Abstract
Background
Failure to rescue (FTR), defined as a postoperative complication leading to death, is a recently described outcome metric used to evaluate treatment quality. However, the predictive factors for FTR, particularly following highly advanced hepatobiliary-pancreatic surgery (HBPS), have not been adequately investigated. This study aimed to identify perioperative predictive factors for FTR following highly advanced HBPS.
Methods
This single-institution retrospective study involved 177 patients at Gifu University Hospital, Japan, who developed severe postoperative complications (Clavien–Dindo classification grades ≥ III) between 2010 and 2022 following highly advanced HBPS. Univariate analysis was used to identify pre-, intra-, and postoperative risks of FTR.
Results
Nine postoperative mortalities occurred during the study period (overall mortality rate, 1.3% [9/686]; FTR rate, 5.1% [9/177]). Univariate analysis indicated that comorbid liver disease, intraoperative blood loss, intraoperative blood transfusion, postoperative liver failure, postoperative respiratory failure, and postoperative bleeding significantly correlated with FTR.
Conclusions
FTR was found to be associated with perioperative factors. Well-coordinated surgical procedures to avoid intra- and postoperative bleeding and unnecessary blood transfusions, as well as postoperative team management with attention to the occurrence of organ failure, may decrease FTR rates.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-03257-6.
Keywords: Failure to rescue, Highly advanced hepatobiliary-pancreatic surgery, Perioperative predictive factors
Background
Failure to rescue (FTR) is defined as a severe postoperative complication leading to death [1–5]. When surgery is a key component of treatment, there may be surgery-related complications that lead to FTR in some cases. In particular, highly advanced hepatobiliary-pancreatic surgery (HBPS) is more likely than general gastroenterological surgery to induce severe complications leading to FTR. The FTR rate can be used as a quality indicator of the management of postoperative complications rather than simply being indicative of complication severity. Thus, FTR is an important outcome to consider when seeking to improve treatment quality.
World Health Organization (WHO) guidelines [6] state that postoperative mortality and complication rates decreased in departments that used the WHO surgical check list [7]. In the USA, use of the National Surgical Quality Improvement Program (NSQIP) has been shown to improve surgical outcomes [8]. The Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS) established systems for board certification in relation to both instructors and training institutions in Japan in 2008, which were reported to have improved highly advanced HBPS mortality rates [9].
Silber et al. suggested that both patient- and hospital-specific factors affect potential prevention of FTR [10]. However, subsequent studies have focused only on hospital-specific risk factors [11–13], whereas patient-specific predictors of FTR in highly advanced HBPS have not been adequately investigated. Recognition of factors significantly associated with FTR may improve protocols that attempt to rescue patients with severe postoperative complications. Therefore, this study aimed to identify perioperative (pre-, intra-, and postoperative) risk factors to help predict FTR following highly advanced HBPS.
Methods
This single-center retrospective study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Gifu University (approval number: 2023–018).
Definition of FTR
The main outcome of this study was FTR, defined as in-hospital mortality after experiencing at least one severe postoperative complication. The numerator was defined as all patients who died after experiencing severe complications. The denominator included all patients who experienced severe complications. A severe postoperative complication was defined as a grade ≥ III complication according the Clavien–Dindo (CD) classification after the surgical procedure. Mortality was defined as death during hospitalization or within 90 days of the surgical procedure.
Pre-, intra-, and postoperative variables
Pre-, intra-, and postoperative variables were included in the analysis. Preoperative variables were patient background (age, sex, body mass index); American Society of Anesthesiologists (ASA) physical status classification, active smoking, a past history of abdominal surgery, and preoperative chemotherapy; prognostic indices (prognostic nutritional index, modified Glasgow prognostic score, and systemic immune inflammation index); and patient comorbidity (Charlson risk index and type of comorbidity). Intraoperative variables were type of surgery (hepatobiliary or pancreatic surgery), operation time, blood loss, and blood transfusion. Postoperative variables were onset time of a postoperative complication, postoperative complications (pancreatic fistula, bile leakage, liver failure, respiratory failure, postoperative bleeding, and reoperation), and blood tests on postoperative day 3 (white blood cell count and C-reactive protein and albumin levels).
Highly advanced HBPS
Highly advanced HBPSs included hepatobiliary surgeries such as hepatic trisegmentectomy, hemihepatectomy, hepatic sectionectomy (except lateral sectionectomy), hepatic segmentectomy (except S4), hepatectomy (S4a + S5 resection or hemihepatectomy) with extrahepatic bile duct resection, extrahepatic bile duct resection for congenital biliary dilatation, and hepatopancreatectomy, in addition to pancreatic surgeries such as total pancreatectomy, pancreaticoduodenectomy, distal pancreatectomy with lymph node dissection, and middle pancreatectomy.
