Abstract
Lamina Associated Domains are large regions of heterochromatin positioned at the nuclear periphery. These domains have been implicated in gene repression, especially in the context of development. In mammals, LAD organization is dependent on nuclear lamins, inner nuclear membrane proteins, and chromatin state. In addition, chromatin readers and modifier proteins have been implicated in this organization, potentially serving as molecular tethers that interact with both nuclear envelope proteins and chromatin. More recent studies have focused on teasing apart the rules that govern dynamic LAD organization and how LAD organization, in turn, relates to gene regulation and overall 3D genome organization. This review highlights recent studies in mammalian cells uncovering factors that instruct the choreography of LAD organization, re-organization, and dynamics at the nuclear lamina, including LAD dynamics in interphase and through mitotic exit, when LAD organization is re-established, as well as intra-LAD subdomain variations.
Keywords: lamina-associated domains, heterochromatin, lamins, 3D genome organization, cell cycle, nuclear lamina, development, chromatin, inner nuclear membrane, CTCF, Hi-C, Dam-ID
Graphical Abstract
In most mammalian nuclei, much of the heterochromatin is positioned near the nuclear envelope, and these chromatin stretches are known as lamina-associated domains (LADs). This review highlights recent studies in mammalian cells uncovering factors that instruct the choreography of LAD organization and dynamics at the nuclear lamina, including LAD dynamics in interphase and through mitotic exit, when LAD organization is re-established.
Introduction
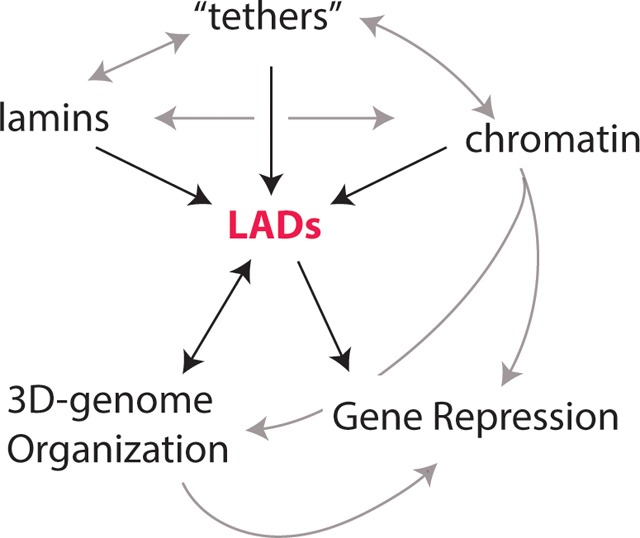
In most mammalian nuclei, a large portion of the heterochromatin is positioned near the nuclear envelope (NE)–referred to as ‘radial’ organization. Given their proximity to the NE and networks of inner nuclear membrane (INM) proteins, nuclear intermediate filaments (lamins), and lamin binding proteins, these chromatin domains are known as lamina associated domains (LADs) [1]. Ranging in length from 0.1–10 Mb, LADs can comprise a large portion of the genome (30–50%, depending on cell type) [1,2]. LADs largely correspond to the heterochromatic B-compartments defined by Hi-C, a chromosome conformation capture technique that monitors the probability of chromatin interactions in 3D [3]. In general, these domains are relatively gene-poor, transcriptionally inactive, and late-replicating. Although many LADs are consistent between cell types (“constitutive” cLADs), some LADs change between cell types (“facultative” fLADs or “variable” vLADs), and these often harbor key developmental genes and cell-type specific genes that are repositioned away from the nuclear lamina (NL) as they become active or poised for activation. These findings gave rise to the hypothesis that LAD organization and re-organization (LAD dynamics) are fundamental to cell development. However, LADs are much more complicated than this simple binary picture. This review highlights recent studies in mammalian cells uncovering factors that instruct the choreography of LAD organization, re-organization, and dynamics at the nuclear lamina. This includes LAD dynamics in interphase and through mitotic exit, when LAD organization is re-established, as well as intra-LAD subdomain variations.
Regulation of LADs
Chromatin and LAD organization and function
The dynamic and spatial organization of eukaryotic genomes has intrigued biologists since the early days of microscopy, especially the non-random localization of heterochromatin [4,5]. Peripheral heterochromatin associated with the nuclear envelope (NE) and the nuclear lamina (NL) is a hallmark of mammalian nuclei. The NL is comprised of intermediate filaments (made from nuclear lamins) and associated proteins, including proteins of the inner nuclear membrane (INM;(described below and reviewed in [6]). Recent advances in measuring genome organization using a variety of chromosome conformation capture techniques, advanced microscopy approaches, and proximity labeling strategies have uncovered underlying principles in genome organization (reviewed in [7]). One recurring question is how spatial localization of chromatin at the NL relates to overall genome architecture as measured by the genome-wide chromosomal conformation capture technique Hi-C. Peripheral heterochromatin domains were first molecularly identified and sequenced using a proximity labeling technique called DNA Adenine Methylation Identification (DamID) by fusing a DNA-methylating enzyme (DNA adenine methyltransferase; Dam) with (typically) a lamin protein. Thus, this technique allows for labeling of DNA regions in close proximity to the NL. Although ChIP-seq (Chromatin Immunoprecipitation) can also be used, DamID, the newer TSA-seq (Tyramide Signal Amplification), and pA-DamID (protein A-mediated DamID) methods avoid the solubilization issues posed by lamin filament networks in standard ChIP assays [8–11]. While DamID, ChIP, and TSA-seq all detect spatial proximity to a protein landmark like the NL, Hi-C identifies both local and long-range chromatin interactions [3,12–16]. Hi-C, therefore, identifies both broad, local self-interacting regions called Topologically Associated Domains (TADs), as well as longer-range chromosome folding into two general compartments: A (active) and B (inactive). These general (A versus B) compartments, originally defined by principal component analysis of Hi-C data, can be further refined to identify functional sub-compartments based on knowing which genes are transcriptionally active (through intersection with transcriptome data), as well as chromatin state (histone ‘marks’) as determined by ChIP-seq to specific post-translational modifications (PTMs) of histone proteins [3]. A/B compartmentalization is now refined into five primary sub-compartments: A1, A2, B1, B2, and B3, with LADs corresponding most highly to the B2 and B3 compartments [3]. In the A-compartment, CCCTC-binding factor (CTCF) and cohesin (among others) physically delimit TAD boundaries and mediate extensive sub-TAD configurations reflecting regulatory interactions, such as enhancer-promoter interactions, at and among local gene loci. LADs, by contrast, are generally depleted of CTCF, except at transitions from LAD to non-LAD boundaries (B to A compartment transitions), and do not exhibit similar local regulatory looping events [1,17–19].
Intriguingly, there are repressed regions within the A-compartment and internally located that, upon cohesin depletion, interact more strongly with the B-compartment and lamina-proximal regions, suggesting that folding of the A-compartment can trap otherwise LAD-like regions in the nuclear interior [20]. Indeed, local accumulation of CTCF and cohesin corresponds with and leads to local detachment of lamina-proximal domains [18,19]. Thus, LADs generally display different principles of genome organization compared to active A-compartment regions (Figure 1).
Figure 1:
Schematic of LAD and genome organization
Lamina-Associated Domains (LADs, red) are regions of heterochromatin that associate with the nuclear lamina (green shading). LADs are part of the B-compartment as determined by Hi-C studies. non-LAD active chromatin (A-compartment, cyan) is organized into active topologically associating domains (active TADs) and sub-TADs through domain anchors CTCF, cohesin, and other factors. TADs can be further folded and looped via intra-TAD regulatory interactions (e.g. enhancer: promoter pairs, CTCF). While LADs are also bordered by CTCF and cohesin, LADs do not form loops like active TADs, nor do they form the same sub-domain loop structures. Indeed, LADs are generally devoid of both CTCF and cohesin except at border regions and in regions that ‘dip’ or loop away. These border regions (transitions) are also enriched for H3K27me3. While LADs can be defined by their proximity to the nuclear lamina and some shared characteristics, LADs display great inter- and intra-LAD heterogeneity. cLADs (constitutive LADs) tend to be gene-poor and show the most consistent and persistent localization to the lamina. Gene-rich LADs comprise the facultative LAD (fLAD) population and can display, depending on cell type, more dynamic interactions with the lamina (depicted as a wavy LAD). In some cell types, these fLADs move away from the lamina and are expressed (and thereforeno longer a LAD). Regions within LADs can also dip away locally (<20Kb regions) to allow expression from a transcription start site (TSS) of a gene, usually with aborted transcripts, or to engage with the A-compartment (e.g. an enhancer looping out). Note that heterochromatin is not restricted to the lamina including some centromeric, telomeric, and nucleolar regions, but can also be found “trapped” in the midst of the A-compartment (gray). While LADs can comprise 30–50% of the genome of a given cell type, not all of those lamina interactions are found in every cell within a population. At least for some cultured cell models, in individual cells only about 1/3 of LADs defined in the cell population actually reside at the lamina at any given time and many of these are stochastically repositioned to intranuclear sites (including perinucleolar regions). Two transition regions are shown as “zoomed in views” to incorporate CTCF and cohesin. Please note that this is an artistic rendering of LAD and genome organization. See text for more details and references. Adapted from [18].
