Abstract
Background:
Fatigue negatively impacts the function and quality of life of people with disabilities (PwD). Mobile health (mHealth) platforms are recognized as effective and accessible approaches to delivering health interventions and may show higher satisfaction by tailoring the information toward personalized needs for PwD.
Objective:
To evaluate the acceptability, feasibility, and participant engagement with a Short Message Service (SMS) text messaging intervention for fatigue self-management and to explore the pre- and post-score health changes in PwD.
Methods:
A total of 27 PwD (multiple sclerosis = 9, spinal cord injury = 9, or stroke = 9) experiencing fatigue in their daily lives participated in a 12-week self-management text messaging intervention. Participants completed a demographic survey and health outcome measures, including patient activation, self-efficacy for managing symptoms, fatigue, sleep, and satisfaction with participation in social roles before and after the intervention. Participants also completed a client satisfaction questionnaire after the intervention. We also tracked the program retention and SMS response rates over the 12-week intervention period.
Results:
Twenty-five participants completed the entire intervention (93% retention rate), and the overall SMS response rate was 84.67%, indicating high acceptability and adherence to the intervention. The mean satisfaction score was 3.18, indicating high satisfaction with the intervention. Despite finding a negligible effect on patient activation, we found a small intervention effect on self-efficacy for managing symptoms (η2 = 0.04) and moderate effects on fatigue (η2 = 0.06–0.12), sleep (η2 = 0.11), and satisfaction with participation in social roles (η2 = 0.08).
Conclusions:
This study provides initial feasibility and health outcome change evidence to support an SMS text messaging intervention to manage fatigue in PwD.
Keywords: Fatigue, mHealth, Stroke, Spinal cord injury, Multiple sclerosis
1. Introduction
Fatigue is a symptom characterized by prolonged periods of exhaustion accompanied by the inability to perform activities to an expected capacity and is commonly experienced by people with disabilities (PwD). Fatigue is two to three times more prevalent in PwD than in the general population and significantly higher than in older adults with other medical conditions.1,2 Three disability groups commonly experiencing fatigue include approximately 80% of people with multiple sclerosis (MS),1 75% of stroke survivors,3 and 50% of people with spinal cord injury (SCI).4 Fatigue often impacts the daily functioning of PwD and negatively impacts their health-related quality of life.5 A study surveying PwD (including people with MS, SCI, and stroke) about their life activities identified fatigue as one of the significant health-related limitations impacting participation in self-care, mobility, and community activities.6 Another survey study of adults aging with a physical disability found that greater fatigue was associated with lower social participation outcomes.2
Approaches to managing fatigue include pharmacologic and non-pharmacologic interventions. Pharmacologic interventions can involve medications with undesirable side effects such as difficulty sleeping, anxiety, and headaches.7 A systematic review suggests that pharmacologic interventions have limited efficacy and are inferior to non-pharmacologic interventions.8 Thus, PwD are increasingly interested in non-pharmacologic approaches to fatigue management, such as exercise, education, or behavioral management techniques.9 However, there is a lack of consensus across research and clinical practitioners on the most effective approach for fatigue management for PwD.5
Mobile health (mHealth) platforms have become an effective and accessible approach to providing care and delivering health interventions.10 Previous research utilizing mHealth technologies to support self-management has shown favorable outcomes for PwD, including increased self-efficacy and perceived ability to manage symptoms.11 Short message service (SMS) text messages have been used to implement health behavior change interventions in a person’s natural environment and provide an ideal platform for increasing patient engagement.12 A systematic review evaluating mobile text messaging interventions indicated that text messaging elicited significant improvements in health outcomes, self-management adherence, and health-related behavior change as an inexpensive and easy-to-use method.12 Further, text messaging is widely accessible and cost-effective and has demonstrated usability with many different populations.13
Though mHealth is a relatively new approach to managing health, several studies have confirmed its feasibility, accessibility, and acceptability in vulnerable populations experiencing fatigue, including PwD. A previous study using mHealth to assess symptoms and deliver health education and self-management strategies in persons with MS suggested that mHealth platforms are beneficial for mitigating fatigue and other symptoms.14 Another study examining the real-time relationship between social activity and post-stroke fatigue, among other symptoms, found that mHealth can feasibly measure the dynamics between post-stroke fatigue and social interactions in real-world contexts.15 This emerging body of research justifies the need to investigate an mHealth approach for fatigue self-management in PwD.
The development of mHealth interventions should be grounded in theory and tailored to the needs of the target population.16 A meta-analysis showed that theory-guided digital self-management programs improve fatigue, depression, anxiety, and self-efficacy among PwD more than those not guided by theory.17 Moreover, studies have shown that patient satisfaction and engagement with traditional self-management programs are low for PwD, illustrating the need to tailor interventions to address personal needs by increasing patient activation.18 Patient activation is defined as a patient demonstrating the skills, knowledge, and confidence needed to manage their health and participate in healthcare decisions effectively.19 A study found that patient activation was related to 12 out of 13 health-related outcomes in patients receiving primary care services. Those with higher patient activation had lower adverse health outcomes, such as emergency department visits, obesity, and smoking.20 Another study found that higher patient activation was associated with improved self-management behaviors, satisfaction with services, quality of life, and functional status in adults with chronic conditions.21 Recent behavioral intervention studies also showed that increased patient activation was related to better self-management of chronic conditions.22 Further evidence suggests that tailored interventions that improve patient activation may improve health outcomes.23–25
We have developed an mHealth fatigue self-management text messaging intervention targeting patient activation for persons with MS, stroke, and SCI.26 The intervention was developed based on feedback from a consumer advisory board (CAB). Each participant received four weekly educational text messages for various fatigue self-management strategies and two weekly check-in messages over 12 weeks. Fig. 1 shows the proposed framework that outlines the content and functionality of the intervention. The overall objective of this study was to test the acceptability and feasibility of this intervention to improve fatigue self-management in PwD. Specifically, our first aim was to evaluate the feasibility and acceptability of the intervention among PwD. Our second aim was to examine participant engagement with the intervention over the 12-week period. Although testing the intervention’s effects was not the critical scope of this study, pre- and post-score changes for each health measure were examined to select optimal endpoints for a future efficacy trial.