Statistical analysis
Continuous and categorical variables are presented as median (range) values and frequencies (percentages), respectively. Fisher’s exact test was used to compare categorical variables between two patient groups, namely, an FTR group and a non-FTR group. A Mann–Whitney U test was used for continuous variables. Youden’s index was used to determine the optimal cutoff value to calculate the specificities and sensitivities in receiver operating characteristic curve analysis. Variables associated with FTR following highly advanced HBPS were assessed using univariate analysis. The limit of statistical significance for all analyses was defined as a two-sided p value < 0.05. All statistical analyses were performed using JMP software (SAS Institute Inc., Cary, NC, USA).
Results
This retrospective study involved 686 patients who had undergone highly advanced HBPS at the Department of Gastroenterological Surgery, Gifu University Hospital, between January 2010 and October 2022. Gifu University Hospital is a JSHBPS-certified training institution. All highly advanced HBPS surgical procedures were conducted by experienced board-certified JSHBPS-qualified surgeons.
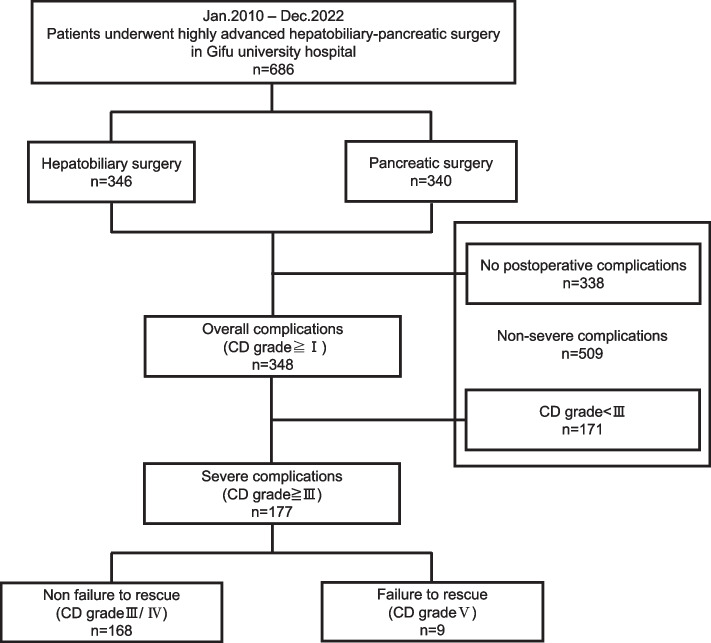
At least one postoperative complication occurred in 348 (50.7%) patients. According to the CD grading system, 42 (6.1%) patients with grade I complications recovered without any treatment, 129 (18.8%) patients with grade II complications required antibiotic therapy, 156 (22.7%) patients with grade III complications needed radiologic intervention or re-operation, and 12 (1.7%) patients with grade IV complications and 9 (1.3%) patients with grade V complications died in the hospital. We excluded 509 patients (no postoperative complications, n = 338; no severe complications, n = 171). In total, 177 (25.8%) patients who experienced at least one postoperative severe complication, defined as grades ≥ III, were included in our study (Fig. 1).
Fig. 1.
Exclusion criteria used in the study
Surgical outcomes according to highly advanced HBPS type
Table 1 summarizes surgical outcomes according to HBPS type. The overall severe complication rate was 25.8% (177 of 686 patients), and the FTR rate was 5.1% (9 of 177 patients). The mortality rate was 1.3% (9 of 686 patients). The severe complication rate was higher in pancreatic surgery than in hepatobiliary surgery (31.8% vs. 19.9%, respectively); however, and in contrast, the FTR rates were 3.7% vs. 7.2%, respectively. Hepatic trisegmentectomy, hepatectomy with extrahepatic bile duct resection, hepatopancreatectomy, pancreaticoduodenectomy, and middle pancreatectomy showed high rates of severe complications in patients with highly advanced HBPS. FTR occurred in hemihepatectomy, hepatic sectionectomy, hepatopancreatectomy, pancreaticoduodenectomy, and distal pancreatectomy with lymph node dissection.
Table 1.
