Abstract
Introduction
Management of the neck in oral cavity squamous cell carcinoma (OCSCC) is essential to oncologic control and survival. The rates of lymph node metastasis (LNM) vary based on oral cavity tumor site and stage and influence treatment decisions. The aim of this paper was to describe clinical LNM for different tumor subsites and stages of surgically managed OCSCC.
Methods
We conducted a retrospective analysis of 25,846 surgically managed OCSCC patients from the National Cancer Database (NCDB) stratified by tumor subsite and clinical T-stage. For cN + patients, rates of pathologic LNM and absence of pathologic LNM were determined. For cN0 patients, outcomes included the rates of elective neck dissection (END) and occult LNM and predictors of occult LNM determined by a multivariable logistic regression model.
Results
A total of 25,846 patients (59.1% male, mean age 61.9 years) met inclusion criteria with primary tumor sites including oral tongue (50.8%), floor of mouth (21.2%), lower alveolus (7.6%), buccal mucosa (6.7%), retromolar area (4.9%), upper alveolus (3.6%), hard palate (2.7%), and mucosal lip (2.5%). Among all sites, clinical N+ rates increased with T-stage (8.9% T1, 28.0% T2, 51.6% T3, 52.5% T4); these trends were preserved across subsites. Among patients with cN + disease, the overall rate of concordant positive pathologic LNM was 80.1% and the rate of discordant negative pathologic LNM was 19.6%, which varied based on tumor site and stage. In the overall cohort of cN0 patients, 59.9% received END, and the percentage of patients receiving END increased with higher tumor stage. Occult LNM among those cN0 was found in 25.1% of END cases, with the highest rates in retromolar (28.8%) and oral tongue (27.5%) tumors. Multivariable regression demonstrated significantly increased rates of occult LNM for higher T stage (T2 OR: 2.1 [1.9–2.4]; T3 OR: 3.0 [2.5–3.7]; T4 OR: 2.7 [2.2–3.2]), positive margins (OR: 1.4 [1.2–1.7]), and positive lymphovascular invasion (OR: 5.1 [4.4–5.8]).
Conclusions
Management of the neck in OCSCC should be tailored based on primary tumor factors and considered for early-stage tumors.
Keywords: Oral cavity, Squamous cell carcinoma, Head and neck, Metastasis, Neck dissection
Introduction
Squamous cell carcinoma (SCC) comprises more than 90% of all primary oral cavity cancers and can originate from the squamous mucosal lining within any of the distinct anatomic subsites (mucosal lip, oral tongue, upper and lower alveolar ridge, floor of mouth, hard palate, buccal mucosa, or retromolar trigone) [1]. The American Joint Committee on Cancer (AJCC) staging system recognizes that there are unique anatomical subsites within the oral cavity but does not delineate between them for the purposes of cancer staging [2]. Similarly, the National Comprehensive Cancer Network (NCCN) guidelines do not include subsite-specific treatment recommendations, specifically in regards to management of the neck [3].
Given the propensity for oral cavity SCCs (OCSCCs) to spread to regional lymph nodes, proper management of the neck is crucial to provide optimal disease control and survival. When there is clinically apparent lymph node metastasis (LNM) noted on physical exam or imaging, therapeutic treatment of the neck with surgery or radiation is recommended. When a patient presents without clinically apparent LNM, the options for management of the neck include an elective neck dissection (END), sentinel lymph node biopsy (SLNB), radiation therapy, or observation [4]. Management of the clinically N0 neck was previously considered controversial, but classically, elective treatment of the neck lymph nodes is indicated when the chance of occult LNM exceeds 15–20% [5–7].
In separate heterogenous studies, tumor characteristics such as tumor thickness, depth of invasion (DOI), perineural invasion (PNI), lymphovascular invasion (LVI), and poorly differentiated tumor grade have all been shown to increase risk of occult LNM [8]. The risk of regional LNM also varies between the primary oral cavity subsite involved, but these differences have not been described in a single comprehensive study. The purpose of the current study was to use the National Cancer Database (NCDB) to analyze the rates of clinical LNM at presentation for surgically treated OCSCC stratified by primary oral cavity subsite and clinical tumor stage. Additionally, we evaluate the concordance between clinical and pathologic node positivity after neck dissection, the rates of END and occult LNM, and predictors of occult LNM.
Materials and Methods
Study Sample
The NCDB dataset is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. This study was determined to be exempt by the University of Pennsylvania Institutional Review Board. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline [9].
Patients with OCSCC recorded in the NCDB between January 2004 and December 2016 who underwent surgery to the primary tumor site were included in this study using oral cavity International Classification of Disease for Oncology, 3rd Edition (ICD-O-3) [10] topography codes. Cases were included with SCC histology (codes 8070, 8071, 8072) and no distant metastasis. Patients were excluded if they had multiple primary malignancies, missing follow-up data, surgery at a distant site, incomplete clinical staging, or incomplete pathologic T staging. OCSCC patients were divided into primary tumor subsites.
Covariates
The clinicodemographic information extracted from the database included age, sex, race, Charlson-Deyo Comorbidity Index score, primary oral cavity subsite, AJCC tumor/node/metastases clinical and pathologic staging, margin status, and LVI. These variables have been defined in previous studies [11, 12]. AJCC clinical staging is based on physical examination and any imaging that was performed. Due to the study period, all patients were staged with either the 6th or 7th edition of the AJCC [13]. The differences between the 6th and 7th staging criteria for OCSCC are negligible; however, these staging systems do not incorporate DOI and extranodal extension which are now included in the 8th edition of the AJCC staging [2].
Management of the neck was determined using the NCDB variable “regional lymph nodes examined” which is the number of regional lymph nodes removed and pathologically evaluated. To distinguish between excisional biopsy and SLNB, patients were classified as having undergone neck dissection if they had ≥3 lymph nodes removed and pathologically evaluated. END was characterized as removal of ≥3 lymph nodes removed and node-negative clinical exam and/or imaging.