Fig. 1.
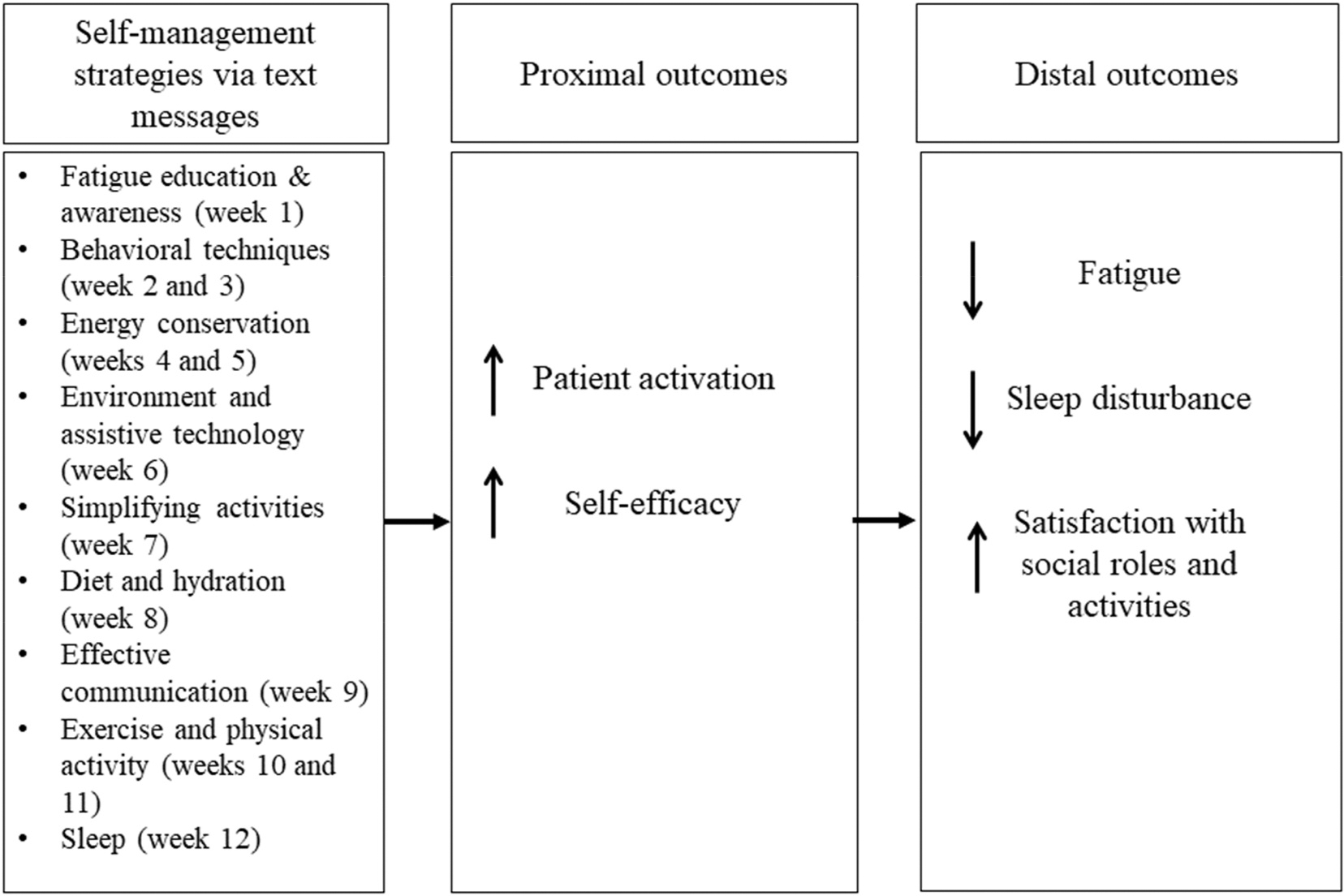
Proposed intervention framework. Solid arrows represent the expected direction of the intervention effect.
2. Methods
2.1. Study design
The study design was a single-arm, pre-post trial of a 12-week fatigue self-management text messaging intervention for PwD. This study was designed to support the early stages of the intervention’s development by investigating the feasibility and acceptability of the intervention before formally testing its effectiveness.27 The study was approved by the university’s Institutional Review Board (#202103191). Before study participation, each participant provided informed consent.
2.2. Participants
The study recruited people with MS, SCI, or stroke living in a Midwestern city in the United States between September and November of 2021. We recruited participants through previous studies conducted by the investigators, a research registry, a recruitment enhancement core, a local independent living center, and word of mouth. We contacted participants in person or by telephone, explained the research study, and screened them if interested to ensure project eligibility. Inclusion criteria included (1) over 18 years old, (2) living with a disability for at least one year, (3) self-reported fatigue impacting their daily routine, (4) ability to read or speak English, and (5) willing to use their phone for SMS text messaging. Exclusion criteria included (1) a self-reported acute medical condition (e.g., pneumonia), (2) sleep apnea, (3) inability to answer questions or provide consent, and (4) terminal cancer.
