Abstract
Trigeminal inflammatory pain is one of the most severe pain-related disorders in humans; however, the underlying mechanisms remain largely unknown. In this study, we investigated the possible contribution of interaction between ten-eleven translocation methylcytosine dioxygenase 1 (TET1) and the voltage-gated K+ channel Kv7.2 (encoded by Kcnq2) to orofacial inflammatory pain in mice. We found that complete Freund’s adjuvant (CFA) injection reduced the expression of Kcnq2/Kv7.2 in the trigeminal ganglion (TG) and induced orofacial inflammatory pain. The involvement of Kv7.2 in CFA-induced orofacial pain was further confirmed by Kv7.2 knockdown or overexpression. Moreover, TET1 knockdown in Tet1flox/flox mice significantly reduced the expression of Kv7.2 and M currents in the TG and led to pain-like behaviors. Conversely, TET1 overexpression by lentivirus rescued the CFA-induced decreases of Kcnq2 and M currents and alleviated mechanical allodynia. Our data suggest that TET1 is implicated in CFA-induced trigeminal inflammatory pain by positively regulating Kv7.2 in TG neurons.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-023-01139-1.
Keywords: Facial pain, KCNQ2 potassium channel, TET1 protein
Introduction
Orofacial pain is an extremely painful condition affecting ~16% of the population worldwide [1]. Trigeminal neuralgia is characterized by recurrent unilateral brief electric shock-like pains [2]. These abnormal events may be attributed to alterations in the activities of nociceptive neurons or the sensitization of trigeminal nociceptors induced by inflammatory mediators [3]. However, current treatments have limited effectiveness, at least partly owing to the unknown underlying mechanisms. Therefore, it is essential to understand the pathogenesis of orofacial pain and develop novel therapeutic strategies to improve the treatment outcomes.
Accumulating evidence indicates that abnormal changes in neural activity and plasticity triggered by tissue or nerve injury/inflammation contribute to pain hypersensitivity [4, 5]. The M-type K+ current conducted by Kv7 channels (encoded by the Kcnq 1–5 genes), one of the major mechanisms controlling the tonic excitability of neurons, has attracted wide attention in recent years. The M-type K+ current is a slowly inactivated or non-inactivated K+ current that maintains substantial control over neuronal excitability. Among the five members of the Kcnq gene family, Kcnq2 and Kcnq3 are critical in regulating neuronal activity owing to their broad expression in the central nervous system, with an almost complete overlap in expression patterns [6]. Functional Kcnq2 and Kcnq3 are involved in various physiological and pathological processes, and mutations in Kcnq2 and Kcnq3 are associated with benign familial neonatal convulsions, an autosomal dominant neonatal epilepsy [7, 8]. Acute inhibition of the M-channel of nociceptors in rodents can lead to depolarization, increased excitability, and nocifensive behaviors [9, 10]. The activation of the K+ channel Kv7.2 in small dorsal root ganglion (DRG) neurons may be an activity-dependent compensatory mechanism to limit the streptozotocin- and partial sciatic nerve ligation-induced hyperexcitability of DRG neurons and the associated pain hypersensitivity [11, 12]. However, whether Kcnq2/Kcnq3 is implicated in orofacial pain and the possible regulatory mechanisms of these Kcnq subunits remain unclear.
DNA methylation is a crucial epigenetic mechanism and is essential for gene silencing and maintaining genome stability [13]. The active DNA demethylation pathway involves oxidizing 5-methylcytosine (5 mC) to 5-hydroxymethylcytosine (5 hmC), followed by transformation into 5-formylcytosine and/or 5-carboxylcytosine [14]. Ten-eleven translocation methylcytosine dioxygenase 1 (TET1), one of the TET family members (TET1–3), promotes DNA demethylation by catalyzing the conversion of 5mC to 5hmC through direct enzymatic removal of the 5-hydroxylated methyl group and replacement of the methylated cytosine base via the DNA base excision repair pathway, thereby inducing gene expression. Wu et al. reported that nerve injury downregulates the Kv1.2 expression in DRG neurons, whereas TET1 overexpression alleviates nerve injury-induced pain by increasing Kv1.2 expression [15]. TET1 may also regulate K2p1.1 [16] and Kcnh2 [17] expression to affect nerve injury-induced neuropathic pain and formalin-induced acute inflammatory pain respectively. However, the role of TET1 in trigeminal pain remains unknown. Given the role of TET1 in regulating ion channels, we hypothesized that TET1 is associated with the Kv7.2 channel in TG neurons.
In the present study, we investigated the role of TET1 in trigeminal inflammatory pain, as well as the potential regulatory mechanism between TET1 and Kcnq2 using a mouse model of trigeminal inflammatory pain. In addition, either overexpression of TET1 or activation of Kv7 channels could change the excitability of trigeminal ganglion neurons, and further relieve the orofacial pain-like behaviors. Our work provides a new insight into the function of the TET1-Kcnq2 pathway during the progression of orofacial pain.
Materials and Methods
Animals
Male C57BL/6 mice (8 weeks old) were purchased from SiPeiFu (Beijing) Biotechnology Co., Ltd. Tet1flox/flox mice were a generous gift from Dr. Guoliang Xu (Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, China). All mice were maintained in a 12/12 h light-dark cycle at 25 °C, with free access to water and food. All the procedures for animals were approved by the Animal Care and Use Committee of Zhengzhou University and were performed in accordance with the guidelines of the International Association for the Study of Pain.
Establishment of the Trigeminal Inflammatory Pain Model
Mice were anesthetized with isoflurane (3% for induction, 1.5% for maintenance) and then received a bilateral injection of 10 µL CFA into the whisker pad to induce orofacial inflammation [3, 18]. Sample sizes were similar in various treatment groups in which animals were randomized and allocated. Prior to the CFA procedure, the baseline thresholds were measured. Behavioral testing was conducted before (baseline) and 3 and 7 days after the CFA injection.
Behavioral Testing
A series of von Frey filaments (0.07 g, 0.16 g, 0.4 g, 0.6 g, 1.0 g, and 1.4 g) was applied to the whisker pad region of the (orofacial) skin innervated by the V2 and V3 branches of the trigeminal nerve, as previously described [19–21]. The stimulus of each von Frey filament was repeated five times with an interval of 6 s, and with an interval of 5 min–10 min when the filaments were changed. The threshold was defined when, from five stimuli, at least three positive responses (head withdrawal, aggressive behavior, and asymmetric facial grooming) were observed. Behavioral tests were performed in a quiet room with a constant temperature of 25 °C.
