor 3D preparations to better understand the interactions between MSCs and distal lung epithelial cells. MSCs induced the production of Epcam+ Sca-1+ derived organoids, enhanced alveolar differentiation, and reduced self-renewal. Based on several animal models of lung damage and MSC therapies, MSC-CM has been suggested to be therapeutic activators of lung healing. MSC-secreted factors may help to prevent illness by replacing damaged signals and activating endogenous epithelial progenitor cells to repair damaged epithelium [30].
The presented results show that MSC-CM may substitute the signals of hyperoxic damage effects, activating alveolar progenitor repair in a mouse model of BPD. The presence of TSP1 in MSC supernatant, together with earlier findings of K. Leeman’s research group, confirms that TSP1 is one mechanism by which MSCs might influence alveolar differentiation [40]. Their findings provide light on the mechanics of lung stem cell alveolar differentiation and open the door to the investigation of innovative treatment approaches to enhance healing by stimulating endogenous progenitor cells. The effectiveness of an in vitro system for examining factors that drive changes in differentiation patterns is emphasized. These findings suggest that in the context of lung injury, incorrect supporting cell communication to alveolar progenitors may occur, which may be corrected by MSC-secreted signals [30].
To assure safety and efficacy, more research is needed to investigate the role of these putative secreted components.
Takeuchi’s group used exosomes from a conditioned medium of bone marrow-derived mesenchymal stem cells (BMSCs) to stimulate bone regeneration by boosting angiogenesis in a rat model with a calvaria bone lesion. Exosomes (Exo), which are components of MSC-CM, convey genetic information to the cells being stimulated and improve recipient cellular paracrine stimulation, which aids in tissue regeneration [31].
The researchers created three groups: MSC-Exo, MSC-CM, and Exo-antiVEGF (MSC-Exo with angiogenesis inhibitor), and they investigated the osteogenic and angiogenic capabilities of MSCs in vitro. Furthermore, they employed a rat model of calvaria bone deficiency and implanted Atelocollagen sponges (Terudermis1, Olympus Terumo Bio-materials Corp., Tokyo, Japan) used as a scaffold, soaked with each sample as per previously described treatment groups. Bone development examinations were performed at the 2 and 4-week mark. The results show that in vitro MSC-Exo improved cellular migration and osteogenic and angiogenic gene expression in MSCs when compared to other groups. In vivo, the study confirms an early bone formation by MSC-Exo. Histologically, the MSC-Exo group had a significant buildup of osteoblast-like cells and vascular endothelial cells; whereas, the Exo-antiVEGF and control groups had fewer cells. Two and four weeks after implantation, disparities in the region between the MSC-Exo group and the Exo-antiVEGF or control groups were significantly expanded. The in vitro results also suggest that MSC-Exo increased the expression of not only osteogenesis-related genes but also angiogenesis-related genes including VEGF, ANG 1, and ANG2, but Exo-antiVEGF lowered the expression of these genes [31]. These findings imply that MSC-Exos have a great osteogenic capacity. MSC-CM exosomes may play essential roles in bone regeneration by increasing angiogenesis in the early phases. MSC-Exo can also be employed as a bioactive agent for bone repair, according to the findings of Takeuchi et al. [31].
Because of MSCs’ high plasticity, it has been reported that by subjecting these cells to an in vitro preconditioning stimulus, it is possible to modify the MSC-conditioned medium baseline composition, resulting in a biological product with a defined combination and ratio of biomolecules specific for a specific pathology [41–42]. Gregorio et al. recently reported that preconditioning human adipose tissue-derived mesenchymal stem cells (AD-MSCs) with the hypoxic mimetic agent deferoxamine (DFX) in vitro resulted in a significant increase in the secretion of potent antioxidant, neuroprotective, angiogenic, and anti-inflammatory factors compared to non-preconditioned cells. Furthermore, when tested in an in vitro model of Diabetic polyneuropathy (DPN), the conditioned medium obtained from DFX preconditioned cells had stronger neuroprotective benefits than the conditioned medium obtained from non-preconditioned MSCs [43].
DPN is the most prevalent and early occurring consequence of diabetes mellitus, and a major contributor to the development of foot ulcers (FU), for which no particular medications are available. DPN is a clinical consequence of diabetes that progresses from functional peripheral nerve deficits in the early stages to structural alterations in the later stages [44].
Gregorio et al. investigated whether systemic administration of conditioned medium derived from DFX-preconditioned human AD-MSCs or non-precon-ditioned AD-MSCs was capable of reversing multiple functional and physical changes associated with DPN and preventing the development of foot ulcers (FU) in BKS db/db mice [32].
DFX has the advantage of having been used in humans for over thirty years with no adverse effects to treat iron overload conditions, and it was already proven that due to its small size, it could be completely removed from the final product in the concentration steps before intravenous administration into animals [45].