2.3. Procedures
Once eligibility was determined, participants were scheduled to complete the baseline assessment before beginning the intervention. Participants completed the evaluation at their homes via an emailed online Research Electronic Data Capture (REDCap). Participants requiring assistance to answer the survey or those without internet access completed the assessment via phone interviews with our project staff. Following the baseline assessment, participants reviewed training materials on how to receive and respond to text messages sent via the intervention’s digital platform, Epharmix. If a participant completed the assessment by phone, the training slides were read to the participant by project staff. All training was conducted remotely using either the REDCap link or phone calls. Participants received their first text message the Monday after training.
We assessed each participant’s patient activation level using the Patient Activation Measure short form (PAM-13) during the baseline assessment.28 The PAM-13 consists of 13 items assessing an individual’s knowledge, skills, and confidence to managing one’s health and healthcare. Participants rated each item from “strongly disagree” to “strongly agree.” Raw PAM scores were transformed into interval-level Rasch scores (0–100), where higher scores represent higher levels of patient activation.29 Participants were classified into one of four levels of patient activation based on their Rasch scores, including: disengaged and overwhelmed (level 1); becoming aware but still struggling (level 2); takes action and gains control (level 3); and maintains behavior and pushes further (level 4). A research license from Insignia Health was purchased to compute final scores based on their proprietary survey scoring algorithm.30 According to Hibbard and colleagues,28 participants with a Rasch score of ≤41 were classified as level 1, 42–47 as level 2, 50–51 as level 3, and ≥52 as level 4.
Participants received text messages based on their baseline patient activation level. Participants in levels 1 or 2 are described as building a knowledge base and growing confidence in taking a role in managing their health and health care.28,31 These participants received a set of 48 text messages for PAM levels 1 and 2, focusing on informing and educating them about fatigue and activities in their daily life that may affect their fatigue. Participants in levels 3 or 4 are described as actively practicing skills and maintaining behaviors in managing their health and health care.28,31 These participants received another set of 48 messages for PAM levels 3 and 4, focusing on providing strategies for implementing changes into daily life that may help manage their fatigue.
The content for the two sets of 48 messages was based on existing evidence and reviewed by a CAB comprising six persons with a disability (MS = 2, SCI = 2, and stroke = 2) and a physician.26 The research team conducted an in-person interview with the physician, during which the physician reviewed the two sets of text messages to determine their accuracy and appropriateness in meeting the fatigue management needs of PwD. The research team modified the text messages based on the physician’s feedback. The research team then sent two refined sets of messages to the CAB members prior to two virtual feedback sessions. The research team conducted two feedback sessions with the members via videoconferencing for approximately 90 min each to obtain their feedback on the refined text messages. During the sessions, CAB members rated the clarity and relevance of each message on a five-point Likert scale, with 5 being the highest rating. They also provided feedback on how to improve those messages. The research team incorporated the feedback to modify items with either clarity or relevant ratings ≤4. Each of the final two sets of text messages covered nine focus areas as self-management tips: fatigue education and awareness, behavioral techniques, energy conservation, environment and assistive technology, simplifying activities, diet/hydration, effective communication, exercise/physical activity, and sleep. Each participant received a daily “educational” text message Monday through Thursday morning providing a fatigue management tip (i.e., four messages per week). The fatigue management tips did not require a response from participants. On Fridays, participants received two “check-in” messages in the morning requesting a response via text messaging. The first message asked the participants to rate the average impact of their fatigue over the last week using a five-point scale, with 1 representing not at all and 5 representing very much. The second message asked participants if they had applied any tips sent in the past week using a four-point scale, with 1 representing not at all and 4 representing nearly every day. Example messages can be found in our previous publication on the development of the intervention.26
Following the completion of the 12-week intervention, participants completed the post-intervention evaluation. Like the baseline evaluation, participants completed the assessment at their own convenience via an online REDCap link or via phone interviews supported by our project staff. Participants willing to share their post-intervention experience also completed interviews via phone calls with project staff.
2.4. Outcome measures
The feasibility of the intervention was determined based on adherence and acceptability data as well as participant ratings of intervention satisfaction. We also utilized different health outcome measures for potential future endpoints.
Feasibility Outcomes:
To assess intervention acceptability and adherence, we tracked the overall retention rate and the SMS text message response rate, respectively. Participants completed the Client Satisfaction Questionnaire-8 (CSQ-8) at the post-intervention evaluation, which includes eight self-reported items as a brief measure of client satisfaction with services received.32 This measure used four response choices, with 1 indicating the lowest degree of satisfaction and 4 indicating the highest. The internal consistency reliability of CSQ-8 is high, with alpha levels of 0.88 and 0.90 for the English and Spanish versions, respectively.
Health Outcomes:
We chose health measures as our future endpoints based on our proposed intervention framework to improve fatigue self-management (Fig. 1). Participants completed the PAM-13 and the Patient-Reported Outcomes Measurement Information System (PROMIS) short form for Self-efficacy for Managing Symptoms as proximal outcomes. They also completed the Modified Fatigue Impact Scale (MFIS) and the PROMIS short forms for Fatigue, Sleep Disturbance, and Satisfaction with Social Roles and Activities as distal outcomes to determine effect sizes that can power future studies. We expected that patient activation, self-efficacy, fatigue, sleep disturbance, and satisfaction with social roles and activities would improve after intervention. Patient activation, the primary mechanism of action underlying the intervention, and self-efficacy, a related construct, were proposed as mediators of the impact of the intervention on distal outcomes based on our framework.
Participant Demographics: This is a self-report questionnaire including participant demographics (e.g., age, gender, and race), education level, income, diagnosis, and date of diagnosis.6 We administered this questionnaire only at baseline.