Intra-TG Injection
Mice were anesthetized with isoflurane (3% for induction, 1%–2% for maintenance). Each animal was placed flat on a surgical table in the prone position. The head was stabilized with one hand, and the most anterior–rostral portion of the zygomatic process of the maxillary bone was palpated. A needle was then inserted medial to the palpated portion of the zygomatic process through the infraorbital foreman. The needle was positioned at an angle of 10° relative to the midline of the head. The tip of the needle, with active electrical stimulation, was advanced ~9 mm along the infraorbital canal. The tip of the needle terminated at the medial aspect of the ganglion near the ophthalmic nerve (V1) division [22, 23]. Recombinant AAV5-CMV-bGlobin-Cre-eGfp was used to knock down TET1 in the TG of Tet1flox/flox mice, with AAV5-CMV-bGlobin-eGfp (GeneChem) as a control. TET1 lentiviral activation particles and the corresponding control particles (Santa Cruz Biotechnology, Santa Cruz, CA) were used for the overexpression of TET1. For each mouse, a volume of 1 μL AAV or lentiviral virus was injected.
Western Blotting
The TGs dissected from mice were rinsed with cold phosphate-buffered saline (PBS) and homogenized on ice in a lysis buffer containing protease inhibitors. Samples were centrifuged at 1000 r/min for 10 min. The supernatant, including plasma protein, was obtained; the nuclear protein was then collected after the nucleus lysis buffer was added to the precipitate for ultrasonication. The protein concentration was quantified with a bicinchoninic acid kit. The same amount of protein was separated on a sodium dodecyl-sulfate polyacrylamide gel (8%; CoWin Biosciences; used for Kv7.2 protein) or a gradient gel (4%–20%; Solarbio; used for TET1 protein) and transferred to polyvinylidene difluoride membranes. The membranes were then blocked in 5% fetal bovine serum for 2 h at room temperature and incubated with a primary antibody against Kv7.2 (1:500; Alomone) or TET1 (1:1000; Abcam) overnight at 4 °C. The membranes were washed with tris-buffered saline with 0.1% Tween 20 and incubated with the corresponding secondary antibodies for 2 h at room temperature. The bands were visualized by an enhanced chemiluminescence kit (ECL Kit; Affinity). Densitometric values were normalized to the internal beta-actin controls, and quantifications were performed with ImageJ software (Bio-Rad).
Quantitative Real-time Polymerase Chain Reaction (PCR)
Mice were deeply anesthetized with isoflurane (3%) and sacrificed, and the TGs were quickly removed. Total RNA was extracted by a total RNA isolation kit (Accurate Biology), and mRNAs were reverse-transcribed into cDNA using a reverse-transcription kit (Accurate Biology). Quantitative PCR was performed with the Q3 Real-Time Quantitative PCR Detection System, in which a 20 μL mixture was used, including 10 μL SYBR Mix, 2 μL cDNA template, 0.8 μL of 10 μmol/L forward and reverse primers, and 7.2 μL RNase-free water. The sequences of the primer pairs for each gene are listed in Table S1 [24].
Immunofluorescence
Mice were anesthetized by isoflurane and transcardially perfused with precooled saline, followed by 4% paraformaldehyde (PFA). TGs were excised and post-fixed in 4% PFA overnight and embedded for paraffin sections. After deparaffinization and retrieval of antigenic sites, sections were washed three times with PBS. They were then blocked with 10% normal goat serum and incubated with the following primary antibodies: rabbit anti-Kv7.2 (1:200, Alomone Labs), rabbit anti-TET1 (1:200, Abcam), mouse anti-TET1 (1:200, Genetex), mouse anti-neurofilament 200 (NF200; 1:200, Sigma), mouse anti-calcitonin gene-related peptide (CGRP, 1:200, Abcam), and FITC-conjugated isolectin B4 (IB4, 1:200, Sigma). The sections were washed thrice with PBS and incubated with secondary antibodies at 37 °C for 2.5 h. Images were captured with a Nikon confocal laser scanning microscope.
Preparation of TG Neurons
The mice were deeply anesthetized with isoflurane (3%) and decapitated. TGs were quickly isolated from the cranial cavity before incubation in Hank’s balanced salt solution containing dispase II (5 mg/mL, Roche) and collagenase I (2 mg/mL, Sigma) at 37 °C for 40 min. TG neurons were subsequently re-suspended in Neurobasal-A medium (containing B-27 and glutamine, ThermoFisher) and plated on coverslips pre-coated with poly-D-lysine (Sigma). Whole-cell patch-clamp recordings were made 2 h to 8 h after cells were maintained in an incubator at 37 °C with 5% CO2.
Electrophysiology
Whole-cell voltage-clamp recordings were made with pipettes (3–5 MΩ by P-97, Sutter Instruments) containing filament. The pipette solution contained the following (in mmol/L): 140 KCl, 2 MgCl2, 4 EGTA, 10 HEPES, and 0.14 CaCl2, pH 7.2 (adjusted with KOH). The bath solution contained (in mmol/L) 145 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose, pH 7.3–7.4 (adjusted with Tris-base).
For M current recording in voltage-clamp mode, neurons were held at −20 mV for 1 s to activate the M-current before a deactivation step at –50 mV. The amplitude of M currents was measured based on the difference in the means between the start (~10 ms) and the end of the hyperpolarizing pulse [25, 26].
For action potentials in current-clamp recording, the bridge was 100% balanced with no holding current applied to neurons. A series of current pulses (40 pA to 600 pA in steps of 40 pA, 200 ms) was injected to generate action potentials. Sweeps were increased if the threshold was >600 pA.
Statistical Analysis
Results are presented as mean values ± SEM. GraphPad Prism 8 was used for statistical comparisons. For two-group analyses, an unpaired Student’s t-test was used whereas a one or two-way analysis of variance followed by Bonferroni post hoc tests was applied, for multiple groups. P <0.05 was considered statistically significant. For the data of electrophysiological recordings, parameters, and M currents were analyzed with Clampfit 10 and MatLab 2014 software.