Several studies have shown that repressive histone modifications are one criterion for LAD organization. Chromatin in LADs is enriched for histone post-translational modifications (PTMs) associated with heterochromatin, particularly histone H3 lysine 9 di- and tri-methylation (H3K9me2/3) and histone H3 lysine 27 tri-methylation (H3K27me3) [1]. While LADs contain H3K9me2/3 throughout their length, H3K27me3 appears at the boundaries of large LADs and completely covers some smaller LADs [17]. LADs are generally transcriptionally inactive (or very low expressing), with little RNA polymerase II (polII) or CTCF occupancy and a relative lack of “active” chromatin modifications, such as histone H3 lysine 4 methylation (H3K4me1 or H3K4me3), histone H3 lysine 27 acetylation (H3K27Ac), among others. In contrast, immediately flanking LADs are very active regions with CTCF and polII occupancy, active chromatin PTMs, and active genes. Thus, these boundary regions are of special interest since they sharply delineate both lamina-associated heterochromatin and adjacent active regions. A series of breakthrough studies implicated H3K9me2/3 in the physical association between LADs and the NL, although this modification does not appear sufficient to drive association in all contexts [17,21,22]. Interference of the G9A methyltransferase, which di-methylates histone H3, either by knockdown or drug treatment in mammalian cells, prevented LAD organization to the NL as shown by monitoring de novo LADs generated by an ectopic insertion or via live cell imaging of marked LADs using a LAD-tracer system [17,22–24].Thus, H3K9me2/3 is both a hallmark of and a requirement for LADs.
Histone H3K27me3 remains a bit of a mystery, with one earlier study showing that ectopic recruitment of LADs, particularly at LAD border regions, relied on this modification in combination with H3K9me2/3, as shown by knockdown of the methyltransferase (in this case EZH2) or treatment with an EZH2 inhibitor [17,25]. However, perinuclear targeting of LADs is not reliant on H3K27me3 in C. elegans [21]. It is important to note that H3K27me3 is enriched in the B1 compartment identified by Hi-C, and that these modifications also occur throughout the A-compartment, but not in combination with H3K9me2 as it does at LAD boundaries and small fLADs [1,17]. Intriguingly, perinucleolar heterochromatin contains the well-studied rDNA genes, as well as other regions called nucleolar-associated domains (NADs). These NADs have fairly extensive genomic overlap with LADs, leading to the hypothesis that the two compartments can serve the same purpose vis a vis genome organization and regulation [26]. However, more in-depth studies have identified different classes of NADs that are either nucleolus-specific or that overlap with LADs, providing a provocative tool to potentially uncover specific targeting modalities for both compartments. One such study found that higher H3K27me3 was found in heterochromatin preferentially associating with NADs (and not LADs) in MEFs, suggesting that this modification might also confer more mobility and nucleolar association [25]. A contrasting study in mESC found that NADs had lower H3K27me3 and higher H3K9me2/3 compared to LADs [27]. Thus, even within a related compartment and directed comparisons, the role of H3K27me3 remains elusive. This could be because this modification serves multiple roles depending on the genomic context. By parsing LADs based on H3K27me3 status, one study elegantly showed that LADs enriched in this modification (whether at the borders of large LADs or across short LADs) correlated with fLADs rather than cLADs, in agreement with previous studies and highlighting the potential importance of this modification to fLADs [28,29]. Depletion of this modification had dramatic consequences: normally H3K27me3-marked regions in non-LADs began associating with the NL, and heterochromatic H3K9me3 modifications spread from LAD border regions (normally enriched in H3K27me3) into adjacent non-LAD regions, suggesting H3K27me3 normally prevents LAD heterochromatin from spreading [29]. Thus, H3K27me3 appears to contribute to LAD regulation in a variety of ways: potentially directing facultative LADs to the lamina and insulating LAD boundaries while also keeping fLADs and border regions more loosely associated with the lamina network than the cLADs. However, these recent studies also highlight the need to interpret results in the context of developmental stage, cell type, and even model system used.
The nuclear lamins and INM proteins
For obvious reasons, LAD associations with nuclear intermediate filaments or lamin-associated complexes at the INM are attractive as potential mechanisms for LAD chromatin localization near the nuclear periphery [30,31]. In somatic differentiated cells, the NL includes both A-type (lamin A, lamin C) and B-type (lamin B1, lamin B2) filaments, whereas early progenitor cells usually lack A-type lamins. LMNA encodes both lamin A and lamin C via alternative splicing, whereas B-type lamins are encoded by different genes [6]. In addition to their roles in genome organization through interactions with the underlying heterochromatic LADs, lamins (directly or indirectly) support many other pathways, including signaling, mechanotransduction, DNA repair, mRNA splicing, as well as nuclear integrity and nuclear positioning, any of which also influence chromatin state and transcription (reviewed in [6]). These proteins also display some functional redundancy, making it challenging to tease apart their individual roles in LAD organization and direct gene regulation. Lamins appear to form spatially separate (and homotypic) networks that are, nonetheless, interdependent since depleting one lamin isotype disrupts the organization of the other lamin isotype networks [32–34]. In mammals, lamin expression is developmentally regulated and ratios of the different lamin isotypes vary across cell types and contribute differentially to nuclear mechanics as well as other lamin-regulated processes [35,36]. Every nucleated mammalian cell expresses at least one B-type lamin, whereas A-type lamins are expressed at very low levels early development and in embryonic stem cells [37,38]. During early development, cells express high levels of lamin B1, lamin B2, and lamin B receptor (LBR), an integral inner nuclear membrane (INM) protein that also binds heterochromatin-stabilizing proteins named HP1 [39–41]. The B-type lamins and LBR have pivotal roles in early development and in certain cell types. Studies in mice have shown that normal organ development is dependent on lamin B expression and expression of LBR, attributed to loss of the sterol C-14 reductase activity of LBR, while A-type lamins appear to function in a coordinated fashion in organogenesis and later developmental stages [42,43].
Work in mouse embryonic stem cells (mESCs) depleted of lamins B1, B2, and lamin A/C (the so-called triple-knockout or TKO) showed that overall LAD organization, as detected by DamID with an INM protein ( Lap2β-DamID), is largely unaffected in these cells, suggesting that lamins are dispensable in very early development for LAD organization [44]. However, using slightly different algorithms to infer local interactions from DamID data combined with 3D DNA-FISH and Hi-C in TKO mESC cells, local chromatin changes in cLADs, in particular, were observed, with TAD-TAD interactions altered [28]. In this study, the authors suggest that lamins act as a framework or cage rather than “tethers”. Because mESCs do not express A-type lamins, the observed effects would presumably be dependent on the loss of lamin B1/2 (and thus LBR interactions). These results suggest that lamin B1 and/or B2 can play a role in defining the LAD landscape. In support of this, earlier work inducing senescence identified loss of LAD integrity and shifting chromatin landscapes coincident with lamin B1 loss [45]. In a breast cancer model, lamin B1 appears to be critical for proper LAD organization to the lamina and long-range compartment organization [46]. In general, DamID maps from cells expressing either Dam-lamin A or Dam-lamin B1 look indistinguishable from each other. However, as previously noted, lamins form microdomains and separate (but interdependent) networks at the NE. In addition, a subset of A-type lamins retain a mitotic phosphorylation and are kept in an unpolymerized state via interactions with Lap2α and thus are localized in the nucleoplasm and associated with active chromatin [32,47,48]. Using ChIP-based approaches, different binding profiles of A- and B-type lamins have been observed, and while there is significant overlap between their LAD profiles, A-type lamins are found in non-LADs as well as LADs [49]. A recent study using HeLa cells to identify lamin interaction domains (LiDs), using both sonication and MNase-based ChIP approaches, identified A/C-only versus A/C plus B-type LiDs. B-type containing LiDs represent cLADs, and the A/C-only enriched demarcate fLADs and euchromatin regions [50]. It should be noted that this study, like many, did not discriminate between lamin A and lamin C. Overall these studies implicate both A-type lamins and B-type lamins as playing a role in sequestering regions to the lamina.
Even though LMNA is mostly restricted to more differentiated cell types and its expression is dispensable for early differentiation, several studies support roles for A-type lamins in heterochromatin organization and regulation in differentiated cells. One early indication that A-type lamins are important for LAD organization came from studies that showed loss of heterochromatin in cells from patients with Hutchinson Gilford Progeria Syndrome (HGPS) who express a dominant variant of lamin A that lacks fifty amino acids and is permanently farnesylated [51]. Since then, numerous studies have tried to pinpoint heterochromatin regulation by A- and B-type lamins, with mixed interpretations [38]. A recent study found that pathogenic LMNA mutations from patients with dilated cardiomyopathy (DCM), introduced via CRISPR into human induced pluripotent stem cells (hiPSCs), led to LAD disruptions in cardiomyocytes, but not hepatocytes or adipocytes [52]. The LAD reorganization correlated with upregulation of non-myogenic genes and a general disruption of nuclear morphology, directly linking specific lamin A mutations to cell type and defects in a subset of LADs. A great advance in our understanding of how lamin A/C might contribute to genome organization came from a study of genome organization in the rod cells of nocturnal animals, where heterochromatin all concentrates in the center of the nucleus, not the periphery. This inverted configuration was caused by the absence of both lamin A/C and LBR [43]. Based on comparing expression profiles and chromatin configurations, these authors suggest that, in most cells, LBR and lamin A/C sequentially sequester LADs to the periphery. In cells engineered to remove lamin A/C, LBR is often upregulated, potentially compensating for its loss. Thus, lamin A/C and lamin B (through LBR) exert different influences on genome organization depending on cell type, their relative expression levels, and differentiation state: generally LBR (hence B-type lamins) regulates LAD organization in early development, while lamin A/C enforces this organization at later stages, either with or without LBR [43]. One important caveat is that this study implicated A-type lamins generally but did not determine which isoform(s) (lamin A, lamin C, lamin AΔ10) was vital. This question was partly answered by a new study: in mouse embryonic fibroblasts that express very low levels of LBR, global LAD organization depended on phosphorylated lamin C in the nucleoplasm, rather than lamin A or lamin B1 at the NE [53]. In this case, the authors suggest that lamins in the nuclear interior prevent aberrant LAD aggregation after mitosis in cycling cells in the nuclear interior and help cage and sequester LADs to the NL (see also Cell Cycle section). It remains unclear if there is a similar role in other cell types or in non-cycling cells.
