Abstract

Nephroprotection or renal rescue is to revive and restore kidney function after damage, with no need for further dialysis. During acute kidney injury (AKI), sudden and recent reductions in kidney functions occur. Causes are multiple, and prompt intervention can be critical to diminish or prevent morbidity. Echinops spinosus (ES) is a curative plant with proven pharmacological and biological effects including anti-inflammatory, antioxidant, and antibacterial competencies. The principal goal of this research is to scrutinize the nephroprotective features of E. spinosa extract (ESE) against glycerol-induced AKI. Male Wistar albino rats were equally divided into five separated groups: negative control rats (vehicle-injected), ESE control rats (ESE-treated rats), positive control rats, glycerol-induced AKI-model rats (single IM injection of 50% glycerol), and 2 groups of diseased rats but pretreated with different concentrations of ESE for 7 days (ESE150 + AKI rats and ESE250 + AKI rats). Kidney tissues were collected and used for histopathology analysis. The relative kidney weight percentage was assessed. ESE effects were investigated via scanning several biomarkers, such as serum urea and creatinine, as kidney function biomarkers. Lactate dehydrogenase (LDH) and creatine kinase (CK) activities were examined as rhabdomyolysis (RM) indicators. Kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin (NGAL) were also examined to investigate kidney injury. Enzymatic and nonenzymatic oxidative stress markers were analyzed, namely, superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), glutathione peroxidase (GPx), malondialdehyde (MDA), nitric oxide (NO), and reduced glutathione GSH. Proinflammatory cytokine [tumor necrosis factor-α (TNF-α) and interleukin-1 β (IL-1β)] and the renal proapoptotic protein (Bax) and antiapoptotic protein (Bcl-2) levels were evaluated. Statistical analysis for the resulting data revealed that ESE pretreatment turned AKI-induced biological antioxidant levels to an extent comparable to normal results. Furthermore, ESE decreased kidney function markers and RM-related biomarkers (LDH, CK, Kim-1, and NGAL) compared to those in untreated AKI-model rats. ESE treatment dropped the apoptotic renal Bax levels, enhanced antiapoptotic Bcl-2 manufacture, and disallowed the release of IL-1β and TNF-α. This study revealed the protective effect of ESE as therapeutic medicine against AKI-encouraged oxidative stress, inflammation, and apoptosis. It can be effectively used as adjuvant therapy, helping in renal rescue, and for kidney healing in cases with risk factors of AKI.
1. Introduction
Kidney injury has become a worldwide public health issue, with poor prognosis and significant morbidity and mortality rates. Acute kidney injury (AKI), commonly known as a “kidney attack”, is a sudden and unexpected decline in kidney function that has an unclear incidence and effect and can occur in the community or during hospitalization. Serious complication leads patients to an intensive care unit (ICU), particularly the elderly.1,2 Another way to describe AKI is if the serum creatinine (sCr) level has increased by 1.5 times from baseline (within 48 h to the previous 7 days) or by an absolute amount above 0.5 mg/dL (44.2 mol/L), or if the urine volume has decreased to less than 0.5 mL/kg/h for at least 6 h.3,4 Many factors can lead to AKI, especially alcohol, diabetes, cardiac dysfunction, volume depletion, and hepatic failure.5,6 AKI can be caused by a variety of factors, including acute tubular necrosis, renal ischemia-reperfusion, drug-induced acute interstitial glomerulonephritis or nephrotoxicity, infection, and hepatorenal syndrome.1,7
The model used to examine this injury most frequently is glycerol-induced AKI. Not all of the pathophysiology has been uncovered.8 AKI pathophysiology is complex and multifactorial, characterized by intrarenal hemodynamic changes, oxidation, endothelial dysfunction, inflammation, intraglomerular thrombosis, and tubule obstruction with necrotic cells and debris. The cases of AKI worsen in different medical situations, such as volume depletion, hypoalbuminemia, and in the presence of acidic urine.2,6,9 Also, AKI is distinguished by renal tubular injury and rhabdomyolysis (leakage of skeletal muscle fiber content into the blood, resulting in myoglobin buildup in renal tubules). Between 10 and 50% of rhabdomyolysis patients get some degree of AKI.7 Oxidative damage depletes antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx), increases oxidative markers (such as nitrotyrosine), and activates proinflammatory pathways such as nuclear factor-B and c-Jun N-terminal kinase (JNK), resulting in tubular necrosis.10 A tiny circulating protein called neutrophil gelatinase-associated lipocalin (NGAL) is significantly altered under a number of disease conditions. After tubular damage, it is one of the greatest, most accurate, and rapidly released markers, especially in acute kidney injury cases.11
For many centuries, herbal medicines have been intended for the prevention, treatment, and cure of diseases. The World Health Organization (WHO) announced that 80% of the world’s population depended in their medicinal health cases on traditional natural compounds.12Echinops spinosus (ES) is one of the curative Worts of the Asteraceae family. Recently, its roots and fruits have been effectively used as adjuvant elements in infections, diarrhea, hemorrhoids, labor pains, heart pain, migraine, and neuralgia. ES’s chief components are alkaloids, polyacetylene thiophene, flavone glycoside, and benzothiophene glycosides besides echinopane, which were isolated from the roots, with verified antioxidant, anti-inflammatory, antiapoptotic, and neuro-modulatory effects.13−15
Traditional medicine frequently treats disorders associated with inflammation using E. spinosus. The inside portion of the inflorescence is employed in the treatment of diabetes mellitus, postpartum care, and renal disorders. The fruits and roots of E. spinosus subsp. bovei are used as a spice in Morocco and Cameroon, as an abortifacient in Algeria, and to relieve labor pains and neuralgia. Pregnant women are given a decoction of the roots in water or olive oil to stimulate uterine contractions.16 Additionally, it is used as a diuretic or depurative for diabetes, to treat liver disorders, and for stomach pain, indigestion, and loss of appetite. These behaviors are closely related to the concentrations of secondary metabolites from a variety of groups, including alkaloids, sesquiterpenes, flavonoids, and polyphenolic chemicals, in various E. spinosus organs.17
According to Deyno et al.’s18 investigation on one of the Echinops species, ES can be used safely at high doses. No toxicity signs were detected in an acute oral dose testing up to 2000 mg/kg per body weight. This work investigated the possibility of using E. spinosus extract (ESE) as a herbal intervention for kidney protection in the case of the glycerol-induced AKI model. The aim is to scan various indicators and determine how much ES can help with renal rescue and also determine whether this action is concentration-dependent.