Patient Activation Measure (PAM-13): The PAM-13 assesses four activation levels. The PAM-13 had acceptable infit and outfit statistics (i.e., within the 0.5–1.5 acceptable range). It also had acceptable reliabilities for persons with chronic conditions (i.e., model person-reliability ≥0.8).26 We administered the PAM-13 at baseline and post-intervention.
PROMIS Short Form v1.0 Self-Efficacy for Managing Symptoms 8a: This measure is an eight-item short form measuring confidence in the ability to control symptoms, manage symptoms in different settings, and keep symptoms from interfering with work, sleep, relationships, or recreational activities.33 This short form is universal rather than disease-specific. Items are rated on a five-point scale ranging from “I am not at all confident” to “I am very confident” and are summed and transformed into a T-score metric. Higher T-scores represent higher self-efficacy for managing symptoms. We administered this measure at baseline and post-intervention.
Modified Fatigue Impact Scale (MFIS): The MFIS is a 21-item shortened version of the 40-item Fatigue Impact scale that assesses the perceived impact of fatigue on physical, cognitive, and psychosocial functioning during the past four weeks. Participants answered using a five-point Likert scale, with 0 representing never and 4 representing almost always. The MFIS is either reported as a total score (0–84) or by each subscale: physical (0–36), cognitive (0–40), and psychosocial functioning (0–8). Higher scores indicate greater fatigue.34 We administered this measure at baseline and post-intervention.
PROMIS Short Form v1.0 Fatigue 8a: This measure evaluates fatigue based on its frequency, duration, and intensity, as well as its impact on physical, mental, and social activities. Items are rated on a five-point scale ranging from “never” to “always” and are summed and transformed to a T-score metric.33 This scale was previously validated in people with chronic conditions and has good precision across different fatigue levels.33 Higher T-scores represent greater fatigue. We administered this measure at baseline and post-intervention.
PROMIS Short Form v1.0 Sleep Disturbance 8a: This measure is an eight-item short form assessing self-reported sleep quality, sleep depth, and difficulty falling and staying asleep over seven days.35 The raw sum score is rescaled on the PROMIS score conversion table to determine the standardized T-score.35 Higher T-scores represent greater sleep disturbance. We administered this measure at baseline and post-intervention.
PROMIS Short Form v1.0 Satisfaction with Participation in Social Roles and Activities 8a: This measure is an eight-item short form assessing self-reported contentment with social roles, such as work and family responsibilities, over the previous seven days.36 Items are rated on a five-point scale ranging from “not at all” to “very much” and are summed and transformed to a T-score metric. Higher T-scores represent greater satisfaction with participation in social roles and activities. We administered this measure at baseline and post-intervention.
After completing post-intervention outcome measures, we invited participants to interview with research staff to collect feedback regarding their experiences with the intervention. We asked if they would again participate in this intervention and why. We also asked if they found any strengths, weaknesses, or areas for improvement for this program.
Participants were compensated for their time and efforts in the study: $30 for the baseline evaluation, $30 for the post-intervention evaluation, and an additional $15 for providing qualitative feedback on their experiences, for up to $75 total.
2.5. Data analysis
We examined feasibility data in a descriptive fashion. We defined high acceptability as 80% retention and high adherence as 70% SMS text message response rate. We defined high satisfaction as an average score for the CSQ of ≥3 (out of 4). We characterized participant engagement with text messaging over a 12-week intervention period by the proportion of weekly SMS text messages to which participants responded (i.e., overall response rate). A response was defined as participants reporting weekly fatigue level or weekly tip usage. We reported their fatigue and tip usage ratings over the intervention period. We also compared the intervention satisfaction and the overall text message response rate between participants receiving text messages for PAM levels 1 and 2 versus levels 3 and 4 using independent t-tests.
We examined pre- and post-score changes for both primary and secondary outcomes to select optimal endpoints for a future efficacy trial. We estimated these pre-post differences by running General Linear Models (GLM) and entering PAM, MFIS, and PROMIS scores as within-subject factors. Due to the small sample size and pilot nature of the study, we reported effect size (η2) to reflect the magnitude of the intervention effect, with 0.01 = small effect, 0.06 = medium effect, and 0.14 = large effect. All analyses were conducted in version 27 of SPSS. For the qualitative data, we reported participants’ responses regarding their perception of the intervention, strengths, and areas of improvement.
3. Results
3.1. Feasibility and acceptability
This study enrolled a total of 27 participants (MS = 9, SCI = 9, and stroke = 9). They were primarily female (63%), with a mean age of 49.7 years (Table 1). Among those, 25 participants (93%) completed the full protocol, suggesting the high acceptability of the intervention. Two persons with stroke discontinued the study (one after the fourth week and another after the tenth week) due to loss of contact. We contacted them several times by phone calls and emails but could not reach them. These two participants did not complete the remaining messages and the post-intervention evaluation.
Table 1.