Surgical outcomes by type of the highly advanced HBPS
| Severe complications (Clavien–Dindo classification ≧ grade III) | Failure to rescue | |||
|---|---|---|---|---|
| Number | Rate | Number | Ratea | |
| Hepatobiliary surgeries | 69 | 19.9% | 5 | 7.2% |
| Hepatic trisegmentectomy | 4 | 50.0% | 0 | 0.0% |
| Hemihepatectomy | 16 | 13.7% | 2 | 12.5% |
| Hepatic sectionectomy | 21 | 19.3% | 2 | 9.5% |
| Hepatic segmentectomy | 5 | 9.3% | 0 | 0.0% |
| Hepatectomy with extrahepatic bile duct resection | 14 | 42.4% | 0 | 0.0% |
| Extrahepatic bile duct resection for congenital biliary dilatation | 2 | 14.3% | 0 | 0.0% |
| Hepatopancreatectomy | 7 | 63.6% | 1 | 14.3% |
| Pancreatic surgeries | 108 | 31.8% | 4 | 3.7% |
| Total pancreatectomy | 4 | 23.5% | 0 | 0.0% |
| Pancreaticoduodenectomy | 86 | 34.3% | 2 | 2.3% |
| Distal pancreatectomy with lymph node dissection | 16 | 23.5% | 2 | 12.5% |
| Middle pancreatectomy | 2 | 50.0% | 0 | 0.0% |
| Total | 177 | 25.8% | 9 | 5.1% |
aFailure to rescue rate (%) = number of all patients who died after experiencing a severe complication / number of all patients who experienced severe complications
Patient characteristics of those with severe postoperative complications
Table 2 summarizes the patient characteristics in those with severe postoperative complications. Patients in the severe complication group were significantly older, more often male, had a higher rate of pancreatic surgery, longer operation time, and more intraoperative blood loss than those in the non-severe complication group. Furthermore, the duration of hospital stay was significantly longer in the severe complication group (Supplemental Table 1).
Table 2.
Patient characteristics of those with postoperative severe complications
| Severe complications group (n = 177) | |
|---|---|
| Age (years) | 70 (24–89) |
| Sex | Male: 121 (68.4%) |
| Female: 56 (31.6%) | |
| BMI (kg/m2) | 22.0 (18.9–30.1) |
| ASA | 1: 24 (13.6%) |
| 2: 133 (75.1%) | |
| 3: 19 (10.7%) | |
| Type of disease | Malignancy: 158 (89.3%) |
| Others: 19 (10.7%) | |
| Type of surgery | Hepatobiliary: 69 (39.0%) |
| Pancreatic: 108 (61.0%) | |
| Open: 175 (98.9%) | |
| Laparoscopic: 2 (1.1%) | |
| Operation time (min) | 417 [161–949] |
| Blood loss (ml) | 730 [55-21800] |
| Blood transfusion | 49 (27.7%) |
| Pancreatic fistula | 76 (42.9%) |
| Bile leakage | 25 (14.1%) |
| Liver failure | 6 (3.4%) |
| Respiratory failure | 10 (5.6%) |
| Postoperative bleeding | 25 (14.1%) |
| Re-operation | 12 (6.8%) |
| Hospital stay (days) | 40 (9–162) |
Data are expressed as median (range) or number of patients
BMI body mass index, ASA American Society of Anesthesiologists physical status classification
Univariate analysis to predict FTR following highly advanced HBPS
In the univariate analysis, FTR following highly advanced HBPS was significantly associated with liver-related comorbidities (p = 0.04), intraoperative blood loss (p < 0.001), intraoperative blood transfusion (p < 0.001), postoperative liver failure (p < 0.001), postoperative respiratory failure (p < 0.001), and postoperative bleeding (p = 0.02) (Table 3).
Table 3.
Univariate analysis of prediction for FTR following highly advanced HBPS
| n | OR | 95%CI | p-value | |
|---|---|---|---|---|
| Age (years) | ||||
| >75 | 56 | 2.86 | 0.73-12.00 | 0.13 |
| <75 | 121 | 1 | ||
| Sex | ||||
| Male | 121 | 1.66 | 0.39-11.37 | 0.52 |
| Female | 56 | 1 | ||
| BMI (kg/m2) | ||||
| >24 | 46 | 0.34 | 0.02-1.94 | 0.26 |
| <24 | 131 | 1 | ||
| ASA | ||||
| 3 | 20 | 4.44 | 0.88-18.53 | 0.07 |
| 1/2 | 157 | 1 | ||
| Smoking | ||||
| Yes | 99 | 4.18 | 0.72-79.29 | 0.12 |
| No | 78 | 1 | ||
| Past abdominal surgery | ||||
| Yes | 76 | 0.65 | 0.13-2.55 | 0.55 |
| No | 101 | 1 | ||
| Preoperative-chemotherapy | ||||
| Yes | 33 | 0.53 | 0.03-3.05 | 0.53 |
| No | 144 | 1 | ||
| PNIa | ||||
| >40 | 100 | 0.35 | 0.05-1.52 | 0.17 |