2. Materials and Methods
2.1. Plant Material Preparation
E. spinosus is a synonym of Echinops spinosissimus.(19) Plants were fetched from a local market in Hail, KSA in October 2020, identified, and authenticated by a taxonomist from the herbarium at Ha’il University’s Botany Department. The whole plant parts20 were purified, dried at room temperature (25 °C ± 2) away from the sun, and then milled to powder by mechanical mills. The dried powder (100 g) of the ES plant was macerated in 70% methanol for 48 h at 23 °C for ESE preparation. Then, the extract was concentrated using rotary evaporation.21 Based on our previous study, high-performance liquid chromatography (HPLC) revealed the presence of 16 peaks showing different phytochemical active constituents as possibly ferulic acid and its derivatives, chlorogenic acid, luteolin, apigenin, rutin and quercetin, and kaempferol.15
2.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
The gas chromatography–mass spectrometry (GC–MS) technique was used in this study to identify the active ingredients presented in E. spinosus extract. GC–MS analysis of this extract was performed using a Thermo Scientific, Trace GC Ultra/ISQ single Quadrupole MS, TG-5MS fused silica capillary column (length 30 mL, inner diameter 0.251 mm, and film thickness 0.1 mm). For GC/MS detection, an electron ionization system with ionization energy of 70 eV was used, and helium gas was used as the carrier gas at a constant flow rate of 1 mL/min. The injector and MS transfer line temperature was set at 280 °C. The oven temperature was programmed from an initial temperature of 40 °C (hold 3 min) to 280 °C as a final temperature at an increasing rate of 5 °C/min (hold 5 min). The quantification of all identified components was investigated using a percent relative peak area. A tentative identification of the compounds was performed based on the comparison of their relative retention time and mass spectra with those of the NIST WILLY Library data of the GC/MS system.
2.3. Chemicals
Glycerol (product number: G6279) was purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals used in the present experiment were analytical grade.
2.4. Acute Toxicity Study
Male Wistar albino rats were used in a study of acute toxicity. Five rats each from the control and ESE groups were separated. Before the study, rats were given a week to acclimatize. Before ESE doses were administered to the rats, they were made to fast for 24 h while providing access to enough water. ESE was given orally to the treated rats at a dose of 2000 mg/kg bwt, while saline was gavaged to the control rats. Within the first 12 h following treatment, each rat from each of the two groups was examined for any indications of toxicity. Following the treatment, 14 days later, the mortality was evaluated, and any deaths were noted. Male rats treated with a single oral dose of ESE (2000 mg/kg bwt) showed no signs of toxicity. Additionally, after 14 days of treatment, no rats died, and there was no discernible difference between the treated rats and the control rats in terms of body weight or the relative weights of the liver, kidney, spleen, or heart (data not shown).
2.5. Experimental Animals
Thirty five adult male (8–10 weeks of age and weighing 180–200 g) Wistar albino rats, “from institutional breeding house”, were acclimatized to typical lab settings (12 h light/dark cycle; 60% humidity; 25 ± 2 °C), with free food and drink access. All experimental protocols and procedures used in this study were approved by the Department of Zoology and Entomology, Faculty of Science, Helwan University (approval no. HU2021/Z/MDA0521-02).
2.6. Study Design
The rats were divided into five equal groups randomly, each with seven animals, as shown in Table 1.
Table 1. Classification of Study Groups.
| con group | negative control rats were injected with 0.9% NaCl intramuscularly (IM), once 24 h before decapitation. |
| ESE250 group | rats were orally gavaged ES extract in a concentration of 250 mg/kg for 7 continuous days.15 |
| AKI group | positive control glycerol-induced AKI-model rats received vehicle for 7 days, and then were injected with single IM dose of 50% glycerol (10 mL/kg) diluted in normal saline (0.9% NaCl) in the hind limbs with water restriction 1 day before injection.22,23 |
| ESE150 + AKI group | diseased rats were injected with 50% glycerol (10 mL/kg) as a single IM dose for the AKI model, but pretreated orally with 150 mg/kg of ESE for 7 days, before the AKI model application. |
| ESE250 + AKI group | diseased rats were injected with 10 mL/kg 50% glycerol as a single IM dose for the AKI model, and pretreated orally with 250 mg/kg of ESE for 7 days as a different drug concentration model. |
After 24 h of the last treatment, rats were narcotized and then euthanized by decapitation. Then, immediately, the kidneys were removed and weighed. For biochemical tests, blood samples were collected. The right kidney was used for performing the biochemical and molecular analyses, while the left kidney was examined for histopathological alterations.
2.7. Kidney Weight Measuring
Kidneys were weighed, and then according to Almeer et al.,24 the following mathematical calculation was used to calculate the relative kidney weight.
2.8. Tissue Homogenate Preparation
Tissue was homogenized with 50 mM Tris–HCl (pH 7.4; product number: 1.08219, Sigma), using 10% (w/v) renal tissue. The homogenate was centrifuged at 4 °C for 10 min at 3000g, and then the supernatant was stored at −80 °C for biochemical analysis. Lowry et al.’s25 method was used to measure the total renal protein content using bovine serum albumin as a reference protein.
2.9. Biochemical Analysis
2.9.1. Kidney Function Biomarker Assessment
Serum samples were used to determine the levels of urea (catalog number: UR9781) and creatinine (SCr, catalog number: CR510) as kidney function biomarkers using Randox/Laboratory (Crumlin, U.K.) kits according to instructions.
2.9.2. Extent of Rhabdomyolysis Measurement
Lactate dehydrogenase (LDH, catalog number: LD401) and creatine kinase (CK, catalog number: Ck110) activities were assessed following the manufacturer’s instructions supplied by Randox/Laboratory (Crumlin, U.K.) kits.
2.9.3. Kidney Injury Molecule-1 (Kim-1) and Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Enzyme-linked immunosorbent assay (ELISA) kits were employed to assess the plasma levels of Kim-1 (R&D Systems, catalog number: AF3689) and NGAL (My Biosource, catalog number: MBS260195) following the instructions of the manufacturer’s kits.
2.9.4. Redox Status
2.9.4.1. Evaluation of Oxidative Stress Markers
The thiobarbituric acid method described by Ohkawa et al.26 was used to assess lipid peroxidation using malondialdehyde (MDA). The nitric oxide (NO) level in the renal samples was assayed using a Griess reagent at 540 nm, as described by Green et al.27 Furthermore, an Elabscience (Toronto, Ontario, Canada) colorimetric assay kit (catalog number: E-BC-K802-M) was used according to the manufacturer’s instructions to detect the level of total oxidant status (TOS) in renal tissue. Moreover, GSH contents were calculated using Ellman’s reagent, and the formed yellow chromogen was measured at 412 nm as a result.28
2.9.4.2. Enzymatic Antioxidant Activity Assessment
The activity of superoxide dismutase (SOD) and renal catalase (CAT) as kidney enzymatic antioxidants was determined using the procedures described by Nishikimi et al.29 and by Aebi,30 respectively. We followed Factor et al.’s31 assay described to study glutathione reductase (GR) activity. Moreover, glutathione peroxidase (GPx) activity was estimated using the Paglia and Valentine technique.32
2.9.5. Estimation of Inflammatory Cytokines
The Sigma-Aldrich (St. Louis, MO) ELISA kit (catalog number: RAB0278) was used for the assessment of renal proinflammatory cytokine levels, IL-1β. Furthermore, the Merck Millipore (Toronto, Ontario, Canada) ELISA kit (catalog number: EZRTNFA) was used according to the manufacturer’s instructions to detect changes in TNF-α levels, and then all results were normalized by total protein level.