Participant demographics.
| Participant Characteristics | Total Sample (N = 27) |
|---|---|
|
| |
| Age in years, range, mean (SD) | 23–73, 49.7 (12.3) |
| Gender, n (%) | |
| Male | 10 (37) |
| Female | 17 (63) |
| Race, n (%)* | |
| Black/African American | 12 (44) |
| White/Caucasian | 13 (48) |
| Others | 3 (11) |
| Education, n (%) | |
| Less than or equal to high school | 5 (19) |
| Some college/trade school/associate degree | 9 (33) |
| Bachelor’s degree or graduate degree | 13 (48) |
| Length of disability in years, range, mean (SD) | 2–32, 10.4 (8.3) |
| Income Personal, n (%) | |
| $14,999 or less | 10 (37) |
| $15,000 – $34,999 | 6 (22) |
| $35,000 – $54,999 | 1 (4) |
| $55,000 – $74,999 | 4 (15) |
| $75,000 or more | 4 (15) |
| I don’t know or prefer not to answer | 2 (7) |
| Income Household, n (%) | |
| $14,999 or less | 9 (33) |
| $15,000 – $34,999 | 3 (11) |
| $35,000 – $54,999 | 2 (7) |
| $55,000 – $74,999 | 4 (15) |
| $75,000 or more | 6 (22) |
| I don’t know or prefer not to answer | 3 (11) |
| Diagnosis, n (%) | |
| Multiple Sclerosis | 9 (33) |
| Spinal Cord Injury | 9 (33) |
| Stroke | 9 (33) |
| Initial patient activation level, n (%) | |
| Level 1 | 2 (7) |
| Level 2 | 5 (19) |
| Level 3 | 5 (19) |
| Level 4 | 15 (56%) |
Note.
Participants could check more than one response; therefore, the total number of respondents for this question adds up to more than 27.
The overall response rate to the SMS text messages of all 27 participants was 83.18%, with response rates ranging from 33.33% to 100%. When examining the 25 participants who completed the protocol, the overall response rate improved to 85.17%, with response rates ranging from 45.83% to 100% (Table 2). Among those who completed the protocol, 80% of participants (20/25) had a 70% or higher weekly response rate. The SMS response rate of participants receiving text messages for PAM levels 1 and 2 (mean = 90.48%, SD = 14.58, n = 7) was larger than participants receiving text messages for PAM levels 3 and 4 (mean = 83.10%, SD = 19.78, n = 18). However, this contrast was not statistically significant (t(23) = 0.892, p = 0.382). The response rates slightly varied between the three diagnostic groups, with participants with MS demonstrating the highest response rate (mean = 96.76%, SD = 4.05%), followed by those with SCI (mean = 82.87%, SD = 16.85%) and those with stroke (mean = 73.21%, SD = 24.28% for seven stroke survivors; mean = 69.91%, SD = 25.33% for nine stroke survivors including the two participants who discontinued the intervention).
Table 2.
Participants’ SMS text message responses over the 12-week intervention period.
| Group | Initial | Overall SMS | Fatigue | Weekly Fatigue Ratings | Tip Usage | Weekly Tip Usage Ratings | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||
| PAM Level | Response Rate | Response Rate | Mean^ | SD | Response Rate | Mean^ | SD | |
|
| ||||||||
| MS | 3 | 100.00% | 100.00% | 2.25 | 0.75 | 100.00% | 2.17 | 1.03 |
| MS | 2 | 100.00% | 100.00% | 2.50 | 0.52 | 100.00% | 2.58 | 0.51 |
| MS | 4 | 100.00% | 100.00% | 1.67 | 0.49 | 100.00% | 2.92 | 0.29 |
| MS | 3 | 91.67% | 91.67% | 2.82 | 0.75 | 91.67% | 1.27 | 0.47 |
| MS | 4 | 95.83% | 100.00% | 2.33 | 0.65 | 91.67% | 3.45 | 0.52 |
| MS | 2 | 91.67% | 91.67% | 2.55 | 0.93 | 91.67% | 1.73 | 0.47 |
| MS | 4 | 100.00% | 100.00% | 2.50 | 1.38 | 100.00% | 1.33 | 0.49 |
| MS | 4 | 91.67% | 83.33% | 4.00 | 0.67 | 100.00% | 3.67 | 0.65 |
| MS | 4 | 100.00% | 100.00% | 2.58 | 1.38 | 100.00% | 2.08 | 0.79 |
| SCI | 3 | 58.33% | 66.67% | 3.25 | 0.89 | 50.00% | 2.00 | 0.00 |
| SCI | 4 | 95.83% | 91.67% | 2.00 | 0.63 | 100.00% | 3.00 | 0.43 |
| SCI | 2 | 100.00% | 100.00% | 2.92 | 0.29 | 100.00% | 2.83 | 0.39 |
| SCI | 4 | 66.67% | 75.00% | 2.78 | 0.67 | 58.33% | 3.43 | 0.53 |
| SCI | 2 | 95.83% | 91.67% | 3.73 | 0.79 | 100.00% | 3.50 | 0.52 |
| SCI | 2 | 58.33% | 58.33% | 1.29 | 0.49 | 58.33% | 1.43 | 0.53 |
| SCI | 1 | 91.67% | 91.67% | 3.27 | 0.79 | 91.67% | 3.18 | 0.75 |
| SCI | 4 | 87.50% | 83.33% | 1.90 | 1.29 | 91.67% | 3.82 | 0.40 |
| SCI | 3 | 91.67% | 91.67% | 3.18 | 0.60 | 91.67% | 2.64 | 0.67 |
| Stroke | 1 | 95.83% | 100.00% | 1.92 | 0.90 | 91.67% | 2.64 | 0.67 |
| Stroke | 4 | 95.83% | 91.67% | 2.82 | 0.60 | 100.00% | 3.75 | 0.45 |
| Stroke | 4 | 33.33% | 25.00% | 1.67 | 1.15 | 41.67% | 1.80 | 0.45 |
| Stroke | 4 | 79.17% | 83.33% | 2.10 | 0.99 | 75.00% | 1.89 | 0.78 |
| Stroke | 3 | 83.33% | 83.33% | 3.60 | 1.17 | 83.33% | 1.90 | 0.32 |
| Stroke | 4 | 45.83% | 41.67% | 2.80 | 0.45 | 50.00% | 3.33 | 0.52 |
| Stroke | 4 | 79.17% | 75.00% | 2.89 | 0.93 | 83.33% | 2.50 | 0.53 |
| Stroke+ | 4 | 83.33% | 83.33% | 2.50 | 1.27 | 83.33% | 3.30 | 0.95 |
| Stroke+ | 4 | 33.33% | 33.33% | 2.00 | 0.82 | 33.33% | 1.50 | 0.58 |
Note:
Two participants who discontinued the study during the intervention.
