Summary
Despite the rapid progress in the field of personalized medicine and the efforts to apply specific treatment strategies to patients based on the presence of pathogenic variants in one, two, or three genes, patient response to the treatment in terms of positive benefit and overall survival remains heterogeneous. However, advances in sequencing and bioinformatics technologies have facilitated the simultaneous examination of somatic variants in tens to thousands of genes in tumor tissue, enabling the determination of personalized management based on the patient’s comprehensive genomic profile (CGP). CGP has the potential to enhance clinical decision-making and personalize innovative treatments for individual patients, by providing oncologists with a more comprehensive molecular characterization of tumors. This study aimed to highlight the utility of CGP in routine clinical practice. Here we present three patient cases with various advanced cancer indicated for CGP analysis using a combination of SOPHiA Solid Tumor Solution (STS, 42 genes) for DNA and SOPHiA RNAtarget Oncology Solution (ROS, 45 genes and 17 gene fusions with any random partners) for RNA. We were able to identify actionable genomic alterations in all three cases, thereby presenting valuable information for future management of these patients. This approach has the potential to transform clinical practice and greatly improve patient outcomes in the field of oncology.
Key words: Comprehensive genomic profile, Personalized medicine
Introduction
Personalized medicine (PM) is a medical model of care which allows for the customization of health care, therapy, and diagnostic procedures taking into account the molecular basis of the disease. This model often uses laboratory testing focused on the selection of appropriate and optimal therapies based on comprehensive molecular and genetic analysis. One of the most important aspects of PM in the field of oncology is the identification of suitable molecular biomarkers that can be used for the estimation of prognosis and prediction of targets for innovative biological therapy [1–3].
Information about the molecular characteristics of individual tumors helps oncologists supplement traditional methods such as tissue localization and tumor histology. PM is in current clinical practice predominantly used in the form of single gene-based assays. However, this approach is being gradually substituted by next-generation sequencing (NGS) [4]. Analysis of tumor DNA using NGS allows for the creation of a comprehensive genomic profile (CGP) through the simultaneous evaluation of tens to thousands of genes at once. The use of a comprehensive gene panel has several advantages over analyzing individual genes as it enables simultaneous detection of all classes of genomic alterations known to drive malignant growth such as single nucleotide variants (SNV), insertions and deletions (indels), copy number variations (CNV), gene rearrangements and fusions [5]. Additionally, there are also more complex biomarkers called genomic signatures, which include microsatellite instability (MSI status) and tumor mutational burden (TMB), that hold potential as valuable instruments in the immunotherapy of cancer patients [6,7].
The integration of PM and biomarker-guided therapy has instigated a paradigm shift in oncology, necessitating the adoption of CGP in routine clinical practice. Furthermore, CGP also offers a more efficient approach by utilizing often small amounts of available biopsy tissue, detecting gene fusions with unknown partners, helping to identify carcinomas of unknown primary, and facilitating the differential diagnosis of various tumors [8–10]. This approach provides oncologists with a comprehensive genomic profile of a patient’s tumor, enhancing their ability to identify targeted therapies that offer a higher likelihood of success with fewer adverse effects.
This trend was recently acknowledged by the European Society for Medical Oncology (ESMO) in their 2020 recommendations regarding the use of NGS for identifying biomarkers in patients with metastatic cancer [11]. ESMO recommends the use of NGS for patients with various types of cancer including non-small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma. Additionally, NGS is recommended for determining TMB in patients with cervical cancer, salivary gland cancer, thyroid cancer, vulvar cancer, and neuroendocrine tumors. In the case of colorectal cancer, NGS may serve as a cost-effective alternative to PCR-based tests. ESMO also recommends and emphasizes the significance of multigene sequencing for clinical facilities as part of streamlining drug research and development [11]. The American Society of Clinical Oncology (ASCO) similarly recommends the use of NGS for identifying biomarkers in all patients whose cancer has at least one specific genetic alteration with regulatory approval for the use or exclusion of a particular drug. ASCO emphasizes the importance of genomic testing for all tumors, with an increasing number of tissue-agnostic drugs approved for cancers with high TMB, microsatellite instability-high (MSI-H), or neurotrophic tropomyosin receptor kinase (NTRK) fusions [12]. Therefore this research aimed to emphasize the importance and applicability of CGP in modern clinical practice.