Study Outcomes
The overall rates of clinical node positive (cN+) and node negative (cN0) status were reported for each tumor subsite and clinical T stage. Patients were then divided into cN+ and cN0 groups for further analysis.
For cN + patients, the rates of pathologic LNM after neck dissection stratified by clinical T stage and oral cavity subsite were reported. This was calculated by taking the number of patients who were pN+ and had received a neck dissection divided by all cN + patients who had received a neck dissection. An additional outcome for the cN + group was the rate of “false positive” nodal disease status defined as cases that were cN + but determined to be pN0 after neck dissection.
For cN0 patients, the rates of END and occult LNM after END were calculated and stratified by clinical T stage and subsite. The rates of END for the cN0 group were calculated by taking the number of patients who were cN0 and had received a neck dissection divided by the total number of cN0 patients. The rates of occult LNM were calculated by taking the number of patients who were pN + who had received an END divided by all patients who had received an END. Lastly, predictors of occult LNM were determined for cN0 patients using a multivariable regression model.
Statistical Analysis
Descriptive statistics were calculated for study outcomes defined above. A multivariable logistic regression was used to determine predictive relationships between covariates and the likelihood of occult LNM. Variables were determined a priori by consensus of co-authors and included patient factors (age, sex, race, Charlson/Deyo score) and primary site pathologic factors (subsite, pathologic T stage, margin status, LVI). Statistical analyses were performed with R v.3.4.1 (https://cran.r-project.org) via RStudio v.1.1.23 (RStudio, Boston, MA, USA). A significance level of 0.05 was used.
Results
A total of 25,846 patients diagnosed with OCSCC between January 2004 and December 2016 met inclusion criteria. Patient demographic information is listed in Table 1. The oral tongue was the most commonly involved primary subsite (51.0%, n = 13,191) followed by the floor of mouth (21.0%, n = 5,434).
Table 1.
Patient demographics
| Total, n (column %) | |
|---|---|
| Patients | 25,846 |
| Age, years | |
| <54 | 7,651 (29.6) |
| 54–64 | 7,467 (28.9) |
| 64–74 | 5,872 (22.7) |
| 74–100 | 4,856 (18.8) |
| Sex | |
| Male | 15,366 (59.5) |
| Female | 10,480 (40.5) |
| Race | |
| White/caucasian | 22,858 (88.4) |
| Black | 1,403 (5.4) |
| Other/unknown | 622 (2.4) |
| Asian | 963 (3.7) |
| Facility type | |
| Community cancer program | 1,064 (4.1) |
| Comprehensive community cancer program | 6,226 (24.1) |
| Academic/research | 14,821 (57.3) |
| Integrated network | 2,306 (8.9) |
| N/A | 1,429 (5.5) |
| Facility geography | |
| Northeast | 4,806 (18.6) |
| South | 6,868 (26.6) |
| Midwest | 9,145 (35.4) |
| West | 3,598 (13.9) |
| N/A | 1,429 (5.5) |
| Charlson/Deyo | |
| 0 | 19,465 (75.3) |
| 1 | 4,827 (18.7) |
| 2 | 1,148 (4.4) |
| 3 | 406 (1.6) |
| Insurance | |
| Private | 11,148 (43.1) |
| None | 1,280 (5.0) |
| Medicaid | 2,256 (8.7) |
| Medicare | 10,378 (40.2) |
| Other government | 398 (1.5) |
| Unknown | 386 (1.5) |
| Housing area | |
| Metro | 20,362 (78.8) |
| Urban | 4,302 (16.6) |
| Rural | 526 (2.0) |
| N/A | 656 (2.5) |
| Education | |
| 21% or more | 4,052 (15.7) |
| 13–20.9% | 6,686 (25.9) |
| 7–12.9% | 8,829 (34.2) |
| Less than 7% | 6,163 (23.8) |
| N/A | 116 (0.4) |
| Income | |
| Less than USD 38,000 | 4,361 (16.9) |
| USD 38,000–USD 47,999 | 6,342 (24.5) |
| USD 48,000–USD 62,999 | 6,880 (26.6) |
| USD 63,000 + | 8,133 (31.5) |
| N/A | 130 (0.5) |
| Clinical T stage | |
| T1 | 10,581 (40.9) |
| T2 | 8,254 (31.9) |
| T3 | 2,115 (8.2) |
| T4 | 4,896 (18.9) |
| Clinical N stage | |
| N0 | 18,934 (73.3) |
| N1 | 2,893 (11.2) |
| N2 | 3,937 (15.2) |
| N3 | 82 (0.3) |
| Pathologic T stage | |
| T1 | 11,756 (45.5) |
| T2 | 6,897 (26.7) |
| T3 | 1,871 (7.2) |
| T4 | 5,322 (20.6) |
| Pathologic N stage | |
| N0 | 12,873 (49.8) |
| N1 | 3,083 (11.9) |
| N2 | 5,140 (19.9) |
| N3 | 90 (0.3) |
| NX | 3,882 (15.0) |
| N/A | 778 (3.0) |
| Primary site | |
| Mucosal lip | 551 (2.1) |
| Oral tongue | 13,191 (51.0) |
| Upper alveolus | 943 (3.6) |
| Lower alveolus | 2,058 (8.0) |
| Floor of mouth | 5,434 (21.0) |
| Hard palate | 647 (2.5) |
| Buccal mucosa | 1,731 (6.7) |
| Retromolar trigone | 1,291 (5.0) |
Patients were stratified by primary subsite and clinical nodal status (cN0 vs. cN+) as listed in Table 2. Overall, a minority of OCSCC patients were cN+ (26.7%, n = 6,912) with highest percentage of cN + found in those with T4 disease (52.5%, n = 2,572). Among the subsites, the retromolar trigone had the highest overall cN + rate (38.3%, n = 495). The highest rate of cN + status among sub-stratification by both subsite and stage was T4 oral tongue tumors (69.3%, n = 470).
Table 2.