2.9.6. Evaluation of Renal Apoptotic Markers
According to the manufacturer’s instructions, a renal proapoptotic protein (Bax) and antiapoptotic protein (Bcl-2) were evaluated using ELISA kits (Cusabio, Wuhan, China) (catalog numbers: CSB-ELOO2573RA and CSB-E08854r, respectively, in order). The protein ratio of Bcl-2/Bax was plotted too.
2.10. Histopathological Assessments
The left kidneys for all examined groups were fixed in 10% neutral buffered formalin for 24 h and then dehydrated and paraffinized. Light microscopy (Nikon Eclipse E200-LED, Tokyo, Japan) was performed at 400× magnification on renal sections (5 μm) stained with hematoxylin and eosin (H&E). Based on Gibson-Corley et al.,33 renal lesions were scored semiquantitatively by a blind pathologist. The lesions, which included glomerular degeneration, tubular dilatation and desquamation, necrosis, and debris deposition in the tubular lumina, were totaled together and quantified at random from each slide for each rat. The lesions were scored on a scale of 0 (normal), 1 < 25%, 2 = 26–50%, 3 = 51–75%, and 4 > 100%.
2.11. Statistical Analysis
For the examination of different groups’ relations, one-way analysis of variance (ANOVA) was employed using the statistical tool SPSS version 23.0, followed by Duncan’s post hoc test. The data were presented as mean ± standard deviation (SD). At p-values of 0.05, the difference is considered statistically significant. Pearson correlation was utilized to evaluate the relationships between variables. Graph Pad Prism (ver. 6.01) for Windows (San Diego, CA) was used to create the histograms.
3. Results
3.1. GC–MS of E. spinosus Extract
The GC–MS results (Figure 1 and Table 2) show that the essential oil contained 37 active ingredients such as omega-3 arachidonic acid methyl ester, adipic acid hexyl 3-methylbutyl ester, alanine, succinic acid, and quinoline. Some of these active ingredients showed anti-inflammatory and immunomodulatory effects like omega-3 arachidonic acid.
Figure 1.
GC–MS chromatogram of active ingredients of E. spinosus.
Table 2. Composition of Active Ingredients of Echinops spinosus Detected by GC–MS.
| component RTa | compound name | molecular weight | [M – H]-(m/z) molecular weight – 1 | formula | area | peak % |
|---|---|---|---|---|---|---|
| 3.3107 | 2-furancarboxaldehyde, 5-methyl- | 110.1106 | 109.1106 | C6H6O2 | 2905039442 | 8.053 |
| 3.6549 | propionaldehyde, diethylhydrazone | 128.22 | 127.22 | C7H16N2 | 4362834733 | 12.094 |
| 4.3394 | benzofuran, 2,3-dihydro- | 120.1485 | 119.1485 | C8H8O | 2313407136 | 6.4128 |
| 4.8316 | 2-methoxy-4-vinylphenol | 150.1745 | 149.1745 | C9H10O2 | 2628539488 | 7.2863 |
| 5.6347 | 5-isopropylidene-4,6-dimethylnona-3,6,8-trien2-ol | 206.32 | 205.32 | C14H22O | 2562587117 | 7.1035 |
| 5.8705 | 3-cyclopentylpropionic acid, tridec-2-ynyl ester | 320.5 | 319.5 | C21H36O2 | 4590450320 | 12.725 |
| 6.0810 | naphthalene, 1,2-dihydro-2,5,8-trimethyl- | 172.2661 | 171.2661 | C13H16 | 2204004417 | 6.1095 |
| 6.8594 | 1,1,4,5,6-pentamethyl-2,3-dihydro-1H-indene | 188.3086 | 187.3086 | C14H20 | 1348519277 | 3.7381 |
| 7.2577 | 2-furanmethanol, tetrahydro-.α.,.α.,5-trimethyl-5-(4-methyl-3-cyclohexen-1-yl)-, [2S-[2.α.,5.β.(R*)]]- | 238.3657 | 237.3657 | C15H26O2 | 1388701674 | 3.8495 |
| 7.7015 | 2,6,8-trimethylbicyclo[4.2.0]oct-2-ene-1,8-diol | 182.26 | 181.26 | C11H18O2 | 846002886 | 2.345127759 |
| 8.0312 | 7-oxabicyclo[4.1.0]heptan-3-ol, 6-(3-hydroxy1-butenyl)-1,5,5-trimethyl- | 226.3120 | 225.312 | C13H22O3 | 1434701595 | 3.9770 |
| 8.4968 | 6-hydroxy-4,4,7a-trimethyl-5,6,7,7atetrahydrobenzofuran-2(4H)-one | 196.2429 | 195.2429 | C11H16O3 | 2708345136 | 7.5076 |
| 8.8169 | omega-3 arachidonic acid methyl ester | 318.5 | 317.5 | C21H34O2 | 842593525 | 2.3357 |
| 9.2337 | cyclopentanecarboxylic acid, 3-pentyl-4-methylene-, methyl ester, trans- | 210.31 | 209.31 | C13H22O2 | 264773614 | 0.7340 |
| 9.7148 | 2-propenoic acid, 3-(3,4-dimethoxyphenyl)-,(E)- | 208.2106 | 207.2106 | C11H12O4 | 408065695 | 1.1312 |
| 10.0563 | adipic acid, hexyl 3-methylbutyl ester | 300.4 | 299.4 | C17H32O4 | 365721979 | 1.0138 |
| 10.5395 | 1H-purine-2,6-dione, 8-[(2-furanylmethyl)amino]-3,9-dihydro-1,3-dimethyl- | 275.26 | 274.26 | C12H13N5O3 | 7905710 | 0.0219 |
| 10.8621 | 2-propen-1-ol, 1-[(1,1-dimethylethyl)dimethylsilyl]- | 172.34 | 171.34 | C9H20OSi | 3804232328 | 10.545 |
| 11.1942 | succinic acid, diamide, N,N′-methyl-N,N′-phenyl | 296.4 | 295.4 | C18H20N2O2 | 217012507 | 0.6016 |
| 11.6221 | alanine, N-phenyl-, ethyl ester | 193.24 | 192.24 | C11H15NO2 | 34455094 | 0.0955 |
| 12.1202 | 2H-cyclohepta[b]furan-2-one, 6-[1-(acetyloxy)-3-oxobutyl]-3,3a,4,7,8,8a-hexahydro-7-methyl3-methylene- | 306.3536 | 305.3536 | C17H22O5 | 282579432 | 0.7833 |
| 12.5506 | 1,7-dimethyl-4-(1-methylethyl)cyclodecane | 210.3987 | 209.3987 | C15H30 | 165797632 | 0.4596 |
| 12.9316 | (E)-1-(4-hydroxy-3-methoxyphenyl)dec-3-en-5-one | 276.3707 | 275.3707 | C17H24O3 | 9106614 | 0.0252 |
| 13.2777 | 1,2-benzenedicarboxylic acid, 4-hydroxy-,dimethyl ester | 210.1834 | 209.1834 | C10H10O5 | 1644187 | 0.0046 |
| 13.6290 | 1-(4-hydroxy-3-methoxyphenyl)dec-4-en-3-one | 276.3707 | 275.3707 | C17H24O3 | 32074245 | 0.0889 |