Methods
Study design
During the period between 1. 9. 2020 to 18. 5. 2023, we conducted a thorough analysis of a cohort comprising 295 patients who were referred for CGP testing to determine their future treatment, prognosis, and further management as part of their personalized care. For the purpose of this study, we selected three unique and interesting cases. Molecular testing was performed at the Department of Medical Genetics of St. Elizabeth Cancer Institute in Bratislava as part of the standard clinical practice. All experiments conformed to the Helsinki Declaration, and all patients provided written informed consent.
Nucleic acid extraction
Nucleic acids required for analysis were extracted from tumor tissue in the form of formalin-fixed paraffin-embedded (FFPE) sections obtained following surgical resection of the tumor. All FFPE samples were reviewed by a pathologist to identify tumor-rich regions. Commercially available FFPE tissue kits – Maxwell® FFPE Plus DNA Kit (Promega) and Maxwell® RSC RNA FFPE Kit (Promega) – were used for nucleic acid isolation. The isolation was performed using the Maxwell® RSC Instrument (Promega) which employs paramagnetic beads for efficient isolation. The concentration of nucleic acids was determined using a Quantus fluorometer (Promega), with a concentration of nucleic acids ≥40 ng/μl being deemed suitable for further analysis.
Comprehensive genomic profiling
CGP analysis was in this study performed by a combination of two multi-gene NGS panels – SOPHiA Solid Tumor Solution (STS) and SOPHiA RNAtarget Oncology Solution (ROS). Both are hybridization capture-based NGS assays targeting genomic alteration involved in the most common solid tumors. STS targets 42 genes in DNA, 6 unique loci to detect MSI status, and gene amplification events in 24 genes. ROS covers transcripts of 45 genes at the RNA level and 17 RNA fusion genes in combination with any random fusion partner. Even though there is a 30-gene overlap between the two panels, we use the ROS gene panel in addition to the STS panel mainly due to its capability of detecting RNA gene fusions with any unknown partner.
To prepare DNA and RNA libraries from fixed FFPE tissue, we utilized the SOPHiA Solid Tumor Solution and SOPHiA RNAtarget Oncology Solution, following the manufacturer’s instructions. Firstly, we transcribed RNA into cDNA using random hexamers. Subsequently, we enzymatically fragmented the DNA into 90 to 250 bp, repaired them by adding adenine to the 3’ end, and labeled them with adapter molecules. Then, we purified libraries of unligated adapters and added universal index sequences to the fragments using universal index primers (UP1–12) to distinguish the samples in the subsequent multiplex sequencing reaction during demultiplexing. Following this, we hybridized overnight a set of oligonucleotide sequences targeting genomic alterations of interest to the libraries prepared in the previous steps. After hybridization, we employed streptavidin-coated magnetic beads to capture the hybridized probes firmly bound to the targeted sequences of DNA and RNA libraries. We washed the beads twice to remove unhybridized nonspecific sequences and eluted the enriched library from the magnetic beads. We repeated the hybridization, capture, and enrichment steps once more to ensure maximum specificity. Subsequently, we amplified and purified the enriched libraries with cleaning beads. Following this step, we quantified the libraries using fluorescence measurement, determined library quality and fragment size using a 2100 Bioanalyzer (Agilent), and normalized the libraries to ensure uniform representation during the analysis of multiple samples simultaneously. Finally, we denatured and diluted the libraries to the final sequencing concentration and sequenced them on a NextSeq 550 analyzer (Illumina).
Bioinformatic data analysis
The data obtained in FASTQ format were analyzed using CGW SOPHiA DDM software (Sophia Genetics).