| <40 | 77 | 1 | ||
| Modified GPS | ||||
| 1/2 | 49 | 0.74 | 0.11-3.17 | 0.70 |
| 0 | 128 | 1 | ||
| SIIb | ||||
| >437 | 88 | 1.26 | 0.32-5.27 | 0.73 |
| <437 | 89 | 1 | ||
| Charlson risk index | ||||
| 2+ | 93 | 1.14 | 0.29-4.73 | 0.85 |
| 0/1 | 84 | 1 | ||
| History of malignancy | ||||
| Yes | 56 | 1.08 | 0.22-4.28 | 0.91 |
| No | 121 | 1 | ||
| Heart-related comorbidity | ||||
| Yes | 30 | 1.43 | 0.21-6.29 | 0.68 |
| No | 147 | 1 | ||
| Respiratory-related comorbidity | ||||
| Yes | 31 | 1.36 | 0.20-6.02 | 0.71 |
| No | 146 | 1 | ||
| Liver-related comorbidity | ||||
| Yes | 29 | 4.58 | 1.07-18.46 | 0.04* |
| No | 148 | 1 | ||
| Cerebrovascular-related comorbidity | ||||
| Yes | 15 | 0.00 | -2.59 | 0.2 |
| No | 162 | 1 | ||
| Diabetes mellitus | ||||
| Yes | 58 | 1.03 | 0.21-4.05 | 0.97 |
| No | 119 | 1 | ||
| Chronic renal dysfunction | ||||
| Yes | 12 | 1.78 | 0.09-11.09 | 0.62 |
| No | 165 | 1 | ||
| Type of surgery | ||||
| Hepatobiliary | 69 | 2.03 | 0.52-8.47 | 0.30 |
| Pancreas | 108 | 1 | ||
| Operative time (min) | ||||
| >420 | 88 | 1.28 | 0.33-5.33 | 0.72 |
| <420 | 89 | 1 | ||
| Blood loss (ml) | ||||
| >1600 | 25 | 71.06 | 12.02-1359.77 | <0.001*** |
| <1600 | 152 | 1 | ||
| Blood transfusion | ||||
| Yes | 48 | 10.84 | 2.51-74.70 | <0.01** |
| No | 129 | 1 | ||
| Onset time of complication (POD) | ||||
| >12 | 32 | 3.97 | 0.93-15.93 | 0.06 |
| <12 | 145 | 1 | ||
| Pancreatic fistula | ||||
| Yes | 76 | 0.36 | 0.05-1.55 | 0.18 |
| No | 101 | 1 | ||
| Bile leakage | ||||
| Yes | 25 | 0.00 | -0.00 | 0.09 |
| No | 152 | 1 | ||
| Liver failure | ||||
| Yes | 6 | 66.4 | 10.6-574.22 | <0.001*** |
| No | 171 | 1 | ||
| Respiratory failure | ||||
| Yes | 10 | 82 | 16.28-530.28 | <0.001*** |
| No | 167 | 1 | ||
| Postoperative bleeding | ||||
| Yes | 25 | 5.6 | 1.30-22.84 | 0.02* |
| No | 152 | 1 | ||
| Re-operation | ||||
| Yes | 12 | 1.78 | 0.09-11.09 | 0.62 |
| No | 165 | 1 | ||
| White blood cell on POD3 (×103µl) | ||||
| >10000 | 73 | 1.84 | 0.47-7.66 | 0.38 |
| <10000 | 104 | 1 | ||
| C-reactive protein on POD3 (mg/dl) | ||||
| >15 | 93 | 0.69 | 0.17-2.71 | 0.59 |
| <15 | 84 | 1 | ||
| Albumin (g/dl) | ||||
| >2.8 | 70 | 1.24 | 0.20-4.85 | 0.76 |
| <2.8 | 107 | 1 | ||
OR odds ratio, 95%CI 95% confidence interval, BMI body mass index, ASA American Society of Anesthesiologists physical status classification, GPS Glasgow prognostic score, POD postoperative day
aPrognostic nutritional index = 10 × albumin (g/dl) + 0.005 × the absolute lymphocyte count
bSystemic Inflammation Index = the absolute platelet count × the absolute neutrophil count / the absolute lymphocyte count
*p < 0.05
**p < 0.01
***p < 0.001
Nine FTR cases following highly advanced HBPS
Table 4 summarizes detailed data concerning nine FTR cases following highly advanced HBP surgery. The diseases requiring surgery were hepatocellular carcinoma (HCC) in 3 (33.3%) patients; pancreatic ductal adenocarcinoma (PDAC) in 3 (33.3%) patients; and intraductal papillary neoplasm of bile duct (IPNB), metastatic pancreatic cancer from renal cancer, and cholangiocarcinoma in one (11.1%) patient each, respectively. The operating time ranged from 228 to 767 min (median, 427 min), and the amount of intraoperative blood loss ranged from 190 to 4920 ml (median, 2640 ml). A total of 7 (77.8%) patients underwent blood transfusion during the surgery. Severe postoperative complications included postoperative bleeding in 4 (44.4%) patients, liver failure in 3 (33.3%) patients, respiratory failure in 2 (22.2%) patients, and intestinal necrosis and pancreatic fistula in one (11.1%) patient each. The onset time of postoperative complications ranged from 1 to 34 days (median, 8 days). Fatal comorbidities included multiple organ failure (MOF) in 6 (66.7%) patients and hemorrhagic shock in 2 (22.2%) patients. The postoperative days to death ranged from 9 to 80 days (median, 35 days).