In addition to LBR, other INM proteins help organize and/or maintain LADs either directly or indirectly, especially proteins with the LEM-domain, first identified in Lap2, Emerin, and MAN1. For example, Lap2β (lamin-associated polypeptide 2, beta), mentioned previously as a proxy for lamins in DamID experiments, also binds and sequesters histone deacetylase 3 (HDAC3), which helps maintain heterochromatin by deacetylating lysines on histone tails [54,55]. Lap2β and HDAC3 have been implicated in the recruitment of an ectopic LAD in an engineered recruitment system in MEFs and in LAD organization and regulation in cardiac cell development [55,56]. Emerin also promotes LAD interactions with the NE in part by binding and activating HDAC3 [57]. However, Lap2β and emerin are each widely expressed and cannot explain the tissue and cell specificity of developmentally regulated LADs. Intriguingly, LBR and emerin (and likely Lap2β) have both overlapping and distinct proteomes (hence, microenvironments) at the NE [58]. Given that LAD organization is cell/tissue-specific for fLADs, the search has continued for INM proteins with cell-type restricted expression patterns; such ‘nuclear envelope transmembrane’ proteins (NETs) are a class of previously unknown INM proteins that were discovered by biochemical isolation or biotinylation from a variety of tissue types. Some are quite restricted in their expression patterns, and a large fraction of novel NETs have roles in tissue-specific chromosome organization or direct targeting of specific loci to the NL. This class of novel proteins offers further mechanisms to explain or regulate the tissue-specificity of LAD organization (reviewed in [59] and [53,60–67]).
Tethers, Readers, and Writers
Early in vitro binding assays hinted at direct lamin:DNA interactions and a more recent study identified regions in the lamin A tail that interact with histone H3 and, particularly, repressive modification (H3K9me2, H3K27me2, and H4K20me) [68–71]. Lamins also interact with a myriad of transcription factors and chromatin binding proteins (reviewed in [72]). Such interactions could serve to sequester these proteins from the genome or, alternatively, provide bridges to scaffold chromatin to the NL. These molecular ‘tethers’ or ‘middlemen’ would presumably connect LAD chromatin with NL proteins by binding to both while potentially reinforcing the heterochromatin compartment. Experimental and modeling evidence clearly suggests that heterochromatin can self-assemble by phase separation dynamics and, by microscopy, LADs appear to self-organize into higher-order domains prior association with the NL after mitosis [53,73–77]. In biophysical models of genome organization, LAD heterochromatin is predicted to localize peripherally only if an affinity for the lamina is included in the model [74]. Hence, the question remains: how are LADs constrained to the NL? Given that heterochromatin-driven phase-separation mechanisms can only explain part of the story, there must be other forces and/or protein:protein interactions contributing to the peripheral localization of LADs.
Of particular interest are proteins that interact with lamins or are localized to the lamina that also have chromatin binding properties (Figure 2). Barrier to autointegration factor (BANF1) has essential roles in bridging segregating daughter chromosomes to ensure capture within the same reforming nucleus [78]. BANF1 also binds histones and recruits membranes (via INM proteins) and lamins to the segregated chromatin surface after mitosis [78–84]. LBR also ‘bridges’ chromatin to lamins by binding heterochromatin protein 1 (HP1α), a chromodomain protein that binds the silent H3K9me3 mark and is important for silencing and long-term stability of heterochromatin [39–41]. HP1α is a key player because it forms large stable self-interacting heterochromatin domains by dimerizing (via the chromoshadow domain) and phase separation [76,77,85]. Intriguingly, a protein originally identified as an important regulator of heterochromatin, proline-rich 14 (PRR14), has both lamina-binding and HP1α binding domains [86,87]. This protein is highly localized to the nuclear periphery and recruits heterochromatin (H3K9me2 regions in particular) to the NL, but it is also found in the nucleoplasm suggesting that this protein localization is regulated and may mediate dynamic interactions [87–89]. Chromodomain proteins regulating lamina association appear to be evolutionarily conserved, since the CEC-4 chromodomain directly mediates perinuclear anchoring in C. elegans [90]. These findings collectively support direct roles for bridging proteins such as HP1α and their partners in LAD organization, although in C. elegans neither HP1 homologue, HPL-1 nor HPL-2, nor the MBT-domain methyl-reader LIN-61, is involved in the CEC-4 anchoring of heterochromatin to the nuclear periphery [90].
Figure 2:
The Growing list of “Middlemen”
LADs are enriched for repressive heterochromatin marks and localization to the lamina is dependent on H3K9me2/3 and, to some degree, H3K27me3. Shown in this schematic are proteins found to interact with either chromatin or chromatin modifiers/interactors as well as the nuclear lamina–i.e. these proteins serve as molecular bridge or tether. Among these tethers ZKSCAN3, PRDM16, MECP2, BANF, AHCTF1, and PRR14 (green), some of which interact with lamin B receptor (LBR) or LEM domain proteins as indicated. Chromatin interactors are shown in pink. Of special note, both TRIM28 (KAP1) and HP1α have also been shown to interact with the lamina or INM proteins but are treated in this figure as indirect (and chromatin-centric) given their repeated appearance in multiple complexes. See text for details and references. Adapted from [91].
In nearly all metazoans, HP1α is also found in heterochromatin domains distant from the NL; while fully consistent with its proposed role in phase-separating heterochromatin, its general association with chromatin fails to explain tissue-specific LAD dynamics. Thus, additional proteins and/or mechanisms are needed to explain the developmental timing and tissue-specificity of fLAD organization. To screen for such mediators, one study used BioID to identify the individual interactomes of LADs, lamins, and lap2β [91]. BioID is a proximity labeling technique that deploys a promiscuous biotin ligase to biotinylate lysine residues on proximal/interacting proteins [91]. By intersecting the ‘laminome’ and ‘LADome’, and focusing on proteins identified in both datasets, several candidate mediators were identified [91]. These proteins included lamins, INM proteins and chromatin interactors such as PRR14, MECP2 (Methyl-CpG Binding Protein 2), Rif1 (Replication Timing Regulatory Factor 1), TRIM28 (Tripartite Motif Containing 28; aka KAP1), AHCTF1 (AT-Hook binding protein), many of which interact with HP1α or are implicated in higher order chromatin organization [92–95]. Notably, MECP2 was subsequently shown to be involved in LAD organization [96]. Intriguingly, AFCTF1 (also known as ELYS) associates with both NPCs and active/poised (H3K27me3-marked) chromatin, suggesting a role in LAD boundaries or loosely-interacting LAD regions [97,98]. The LAD-only interactome was enriched in repressive chromatin modifiers and regulators such as EZH2 (Enhancer of Zeste 2), WIZ (WIZ Zinc Finger), SMCHD1 (Structural Maintenance of Chromosomes flexible Hinge Domain Containing 1), HP1α, and HP1β. Additional studies have found that protein:protein interactions at the interface of the lamina and chromatin rely on chromatin interactors and modifiers to maintain both heterochromatin state and lamina proximity. In one study, ZKSCAN3 (Zinc Finger With KRAB And SCAN Domains) was found to interact with lamin B1, LBR, and HP1α via KAP1 (TRIM28) to counteract LAD and heterochromatin loss in a senescence model [99]. In a different study in fibro-adipogenic progenitors (FAPs), Prdm16 (PR domain-containing 16) displayed a striking localization to the NL where it interacts with the methyltransferase G9a (which is responsible for H3K9me2) to control the chromatin state and localization of specific myogenic genes, thus representing a cell-type specific pathway to attenuate LAD chromatin state and organization [100].
LAD Dynamics
Intra-LAD dynamics and heterogeneity
LADs can change organization between cell types, but there is also significant heterogeneity between LADs and within a single LAD in a given cell type. Single cell DamID (scDamID) and live cell imaging, for instance, have found that there is some cell-to-cell heterogeneity in LAD organization for some LADs [23,101,102]. A recent study generated an atlas of lamin B1 occupancy using ChIP-seq across twelve human cell types, including embryonic stem cells and differentiated ES cells from all three germ layers [103]. These data revealed that two distinct LAD subtypes can be found throughout all twelve cell types that were termed T1- and T2-LADs. LADs designated T1 had the highest lamin B1 and H3K9me2 occupancy and less chromatin accessibility. Furthermore, T1-LADs were enriched for late-replicating DNA and possessed lower gene content. Conversely, T2-LADs had intermediate levels of lamin B1 and H3K9me2 occupancy, relative to T1-LADs and non-LADs, and exhibited greater chromatin accessibility. T2-LADs were also enriched for replication timing switch domains and had a higher density of genes [103].The T2-LADs are reminiscent of earlier findings that H3K27me3 is coincident with LAD borders, gene-rich smaller fLADs, and a looser association with the lamina [17,29]. Heterogeneity within LADs is also evident and linked with gene regulation. A study investigating the response of repression-sensitive promoters inserted at thousands of different locations within LADs found that most were generally repressed when embedded in a LAD, but to different extents—there was significant heterogeneity in expression related to chromatin context and frequency of contact with the lamina [18,104]. It is important to note that different promoters display different sensitivities to similar LAD environments, suggesting that transcription from a given LAD-embedded promoter is regulated by a combination of factors, including chromatin state, promoter-specific qualities, and extent and/or frequency of lamina contact.