The administration of conditioned media of DFX-preconditioned, non-preconditioned ADMSCs or saline (control group) was repeated every two weeks for a total of four treatments, and numerous functional and structural characteristics of DPN were examined. Ultimately, the rate of wound healing, re-epithelia-lization, angiogenesis, and cell proliferation were studied on the dorsal surface of both feet. The data presented show that systemic administrations of conditioned medium derived from DFX-preconditioned MSCs and non-preconditioned MSCs considerably relieved thermal and mechanical hypoalgesia in diabetic mice, thereby restoring the normal sensitivity limits compared to untreated diabetic mice.
Furthermore, in vivo, treatment of conditioned media produced from DFX-preconditioned AD-MSCs or non-preconditioned AD-MSCs dramatically decreased neuron and Schwann cell death. The administration of a conditioned medium derived from DFX-preconditioned AD-MSCs, which is significantly enriched in angiogenic factors, induced a better angiogenic response compared to non-preconditioned AD-MSC-conditioned medium-treated animals. The findings suggest that administering MSCs modifies the inflammatory response by altering the cytokine profile toward an anti-inflammatory phenotype [32].
This study suggests that the cocktail of bioactive mediators found in the conditioned medium of AD-MSCs may be a great and new treatment strategy for reversing the early stages of DPN, preventing the development of FU, and lowering the risk of lower limb amputation.
MSC-CM is a promising therapy option for another diabetic comorbidity; according to Dr. Yang’s research, MSC-CM has a high potential in the treatment of non-alcoholic fatty liver disease (NAFLD). MSC-CM not only restored insulin resistance and sensitivity in T2DM mice, but it also alleviated liver dysfunction and dyslipidemia. MSC-CM also decreased liver inflammation and cell death while increasing antioxidant capacity by preserving mitochondrial activity. MSC-CM increased mitochondrial activity in the liver while decreasing inflammation and apoptosis in both in vivo and in vitro studies [33].
Diabetes-associated NAFLD is caused by excessive lipid buildup in the liver, and the presence of NAFLD is a signal of insulin resistance, which is a prominent feature of T2DM. The molecular processes underpinning the progression from steatosis and steatohepatitis to severe liver disease are not completely clarified, however, mitochondria have been shown to play an important part [46,47].
Yang et al. investigated the link between MSC-CM, NAFLD, and Sirtuin 1 (SIRT1) and discovered that MSC-CM may function as an agonist of SIRT1, considerably upregulating the expression level of SIRT1 in liver cells, thus improving NAFLD. In brief, the current findings showed that MSC-CM effectively decreased NAFLD by improving mitochondrial biological processes, decreasing inflammation, and preventing cell apoptosis both in vivo and in vitro and that these beneficial effects were strongly linked with SIRT1 upregulation.
MSC-CM decreased insulin resistance in diabetic mice, altered the diseased structure of the liver, increased overall antioxidant abilities and mitochondrial activity, and decreased inflammation and cell death. The researchers also confirmed that SIRT1 was important in mediating the protective effect of MSC-CM. These findings give new evidence that MSC-CM can therapeutically treat T2DM patients with NAFLD [33].
The research team of Dr. Dorronsoro investigated MSC-CM application in anti-aging effects of BMSCs and EVs derived from BMSCs and human embryonic stem cell-derived MSCs (hESC- MSC). Aging is characterized by a decrease in regenerative capacity, which results in a reduced ability to adapt to stress and, as a result, increased morbidity and mortality. This contributed to the theory that aging is caused in part by the loss of function of adult stem cells required for tissue homeostasis management. Indeed, mice above the age of two exhibit a considerable decrease in the quantity and proliferative potential of many different adult stem cells.
The researchers have previously disclosed that aging has a negative impact on muscle-derived stem/progenitor cells (MDSPC). MDSPCs isolated from old and Ercc1/progeroid mice display decreased proliferative ability and decreased differentiative capacity, and this dysfunction leads directly to age-related deterioration, given that transplantation of young MDSPCs extended longevity and life span in ERCC1-deficient progeroid mouse models. Transplanted MDSPCs did not differentiate or move from the site of administration, suggesting that the therapeutic action of MDSPCs is controlled by systemic secreted factors. Similarly, the co-culture of youthful MDSPCs with old MDSPCs resulted in the renewal of old MDSPC proliferative and differentiative capacity, although the variables responsible for the rejuvenation of aged MDSPCs remained unresolved [48]. The most recent findings revealed BMSCs from young animals, as well as lineage-directed hESC-derived BMSC surrogates, as a unique source of EVs with senotherapeutic potential. The researchers show that transplanting BMSCs from young mice, but not from elderly mice, increases life duration and health span in ERCC1-deficient animals. Furthermore, CM from youthful BMSCs restored the function of old, senescent stem cells and senescent murine embryonic fibroblasts (MEFs) in culture. Furthermore, the senotherapeutic action of CM was co-purified with EVs generated by young, but not old, MSCs and muscle-derived stem/progenitor cells (MDSPC). Importantly, IP injection of EVs from BMSCs from young mice increased the life duration of ERCC1-deficient animals [34]. Treatment with EVs isolated from human embryonic stem cell-derived MSCs (hESC-MSC) was also capable of substantially decreasing the expression of senescence markers in cultured senescent fibroblasts in addition to naturally aged wild-type and Ercc1/mice, as well as enhancing healthspan measures in vivo. These ground-breaking findings revealed EVs as crucial components secreted by youthful, functioning stem cells that can reverse cellular senescence and stem cell failure in vitro as well as decrease senescent cell load in vivo. As a result, functional stem cell-derived EVs constitute a possible treatment strategy for reducing senescent cell load and extending good health [34].