Classification of the detected variants
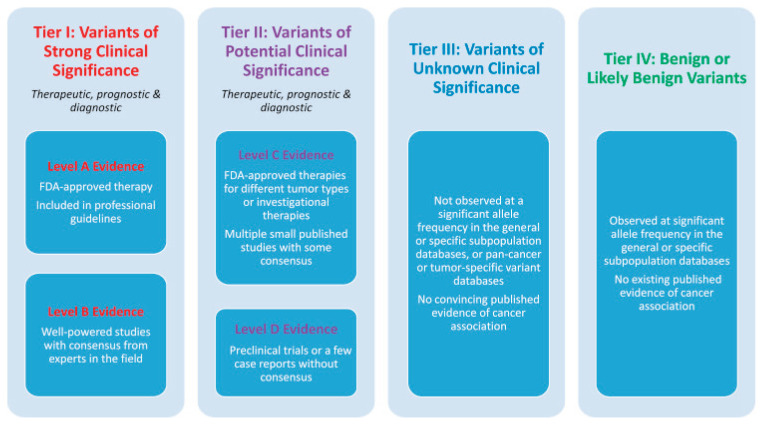
The detected variants were described according to HGVS (Human Genome Variation Society) [13]. The pathogenicity of the variants was classified based on valid ACMG criteria [14], and the clinical significance was defined according to TIER criteria [15]. The interpretation of variants is based on data published in databases and scientific publications and is conducted following current ACMG and TIER criteria. The results do not include benign variants (Class 1), likely benign variants (Class 2), and variants with unknown significance (Class 3) according to the ACMG criteria. Likewise, variants with unknown clinical significance (Class III) and benign/likely benign variants (Class IV) according to the TIER criteria (Fig. 1) are not included in the results but are part of the laboratory protocol. The results are valid only within the limits of the detection methods used.
Fig. 1.
Categorization of somatic variants based on their clinical significance. The figure presents an evidence-based categorization of somatic variants according to their clinical significance in cancer diagnosis, prognosis, and therapeutics. The classification system is structured into four tiers, each indicating different levels of clinical importance. Variants in tier I are of the strongest clinical significance, and variants in tier IV are benign or likely benign variants [15].
Results
As part of personalized care, patients referred for comprehensive genomic profiling (CGP) testing underwent a molecular genetic analysis using a panel of genes to aid in determining their future treatment, prognosis, and management. For this study, we identified and analyzed three intriguing case studies, including rare cases. The cohort consisted of patients with a malignant brain tumor, axillary metastases with the suspected origin of high-grade serous ovarian carcinoma, and unspecified fibromatosis.
Case 1
A 69-year-old male patient with an unspecified malignant brain tumor was referred for CGP analysis with the primary objective of identifying potential treatment options and optimizing his future clinical management. NGS of DNA and RNA obtained from tumor tissue was performed to identify possible actionable genomic alterations.
In the sample of DNA isolated from tumor tissue, we detected the presence of a somatic variant c.-146C>T in the TERT gene. The variant has unknown significance according to internationally accepted criteria (ACMG) and a potential clinical significance of IID (TIER) (Table 1). The somatic variant c.-146C>T (sometimes referred to as C250T) identified in the DNA sample is located in the promoter region of the TERT gene and was present in the tumor DNA sample at a frequency of 19.5 %. Interestingly, we also concurrently identified the presence of FGFR3-TACC3 (F3T3) gene fusion in the RNA sample extracted from the tumor tissue. Applying the internationally accepted ACMG criteria, we classified this genomic alteration as pathogenic. According to the TIER criteria, we assigned this variant a classification of having potential clinical significance (IIC). Furthermore, the sample was MSS (microsatellite stable) and no somatic CNVs were detected.
Table 1.
Detected somatic variant in the DNA sample of tumor tissue (case 1).
| Gene | LRG sequence | Reference sequence | HGVS nomenclature | Depth | VAF | TIER classification | ACMG classification |
|---|---|---|---|---|---|---|---|
| TERT | LRG_343t1 | NM_198253.2 | c.-146C>T p.(C250T) |
1681 | 19.5 % | IID | Variant of unknown significance |
LRG, Locus Reference Genomic; HGVS, Human Genome Variation Society; VAF, Variant allele frequency.
Case 2
A 62-year-old female patient presented with axillary metastases and suspected high-grade serous ovarian carcinoma (HGSOC). The primary objective of the patient’s referral for CGP analysis was to assess potential treatment options with TRK inhibitors or PARP inhibitors, based on the identification of NTRK fusions and any potential pathogenic variants within the BRCA1/BRCA2 genes respectively. NGS of DNA and RNA obtained from the patient’s tumor tissue was performed to identify actionable genomic alterations.