Development and Differentiation
As has been discussed, some LADs display developmental regulation and can be classed into fLADs and cLADs. Early studies identified dynamic association with the NL as a potential mechanism for gene regulation at the immunoglobulin heavy and light chain loci (IgH and IgK, respectively) and the T-cell receptor beta locus (TCRβ). At that time, even though genome-wide LADs (and TADs) had not yet been identified, these particular loci were visualizable by 3D immuno-FISH and seen to undergo changes in locus looping (figure 1) and lamina association that correlated directly with developmentally-regulated gene activity [55,105–111]. Later genome-wide assays, such as DamID and Hi-C, verified and refined these early models. While LADs are generally gene poor, they are enriched in key developmentally regulated genes located in facultative LADs (fLADs) that organize away from the lamina in certain cell types even though most LADs (cLADs) are conserved between cell types (figure 1) [1,112]. Ongoing studies continue to map LAD organization in a variety of cell types to elucidate and determine which LADs are conserved between cell types (cLADs), those that differ (fLADs), and to understand how they are differentially regulated [103]. fLADs are enriched in developmentally regulated and cell-type specific genes, suggesting a role for LAD organization in regulating these loci. For example, despite the strong conservation in large-scale genome organization across cell types, as embryonic stem cells differentiate towards different lineages, contacts between the NL and hundreds of genes are reconfigured [113]. During the processes of neurogenesis and myogenesis, for example, 5%−15% of genes shift from the nuclear periphery to the interior, and another 5%−10% move toward the NE; these general findings have been confirmed in other studies [62,113,114]. Thus, even though cLADs are consistent between cell types, LAD interactions that do change involve gene loci with pivotal roles in cell identity.
The robust overlap between LADs and (inactive) TAD regions, as defined by Hi-C, highlights the link between overall genome organization and LAD organization [101,115–117]. T-cell activation is a rapid process accompanied by global genome reorganization and changes in gene expression at both early and later time points, providing a great model system to monitor LAD reorganization. By monitoring both LAD reorganization and TAD A/B-compartment organization in this dynamic model, a study showed that LADs reorganize within the existing TAD framework, with LADs moving on or off the lamina in units delimited by TADs, allowing genes and their enhancers to disassociate from the NE together during activation/induction, with some distal enhancers looping out (into the interior) to contact the A2 compartment [118]. In so doing, each LAD/TAD region functions as a cohesive unit when migrating from the B-compartment (LAD) to the A-compartment (interior) and inducing both the previously-’LADed’ genes and their cis-regulatory elements. An independent study removed a LAD border enriched in CTCF sites (i.e., also a TAD border) that separates inactive regions of the TCRβ locus from the active recombination center [119]. As a result, the removal of this border allowed the active H3K27Ac mark to spread, leading to aberrant activation and recombination of normally silent TCRβ segments. Taken together, these newer studies highlight the importance of dynamic regions at the lamina for development and cellular transitions.
Cell Cycle
LAD reorganization is not restricted to development or cellular activation. In late prophase, the spatial organization of the genome (TADs and LADs) and NL/NE organization of the interphase nucleus are lost as chromosomes adopt a condensed mitotic configuration and the NE and lamina networks disassemble [6,120]. Tissue-specific 3D-LAD organization is fully re-established by mid-G1 of the next interphase, suggesting re-assembly is regulated and efficient [18,23,53,120–122]. Thus, these organizational changes provide an excellent opportunity to explore fundamental mechanisms of LAD organization and regulation while gaining deeper insight into nuclear assembly. As cells enter anaphase, super-resolution imaging, and single cell Hi-C both show that LAD regions of chromosomes begin self-aggregating as globular “compartments” before lamins are incorporated into the reforming NE [18,23,53,123,124]. These LAD aggregates slowly make their way to the nascent NE during early G1, with some associating instead with nucleoli, and ultimately “spread” across the lamina as they approach and interact with the nuclear periphery [23,53,123,124].
Exit from mitosis, therefore, is a time when LAD organization is quite dynamic and serves as a model for discovery of basic principles and spatial dynamics of LAD organization. In a clever adaptation of the original DamID technique, called pA-DamID, a protein-A coupled Dam enzyme added to cells that have been incubated with an antibody to a NL protein (i.e. a hybrid between DamID and the CUT & RUN method) [11,125]. This technique allows for rapid snapshots of LAD organization which is not feasible with the original DamID technique. Using a lamin B2 antibody, snapshots of synchronized cells (HAP-1, HTC116, hTERT-RPE, and K562) at mitotic exit and into G1 showed that, in these human cell lines, telomere proximal LADs organize to the lamina first, with centromere proximal LADs organizing to the lamina last, suggesting a polarization in the timing of interactions. It is unclear if these preferred association timings are related to the mitotic chromosome structure/organization. While LAD organization generally appears to be constrained at the lamina throughout interphase, some LAD-NL interactions become stronger while others become weaker, as measured by pA-DamID interaction levels. These modulations in association take place largely within the first few hours of interphase, with some mobility persisting. Interestingly, LADs that underwent a weakening of NL interaction did not exhibit lower pA-DamID scores relative to stable LADs in the time points shortly after division. This suggests that their later detachment from the NL is not due to a weaker initial association. The LADs that displayed this gradual release from the NL tended to be smaller and more gene-rich than the LADs that saw a gradual strengthening in interaction [11]. These findings are in agreement with variability in LAD organization and chromatin content, with border regions and some fLADs exhibiting decreased interactions (and perhaps higher H3K27me3). Considering the global architecture of chromosomes, weakening LADs were generally found near the ends of chromosomes (‘distal’; within ~25 Mb of telomeres), whereas strengthening LADs were closer to centromeres. One of the most striking features of this configuration is that, as mentioned above, distal LADs arrive at the lamina first and therefore have more/stronger contacts than the more internal LADs at earlier time points. Soon after division however, distal LADs weaken in their interaction with the lamina and central LADs increase interactions. Therefore, chromosomes appear to adjust positioning as cells move through interphase, once again highlighting the dynamic nature of these domains [11].
Lamins have a defined order of recruitment to the reforming NE, with membrane-associated B-type lamins arriving first, followed by NPC-dependent import and assembly of A-type lamins [6]. To understand the roles of individual lamins in LAD ‘assembly’ during mitotic exit, mouse embryonic fibroblasts, which express little-to-no LBR, were subjected to shRNA-mediated downregulation of lamin A-only (shA), lamin C-only (shC), lamin A and lamin C (shAC), or lamin B1 (shB1) [53]. Reorganization of LADs to the NL was followed in single cells by microscopy using a cell-cycle- LAD-tracer system to monitor LAD reorganization from one cell cycle to the next [21,46,84]. LADs first self-associated in anaphase, forming 1–2 distinct ‘blobs’ per chromosome in the nuclear interior that persisted for the first 2–3 hours of the next G1-phase [53,73]. While some LADs became lamina-proximal early, most displayed delayed recruitment to the lamina, in agreement with previous findings [23]. The surprise was that lamin C also localized in the nucleoplasm, between coalesced LAD ‘blobs’, during this same time, when lamin A was already obvious at the NE. Furthermore, global LAD reorganization to the lamina was grossly disrupted in cells that lacked lamin C (shC) but was only subtly/mildly affected in cells lacking lamin A (shA) or lamin B (shB1). Interestingly, lamin C and LADs also showed parallel delayed kinetics of recruitment to the lamina. All lamins are phosphorylated upon entry into mitosis to enable mitotic breakdown. During nuclear reassembly, lamin A and B are rapidly dephosphorylated and only a fraction of lamin A remains phosphorylated. By contrast, nearly all lamin C remains phosphorylated and nucleoplasmic well into G1, and this nucleoplasmic, and euchromatic, pool of lamin C is essential for LAD reorganization after mitosis [53,126]. Without lamin C, nucleoplasmic LADs appear to merge and interact with LADs from other chromosomes as they make their way to the lamina, consistent with models in which lamins ‘shepherd’ or cage LADs to block aberrant interactions. In this model, LADs are sandwiched between nucleoplasmic lamin C and peripheral lamin A, in agreement with the proposal that lamins cage LADs rather than directly tethering them [28]. It should be noted that transgenic expression of lamin A/C in mouse rod cells does not lead to ‘rescue’ of the inverted chromatin phenotype. Thus, lamin C alone is not sufficient for repositioning LADs which suggests additional partners or cell-type specific requirements are needed (reviewed in [127]).