Results
CFA Injection Leads to Mechanical Allodynia in the Whisker Pad Region and Altered M Currents in TG Neurons in Mice
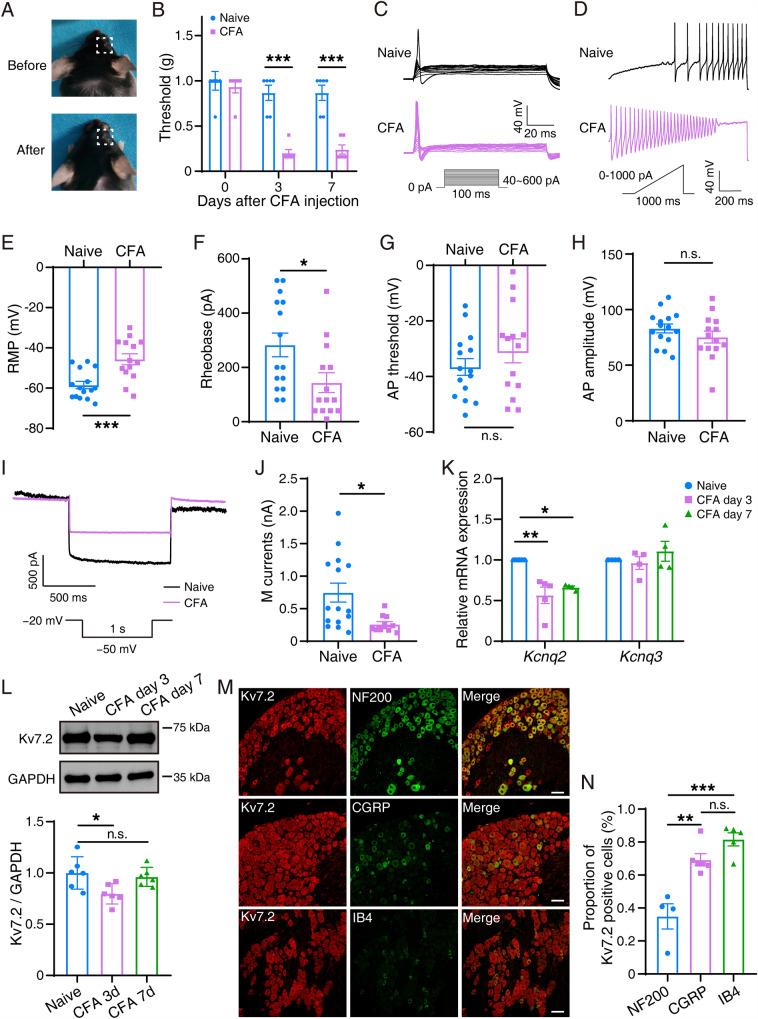
After CFA injection, the whisker pad in model mice was swollen compared with that in control mice (Fig. 1A). To evaluate whether CFA induced orofacial pain hypersensitivity, calibrated von Frey filaments were applied to the whisker pad to measure the withdrawal threshold of mechanical stimulation. The threshold was significantly lower at either 3 (0.20 ± 0.04 g) or 7 (0.24 ± 0.051 g) days after CFA in model mice than in controls (0.867 ± 0.084 g) (Fig. 1B). We then determined the action potential (AP) firing of TG neurons in the naïve and CFA groups (Fig. 1C, D). The resting membrane potentials (RMPs) of TG neurons were −58.44 ± 1.838 mV in the naïve group but were significantly depolarized to −45.62 ± 2.722 mV in the CFA group (Fig. 1E). The rheobase for AP initiation in TG neurons in the CFA group (143.6 ± 36.52 pA) had a significantly decreasing trend compared with that in the naïve group (282.7 ± 43.38 pA) (Fig. 1F). Besides, no significant difference was detected in AP threshold (Fig. 1G) or AP amplitude (Fig. 1H) between the naïve and CFA groups. In addition to the AP, we recorded the M currents in TG neurons. The results showed that the amplitude of M currents in the CFA group was 312.9 ± 38.45 pA, which was significantly lower (P = 0.0076) than that in the naïve group (746.2 ± 145.6 pA) (Fig. 1I, J).
Fig. 1.
Mechanical allodynia in the whisker pad region in CFA model mice and alterations in M currents and Kcnq2. A The animal before (upper) and after (lower) CFA injection in the whisker pad. B Mechanical allodynia was tested on naïve mice (n = 6) and on model mice on day 3 or day 7 after CFA injection (n = 6). The data are presented as the mean ± SEM. C, D Two sets of sample traces of CFA (purple) and naïve (black) mice depicting the action potential (AP) of TG neurons. E–H Summary data of resting membrane potential (RMP; P = 0.0005, E), rheobase (P = 0.0218, F), AP threshold (P = 0.2720, G), and AP amplitude (P = 0.2514, H) in the TG neurons in naïve (n = 15) and CFA (n = 14) mice. Data are presented as the mean ± SEM. I Two sample traces depicting the M current recorded in the TG neurons in the CFA group (purple) vs the naïve group (black). J Summary data of M currents in the TG neurons in naïve (n = 15) and CFA (n = 15) mice (P = 0.0076). The data are presented as the mean ± SEM. K Relative mRNA expression of Kcnq2 and Kcnq3 in the trigeminal ganglia in naïve and CFA mice at day 3 and day 7. Data are presented as the mean ± SEM. L Representative western blots and summarized results of Kv7.2 protein in the trigeminal ganglia in naïve and CFA mice at day 3 and day 7. The data are presented as the mean ± SEM. M, N TG slices double-stained with Kv7.2 (red) and NF200/CGRP/Ib4 (green) antibodies. Scale bars, 50 µm.
Decreased Expression of Kcnq2 in the TG in CFA Model Mice
Based on the alteration of M currents, we further determined if the expression of Kcnq2 changed after CFA injection. The relative mRNA expression of Kcnq2, but not Kcnq3, was decreased in TG neurons extracted from CFA model mice compared with those from naïve mice (Fig. 1K). Similarly, western blotting revealed that Kv7.2 protein expression significantly decreased in the CFA model (3 days) (Fig. 1L). In addition, dual immunofluorescence revealed a more extensive colocalization between Kcnq2 and smaller-diameter neurons labeled by IB4 and CGRP (Fig. 1M, N). In summary, Kcnq2 expression was significantly decreased in TG neurons of CFA model mice, especially on day 3 after CFA injection.