Clinical nodal status stratified by oral cavity subsite and clinical T stage
| Primary site and clinical T stage | Total, n (%)a | Clinical node negative (cN0), n (%)b | Clinical node positive (cN+), n (%)b |
|---|---|---|---|
| All sites | |||
| Overall | 25,846 (100.0) | 18,934 (73.3) | 6,912 (26.7) |
| T1 | 10,581 (40.9) | 9,642 (91.1) | 939 (8.9) |
| T2 | 8,254 (31.9) | 5,944 (72.0) | 2,310 (28.0) |
| T3 | 2,115 (8.2) | 1,024 (48.4) | 1,091 (51.6) |
| T4 | 4,896 (18.9) | 2,324 (47.5) | 2,572 (52.5) |
| Mucosal lip | |||
| Overall | 551 (100.0) | 501 (90.9) | 50 (9.1) |
| T1 | 346 (62.8) | 340 (98.3) | 6 (1.7) |
| T2 | 144 (26.1) | 124 (86.1) | 20 (13.9) |
| T3 | 31 (5.6) | 19 (61.3) | 12 (38.7) |
| T4 | 30 (5.4) | 18 (60.0) | 12 (40.0) |
| Tongue | |||
| Overall | 13,191 (100.0) | 10,216 (77.4) | 2,975 (22.6) |
| T1 | 6,573 (49.8) | 6,061 (92.2) | 512 (7.8) |
| T2 | 4,729 (35.9) | 3,405 (72.0) | 1,324 (28.0) |
| T3 | 1,211 (9.2) | 542 (44.8) | 669 (55.2) |
| T4 | 678 (5.1) | 208 (30.7) | 470 (69.3) |
| Upper alveolus | |||
| Overall | 943 (100.0) | 770 (81.7) | 173 (18.3) |
| T1 | 305 (32.3) | 288 (94.4) | 17 (5.6) |
| T2 | 229 (24.3) | 194 (84.7) | 35 (15.3) |
| T3 | 42 (4.5) | 29 (69.0) | 13 (31.0) |
| T4 | 367 (38.9) | 259 (70.6) | 108 (29.4) |
| Lower alveolus | |||
| Overall | 2,058 (100.0) | 1,425 (69.2) | 633 (30.8) |
| T1 | 467 (22.7) | 431 (92.3) | 36 (7.7) |
| T2 | 409 (19.9) | 313 (76.5) | 96 (23.5) |
| T3 | 85 (4.1) | 53 (62.4) | 32 (37.6) |
| T4 | 1,097 (53.3) | 628 (57.2) | 469 (42.8) |
| Floor of mouth | |||
| Overall | 5,434 (100.0) | 3,554 (65.4) | 1,880 (34.6) |
| T1 | 1,890 (34.8) | 1,641 (86.8) | 249 (13.2) |
| T2 | 1,568 (28.9) | 1,070 (68.2) | 498 (31.8) |
| T3 | 381 (7.0) | 167 (43.8) | 214 (56.2) |
| T4 | 1,595 (29.4) | 676 (42.4) | 919 (57.6) |
| Hard palate | |||
| Overall | 647 (100.0) | 472 (73.0) | 175 (27.0) |
| T1 | 163 (25.2) | 145 (89.0) | 18 (11.0) |
| T2 | 157 (24.3) | 129 (82.2) | 28 (17.8) |
| T3 | 61 (9.4) | 42 (68.9) | 19 (31.1) |
| T4 | 266 (41.1) | 156 (58.6) | 110 (41.4) |
| Buccal mucosa | |||
| Overall | 1,731 (100.0) | 1,200 (69.3) | 531 (30.7) |
| T1 | 537 (31.0) | 482 (89.8) | 55 (10.2) |
| T2 | 637 (36.8) | 456 (71.6) | 181 (28.4) |
| T3 | 211 (12.2) | 128 (60.7) | 83 (39.3) |
| T4 | 346 (20.0) | 134 (38.7) | 212 (61.3) |
| Retromolar trigone | |||
| Overall | 1,291 (100.0) | 796 (61.7) | 495 (38.3) |
| T1 | 300 (23.2) | 254 (84.7) | 46 (15.3) |
| T2 | 381 (29.5) | 253 (66.4) | 128 (33.6) |
| T3 | 93 (7.2) | 44 (47.3) | 49 (52.7) |
| T4 | 517 (40.0) | 245 (47.4) | 272 (52.6) |
aPercentages correspond to the proportions of different T stages within each oral cavity subsite.
bPercentages correspond to the proportions of cN0 and cN + patients across a given T stage and oral cavity subsite.
Table 3 describes the rates of pathologic LNM and false-positive LNM for cN + patients who underwent primary surgery and neck dissection stratified by tumor stage and subsite. Overall, the rate of false-positive LNM (i.e., cases that were cN + but determined to be pN0 after neck dissection) was 19.6%, highest in T4 disease at 21.9%, and variable between subsites.
Table 3.