How do chromatin modifications and protein partners function during this post-mitotic reorganization? One interesting study identified the H3K9me2 modification as a ‘bookmark’ for LAD re-localization to the lamina during mitotic exit. A nearby mitotic modification, Histone H3 serine 10 phosphorylation (H3S10P), releases LADs from the NL and must be removed to allow re-association [128]. This study implicated early attachment of all H3K9me2 marked LADs at the INM lamina and suggested a mechanism whereby H3S10P modifications ‘mask’ the H3K9me2 ‘bookmark’ [128]. Removal of H3S10P is proposed to ‘unmask’ the H3K9me2 bookmark, allowing early association of H3K9me2-marked LADs at the INM lamina [128][88,89]. At least one of the factors mediating this relocation is the previously discussed PRR14 (and HP1α,) which also has roles in organizing LADs in interphase [88,89]. While this model provides a tempting mechanism suggesting how LADs are re-organized after mitosis, the findings are somewhat contradictory with other recent studies showing LAD reorganization takes some time into G1 to fully relocate and that many regions instead localize to perinucleolar regions, thus it will be important to test these findings in other systems. Taken together, these data suggest that chromatin state, lamins, and lamin interacting proteins dynamically regulate the reorganization of LADs to the lamina at and shortly after mitotic exit.
Circadian Rhythm
Thousands of genes display oscillatory expression with defined periodicities over a 24-hour period in virtually all organs. This periodicity is regulated by circadian patterns of transcription factor binding, histone modifications, and 3D chromosomal interactions. This internal ‘clock’ is controlled by light, food, and other zeitgebers (environmental cues to entrain the internal clock). Intriguingly, one of the first identified chromatin changes associated with circadian gene regulation was histone H3 serine 10 phosphorylation of activated clock genes in the hypothalamic suprachiasmatic nucleus (SCN) of mice, the central pacemaker that regulates most circadian rhythms throughout the organism [129]. This finding poses particular interest now in light of the mechanisms of LAD dynamics through the cell cycle and the role of H3S10P, HP1α, and PRR14 in that process, indicating a potential mechanism for rapid relocalization to and from the NL [128]. Since that initial finding, changes in methylation and other PTMs have been implicated in the regulation of cyclic transcription, including dynamic histone acetylation via histone acetylases/deacetylases (HATs and HDACs) and/or distinct histone methylation patterns, all of which have been shown to oscillate at multiple genomic regions (reviewed in. [130]). Indeed, there is growing evidence that the 3D-organization of clock-regulated genes oscillates and is correlated with gene regulation, but the role of LADs in this process still remains unclear [130–133]. Given the relationship between chromatin state, 3D-genome organization, and LAD organization, it is tempting to speculate that LADs might also have a role in regulating circadian rhythms. Indeed, MAN1, an inner nuclear membrane protein, was found to interact with a key clock regulator, the transcription factor BMAL1, raising the possibility that the laminas might play a role in the regulation of clock genes either through sequestration of key clock regulators or reorganizing their target genes to the lamina [133,134]. In addition, certain INM proteins have been implicated in dynamic deacetylation via their interactions with HDAC3, suggesting that the environment near the NL and organization into a LAD could facilitate regulation at these loci [55–57]. In support of this, an earlier study implicated oscillations of clock-regulated genes in colon cells to the NL in their transcriptional repression, with H3K9me2 accumulating only after association with LADs. In this study, the global genome organizer CTCF and PARP1 served to regulate clock gene loci in response to serum shock [135]. It should be noted that 3D-genome organization and association with LADs were measured by 4C and 3D-immunoFISH and not DamID or ChIP methodologies. Conversely, a more recent study found that, in mouse livers, clock entrainment by starvation led to re-organization of some LADs, particularly LAD border regions, but that clock gene regulation was uncoupled from these changes [136]. Indeed, by using ChIP to lamin B1, the authors found that clock genes, at all time points tested, resided in interLAD regions. The differences in the findings between these studies could be due to either the model system used (human embryonic stem cells, human embryoid bodies, and HT116 liver cells versus whole mouse livers) or in identifying LAD “association” (imaging and proximity versus ChIP). Thus, it remains unclear to what extent reorganization into LADs or the larger LAD environment might influence circadian gene regulation.
Conclusion & Future Directions
Although chromatin in LADs is generally transcriptionally inactive, these regions are far from inert and static. LADs show many levels of dynamic organization, including developmentally-regulated reorganization to enable cell-type specific gene programs, shifting landscapes in response to stimuli, and different interaction frequencies with the lamina within a given LAD. Although recent studies have uncovered numerous chromatin proteins important for LAD organization at the INM/lamina/LAD interface, it is unclear if these are acting as molecular “tethers”, directly linking chromatin to the NE, or are instead (or also) enforcing/reinforcing a chromatin state required for lamina-proximity–a proximity that may depend more on biophysical separation of heterochromatin and/or caging by lamins. Further studies on protein:protein, protein:DNA, and chromatin interactions at the NL/chromatin interface, particularly at rapid time-scales, will lead to a greater understanding of the mechanisms governing LAD organization and how this relates to and regulates global genome organization and function. In addition, many studies have leveraged cell culture lines and findings in one cell-type, culture system, developmental stage, or even model organism may not always be broadly applicable. The use of ever more precise tools to perturb lamina-chromatin association in a variety of systems, for instance rapid degradation of cell-type specific interface proteins, will help us understand how the LAD environment contributes to cellular identity, gene regulation, and disease.
Acknowledgements
We thank Joan M. Sobo for her valuable contributions in providing us with insight in discussing this review. Our work has been funded by The National Institutes of Health NIGMS grants RO1GM132427 (N.S.A., T.I.T., K.L.W., and K.L.R). and R25GM109441 (T.I.T).
Abbreviations
- LADs
Lamina-Associated Domains
- cLADs
constitutive LADs
- fLADs
facultative LADs
- NL
nuclear lamina
- NE
nuclear envelope
- INM
inner nuclear membrane
- Lids
Lamin interaction domains
- DamID
DNA adenine methyltransferase identification
- ChIP-seq
Chromatin Immunoprecipitation
- TSA-seq
Tyramide Signal Amplification
- TADs
Topologically Associated Domains
- PTMs
Post-translational Modifications
- H3K9me2/3
histone H3 lysine 9 di- and tri-methylation
- H3K27me3
histone H3 lysine 27 tri-methylation
- polII
RNA polymerase II
- CTCF
CCCTC-binding factor
- NADs
Nucleolar-associated domains
- MEFs
Mouse Embryonic Fibroblasts
- mESC
Mouse Embryonic Stem Cells
- LBR
Lamin B Receptor
- Lap2β
Lamin-associated polypeptide 2, beta
- HGPS
Hutchinson Gilford Progeria Syndrome
- DCM
Dilated Cardiomyopathy
- hiPSCs
Human induced Pluripotent Stem Cells
- NETs
Nuclear envelope transmembrane
- BANF1
Barrier to autointegration factor
- PRR14
Proline-rich 14
- HP1
Heterochromatin protein 1
- MECP2
Methyl-CpG Binding Protein 2
- Rif1
Replication Timing Regulatory Factor 1
- TRIM28
Tripartite Motif Containing 28
- AHCTF1
AT-Hook Binding Protein
- EZH2
Enhancer of Zeste 2
- WIZ
WIZ Zinc Finger
- SMCHD1
Structural Maintenance of Chromosomes Flexible Hinge Domain Containing 1
- FAPs
fibro-adipogenic progenitors
- Prdm16
PR domain-containing 16
- scDamID
Single cell DamID
- TCRB
T-cell receptor beta locus
- H3S10P
Histone H3 serine 10 phosphorylation
Bibliography
- 1.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W & van Steensel B (2008) Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951. [DOI] [PubMed] [Google Scholar]
- 2.Pope BD, Ryba T, Dileep V, Yue F, Wu W, Denas O, Vera DL, Wang Y, Hansen RS, Canfield TK, Thurman RE, Cheng Y, Gülsoy G, Dennis JH, Snyder MP, Stamatoyannopoulos JA, Taylor J, Hardison RC, Kahveci T, Ren B & Gilbert DM (2014) Topologically associating domains are stable units of replication-timing regulation. Nature 515, 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao SSP, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, Sanborn AL, Machol I, Omer AD, Lander ES & Aiden EL (2014) A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitz E (1929) Heterochromatin, Chromocentren, Chromomeren. Ber Botan Ges 47, 274–284. [Google Scholar]
- 5.Cremer T & Cremer C (2006) Rise, fall and resurrection of chromosome territories: a historical perspective. Part I. The rise of chromosome territories. Eur J Histochem 50, 161–76. [PubMed] [Google Scholar]
- 6.Wong X, Melendez-Perez AJ & Reddy KL (2022) The Nuclear Lamina. Cold Spring Harbor Perspectives in Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerkovic I & Cavalli G (2021) Understanding 3D genome organization by multidisciplinary methods. Nat Rev Mol Cell Biol 22, 511–528. [DOI] [PubMed] [Google Scholar]
- 8.Greil F, Moorman C & van Steensel B (2006) DamID: mapping of in vivo protein-genome interactions using tethered DNA adenine methyltransferase. Methods Enzymol 410, 342–359. [DOI] [PubMed] [Google Scholar]