Mean rating of each participant over the 12-week intervention period.
Based on results from the CSQ, the mean satisfaction score was 3.18 (SD = 0.45), suggesting high overall satisfaction with the intervention. The majority of participants (92%) were “very” or “mostly” satisfied with the program, and 92% would recommend the intervention to a friend. Approximately 88% of participants said the intervention helped them deal more effectively with their problems, and 84% believed the program to be “excellent” or “good quality.” Finally, 87.5% of participants reported they would participate again, supporting the intervention’s acceptability. The overall satisfaction score of participants who received text messages for PAM levels 1 and 2 (mean = 2.96, SD = 0.45, n = 7) was lower than participants who received text messages for PAM levels 3 and 4 (mean = 3.26, SD = 0.43, n = 18). However, this contrast was not statistically significant (t(23) = −1.544, p = 0.136).
3.2. Participant engagement
Figs. 2 and 3 show the weekly fatigue level and tip use over the intervention period. We did not conduct any time-series analysis, as it was not within the study’s scope. However, a visual inspection of the weekly fatigue ratings (Fig. 2) showed variations in self-reported fatigue across three diagnostic groups over time. Visual inspection of the weekly tip application ratings (Fig. 3) indicated that participants with SCI had the highest tip use over time, followed by those with stroke and MS.
Fig. 2.

Weekly average fatigue by diagnosis.
Fig. 3.

Weekly average tip usage by diagnosis.
3.3. Health outcomes
Results of the GLM revealed a range of effect sizes among health outcomes (Table 3). The PAM model showed a negligible effect size, while Self-Efficacy for Managing Symptoms (η2 = 0.04) demonstrated a small effect size. The two fatigue measures showed moderate effect sizes of reducing fatigue (MFIS: η2 = 0.06 and PROMIS Fatigue: η2 = 0.12). The PROMIS Sleep Disturbance (η2 = 0.11) and Satisfaction with Participation in Social Roles and Activities (η2 = 0.08) also showed moderate effect sizes.
Table 3.
Results of general linear models for outcomes before and after the intervention (n = 25).
| Before Intervention Mean (SD) | After Intervention Mean (SD) | Partial Eta Squared | F | p | |
|---|---|---|---|---|---|
|
| |||||
| Patient Activation Measure (PAM)-13 | 30.08 (8.57) | 30.40 (9.63) | 0.001 | 0.014 | 0.907 |
| Modified Fatigue Impact Scale (MFIS) | 32.52 (14.67) | 29.04 (12.90) | 0.060 | 1.543 | 0.230 |
| PROMIS Fatigue | 54.99 (7.18) | 52.97 (7.56) | 0.115 | 3.119 | 0.090 |
| PROMIS Satisfaction with Participation in Social Roles & Activities | 45.52 (7.46) | 48.04 (9.48) | 0.075 | 1.937 | 0.177 |
| PROMIS Self-Efficacy for Managing Symptoms | 46.38 (6.91) | 47.74 (8.84) | 0.042 | 1.064 | 0.313 |
| PROMIS Sleep Disturbance | 51.98 (10.16) | 49.88 (8.88) | 0.105 | 2.805 | 0.107 |
Note: PROMIS: Patient Reported Outcomes Measurement Information System. Baseline and post-intervention scores of above outcome measures were entered as tests of within-subjects contrasts.
3.4. Qualitative feedback
Participants’ qualitative feedback was summarized into four primary themes: 1) the relevance of the content delivered throughout the intervention, 2) the specific reasons why participants found the intervention helpful, 3) strengths of the intervention, and 4) weaknesses of the intervention. First, five participants identified the week of text messaging content dedicated to sleep as the most helpful. Four participants described the first week focusing on general fatigue education and awareness as the most valuable content. The vast majority of participants (n = 24) reported there were no other weekly tip topics they thought would have been helpful to include. When asked why they would or would not participate in a similar future intervention, fifteen participants responded that they found the experience helpful or informative in some way. Two participants described how they enjoyed being checked in on or feeling motivated by the daily text message: “It was motivating first thing in the morning; it was nice to wake up to the messages.” Finally, participants perceptions of the intervention’s strengths and weaknesses centered around repetition and simplicity. Seven participants pointed to the intervention’s simplicity and “easy to access” text messages, while five participants explained how they benefitted from the consistency and repetition of the text messages throughout the week as reinforcement of positive behavior changes. For example, one participant noted, “daily reinforced tips helped make sticking to a plan easier.” Repetitiveness, however, was also identified as a weakness of the intervention, with four participants reporting that some of the content across weeks seemed to overlap too much. Three participants expressed wanting less “vague” and more action-oriented, in-depth, and tailored content specific to their disability: “It might be helpful if some of the tips were more immediate: try this stretch, watch this quick video, answer these questions.”