In the sample of DNA isolated from tumor tissue, we were able to detect somatic pathogenic variant c.595G>T in the TP53 gene according to internationally accepted criteria (ACMG) and with a potential clinical significance of IIC (TIER). The somatic variant c.595G>T analyzed from the DNA sample of tumor tissue is located in exon 6 of the TP53 gene. This variant was present in the tumor DNA sample at a frequency of 9.4 %. Due to its presence, a reading frame shift occurs, and a premature STOP codon is incorporated, which is likely to lead to the formation of a non-functional or truncated protein. The variant is classified as pathogenic in the ClinVar database and was detected in lung, breast, gastrointestinal, pancreatic, and esophageal cancers in the somatic variant database Cosmic. Classification software, such as Varsome and Franklin, classify it as pathogenic with potential clinical significance. Based on internationally accepted ACMG criteria, we classify this variant as pathogenic. According to TIER criteria, we classify variant c.595G>T as a variant with potential clinical significance (IIC).
In the sample of RNA isolated from tumor tissue, we were able to detect the same variant (c.595G>T in the TP53 gene) and also another somatic pathogenic variant c.3018_3021del in the BRCA1 gene. The somatic variant c.3018_3021del is located in exon 11 of the BRCA1 gene. This variant was present in the tumor RNA sample at a frequency of 58.0 % (Table 2). Due to its presence, a reading frame shift occurs, and a premature STOP codon is incorporated, which is likely to lead to the formation of a non-functional or truncated protein. The variant is classified as pathogenic in the ClinVar database. Classification software, such as Varsome and Franklin, classify it as pathogenic with strong clinical significance. Based on internationally accepted ACMG criteria, we classify this variant as pathogenic. According to TIER criteria, we classify variant c.3018_3021 as a variant with strong clinical significance (IA). The analyzed sample was MSS and no somatic CNVs were detected.
Table 2.
Detected somatic variants in samples of tumor tissue isolated from DNA or RNA (case 2).
| Gene (DNA/RNA) | LRG sequence | Reference sequence | HGVS nomenclature | Depth | VAF | TIER classification | ACMG classification |
|---|---|---|---|---|---|---|---|
| TP53 (DNA) | LRG_321 | NM_000546 | c.595G>T p.(Gly199*) |
3318 | 9.4 % | IIC | Pathogenic variant |
| TP53 (RNA) | LRG_321 | NM_000546 | c.595G>T p.(Gly199*) |
4359 | 9.1 % | IIC | Pathogenic variant |
| BRCA1 (RNA) | LRG_292 | NM_007294 | c.3018_3021del p.(His1006Glnfs*17) |
1205 | 58.0 % | IA | Pathogenic variant |
LRG, Locus Reference Genomic; HGVS, Human Genome Variation Society; VAF, Variant allele frequency.
Case 3
A 61-year-old female patient with unspecified fibromatosis in the thigh region was referred for CGP analysis with the primary objective of this analysis being evaluation of the CTNNB1 gene for differential diagnosis of desmoid tumors. NGS of DNA and RNA obtained from tumor tissue was performed to identify possible actionable genomic alterations.
In the sample of DNA and RNA isolated from tumor tissue, we were able to detect likely pathogenic somatic variant c.133T>C in the CTNNB1 gene according to internationally accepted criteria (ACMG) with a potential clinical significance of IIC (TIER). The somatic variant c.133T>C identified from the DNA sample of tumor tissue is located in exon 3 of the CTNNB1 gene. This variant was present in the tumor DNA sample at a frequency of 20.8 % (Table 3). The variant c.133T>C is described in the ClinVar database as pathogenic (3×) and as likely pathogenic (11×). It is also described in the somatic variant database Cosmic in patients with liver carcinoma, soft tissue tumors, adrenal gland, kidney, and colon cancer. The classification software Varsome classifies it as a likely pathogenic variant with potential clinical significance (IIC). According to TIER criteria, we classify the variant as having potential clinical significance (IIC). The examined sample was MSS and no somatic CNVs were identified.