- 9.Orian A, Abed M, Kenyagin-Karsenti D & Boico O (2009) DamID: a methylation-based chromatin profiling approach. Methods Mol Biol 567, 155–69. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Zhang Y, Wang Y, Zhang L, Brinkman EK, Adam SA, Goldman R, van Steensel B, Ma J & Belmont AS (2018) Mapping 3D genome organization relative to nuclear compartments using TSA-Seq as a cytological ruler. J Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Schaik T, Vos M, Peric-Hupkes D, Hn Celie P & van Steensel B (2020) Cell cycle dynamics of lamina-associated DNA. EMBO Rep 21, e50636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES & Dekker J (2009) Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS & Ren B (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips-Cremins JE, Sauria MEG, Sanyal A, Gerasimova TI, Lajoie BR, Bell JSK, Ong C-T, Hookway TA, Guo C, Sun Y, Bland MJ, Wagstaff W, Dalton S, McDevitt TC, Sen R, Dekker J, Taylor J & Corces VG (2013) Architectural Protein Subclasses Shape 3D Organization of Genomes during Lineage Commitment. Cell 153, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belton J-M, McCord RP, Gibcus JH, Naumova N, Zhan Y & Dekker J Hi– C: A comprehensive technique to capture the conformation of genomes. Methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belaghzal H, Dekker J & Gibcus JH (2017) Hi-C 2.0: An optimized Hi-C procedure for high-resolution genome-wide mapping of chromosome conformation. Methods 123, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harr JC, Luperchio TR, Wong X, Cohen E, Wheelan SJ & Reddy KL (2015) Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol 208, 33–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luperchio TR, Sauria MEG, Wong X, Gaillard M-C, Tsang P, Pekrun K, Ach RA, Alice Yamada N, Taylor J & Reddy KL (2017) Chromosome Conformation Paints Reveal the Role of Lamina Association in Genome Organization and Regulation. bioRxiv, 122226. [Google Scholar]
- 19.van Schaik T, Liu NQ, Manzo SG, Peric-Hupkes D, de Wit E & van Steensel B (2022) CTCF and cohesin promote focal detachment of DNA from the nuclear lamina. Genome Biol 23, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzer W, Abdennur N, Goloborodko A, Pekowska A, Fudenberg G, Loe-Mie Y, Fonseca NA, Huber W, Haering CH, Mirny L & Spitz F (2017) Two independent modes of chromatin organization revealed by cohesin removal. Nature 551, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towbin BD, González-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P & Gasser SM (2012) Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947. [DOI] [PubMed] [Google Scholar]
- 22.Bian Q, Khanna N, Alvikas J & Belmont AS (2013) β-Globin cis-elements determine differential nuclear targeting through epigenetic modifications. J Cell Biol 203, 767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA & van Steensel B (2013) Single-cell dynamics of genome-nuclear lamina interactions. Cell 153, 178–192. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Yammine S, Shi C, Tark-Dame M, Göndör A & Ohlsson R (2014) The visualization of large organized chromatin domains enriched in the H3K9me2 mark within a single chromosome in a single cell. Epigenetics 9, 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vertii A, Ou J, Yu J, Yan A, Pagès H, Liu H, Zhu LJ & Kaufman PD (2019) Two contrasting classes of nucleolus-associated domains in mouse fibroblast heterochromatin. Genome Res 29, 1235–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Koningsbruggen S, Gierlinski M, Schofield P, Martin D, Barton GJ, Ariyurek Y, den Dunnen JT & Lamond AI (2010) High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli. Mol Biol Cell 21, 3735–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bersaglieri C, Kresoja-Rakic J, Gupta S, Bär D, Kuzyakiv R, Panatta M & Santoro R (2022) Genome-wide maps of nucleolus interactions reveal distinct layers of repressive chromatin domains. Nat Commun 13, 1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Hu J, Yue S, Kristiani L, Kim M, Sauria M, Taylor J, Kim Y & Zheng Y (2018) Lamins Organize the Global Three-Dimensional Genome from the Nuclear Periphery. Mol Cell 71, 802–815.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegenfeld AP, Roseman SA, Roh H, Lue NZ, Wagen CC, Zhou E, Johnstone SE, Aryee MJ & Liau BB (2022) Polycomb-lamina antagonism partitions heterochromatin at the nuclear periphery. Nat Commun 13, 4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzo SG, Dauban L & van Steensel B (2022) Lamina-associated domains: Tethers and looseners. Curr Opin Cell Biol 74, 80–87. [DOI] [PubMed] [Google Scholar]
- 31.Gruenbaum Y & Foisner R (2015) Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem 84, 131–164. [DOI] [PubMed] [Google Scholar]
- 32.Shimi T, Kittisopikul M, Tran J, Goldman AE, Adam SA, Zheng Y, Jaqaman K & Goldman RD (2015) Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol Biol Cell 26, 4075–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie W, Chojnowski A, Boudier T, Lim JS, Ahmed S, Ser Z, Stewart C & Burke B (2016) A-type Lamins Form Distinct Filamentous Networks with Differential Nuclear Pore Complex Associations. Curr Biol 26, 2651–2658. [DOI] [PubMed] [Google Scholar]
- 34.Nmezi B, Xu J, Fu R, Armiger TJ, Rodriguez-Bey G, Powell JS, Ma H, Sullivan M, Tu Y, Chen NY, Young SG, Stolz DB, Dahl KN, Liu Y & Padiath QS (2019) Concentric organization of A- and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina. Proc Natl Acad Sci U S A 116, 4307–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK & Spann TP (2002) Nuclear lamins: building blocks of nuclear architecture. Genes Dev 16, 533–547. [DOI] [PubMed] [Google Scholar]
- 36.Vahabikashi A, Sivagurunathan S, Nicdao FAS, Han YL, Park CY, Kittisopikul M, Wong X, Tran JR, Gundersen GG, Reddy KL, Luxton GWG, Guo M, Fredberg JJ, Zheng Y, Adam SA & Goldman RD (2022) Nuclear lamin isoforms differentially contribute to LINC complex-dependent nucleocytoskeletal coupling and whole-cell mechanics. Proc Natl Acad Sci U S A 119, e2121816119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckersley-Maslin MA, Bergmann JH, Lazar Z & Spector DL Lamin A/C is expressed in pluripotent mouse embryonic stem cells. Nucleus 4, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong X & Stewart CL (2020) The Laminopathies and the Insights They Provide into the Structural and Functional Organization of the Nucleus. Annu Rev Genomics Hum Genet 21, 263–288. [DOI] [PubMed] [Google Scholar]
- 39.Polioudaki H, Kourmouli N, Drosou V, Bakou A, Theodoropoulos PA, Singh PB, Giannakouros T & Georgatos SD (2001) Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. EMBO Rep 2, 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye Q, Callebaut I, Pezhman A, Courvalin JC & Worman HJ (1997) Domain-specific interactions of human HP1-type chromodomain proteins and inner nuclear membrane protein LBR. J Biol Chem 272, 14983–14989. [DOI] [PubMed] [Google Scholar]
- 41.Ye Q & Worman HJ (1996) Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J Biol Chem 271, 14653–14656. [DOI] [PubMed] [Google Scholar]
- 42.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan C-M, Gaiano N, Ko MSH & Zheng Y (2011) Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 334, 1706–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H & Joffe B (2013) LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152, 584–598. [DOI] [PubMed] [Google Scholar]
- 44.Amendola M & Steensel B (2015) Nuclear lamins are not required for lamina-associated domain organization in mouse embryonic stem cells. EMBO reports 16, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T, Gregory BD, Adams PD & Berger SL (2013) Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev 27, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang L, Li M, Shao S, Li C, Ai S, Xue B, Hou Y, Zhang Y, Li R, Fan X, He A, Li C & Sun Y (2022) Nuclear peripheral chromatin-lamin B1 interaction is required for global integrity of chromatin architecture and dynamics in human cells. Protein Cell 13, 258–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimi T, Pfleghaar K, Kojima S-I, Pack C-G, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T & Goldman RD (2008) The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 22, 3409–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naetar N, Georgiou K, Knapp C, Bronshtein I, Zier E, Fichtinger P, Dechat T, Garini Y & Foisner R (2021) LAP2alpha maintains a mobile and low assembly state of A-type lamins in the nuclear interior. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gesson K, Rescheneder P, Skoruppa MP, von Haeseler A, Dechat T & Foisner R (2016) A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res 26, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forsberg F, Brunet A, Ali TML & Collas P (2019) Interplay of lamin A and lamin B LADs on the radial positioning of chromatin. Nucleus 10, 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW & Collins FS (2003) Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah PP, Lv W, Rhoades JH, Poleshko A, Abbey D, Caporizzo MA, Linares-Saldana R, Heffler JG, Sayed N, Thomas D, Wang Q, Stanton LJ, Bedi K, Morley MP, Cappola TP, Owens AT, Margulies KB, Frank DB, Wu JC, Rader DJ, Yang W, Prosser BL, Musunuru K & Jain R (2021) Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Cell Stem Cell 28, 938–954.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong X, Hoskins VE, Melendez-Perez AJ, Harr JC, Gordon M & Reddy KL (2021) Lamin C is required to establish genome organization after mitosis. Genome Biol 22, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somech R, Shaklai S, Geller O, Amariglio N, Simon AJ, Rechavi G & Gal-Yam EN (2005) The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci 118, 4017–4025. [DOI] [PubMed] [Google Scholar]