| 13.9418 | benzene, (butoxymethyl)- | 164.2441 | 163.2441 | C11H16O | 147274677 | 0.4082 |
| 14.4042 | benzene, 1,1′-(1,2-dimethyl-1,2-ethanediyl)bis-, (R*,S*)- | 210.3141 | 209.3141 | C16H18 | 82655983 | 0.2291 |
| 14.8433 | benzimidazo[2,1-a]isoquinoline | 218.25 | 217.25 | C15H10N2 | 9458298 | 0.0262 |
| 15.2583 | quinoline-2-carboxamide, N-(2-furfuryl)- | 252.27 | 251.27 | C15H12N2O2 | 7143676 | 0.0198 |
| 15.8324 | (E)-3,3′-dimethoxy-4,4′-dihydroxystilbene | 272.29 | 271.29 | C16H16O4 | 54288941 | 0.1505 |
| 16.3179 | 2-((5R,6R,8S,8aS)-6-butyl-5-propyloctahydroindolizin-8-yl)ethanol | 267.4 | 266.4 | C17H33NO | 2613075 | 0.0072 |
| 17.0212 | succinic acid, 3-methylbut-2-ylpentafluorophenyl ester | 354.27 | 353.27 | C15H15F5O4 | 1139851 | 0.0032 |
| 17.6524 | 3,4-divanillyltetrahydrofuran | 344.4 | 343.4 | C20H24O5 | 33584083 | 0.0931 |
| 18.1102 | trans-2,7-dimethyl-4,6-octadien-2-ol | 154.25 | 153.25 | C10H18O | 4354764 | 0.0121 |
| 18.4653 | 2-propenoic acid, 3-(3,4,5-trimethoxyphenyl)- | 238.24 | 237.24 | C12H14O5 | 2063798 | 0.0057 |
| 19.3356 | benzamide, N-(3-[1,3]dioxan-2-ylphenyl)-3,4-dimethoxy- | 343.4 | 342.4 | C19H21NO5 | 777188 | 0.0022 |
| 19.8190 | benzenamine, N,N-diphenyl-4-[2-phenylethenyl]- | 347.4 | 346.4 | C26H21N | 466598 | 0.0013 |
RT: retention time.
3.2. Effect of ESE on RKW % Following Glycerol Injection
This study intended to apply the AKI model by injecting 50% glycerol leading to RM and then AKI was developed. Both kidney weight (23.44%) and RKW (25.70%) showed significant increment in the AKI group (p < 0.05) as compared to the negative control group (Figure 2). However, kidney weight and RKW were significantly diminished in both the ESE150 + AKI group and ESE250 + AKI group compared to the AKI group. Furthermore, Pearson r values revealed significant strong positive correlations between kidney weight and RKW % (Table 3).
Figure 2.
Effects of E. spinosus extract (ESE) on kidney weight and relative kidney weight (RKW %) in glycerol-induced acute kidney injury. Results are presented as mean ± SD (n = 7); ψ and Δ represent significant changes at p < 0.05 between the untreated control and AKI groups, respectively.
Table 3. Correlation Studies between Kidney Weight, Kidney Function Parameters, RM-Related Markers, and the Newly Advanced Kidney Injury Markers in the Studied Groups.
| correlations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| KW | RKW | Ur | Cr | LDH | CK | Kim-1 | NAGL | ||
| KW | Pearson correlation | 1 | 0.989a | 0.779a | 0.782a | 0.801a | 0.833a | 0.815a | 0.828a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| RKW | Pearson correlation | 0.989a | 1 | 0.789a | 0.808a | 0.816a | 0.838a | 0.836a | 0.858a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Ur | Pearson correlation | 0.779a | 0.789a | 1 | 0.912a | 0.939a | 0.944a | 0.951a | 0.924a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Cr | Pearson correlation | 0.782a | 0.808a | 0.912a | 1 | 0.906a | 0.928a | 0.942a | 0.938a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| LDH | Pearson correlation | 0.801a | 0.816a | 0.939a | 0.906a | 1 | 0.938a | 0.930a | 0.899a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| CK | Pearson correlation | 0.833a | 0.838a | 0.944a | 0.928a | 0.938a | 1 | 0.977a | 0.941a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Kim | Pearson correlation | 0.815a | 0.836a | 0.951a | 0.942a | 0.930a | 0.977a | 1 | 0.961a |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| NAGL | Pearson correlation | 0.828a | 0.858a | 0.924a | 0.938a | 0.899a | 0.941a | 0.961a | 1 |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
Correlation is significant at the 0.01 level (2-tailed).
3.3. Effect of ESE on Kidney Function (AKI Model-Related) Biomarkers
The AKI model group showed significant magnification (p < 0.05) in the serum urea (210.27%) and creatinine (134.04%) levels as compared to both the control and ESE250 groups, but we can find a significant decline (p < 0.05) in the pretreated ESE150 + AKI and ESE250 + AKI groups when compared with the AKI group (Figure 3). Furthermore, Pearson r values revealed significant moderate positive correlations between traditional kidney function parameters and the kidney weight (Table 3).
Figure 3.
Effects of E. spinosus extract (ESE) on kidney function (urea and creatinine), rhabdomyolysis (RM)-related (LDH and CK) markers, kidney injury molecule-1 (Kim-1), and neutrophil gelatinase-associated lipocalin (NGAL) in glycerol-induced acute kidney injury. Results are presented as mean ± SD (n = 7); ψ and Δ represent significant changes at p < 0.05 between the untreated control and AKI groups, respectively.
3.4. Effect of ESE on Rhabdomyolysis (RM)-Related Markers After Glycerol Injection
CK and LDH were determined in the present study to prove the onset of RM. Both markers LDH (149.79%) and CK (349.43%) exhibited significant upsurges (p < 0.05) as compared to both control and ESE250 groups (Figure 3). However, these markers showed a significant decline (p < 0.05) in the pretreated ESE150 + AKI and ESE250 + AKI groups as compared to the AKI group. Results are comparable to those of the normal control group.