Pathologic nodal status of clinical node positive (cN+) patients after neck dissection
| Primary site and clinical T stage | cN+, n | Pathologic LNM, n (%)a | False positive (pathologically N0) LNM, n (%)a |
|---|---|---|---|
| All sites | |||
| Overall | 6,612 | 5,297 (80.1) | 1,299 (19.6) |
| T1 | 851 | 707 (83.1) | 143 (16.8) |
| T2 | 2,199 | 1,781 (81.0) | 414 (18.8) |
| T3 | 1,053 | 859 (81.6) | 192 (18.2) |
| T4 | 2,509 | 1,950 (77.7) | 550 (21.9) |
| Mucosal lip | |||
| Overall | 47 | 32 (68.1) | 14 (29.8) |
| T1 | 5 | 3 (60.0) | 2 (40.0) |
| T2 | 18 | 13 (72.2) | 4 (22.2) |
| T3 | 12 | 7 (58.3) | 5 (41.7) |
| T4 | 12 | 9 (75.0) | 3 (25.0) |
| Tongue | |||
| Overall | 2,813 | 2,310 (82.1) | 500 (17.8) |
| T1 | 454 | 373 (82.2) | 80 (17.6) |
| T2 | 1,256 | 1,013 (80.7) | 243 (19.3) |
| T3 | 641 | 535 (83.5) | 105 (16.4) |
| T4 | 462 | 389 (84.2) | 72 (15.6) |
| Upper alveolus | |||
| Overall | 167 | 120 (71.9) | 47 (28.1) |
| T1 | 16 | 14 (87.5) | 2 (12.5) |
| T2 | 32 | 23 (71.9) | 9 (28.1) |
| T3 | 13 | 10 (76.9) | 3 (23.1) |
| T4 | 106 | 73 (68.9) | 33 (31.1) |
| Lower alveolus | |||
| Overall | 618 | 455 (73.6) | 155 (25.1) |
| T1 | 33 | 27 (81.8) | 6 (18.2) |
| T2 | 92 | 73 (79.3) | 16 (17.4) |
| T3 | 32 | 24 (75.0) | 8 (25.0) |
| T4 | 461 | 331 (71.8) | 125 (27.1) |
| Floor of mouth | |||
| Overall | 1,817 | 1,457 (80.2) | 357 (19.6) |
| T1 | 232 | 198 (85.3) | 34 (14.7) |
| T2 | 482 | 387 (80.3) | 95 (19.7) |
| T3 | 209 | 167 (79.9) | 41 (19.6) |
| T4 | 894 | 705 (78.9) | 187 (20.9) |
| Hard palate | |||
| Overall | 163 | 123 (75.5) | 40 (24.5) |
| T1 | 17 | 14 (82.4) | 3 (17.6) |
| T2 | 25 | 23 (92.0) | 2 (8.0) |
| T3 | 18 | 15 (83.3) | 3 (16.7) |
| T4 | 103 | 71 (68.9) | 32 (31.1) |
| Buccal mucosa | |||
| Overall | 507 | 413 (81.5) | 94 (18.5) |
| T1 | 51 | 41 (80.4) | 10 (19.6) |
| T2 | 168 | 142 (84.5) | 26 (15.5) |
| T3 | 81 | 66 (81.5) | 15 (18.5) |
| T4 | 207 | 164 (79.2) | 43 (20.8) |
| Retromolar trigone | |||
| Overall | 480 | 387 (80.6) | 92 (19.2) |
| T1 | 43 | 37 (86.0) | 6 (14.0) |
| T2 | 126 | 107 (84.9) | 19 (15.1) |
| T3 | 47 | 35 (74.5) | 12 (25.5) |
| T4 | 264 | 208 (78.8) | 55 (20.8) |
aThe sum of “pathologic LNM” and “false positive LNM” may be less than 100% due to some patients being documented as unknown pathologic nodal staging after neck dissection.
END and occult LNM rates for cN0 patients varied between different oral cavity subsites as shown in Table 4. END was performed in 42.5%, 74.3%, 84.5%, and 84.1% of T1, T2, T3, and T4 tumors, respectively. Patients with primary tumors of the retromolar trigone had the highest rate of END performed for early-staged cancers (48.0% for T1 and 83.8% for T2). Rates of END were lowest for patients with primary tumors of the mucosal lip (5.6% for T1 and 34.7% for T2) and the hard palate (11.0% for T1 and 31.8% for T2).
Table 4.
Rates of END and occult LNM in clinical node negative (cN0) patients
| Primary site and clinical T stagea | Total cN0 patients, n | END, n (%) | Occult LNM, n (%) |
|---|---|---|---|
| All sites | |||
| Overall | 18,934 | 11,334 (59.9) | 2,849 (25.1) |
| T1 | 9,642 | 4,101 (42.5) | 680 (16.6) |
| T2 | 5,944 | 4,414 (74.3) | 1,337 (30.3) |
| T3 | 1,024 | 865 (84.5) | 327 (37.8) |
| T4 | 2,324 | 1,954 (84.1) | 505 (25.8) |
| Mucosal lip | |||
| Overall | 501 | 80 (16.0) | 14 (17.5) |
| T1 | 340 | 19 (5.6) | 3 (15.8) |
| T2 | 124 | 43 (34.7) | 8 (18.6) |
| T3 | 19 | 7 (36.8) | 2 (28.6) |
| T4 | 18 | 11 (61.1) | 1 (9.1) |
| Tongue | |||
| Overall | 10,216 | 6,109 (59.8) | 1,683 (27.5) |
| T1 | 6,061 | 2,763 (45.6) | 479 (17.3) |
| T2 | 3,405 | 2,672 (78.5) | 897 (33.6) |
| T3 | 542 | 479 (88.4) | 220 (45.9) |
| T4 | 208 | 195 (93.8) | 87 (44.6) |
| Upper alveolus | |||
| Overall | 770 | 246 (31.9) | 52 (21.1) |
| T1 | 288 | 50 (17.4) | 5 (10.0) |
| T2 | 194 | 67 (34.5) | 18 (26.9) |
| T3 | 29 | 13 (44.8) | 2 (15.4) |
| T4 | 259 | 116 (44.8) | 27 (23.3) |
| Lower alveolus | |||
| Overall | 1,425 | 1,072 (75.2) | 198 (18.5) |
| T1 | 431 | 188 (43.6) | 28 (14.9) |
| T2 | 313 | 245 (78.3) | 53 (21.6) |
| T3 | 53 | 45 (84.9) | 8 (17.8) |
| T4 | 628 | 594 (94.6) | 109 (18.4) |
| Floor of mouth | |||
| Overall | 3,554 | 2,354 (66.2) | 533 (22.6) |
| T1 | 1,641 | 761 (46.4) | 109 (14.3) |
| T2 | 1,070 | 824 (77.0) | 188 (22.8) |
| T3 | 167 | 149 (89.2) | 49 (32.9) |
| T4 | 676 | 620 (91.7) | 187 (30.2) |
| Hard palate | |||
| Overall | 472 | 147 (31.1) | 32 (21.8) |
| T1 | 145 | 16 (11.0) | 2 (12.5) |
| T2 | 129 | 41 (31.8) | 15 (36.6) |
| T3 | 42 | 23 (54.8) | 8 (34.8) |
| T4 | 156 | 67 (42.9) | 7 (10.4) |
| Buccal mucosa | |||
| Overall | 1,200 | 715 (59.6) | 161 (22.5) |
| T1 | 482 | 182 (37.8) | 25 (13.7) |
| T2 | 456 | 310 (68.0) | 91 (29.4) |
| T3 | 128 | 106 (82.8) | 22 (20.8) |
| T4 | 134 | 117 (87.3) | 23 (19.7) |
| Retromolar trigone | |||
| Overall | 796 | 611 (76.8) | 176 (28.8) |
| T1 | 254 | 122 (48.0) | 29 (23.8) |
| T2 | 253 | 212 (83.8) | 67 (31.6) |
| T3 | 44 | 43 (97.7) | 16 (37.2) |
| T4 | 245 | 234 (95.5) | 64 (27.4) |
aThe clinical T stage for each oral cavity subsite where risk of occult LNM exceeds 15% is underlined.