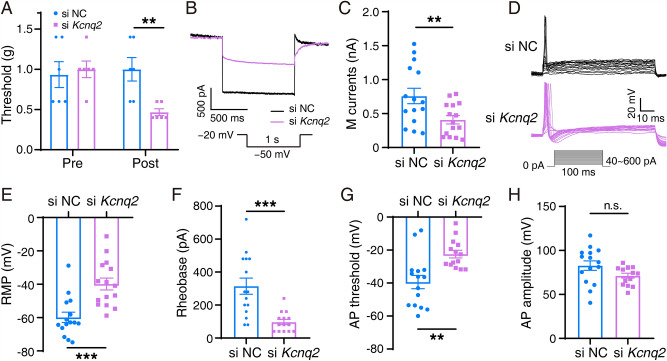
Kcnq2 Affects the Procession of Orofacial Inflammatory Pain
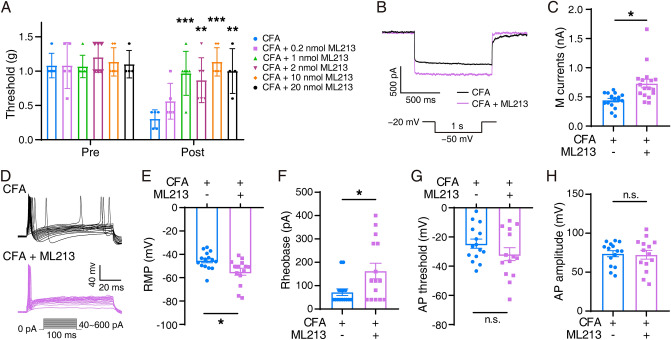
Based on the earlier data, we hypothesized that Kcnq2 plays a vital role in regulating trigeminal inflammatory pain in mice. Behavioral analyses revealed that si-Kcnq2 mice exhibited greater hypersensitivity than negative controls (Fig. 2A). M currents were significantly decreased (349.9 pA ± 128.5 pA) in the si-Kcnq2 group (Fig. 2B, C). Furthermore, the RMP in the TG neurons in the si-Kcnq2 group was −41.89 ± 3.146 mV, which was more depolarized than that in the control group (−59.85 ± 3.101 mV) (Fig. 2E). The rheobase in TG neurons in the si-Kcnq2 group (97.14 ± 16.08 pA) was significantly lower than that in controls (314.7 pA ± 48.89 pA) (Fig. 2F). The AP threshold (Fig. 2G) had similar trend with RMP, but no significant change was found in AP amplitude (Fig. 2H). The Kv7.2 agonist ML213 attenuated inflammatory pain after CFA (Fig. 3). Various doses of ML213, except for 0.2 nmol, relieved orofacial pain (compared with controls) in CFA mice (Fig. 3A). M currents and TG neuronal excitability simultaneously changed after the administration of ML213. ML213 significantly increased M currents in TG neurons in CFA mice (from 443.8 ± 30.38 pA to 729.6 ± 73.06 pA) (Fig. 3B, C). The RMP in the ML213 + CFA group was −54.89 ± 2.982 mV, which was more hyperpolarized than that in the control group (−45.31 ± 1.924 mV) (Fig. 3E). The rheobase in TG neurons was significantly increased by 93.71 ± 37.53 pA following administration of ML213 (Fig. 3F). The AP threshold and amplitude were not significantly altered (Fig. 3G, H). The above results showed that ML213 reverses the trigeminal neuronal M currents and activity of mice with trigeminal inflammatory pain.
Fig. 2.
Behavior, M currents, and APs in si-Kcnq2 and si-scrambled mice. A Mechanical allodynia was tested in si-Kcnq2 (n = 6) and si-scrambled (n = 6) mice. The data are presented as the mean ± SEM. B Two sample traces depicting the M currents recorded in TG neurons in the si-Kcnq2 group (purple) vs the control group (black). C Summary data of M currents in the TG neurons in si-scrambled (n = 15) and si-Kcnq2 (n = 15) mice (P = 0.0110). The data are presented as the mean ± SEM. D Two sets of sample traces of si-Kcnq2 (purple) and control (black) mice depicting the action potential (AP) in TG neurons. E–H Summary data of RMP (P = 0.0004, E), rheobase (P = 0.0003, F), AP threshold (P = 0.0013, G), and AP amplitude (P = 0.0749, H) in the TG neurons in si-scrambled (n = 15) and si-Kcnq2 (n = 14) mice. The data are presented as the mean ± SEM.
Fig. 3.
Behavior, M currents, and APs in mice with Kv7.2 agonist ML213 injection after CFA injection. A Mechanical allodynia tested in ML213 (0.2, 1, 2, 10, 20 nmol) + CFA mice and CFA mice (n = 6). The data are presented as the mean ± SEM. B Two sample traces depicting the M currents recorded in TG neurons in the ML213 (2 nmol) + CFA group (purple) vs the CFA group (black). C Summary data of M currents in TG neurons in the CFA (n = 17) and ML213 (2 nmol) + CFA (n = 17) mice (P = 0.0010). The data are presented as the mean ± SEM. D Two sets of sample traces of ML213 (2 nmol) + CFA (purple) and control (black) mice depicting the action potential (AP) in TG neurons. E–H Summary data of RMP (P = 0.0109, E), rheobase (P = 0.0189, F), AP threshold (P = 0.1913, G), and AP amplitude (P = 0.8500, H) in the TG neurons in CFA (n = 15) and ML213 (2 nmol) + CFA (n = 14) mice. The data are presented as the mean ± SEM.
Moreover, we compared the trigeminal neuronal M currents and excitability of the Naïve and ML213-treated naïve mice. Although a slight trend of hyperpolarization in RMP was found in the naïve+ML213 group, no significant difference was detected between the Naïve and Naïve + ML213 groups, indicating that ML213 treatment did not change the M channel activity and baseline excitability in TG neurons (Fig. S1). We consider such a result may be due to the homeostasis of the healthy nervous system that keeps the K+ channel and neuronal activity in a relatively stable condition.
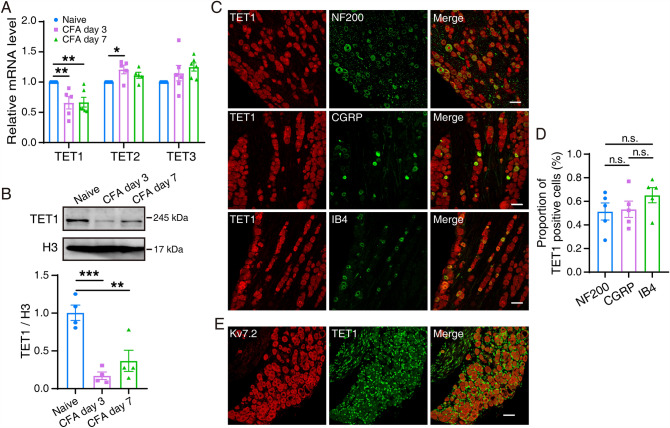
CFA Injection Decreases the Expression of TET1
Our findings suggest that Kcnq2 has a significant effect on the regulation of orofacial inflammatory pain and TG neuron excitability. To further reveal whether TET1 regulates Kcnq2, we first determined the expression of TET1 using western blotting and qPCR analysis. The results (Fig. 4A, B) showed that the protein expression of TET1 and the mRNA expression of TET1 but not TET2 and TET3 in the TG significantly decreased after CFA. Immunofluorescence revealed the colocalization between TET1 and different diameters of TG neurons labeled by NF200/CGRP/Ib4 (Fig. 4C, D). Subsequently, we examined the co-localization of TET1 and Kv7.2 using double-immunofluorescence staining. The results revealed that TET1 distinctly co-localized with Kv7.2 in mouse TG (Fig. 4E).
Fig. 4.
Change in TET1 expression levels and coexpression of TET1 and Kcnq2. A Relative mRNA expression of TET1–3 in the trigeminal ganglia in naïve and CFA mice. The data are presented as the mean ± SEM. B Representative western blots and summarized results of TET1 protein in the trigeminal ganglia in naïve and CFA mice. The data are presented as the mean ± SEM. C, D TG sections from mice doubly stained with anti-TET1 (red) and anti-NF200/CGRP/Ib4 (green) antibodies. Scale bars, 50 µm. E TG sections were doubly stained with Kv7.2 (red) and TET1 (green) antibodies. Scale bar, 50 µm.