3.5. Kidney Injury Molecule-1 and Neutrophil Gelatinase-Associated Lipocalin
As shown in Figure 3, the AKI model group revealed significant intensive magnification (p < 0.05) in both kidney markers, namely, Kim-1 (2079.31%) and NGAL (101.69%) as compared to the control group. On the contrary, pretreating with ESE150 mg and ESE250 mg astonishingly decreased this elevation (p < 0.05) as compared to the AKI model following glycerol injection in the AKI group.
3.6. Effect of ESE on Oxidative Stress Biomarkers and Antioxidant Molecules Following AKI Model Induction
In all experimental groups, the renal tissue’s redox status was evaluated at the level of both pro-oxidant and antioxidant molecules. It was shown that the AKI group model amplified renal lipid peroxidation via the MDA (50.91%) level and boosted NO (102.03%) production. As a result, the TOS (58.49%) level in renal tissue was significantly increased (p < 0.05) in the AKI group compared to the control group. On conflict, reduction in the cellular antioxidant capacity (p < 0.05) was distinguished in renal GSH (−40.06%) level, GR (−41.73%), GPx (−32.0%), CAT (−32.99%), and SOD (−30.39%) activities as compared to the control group (Figure 4).
Figure 4.
Effects of E. spinosus extract (ESE) on the renal levels of oxidative stress biomarkers [malondialdehyde (MDA), nitric oxide (NO), and total oxidant status (TOS)] and enzymatic and nonenzymatic molecules [glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR)] in glycerol-induced acute kidney injury. Results are presented as mean ± SD (n = 7); ψ and Δ represent significant changes at p < 0.05 between the untreated control and AKI groups, respectively.
ESE proved to have surprising results when comparing ESE150 + AKI and ESE250 + AKI groups to the AKI group. TOS, MDA, and NO production levels were lowered; their levels came nearly to normal levels. ESE was able to heal the induced oxidative injury in both ESE150 + AKI and ESE250 + AKI groups compared to the AKI group (Figure 4). On the other hand, GSH levels, GR, GPx, CAT, and SOD activities were intensely improved in both pretreated ESE150 + AKI and ESE250 + AKI groups, and the levels were comparable to control levels.
3.7. Effect of ESE on Inflammatory Mediators after AKI Model Induction Using Glycerol
The levels of proinflammatory cytokines TNF-α (136.43%) and IL-1β (60.94%) in renal tissue were significantly increased (p < 0.05) in the AKI group. The ESE pretreatment groups, the ESE150 + AKI group and ESE250 + AKI group, had normalized levels of these proinflammatory mediators as compared to the AKI group (Figure 5).
Figure 5.
Effects of E. spinosus extract (ESE) on the levels of proinflammatory cytokines [tumor necrosis factor-α (TNF-α) and interleukin-1 β (IL-1β)] in glycerol-induced acute kidney injury. Results are presented as mean ± SD (n = 7); ψ and Δ represent significant changes at p < 0.05 between the untreated control and AKI groups, respectively.
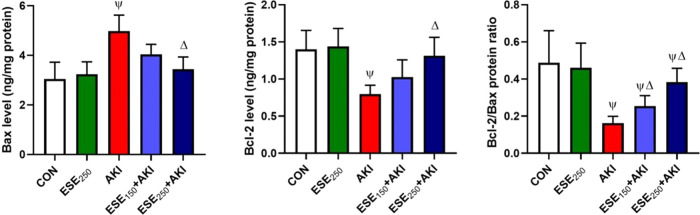
3.8. Influence in Proapoptotic and Antiapoptotic Proteins under the Effect of ESE
By measuring Bax and Bcl-2 levels as proapoptotic and antiapoptotic markers, it was found that the AKI group increased renal apoptosis by raising the proapoptotic Bax (66.03%) protein and lessening the antiapoptotic protein Bcl-2 (−43.14%) levels in comparison to both control and ESE250 groups. In the ESE150 and ESE250 pretreated groups, we noticed significant suppression (p < 0.05) in the Bax levels accompanied by amplified Bcl-2 levels as compared to the AKI group (Figure 6). The Bcl-2/Bax protein ratio was significantly decreased (p < 0.05) in the AKI group as compared to both control and ESE250 groups. However, ESE150 + AKI and ESE250 + AKI groups showed elevated values (p < 0.05) comparable to normal levels (Figure 6).
Figure 6.
Effects of E. spinosus extract (ESE) on the levels of apoptotic proteins [Bcl-2-associated X-protein (Bax), B-cell lymphoma 2 (Bcl-2), and Bcl-2/Bax ratio] in glycerol-induced acute kidney injury. Results are presented as mean ± SD (n = 7); ψ and Δ represent significant changes at p < 0.05 between the untreated control and AKI groups, respectively.
3.9. Histopathological Changes Associated with ESE
Normal tissue architecture of the renal cortex was visible upon microscopic examination of stained renal slices from the control and ESE groups (Figure 7a,b, respectively). However, severe tissue damage was visible in the glycerol-treated group’s sections as evidenced by glomerular degeneration, tubular dilatation and desquamation, vacuolation, necrosis, and debris buildup in the tubular lumina (Figure 7c). However, renal sections from rats given high and low dosages of ESE demonstrated some amelioration of renal histological abnormalities (Figure 7d,7e, respectively). Surprisingly, the renal tissue changes markedly improved with the addition of 250 mg of ESE (Figure 7e). According to semiquantitative statistical analysis of renal lesion scores, animals treated with glycerol had significantly higher renal lesion scores than rats in the control group. However, animals in the ESE150 + AKI and ESE250 + AKI groups had a substantial decrease in the renal lesion score compared to rats in the glycerol group (Figure 7f).
Figure 7.
Effects of E. spinosus extract (ESE) on the histopathology of renal tissue in glycerol-induced acute kidney injury. (a) Control showing normal tissue architecture of the renal cortex, (b) ESE 250 mg showing the cortex area with normal structure and healthy glomeruli, (c) AKI showing severe tissue damage as evidenced by glomerular degeneration (white star), tubular dilatation and desquamation (blue arrow), hemorrhage (white arrow), vacuolation, necrosis, and debris buildup in the tubular lumina (green arrow), (d) ESE150 + AKI showing some amelioration of the renal histological abnormalities, (e) ESE250 + AKI showing a marked improvement in the renal tissue appearance, and (f) semiquantitative scoring of renal lesions. Hematoxylin and eosin (H&E). Scale bar = 100 μm. Results are presented as mean ± SD (12 fields per kidney, 2 kidneys per animal, and 3 animals per group were used in the analyses); ψ and Δ represent significant changes at p < 0.05 between the untreated control and AKI groups, respectively.