Table 3.
Detected somatic variant in the DNA sample of tumor tissue (case 3).
| Gene | LRG sequence | Reference sequence | HGVS nomenclature | Depth | VAF | TIER classification | ACMG classification |
|---|---|---|---|---|---|---|---|
| CTNNB1 | LRG_1108t1 | NM_001904.4 | c.133T>C p.(S45P) |
4300 | 20.8 % | IIC | Likely pathogenic variant |
LRG, Locus Reference Genomic; HGVS, Human Genome Variation Society; VAF, Variant allele frequency.
Discussion
Personalized medicine based on patients’ actionable genomic alterations is a promising and rapidly advancing area in the field of modern oncology. The development of targeted biological drugs tailored against specific mutations in tumors is slowly replacing nonselective conventional standard therapies. The proven clinical benefits associated with certain specific targeted therapies, the rapidly increasing number of actionable biomarkers, and the declining cost of comprehensive genomic profiling (CGP) have resulted in a greater demand for these types of assays.
In this study, we describe our experience with the clinical use of CGP through three patient case studies. The first case involved the identification of a somatic variant c.-146C>T in the TERT gene (chromosome 5) promoter, a hotspot mutation commonly found in oligodendro-gliomas with 1p and 19q deletions, as well as in most glioblastomas. In the scientific literature, the occurrence of this variant has been described in patients with brain cancer (among others, also in patients with thyroid cancer and bladder cancer) [16–18]. In diffuse infiltrating gliomas, the presence of this mutation, in the absence of IDH mutations, is associated with shorter overall patient survival [19]. Arita et al. demonstrated the clinical significance of testing the combination of TERT mutation and MGMT (O(6)-methylguanine-DNA methyltransferase) methylation status in glioblastoma patients. TERT mutated-MGMT unmethylated tumors were associated with the poorest prognosis, highlighting TERT as a potential therapeutic target. Integrating TERT mutation as an additional biomarker enhances diagnostic accuracy, prognostication, and treatment selection for glioma patients, particularly for those with TERT-mutated and MGMT-unmethylated glioblastoma who have limited response to standard treatments [20].
Furthermore, we identified a FGFR3-TACC3 (F3T3) gene fusion in this patient through the RNA portion of the CGP analysis. This gene fusion is listed in the ChimerDB fusion gene database and has been previously reported in patients with brain cancer, as well as in patients with bladder cancer and non-small cell lung cancer. The same fusion was identified in a 2020 study by Di Stefano et al. [21], which showed that patients with IDH wild-type glioblastoma and the F3T3 fusion had better response and overall survival with standard radiochemotherapy than patients without the fusion. The study conducted by Di Stefano et al. offers a thorough characterization of F3T3-positive gliomas, highlighting their unique molecular, radiological, and clinical attributes in comparison to IDH wild type gliomas. The results of their findings suggest that F3T3-positive gliomas should be recognized as a distinct subgroup of brain tumors, warranting specialized approaches for accurate diagnosis, prognostication, and therapeutic interventions. Patients with metastatic urothelial carcinoma and FGFR3 alterations may also benefit from treatment with FGFR tyrosine kinase inhibitors (Erdafitinib) after progression on platinum-based treatment [22]. The literature even describes a very specific case of a patient with papillary glioneuronal tumor (IDH wild-type, F3T3 fusion, TERT promoter mutation c.-124C>T (C228T)), who was treated with FGFR tyrosine kinase inhibitor (Erdafitinib) after disease progression following the third resection, but without observed response to the treatment [23]. F3T3 fusions demonstrate increased sensitivity to FGFR-targeted therapies compared to other FGFR aberrations. This finding carries clinical significance, as patients with aggressive tumors such as glioblastoma and bladder cancer, where T3F3 fusions have been identified, have limited treatment options. Although F3T3 fusions are rare, they are present in a wide range of solid tumor types, emphasizing the importance of including their analysis in screening procedures for FGFR-targeted trials in solid tumors [24]. CGP analysis of this patient’s tumor identified important genomic alterations with potential for prognosis and use in future patient management.