- 55.Zullo JM, Demarco IA, Piqué-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL & Singh H (2012) DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 149, 1474–1487. [DOI] [PubMed] [Google Scholar]
- 56.Poleshko A, Shah PP, Gupta M, Babu A, Morley MP, Manderfield LJ, Ifkovits JL, Calderon D, Aghajanian H, Sierra-Pagán JE, Sun Z, Wang Q, Li L, Dubois NC, Morrisey EE, Lazar MA, Smith CL, Epstein JA & Jain R (2017) Genome-Nuclear Lamina Interactions Regulate Cardiac Stem Cell Lineage Restriction. Cell 171, 573–587.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demmerle J, Koch AJ & Holaska JM (2012) The nuclear envelope protein emerin binds directly to histone deacetylase 3 (HDAC3) and activates HDAC3 activity. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng L-C, Zhang X, Abhinav K, Nguyen JA, Baboo S, Martinez-Bartolomé S, Branon TC, Ting AY, Loose E, Yates JR 3rd & Gerace L (2022) Shared and Distinctive Neighborhoods of Emerin and Lamin B Receptor Revealed by Proximity Labeling and Quantitative Proteomics. J Proteome Res 21, 2197–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong X, Luperchio TR & Reddy KL (2014) NET gains and losses: the role of changing nuclear envelope proteomes in genome regulation. Curr Opin Cell Biol 28, 105–120. [DOI] [PubMed] [Google Scholar]
- 60.Schirmer EC, Florens L, Guan T, Yates JR 3rd & Gerace L (2003) Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 301, 1380–1382. [DOI] [PubMed] [Google Scholar]
- 61.Wilkie GS, Korfali N, Swanson SK, Malik P, Srsen V, Batrakou DG, de las Heras J, Zuleger N, Kerr ARW, Florens L & Schirmer EC (2011) Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol Cell Proteomics 10, M110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robson MI, de Las Heras JI, Czapiewski R, Lê Thành P, Booth DG, Kelly DA, Webb S, Kerr ARW & Schirmer EC (2016) Tissue-Specific Gene Repositioning by Muscle Nuclear Membrane Proteins Enhances Repression of Critical Developmental Genes during Myogenesis. Mol Cell 62, 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Las Heras JI, Zuleger N, Batrakou DG, Czapiewski R, Kerr ARW & Schirmer EC (2017) Tissue-specific NETs alter genome organization and regulation even in a heterologous system. Nucleus 8, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Las Heras JI, Meinke P, Batrakou DG, Srsen V, Zuleger N, Kerr AR & Schirmer EC (2013) Tissue specificity in the nuclear envelope supports its functional complexity. Nucleus 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng L-C, Zhang X, Baboo S, Nguyen JA, Martinez-Bartolomé S, Loose E, Diedrich J, Yates JR 3rd & Gerace L (2023) Comparative membrane proteomics reveals diverse cell regulators concentrated at the nuclear envelope. Life Sci Alliance 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Todorow V, Hintze S, Schoser B & Meinke P (2022) Nuclear envelope transmembrane proteins involved in genome organization are misregulated in myotonic dystrophy type 1 muscle. Front Cell Dev Biol 10, 1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramirez-Martinez A, Zhang Y, Chen K, Kim J, Cenik BK, McAnally JR, Cai C, Shelton JM, Huang J, Brennan A, Evers BM, Mammen PPA, Xu L, Bassel-Duby R, Liu N & Olson EN (2021) The nuclear envelope protein Net39 is essential for muscle nuclear integrity and chromatin organization. Nat Commun 12, 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shoeman RL & Traub P (1990) The in vitro DNA-binding properties of purified nuclear lamin proteins and vimentin. J Biol Chem 265, 9055–9061. [PubMed] [Google Scholar]
- 69.Luderus ME, den Blaauwen JL, de Smit OJ, Compton DA & van Driel R (1994) Binding of matrix attachment regions to lamin polymers involves single-stranded regions and the minor groove. Mol Cell Biol 14, 6297–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stierlé V, Couprie J, Ostlund C, Krimm I, Zinn-Justin S, Hossenlopp P, Worman HJ, Courvalin J-C & Duband-Goulet I (2003) The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochemistry 42, 4819–4828. [DOI] [PubMed] [Google Scholar]
- 71.Schibler AC, Jevtic P, Pegoraro G, Levy DL & Misteli T (2023) Identification of epigenetic modulators as determinants of nuclear size and shape. Elife 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pawar S & Kutay U (2021) The Diverse Cellular Functions of Inner Nuclear Membrane Proteins. Cold Spring Harb Perspect Biol 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luperchio TR, Sauria MEG, Hoskins VE, Wong X, DeBoy E, Gaillard M-C, Tsang P, Pekrun K, Ach RA, Yamada NA, Taylor J & Reddy KL (2018) The repressive genome compartment is established early in the cell cycle before forming the lamina associated domains. bioRxiv, 481598. [Google Scholar]
- 74.Falk M, Feodorova Y, Naumova N, Imakaev M, Lajoie BR, Leonhardt H, Joffe B, Dekker J, Fudenberg G, Solovei I & Mirny LA (2019) Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature 570, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiang M, Michieletto D, Brackley CA, Rattanavirotkul N, Mohammed H, Marenduzzo D & Chandra T (2019) Polymer Modeling Predicts Chromosome Reorganization in Senescence. Cell Rep 28, 3212–3223.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X & Karpen GH (2017) Phase separation drives heterochromatin domain formation. Nature 547, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S & Narlikar GJ (2017) Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samwer M, Schneider MWG, Hoefler R, Schmalhorst PS, Jude JG, Zuber J & Gerlich DW (2017) DNA Cross-Bridging Shapes a Single Nucleus from a Set of Mitotic Chromosomes. Cell 170, 956–972.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai M, Huang Y, Ghirlando R, Wilson KL, Craigie R & Clore GM (2001) Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. EMBO J 20, 4399–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haraguchi T, Koujin T, Segura-Totten M, Lee KK, Matsuoka Y, Yoneda Y, Wilson KL & Hiraoka Y (2001) BAF is required for emerin assembly into the reforming nuclear envelope. J Cell Sci 114, 4575–4585. [DOI] [PubMed] [Google Scholar]
- 81.Margalit A, Segura-Totten M, Gruenbaum Y & Wilson KL (2005) Barrier-to-autointegration factor is required to segregate and enclose chromosomes within the nuclear envelope and assemble the nuclear lamina. Proc Natl Acad Sci U S A 102, 3290–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montes de Oca R, Shoemaker CJ, Gucek M, Cole RN & Wilson KL (2009) Barrier-to-autointegration factor proteome reveals chromatin-regulatory partners. PLoS One 4, e7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Montes de Oca R, Andreassen PR & Wilson KL (2011) Barrier-to-Autointegration Factor influences specific histone modifications. Nucleus 2, 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montes de Oca R, Lee KK & Wilson KL (2005) Binding of barrier to autointegration factor (BAF) to histone H3 and selected linker histones including H1.1. J Biol Chem 280, 42252–42262. [DOI] [PubMed] [Google Scholar]
- 85.Keenen MM, Brown D, Brennan LD, Renger R, Khoo H, Carlson CR, Huang B, Grill SW, Narlikar GJ & Redding S (2021) HP1 proteins compact DNA into mechanically and positionally stable phase separated domains. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poleshko A, Kossenkov AV, Shalginskikh N, Pecherskaya A, Einarson MB, Marie Skalka A & Katz RA (2014) Human factors and pathways essential for mediating epigenetic gene silencing. Epigenetics 9, 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dunlevy KL, Medvedeva V, Wilson JE, Hoque M, Pellegrin T, Maynard A, Kremp MM, Wasserman JS, Poleshko A & Katz RA (2020) The PRR14 heterochromatin tether encodes modular domains that mediate and regulate nuclear lamina targeting. J Cell Sci 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poleshko A, Mansfield KM, Burlingame CC, Andrake MD, Shah NR & Katz RA (2013) The Human Protein PRR14 Tethers Heterochromatin to the Nuclear Lamina during Interphase and Mitotic Exit. Cell Rep 5, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiseleva AA, Cheng Y-C, Smith CL, Katz RA & Poleshko A (2023) PRR14 organizes H3K9me3-modified heterochromatin at the nuclear lamina. Nucleus 14, 2165602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez-Sandoval A, Towbin BD, Kalck V, Cabianca DS, Gaidatzis D, Hauer MH, Geng L, Wang L, Yang T, Wang X, Zhao K & Gasser SM (2015) Perinuclear Anchoring of H3K9-Methylated Chromatin Stabilizes Induced Cell Fate in C. elegans Embryos. Cell 163, 1333–1347. [DOI] [PubMed] [Google Scholar]
- 91.Wong X, Cutler JA, Hoskins VE, Gordon M, Madugundu AK, Pandey A & Reddy KL (2021) Mapping the micro-proteome of the nuclear lamina and lamina-associated domains. Life Sci Alliance 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agarwal N, Hardt T, Brero A, Nowak D, Rothbauer U, Becker A, Leonhardt H & Cardoso MC (2007) MeCP2 interacts with HP1 and modulates its heterochromatin association during myogenic differentiation. Nucleic Acids Res 35, 5402–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hiraga S-I, Ly T, Garzón J, Hořejší Z, Ohkubo Y-N, Endo A, Obuse C, Boulton SJ, Lamond AI & Donaldson AD (2017) Human RIF1 and protein phosphatase 1 stimulate DNA replication origin licensing but suppress origin activation. EMBO Rep 18, 403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foti R, Gnan S, Cornacchia D, Dileep V, Bulut-Karslioglu A, Diehl S, Buness A, Klein FA, Huber W, Johnstone E, Loos R, Bertone P, Gilbert DM, Manke T, Jenuwein T & Buonomo SCB (2016) Nuclear Architecture Organized by Rif1 Underpins the Replication-Timing Program. Mol Cell 61, 260–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vouzas AE & Gilbert DM (2023) Replication timing and transcriptional control: beyond cause and effect - part IV. Curr Opin Genet Dev 79, 102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ibrahim A, Papin C, Mohideen-Abdul K, Le Gras S, Stoll I, Bronner C, Dimitrov S, Klaholz BP & Hamiche A (2021) MeCP2 is a microsatellite binding protein that protects CA repeats from nucleosome invasion. Science 372. [DOI] [PubMed] [Google Scholar]
