Abstract
Objective
Anti-dsDNA antibodies (anti-dsDNA) are a component of all classification schemes in SLE and comprise one of the domains in validated activity indices. Anti-dsDNA is frequently measured commercially by an enzyme immunoassay (EIA) or Crithidia luciliae immunofluorescence test (CLIFT). To address the clinical impact of measuring these antibodies by two different assays, this study leveraged a well-phenotyped multiethnic/racial cohort.
Methods
All patients fulfilled the classification criteria for SLE by at least one of the validated schemes: American College of Rheumatology, Systemic Lupus Erythematosus International Collaborating Clinics and/or American College of Rheumatology/European League Against Rheumatism classification criteria. Patients with one or more simultaneously paired anti-dsDNA by multiplex EIA and CLIFT were identified. Analysis of concordance or discordance, titre comparability of assays and association with hybrid SLE Disease Activity Index score, prevalence of lupus nephritis (LN), ability to predict a flare and classification criteria was performed.
Results
207 patients were simultaneously tested by EIA and CLIFT at least once for anti-dsDNA, generating 586 paired results. 377 pairs were concordant and 209 were discordant. 41 of 207 patients always had discordant paired results and 39 patients always had results with titre discordance. In 100 patients with LN, 60 were positive by EIA and 72 by CLIFT. Sensitivities and specificities for patients with LN versus patients without LN were EIA 60% and 47%, and CLIFT 72% and 37%, respectively. 42 patients had flare assessment within 90 days of their paired result. Six of seven patients with mild flares and all four patients with severe flares had concordant positive results.
Conclusion
Our data demonstrate that discordance of positivity between both assays for anti-dsDNA is relatively common, occurring in a fifth of patients overall and a third of visits. EIA positivity is associated with LN less often than CLIFT positivity. With the significant discordance of results between anti-dsDNA assays, obtaining both CLIFT and EIA assays may be beneficial for classification and routine monitoring of SLE.
Keywords: Antibodies; Autoantibodies; Autoimmune Diseases; Lupus Erythematosus, Systemic; Lupus Nephritis
WHAT IS ALREADY KNOWN ON THIS TOPIC
A gold standard assay for anti-dsDNA antibodies (anti-dsDNA) antibody detection in SLE diagnosis and disease activity monitoring does not exist.
Of the two most used assays, enzyme immunoassays (EIAs) are considered to be more sensitive and less specific compared with Crithidia luciliae immunofluorescence test (CLIFT).
WHAT THIS STUDY ADDS
Our study reveals high discordance of assay results in a multiethnic SLE cohort which had simultaneous EIA and CLIFT assays performed during an encounter.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Clinicians should consider using more than one anti-dsDNA assay for diagnosis and routine monitoring of SLE.
Introduction
SLE is characterised by autoantibody production. Anti-dsDNA antibodies (anti-dsDNA) are highly SLE-specific and are included in the diagnostic criteria for SLE.1 2 Anti-dsDNA is detected using various assays with different methods for quantifying immunological responses to native DNA. However, autoimmune responses to DNA among individuals are diverse and can produce different results depending on the anti-dsDNA assay used. Inconsistent results can have clinical implications as positive anti-dsDNA is an important component of the classification criteria for diagnosing SLE, and serial measurement of anti-dsDNA is often used to monitor lupus disease activity, especially lupus nephritis (LN).3–5
Currently, two prominently used anti-dsDNA assays are enzyme immunoassays (EIA), such as the multiplex flow immunoassay, and the Crithidia luciliae indirect immunofluorescence test (CLIFT). CLIFT requires fluorescence microscopy and slide interpretation, a more intensive technique than EIA. It detects medium to high avidity antibodies using the substrate Crithidia luciliae. Overall, CLIFT is considered to have relatively high specificity for SLE but lower sensitivity compared with EIA.6–8 EIA uses a more automated methodology that eliminates technician variability and is less cumbersome to perform than CLIFT. It detects low and high-avidity antibodies using different antigenic DNA sources depending on the EIA used.9 Traditionally, EIA is considered more sensitive for anti-dsDNA in SLE and has been recommended as the primary method of anti-dsDNA detection in SLE.7 10
In the present study, a large multiracial and multiethnic lupus registry was leveraged to study the relationship between EIA and CLIFT anti-dsDNA results, including an analysis of the absolute concordance, discordance and titre comparability of assays and their association with hybrid SLE Disease Activity Index (SLEDAI) score, prevalence of LN, ability to predict subsequent flares and classification criteria.