Table 4.
Nine FTR cases following highly advanced HBP surgery
| FTR case | Age (years) | Sex | Disease | Surgical procedure | Operation time (min) | Blood loss (ml) | Blood transfusion (ml) | Postoperative severe complications | Onset time of complication (days) | Fatal comorbidity | Postoperative days to death (days) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | Male | IPNB | Right hemihepatectomy | 763 | 2640 |
PRBC 280 FFP 160 |
Postoperative bleeding Liver failure |
5 | MOF | 57 |
| 2 | 67 | Male | Metastatic pancreatic cancer | Pancreaticoduodenectomy | 466 | 4200 |
PRBC 1680 FFP 1920 |
Postoperative bleeding | 8 | Hemorrhagic shock | 67 |
| 3 | 59 | Male | HCC | Right hemihepatectomy | 443 | 3315 |
PRBC 840 FFP 1200 |
Liver failure | 34 | MOF | 68 |
| 4 | 77 | Female | PDAC | Distal pancreatectomy with lymph node dissection | 228 | 190 | None | Respiratory failure | 15 | MOF | 30 |
| 5 | 77 | Female | PDAC | Pancreaticoduodenectomy | 767 | 4920 |
PRBC 1120 FFP 720 |
Intestinal necrosis | 1 | MOF | 80 |
| 6 | 79 | Male | HCC | Hepatic sectionectomy | 334 | 1630 |
PRBC 560 FFP 1200 |
Liver failure | 5 | MOF | 35 |
| 7 | 67 | Male | PDAC | Distal pancreatectomy with lymph node dissection | 262 | 1840 |
PRBC 1120 FFP 960 |
Postoperative bleeding | 12 | Hemorrhagic shock | 12 |
| 8 | 82 | Male | HCC | Hepatic sectionectomy | 407 | 3745 | PRBC 2520 | Respiratory failure | 16 | MOF | 35 |
| 9 | 72 | Male | Cholangiocarcinoma | Hepatopancreatectomy | 427 | 1700 | None |
Postoperative bleeding Pancreatic fistula |
7 | Hemorrhagic shock | 9 |
PRBC packed red blood cells, FFP fresh frozen plasma, IPNB intraductal papillary neoplasm of bile duct, HCC hepatocellular carcinoma, PDAC pancreatic ductal adenocarcinoma, PD pancreaticoduodenectomy, MOF multiple organ failure
Discussion
FTR is defined as a postoperative complication leading to death [1–5]. While a higher complication rate might appear likely to lead to an increased postoperative mortality rate, Ghaferi et al. showed that differences in mortality rates were not associated with large differences in postoperative complication rates [12, 14], but with the ability of hospitals to effectively rescue patients from complications. Therefore, FTR can be considered a quality indicator of the management of postoperative complications rather than of the extent of postoperative complications alone. FTR rates have been reported to vary widely across hospitals for all procedures and are highly correlated with postoperative mortality. Hospital bed size, intensive care unit (ICU) availability, rapid response system (RRS) availability, hospital technology, nurse-to-patient ratios, average daily census, and teaching status have been found to be associated with differences in FTR rates between hospitals with very low and very high mortality rates [11–15]. These findings suggest that FTR rates might be influenced by the extent to which hospitals have well-organized multi-disciplinary teams enabling early intervention through involving endoscopists, radiologists, infection control doctors, and intensivists. Our institution, with > 600 beds and classified as a high-volume center, is a university-affiliated hospital with highly advanced technology as well as being a JSHBPS-certified training institution. Furthermore, endoscopists, radiologists, infection control doctors, and intensivists are on staff. Both ICU and RRS are available, and nursing care is provided at a ratio of 7:1. These specific characteristics of our institution are likely to have contributed to the lower FTR rates than those reported in previous studies [16–21]. Therefore, analysis of data obtained in this highly technical and well-equipped medical environment may help identify issues that need to be addressed to further reduce FTR rates following highly advanced HBPS.
Some studies have reported non-hospital-related risk factors for FTR following highly advanced HBPS. Elfrink et al. showed that factors independently associated with FTR following liver resection were age (65–80 years), an ASA physical status classification of 3, liver cirrhosis, biliary cancer, major liver resection, postoperative liver failure, cardiac complications, and thromboembolic complications [16]. Lei et al. reported that the factors predicting postoperative mortality following liver resection for hepatocellular carcinoma were the Child–Pugh score, intraoperative blood loss, and postoperative liver failure [22]. Gleeson et al. identified the following independent risk factors in FTR following PD: age, ≥ 65 years; albumin level, < 3.5 g/dl; and the development of shock, postoperative renal failure, or postoperative respiratory failure [19]. Endo et al. found that major liver resection and blood transfusion were independently associated with FTR following hepatopancreatectomy [23]. The results of this study are consistent with those of previous reports concerning perioperative predictive factors for FTR following highly advanced HBPS.