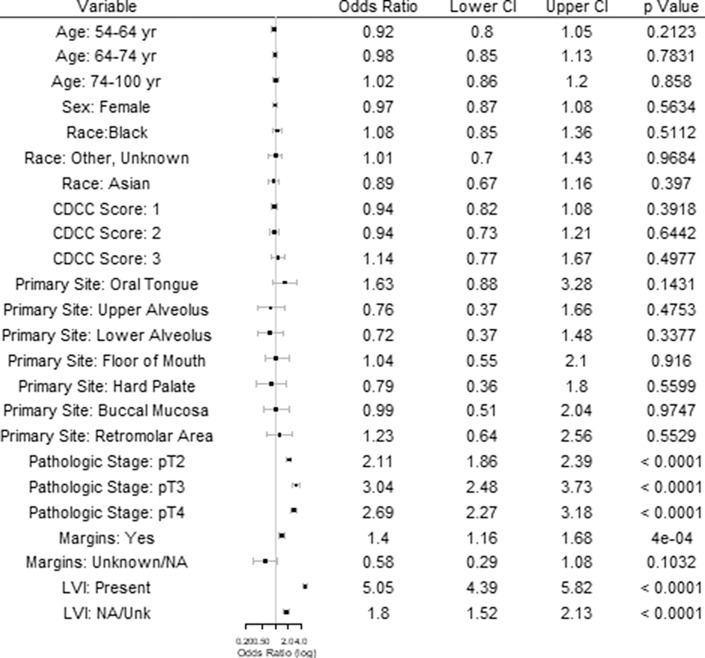
When an END was performed, the overall rate of occult LNM was 16.6%, 30.3%, 37.8%, and 25.8% for T1, T2, T3, and T4 tumors, respectively (Table 4). The likelihood of an END diagnosing occult LNM was highest for tumors involving the oral tongue (17.3% for T1 and 33.6% for T2) and the retromolar trigone (23.8% for T1 and 31.6% for T2). Multivariable regression for predictors of occult LNM demonstrated significantly increased rates of occult LNM for higher pathologic T stage (T2 OR: 2.1 [1.9–2.4]; T3 OR: 3.0 [2.5–3.7]; T4 OR: 2.7 [2.2–3.2]), positive margins (OR: 1.4 [1.2–1.7]), and positive LVI (OR: 5.1 [4.4–5.8]) (Fig. 1).
Fig. 1.
Predictors of occult LNM. a References include age <54, male sex, white race, CDCC score 0, mucosal lip primary site, pT1 pathologic stage, negative margins, and negative LVI.
Discussion
This large national analysis provides a comprehensive description of clinical nodal disease in surgically managed OCSCC for different oral cavity subsites and clinical tumor stages. We report the rates of END which highlights the surgical practice patterns in NCDB-participating centers. Lastly, we describe the rates of occult LNM across subsites and predictive factors that should be considered when determining the role of END in OCSCC management.
Our comparison of clinical and pathologic node-positive status evaluates the accuracy of clinical nodal staging in predicting pathologic LNM. The discrepancies between clinical and pathologic nodal staging highlights the fact that clinical staging may over or underestimate nodal disease in a portion of cases. Specifically, cN + patients who received a neck dissection were found to not have pathologic LNM in approximately 19.6% of cases. The highest rate of falsely predicting positive nodal disease was seen for oral tongue, with some subtle differences in the rates between other subsites. This is generally in line with prior studies showing discrepancies between clinical and pathological staging [14].
It is generally accepted that patients with advanced tumor stage (T3 and T4) should have a lower threshold for treatment of the neck due to their elevated risk of regional metastasis; however, controversy exists in the role of END for some early stage OCSCC tumors without clinical lymphadenopathy as the therapeutic benefit and morbidity must be weighed. Across all subsites in our study, ENDs were performed in 42.5% of T1 and 74.3% of T2 tumors and occult LNM were found in 16.6% and 30.3%, respectively. Historically, END has been recommended for cN0 OCSCC when the risk of occult LNM reaches 15–20% [5–7]. Table 4 highlights the clinical T stage for each oral cavity subsite where the risk of occult LNM exceeds 15% and END should be considered.
There are several studies that have suggested that specific factors are associated with occult LNM such as tumor thickness, DOI, PNI, LVI, and poorly differentiated tumor grade [8, 15–18]. Our multivariable model for predictors of occult disease showed increased odds for higher pathologic T stage, positive margins, and presence of LVI. These data suggest that END should be more strongly considered in patients with these pathologic factors after primary site surgery. Interestingly, we found no statistically significant associations between primary tumor subsites and occult LNM when controlling for other factors.