Patients/methods
Subjects from the diverse New York University Lupus Cohort of patients with SLE seen at NYU Langone Health (NYULH) and Bellevue Hospital Center since 2014 were used. Inclusion was based on the diagnosis of SLE by the revised American College of Rheumatology (ACR), Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) and/or 2019 ACR/European League Against Rheumatism (EULAR) classification criteria.11–13 Consent to participate in the research cohort was provided in English, Spanish or Mandarin. Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research. Within this cohort, we identified patients who, at the discretion of the treating provider, had one or more simultaneous paired anti-dsDNA results by both multiplex EIA and CLIFT. Out of 207 patients, 204 had 581 paired results performed by the NYULH laboratory facilities, where the upper limit of normal for EIA is 10 IU/mL.14 Three of the 207 patients had five paired results performed by Bellevue Hospital facilities with an upper limit of normal of 75 IU/mL.15 The specific EIA assay at NYULH was Biorad’s Bioplex 2200 using reagent catalogue number 6651150, and at Bellevue Hospital was Inova Diagnostics QUANTA Lite SC dsDNA. CLIFT results were performed by Associated Regional and University Pathologists, Inc. (ARUP Laboratories), a Salt Lake City, Utah-based non-profit laboratory at the University of Utah’s Department of Pathology using the same method for both locations with an upper limit of normal of 1:10.16
We report a number and percentage of discordance for the 207 patients and for the 586 visits with paired results. Paired results were either concordant or discordant for each visit. Patients with more than two results were classified as having always concordant, fluctuating concordance or always discordant paired results. For the subset of concordant results where both assays were positive, we report a number and percentage for discrepancy by titre for both patients and paired results. Tertiles for EIA and CLIFT were defined as low positive (11–50 (NYULH), 75–375 (Bellevue) and 1:10–1:40), moderate positive (51–200 (NYULH), 376–1500 (Bellevue) and 1:80–1:320) and high positive (>200 (NYULH), >1500 (Bellevue) and >1:640) and compared for each positive concordant paired result. When possible, information on disease activity at the time of paired result as measured by the hybrid SLEDAI score (excluding the anti-dsDNA domain), renal involvement and the presence of a low C3 and or C4 was provided to assess assay positivity in the context of disease activity.17
The ability to classify as SLE based on anti-dsDNA positivity was evaluated using retrospective medical record review. LN was identified in a subset of patients who had either biopsy-proven LN, persistent proteinuria ≥500 mg/gm per day, and or cellular casts as defined by ACR classification criteria for LN.11 Paired results independent of renal activity were used to assess variability in assay positivity in the context of kidney disease. Patients with LN with always negative EIA and positive CLIFT results were compared with those with always positive EIA and negative CLIFT results in ISN/RPS class, average urine protein to creatinine ratio (uPCR) at the visit with paired results and presence of hypocomplementemia.
Investigation of each assay’s association with a mild or severe SLE flare in the subsequent 90 days was made. Patients who had flares within 90 days of their paired result were identified using the SELENA-SLEDAI Flare Index.18 19 Analysis of assay positivity prior to subsequent flare was done in the context of hypocomplementemia.
Statistical analysis
The sensitivity, specificity, positive predictive value and negative predictive value of anti-dsDNA as measured by EIA and CLIFT were calculated for LN versus non-LN cohorts. A two-tailed sample t-test with unequal variance was used to compare the significance of uPCR difference in always positive EIA and negative CLIFT versus negative EIA and positive CLIFT in LN group analysis.