In the case of a second patient with axillary metastases with suspected HGSOC origin, we conducted CGP to determine potential treatment options with TRK or PARP inhibitors based on the identification of NTRK fusions and any potential pathogenic variants within the BRCA1/BRCA2 genes respectively. In the sample of DNA and RNA isolated from tumor tissue, we were able to detect a c.595G>T variant in the TP53 gene. This variant is located in the DNA binding domain where approximately 80 % of all identified pathogenic TP53 variants are located, which is important for binding to AXIN1 [25]. TP53 is the most frequently mutated gene in human cancer, with a prevalence of 40–50 % across various cancer types. In HGSOC, TP53 mutations are even more prevalent, occurring in approximately 95 % of cases [26]. Wong et al. demonstrated that patients with TP53 wild-type HGSOC had poorer survival and increased chemoresistance compared to those with TP53 mutations [27]. Conversely, Ghezelayagh et al. found no overall survival benefit associated with TP53 mutations in HGSOC, but these mutations were linked to increased sensitivity to platinum-based chemotherapy [28]. However, arguably more important was the identification of another somatic variant c.3018_3021del in the BRCA1 gene. This variant was present in the tumor RNA sample at a frequency of 58.0 % which suggests the possibility that it could be a germline variant, and we recommend considering verification of its germline status by analyzing DNA from peripheral blood [29]. Patients with HGSOC, prostate, pancreatic, triple-negative breast cancer, and with pathogenic variants in the BRCA1 gene may benefit from treatment with PARP inhibitors.
The third presented case was a 61-year-old female patient with unspecified fibromatosis who was referred for CGP analysis with the primary objective of this analysis being evaluation of the CTNNB1 gene for differential diagnosis of desmoid tumors. The differential diagnosis of fibromatosis and desmoid tumors can be challenging because they are similar in their clinical presentation and histological features. However, there are some key differences that can aid in the diagnosis. Desmoid fibromatosis is a locally aggressive neoplasm that affects young to middle-aged adults and occasionally children. It can occur sporadically or as part of familial adenomatous polyposis (FAP) syndrome. The activation of the canonical Wnt/beta-catenin signaling pathway plays a key role in both situations. In sporadic cases, mutations occur in CTNNB1 with the most common types being point mutations involving phosphorylation sites encoded by exon 3, while in FAP, germline mutations occur in the APC tumor suppressor gene. Both mutations result in the accumulation of non-phosphorylated beta-catenin in the cytoplasm and its translocation to the nucleus, activating the transcription of genes that promote proliferation and increased cell survival [30]. In the sample of DNA and RNA isolated from tumor tissue, we were able to detect somatic variant c.133T>C located in exon 3 of the CTNNB1 gene. Approximately 85 % of patients with desmoid tumors have been identified with the presence of a somatic pathogenic (or likely pathogenic) variant in the CTNNB1 gene, with the c.133T>C variant occurring at a frequency of 8 % [31]. Desmoid tumors present a highly variable prognosis, characterized by an unpredictable disease course. Notably, approximately 20–30 % of patients experience spontaneous regressions within a 2 to 3-year monitoring period. Following an initial growth phase, these tumors often stabilize. However, it is important to acknowledge the high recurrence rate associated with desmoid tumors, which significantly diminishes the effectiveness of surgical intervention. Given the uncertain disease trajectory and the potential for spontaneous regressions, the current preferred management approach for patients with desmoid tumors is active surveillance, prioritizing close monitoring rather than immediate surgical intervention [32]. Furthermore, oral administration of Vinorelbine is an effective and well-tolerated therapy in patients with advanced or progressive desmoid fibromatosis, with prolonged activity observed in patients with tumors carrying the c.134C>T or c.133T>C variants in the CTNNB1 gene [33].