A study report was published, in which authors induced tumor-suppressing (iTS) MSCs, which then protected bone from breast cancer metastases formation. Sun et al.’s work provides an MSC CM-based treatment approach that inhibits not only tumor growth but also osteoclast production. While this work used in vitro, ex vivo, and in vivo, models with breast and prostate cancer cells, the response to MSC CMs and the degree of inhibitory effects may vary depending on cancer cell type, hormone receptor status, and p53 mutations. It will also be interesting to see whether any other genes can be overexpressed to create iTS cells and if those alternatives have any particular efficacies. It’s also worth wondering if the selected genes can convert other cells besides MSCs to iTS cells. In summary, the gene overexpression strategy and systemic delivery of MSC CMs significantly prevented the development of mammary tumors and tumor-induced osteolysis in the two mice models. Further research is needed to understand the regulatory basis of altered MSC activity and to assess the viability of clinical translation for the treatment of tumor-invaded bone [35].
This study found that by overexpressing Lrp5, -catenin, Snail, or Akt, MSCs might become tumor suppressors. Amplification of these genes increased the levels of Hsp90ab1, calreticulin, Ppib, Trail, and p53 in their CMs while decreasing tumor-promoting cytokines including CXCL2 and LIF as well as PDL1, a target of anti-PD1 immunotherapy. Systemic CM injection inhibited the development of breast tumors as well as tumor-induced bone loss. Furthermore, these CMs prevented osteoclasto-genesis by downregulating NFATc1, a master transcription factor for osteoclasto-genesis, as well as cathepsin K, a bone degradation protease.
More research is needed to understand the regulatory foundation for altered MSC activity and to assess the viability of clinical translation into the therapy of tumor-invaded bone [35].
Clinical Reports utilizing MSC-derived CM
In this paper, we analyze three published data from clinical trials using MSC-CM, focusing on the safety of this unique treatment method and its potential impact on several disorders. The first clinical study to discuss was published by Harell et al., who looked at the possibility of MSC secretome for the treatment of inflammatory lung illnesses such as chronic obstructive pulmonary disease (COPD) [36]. They studied the effects of a newly developed MSC-derived product “Exosome-derived Multiple Allogeneic Protein Paracrine Signaling (Exo-d-MAPPS)” in the reduction of chronic airway inflammation using an animal model of chronic obstructive pulmonary disease (COPD) (induced by chronic exposure to cigarette smoke (CS)) and clinical data obtained from Exo-d-MAPPS-treated COPD patients, because most of the MSC-mediated beneficial outcomes were the result of their paracrine activity. For these reasons, Exo-d-MAPPS was given intraperitoneally to mice and inhaled by COPD patients [36]. To summarize, we feel that Exo-d-MAPPS might be a potentially novel therapeutic agent in the treatment of chronic inflammatory lung disorders and that its safety and effectiveness need to be investigated further in major clinical studies [36].
In summary, the data suggest that the primary mode of action for Exo-d-MAPPS-based COPD relief was dependent on the anti-inflammatory effects of soluble messengers (sTNFRI and II, IL-1Ra, and sRAGE), which blocked the influx of inflammatory leukocytes while assisting the expansion of immunosuppressive cells in the lungs. Changes in the cellular constitution of the lungs lead to the formation of an anti-inflammatory milieu, allowing for greater tissue repair and regeneration as well as improved pulmonary activity in CS-exposed mice and COPD patients. The researchers also believe that sTNFRI, sTNFRII, and IL-1Ra, which were abundant in the Exo-d-MAPPS sample, contributed to the lower serum levels of TNF- and IL-1, as well as the considerably decreased levels of inflammatory TNF-producing macrophages and TNF- and IL-1-producing neutrophils in the lungs of CS+Exo-d-MAPPS-treated animals. The data also show that the reduction of T cell-driven airway inflammation as well as damage of alveolar epithelial cells seen in CS+Exo-d-MAPPS-treated animals was caused by the Exo-d-MAPPS-induced expansion of lung-infiltrated IL-10-producing macrophages, DCs, and Tregs, as well as elevated serum IL-10 levels. Exo-d-MAPPS therapy dramatically improved the pulmonary status and quality of life of COPD patients, as demonstrated in an animal model. Importantly, Exo-d-MAPPS was well tolerated, as none of the 30 COPD patients experienced any side effects after receiving Exo-d-MAPPS [36].