Results
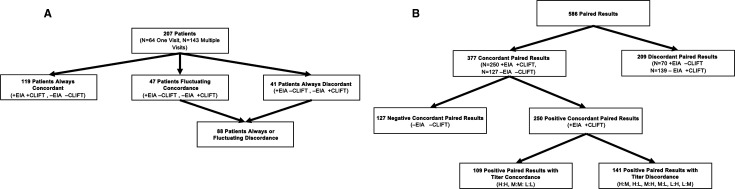
The cohort demographics of the 207 patients analysed were self-identified by patients and consisted of 92% female, 22% Hispanic ethnicity, 24% black, 16% Asian, 49% white and 10% other. Among the 207 patients, 64 had one visit with paired tests and could only be categorised as always concordant or always discordant. Of these 64 patients, 46 had concordant results: 18 had both positive EIA and CLIFT, and 28 had negative EIA and CLIFT. The remaining 18 patients had discordance: 8 had a positive EIA and negative CLIFT, and 10 had a negative EIA and positive CLIFT. The remaining 143 patients had two or more visits with paired tests and were categorised as always concordant, always discordant or as having fluctuating concordance if their paired results varied between concordant and discordant. The concordance of these 143 patients was as follows: 73 always, 23 never and 47 fluctuating. Thus, of the overall cohort of 207 patients, 41 (20%) patients were always discordant; 18 with one visit, 23 with two or more visits. Including the 47 patients with fluctuating concordance, 88 patients had discordant results in at least one visit (figure 1A).
Figure 1.
Flow chart depicting concordance and discordance of EIA and CLIFT by (A) patients and (B) the 586 paired results. CLIFT, Crithidia luciliae immunofluorescence test; EIA, enzyme immunoassay.
The 207 patients generated 586 paired results for analysis (table 1, figure 1B). Of the 586 paired results, 377 (64%) pairs were concordant: 250 (42%) of paired results had both positive EIA and CLIFT, and 127 (22%) had negative results for both. The remaining 209 (36%) were discordant; 70 (12%) had a positive EIA and negative CLIFT, and 139 (24%) had a negative EIA and positive CLIFT.
Table 1.
SLE disease activity during the time of paired result analysed by anti-dsDNA titre tertiles
| dsDNA titre (EIA/CLIFT) | Total visits | Available mean SLEDAI score (range) (n=490) | Renal (uPCR >0.5) at visit (%) |
Low complements at visit (%) |
| H/H | 19 | 6.46 (0–16) (n=13) | 9 (47%) | 18 (95%) |
| H/M | 12 | 4.72 (0–16) (n=11) | 4 (33%) | 10 (83%) |
| H/L | 4 | 3.00 (0–6) (n=2) | 1 (25%) | 3 (75%) |
| M/H | 22 | 7.00 (0–18) (n=15) | 15 (68%) | 17 (77%) |
| M/M | 43 | 3.55 (0–16) (n=33) | 12 (28%) | 29 (67%) |
| M/L | 11 | 3.60 (0–14) (n=10) | 2 (18%) | 4 (36%) |
| M/N | 4 | 0 (0–0) (n=3) | 0 (0%) | 0 (0%) |
| L/H | 20 | 4.15 (0–14) (n=20) | 8 (40%) | 13 (65%) |
| L/M | 72 | 2.11 (0–16) (n=59) | 11 (15%) | 33 (46%) |
| L/L | 47 | 2.74 (0–14) (n=39) | 12 (26%) | 15 (32%) |
| L/N | 66 | 2.02 (0–10) (n=50) | 6 (9%) | 24 (36%) |
| N/H | 11 | 2.45 (0–6) (n=11) | 3 (27%) | 6 (55%) |
| N/M | 68 | 2.57 (0–18) (n=61) | 11 (16%) | 30 (44%) |
| N/L | 60 | 1.29 (0–10) (n=55) | 2 (3%) | 25 (42%) |
| N/N | 127 | 1.98 (0–12) (n=108) | 22 (17%) | 48 (38%) |
anti-dsDNA, anti-dsDNA antibodies; H, high positive; L, low positive; M, moderate positive; N, negative; SLEDAI, SLE Disease Activity Index; uPCR, urine protein to creatinine ratio.