First, intraoperative blood loss of > 1600 ml and blood transfusion have been found to be predictive factors for FTR. Intraoperative blood loss is an essential consideration in surgery and has been reported to have both short- and long-term outcomes [22–25]. Nonami et al. [26] reported that blood loss was independently associated with postoperative liver failure and mortality. A strong correlation has been observed between intraoperative blood loss and blood transfusions. Yamamoto et al. [27] reported that all patients with blood loss > 1500 ml received blood transfusions in their study, including 251 liver resection cases. Therefore, considering the cutoff value for blood loss calculated in this study, it is possible to conclude that both massive blood loss and transfusion are risk factors for FTR. Homologous blood transfusion is known to increase the rate of postoperative infectious complications, owing ostensibly to immunosuppression [28, 29], with homologous blood transfusion being a significant risk factor for bacterial infection and a possible risk factor for FTR in surgically treated patients. Therefore, surgeons should maximize their efforts to decrease intraoperative blood loss and avoid unnecessary blood transfusions through the application of sophisticated surgical skills and communication with anesthesiologists.
Second, postoperative organ failure is one of the most serious postoperative complications that can lead to poor outcomes in all surgeries [30–32]. Organ failure involves organ dysfunction to such a degree that homeostasis cannot be maintained without external clinical intervention. MOF is defined as the involvement of two or more organ systems. Postoperative organ dysfunction can occur in any organ; however, the pulmonary, hepatic, cardiac, renal, and cerebral vessels are more commonly involved. In this study, we selected liver and respiratory failure as risk factors for FTR owing to their high rate of postoperative organ failure. Both types of organ failure significantly correlated with FTR in the univariate analysis. Postoperative liver failure has previously been reported to be an independent risk factor for FTR following liver resection [16–18, 22]. In addition, massive intraoperative bleeding and liver-related disease comorbidities are known causes of postoperative liver failure [16, 26], and the confounding relationship between these factors may have influenced our results.
Postoperative respiratory failure is a significant risk factor for FTR [32–34]. Postoperative respiratory failure is defined as an unplanned postoperative reintubation or prolonged postoperative intubation. Several previous studies have reported postoperative respiratory failure incidence rates ranging from 2.7 to 3.4%. Older age, ASA, pulmonary-related disease comorbidity, longer surgery, pneumonia, abdominal surgery, and diaphragmatic dysfunction have also been reported to be risk factors [32–35]. Diaphragmatic dysfunction may develop following prolonged mechanical ventilation, damage to the muscles and nerves of the diaphragm, and irritation from subdiaphragmatic abscesses or thoracoabdominal effusions. More caution may be needed to avoid respiratory failure for patients with complications that may lead to diaphragmatic dysfunction.
This study had some limitations. First, this single-center retrospective study involved a small number of FTR events, which may have resulted in selection bias and multiplicity issues in the statistical analysis. A multicenter study with a larger number of patients is required to obtain more accurate results. However, multicenter studies may show a large effect on inter-institutional disparities in terms of FTR rates. Therefore, our study concerning FTR at a single institution with a well-developed medical environment and uniform surgical indications may be of particular value. Second, this study included all patients with highly advanced HBPS and hepatobiliary and pancreatic surgeries. Each type of surgery may be associated with different risk factors for FTR. This limitation should be considered when evaluating our study results.
Conclusions
FTR was shown to be associated with perioperative factors. Well-coordinated surgical procedures to avoid intra- and postoperative bleeding and unnecessary blood transfusions, as well as postoperative team management with attention to the occurrence of organ failure, may decrease FTR rates.
Supplementary Information
Additional file 1: Supplemental Table 1. Comparison of patient characteristics between patients with and without postoperative severe complications.
Acknowledgements
The authors thank the medical staff of the Department of Gastroenterological Surgery at Gifu University Hospital for their participation in this study. We could not have completed this study without their diligence or support. We would also like to thank Editage (www.editage.jp) for the English language editing.
Abbreviations
- ASA
American Society of Anesthesiologists
- CI
Confidence interval
- FTR
Failure to rescue
- HBPS
Hepatobiliary-pancreatic surgery
- ICU
Intensive care unit
- JSHBPS
Japanese Society of Hepato-Biliary-Pancreatic Surgery
- NSQIP
The National Surgical Quality Improvement Program
- OR
Odds ratio
- PD
Pancreaticoduodenectomy
- RSS
Rapid response system
- WHO
World Health Organization
- MOF
Multiple organ failure
- HCC
Hepatocellular carcinoma
- PDAC
Pancreatic ductal adenocarcinoma
- IPNB
Intraductal papillary neoplasm of bile duct
- CD
Clavien–Dindo
Authors’ contributions
MF conceived the study concept, planned the study design as the principal investigator, interpreted the results, and drafted the manuscript. NM revised the manuscript, added intellectual content, and provided critical advice. MF, KM, TH, IY, YS, JT, SK, YT, NO, and NM obtained the data, provided critical comments to improve the manuscript, and approved its final submission.