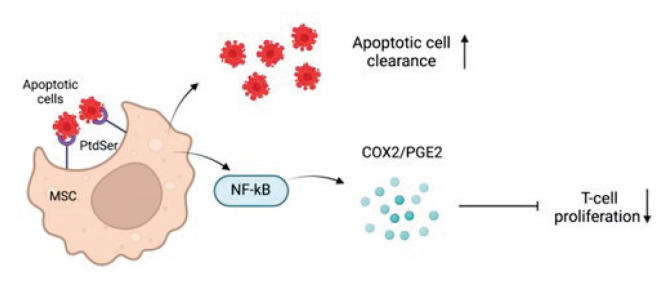
Next clinical trial to discuss delivered interesting data regarding the application of MSC-CM in the treatment of systemic lupus erythematosus (SLE). Apoptotic cell (AC) clearance failure has been implicated in the etiology of SLE. MSCs show promising therapeutic benefits in SLE, although it is uncertain if MSCs phagocytose ACs and contribute to the underlying process in SLE therapy [37]. Buildup ACs contribute to autoantigen exposure to the immune system, which eventually results in autoimmunity such as SLE.
Zhang et al. demonstrated that umbilical cord MSCs (UCMSCs) could engulf ACs and that these MSCs displayed improved immunosuppressive action via the activation release of prostaglandin (PG)E2, which engaged the NF-κB signaling pathway. Additionally, after MSC transplantation, individuals with SLE with reduced ACs had higher levels of PGE2 metabolites (PGEM). Both modes of action are depicted in Figure 1. The researchers observed that UCMSCs could consume ACs both in vitro and in vivo. Furthermore, phosphatidylserine (PtdSer), the best studied ‘eat me’ signal during phagocytosis, facilitated the contact amongst UCMSCs and ACs, confirming phagocytosis as a shared activity of MSCs. Thus, the transplantation of UCMSCs, a new non-professional phagocyte, might offer a different path to improve AC elimination during the therapy of SLE, where poor clearance of ACs was found and related to the breakdown of self-tolerance. The data reveals that after being subjected to ACs, MSCs secreted significantly more PGE2. PGE2 is an essential mediator in MSC immunoregulatory actions, particularly T cell proliferation inhibition, T cell and DC differentiation, and cytokine generation [37].
Fig. 1.
Scheme of MSC promoting AC clearance and induction of immunosuppression.
A modest rise was also found in the transwell condition when phagocytosis was prevented in this investigation. Thus, in addition to phagocytosis, there may be other substances released by ACs that encourage MSCs to create PGE2, which needed to be investigated further. The signaling mechanisms that relate AC engulfment to the production of anti-inflammatory mediators in professional phagocytes remain unknown. In the case of MSCs, it was discovered that activation of the NF-κB signaling pathway was essential for the amplification of COX2/PGE2 when ACs were consumed.
Importantly, after MSC therapy, individuals with lower ACs had greater PGEM levels. Thus, more research and clinical trials are needed to determine if PGEM can be used to predict the success of MSC therapy in SLE [37].
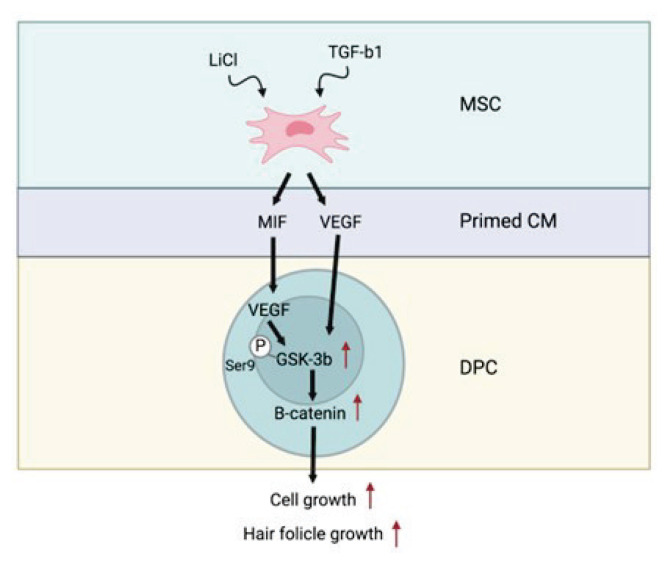
The purpose of the investigation, performed by Oh et al., was to evaluate the use of conditioned medium (CM) obtained from human umbilical cord blood-derived MSCs (hUCB-MSCs) for enhancing hair development and to create a methodology to dependably produce this optimal CM. The influences of primed MSC-derived CM (P-CM) with combinations of TGF-β1 and LiCl on the cell viability of dermal papilla cells (DPCs) were compared. Moreover, clinical trials have revealed that 5 % P-CM improved androgenetic alopecia by increasing hair density, thickness, and growth rate, indicating that this topical drug may be a unique and effective therapeutic option for individuals suffering from androgenetic alopecia [38].