The 250 positive concordant paired results came from 98 patients and were analysed for titre concordance using the previously defined tertiles of high positive, moderate positive and low positive titres. Of those results, 109 (44%) had titre concordance and 141 (56%) had titre discordance. Of the 98 patients, 35 (36%) always had titre concordance, 24 (24%) had fluctuating titre concordance and 39 (40%) always had titre discordance.
EIA and CLIFT association with hybrid SLEDAI score
Available mean SLEDAI scores, excluding the anti-dsDNA domain, were reported for every possible combination of titre tertile for paired results (table 1). Mean SLEDAI scores >4 were associated with pairs when both assay results were high titre, one result was high titre and the other moderate titre and the combination of low titre EIA and high titre CLIFT. The opposite pairing of a high titre EIA and low titre CLIFT had a mean SLEDAI of only 3. Thus, of the results where both assays were positive, mean SLEDAI was elevated (>4) in all pairs when CLIFT was high, regardless of the EIA tertile. Low positive or negative EIA associated with a mean SLEDAI <3 regardless of CLIFT titre in all cases, except low titre EIA and high titre CLIFT. Although low titre, the mean SLEDAI was above zero in 139 visits with a negative EIA but high, moderate or low positive CLIFT.
EIA and CLIFT association with LN
Of the 207 patients, 100 met the criteria for LN and had simultaneous anti-dsDNA results independent of their current LN activity. Seventy-six patients had biopsy-proven LN and 24 had LN as defined by the ACR classification criteria (ie, either persistent proteinuria ≥500 mg/gm Cr per day and or cellular casts). Of those patients with LN, 60 had at least one positive EIA result and 72 had at least one positive CLIFT result of the visits with paired results (table 2). Of the remaining 107 patients without LN, 57 had at least one positive EIA result and 67 had at least one positive CLIFT result. Between the LN and non-LN subgroups, sensitivity and specificity for each test was as follows: EIA 60% (95% CI 50% to 70%) and 47% (95% CI 37% to 57%), and CLIFT 72% (95% CI 62% to 81%) and 37% (95% CI 28% to 47%). Further, the positive and negative predictive values were 51% (95% CI 42% to 61%) and 55% (95% CI 45% to 66%) for EIA, and 52% (95% CI 43% to 60%) and 59% (95% CI 46% to 71%) for CLIFT, respectively. Regardless of LN activity at the time of paired result, hypocomplementemia was present in 88% of patients with positive EIA results and 89% with positive CLIFT results.
Table 2.
Relationship between EIA, CLIFT and hypocomplementemia with LN
| >1 positive EIA result | Only negative EIA results | >1 positive CLIFT result | Only negative CLIFT results | |
| Patients with LN (n=100) | 60 | 40 | 72 | 28 |
| Patients without LN (n=107) | 57 | 50 | 67 | 40 |
| Low complements ever (%) | 53 (88%) | 26 (65%) | 64 (89%) | 15 (54%) |
CLIFT, Crithidia luciliae immunofluorescence test; EIA, enzyme immunoassay; LN, lupus nephritis.
Of the patients with LN with always discordant results, 5 had paired results that were always positive EIA and negative CLIFT, and 10 had results of always negative EIA and positive CLIFT (table 3). Patients with one or more of these paired results were included for a total of 31 paired visits. Patients with always negative EIA and positive CLIFT paired results had a mean uPCR of 1.393 (0.386 SE) at the time of paired results compared with 0.363 (0.110 SE) of those with always positive EIA and negative CLIFT results. A two-tailed sample t-test with unequal variance had a p value of 0.02 and was used to assess the significance of the difference in uPCR at each visit between groups. Hypocomplementemia was present in 45% of visits from patients with always negative EIA and positive CLIFT results and 30% of visits from patients with always positive EIA and negative CLIFT results.