CGP has emerged as a potent tool that provides oncologists with a more comprehensive molecular characterization of tumors, enabling enhanced clinical decision-making and the personalization of innovative biological therapies for individual patients. Nevertheless, despite its potential benefits, CGP still presents challenges that require careful consideration. One such challenge pertains to the high initial cost associated with acquiring an NGS-capable genetic analyzer. Additionally, accurate classification of somatic variants necessitates the high expertise of molecular biologists and geneticists and requires competent personnel. In addition, it is essential to conduct further research to determine the optimal panel size for routine clinical practice. While complex genomic signatures such as TMB hold great promise in the field of personalized oncology, it necessitates the analysis of hundreds of genes. Therefore, it is crucial to consider, whether the potential benefits of analyzing these complex biomarkers outweigh the costs and practicality of using smaller gene panels in routine clinical practice. Furthermore, it is crucial to evaluate whether universal testing or targeted testing of specific patient groups would deliver the greatest benefit. This approach has the potential to transform clinical practice and greatly improve patient outcomes in the field of clinical oncology.
Conclusions
Comprehensive genomic profiling (CGP) in personalized medicine holds promise to improve clinical decision-making and enhance treatment outcomes for cancer patients. This study highlights three cases where CGP analysis successfully identified actionable genomic alterations, demonstrating the transformative impact of this approach on clinical practice and patient management in oncology.
Acknowledgements
We acknowledge the funding provided by VEGA-1/0405/22.
Footnotes
Conflict of interest
There is no conflict of interest.
References
- 1.Di Sanzo M, Cipolloni L, Borro M, La Russa R, Santurro A, Scopetti M, Simmaco M, et al. Clinical Applications of Personalized Medicine: A New Paradigm and Challenge. Curr Pharm Biotechnol. 2017;18:194–203. doi: 10.2174/1389201018666170224105600. [DOI] [PubMed] [Google Scholar]
- 2.Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018;109:952–963. doi: 10.1016/j.fertnstert.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambardella V, Tarazona N, Cejalvo JM, Lombardi P, Huerta M, Roselló S, Fleitas T, et al. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers (Basel) 2020;12:1009. doi: 10.3390/cancers12041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malone ER, Oliva M, Sabatini PJB, Stockley TL, Siu LL. Molecular profiling for precision cancer therapies. Genome Med. 2020;12:8. doi: 10.1186/s13073-019-0703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DM, Hackett JM, Plestina S. Unlocking Access to Broad Molecular Profiling: Benefits, Barriers, and Policy Solutions. Public Health Genomics. 2021;25:70–79. doi: 10.1159/000520000. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: A predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. 2018;26:e15–e21. doi: 10.1097/PAI.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 7.Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–1825. doi: 10.1158/2159-8290.CD-20-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penault-Llorca F, Kerr KM, Garrido P, Thunnissen E, Dequeker E, Normanno N, Patton S, et al. Expert opinion on NSCLC small specimen biomarker testing - Part 1: Tissue collection and management. Virchows Arch. 2022;481:335–350. doi: 10.1007/s00428-022-03343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross JS, Sokol ES, Moch H, Mileshkin L, Baciarello G, Losa F, Beringer A, et al. Comprehensive Genomic Profiling of Carcinoma of Unknown Primary Origin: Retrospective Molecular Classification Considering the CUPISCO Study Design. Oncologist. 2021;26:e394. doi: 10.1002/onco.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JS, Ali SM, Fasan O, Block J, Pal S, Elvin JA, Schrock A, et al. ALK Fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncologist. 2017;22:1444–1450. doi: 10.1634/theoncologist.2016-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, Normanno N, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31:1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarty D, Johnson A, Sklar J, Lindeman NI, Moore K, Ganesan S, Lovly C, et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. J Clin Oncol. 2022;40:1231–1258. doi: 10.1200/JCO.21.02767. [DOI] [PubMed] [Google Scholar]