4. Discussion
This study assessed the acceptability, feasibility, and participant engagement with an SMS text messaging fatigue self-management intervention and to explore the pre- and post-score changes for health outcomes in PwD, including people with MS, stroke, and SCI. Establishing the feasibility and acceptability of the intervention for PwD is an essential first step before researchers and clinicians formally examine its efficacy.27 Our intervention demonstrated both a high retention rate (93%) and overall response rate (85.17% of those who completed the protocol). This response rate exceeded our pre-defined benchmark (70%), suggesting high adherence to text messaging for intervention delivery in PwD. We also found high overall satisfaction with the intervention. Participants noted positive features of the intervention such as its simplicity, quick and easy access, and helpfulness of the tips. mHealth interventions are becoming increasingly popular,10 and studies using similar mHealth interventions have found similarly high feasibility and acceptability rates with strengths such as ease of access, convenience, and cost effectiveness.13,14,37 For example, a prior mHealth study assessing the survey response rate via SMS text messaging reported an 80% response rate by using shortened surveys.38 Our study requested that participants only provide responses on Fridays to two different questions, while the SMS messages Mondays through Thursdays were intended to be informational. Using less frequent and short surveys may be more appropriate for PwD who experience physical and cognitive limitations and for those with low health literacy.13,39
Furthermore, participants’ SMS data demonstrated temporal variability of symptoms and tips used across diagnoses (Figs. 1 and 2). The variability in fatigue levels demonstrates the dynamic nature of health outcomes. This information collected via SMS text messages would provide helpful information to clinicians in structuring their patient’s schedules. For example, clinicians can identify what time of day the patient feels least fatigued and recommend they perform important activities during that time window. Future studies may test this SMS-based activity scheduling approach as a new treatment strategy to improve self-management behaviors for PwD.
Our final aim was to examine the pre- and post-score changes following the intervention for patient activation, self-efficacy, fatigue, and other health outcomes in PwD. We found a negligible effect on patient activation. One recommendation to promote patient activation is tailoring the intervention to an individual’s needs.20,24 Though our intervention was tailored to fatigue self-management behaviors by providing content matched to the activation level of each participant, our findings did not show any changes in patient activation levels. However, a review of mHealth interventions found significant increases in physical and mental health, including reductions in anxiety, stress, and depression.37 Similar to prior studies that found improved health outcomes for interventions targeting patient activation,20,21 participants from our study reported decreased fatigue and sleep disturbances, and increased self-effiacy and social participation following the text messaging intervention. Due to the pilot nature and small sample size of this study, it is premature to draw any conclusions on the intervention effect. A future randomized controlled trial (RCT) with an appropriate comparator and a sufficient sample size is warranted to test the efficacy of an SMS text messaging intervention on patient activation and other health outcomes. Further, previous research on increasing patient activation has been conducted with telephone coaching interventions40 and clinic-based interventions,23 yet there is limited literature connecting SMS text messaging interventions and patient activation. Future research comparing other delivery methods affecting patient activation may also be warranted. Finally, patient activation is a complex construct composed of skills, knowledge, and confidence needed to manage one’s health and participate in healthcare decisions.19 The change of this complex construct may require a more multifaceted or sustained intervention approach.
Regarding our study’s limitations, our small sample size of 27 participants from one Midwestern city may limit the generalizability of our findings. We recruited individuals from previous research and an independent living center; these individuals were generally higher functioning and may not have been representative of the larger population of each diagnosis. Furthermore, we tracked the check-in text message responses on Fridays but did not track whether participants read daily self-management tips. Future studies should examine whether tracking additional information affects response rates.
5. Conclusion
Our findings showed high acceptability, feasibility, and participant engagement with an SMS text messaging fatigue self-management intervention. The study also showed the preliminary effects of the intervention to improve self-efficacy for managing symptoms, fatigue, sleep, and social participation. Future trials that include appropriate control arms and a larger sample size are needed to test the intervention efficacy. Our research team will use a study published by Freedland and colleagues to guide the selection of comparators in future RCTs.41 This project supports further study of mHealth strategies to improve health outcomes in various populations, such as individuals with MS, stroke, and SCI.
Acknowledgments
The authors would like to thank the Stephen A. Orthwein Center at Paraquad for their collaboration and Megen Devine for her assistance with editing the manuscript.
Financial support
The contents of this manuscript were developed under a grant from The Foundation for Barnes-Jewish Hospital Clinical Translational Research ICTS (Award #5562). The National Center for Medical Rehabilitation Research (K01HD095388) supported a portion of the first author’s effort for the write-up. The funders did not have a role in planning (e.g., identifying the study design) or executing (e.g., collection, analysis or interpretation of the data) the study described in this manuscript. The funders also did not have a role in writing the manuscript or deciding to submit the manuscript.
Footnotes
Declaration of competing interest
There are no conflicts of interest to disclose.
Submission declaration
The manuscript, or any part of it, has not been published and will not be submitted elsewhere for publication while being considered by your journal.