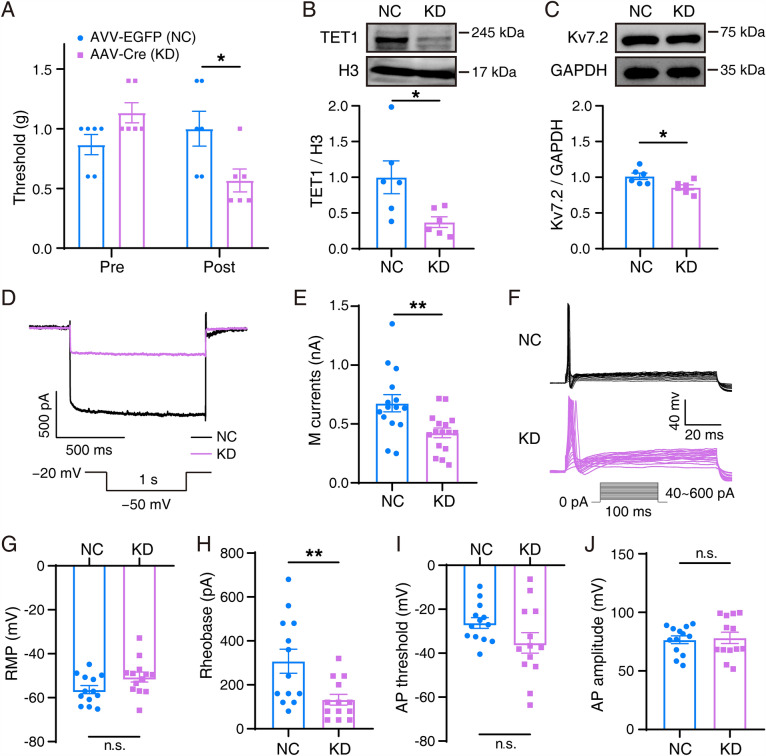
Decreased Kcnq2 Expression in the TG of TET1 Knockdown Mice
We further investigated whether deletion of TET1 in the TG contributes to the decrease in Kcnq2 expression and affects the mechanical threshold of pain. To construct a TET1 knockdown model, we used TET1flox/flox mice and pAAV-CMV bGlobin-Cre virus. TET1 knockdown mice were more hypersensitive to pain in the whisker pad than controls (Fig. 5A). Kv7.2 protein expression significantly decreased owing to TET1 knockdown in the TG (Fig. 5B, C). Meanwhile, the excitability of TG neurons was remarkably raised following TET1 deletion. A significant increase in M currents was found in TG neurons in TET1 knockdown mice (423.9 ± 41.40 pA) when compared with the control (676.2 ± 72.68 pA; Fig. 5D, E). The RMPs in TG neurons were depolarized from −56.34 ± 1.829 mV to −50.63 ± 2.247 mV after deletion of TET1 (Fig. 5G). Meanwhile, the rheobase for AP initiation showed a significant decreasing trend (Fig. 5H).
Fig. 5.
Behavior, Kv7.2 protein, M currents, and APs in selective TET1 knockdown mice. A Mechanical allodynia in TET1 knockdown and negative-control mice. The data are presented as the mean ± SEM. B, C Representative western blots and summarized results of TET1 and Kv7.2 protein in the trigeminal ganglia in TET1 knockdown and negative control mice. The data are presented as the mean ± SEM. D Two sample traces depicting the M currents recorded in TG neurons in the TET1 knockdown group (purple) vs the control group (black). E Summary data of M currents in TG neurons in TET1 knockdown (n = 16) and control (n = 15) mice (P = 0.0047). The data are presented as the mean ± SEM. F Two sets of sample traces of TET1 knockdown (purple) and control (black) mice depicting the action potential (AP) in the TG neurons. G–J Summary data of RMP (P = 0.0600, G), rheobase (P = 0.0075, H), AP threshold (P = 0.0990, I), and AP amplitude (P = 0.7819, J) in TG neurons in TET1 knockdown (n = 13) and control (n = 13) mice. The data are presented as the mean ± SEM.
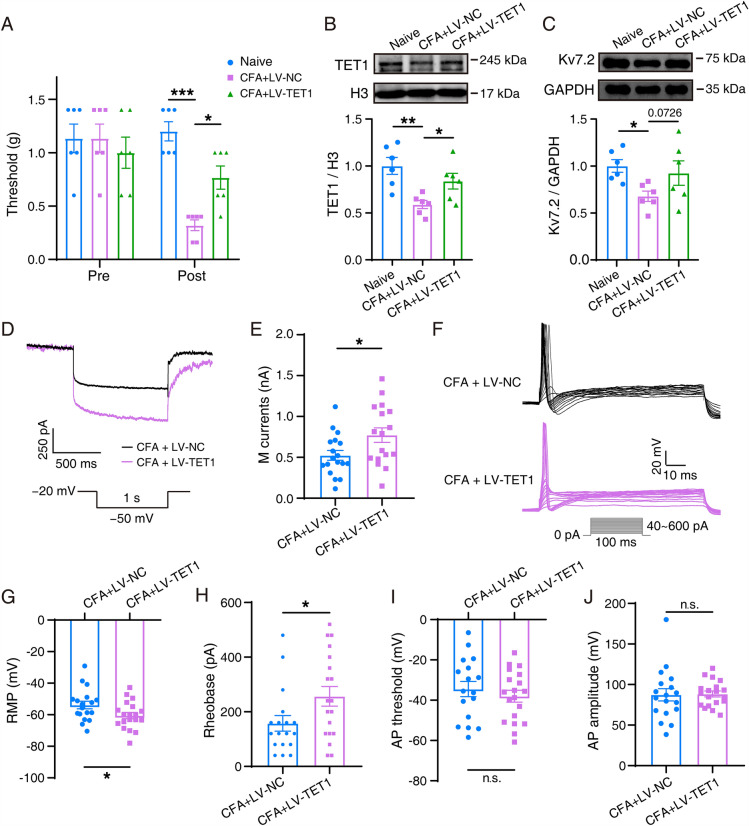
TET1 Overexpression in the TG Attenuates Pain Through Kcnq2 in the Whisker Pad in CFA Mice
To determine whether TET1 overexpression can alleviate orofacial inflammatory pain by regulating the expression of Kv7.2, we constructed TET1 overexpression in CFA model mice. TET1 lentiviral activation particles (Santa Cruz Biotechnology) were injected into the trigeminal ganglion of mice to overexpress TET1. Overexpression of TET1 attenuated the pain hypersensitivity in the whisker pad elicited by CFA (Figure 6A). Western blotting analysis revealed that Kv7.2 protein expression had an increasing trend in the TET1 overexpression + CFA group compared with that in the control virus + CFA group; however, the difference was not statistically significant (Fig. 6B, C). The amplitude of M currents increased by 247.9 ± 105.7 pA after TET1 overexpression (Fig. 6D, E). In TG neurons of control mice, the mean rheobase was 157.8 ± 28.28 pA, and the mean RMP was −53.90 ± 2.418 mV. Overexpression of TET1 significantly changed the membrane potential to −60.54 ± 2.004 mV and increased the rheobase to 256.8 ± 35.84 pA (Fig. 6G, H).