Funding
Not applicable.
Availability of data and materials
The datasets used in this study are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the World Medical Association Declaration of Helsinki and approved by the Ethics Committee of Gifu University (approval number “2023–018”). This retrospective study did not include any potentially identifiable patient data; therefore, the need for informed consent was waived by the Ethics Committee of Gifu University. This retrospective study was approved by the relevant Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.van Rijssen LB, Zwart MJ, van Dieren S, de Rooij T, Bonsing BA, Bosscha K, et al. Variation in hospital mortality after pancreatoduodenectomy is related to failure to rescue rather than major complications: a nationwide audit. HPB (Oxford) 2018;20:759–767. doi: 10.1016/j.hpb.2018.02.640. [DOI] [PubMed] [Google Scholar]
- 2.Staiger RD, Gerns E, Castrejón Subirà M, Domenghino A, Puhan MA, Clavien PA. Can early postoperative complications predict high morbidity and decrease failure to rescue following major abdominal surgery? Ann Surg. 2020;272:834–839. doi: 10.1097/SLA.0000000000004254. [DOI] [PubMed] [Google Scholar]
- 3.Henneman D, Snijders HS, Fiocco M, van Leersum NJ, Kolfschoten NE, Wiggers T, et al. Hospital variation in failure to rescue after colorectal cancer surgery: results of the Dutch Surgical Colorectal Audit. Ann Surg Oncol. 2013;20:2117–2123. doi: 10.1245/s10434-013-2896-7. [DOI] [PubMed] [Google Scholar]
- 4.Henneman D, van Leersum NJ, Ten Berge M, Snijders HS, Fiocco M, Wiggers T, et al. Failure-to-rescue after colorectal cancer surgery and the association with three structural hospital factors. Ann Surg Oncol. 2013;20:3370–3376. doi: 10.1245/s10434-013-3037-z. [DOI] [PubMed] [Google Scholar]
- 5.Busweiler LA, Henneman D, Dikken JL, Fiocco M, van Berge Henegouwen MI, Wijnhoven BP, et al. Failure-to-rescue in patients undergoing surgery for esophageal or gastric cancer. Eur J Surg Oncol. 2017;43:1962–1969. doi: 10.1016/j.ejso.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 6.World Alliance for Patient Safety . WHO guidelines for safe surgery. Geneva: World Health Organization; 2008. [Google Scholar]
- 7.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 8.Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–376. doi: 10.1097/SLA.0b013e3181b4148f. [DOI] [PubMed] [Google Scholar]
- 9.Otsubo T, Kobayashi S, Sano K, Misawa T, Katagiri S, Nakayama H, et al. A nationwide certification system to increase the safety of highly advanced hepatobiliary-pancreatic surgery. J Hepatobil Pancreat Sci. 2023;30:60–71. doi: 10.1002/jhbp.1186. [DOI] [PubMed] [Google Scholar]
- 10.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in hospital volume and failure to rescue for pancreatic surgery. J Gastrointest Surg. 2015;19:1581–1592. doi: 10.1007/s11605-015-2800-9. [DOI] [PubMed] [Google Scholar]
- 12.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211:325–330. doi: 10.1016/j.jamcollsurg.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Brooke BS, Dominici F, Pronovost PJ, Makary MA, Schneider E, Pawlik TM. Variations in surgical outcomes associated with hospital compliance with safety practices. Surgery. 2012;151:651–659. doi: 10.1016/j.surg.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 15.Scarborough JE, Pappas TN, Bennett KM, Lagoo-Deenadayalan S. Failure-to-pursue rescue: explaining excess mortality in elderly emergency general surgical patients with preexisting “do-not-resuscitate” orders. Ann Surg. 2012;256:453–461. doi: 10.1097/SLA.0b013e31826578fb. [DOI] [PubMed] [Google Scholar]
- 16.Elfrink AKE, Olthof PB, Swijnenburg RJ, den Dulk M, de Boer MT, Mieog JSD, et al. Factors associated with failure to rescue after liver resection and impact on hospital variation: a nationwide population-based study. HPB (Oxford) 2021;23:1837–1848. doi: 10.1016/j.hpb.2021.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Ardito F, Famularo S, Aldrighetti L, Grazi GL, DallaValle R, Maestri M, et al. The impact of hospital volume on failure to rescue after liver resection for hepatocellular carcinoma: analysis from the HE.RC.O.LE.S. Italian registry. Ann Surg. 2020;272:840–6. doi: 10.1097/SLA.0000000000004327. [DOI] [PubMed] [Google Scholar]