Oral Tongue
Despite the high relative incidence of oral tongue SCC and considerable amount of published literature compared to other oral cavity subsites, controversy still exists regarding management of the neck for this subsite. Within our NCDB analysis, the oral tongue was the most frequently involved subsite for OCSCC, encompassing 50.8% of all patients, and clinically apparent nodal disease was present in 22.6%. Oral tongue had one of the highest rates of END for early-stage tumors at 45.6% and 78.5% for T1 and T2 tumors, respectively. The rates of occult LNM for early stage tumors was also one of the highest for the oral tongue subsite at 17.3% for T1 and 33.6% for T2 tumors in agreement with prior studies [19–25]. Our data corroborate previous literature showing that small primary OCSCCs of the oral tongue can spread to regional lymph nodes at non-negligible rates and emphasize the need to consider END for even T1 oral tongue SCC [26].
Floor of Mouth
The floor of mouth is known to contain a rich lymphatic network, increasing the ability of cancers to spread to regional lymph nodes in early staged tumors [27, 28]. In our study, ENDs were performed in 46.4% of T1 and 77.0% of T2 tumors with occult LNM identified in 14.3% and 22.8%, respectively. These data suggest that END should be strongly considered in T2 patients, and that additional evidence is needed to inform elective neck management in T1 lesions, especially regarding potential survival advantage [18, 29, 30] not analyzed in this study.
Mucosal Lip
Mucosal lip accounted for the lowest percentage of OCSCC cancers at 2.5% and most cases were early T stage (67.9% T1 tumors). Clinical T stages above T1 had high rates of presenting with clinical nodal disease at 34.7%, 38.7%, and 40.0% for T2, T3, and T4 tumors, respectively. ENDs were performed for lip SCC at 16.0% overall and occult LNM were identified in 15.8% and 18.6% of T1 and T2 tumors, respectively. Given that the rates of occult LNM fall between 15% and 20%, the utility of performing an END in the setting of early-stage mucosal lip SCC should factor in a patient’s own preferences regarding risks and benefits of the procedure and additional studies should be performed. Unfortunately, the low number of patients with T3 and T4 tumors makes it difficult to draw specific conclusions for best practices in advanced mucosal lip tumor stage.
Buccal Mucosa
Within our NCDB study, END was performed in 37.8% and 68.0% of T1 and T2 buccal tumors with occult LNM rates of 13.7% and 29.4%, respectively. One series of 119 patients with various stages of buccal mucosa OCSCC showed occult LNM in 26% of patients who underwent END [31]. A separate review of 125 patients treated with END for clinical T1 buccal mucosa OCSCC showed that 8% had occult LNM, with no clear survival benefit for END versus no treatment of the neck [32]. Based on our relatively low rates of occult LNM for T1 tumors and data from Fang et al. [32] showing no survival benefit of END for T1 tumors, it is reasonable to consider a “watchful-waiting” approach for the neck in T1 buccal tumors; however, additional studies are necessary, especially given the high rates of locoregional recurrence for buccal OCSCC [33].
Hard Palate and Alveolar Ridge
Within our NCDB surgical cohort, ENDs were performed in 31.1% of hard palate and 31.9% of upper alveolus SCCs which is consistent with findings from previous studies [34]. There was a low rate of occult LNM in hard palate SCC for T1 tumors at 12.5% which increased to 36.6% for T2 tumors. For upper alveolus SCC, occult LNM rates followed a similar trend at 10.0% and 26.9% for T1 and T2 tumors, respectively. Although historically managed expectantly in many cases, this would argue that END should be considered in T2N0 hard palate and upper alveolus cancers based on our calculated occult LNM rate, though evaluation of survival impacts is lacking.
A previous Surveillance, Epidemiology, and End Results (SEER) database study showed that OCSCC of the hard palate and maxillary alveolus presented with cervical nodal metastasis in less than 15% of patients for T1–T3 tumors and 24.7% for T4 tumors. The authors concluded that patients with T4N0 SCC of the hard palate or maxillary alveolus should undergo END due to the high rates of cervical metastasis; however, no specific conclusion was drawn for T1–3 primary tumors without clinically positive nodal disease [35]. This study has been supported by other work suggesting END is beneficial for T4N0 tumors and clinical surveillance is a reasonable alternative for T1–T3N0 tumors [36, 37]. Contrastingly, others have suggested that, despite potentially lower rates of occult LNM with early staged OCSCC of the maxilla, END is important for initial management of the neck due to high rates of locoregional recurrence [38, 39].
Retromolar Trigone
Multiple cancer centers have published data on the utility of primary radiation therapy for tumors of the retromolar trigone [40, 41], potentially relating to the fact that less than 27% of all retromolar OCSCCs are isolated to the subsite and often there is overlap/extension into the oropharynx [42–44]. These factors likely contribute to the lack of published data on END and occult disease in surgically managed retromolar trigone OCSCC. In our study of surgical patients, the retromolar trigone had the highest rates of END (48.0% and 83.8% for T1 and T2) and occult LNM (23.8% and 31.6% for T1 and T2). While awaiting additional studies on predictors of regional metastasis, END should be strongly considered for all OCSCC of the retromolar trigone treated with primary surgery.
Limitations
There are several limitations to our study including those inherent to retrospective database analyses. First, it should be noted that the NCDB does not include all high-risk pathologic variables such as PNI and DOI which may increase the risk of LNM but could not be included in our multivariate model of occult LNM. DOI is now included in the most recent AJCC staging edition [2] and has been shown to be associated with survival outcomes in surgically managed OCSCC [45]. The NCDB also does not provide specific information on the methods for clinical staging for each individual case including if imaging was utilized. Additional limitations include the lack of generalizability given the low proportions of non-academic centers and non-white patients and potential selection biases (such as from some patients not receiving therapeutic neck dissections for clinically apparent nodal disease or advanced T stage and differences in the rates of incomplete pathologic nodal staging after neck dissection). Additionally, the NCDB only contains a single ICD-O-3 code for the primary tumor site which does not account for the fact that many oral cavity tumors involve multiple subsites at presentation or may have unclear initial site of origin.