4. Discussion
Herbal medicines have attracted the world’s attention, owing to their astonishing biological activities, economic facts, and easily accessed sources. One of the unprecedented herbal cotherapeutic agents with outstanding effects that has been found is E. spinosus. From leaves to roots, this herbal medicine has succeeded to prove dazzling cotherapeutic effects in different medicinal cases. Scientific investigations proved that ES effectively has antioxidant, anti-inflammatory, and antibacterial qualities and it can be used as adjuvant therapy.19 It has many antioxidant phytochemicals such as phenols, terpenes, alkaloids, flavonoids, steroids, cardiac glycosides, and coumarins characterized by anti-inflammatory, anticancer, and antiaging actions and free radical scavenging abilities.13,34 In the case of AKI, a sudden and recent decrease in kidney functions occurs. Causes are multiple, and prompt intervention can be critical to diminish or prevent morbidity.5 In this study, we linked the previously supplied information about ESE’s effects and investigated the possible renal-protective activities in the case of glycerol-induced acute kidney injury in an animal model, in a concentration-dependent manner. The study examined the extent to which ESE can help renal cells to survive, as we can use ES as herbal adjuvant therapy in cases with the risk of AKI. A single dose of the nephrotoxic component (glycerol) was injected intramuscularly to trigger AKI. As mentioned by AlBasher et al.,35 this model leads to AKI via induction of rhabdomyolysis. When skeletal muscles leak their contents in circulation, this debris clogs the renal tubule. After 24 h of glycerol injection (exogenous-toxin), the primary end point was developed as an absolute increase in the SCr level (≥0.5 mg/dL) from the baseline.4
A significant upsurge in the kidneys’ weight and relative kidney weight percentage (RKW %) in our finding, in addition to the elevation of other renal biomarkers, agrees with Al Asmari et al.36 This kidney enlargement elucidates pathophysiological edema, which results in RM following toxin exposure and AKI development. This is mainly explained by glomeruli and renal tubule damage. However, the significant rise in RM-related markers (LDH and CK) confirms skeletal muscle crumbling and infiltration into blood circulation. CK is a marker for myocyte damage that coexists with muscle problems; however, LDH is a biomarker of muscle injury and will be significantly elevated in individuals with RM. Furthermore, LDH levels have been found to be raised during cell necrosis. It has been proven that LDH is a sensitive marker for the onset of AKI as a complication of RM.37
Oxidative stress refers to a state of imbalance between oxidation and antioxidants in vivo, which plays an important role in the development of AKI.38 Nephron’s death was exacerbated due to changes in both enzymatic and nonenzymatic antioxidants. The reaction of both ROS and RNS on cellular biological macromolecules on the biofilm resulted in lipid peroxidation products, such as MDA and 4-hydroxynonenoic acid (4-HNE). According to Rizk et al.,39 nitric oxide reacts with superoxide (O2•–) resulting in the production of peroxynitrite (ONOO•–), the main cause of NO’s toxic effects. This is also the main cause of tissue injury, following acute nephrotoxicity. A decrease in excessive ONOO•– is suggested to be the cause of diminished nephron damage. Oxygen free radicals damage cells and result in an upsurge of excessive MDA content. Then, antioxidant enzymes including SOD, CAT, GR, and GPx were extensively consumed to remove excess free radicals.40 As a result of AKI induction in this study, acute oxidative damage was evinced by increased ROS formation, lipid peroxidation through MDA levels, and NO generation, while the antioxidant markers, GSH, GPx, SOD, and CAT were dropped. AKI is mainly manifested by an increase in serum creatinine and urea nitrogen as a cause of glomerular filtration rate reduction.40,41 This information matches our results. Therefore, the amplification of kidney function as serum creatinine and urea nitrogen proves the presence of an effective AKI model. On the other hand, ESE supplementation was able to influence redox changes in the AKI model, as seen by the suppression of TOS, MDA, and NO production as compared with the nonpretreated AKI model. Bouzabata et al.16 analyzed E. spinosum extracts. The identified compounds were found in quinic acid, campesterol, stigmasterol, apigenin glucoside derivatives, brassicasterol, cynarin, rutin, chlorogenic acid, and its derivatives. They confirmed our results that ES can be effectively used as a powerful natural source rich in polyphenols as antioxidants, especially root methanol extract, with the highest reducing activity. They confirmed that ES contains ‘di and tricaffeoyl-altraric acid’, which exhibited the best antioxidant capacity and reasonable pharmacokinetic and pharmacodynamic ES effects, with a significantly safe profile.16
Furthermore, a large number of studies have demonstrated that NGAL as a renal injury biomarker is rapidly released in response to tubular injury. It is involved in antibacterial defense through iron sequestration and is considered a traditional marker of kidney injury. The relative level of serum NGAL in patients with AKI correlates with the severity of renal damage and the risk of mortality. Both NGAL and Kim-1 are sensitive, specific, and highly predictive markers of AKI.42 However, ESE treatment preserve kidney tissues form glycerol-induced AKI. Hegazy et al.19 corroborated that ESE diminished oxidative damage by decreasing NADPH oxidase 2 activity and ROS production. By diminishing lipid peroxidation and NO formation, in addition to enhancing the pool of accessible GSH and upstream antioxidants in renal tissues, ESE proved to have the ability to rescue renal tissue in the glycerol-induced AKI model.
ROS can trigger inflammation via the stimulation of TNF-α production. This excessive release of TNF-α led to the extra production of other inflammatory factors, such as IL-1β and IL-6. These results were confirmed by Hegazy et al.19 as they measured the effect of ES on induced renal inflammation. Finally, these oxidative stress and inflammatory mediators help each other and start damaging the kidney tissue, forming AKI.2 According to the findings, in our case, renal stimulation using glycerol enhanced the proinflammatory cytokine release via overproduction of both renal TNF-α and IL-1β. Studies have demonstrated that active components found in ESE inhibited the synthesis of TNF-α as well as the creation of IL-1β. Furthermore, ESE demonstrated immunomodulatory effects and lowered elevated proinflammatory markers in animal models of renal inflammation.19 In animals, the glycerol-induced AKI model was linked to a rise in proapoptotic protein expression (Bax) as well as a decline in antiapoptotic protein expression (Bcl-2). AKI is characterized by renal tubular cell injury or death, apoptosis, inflammation, and vascular dysfunction. Pretreatment with ESE considerably modulated and reversed oxidative stress, apoptosis, inflammation, and AKI and RM-related markers to nearly normal levels. This confirms the ESE’s renal-protective properties as well as its role in cell content stabilization. As a result, these findings suggest that ESE is a promising therapy that we can depend on, especially in risk factor-exposed persons. ESE can reverse the damage to kidney cells.
The glycerol-induced acute kidney injury model rats were characterized by glomerular degeneration, tubular dilatation and desquamation, vacuolation, necrosis, and debris buildup in the tubular lumina. In the case of chronic interstitial nephritis, early detection is especially important because it is difficult to diagnose until most of the functionality of the kidneys is destroyed. Similar changes were also reported by Wu et al.,43 Li et al.,23 and others demonstrating structural changes in renal tissue of glycerol-treated animals and its protection by various agents. Administration of ESE reversed kidney damage, with especially a marked reduction in tubular damage induced by AKI. The nephroprotective effect of ESE perhaps by improving antioxidant defense system and restraining inflammation and apoptosis.