Fig. 6.
Behavior, Kv7.2 protein, M currents, and APs in TET1-overexpressing and CFA mice. A Mechanical allodynia. The data are presented as the mean ± SEM. B, C Representative western blots and summarized results of TET1 and Kv7.2 protein in the trigeminal ganglia. The data are presented as the mean ± SEM. D Two sample traces depicting the M currents recorded in the TG neurons in the TET1-overexpressing group (purple) vs the control group (black). E Summary data of M currents in the TG neurons in TET1-overexpressing (n = 17) and control (n = 18) mice (P = 0.0252). The data are presented as the mean ± SEM. F Two sets of sample traces of TET1-overexpressing mice (purple) and control mice (black) depicting the action potential (AP) in the TG neurons. G–J Summary data of RMP (P = 0.0408, G), rheobase (P = 0.0382, H), AP threshold (P = 0.4559, I), and AP amplitude (P = 0.8986, J) in the TG neurons in TET1-overexpressing (n = 19) and control (n = 18) mice. The data are presented as the mean ± SEM.
Discussion
Orofacial inflammatory pain is excruciating, and its treatment is complex. The underlying mechanisms remain unclear. In the present study, we demonstrated that the down-regulated expression of Kv7.2, a member of the M channel family, was modulated by the DNA hydroxymethylase TET1 in orofacial inflammatory pain; the ablation of TET1 downregulated the expression of Kv7.2, led to an increase in the excitability of the trigeminal ganglia neurons, and mimicked trigeminal inflammatory pain-like behavior, whereas overexpression of TET1 rescued the M currents and mitigated the CFA-induced trigeminal inflammatory pain.
Accumulating evidence indicates that activation of the M channel is essential for the potential treatment of inflammatory pain [9, 27, 28], neuropathic pain [29–31], osteoarthritic pain [32, 33], bone cancer pain [26, 34], paclitaxel-induced peripheral neuropathy [35], chronic visceral pain [36], and diabetic peripheral neuropathic pain [37]. In addition, the M channel opener QO58-lysine has anti-nociceptive effects on inflammatory pain in rodent models [28], and activation of neuronal M channels might have therapeutic potential for temporomandibular disorders [27]. Notably, John et al. reported that inhibiting the M current in sensory neurons might mediate inflammatory nociception [9]. Moreover, the M channel agonists retigabine and flupirtine exhibit analgesic efficacy in osteoarthritic pain [32, 33], and spinal M channels activated by retigabine inhibit the hyperexcitability of dorsal horn wide dynamic range neurons induced by bone cancer as well as alleviating bone cancer pain [26, 34]. In this study, we identified a decreasing trend of M current and Kcnq2 in TG neurons in a mouse model of orofacial inflammatory pain. The role of the M channel in pain in our study is consistent with that in the reports described above.
Kcnq2 is the major Kcnq gene transcript in the DRG, and neuropathic injury induces substantial downregulation of Kcnq2 mRNA expression in the DRG [11]. Wu et al. demonstrated that a potent and efficacious Kcnq2 opener, acrylamide (S)-6, activates Kcnq2 and alleviates the pain in two models of neuropathic pain (spinal nerve ligation and diabetic neuropathy) and in the formalin test, suggesting that Kcnq2 openers may be helpful in the treatment of neuropathic pain [38]. Abdrrahman et al. and Mariusz et al. showed that Kcnq2/3 are reportedly valid targets for the treatment of pain [39] and are essential in the control of the excitability of neurons [40]. In this study, we revealed the potential role of Kcnq2 in TG neurons in orofacial inflammatory pain. The injection of CFA decreased the expression of Kv7.2 protein on day 3. However, we found a slight recovery in the Kv7.2 protein after 7 days following CFA injection, although the pain remained and the expression of TET1 protein was still maintained at a lower level compared with the control. This phenomenon suggests that the Kv7.2 protein has an essential effect at the early stage of inflammatory pain. The function of Kcnq2 in the TG requires further investigation.
The agonists of M channels include retigabine, flupirtine, QO-58 [41], and ML213. ML213, used in the present study, is an activator of Kv7.2 and Kv7.4 channels and exhibits more pronounced effects than retigabine [42]. The selective Kv7.2 activator has not been identified in previous studies. To mimic the treatment in the human body, we administered a highly selective agonist, ML213, in the mouse model of orofacial inflammatory pain.
This study is the first to report the role of TET1 in orofacial inflammatory pain. We revealed that the expression of TET1 decreased in the TG of mice in CFA-induced orofacial pain compared with the control. Previous studies suggested that TET1 is involved in neuropathic pain [15, 16, 43–45] and paw inflammatory pain [17]. Notably, some studies demonstrated that TET1 mitigates the pain. Our findings are consistent with those in a previous study, which revealed that TET1 overexpression rescues Kv1.2 in DRG neurons to mitigate neuropathic pain [15]. Overexpression of TET1 increased the expression of Kv1.2 by downregulating the level of 5 mC and upregulating the level of 5 hmC in the promoter region of the Kcna2 gene (encoding Kv1.2) in the DRG ipsilateral to spinal nerve ligation. In addition, TET1 overexpression reportedly attenuates paclitaxel-induced neuropathic pain by regulating K2p1.1 expression in the primary sensory neurons of rats [16]. TET1 overexpression rescues the expression of K2p1.1 by reducing the amount of 5 mC and simultaneously increasing the level of 5 hmC within the K2p1.1 promoter region in the DRG of rats with paclitaxel-induced neuropathic pain. Nevertheless, other studies have reported relatively different results. For example, Pan et al. suggested that the upregulation of TET1 in the spinal cord leads to pain hypersensitivity [44]. By knocking down or conditionally deleting spinal METTL3, the levels of m6A in Tet1 mRNA increased, resulting in pain hypersensitivity. The TET1-mediated promoter demethylation of SOX10 in the spinal dorsal horn might contribute to oxaliplatin-induced neuropathic pain [45], and nerve injury-induced decrease in miR-489-3p expression increases Tet1 expression in the dorsal horn [43]. Moreover, overexpression of spinal TET1 or TET3 increases 5hmC in the miR-365-3p promoter and induces formalin-induced inflammatory pain behavior via Kcnh2 [17]. Our study focused mainly on the relationship between TET1 and Kcnq2 in TG neurons in CFA model mice. According to these different views of previous studies and our research, we hypothesized, and also have verified, that in the primary sensory neurons, the increase of the amount of TET1 may relieve pain, whereas the augmentation of spinal TET1 accelerates the process of pain.