Table 3.
Comparison of patients with LN with results either always −EIA/+CLIFT or always +EIA/−CLIFT
| Always −EIA/+CLIFT |
Always +EIA/−CLIFT |
|
| Patients with LN (n) | n=10 | n=5 |
| Visits with paired results (n) | 21 | 10 |
| ISN class: | n=8 | n=4 |
| Class I | 0 | 0 |
| Class II | 3 | 0 |
| Class III | 1 | 2 |
| Class IV | 0 | 0 |
| Class V | 2 | 2 |
| Mixed IV/V | 1 | 0 |
| Class not assigned | 1 | 0 |
| Mean uPCR at visit (SE) | 1.393 (0.386) | 0.363 (0.110) |
| Low C3 or C4 at paired visit | 9 (45%) (n=20) | 3 (30%) |
CLIFT, Crithidia luciliae immunofluorescence test; EIA, enzyme immunoassay; LN, lupus nephritis; uPCR, urine protein to creatinine ratio.
EIA and CLIFT prediction of SLE flares
Fifty-one visits from 42 patients had paired anti-dsDNA results and a SELENA-SLEDAI Flare Index assessment within 90 days. Six of seven patients had concordant paired results prior to a mild flare at their subsequent encounter: three with positive concordant paired results, three with negative concordant paired results and one with discordant results (table 4). All four patients with severe flares had concordant paired results at their prior visit: three with positive paired results and one with negative paired results. Low C3 and or C4 occurred in one of seven (14%) patients with mild flares and four of four (100%) with severe flares.
Table 4.
Relationship between EIA, CLIFT, hypocomplementemia and flare within 90 days
| dsDNA titres at baseline visit (non-flare) (EIA/CLIFT) | Mild flare at next encounter ≤90 days | Severe flare at next encounter ≤90 days | ||
| Patients (n=7) | Low complements | Patients (n=4) | Low complements | |
| H/H | 1 | 0 | 0 | 0 |
| H/M | 0 | 0 | 2 | 2 |
| L/M | 1 | 1 | 0 | 0 |
| L/L | 1 | 0 | 1 | 1 |
| L/N | 1 | 0 | 0 | 0 |
| N/N | 3 | 0 | 1 | 1 |
CLIFT, Crithidia luciliae immunofluorescence test ; EIA, enzyme immunoassay; H, high positive; L, low positive; M, moderate positive.
EIA and CLIFT association with classification criteria
Among the 41 patients with always discordant EIA and CLIFT results, analysis was performed to explore if anti-dsDNA positivity was required to satisfy classification criteria for SLE. Two patients with a low titre positive EIA and negative CLIFT would not have met the classification criteria for diagnosis of SLE by ACR, SLICC, or 2019 ACR/EULAR without the positive anti-dsDNA result by EIA. Neither patient had LN.
Discussion
Anti-dsDNA is an SLE-specific autoantibody that is critically important to the diagnosis and disease activity monitoring of SLE. Our study evaluated the concordance of widely used commercial methods of assessing anti-dsDNA: EIA and CLIFT assays. Fifty-seven per cent of patients had concordant paired results, 23% had fluctuating results and 20% had discordant results. Of the paired results, 64% were concordant, and 36% were discordant. Titre discordance was present in 56% of positive concordant paired results and high positive CLIFT was more often associated with a high hybrid SLEDAI score. Within the cohort of patients with LN, CLIFT positivity was associated with LN more often than EIA positivity. Flares were infrequent and associated with either EIA or CLIFT positivity, with severe flares more likely if accompanied by hypocomplementemia.