References
- 1.Barin L, Salmen A, Disanto G, et al. The disease burden of multiple sclerosis from the individual and population perspective: which symptoms matter most? Multiple sclerosis and related disorders. 2018;25:112–121. [DOI] [PubMed] [Google Scholar]
- 2.Morgan KA, Putnam M, Espin-Tello SM, et al. Aging with long-term physical disability: cohort analysis of survey sample in the US. F1000Research. 2022;11:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi-Kwon S, Kim JS. Poststroke fatigue: an emerging, critical issue in stroke medicine. Int J Stroke. 2011;6:328–336. [DOI] [PubMed] [Google Scholar]
- 4.Anton H, Miller W, Townson A, Imam B, Silverberg N, Forwell S. The course of fatigue after acute spinal cord injury. Spinal Cord. 2017;55:94–97. [DOI] [PubMed] [Google Scholar]
- 5.Amatya B, Young J, Khan F. Non-pharmacological interventions for chronic pain in multiple sclerosis. Ann Phys Rehabil Med. 2018;61:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray DB, Hollingsworth HH, Stark SL, Morgan KA. Participation survey/mobility: psychometric properties of a measure of participation for people with mobility impairments and limitations. Arch Phys Med Rehabil. 2006;87:189–197. [DOI] [PubMed] [Google Scholar]
- 7.Levine J, Greenwald BD. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Physical Medicine and Rehabilitation Clinics. 2009;20:347–361. [DOI] [PubMed] [Google Scholar]
- 8.Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Multiple sclerosis international. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su Y, Yuki M, Otsuki M. Non-pharmacological interventions for post-stroke fatigue: systematic review and network meta-analysis. J Clin Med. 2020;9:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellbeloved-Stone CA, Weppner JL, Valdez RS. A systematic review of telerehabilitation and mHealth interventions for spinal cord injury. Current Physical Medicine and Rehabilitation Reports. 2016;4:295–311. [Google Scholar]
- 11.Dicianno BE, Henderson G, Parmanto B. Design of mobile health tools to promote goal achievement in self-management tasks. JMIR mHealth and uHealth. 2017;5, e7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Publ Health. 2015;36:393–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergner EM, Nelson LA, Rothman RL, Mayberry L. Text messaging may engage and benefit adults with type 2 diabetes regardless of health literacy status. HLRP: Health Literacy Research and Practice. 2017;1:e192–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newland P, Oliver B, Newland JM, Thomas FP. Testing feasibility of a mobile application to monitor fatigue in people with multiple sclerosis. J Neurosci Nurs. 2019;51:331–334. [DOI] [PubMed] [Google Scholar]
- 15.Neff AJ, Lee Y, Metts CL, Wong AW. Ecological momentary assessment of social interactions: associations with depression, anxiety, pain, and fatigue in individuals with mild stroke. Arch Phys Med Rehabil. 2021;102:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AWK, Fong MWM, Munsell EGS, et al. Using intervention mapping and behavior change techniques to develop a digital intervention for self-management in stroke: development study. JMIR Hum Factors. 2023;10, e45099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau SC, Bhattacharjya S, Fong MW, et al. Effectiveness of theory-based digital self-management interventions for improving depression, anxiety, fatigue and self-efficacy in people with neurological disorders: a systematic review and meta-analysis. J Telemed Telecare. 2022;28:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsche RC, Williams B, Jones A, Manns P. Chronic disease self-management for individuals with stroke, multiple sclerosis and spinal cord injury. Disabil Rehabil. 2011;33:1136–1146. [DOI] [PubMed] [Google Scholar]
- 19.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosen DM, Schmittdiel J, Hibbard J, Sobel D, Remmers C, Bellows J. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manag. 2007;30:21–29. [DOI] [PubMed] [Google Scholar]
- 22.Regeer H, van Empelen P, Bilo HJ, de Koning EJ, Huisman SD. Change is possible: how increased patient activation is associated with favorable changes in well-being, self-management and health outcomes among people with type 2 diabetes mellitus: a prospective longitudinal study. Patient Educ Counsel. 2022;105:821–827. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient–physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47:1201–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff. 2013;32:207–214. [DOI] [PubMed] [Google Scholar]
- 25.Hibbard JH. Patient activation and the use of information to support informed health decisions. Patient Educ Counsel. 2017;100:5–7. [DOI] [PubMed] [Google Scholar]
- 26.Morgan KA, Wong AWK, Walker K, Desai RH, Knepper TM, Newland PK. A mobile phone text messaging intervention to manage fatigue for people with multiple sclerosis, spinal cord injury, and stroke: development and usability testing. JMIR Form Res. 2022;6, e40166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011;45:626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stepleman L, Rutter M-C, Hibbard J, Johns L, Wright D, Hughes M. Validation of the patient activation measure in a multiple sclerosis clinic sample and implications for care. Disabil Rehabil. 2010;32:1558–1567. [DOI] [PubMed] [Google Scholar]
- 30.Insignia Health - a Phreesia Company. Patient activation measure (PAM). https://www.insigniahealth.com/pam/; 2023.
- 31.Packer TL, Kephart G, Ghahari S, Audulv Å, Versnel J, Warner G. The Patient Activation Measure: a validation study in a neurological population. Qual Life Res. 2015;24:1587–1596. [DOI] [PubMed] [Google Scholar]
- 32.Attkisson CC, Zwick R. The Client Satisfaction Questionnaire: psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Progr Plann. 1982;5:233–237. [DOI] [PubMed] [Google Scholar]
- 33.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18:S79–S83. [DOI] [PubMed] [Google Scholar]
- 35.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2012;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn EA, Beaumont JL, Pilkonis PA, et al. The PROMIS satisfaction with social participation measures demonstrated responsiveness in diverse clinical populations. J Clin Epidemiol. 2016;73:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathbone AL, Prescott J. The use of mobile apps and SMS messaging as physical and mental health interventions: systematic review. J Med Internet Res. 2017;19:e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SSS, Xin X, Lee WP, et al. The feasibility of using SMS as a health survey tool: an exploratory study in patients with rheumatoid arthritis. Int J Med Inf. 2013;82:427–434. [DOI] [PubMed] [Google Scholar]
- 39.Islam SMS, Peiffer R, Chow CK, et al. Cost-effectiveness of a mobile-phone text messaging intervention on type 2 diabetes—a randomized-controlled trial. Health Policy and Technology. 2020;9:79–85. [Google Scholar]
- 40.Boyle F, Mutch A, Dean J, Dick M-L, Del Mar C. The Contribution of Consumer Health Organisations to Chronic Disease Self Management in the Context of Primary Care. 2007. [Google Scholar]
- 41.Freedland KE, King AC, Ambrosius WT, et al. The selection of comparators for randomized controlled trials of health-related behavioral interventions: recommendations of an NIH expert panel. J Clin Epidemiol. 2019;110:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