To isolate patients who received a neck dissection and not those who underwent SLNB alone, we defined receipt of neck dissection as having ≥3 lymph nodes removed and evaluated pathologically; however, some patients may have received an SLNB with ≥3 lymph nodes and thus been misclassified as having received a neck dissection and/or may have received SLNB followed by other treatment (i.e., radiation or therapeutic neck dissection). The different methods used for pathologic evaluation of lymph nodes have varying degrees of sensitivity for detecting occult LNM which could not be evaluated or controlled for in this study [46]. We chose not to perform a survival analysis for END due to concerns with comparisons to a group that did not receive END. Specifically, those who did not receive END may have received other treatment to the neck including radiation therapy which is not clearly described in the NCDB.
Conclusion
The rates of regional nodal disease at presentation, END, and occult LNM vary between different subsites and tumor stages for surgically managed OCSCC. Given the increased risk of occult disease for higher pathologic T stage, positive margins, and LVI, these patients should be considered for END following primary site surgery. Additional studies are necessary to determine the oncologic benefit of END across the spectrum of OCSCC disease presentations.
Statement of Ethics
This study protocol was reviewed and the need for ethics approval was determined to be exempt by University of Pennsylvania Institutional Review Board.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no financial disclosures relevant to this work.
Author Contributions
Ryan M. Carey: conception of work, data analysis, manuscript preparation, manuscript revision, final approval. Vincent J. Anagnos: conception of work, manuscript preparation, manuscript revision, final approval. Aman Prasad and Neel R. Sangal: data analysis, manuscript preparation, manuscript revision, final approval. Karthik Rajasekaran, Rabie M. Shanti, Steven B. Cannady, Jason G. Newman, Jason A. Brant, and Robert M. Brody: conception of work, manuscript revision, final approval.
Funding Statement
The authors have no financial disclosures relevant to this work.
Data Availability Statement
The data that support the findings of this study are openly available via the American College of Surgeons National Cancer Database, found at: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/. Further inquiries can be directed to the corresponding author.
References
- 1. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on Epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016 Mar;91(3):386–96. [DOI] [PubMed] [Google Scholar]
- 2. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. American joint committee on cancer, American cancer society, AJCC cancer staging manual. 8th ed. New York, NY: American Joint Committee on Cancer, Springer; 2017. [Google Scholar]
- 3. National Comprehensive Cancer Network . Head and neck cancers (version 2.2019). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [accessed 16 August 2019]. [Google Scholar]
- 4. den Toom IJ, Boeve K, Lobeek D, Bloemena E, Donswijk ML, de Keizer B, et al. Elective neck dissection or sentinel lymph node biopsy in early stage oral cavity cancer patients: the Dutch experience. Cancers. 2020 Jul 3;12(7):1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao M, Epstein JB, Modi BJ, Pytynia KB, Mundt AJ, Feldman LE. Current surgical treatment of squamous cell carcinoma of the head and neck. Oral Oncol. 2007 Mar;43(3):213–23. [DOI] [PubMed] [Google Scholar]
- 6. Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994 Jul;120(7):699–702. [DOI] [PubMed] [Google Scholar]
- 7. Pitman KT. Rationale for elective neck dissection. Am J Otolaryngol. 2000 Jan-Feb;21(1):31–7. [DOI] [PubMed] [Google Scholar]
- 8. Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004 Oct;131(4):472–6. [DOI] [PubMed] [Google Scholar]
- 9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. Oct 20 2007;335(7624):806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fritz AG, Percy C, Jack A, Shanmugartnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology: ICD-O. 3rd ed. First revision. Geneva: World Health Organization; 2013. p. 242. [Google Scholar]
- 11. Carey RM, Fathy R, Shah RR, Rajasekaran K, Cannady SB, Newman JG, et al. Association of type of treatment facility with overall survival after a diagnosis of head and neck cancer. JAMA Netw Open. 2020;3(1):e1919697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prasad A, Carey RM, Brody RM, Bur AM, Cannady SB, Ojerholm E, et al. Postoperative radiation therapy refusal in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Laryngoscope. 2021 Jul 13;132(2):339–348. [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Data Base Participant User File (PUF) . Data dictionary 2016. American College of Surgeons. https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/puf_data_dictionary_2016.ashx [accessed 31 December 2020]. [Google Scholar]
- 14. Kakei Y, Komatsu H, Minamikawa T, Hasegawa T, Teshima M, Shinomiya H, et al. Extent of neck dissection for patients with clinical N1 oral cancer. Int J Clin Oncol. Jun 2020;25(6):1067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19(3):205–10. [DOI] [PubMed] [Google Scholar]
- 16. Abu-Ghanem S, Yehuda M, Carmel NN, Leshno M, Abergel A, Gutfeld O, et al. Elective neck dissection vs observation in early-stage squamous cell carcinoma of the oral tongue with No clinically apparent lymph node metastasis in the neck: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2016;142(9):857–65. [DOI] [PubMed] [Google Scholar]
- 17. Feng Z, Li JN, Li CZ, Guo CB. Elective neck dissection versus observation in the management of early tongue carcinoma with clinically node-negative neck: a retrospective study of 229 cases. J Craniomaxillofac Surg. 2014;42(6):806–10. [DOI] [PubMed] [Google Scholar]