In addition to multiplex EIA and CLIFT, there are several other anti-dsDNA assays commercially available such as radioactive assays (Farr and polyethylene glycol), immunoblot assays and other EIA assays (ELISA, enzyme fluoroimmunoassay, chemiluminescence immunoassay). In the literature, studies show concordant and discordant results comparing EIA and CLIFT; thus, there is no established gold standard method for anti-dsDNA detection. Different sources of DNA likely explain the discordance between anti-dsDNA assays. As a highly charged polymer substrate with structural heterogeneity, DNA antigenicity varies by DNA origin, size, conformation and mobility, affecting anti-dsDNA autoantigens.20 21 Alternatively, discordance could be caused by circulating factors that modify the antigen target and produce false positives (ie, LDL/IgG complexes that non-specifically bind to the Crithidia flagellate).22 Our study found significant discordance between paired results. Of the 209 discordant pairs, 67% were EIA negative and CLIFT positive. However, the finding of two patients that were always EIA positive and CLIFT negative and would not have met the criteria for SLE diagnosis by ACR, EULAR or SLICC criteria without anti-dsDNA positivity is also of note. While acknowledging the low titre positivity of EIA in these two patients, this finding is consistent with EIA having greater sensitivity for SLE diagnosis. These results are in agreement with other studies demonstrating that EIA has a higher sensitivity for SLE diagnosis than CLIFT and that recommend using multiple anti-dsDNA assays for diagnosis.7 8 10 23 24 Viriyataveekul et al researched anti-dsDNA detection in EIAs, CLIFTs and combined EIA and CLIFT testing and found the highest sensitivity for anti-dsDNA in combined assays.25 In aggregate, the significant discordance of results in the present study aligns with research recommending the utilisation of more than one method of anti-dsDNA measurement in the diagnosis and routine monitoring of SLE.
To our knowledge, the assessment of titre discordance within positive concordant paired results has not yet been studied. Within our arbitrarily defined tertiles of high, moderate and low positive titres, we found 141 of 250 positive concordant paired results to have titre discordance. Forty percent of the patients contributing to the 250 results, always had titre discordance. This finding further demonstrates the discrepancy between assay results and, depending on the assay used, could have potential implications in the disease management of patients or clinical trial results if inclusion criteria or assessment of activity is defined by a specific anti-dsDNA titre.
Interestingly, within our study’s cohort of patients with LN, 72% had at least one positive CLIFT result of the paired results while only 60% had EIA positivity. Compared to patients with SLE but without LN, sensitivities and specificities were 60% and 47% for EIA, and 72% and 37% for CLIFT, respectively. Additionally, CLIFT positivity was associated with a greater magnitude of proteinuria. These results are similar to the findings in a study by López-Hoyos et al demonstrating that CLIFT positivity was slightly more sensitive for LN than EIA.26 However, this contrasts with research by Zhao et al and Hernando et al which found EIAs to be more sensitive for LN than CLIFT.24 27 The discrepancy of results between EIA and CLIFT for LN supports the recommendation of Jaekell et al to use more than one assay for anti-dsDNA measurement in routine monitoring and diagnosis of LN.28
Our study has several limitations. As discussed, there are different EIA methods of anti-dsDNA measurement, and we only studied the use of the multiplex EIA. Because each EIA uses a distinct method for detecting immunological responses to DNA, greater insight into the variance between assays could have been provided with a broader assay assessment. The choice of anti-dsDNA tests was not protocolised, and paired results were at the discretion of the ordering provider. Specifically, combined testing was uncommon during the initial presentation for diagnosis, and most often, dual testing typically occurred during disease management. Therefore, only 2 of the 207 patients met one of the classification criteria based on only one positive anti-dsDNA, in this case, EIA, which may underestimate the value of combined testing at the time of diagnosis. Moreover, tertiles evaluating titre concordance were created arbitrarily and further research is required to assess their effective categorisation within paired results. Additionally, LN was defined by renal biopsy diagnosis or by the ACR classification criteria for LN and did not necessarily account for renal activity during the time of paired result. Therefore, LN classification could have represented prior activity and/or renal damage instead of ongoing nephritis. Furthermore, not all patients with LN had both tests, so these findings may not be generalisable across all patients with kidney disease. Our report is also limited to the association between anti-dsDNA testing methods and LN, given it’s pathogenic role in this disease manifestation. Future studies can investigate the relationship between anti-dsDNA testing methods and other clinical features, such as mucocutaneous, musculoskeletal, hematological, etc. Finally, only 9% of visits with paired results had another encounter within 90 days that could be evaluated for a flare defined by the SELENA-SLEDAI flare index. Of these visits, 11 were classified as flares, limiting our power to demonstrate a meaningful difference in each anti-dsDNA assay’s ability to predict SLE flares.