MSCs were initially subjected in vitro to an arti-ficial environment resembling an alopecia condition in hair follicles (“priming”) for pretreatment. Human hair follicles that have been afflicted by androgenic alopecia have significantly reduced vitality and proliferation. Dermal papilla cells (DPCs) on hair follicles regulate the hair follicle cycle by triggering cell proliferation signaling factors. DPC stimulus to restore hair induction has been suggested as a promising therapeutic for hair loss. In this investigation, we found that conditioned medium (CM) derived from MSCs primed with TGF-β1 and LiCl increased DPC viability and hair development. MIF, which was released by primed MSCs, produced VEGF in DPCs by blocking GSK-3, which causes -catenin phosphor-rylation (Fig. 2) [38].
Fig. 2.
Scheme of MSC-CM activity on DPC proliferation and hair follicle growth.
In terms of density of hair, the benefits generated by 5 % v/v P-CM therapy were considerably larger than those found in placebo-treated locations at 4, 8, and 16 weeks. The current study provides the framework for subsequent research, which will require a greater number of participants to corroborate the clinical data’s inherent limitations. The results obtained show that MIF is essential for P-CM effects and acts as a critical regulator of the hair growth-related protein, VEGF, in DPCs. The -catenin and p-GSK-3 [SER9] signaling pathways facilitate this connection. These findings imply that P-CM can prevent hair loss and may be a viable treatment medication for hair restoration. Furthermore, the pilot clinical study findings indicate that P-CM produced from MSCs is a safe and efficient therapy for androgenetic alopecia. Emerging and future MSC-based combination therapies may usher in a new era in the treatment of androgenetic alopecia [38].
Dahbour et al. report safely injecting the largest recorded dosage of autologous MSCs into MS patients through the intrathecal route in this experiment. For the first time in any human neurological illness, a cellular therapy approach was applied in this investigation. There were no significant adverse effects recorded following BMSC injection, with temporary localized discomfort and headache being the most prevalent symptoms. MSCs were provided promptly after growth in this investigation to prevent cell death caused by cryopreservation [39].
It was not able to obtain statistical significance and make definitive findings due to the limited number and variability of included patients in terms of MS type, illness duration, and baseline Expanded Disability Status Scale (EDSS) score. A tendency of lower EDSS among individuals who failed initial first- or second-line treatment, with a rate of 60 % stability or improvement, is favorable. The EDSS of two individuals decreased dramatically.
Furthermore, while some patients improved in these outcome measures, their overall rating remained unchanged or worse. This could be due to (i) the heterogeneity of the patients who participated in terms of MS type, regions of concern, and magnitude, (ii) the different homing and therapeutic capacity of the MSCs administered, and (iii) the differing makeup of MSC-CM and the potential it has to reverse various disease aspects examined in this research [39].
In conclusion, both BMSC and MSC-CM are safe and effective at regulating illness and reversing symptoms. The findings motivate us and others to do bigger clinical trials to more accurately assess the efficacy of MSCs and their MSC-CM, both together and separately. Research like this one, which looks at the cellular components utilized to treat MS, is essential for comprehending the various reactions in patients and improving results.
A disadvantage of this cellular treatment for MS is the absence of a control group, which is owing to the clinical and pathological heterogeneity of MS patients, makes it impossible to locate a matched group [39].
Discussion
Recent research has shown that stem cells are effective in tissue mostly through the paracrine impact. The chemicals released by stem cells play an important function in modulating cross-talk interactions between the cells and the surrounding tissues [9,49]. Based on the paracrine signaling mechanism, MSC-CM, also known as the MSC secretome, is a rich source of paracrine factors and is being studied extensively for a variety of regenerative therapies including bone regeneration, stroke, myocardial infarction, hair growth, etc.
The use of CM in tissue repair has been shown to alleviate problems found during stem cell treatment, such as transplantation rejection and hyperimmuno-genicity. Because MSC-CM-based therapy is still in its initial phases, a detailed analysis of the culture conditions, selection of suitable stem cell sources, evaluation of the number of paracrine factors, establishing a suitable dose, and addressing safety issues related to the use of MSC-CM has yet to be completed. Such research would confirm the significance of stem cell CM as a potential option in regenerative medicine.
This new area of study already claims many significant benefits over cell-based applications: (a) uses protein administration rather than entire cell administration as a new treatment alternative in regenerative medicine (b) CM may be preserved for an extended length of time without the need of hazardous cryopreservatives such as DMSO (c) CM preparation is more cost-effective since it can be mass-produced from accessible MSC populations under cGMP conditions (d) CM safety and efficacy evaluation will be considerably easier and equivalent to traditional pharmaceutical drugs [50].