- 18. Kelner N, Vartanian JG, Pinto CA, Coutinho-Camillo CM, Kowalski LP. Does elective neck dissection in T1/T2 carcinoma of the oral tongue and floor of the mouth influence recurrence and survival rates? Br J Oral Maxillofac Surg. 2014;52(7):590–7. [DOI] [PubMed] [Google Scholar]
- 19. Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth: treatment rationale. Head Neck Surg. 1984;7(1):15–21. [DOI] [PubMed] [Google Scholar]
- 20. Cunningham MJ, Johnson JT, Myers EN, Schramm VL Jr, Thearle PB. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am J Surg. 1986 Oct;152(4):361–6. [DOI] [PubMed] [Google Scholar]
- 21. Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989 Oct;158(4):309–13. [DOI] [PubMed] [Google Scholar]
- 22. Ho CM, Lam KH, Wei WI, Lau SK, Lam LK. Occult lymph node metastasis in small oral tongue cancers. Head Neck. 1992 Sep-Oct;14(5):359–63. [DOI] [PubMed] [Google Scholar]
- 23. Lydiatt DD, Robbins KT, Byers RM, Wolf PF. Treatment of stage I and II oral tongue cancer. Head Neck. 1993;15(4):308–12. [DOI] [PubMed] [Google Scholar]
- 24. Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK, et al. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg. 1999;177(1):90–2. [DOI] [PubMed] [Google Scholar]
- 25. Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the treatment of early oral tongue carcinoma. Head Neck. 1997;19(7):583–8. [DOI] [PubMed] [Google Scholar]
- 26. Ren ZH, Xu JL, Li B, Fan TF, Ji T, Zhang CP. Elective versus therapeutic neck dissection in node-negative oral cancer: evidence from five randomized controlled trials. Oral Oncol. 2015;51(11):976–81. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Ow TJ, Myers JN. Pathways for cervical metastasis in malignant neoplasms of the head and neck region. Clin Anat. 2012;25(1):54–71. [DOI] [PubMed] [Google Scholar]
- 28. Shaha AR, Spiro RH, Shah JP, Strong EW. Squamous carcinoma of the floor of the mouth. Am J Surg. 1984;148(4):455–9. [DOI] [PubMed] [Google Scholar]
- 29. Wushou A, Yibulayin F, Sheng L, Luo Y, Yang ZC. Elective neck dissection improves the survival of patients with T2N0M0 oral squamous cell carcinoma: a study of the SEER database. BMC Cancer. 2021 Dec 7;21(1):1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu JY, Chen CF, Bai CH. Elective neck dissection versus observation in early-stage (cT1/T2N0) oral squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2019 Oct;4(5):554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diaz EM Jr, Holsinger FC, Zuniga ER, Roberts DB, Sorensen DM. Squamous cell carcinoma of the buccal mucosa: one institution’s experience with 119 previously untreated patients. Head Neck. 2003 Apr;25(4):267–73. [DOI] [PubMed] [Google Scholar]
- 32. Fang Q, Gao H, Gao Q, Sun J, Li P, Cui M, et al. Elective neck dissection versus wait-and-see policy in cT1N0 buccal squamous cell carcinoma. BMC Cancer. 2020 Jun 9;20(1):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sieczka E, Datta R, Singh A, Loree T, Rigual N, Orner J, et al. Cancer of the buccal mucosa: are margins and T-stage accurate predictors of local control? Am J Otolaryngol. 2001 Nov-Dec;22(6):395–9. [DOI] [PubMed] [Google Scholar]
- 34. Brown JS, Bekiroglu F, Shaw RJ, Woolgar JA, Rogers SN. Management of the neck and regional recurrence in squamous cell carcinoma of the maxillary alveolus and hard palate compared with other sites in the oral cavity. Head Neck. 2013 Feb;35(2):265–9. [DOI] [PubMed] [Google Scholar]
- 35. Lin HW, Bhattacharyya N. Survival impact of nodal disease in hard palate and maxillary alveolus cancer. Laryngoscope. 2009 Feb;119(2):312–5. [DOI] [PubMed] [Google Scholar]
- 36. Yang Z, Deng R, Sun G, Huang X, Tang E. Cervical metastases from squamous cell carcinoma of hard palate and maxillary alveolus: a retrospective study of 10 years. Head Neck. 2014 Jul;36(7):969–75. [DOI] [PubMed] [Google Scholar]
- 37. Poeschl PW, Seemann R, Czembirek C, Russmueller G, Sulzbacher I, Selzer E, et al. Impact of elective neck dissection on regional recurrence and survival in cN0 staged oral maxillary squamous cell carcinoma. Oral Oncol. 2012 Feb;48(2):173–8. [DOI] [PubMed] [Google Scholar]
- 38. Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head Neck. 2011 Jun;33(6):824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montes DM, Carlson ER, Fernandes R, Ghali GE, Lubek J, Ord R, et al. Oral maxillary squamous carcinoma: an indication for neck dissection in the clinically negative neck. Head Neck. 2011 Nov;33(11):1581–5. [DOI] [PubMed] [Google Scholar]
- 40. Lo K, Fletcher GH, Byers RM, Fields RS, Peters LJ, Oswald MJ. Results of irradiation in the squamous cell carcinomas of the anterior faucial pillar-retromolar trigone. Int J Radiat Oncol Biol Phys. 1987 Jul;13(7):969–74. [DOI] [PubMed] [Google Scholar]
- 41. Hao SP, Tsang NM, Chang KP, Chen CK, Huang SS. Treatment of squamous cell carcinoma of the retromolar trigone. Laryngoscope. 2006 Jun;116(6):916–20. [DOI] [PubMed] [Google Scholar]
- 42. Byers RM, Anderson B, Schwarz EA, Fields RS, Meoz R. Treatment of squamous carcinoma of the retromolar trigone. Am J Clin Oncol. 1984 Dec;7(6):647–52. [DOI] [PubMed] [Google Scholar]
- 43. Kowalski LP, Hashimoto I, Magrin J. End results of 114 extended “commando” operations for retromolar trigone carcinoma. Am J Surg. 1993 Oct;166(4):374–9. [DOI] [PubMed] [Google Scholar]
- 44. Huang CJ, Chao KS, Tsai J, Simpson JR, Haughey B, Spector GJ, et al. Cancer of retromolar trigone: long-term radiation therapy outcome. Head Neck. 2001 Sep;23(9):758–63. [DOI] [PubMed] [Google Scholar]
- 45. D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015 Aug 6;373(6):521–9. [DOI] [PubMed] [Google Scholar]
- 46. Trivedi NP, Ravindran HK, Sundram S, Iyer S, Kekatpure V, Durah S, et al. Pathologic evaluation of sentinel lymph nodes in oral squamous cell carcinoma. Head Neck. 2010;32(11):1437–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available via the American College of Surgeons National Cancer Database, found at: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/. Further inquiries can be directed to the corresponding author.