In summary, the discordance of positivity between EIA and CLIFT assays for anti-dsDNA occurred in 20% of patients and 36% of visits. In 56% of visits and 40% of patients, the anti-dsDNA titres of paired results were discordant. Future studies relying on protocolised prospective dual testing can better clarify the benefit of satisfying classification criteria, identifying or anticipating LN, predicting extra-renal flares, and confirming serological response. Regardless, our results align with prior studies demonstrating a high prevalence of discordance between prominent anti-dsDNA assays, supporting the utility of combined testing in diagnosis and routine monitoring for SLE activity.
Footnotes
Contributors: All authors listed above contributed to the manuscript. HMB is the guarantor for this research.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JPB is an Editorial Board Member at Lupus Science & Medicine. DZ, HMB, AS and PI have no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by NYULH IRB: i14-00487. Participants gave informed consent to participate in the study before taking part.
References
- 1. Kavanaugh AF, Solomon DH, American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines . Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-DNA antibody tests. Arthritis Rheum 2002;47:546–55. 10.1002/art.10558 [DOI] [PubMed] [Google Scholar]
- 2. Kunkel HG. Advances in immunology. New York London: Academic Press, 1982. [Google Scholar]
- 3. ter Borg EJ, Horst G, Hummel EJ, et al. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum 1990;33:634–43. 10.1002/art.1780330505 [DOI] [PubMed] [Google Scholar]
- 4. Ghirardello A, Villalta D, Morozzi G, et al. Diagnostic accuracy of currently available anti-double-stranded DNA antibody assays. Clin Exp Rheumatol 2011;29:50–6. [PubMed] [Google Scholar]
- 5. Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med 1967;126:607–24. 10.1084/jem.126.4.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Compagno M, Jacobsen S, Rekvig OP, et al. Low diagnostic and predictive value of anti-dsDNA antibodies in unselected patients with recent onset of rheumatic symptoms: results from a long-term follow-up Scandinavian multicentre study. Scand J Rheumatol 2013;42:311–6. 10.3109/03009742.2013.765032 [DOI] [PubMed] [Google Scholar]
- 7. Antico A, Platzgummer S, Bassetti D, et al. Diagnosing systemic lupus erythematosus: new-generation immunoassays for measurement of anti-dsDNA antibodies are an effective alternative to the Farr technique and the Crithidia Luciliae Immunofluorescence test. Lupus 2010;19:906–12. 10.1177/0961203310362995 [DOI] [PubMed] [Google Scholar]
- 8. Kim KH, Han JY, Kim JM, et al. Clinical significance of ELISA positive and immunofluorescence negative anti-dsDNA antibody. Clin Chim Acta 2007;380:182–5. 10.1016/j.cca.2007.02.010 [DOI] [PubMed] [Google Scholar]
- 9. Ahsan H. Monoplex and multiplex immunoassays: approval, advancements, and alternatives. Comp Clin Path 2022;31:333–45. 10.1007/s00580-021-03302-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janyapoon K, Jivakanont P, Choosang K, et al. Comparative study of anti-double stranded DNA detection by ELISA and Crithidia Luciliae Immunofluorescence. Southeast Asian J Trop Med Public Health 2003;34:646–50. [PubMed] [Google Scholar]
- 11. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 12. Aringer M, Costenbader K, Daikh D, et al. European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. 10.1002/art.40930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus International collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Houser B. Bio-Rad’s bio-Plex® suspension array system, xMAP technology overview. Arch Physiol Biochem 2012;118:192–6. 10.3109/13813455.2012.705301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anti-DS DNA - NWH test compendium. Available: https://labs.northwell.edu/test/655995 [Accessed 05 Jul 2023].