Lately, tissue engineering research has recommended the use of cell-free treatments due to the ability to circumvent the ethical difficulties associated with the use of entire cells. Furthermore, when implanted in vivo, MSCs have shown low survival [51–53]. MSCs, as previously documented, produce and release a variety of growth factors, proteins, and free nucleic acids into the CM. It was shown that PDLSC-derived CM includes a variety of cytokines, including interleukins and TGF-β. Exos and EVs in particular may play important roles in cell-free therapy [16,54,55]. MSCs release vesicles containing proteins, lipids, mRNA, microRNA, and cytokines. The materials within these vesicles are released into target cells, altering their activity and perhaps eliciting restorative processes [56]. Besides, because of their immunomodulatory properties, EVs are appropriate for both autologous and allogeneic treatments [57]. Finally, EVs are simple to obtain. They can be separated from MSCs of various sources.
To properly use MSC-CM for a given problem, the paracrine elements involved in correcting the sick state must be thoroughly recognized. It should be emphasized that the optimal dose of the CM cocktail provided for different illnesses may differ since the homing signals of a sick organ may differ under different clinical settings. Furthermore, depending on the illness situation, the CM delivery mechanism must be adjusted. Aside from all of the above, the appropriate stem cell source that secretes the paracrine factors necessary for the individual disorders should be identified [58,59]. It is also vital to examine the modification of paracrine factor secretion of each individual in terms of the secretion of the concentration of paracrine factors to the medium when grown ex vivo. Information acquired from in vitro research on optimum treatment doses may be utilized to identify the personal responsibility of each individual to the supplemented MSC-CM and will be a necessity to further build a treatment strategy. Appropriate modulation of the secretion levels of paracrine substances can improve the efficacy of a certain therapy. Because of the short half-lives and the consumption of paracrine components within the body upon delivery, the dosage and frequency of CM must be carefully determined [29,60].
The use of MSC-CM in regenerative medicine is in its early stages. To investigate the influence of MSC-CM on the regeneration process, appropriate animal models must be constructed. The key question that has to be answered when adopting MSC-CM-based treatment is its safety and therapeutic effectiveness validation in humans. According to research, treating tumor cells with human MSC-CM has the same potential for triggering tumor development as using stem cells. The study also demonstrated that the existence of stem cells in the body is not essential for MSC-mediated tumor development. The ineffectiveness of ultracentrifugation in removing tumorigenic effects suggests that the soluble chemicals present in CM spread tumor activity via a paracrine mechanism [61]. Additional research is needed to solve this issue.
Thanks to so many MSC-based clinical studies approved by national authorities, gaining regulatory approval for cell-free treatment using MSC-CM should be quite simple. Yet various difficulties with MSC-CM must be resolved at this time. Some of these include a lack of cGMP-compliant methods for MSC secretome production, storage, product shelf-life, product stability, and quality control parameters, all of which are required to prove the safety and efficacy of MSC-CM. Further investigations concentrating on the ideal protein content of the dosage, administration frequency, and injection volume might add to the therapeutic effectiveness of MSC-CM.
Acknowledgements
This publication is the result of the project implementation CEMBAM—Centre for Medical Bio-Additive Manufacturing and Research, supported by the Operational Programme Integrated Infrastructure funded by the European Regional Development Fund [ITMS2014+: 313011V358].
Footnotes
Conflict of interest
There is no conflict of interest.
References
- 1.Daley GQ. Stem cells and the evolving notion of cellular identity. Philos Trans R Soc B Biol Sci. 2015:370. doi: 10.1098/rstb.2014.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dulak J, Szade K, Szade A, Nowak W, Józkowicz A. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62:329–337. doi: 10.18388/abp.2015_1023. [DOI] [PubMed] [Google Scholar]
- 3.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the Global Burden of Periodontal Diseases on Health, Nutrition and Wellbeing of Mankind: a Call for Global Action Maurizio. J Clin Periodontol. 2017;38:42–49. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- 4.Adler CJ, Dobney K, Weyrich LS, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45:450–455. doi: 10.1038/ng.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 6.Galipeau J, Krampera M, Barrett J, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18(2):151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y, Wang X, Xu C, et al. Xenotransplantation of Long-Term-Cultured Swine Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells. 2007;25:612–620. doi: 10.1634/stemcells.2006-0168. [DOI] [PubMed] [Google Scholar]