- 13.den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, Roux A, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37:564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 14.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody W, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, Tsimberidou A, et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panebianco F, Nikitski AV, Nikiforova MN, Nikiforov YE. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med. 2019;8:5831–5839. doi: 10.1002/cam4.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan S, Liu X, Hua W, Xi M, Zhou Y, Wan Y. The role of telomerase reverse transcriptase (TERT) promoter mutations in prognosis in bladder cancer. Bioengineered. 2021;12:1495–1504. doi: 10.1080/21655979.2021.1915725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powter B, Jeffreys SA, Sareen H, Cooper A, Brungs D, Po J, et al. Human TERT promoter mutations as a prognostic biomarker in glioma. J Cancer Res Clin Oncol. 2021;147:1007–1017. doi: 10.1007/s00432-021-03536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H, Tanaka S, et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun. 2016;4:79. doi: 10.1186/s40478-016-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Stefano AL, Picca A, Saragoussi E, Bielle F, Ducray F, Villa C, Eoli M, et al. Clinical, molecular, and radiomic profile of gliomas with FGFR3-TACC3 fusions. Neuro Oncol. 2020;22:1614–1624. doi: 10.1093/neuonc/noaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, Necchi A, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:244–258. doi: 10.1016/j.annonc.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 23.McDonald MF, Athukuri P, Anand A, Gopakumar S, Jalali A, Patel AJ, Rao G, et al. Varied histomorphology and clinical outcomes of FGFR3-TACC3 fusion gliomas. Neurosurg Focus. 2022;53:E16. doi: 10.3171/2022.9.FOCUS22420. [DOI] [PubMed] [Google Scholar]
- 24.Costa R, Carneiro BA, Taxter T, Tavora FA, Kalyan A, Pai SA, Chae Y, et al. FGFR3-TACC3 fusion in solid tumors: mini review. Oncotarget. 2016;7:55924–55938. doi: 10.18632/oncotarget.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, Olivier M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum Mutat. 2016;37:865–876. doi: 10.1002/humu.23035. [DOI] [PubMed] [Google Scholar]
- 26.Shajani-Yi Z, de Abreu FB, Peterson JD, Tsongalis GJ. Frequency of somatic TP53 mutations in combination with known pathogenic mutations in colon adenocarcinoma, non-small cell lung carcinoma, and gliomas as identified by next-generation sequencing. Neoplasia. 2018;20:256–262. doi: 10.1016/j.neo.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong KK, Izaguirre DI, Kwan SY, King ER, Deavers MT, Sood AK, Mok S, et al. Poor survival with wild-type TP53 ovarian cancer? Gynecol Oncol. 2013;130:565–569. doi: 10.1016/j.ygyno.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghezelayagh TS, Pennington KP, Norquist BM, Khasnavis N, Radke MR, Kilgore MR, Garcia R, et al. Characterizing TP53 mutations in ovarian carcinomas with and without concurrent BRCA1 or BRCA2 mutations. Gynecol Oncol. 2021;160:786–792. doi: 10.1016/j.ygyno.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandelker D, Donoghue M, Talukdar S, Bandlamudi C, Srinivasan P, Vivek M, Jezdic S, et al. Germline-focussed analysis of tumour-only sequencing: recommendations from the ESMO Precision Medicine Working Group. Ann Oncol. 2019;30:1221–1231. doi: 10.1093/annonc/mdz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang WL, Nero C, Pappo A, Lev D, Lazar AJ, López-Terrada D. CTNNB1 genotyping and APC screening in pediatric desmoid tumors: a proposed algorithm. Pediatr Dev Pathol. 2012;15:361–367. doi: 10.2350/11-07-1064-OA.1. [DOI] [PubMed] [Google Scholar]
- 31.Lazar AJF, Tuvin D, Hajibashi S, Habeeb S, Bolshakov S, Mayordomo-Aranda E, Warneke C, et al. Specific Mutations in the β-Catenin Gene (CTNNB1) Correlate with Local Recurrence in Sporadic Desmoid Tumors. Am J Pathol. 2008;173:1518. doi: 10.2353/ajpath.2008.080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedel RF, Agulnik M. Evolving strategies for management of desmoid tumor. Cancer. 2022;128:3027–3040. doi: 10.1002/cncr.34332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mir O, Honoré C, Chamseddine AN, Dômont J, Dumont SN, Cavalcanti A, Faron M, et al. Long-term Outcomes of Oral Vinorelbine in Advanced, Progressive Desmoid Fibromatosis and Influence of CTNNB1 Mutational Status. Clin Cancer Res. 2020;26:6277–6283. doi: 10.1158/1078-0432.CCR-20-1847. [DOI] [PubMed] [Google Scholar]