- 16. Chiaro TR, Davis KW, Wilson A, et al. Significant differences in the analytic concordance between anti-dsDNA IgG antibody assays for the diagnosis of systemic lupus erythematosus--implications for inter-laboratory testing. Clin Chim Acta 2011;412:1076–80. 10.1016/j.cca.2011.02.025 [DOI] [PubMed] [Google Scholar]
- 17. Touma Z, Gladman DD, Ibañez D, et al. Development and initial validation of the systemic lupus erythematosus disease activity index 2000 responder index 50. J Rheumatol 2011;38:275–84. 10.3899/jrheum.100724 [DOI] [PubMed] [Google Scholar]
- 18. Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med 2005;142:953–62. 10.7326/0003-4819-142-12_part_1-200506210-00004 [DOI] [PubMed] [Google Scholar]
- 19. Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999;8:685–91. 10.1191/096120399680411281 [DOI] [PubMed] [Google Scholar]
- 20. Pisetsky DS. Anti-DNA antibodies--Quintessential biomarkers of SLE. Nat Rev Rheumatol 2016;12:102–10. 10.1038/nrrheum.2015.151 [DOI] [PubMed] [Google Scholar]
- 21. Pisetsky DS. Standardization of anti-DNA antibody assays. Immunol Res 2013;56:420–4. 10.1007/s12026-013-8415-x [DOI] [PubMed] [Google Scholar]
- 22. Kumar V, Krasny S, Beutner EH. Specificity of the Crithidia Luciliae method for detecting anti-DNA antibodies. Effect of absorption for lipoproteins. Immunol Invest 1985;14:199–210. 10.3109/08820138509076144 [DOI] [PubMed] [Google Scholar]
- 23. Chiaro TR, Davis KW, Wilson A, et al. Significant differences in the analytic concordance between anti-dsDNA IgG antibody assays for the diagnosis of systemic lupus erythematosus—implications for inter-laboratory testing. Clinica Chimica Acta 2011;412:1076–80. 10.1016/j.cca.2011.02.025 [DOI] [PubMed] [Google Scholar]
- 24. Hernando M, González C, Sánchez A, et al. Clinical evaluation of a new automated anti-dsDNA fluorescent immunoassay. Clin Chem Lab Med 2002;40:1056–60. 10.1515/CCLM.2002.185 [DOI] [PubMed] [Google Scholar]
- 25. Viriyataveekul R, Kobkitjaroen J, Jaiyen J, et al. Evaluation of five commercial assays for the detection of anti-dsDNA antibodies: three Crithidia Luciliae indirect Immunofluorescence test kits and two enzyme immunoassay kits. J Med Assoc Thai 2014;97:220–4. [PubMed] [Google Scholar]
- 26. López-Hoyos M, Cabeza R, Martínez-Taboada VM, et al. Clinical disease activity and titers of anti-dsDNA antibodies measured by an automated Immunofluorescence assay in patients with systemic lupus erythematosus. Lupus 2005;14:505–9. 10.1191/0961203305lu2130oa [DOI] [PubMed] [Google Scholar]
- 27. Zhao J, Wang K, Wang X, et al. The performance of different anti-dsDNA autoantibodies assays in Chinese systemic lupus erythematosus patients. Clin Rheumatol 2018;37:139–44. 10.1007/s10067-017-3771-x [DOI] [PubMed] [Google Scholar]
- 28. Jaekell HP, Trabandt A, Grobe N, et al. Anti-dsDNA antibody subtypes and anti-C1Q antibodies: toward a more reliable diagnosis and monitoring of systemic lupus erythematosus and lupus nephritis. Lupus 2006;15:335–45. 10.1191/0961203306lu2308oa [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.