5. Conclusions
ESE has generous nephron-protective aids against the glycerol-induced AKI group in rats. This plant effectively inhibited renal oxidative stress, inflammation, and apoptotic insults, helping kidneys to survive. Preadministration of ESE 250 mg for 7 days helps kidneys to survive, decrease inflammation, and prevent apoptosis. These data suggested that ES could reduce renal damage in patients with risk factors of AKI. Further studies are required to understand more mechanisms concerned with the action of ESE in the rat model.
Acknowledgments
This study was supported by the Researchers Supporting Project (RSP2023R508), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
All of the data sets generated or analyzed during this study are included in this published article.
Author Contributions
A.A., A.E.A.M., and M.A.D. designed the project. S.R., F.A.T., R.A., and A.E.A.M. performed the experiments and edited the manuscript. A.A., M.A.M., M.A.A.A., and S.R. analyzed and interpreted the data and drafted and edited the manuscript. All authors approved the final draft.
The authors declare no competing financial interest.
Notes
The authors declare no competing financial interest.
Notes
Institutional Review Board Statement All experimental protocols and procedures used in this study were approved by the Department of Zoology and Entomology, Faculty of Science, Helwan University (approval no. HU2021/Z/MDA0521-02).
References
- Bunel V.; Qu F.; Duez P.; Xu Q. Herbal Medicines for Acute Kidney Injury: Evidence, Gaps and Frontiers. World J. Tradit. Chin. Med. 2015, 1, 47–66. 10.15806/j.issn.2311-8571.2015.0019. [DOI] [Google Scholar]
- Yang Q.; Wang Y.; Chen H.; Fan H.; Zhang X.; Bello B. K.; Liu G.; Feng X.; Teng D.; Chen Y.; et al. Protective activities of scutellarin against alcohol-induced acute kidney injury. Chem. Biodiversity 2022, 19, e202200254 10.1002/cbdv.202200254. [DOI] [PubMed] [Google Scholar]
- Matuszkiewicz-Rowińska J.; Malyszko J. Acute kidney injury, its definition, and treatment in adults: guidelines and reality. Pol. Arch. Intern. Med. 2020, 130 (12), 1074–1080. 10.20452/pamw.15373. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xu Z.; He W.; Lin Z.; Liu Y.; Dai Y.; Chen W.; Chen W.; He W.; Duan C.; et al. Elevated Serum Uric Acid/Albumin Ratio as a Predictor of Post-Contrast Acute Kidney Injury After Percutaneous Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction. J. Inflammation Res. 2022, 15, 5361–5371. 10.2147/JIR.S377767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowle J. R.; Forni L. G.; Bell M.; Chew M. S.; Edwards M.; Grams M. E.; Grocott M. P. W.; Liu K. D.; McIlroy D.; Murray P. T.; et al. Postoperative acute kidney injury in adult non-cardiac surgery: joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat. Rev. Nephrol. 2021, 17 (9), 605–618. 10.1038/s41581-021-00418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjou A.; Agarwal A. Sepsis and Acute Kidney Injury. J. Am. Soc. Nephrol. 2011, 22 (6), 999–1006. 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- Rui Y.; Li S.; Luan F.; Li D.; Liu R.; Zeng N. Several Alkaloids in Chinese Herbal Medicine Exert Protection in Acute Kidney Injury: Focus on Mechanism and Target Analysis. Oxid. Med. Cell. Longevity 2022, 2022, 2427802 10.1155/2022/2427802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M.; Jiang N.; Guo L.; Ni Z.; Al-Brakati A. Y.; Othman M. S.; Abdel Moneim A. E.; Kassab R. B. Oleuropein suppresses oxidative, inflammatory, and apoptotic responses following glycerol-induced acute kidney injury in rats. Life Sci. 2019, 232, 116634 10.1016/j.lfs.2019.116634. [DOI] [PubMed] [Google Scholar]
- Sepúlveda R. A.; Anghileri F.; Huidobro E. J.; Julio R.; Ávila E.; Figueroa C. Acute kidney injury associated to sulfamethoxazole urine crystal: The importance of clinical suspicion. Clin. Nephrol. Case Stud. 2022, 10, 71–75. 10.5414/cncs110931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis N. G.; Francescato H. D. C.; de Almeida L. F.; Silva C.; Costa R. S.; Coimbra T. M. Protective effect of calcitriol on rhabdomyolysis-induced acute kidney injury in rats. Sci. Rep. 2019, 9 (1), 7090 10.1038/s41598-019-43564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonafine M.; Martinez-Martinez E.; Jaisser F. More than a simple biomarker: the role of NGAL in cardiovascular and renal diseases. Clin. Sci. 2018, 132 (9), 909–923. 10.1042/CS20171592. [DOI] [PubMed] [Google Scholar]
- Organization W. H.WHO Establishes the Global Centre for Traditional Medicine in India. https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india (accessed Sep 21, 2023).