This study has several limitations. Although we confirmed that Kcnq2 regulated by TET1 was involved in trigeminal inflammatory pain, other voltage-gated K+ channels mediated by TET1 could not be excluded. In addition, we mainly focused on the function of TET1 and Kcnq2 in the acute period of orofacial inflammatory pain while the behavioral changes after 7 days following modeling were not tested. The role of TET1 and Kcnq2 in the chronic period of orofacial inflammatory pain remains to be further investigated. Moreover, the agonist ML213, whose selectivity is relatively higher than other activators, also has an activation effect on Kv7.4 (EC50 = 510 nmol/L), which is slightly milder than the effect on Kv7.2 (EC50 = 230 nmol/L) [46]. However, Kv7.4 has hitherto been considered to be expressed only in discrete areas of the nervous system such as the cochlea, substantia nigra pars compacta, and ventral tegmental area [47]. No evidence of Kv7.4 expression in trigeminal ganglia has been reported until now. Nevertheless, we neither verified nor ruled out, any potential contribution of Kv7.4 to trigeminal inflammatory pain. Moreover, although our findings provide strong evidence for the therapeutic effects of modulating the TET1/Kcnq2 pathway on trigeminal inflammatory pain, whether such a therapeutic schedule could be working in other types of pain needs to be further investigated.
In conclusion, we investigated the effect of Kcnq2 modulated by TET1 on the orofacial inflammatory pain in the whisker pad region in mice. Our findings shed light on a potential therapy for orofacial pain.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Dr. Guoliang Xu (Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, China) for the gift of the Tet1flox/flox mice. This work was supported by the National Natural Science Foundation of China (81771195 and 81971061), and the Program for Innovative Research Team in Universities of Henan Province (22IRTSTHN028).
Conflict of interest
All authors claim that there are no conflicts of interest.
Footnotes
Dengcheng Zhan and Jingjing Zhang contributed equally to this work.
Contributor Information
Weidong Zang, Email: zwd@zzu.edu.cn.
Jing Cao, Email: caojing@zzu.edu.cn.
References
- 1.Horst OV, Cunha-Cruz J, Zhou L, Manning W, Mancl L, DeRouen TA. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. J Am Dent Assoc. 2015;146:721–728.e3. doi: 10.1016/j.adaj.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33: 629–808. [DOI] [PubMed]
- 3.Aczél T, Kecskés A, Kun J, Szenthe K, Bánáti F, Szathmary S, et al. Hemokinin-1 gene expression is upregulated in trigeminal Ganglia in an inflammatory orofacial pain model: Potential role in peripheral sensitization. Int J Mol Sci. 2020;21:E2938. doi: 10.3390/ijms21082938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 5.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 6.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 7.Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- 8.Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, et al. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 9.Linley JE, Rose K, Patil M, Robertson B, Akopian AN, Gamper N. Inhibition of M current in sensory neurons by exogenous proteases: A signaling pathway mediating inflammatory nociception. J Neurosci. 2008;28:11240–11249. doi: 10.1523/JNEUROSCI.2297-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Linley JE, Du X, Zhang X, Ooi L, Zhang H, et al. The acute nociceptive signals induced by bradykinin in rat sensory neurons are mediated by inhibition of M-type K+ channels and activation of Ca2+-activated Cl- channels. J Clin Invest. 2010;120:1240–1252. doi: 10.1172/JCI41084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose K, Ooi L, Dalle C, Robertson B, Wood IC, Gamper N. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 2011;152:742–754. doi: 10.1016/j.pain.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djouhri L, Zeidan A, Abd El-Aleem SA. Changes in expression of Kv7.5 and Kv7.2 channels in dorsal root ganglion neurons in the streptozotocin rat model of painful diabetic neuropathy. Neurosci Lett. 2020;736:135277. doi: 10.1016/j.neulet.2020.135277. [DOI] [PubMed] [Google Scholar]
- 13.Poetsch AR, Plass C. Transcriptional regulation by DNA methylation. Cancer Treat Rev. 2011;37:S8–12. doi: 10.1016/j.ctrv.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh MC, Ho YC, Lai CY, Chou D, Wang HH, Chen GD, et al. Melatonin impedes Tet1-dependent mGluR5 promoter demethylation to relieve pain. J Pineal Res. 2017 doi: 10.1111/jpi.12436. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Wei G, Ji F, Jia S, Wu S, Guo X, et al. TET1 overexpression mitigates neuropathic pain through rescuing the expression of μ-opioid receptor and Kv1.2 in the primary sensory neurons. Neurotherapeutics. 2019;16:491–504. doi: 10.1007/s13311-018-00689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia S, Wei G, Bono J, Pan Z, Zheng B, Wang B, et al. TET1 overexpression attenuates paclitaxel-induced neuropathic pain through rescuing K2p1.1 expression in primary sensory neurons of male rats. Life Sci. 2022;297:120486. doi: 10.1016/j.lfs.2022.120486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Z, Zhang M, Ma T, Xue ZY, Li GF, Hao LY, et al. Hydroxymethylation of microRNA-365-3p regulates nociceptive behaviors via Kcnh2. J Neurosci. 2016;36:2769–2781. doi: 10.1523/JNEUROSCI.3474-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Chen Y, Liedtke W, Wang F. Lack of evidence for ectopic sprouting of genetically labeled Aβ touch afferents in inflammatory and neuropathic trigeminal pain. Mol Pain. 2015;11:18. doi: 10.1186/s12990-015-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui WQ, Zhang WW, Chen T, Li Q, Xu F, Mao-Ying QL, et al. Tacr3 in the lateral habenula differentially regulates orofacial allodynia and anxiety-like behaviors in a mouse model of trigeminal neuralgia. Acta Neuropathol Commun. 2020;8:44. doi: 10.1186/s40478-020-00922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Bian C, Yang J, Arora V, Gao Y, Wei F, et al. Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. eNeuro. 2020 doi: 10.1523/ENEURO.0118-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–887. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju YY, Jiang M, Xu F, Wang D, Ding B, Ma LJ, et al. CXCL10 and CXCR3 in the trigeminal ganglion contribute to trigeminal neuropathic pain in mice. J Pain Res. 2021;14:41–51. doi: 10.2147/JPR.S288292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Res Brain Res Protoc. 2005;15:119–126. doi: 10.1016/j.brainresprot.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, et al. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci. 2015;18:1746–1755. doi: 10.1038/nn.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crozier RA, Ajit SK, Kaftan EJ, Pausch MH. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J Neurosci. 2007;27:4492–4496. doi: 10.1523/JNEUROSCI.4932-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han JS, et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Wu Y, Bi Y, Tan L, Gan Y, Wang K. Activation of voltage-gated KCNQ/Kv7 channels by anticonvulsant retigabine attenuates mechanical allodynia of inflammatory temporomandibular joint in rats. Mol Pain. 2010;6:1744–8069. doi: 10.1186/1744-8069-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng BC, Song Y, Zhang F, Ma TY, Qi JL, Zhang HL, et al. Activation of neuronal Kv7/KCNQ/M-channels by the opener QO58-lysine and its anti-nociceptive effects on inflammatory pain in rodents. Acta Pharmacol Sin. 2016;37:1054–1062. doi: 10.1038/aps.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Bian X, Wang K. Pharmacological activation of neuronal voltage-gated Kv7/KCNQ/M-channels for potential therapy of epilepsy and pain. Handb Exp Pharmacol. 2021;267:231–251. doi: 10.1007/164_2021_458. [DOI] [PubMed] [Google Scholar]
- 30.Abd-Elsayed A, Jackson M, Gu SL, Fiala K, Gu J. Neuropathic pain and Kv7 voltage-gated potassium channels: The potential role of Kv7 activators in the treatment of neuropathic pain. Mol Pain. 2019;15:1744806919864256. doi: 10.1177/1744806919864256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Liu Y, Hu F, Yang J, Guo X, Hou X, et al. Activation of neuronal voltage-gated potassium Kv7/KCNQ/M-current by a novel channel opener SCR2682 for alleviation of chronic pain. J Pharmacol Exp Ther. 2021;377:20–28. doi: 10.1124/jpet.120.000357. [DOI] [PubMed] [Google Scholar]
- 32.Zhang F, Liu S, Jin L, Tang L, Zhao X, Yang T, et al. Antinociceptive efficacy of retigabine and flupirtine for gout arthritis pain. Pharmacology. 2020;105:471–476. doi: 10.1159/000505934. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Wang F, Wang X, Sun R, Chen J, Chen B, et al. Antinociceptive efficacy of retigabine in the monosodium lodoacetate rat model for osteoarthritis pain. Pharmacology. 2015;95:251–257. doi: 10.1159/000381721. [DOI] [PubMed] [Google Scholar]
- 34.Cai J, Fang D, Liu XD, Li S, Ren J, Xing GG. Suppression of KCNQ/M (Kv7) potassium channels in the spinal cord contributes to the sensitization of dorsal horn WDR neurons and pain hypersensitivity in a rat model of bone cancer pain. Oncol Rep. 2015;33:1540–1550. doi: 10.3892/or.2015.3718. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Li J, Zuo Y, Dang D, Frost JA, Yang Q. Activation of KCNQ channels prevents paclitaxel-induced peripheral neuropathy and associated neuropathic pain. J Pain. 2019;20:528–539. doi: 10.1016/j.jpain.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peiris M, Hockley JR, Reed DE, Smith ESJ, Bulmer DC, Blackshaw LA. Peripheral KV7 channels regulate visceral sensory function in mouse and human colon. Mol Pain. 2017;13:1744806917709371. doi: 10.1177/1744806917709371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djouhri L, Malki MI, Zeidan A, Nagi K, Smith T. Activation of Kv7 channels with the anticonvulsant retigabine alleviates neuropathic pain behaviour in the streptozotocin rat model of diabetic neuropathy. J Drug Target. 2019;27:1118–1126. doi: 10.1080/1061186X.2019.1608552. [DOI] [PubMed] [Google Scholar]
- 38.Wu YJ, Conway CM, Sun LQ, Machet F, Chen J, Chen P, et al. Discovery of (S, E)-3-(2-fluorophenyl)-N-(1-(3-(pyridin-3-yloxy)phenyl)ethyl)-acrylamide as a potent and efficacious KCNQ2 (Kv7.2) opener for the treatment of neuropathic pain. Bioorg Med Chem Lett. 2013;23:6188–6191. doi: 10.1016/j.bmcl.2013.08.092. [DOI] [PubMed] [Google Scholar]
- 39.Surur AS, Bock C, Beirow K, Wurm K, Schulig L, Kindermann MK, et al. Flupirtine and retigabine as templates for ligand-based drug design of KV7.2/3 activators. Org Biomol Chem. 2019;17:4512–4522. doi: 10.1039/C9OB00511K. [DOI] [PubMed] [Google Scholar]
- 40.Mucha M, Ooi L, Linley JE, Mordaka P, Dalle C, Robertson B, et al. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J Neurosci. 2010;30:13235–13245. doi: 10.1523/JNEUROSCI.1981-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang F, Mi Y, Qi JL, Li JW, Si M, Guan BC, et al. Modulation of K(v)7 potassium channels by a novel opener pyrazolo[1, 5-a]pyrimidin-7(4H)-one compound QO-58. Br J Pharmacol. 2013;168:1030–1042. doi: 10.1111/j.1476-5381.2012.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanyo R, Wang CK, Locskai LF, Li J, Allison WT, Kurata HT. Functional and behavioral signatures of Kv7 activator drug subtypes. Epilepsia. 2020;61:1678–1690. doi: 10.1111/epi.16592. [DOI] [PubMed] [Google Scholar]
- 43.Lai CY, Hsieh MC, Yeh CM, Yang PS, Cheng JK, Wang HH, et al. MicroRNA-489-3p attenuates neuropathic allodynia by regulating oncoprotein DEK/TET1-dependent epigenetic modification in the dorsal horn. Neuropharmacology. 2022;210:109028. doi: 10.1016/j.neuropharm.2022.109028. [DOI] [PubMed] [Google Scholar]
- 44.Pan Z, Zhang Q, Liu X, Zhou H, Jin T, Hao LY, et al. Methyltransferase-like 3 contributes to inflammatory pain by targeting TET1 in YTHDF2-dependent manner. Pain. 2021;162:1960–1976. doi: 10.1097/j.pain.0000000000002218. [DOI] [PubMed] [Google Scholar]
- 45.Deng J, Ding HH, Long JL, Lin SY, Liu M, Zhang XQ, et al. Oxaliplatin-induced neuropathic pain involves HOXA6 via a TET1-dependent demethylation of the SOX10 promoter. Int J Cancer. 2020;147:2503–2514. doi: 10.1002/ijc.33106. [DOI] [PubMed] [Google Scholar]
- 46.Yu H, Wu M, Townsend SD, Zou B, Long S, Daniels JS, et al. Discovery, synthesis, and structure activity relationship of a series of N-aryl-bicyclo[2.2.1]heptane-2-carboxamides: Characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem Neurosci. 2011;2:572–577. doi: 10.1021/cn200065b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.