- 8.Von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 9.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:1–14. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessio N, Özcan S, Tatsumi K, et al. The secretome of MUSE cells contains factors that may play a role in regulation of stemness, apoptosis and immunomodulation. Cell Cycle. 2017;16:33–44. doi: 10.1080/15384101.2016.1211215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kološa K, Motaln H, Herold-Mende C, Koršič M, Lah TT. Paracrine effects of mesenchymal stem cells induce senescence and differentiation of glioblastoma stem-like cells. Cell Transplant. 2015;24:631–644. doi: 10.3727/096368915X687787. [DOI] [PubMed] [Google Scholar]
- 12.Maguire G. Stem cell therapy without the cells. Commun Integr Biol. 2013;6:1–3. doi: 10.4161/cib.26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng - Part A. 2012;18:1479–1489. doi: 10.1089/ten.tea.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pawitan JA. Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Res Int. 2014;2014:7–9. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: Can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies. Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 16.LPK, Kandoi S, Misra R, Vijayalakshmi S, Rajagopal K. Cytokine and Growth Factor Reviews The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Kupcova Skalnikova H. Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie. 2013;95:2196–2211. doi: 10.1016/j.biochi.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Chuang TJ, Lin KC, Chio CC, Wang CC, Chang CP, Kuo JR. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J Trauma Acute Care Surg. 2012;73:1161–1167. doi: 10.1097/TA.0b013e318265d128. [DOI] [PubMed] [Google Scholar]
- 19.Eiró N, Sendon-Lago J, Seoane S, et al. Potential therapeutic effect of the secretome from human uterine cervical stem cells against both cancer and stromal cells compared with adipose tissue stem cells. Oncotarget. 2014;5:10692–10708. doi: 10.18632/oncotarget.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assoni A, Coatti G, Valadares MC, et al. Different Donors Mesenchymal Stromal Cells Secretomes Reveal Heterogeneous Profile of Relevance for Therapeutic Use. Stem Cells Dev. 2017;26:206–214. doi: 10.1089/scd.2016.0218. [DOI] [PubMed] [Google Scholar]
- 21.Vieira NM, Zucconi E, Bueno CR, et al. Human Multipotent Mesenchymal Stromal Cells from Distinct Sources Show Different In Vivo Potential to Differentiate into Muscle Cells When Injected in Dystrophic Mice. Stem Cell Rev Reports. 2010;6:560–566. doi: 10.1007/s12015-010-9187-5. [DOI] [PubMed] [Google Scholar]
- 22.Pires AO, Mendes-Pinheiro B, Teixeira FG, et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue derived Stem Cells and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016;1:1–37. doi: 10.1089/scd.2016.0048. [DOI] [PubMed] [Google Scholar]
- 23.Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95:2212–2221. doi: 10.1016/j.biochi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Dabrowski FA, Burdzinska A, Kulesza A, et al. Comparison of the paracrine activity of mesenchymal stem cells derived from human umbilical cord, amniotic membrane and adipose tissue. J Obstet Gynaecol Res. 2017;43:1758–1768. doi: 10.1111/jog.13432. [DOI] [PubMed] [Google Scholar]
- 25.Rocha B, Calamia V, Blanco FJ, Ruiz-Romero C. Identification of factors produced and secreted by mesenchymal stromal cells with the SILAC method. Methods Mol Biol. 2016;1416:551–565. doi: 10.1007/978-1-4939-3584-0_33. [DOI] [PubMed] [Google Scholar]
- 26.Riis S, Stensballe A, Emmersen J, et al. Mass spectrometry analysis of adipose-derived stem cells reveals a significant effect of hypoxia on pathways regulating extracellular matrix. Stem Cell Res Ther. 2016;7:1–14. doi: 10.1186/s13287-016-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashiba K, Terunuma A, Terunuma H, et al. Immortalized mesenchymal stem cells producing conditioned medium in a large scale for therapeutic usage. Inflamm Regen. 2015;35:057–060. doi: 10.2492/inflammregen.35.057. [DOI] [Google Scholar]
- 28.Panchalingam KM, Jung S, Rosenberg L, Behie LA. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review Mesenchymal Stem/Stromal Cells - An update. Stem Cell Res Ther. 2015;6:1–10. doi: 10.1186/s13287-015-0228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teixeira FG, Panchalingam KM, Assunção-Silva R, et al. Modulation of the Mesenchymal Stem Cell Secretome Using Computer-Controlled Bioreactors: Impact on Neuronal Cell Proliferation, Survival and Differentiation. Sci Rep. 2016;6:1–14. doi: 10.1038/srep27791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leeman KT, Pessina P, Lee J, Kim CF. Mesenchymal stem cells increase alveolar differentiation in lung progenitor organoid cultures. Sci Rep. 2019:1–10. doi: 10.1038/s41598-019-42819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeuchi R, Katagiri W, Endo S, Kobayashi T. Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis. 2019:1–19. doi: 10.1371/journal.pone.0225472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeGregorio C, Contador D, Díaz D, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. 2020;0:1–21. doi: 10.1186/s13287-020-01680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Cui Y, Song J, et al. Mesenchymal stem cell-conditioned medium improved mitochondrial function and alleviated inflammation and apoptosis in non-alcoholic fatty liver disease by regulating SIRT1. Biochem Biophys Res Commun. 2021;546:74–82. doi: 10.1016/j.bbrc.2021.01.098. [DOI] [PubMed] [Google Scholar]