- Dong M.; Cong B.; Yu S. H.; Sauriol F.; Huo C. H.; Shi Q. W.; Gu Y. C.; Zamir L. O.; Kiyota H. Echinopines A and B: sesquiterpenoids possessing an unprecedented skeleton from Echinops spinosus. Org. Lett. 2008, 10 (5), 701–704. 10.1021/ol702629z. [DOI] [PubMed] [Google Scholar]
- Bouattour E.; Fakhfakh J.; Frikha Dammak D.; Jabou K.; Damak M.; Mezghani Jarraya R. Hexane Extract of Echinops spinosissimus Turra subsp. spinosus from Tunisia: A Potential Source of Acetylated Sterols—Investigation of its Biological Activities. Chem. Biodiversity 2016, 13 (12), 1674–1684. 10.1002/cbdv.201600118. [DOI] [PubMed] [Google Scholar]
- Alkhudhayri A.; Abdel Moneim A. E.; Rizk S.; Bauomy A. A.; Dkhil M. A. The Neuroprotective Effect Associated with Echinops spinosus in an Acute Seizure Model Induced by Pentylenetetrazole. Neurochem. Res. 2023, 48 (1), 273–283. 10.1007/s11064-022-03738-2. [DOI] [PubMed] [Google Scholar]
- Bouzabata A.; Montoro P.; Gil K. A.; Piacente S.; Youssef F. S.; Al Musayeib N. M.; Cordell G. A.; Ashour M. L.; Tuberoso C. I. G. HR-LC-ESI-Orbitrap-MS-Based Metabolic Profiling Coupled with Chemometrics for the Discrimination of Different Echinops spinosus Organs and Evaluation of Their Antioxidant Activity. Antioxidants 2022, 11 (3), 453 10.3390/antiox11030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouattour E.; Fakhfakh J.; Affes M.; Chawech R.; Damak M.; Jarraya R. Chemical Constituents of Echinops spinosus from Tunisia. Chem. Nat. Compd. 2017, 53 (5), 984–987. 10.1007/s10600-017-2179-9. [DOI] [Google Scholar]
- Deyno S.; Tola M. A.; Bazira J.; Makonnen E.; Alele P. E. Acute and repeated-dose toxicity of Echinops kebericho Mesfin essential oil. Toxicol. Rep. 2021, 8, 131–138. 10.1016/j.toxrep.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegazy M. G. A.; Emam M. A.; Khattab H. I.; Helal N. M. Biological activity of Echinops spinosus on inhibition of paracetamol-induced renal inflammation. Biochem. Cell Biol. 2019, 97 (2), 176–186. 10.1139/bcb-2018-0212. [DOI] [PubMed] [Google Scholar]
- Bitew H.; Hymete A. The Genus Echinops: Phytochemistry and Biological Activities: A Review. Front. Pharmacol. 2019, 10, 1234. 10.3389/fphar.2019.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman M. S.; Khaled A. M.; Al-Bagawi A. H.; Fareid M. A.; Hameed R. A.; Zahra F. A. A.; Moneim A. E. A. Echinops spinosus effect against diabetes and its hepatorenal complications: total extract and flavonoids fraction. Environ. Sci. Pollut. Res. Int. 2022, 29, 38606. 10.1007/s11356-022-18824-9. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Lee S. S.; Jung M. H.; Yeo H. D.; Kim H. J.; Yang J. I.; Roh G. S.; Chang S. H.; Park D. J. N-acetylcysteine attenuates glycerol-induced acute kidney injury by regulating MAPKs and Bcl-2 family proteins. Nephrol., Dial., Transplant. 2010, 25 (5), 1435–1443. 10.1093/ndt/gfp659. [DOI] [PubMed] [Google Scholar]
- Li Y. F.; Xu B. Y.; An R.; Du X. F.; Yu K.; Sun J. H.; Zhang G. H.; Wang W.; An L. P.; Wu G. L. Protective effect of anisodamine in rats with glycerol-induced acute kidney injury. BMC Nephrol. 2019, 20 (1), 223. 10.1186/s12882-019-1394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeer R. S.; AlBasher G. I.; Alarifi S.; Alkahtani S.; Ali D.; Abdel Moneim A. E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9 (1), 5825 10.1038/s41598-019-42368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H.; Rosebrough N. J.; Farr A. L.; Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193 (1), 265–275. 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Ohkawa H.; Ohishi N.; Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95 (2), 351–358. 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Green L. C.; Wagner D. A.; Glogowski J.; Skipper P. L.; Wishnok J. S.; Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126 (1), 131–138. 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]; Research Support, U.S. Gov’t, P.H.S.
- Ellman G. L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82 (1), 70–77. 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Nishikimi M.; Appaji N.; Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46 (2), 849–854. 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Factor V. M.; Kiss A.; Woitach J. T.; Wirth P. J.; Thorgeirsson S. S. Disruption of redox homeostasis in the transforming growth factor-α/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem. 1998, 273 (25), 15846–15853. 10.1074/jbc.273.25.15846. [DOI] [PubMed] [Google Scholar]
- Paglia D. E.; Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70 (1), 158–169. [PubMed] [Google Scholar]
- Gibson-Corley K. N.; Olivier A. K.; Meyerholz D. K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50 (6), 1007–1015. 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A.; Prasad S. K.; Joshi V. K.; Hemalatha S. Phytochemical standardization, antioxidant, and antibacterial evaluations of Leea macrophylla: A wild edible plant. J. Food Drug Anal. 2016, 24 (2), 324–331. 10.1016/j.jfda.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlBasher G.; Alfarraj S.; Alarifi S.; Alkhtani S.; Almeer R.; Alsultan N.; Alharthi M.; Alotibi N.; Al-Dbass A.; Abdel Moneim A. E. Nephroprotective Role of Selenium Nanoparticles Against Glycerol-Induced Acute Kidney Injury in Rats. Biol. Trace Elem. Res. 2020, 194 (2), 444–454. 10.1007/s12011-019-01793-5. [DOI] [PubMed] [Google Scholar]
- Al Asmari A. K.; Al Sadoon K. T.; Obaid A. A.; Yesunayagam D.; Tariq M. Protective effect of quinacrine against glycerol-induced acute kidney injury in rats. BMC Nephrol. 2017, 18 (1), 41. 10.1186/s12882-017-0450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Młynarska E.; Krzemińska J.; Wronka M.; Franczyk B.; Rysz J. Rhabdomyolysis-Induced AKI (RIAKI) Including the Role of COVID-19. Int. J. Mol. Sci. 2022, 23, 8215 10.3390/ijms23158215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W. J.; Vernieri C.; Foiani M.; Jiang J. D. Berberine in the treatment of metabolism-related chronic diseases: A drug cloud (dCloud) effect to target multifactorial disorders. Pharmacol. Ther. 2020, 209, 107496 10.1016/j.pharmthera.2020.107496. [DOI] [PubMed] [Google Scholar]
- Rizk S.; Taha H.; Abdel Moneim A. E.; Amin H. K. Neuroprotective effect of green and roasted coffee bean extracts on cerebral ischemia-induced injury in rats. Metab. Brain Dis. 2021, 36 (7), 1943–1956. 10.1007/s11011-021-00769-6. [DOI] [PubMed] [Google Scholar]
- Xue J. L.; Liu B. Y.; Zhao M.; Zhang M. Y.; Wang M. Y.; Gu Q. Q.; Zhang X. Y.; Qin S. C. Inhalation of 4% and 67% hydrogen ameliorates oxidative stress, inflammation, apoptosis, and necroptosis in a rat model of glycerol-induced acute kidney injury. Med. Gas Res. 2023, 13 (2), 78–88. 10.4103/2045-9912.345169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousleh R.; Al Laham S.; Al-Manadili A. The Preventive Role of Pioglitazone in Glycerol-Induced Acute Kidney Injury in Rats during Two Different Treatment Periods. Iran J. Med. Sci. 2018, 43 (2), 184–194. 10.30476/ijms.2018.40527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümpers P.; Hafer C.; Lukasz A.; Lichtinghagen R.; Brand K.; Fliser D.; Faulhaber-Walter R.; Kielstein J. T. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Critical Care 2010, 14 (1), R9. 10.1186/cc8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Pan X.; Fu H.; Zheng Y.; Dai Y.; Yin Y.; Chen Q.; Hao Q.; Bao D.; Hou D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017, 7 (1), 10114 10.1038/s41598-017-10693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data sets generated or analyzed during this study are included in this published article.