- 97.Gozalo A, Duke A, Lan Y, Pascual-Garcia P, Talamas JA, Nguyen SC, Shah PP, Jain R, Joyce EF & Capelson M (2020) Core Components of the Nuclear Pore Bind Distinct States of Chromatin and Contribute to Polycomb Repression. Mol Cell 77, 67–81.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shevelyov YY (2020) The Role of Nucleoporin Elys in Nuclear Pore Complex Assembly and Regulation of Genome Architecture. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu H, Ji Q, Song M, Ren J, Liu Z, Wang Z, Liu X, Yan K, Hu J, Jing Y, Wang S, Zhang W, Liu G-H & Qu J (2020) ZKSCAN3 counteracts cellular senescence by stabilizing heterochromatin. Nucleic Acids Res 48, 6001–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Biferali B, Bianconi V, Perez DF, Kronawitter SP, Marullo F, Maggio R, Santini T, Polverino F, Biagioni S, Summa V, Toniatti C, Pasini D, Stricker S, Di Fabio R, Chiacchiera F, Peruzzi G & Mozzetta C (2021) Prdm16-mediated H3K9 methylation controls fibro-adipogenic progenitors identity during skeletal muscle repair. Sci Adv 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kind J, Pagie L, de Vries SS, Nahidiazar L, Dey SS, Bienko M, Zhan Y, Lajoie B, de Graaf CA, Amendola M, Fudenberg G, Imakaev M, Mirny LA, Jalink K, Dekker J, van Oudenaarden A & van Steensel B (2015) Genome-wide maps of nuclear lamina interactions in single human cells. Cell 163, 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Luca KL & Kind J (2021) Single-Cell DamID to Capture Contacts Between DNA and the Nuclear Lamina in Individual Mammalian Cells. In Capturing Chromosome Conformation: Methods and Protocols (Bodega B & Lanzuolo C, eds), pp. 159–172. Springer; US, New York, NY. [DOI] [PubMed] [Google Scholar]
- 103.Shah PP, Keough KC, Gjoni K, Santini GT, Abdill RJ, Wickramasinghe NM, Dundes CE, Karnay A, Chen A, Salomon REA, Walsh PJ, Nguyen SC, Whalen S, Joyce EF, Loh KM, Dubois N, Pollard KS & Jain R (2023) An atlas of lamina-associated chromatin across twelve human cell types reveals an intermediate chromatin subtype. Genome Biol 24, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leemans C, van der Zwalm MCH, Brueckner L, Comoglio F, van Schaik T, Pagie L, van Arensbergen J & van Steensel B (2019) Promoter-Intrinsic and Local Chromatin Features Determine Gene Repression in LADs. Cell 177, 852–864.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reddy KL, Zullo JM, Bertolino E & Singh H (2008) Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 452, 243–247. [DOI] [PubMed] [Google Scholar]
- 106.Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, Winandy S & Singh H (2008) Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol 9, 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schlimgen RJ, Reddy KL, Singh H & Krangel MS (2008) Initiation of allelic exclusion by stochastic interaction of Tcrb alleles with repressive nuclear compartments. Nat Immunol 9, 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bertolino E, Reddy K, Medina KL, Parganas E, Ihle J & Singh H (2005) Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat Immunol 6, 836–843. [DOI] [PubMed] [Google Scholar]
- 109.Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E & Busslinger M (2004) Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene 10.1101/gad.291504. Genes Dev 18, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fitzsimmons SP, Bernstein RM, Max EE, Skok JA & Shapiro MA (2007) Dynamic changes in accessibility, nuclear positioning, recombination, and transcription at the Ig kappa locus. J Immunol 179, 5264–73. [DOI] [PubMed] [Google Scholar]
- 111.Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG & Singh H (2002) Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296, 158–162. [DOI] [PubMed] [Google Scholar]
- 112.Luperchio TR, Wong X & Reddy KL (2014) Genome regulation at the peripheral zone: lamina associated domains in development and disease. Curr Opin Genet Dev 25C, 50–61. [DOI] [PubMed] [Google Scholar]
- 113.Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SWM, Solovei I, Brugman W, Gräf S, Flicek P, Kerkhoven RM, van Lohuizen M, Reinders M, Wessels L & van Steensel B (2010) Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell 38, 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lochs SJA, Kefalopoulou S & Kind J (2019) Lamina Associated Domains and Gene Regulation in Development and Cancer. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV & Ren B (2012) A map of the cis-regulatory sequences in the mouse genome. Nature 488, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schoenfelder S, Furlan-Magaril M, Mifsud B, Tavares-Cadete F, Sugar R, Javierre B-M, Nagano T, Katsman Y, Sakthidevi M, Wingett SW, Dimitrova E, Dimond A, Edelman LB, Elderkin S, Tabbada K, Darbo E, Andrews S, Herman B, Higgs A, LeProust E, Osborne CS, Mitchell JA, Luscombe NM & Fraser P (2015) The pluripotent regulatory circuitry connecting promoters to their long-range interacting elements. Genome Res 25, 582–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jabbari K & Bernardi G (2017) An Isochore Framework Underlies Chromatin Architecture. PLoS One 12, e0168023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robson MI, de Las Heras JI, Czapiewski R, Sivakumar A, Kerr ARW & Schirmer EC (2017) Constrained release of lamina-associated enhancers and genes from the nuclear envelope during T-cell activation facilitates their association in chromosome compartments. Genome Res 27, 1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen S, Luperchio TR, Wong X, Doan EB, Byrd AT, Roy Choudhury K, Reddy KL & Krangel MS (2018) A Lamina-Associated Domain Border Governs Nuclear Lamina Interactions, Transcription, and Recombination of the Tcrb Locus. Cell Rep 25, 1729–1740.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gibcus JH, Samejima K, Goloborodko A, Samejima I, Naumova N, Nuebler J, Kanemaki MT, Xie L, Paulson JR, Earnshaw WC, Mirny LA & Dekker J (2018) A pathway for mitotic chromosome formation. Science 359, eaao6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Güttinger S, Laurell E & Kutay U (2009) Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol 10, 178–191. [DOI] [PubMed] [Google Scholar]
- 122.Gerace L & Blobel G (1980) The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell 19, 277–287. [DOI] [PubMed] [Google Scholar]
- 123.Nagano T, Wingett SW & Fraser P (2017) Capturing Three-Dimensional Genome Organization in Individual Cells by Single-Cell Hi-C. Methods Mol Biol 1654, 79–97. [DOI] [PubMed] [Google Scholar]
- 124.Zhang H, Emerson DJ, Gilgenast TG, Titus KR, Lan Y, Huang P, Zhang D, Wang H, Keller CA, Giardine B, Hardison RC, Phillips-Cremins JE & Blobel GA (2019) Chromatin structure dynamics during the mitosis-to-G1 phase transition. Nature 576, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Skene PJ & Henikoff S (2017) An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikegami K, Secchia S, Almakki O, Lieb JD & Moskowitz IP (2020) Phosphorylated Lamin A/C in the Nuclear Interior Binds Active Enhancers Associated with Abnormal Transcription in Progeria. Dev Cell 52, 699–713.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feodorova Y, Falk M, Mirny LA & Solovei I (2020) Viewing Nuclear Architecture through the Eyes of Nocturnal Mammals. Trends Cell Biol 30, 276–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Poleshko A, Smith CL, Nguyen SC, Sivaramakrishnan P, Wong KG, Murray JI, Lakadamyali M, Joyce EF, Jain R & Epstein JA (2019) H3K9me2 orchestrates inheritance of spatial positioning of peripheral heterochromatin through mitosis. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crosio C, Cermakian N, Allis CD & Sassone-Corsi P (2000) Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci 3, 1241–1247. [DOI] [PubMed] [Google Scholar]
- 130.Pacheco-Bernal I, Becerril-Pérez F & Aguilar-Arnal L (2019) Circadian rhythms in the three-dimensional genome: implications of chromatin interactions for cyclic transcription. Clin Epigenetics 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim YH, Marhon SA, Zhang Y, Steger DJ, Won K-J & Lazar MA (2018) Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science 359, 1274–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 18, 164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu Y, Guo W, Li P, Zhang Y, Zhao M, Fan Z, Zhao Z & Yan J (2016) Long-Range Chromosome Interactions Mediated by Cohesin Shape Circadian Gene Expression. PLoS Genet 12, e1005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin S-T, Zhang L, Lin X, Zhang LC, Garcia VE, Tsai C-W, Ptáček L & Fu Y-H (2014) Nuclear envelope protein MAN1 regulates clock through BMAL1. Elife 3, e02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao H, Sifakis EG, Sumida N, Millán-Ariño L, Scholz BA, Svensson JP, Chen X, Ronnegren AL, Mallet de Lima CD, Varnoosfaderani FS, Shi C, Loseva O, Yammine S, Israelsson M, Rathje L-S, Németi B, Fredlund E, Helleday T, Imreh MP & Göndör A (2015) PARP1- and CTCF-Mediated Interactions between Active and Repressed Chromatin at the Lamina Promote Oscillating Transcription. Mol Cell 59, 984–997. [DOI] [PubMed] [Google Scholar]
- 136.Brunet A, Forsberg F, Fan Q, Sæther T & Collas P (2019) Nuclear Lamin B1 Interactions With Chromatin During the Circadian Cycle Are Uncoupled From Periodic Gene Expression. Front Genet 10, 917. [DOI] [PMC free article] [PubMed] [Google Scholar]