- 18.Baum P, Diers J, Lichthardt S, Kastner C, Schlegel N, Germer CT, et al. Mortality and complications following visceral surgery: a nationwide analysis based on the diagnostic categories used in German Hospital invoicing data. Dtsch Ärztebl Int. 2019;116:739–746. doi: 10.3238/arztebl.2019.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleeson EM, Clarke JR, Morano WF, Shaikh MF, Bowne WB, Pitt HA. Patient-specific predictors of failure to rescue after pancreaticoduodenectomy. HPB (Oxford) 2019;21:283–290. doi: 10.1016/j.hpb.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Tamirisa NP, Parmar AD, Vargas GM, Mehta HB, Kilbane EM, Hall BL, et al. Relative contributions of complications and failure to rescue on mortality in older patients undergoing pancreatectomy. Ann Surg. 2016;263:385–391. doi: 10.1097/SLA.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varley PR, Geller DA, Tsung A. Factors influencing failure to rescue after pancreaticoduodenectomy: a national surgical quality improvement project perspective. J Surg Res. 2017;214:131–139. doi: 10.1016/j.jss.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Lei GY, Shen L, Junnarkar SP, Huey CT, Low J, Shelat VG. Predictors of 90-day mortality following hepatic resection for hepatocellular carcinoma. Visc Med. 2021;37:102–109. doi: 10.1159/000510811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endo I, Hirahara N, Miyata H, Yamamoto H, Matsuyama R, Kumamoto T, et al. Mortality, morbidity, and failure to rescue in hepatopancreatoduodenectomy: an analysis of patients registered in the National Clinical Database in Japan. J Hepatobil Pancreat Sci. 2021;28:305–316. doi: 10.1002/jhbp.918. [DOI] [PubMed] [Google Scholar]
- 24.Fischer D, Neb H, Choorapoikayil S, Zacharowski K, Meybohm P. Red blood cell transfusion and its alternatives in oncologic surgery-a critical evaluation. Crit Rev Oncol Hematol. 2019;134:1–9. doi: 10.1016/j.critrevonc.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Choi M, Hwang HK, Rho SY, Lee WJ, Kang CM. Intraoperative transfusion is independently associated with a worse prognosis in resected pancreatic cancer-a retrospective cohort analysis. J Clin Med. 2020;9:689. doi: 10.3390/jcm9030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nonami T, Nakao A, Kurokawa T, Inagaki H, Matsushita Y, Sakamoto J, et al. Blood loss and ICG clearance as best prognostic markers of post-hepatectomy liver failure. Hepatogastroenterology. 1999;46:1669–1672. [PubMed] [Google Scholar]
- 27.Yamamoto Y, Shimada K, Sakamoto Y, Esaki M, Nara S, Kosuge T. Preoperative identification of intraoperative blood loss of more than 1,500 mL during elective hepatectomy. J Hepatobil Pancreat Sci. 2011;18:829–838. doi: 10.1007/s00534-011-0399-0. [DOI] [PubMed] [Google Scholar]
- 28.Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M, et al. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet. 1993;342:1328–1333. doi: 10.1016/0140-6736(93)92247-q. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen HJ. Detrimental effects of perioperative blood transfusion. Br J Surg. 1995;82:582–587. doi: 10.1002/bjs.1800820505. [DOI] [PubMed] [Google Scholar]
- 30.Toro-Pérez J, Rodrigo R. Contribution of oxidative stress in the mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep. 2021;26:35–44. doi: 10.1080/13510002.2021.1891808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, Lee HJ, Oh SY, Park SH, Berlth F, Son YG, et al. Prediction of postoperative mortality in patients with organ failure after gastric cancer surgery. World J Surg. 2020;44:1569–1577. doi: 10.1007/s00268-020-05382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attaallah AF, Vallejo MC, Elzamzamy OM, Mueller MG, Eller WS. Perioperative risk factors for postoperative respiratory failure. J Perioper Pract. 2019;29:49–53. doi: 10.1177/1750458918788978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rujirojindakul P, Geater AF, McNeil EB, Vasinanukorn P, Prathep S, Asim W, et al. Risk factors for reintubation in the post-anaesthetic care unit: a case-control study. Br J Anaesth. 2012;109:636–642. doi: 10.1093/bja/aes226. [DOI] [PubMed] [Google Scholar]
- 34.Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–580. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 35.Fu X, Wang Z, Wang L, Lv G, Cheng Y, Wang B, et al. Increased diaphragm echodensity correlates with postoperative pulmonary complications in patients after major abdominal surgery: a prospective observational study. BMC Pulm Med. 2022;22:400. doi: 10.1186/s12890-022-02194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Comparison of patient characteristics between patients with and without postoperative severe complications.
Data Availability Statement
The datasets used in this study are available from the corresponding author upon request.