- 34.Dorronsoro A, Santiago FE, Grassi D, et al. Mesenchymal stem cell- - derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. 2021:1–14. doi: 10.1111/acel.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun X, Li K, Zha R, et al. Preventing tumor progression to the bone by induced tumor-suppressing. MSCs. 2021:11. doi: 10.7150/thno.58779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrell CR, Miloradovic D, Sadikot R, et al. Molecular and cellular mechanisms responsible for beneficial effects of mesenchymal stem cell-derived product “Exo-d-MAPPS” in attenuation of chronic airway inflammation. 2020:2020. doi: 10.1155/2020/3153891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Huang S, Wu S, et al. Medicine Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine. 2019;45:341–350. doi: 10.1016/j.ebiom.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh HA, Kwak J, Kim BJ, et al. Migration inhibitory factor in conditioned medium from human umbilical cord blood-derived mesenchymal stromal cells stimulates hair growth. 2020 doi: 10.3390/cells9061344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dahbour S, Jamali F, Alhattab D, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. 2017:866–874. doi: 10.1111/cns.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Bhang DH, Beede A, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia preconditioned human adipose derived mesenchymal stem cells enhance angiogenic potential via secretion of increased VEGF and bFGF. Cell Biol Int. 2013;37:551–560. doi: 10.1002/cbin.10097. [DOI] [PubMed] [Google Scholar]
- 42.Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res. 2013;4:76–88. doi: 10.1007/s12975-012-0251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oses C, Olivares B, Ezquer M, et al. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS One. 2017;12:1–22. doi: 10.1371/journal.pone.0178011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boucek P. Advanced diabetic neuropathy: a point of no return? Rev Diabet Stud. 2006;3:143–143. doi: 10.1900/RDS.2006.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howland MA. Risks of parenteral deferoxamine for acute iron poisoning. Clin Toxicol. 1996;34:491–497. doi: 10.3109/15563659609028006. [DOI] [PubMed] [Google Scholar]
- 46.Pathania D, Millard M, Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev. 2009;61:1250–1275. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Metab Med Surg. 2014;50:115–132. doi: 10.1201/b17616. [DOI] [PubMed] [Google Scholar]
- 48.Lavasani M, Robinson AR, Lu A, et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun. 2012;3:608–612. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28:329–343. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 50.Bermudez MA, Sendon-Lago J, Eiro N, et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Investig Ophthalmol Vis Sci. 2015;56:983–992. doi: 10.1167/iovs.14-15859. [DOI] [PubMed] [Google Scholar]
- 51.Manassero M, Paquet J, Deschepper M, Viateau V, Retortillo J, Bensidhoum M, Logeart-Avramoglou D, Petite H. Comparison of survival and osteogenic ability of human mesenchymal stem cells in orthotopic and ectopic sites in mice. Tissue Eng Part A. 2016;22:534–544. doi: 10.1089/ten.TEA.2015.0346. [DOI] [PubMed] [Google Scholar]
- 52.Giannoni P, Scaglione S, Daga A, Ilengo C, Cilli M, Quarto R. Short-time survival and engraftment of bone marrow stromal cells in an ectopic model of bone regeneration. Tissue Eng - Part A. 2010;16:489–499. doi: 10.1089/ten.tea.2009.0041. [DOI] [PubMed] [Google Scholar]
- 53.Becquart P, Cambon-Binder A, Monfoulet LE, et al. Ischemia is the prime but not the only cause of human multipotent stromal cell death in tissue-engineered constructs in vivo. Tissue Eng - Part A. 2012;18:2084–2094. doi: 10.1089/ten.tea.2011.0690. [DOI] [PubMed] [Google Scholar]
- 54.Bogatcheva NV, Coleman ME. Conditioned medium of mesenchymal stromal cells: a new class of therapeutics. Biochem. 2019;84:1375–1389. doi: 10.1134/S0006297919110129. [DOI] [PubMed] [Google Scholar]
- 55.Nagata M, Iwasaki K, Akazawa K, et al. Conditioned medium from periodontal ligament stem cells enhances periodontal regeneration. Tissue Eng - Part A. 2017;23:367–377. doi: 10.1089/ten.tea.2016.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nawaz M, Fatima F, Vallabhaneni KC, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016:2016. doi: 10.1155/2016/1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trubiani O, Marconi GD, Pierdomenico SD, Piattelli A, Diomede F, Pizzicannella J. Human oral stem cells, biomaterials and extracellular vesicles: A promising tool in bone tissue repair. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20204987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makridakis M, Roubelakis MG, Vlahou A. Stem cells: Insights into the secretome. Biochim Biophys Acta - Proteins Proteomics. 2013;1834:2380–2384. doi: 10.1016/j.bbapap.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 59.Perin EC, Geng YJ, Willerson JT. Adult stem cell therapy in perspective. Circulation. 2003;107:935–938. doi: 10.1161/01.CIR.0000057526.10455.BD. [DOI] [PubMed] [Google Scholar]
- 60.Khosravi A, Cutler CM, Kelly MH, et al. Determination of the elimination half-life of fibroblast growth factor-23. J Clin Endocrinol Metab. 2007;92:2374–2377. doi: 10.1210/jc.2006-2865. [DOI] [PubMed] [Google Scholar]
- 61.Zhu W, Huang L, Li Y, et al. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10:3198–3207. doi: 10.4161/cc.10.18.17638. [DOI] [PubMed] [Google Scholar]